Abstract
Background:
Variation in cancer care coordination may impact care quality and patient outcomes. We sought to characterize the impact of geographic access to and dispersion of cancer care providers on variation in care coordination.
Methods:
Using electronic health record data from 2,507 women diagnosed with breast cancer at a National Cancer Institute Comprehensive Cancer Center from April 2011 to September 2015, a breast cancer patient-sharing physician network was constructed. Patient “care networks” represent the sub-networks of physicians with whom the focal patient had a clinical encounter. Patient care networks were analyzed to generate two measures of care coordination, care density (ratio of observed vs potential connections between physicians) and clustering (extent to which physicians form connected triangles).
Results:
The breast cancer physician network included 667 physicians. On average, the physicians shared patients with 12 other physicians. Patients saw an average of 8 physicians during active treatment. In multivariable models adjusting for patient sociodemographic and clinical characteristics, we observed that greater travel burden (>2 hours) and lower geographic dispersion was associated with higher care density (p<0.05 and p<0.001, respectively) but lower care network clustering (p<0.05).
Conclusions:
Variation in network-based measures of care coordination is partially explained by patient travel burden and geographic dispersion of care.
Impact:
Improved understanding of factors driving variation in patient care networks may identify patients at risk of receiving poorly coordinated cancer care.
Keywords: breast cancer, network analysis, care coordination, travel time, rural cancer care
Introduction
Cancer care is incredibly complex and coordinated by a multidisciplinary care team that often span multiple facilities (1,2). Suboptimal care coordination is a persistent barrier to high quality cancer care and improving coordination among providers is a national priority (2). Many initiatives to improve care coordination focus on the provider team, such as establishing a multidisciplinary care team or assigning a care coordinator (3,4). Identifying patients at high risk of receiving poorly coordinated care may offer novel strategies for allocating available resources to improve care quality and patient outcomes.
Cancer patients often make multiple inpatient and outpatient visits for diagnosis and treatment (5). Adhering to a treatment plan thus poses particular challenges for patients whose geographic access to cancer care is limited, such as patients residing in rural areas (6). For example, increasing travel burden is associated with more advanced disease at diagnosis, delays in treatment initiation, a worse quality of life, and worse outcomes (5–10). Physicians may attempt to alleviate patient travel burden by referring rural patients to rural providers, but this introduces challenges in coordinating care among a team of physicians who do not typically work together. It is therefore possible that both limited geographic access to care and geographic dispersion of providers contribute independently to reduced care coordination among providers.
Care coordination can be indirectly measured using administrative data and network analysis to calculate the extent to which a set of physicians typically shares patients (11). Using this approach, the “physician network” can be represented by the set of physicians who care for a defined patient cohort and the patient-sharing relationships between them. This method assumes that physicians who frequently share patients are more likely to communicate and coordinate. “Patient care networks” represent the sub-networks of physicians with whom a focal patient had a clinical encounter (11). Care network density has been proposed as a network-based measure of care coordination, which represents the extent to which pairs of physicians in the patient care network share patients, adjusting for the total number of possible pairs (11–14). High care density has been associated with lower cost of care, fewer hospitalizations, and higher levels of some measures of care quality, yet little is known about what causes variation in care density (11,14,15).
Because breast cancer care teams require teamwork across multiple specialties, including medical oncology, (breast) surgical oncology, plastic surgery, radiation oncologists, and primary care, among others, we also consider care network transitivity in an alternative measurement of care coordination in addition to care density. We measure transitivity using the global clustering coefficient (16,17) to describe the extent to which physician’s connections are also connected to each other. Social networks tend to exhibit high levels of clustering, and in this context high levels of clustering among a group of physicians would occur when a team of at least 3 physicians frequently cares for the same patients.
We sought to characterize breast cancer patient care networks and examine the extent to which observed variation in these networks is explained by geospatial factors of patient travel burden and geographic dispersion of providers We hypothesized that teamwork among a breast cancer care team may be negatively impacted by patient travel burden and geographic dispersion of care due to increased challenges patients and physicians face in scheduling care and communicating across facilities.
Methods
Overview of data and subjects
Our study uses electronic health record (EHR) and institutional tumor registry data for breast cancer patients treated at the Norris Cotton Cancer Center, a National Cancer Institute (NCI) Comprehensive Cancer Center in northern New England. This cancer center is one of two NCI Cancer Centers located in a rural setting in the United States, and it works with 30 regional centers and affiliates to coordinate cancer care. We obtained clinical, demographic, and geographic data and records from all clinical encounters occurring within the cancer center and regional centers and affiliates for 2,507 patients with incident breast cancer who received care at the cancer center from 2011-15. This study protocol was approved by the institutional review board of Geisel School of Medicine at Dartmouth.
Study outcomes
The primary outcomes of interest of this study are network-based measures of cancer care coordination:
-
(1)
the patient care network density, which measures the number of ties between physician pairs accounting for the total number of possible pairs.
-
(2)
the patient care network global clustering coefficient, which measures the extent to which physicians in the care network form tightly connected clusters
Breast cancer physician network analysis
A breast cancer physician network was built to represent the network involved in the “active treatment” phase of care (within the 12 months following diagnosis). The network “nodes” are physicians, and two physicians are connected if they both had clinical encounters with at least two shared patients during active treatment. These connections are referred to as the network “ties”. The encounters all took place within the cancer center or one of the 30 regional centers or affiliates, and all physicians are therefore Dartmouth-affiliated. The threshold of two shared patients was used to minimize potential noise due to physician pairs with only one shared patient.
We then created patient “care networks” to represents the subset of physicians with whom a focal patient had clinical encounters within 12 months of diagnosis (Figure 1A). The ties among physicians in the patient care network are based on the care received by all breast cancer patients in the cohort. In other words, patient care networks capture how likely the set of physicians seen by the focal patient typically share patients with each other.
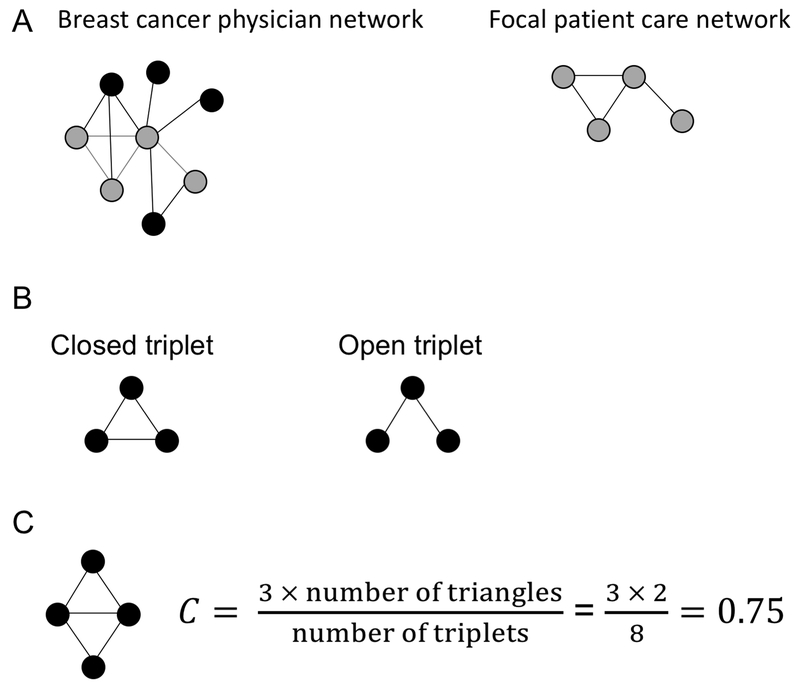
Figure 1. Illustration of network analysis.
(A) Identification of a patient care network from the full physician network. The physicians seen by the focal patient and their network ties are colored gray and extracted from the full network to form an individual patient care network (left), which are unique to each patient. (B) Illustration of a closed and open triplet. Circles represent network nodes and edges between nodes are represented by lines. (C) Calculation of global clustering coefficient.
We first calculate care density for each patient care network, D, defined as:
which is equivalent to the measure evaluated by Pollack and colleagues (11) and others (13). Density values range from 0 to 1, with 0 representing an empty network (no observed ties) and 1 representing a network where ties are observed between all possible pairs. In our calculation of density, the focal patient does not contribute to the ties among physicians in her own care network. We then consider the extent of connections among triplets of physicians for each care network. To this end, we calculated the global clustering coefficient, C, which is defined for each patient network as:
The number of closed triplets is equal to 3 × number of closed triangles (Figure 1B-C). In this study, a closed triplet would occur when three physicians all share patients with each other. Global clustering coefficients range from 0 to 1, with 0 representing a network with no observed triplets and 1 representing a network where all triplets are closed. Care density was measured for all patients with at least two physicians in her care network whereas the global clustering coefficient requires at least three physicians. Network measures were calculated using the igraph package (18) in R (19). The breast cancer physician network and the patient care networks were visualized using the Kamada-Kawai force-directed algorithm (20), which positions nodes to provide a graph with relatively uniform edge length, vertex distribution, minimum crossing of edges, avoidance of nodes lying atop one another, and symmetry.
We conducted a sensitivity analysis by reducing the timeframe of clinical encounters used to build the network from 12 to 6 months following diagnosis, hypothesizing that the shorter timeframe would also reduce the number of non-cancer based encounters. Care density and the global clustering coefficient were then calculated for the 6-month patient care networks.
Travel burden to surgical oncologist
For each patient, we calculated the travel burden to her surgical oncologist. The surgical oncologist was chosen as the geographic anchor because: (i) surgical care is the definitive treatment for breast cancer, (ii) surgical oncologists were more prevalent in patient care networks than medical or radiation oncologists, and (iii) travel burden was greatest to the surgical oncologist compared with medical or radiation oncologists or general surgeons, who practice in the regional facilities.
Travel time is calculated as the time for road-based travel between the population centroid of patient’s ZCTA tabulation area (ZCTA) of residence at the time of diagnosis and the population centroid of the ZCTA corresponding to location of the encounters with the surgical oncologist. If a surgical oncologist performs surgery at multiple locations, the location specific to the encounters with the patient was used to calculate the patient’s travel burden. Road-based travel was calculated through the ArcGIS Network Analyst module for Desktop (Environmental Systems Research Institute), that uses current road data layers, along with associated speed limits (TeleAtlas, Lebanon, NH), using the closest facility algorithm (21). The travel time categories were defined as: <30 minutes, 30 minutes to <1 hour, 1 hour to <2 hours, and > 2 hours (6).
Geospatial dispersion of cancer care
For each patient, the number of unique ZCTA in which the patient received care during the 12 months following diagnosis was calculated to measure geographic dispersion of care. The ZCTAs correspond to the clinical department associated with the patient’s clinical encounters. Due to the rural setting of care, the facility:ZCTA ratio is essentially 1:1.
Study variables
In addition to the travel time, our analyses included patient age at diagnosis (grouped as <55, 55 to <65, 65 to <75, and >75 years), marital status, primary payer type, breast cancer stage, and number of comorbidities at the time of diagnosis (grouped as 0, 1, or 2+). These variables were obtained from the institutional tumor registry. In subsequent models, we included the number of physicians in the patient’s care network and the mean number of encounters per physician. To account for whether effort was made by members of the care team to meet with the patient at one location within the same day, we also created an indicator variable, herein referred to as “multidisciplinary care”, to denote whether the patient met with two of the following specialties on the same day within two months of diagnosis: surgical oncology or general surgery, medical oncology, radiation oncology, or primary care.
Statistical analyses
We used descriptive statistics and bivariate analyses to evaluate patient characteristics by travel time category. The adjusted effects of travel burden and geographic dispersion of care on network-based coordination measures were estimated using multivariable linear regressions. The first model included patient socio-demographic characteristics as additional covariates (age, marital status, payer type), clinical characteristics (breast cancer stage, number of comorbidities) and year of diagnosis. The second model added care network size (i.e., number of physicians seen by patient), mean number of clinical encounters per physician, and the indicator for whether effort was made to coordinate care on the same day as covariates. Further, considering network properties such as density and clustering are typically correlated, we include care network density as a covariate in the second model for global clustering coefficient. This allows us to predict relationships with network clustering while adjusting for not only the size of the network but also the number of connected pairs. The estimates can be interpreted as the change in the expected value of the outcome (in this case, the care coordination measure) with a 1-unit increase in the predictor. Statistical analyses were performed in the R programming environment.
Results
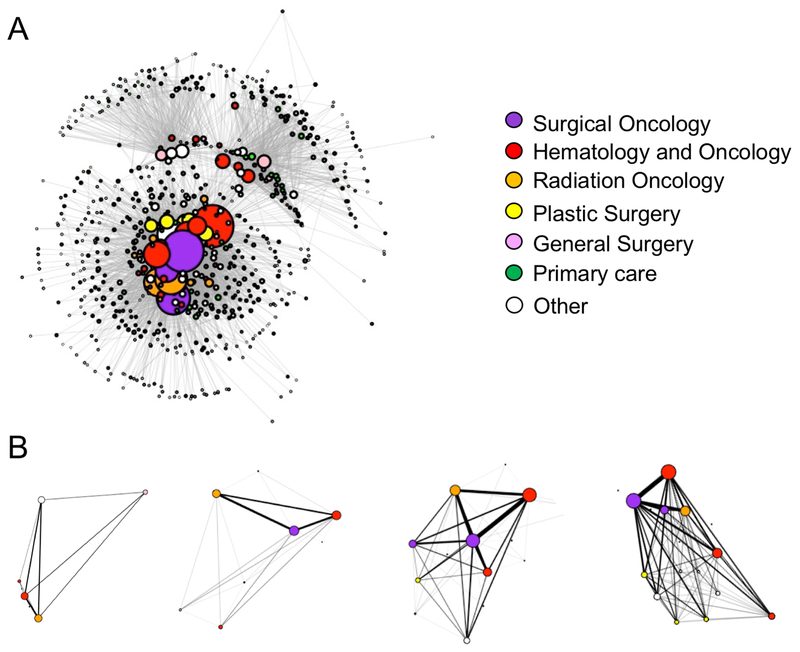
A total of 2,507 patients were diagnosed with breast cancer and treated within the healthcare system between April 2011 and September 2015. These patients were linked to the physicians with whom they had clinical encounters within 12 months of their diagnosis date. The total number of encounters was 46,026; 48% of encounters were with medical oncologists, 23% with surgeons, 8% with radiation oncologists, 8% with primary care, and 13% with other specialties. The resulting breast cancer physician network graph is illustrated in Figure 2A, with network summary statistics for both the entire physician network and the patient-determined sub-networks presented in Table 1. The breast cancer physician network includes 667 physicians. On average, physicians shared patients with 12 other physicians in the network (Table 1).
Figure 2. Visualization of the breast cancer physician network.
Each node (circle) represents a physician and the thickness between physicians (weight) represents the extent of patient-sharing occurring between them. Node size corresponds to the number of ties the physician has to others in the network. (A) The complete breast cancer physician network based on having shared patients within the first 12 months of the patient’s breast cancer diagnosis. (B) Examples of four patient care networks.
Table 1.
Summary statistics for the breast cancer physician network and patient care networks
| Network characteristic | |
|---|---|
| Breast cancer physician network, na | 667 |
| Physician degree (number of ties to other physicians), mean (sd) | 12 (28) |
| Shared patients for each physician pair, mean (sd) | 5 (12) |
| Density (number of observed ties / total number of possible ties) | 0.02 |
| Global clustering coefficient (number of observed triplets / total number of possible triplets) | 0.15 |
| Patient care networksb, mean (sd) | |
| Size (number of physicians) | 8 (6) |
| Density | 0.72 (0.24) |
| Global clustering coefficient | 0.69 (0.27) |
Breast cancer physician network based on all physicians who shared at least two breast cancer patients with another physician.
Patient care network represents the sub-network of the breast cancer physician network including only those physicians who had a clinical encounter with the focal patient.
Patient care networks were identified for each patient. On average, the patients had at least one clinical encounter with 8 different physicians in the 12 months following a breast cancer diagnosis. Patient care networks varied in size (number of physicians) and the extent of connections among the physicians. Examples of four patient care networks are presented in Figure 2B. Patient care network size is inversely correlated with care density (Spearman’s rho = −0.77) and global clustering coefficient (rho = −0.45).
Travel time to a surgical oncologist was measurable for 1,112 patients. Reasons for missing patient travel time data include not having any clinical encounters with a surgical oncologist (n=1,314) and a residential ZCTA outside of New Hampshire or Vermont (n=81). When assessed as a continuous variable, the mean travel time to surgery was 63 minutes with a standard deviation of 34 minutes. Geographic dispersion, measured as the number of ZCTAs corresponding to patient encounters during active treatment, ranged from 1 to 5 with a mean of 1.5 and a standard deviation of 0.73. Patient clinical and sociodemographic characteristics by travel time category are described in Table 2. Patients did not differ by age, marital status, breast cancer stage, or year of diagnosis by travel time category. Patients with the greatest travel times were significantly more likely to have Medicare as their primary payer, have fewer comorbidities, see fewer physicians, and have fewer encounters per physician (Table 2).
Table 2.
Descriptive characteristics of women with breast cancer treated at the NCI Comprehensive Cancer Center, 2011-15.
| Characteristic | Travel time category | ||||
|---|---|---|---|---|---|
| <30 min | 30 - <60 min | 60 - <120 min | 120+ min | p-valueψ | |
| Patients, n | 242 | 280 | 524 | 66 | |
| Age at diagnosis, mean (sd) | 61 (13) | 61 (13) | 59 (11) | 62 (14) | 0.31 |
| Marital status, n (%) | |||||
| Married | 153 (63) | 183 (65) | 342 (65) | 34 (52) | 0.27 |
| Unmarried/unknown | 89 (37) | 97 (35) | 182 (34) | 32 (48) | |
| Payer type | |||||
| Private insurance | 69 (29) | 56 (20) | 145 (28) | 17 (26) | 0.02 |
| Medicare | 91 (38) | 112 (40) | 170 (32) | 32 (48) | |
| Other/unknown | 82 (34) | 112 (40) | 209 (40) | 17 (26) | |
| Stage, n (%) | |||||
| 0 | 43 (18) | 61 (22) | 90 (17) | 11 (17) | 0.20 |
| I | 99 (41) | 108 (39) | 234 (45) | 28 (42) | |
| II | 65 (27) | 69 (25) | 115 (22) | 17 (26) | |
| III | 16 (7) | 26 (9) | 41 (8) | n.r. | |
| IV | n.r. | n.r. | n.r. | n.r. | |
| Missing | n.r. | n.r. | n.r. | n.r. | |
| Comorbidities, n (%) | |||||
| 0 | 78 (32) | 111 (40) | 331 (63) | 39 (59) | <0.01 |
| 1 | 15 (6) | 37 (13) | 48 (9) | n.r. | |
| 2+ | 149 (62) | 132 (47) | 145 (28) | n.r. | |
| Year of diagnosis, n (%) | |||||
| 2011 | 33 (14) | 44 (16) | 83 (16) | 17 (26) | 0.16 |
| 2012 | 51 (21) | 59 (21) | 123 (23) | n.r. | |
| 2013 | 67 (28) | 69 (25) | 125 (24) | 17 (26) | |
| 2014 | 55 (23) | 54 (19) | 123 (23) | 17 (26) | |
| 2015 | 36 (15) | 54 (19) | 70 (13) | n.r. | |
| Multidisciplinary care, n (%) | 70 (26) | 71 (26) | 82 (17) | 16 (28) | 0.12 |
| Number of physicians seen, mean (sd) | 13 (7) | 11 (7) | 7 (5) | 5 (3) | <0.01 |
| Encounters per physician, mean (sd) | 42 (39) | 31 (32) | 18 (20) | 13 (15) | <0.01 |
| Number of encounter ZCTAs, mean (sd) | 1.4 (0.5) | 1.3 (0.5) | 1.3 (0.6) | 1.7 (0.7) | 0.27 |
n.r. = Not reported due to small sample size to protect patient confidentiality.
One-way ANOVA tests and Pearson Chi-squared tests. Percentages may not add up to 100 due to rounding.
The adjusted estimated effects of travel burden and geographic dispersion on network-based care coordination measures are presented in Table 3. Adjusting only for patient sociodemographic and clinical characteristics, we observed that higher levels of care density was observed for patients with greater travel burden (p<0.001) and lower geographic dispersion of care (p<0.001) (Table 3). These associations were consistent when analyzing patient care network clustering. (Table 3).
Table 3.
Adjusted estimated effects of study variables on network-based care coordination measures.
| Care network density N = 1,112 Mean = 0.72 SD = 0.24 Estimate (std. err.) |
Global clustering coefficient N = 951 Mean = 0.70 SD = 0.27 Estimate (std. err.) |
|||
|---|---|---|---|---|
| Variable | Model 1 | Model 2 | Model 1 | Model 2 |
| Travel time category | ||||
| <30 min | referent | referent | referent | referent |
| 30 - <60 min | 0.03 (0.02)* | −0.0003 (0.01) | 0.02 (0.02) | 0.001 (0.01) |
| 60 - <120 min | 0.11 (0.02)** | 0.01 (0.01) | 0.04 (0.02)* | −0.01 (0.01) |
| 120+ min | 0.23 (0.03)** | 0.06 (0.02)* | 0.10 (0.03)* | −0.06 (0.02)* |
| Geographic dispersion | −0.09 (0.01)** | −0.05 (0.01)** | −0.05 (0.01)** | 0.02 (0.01)* |
| Age at diagnosis | ||||
| <55 | referent | referent | referent | referent |
| 55 – 64 | −0.02 (0.02) | −0.02 (0.01)* | −0.03 (0.02) | 0.01 (0.01) |
| 65 – 74 | −0.04 (0.03) | −0.04 (0.02)* | −0.04 (0.03) | 0.01 (0.02) |
| 75+ | −0.05 (0.03) | −0.06 (0.02)* | −0.08 (0.03)* | 0.01(0.02) |
| Marital status | ||||
| Married | referent | referent | referent | referent |
| Unmarried | −0.01 (0.01) | −0.004 (0.01) | −0.01 (0.01) | 0.003 (0.01) |
| Unknown | −0.09 (0.06) | −0.06 (0.04) | −0.01 (0.06) | 0.08 (0.04) |
| Payer type | ||||
| Medicare | referent | referent | referent | referent |
| Private insurance | −0.01 (0.03) | 0.005 (0.02) | 0.01 (0.03) | 0.02 (0.02) |
| Other/unknown | −0.04 (0.03) | −0.02 (0.02) | −0.02 (0.03) | 0.02 (0.02) |
| Stage | ||||
| 0 | referent | referent | referent | referent |
| I | 0.05 (0.02)* | 0.04 (0.01)** | 0.12 (0.02)** | 0.04 (0.01)* |
| II | 0.03 (0.02) | 0.05 (0.01)* | 0.11 (0.02)** | 0.03(0.01)* |
| III | 0.04 (0.02) | 0.08 (0.02)** | 0.14 (0.03)** | 0.02 (0.02) |
| IV | −0.04 (0.04) | 0.02 (0.03) | 0.06 (0.05) | 0.04 (0.03) |
| Comorbidities | ||||
| 0 | referent | referent | referent | referent |
| 1 | −0.02 (0.02) | −0.001 (0.02) | −0.02 (0.02) | −0.001 (0.01) |
| 2+ | −0.13 (0.01)** | −0.05 (0.01)** | −0.12 (0.02)** | −0.02 (0.01)* |
| Year of diagnosis | ||||
| 2011 | referent | referent | referent | referent |
| 2012 | 0.05 (0.02) | 0.02 (0.01) | 0.03 (0.02) | −0.01 (0.01) |
| 2013 | 0.09 (0.02)** | 0.02 (0.01) | 0.05 (0.02)* | −0.01 (0.01) |
| 2014 | 0.10 (0.02)** | 0.03 (0.01) | 0.06 (0.02)* | −0.02 (0.01) |
| 2015 | 0.18 (0.02)** | 0.02 (0.02) | 0.09 (0.02)** | −0.02 (0.02) |
| Multidisciplinary care | NA | 0.03 (0.01)* | NA | 0.02 (0.01) |
| Number of physicians | NA | −0.02 (0.001)** | NA | 0.01 (0.001)** |
| Encounters per physician | NA | 0.01 (0.002)** | NA | 0.003 (0.002) |
| Care density | NA | NA | NA | 1.05 (0.03)** |
p < 0.05.
p < 0.001.
Model 1 is adjusted for patient clinical and sociodemographic characteristics and Model 2 includes number of physicians, mean encounters per physician, care network density (if applicable), and an indicator for multidisciplinary care (whether the patient saw two physicians on the same day within 2 months of diagnosis) as additional covariates. The size of the effect can be gauged by comparing the estimate with the outcome’s standard deviation shown at the top. SD = standard deviation.
After adding number of physicians seen, mean number of encounters per physician, and an indicator for multidisciplinary care as additional covariates, we observed that higher care density remained significantly associated with greater travel burden (>2 hours, p<0.05) and lower geographic dispersion of care (p<0.001) (Table 3). Conversely, in the fully adjusted model, care network clustering showed the opposite trend: lower levels of care network clustering were observed for patients with greater travel burden (>2 hours, p<0.05) and lower geographic dispersion (p<0.05) (Table 3).
Other study variables associated with increased levels of care density were younger age, breast cancer stage, fewer comorbidities, receiving multidisciplinary care, seeing fewer physicians, and having more encounters per physician. Other study variables associated with increased levels of care network clustering were breast cancer stage, fewer comorbidities, seeing more physicians, and care density (Table 3).
In sensitivity analyses, we examined whether the estimates were robust to variation in the definition of active cancer treatment by comparing the 12 month post-diagnosis care networks to care networks based on clinical encounters within 6 months of diagnosis. The patient care network density and global clustering coefficients were highly consistent between these two approaches (rho = 0.82 and 0.78, respectively). The adjusted estimated effects of travel time and other covariates on care density and global clustering coefficient using the 6-month care networks were found to be similar (Supplemental Table 1).
Discussion
Identifying patients at higher risk of receiving less coordinated care may help guide initiatives and allocate resources to improve care quality and patient outcomes. In this study, we have examined the extent to which two network-based measures of cancer care coordination, density and global clustering coefficient, are impacted by geospatial variation in a largely rural population.
Our results indicate that patients with the greatest travel burden to specialized services (>2 hours travel time) have higher care density compared with patients with the lowest travel burden. This was contrary to our hypothesis and may be uncovering established referral pathways between provider pairs that serve patients with the greatest travel burden. On the other hand, we did find that patients with the greatest travel burden had lower levels of care network clustering, suggesting that these patients may be less likely to receive care from a cancer care team (including at least three physicians) that typically share patients.
We also observed that patients with greater geographic dispersion of care had lower care density, which was consistent with our hypothesis. Care networks spanning multiple facilities may face additional barriers to sharing information across all relevant providers. These results suggest that patients may consider receiving care within the same facility to consolidate the location of their care, especially for patients with reduced travel burden. Overall, these factors account for only a portion of the variation in care network structure. Future work identifying additional factors driving variation in patient care networks will be important for developing innovative approaches to improve cancer care coordination.
Our study is subject to several limitations: First, the electronic health record data analyzed in this study is limited to clinical encounters that occurred within the cancer center or one of the 30 regional centers and affiliates, so we cannot capture visits with providers outside of this system. Second, although patient-sharing ties have been demonstrated to associate with referral and advice seeking relationships (23), network-based measures of patient-sharing among physicians are not capable of fully capturing all aspects of care coordination. A better understanding of whether network-based measures of coordination correlate with patient-reported measures of care coordination will improve upon interpretation of studies using this approach. For instance, an alternative implication of small, tightly knit physician networks is that the care providers are insular which may create access barriers to optimal care due to narrow referral networks (22). Third, this study was also unable to address whether the importance of coordinating care between different combinations of specialties varies according to the health care needs of individual patients. A patient with a very severe condition or multiple comorbidities may require a larger or more diverse network of physicians who are otherwise unlikely to be connected. However, this may still be preferable to small care teams that appear to have high levels of coordination but are missing key specialists, such as primary care or specialists involved in other chronic conditions. Fourth, our results cannot be interpreted as causal. Travel burden and geographic dispersion may indirectly impact care coordination through care utilization, which cannot be addressed in this study. Fifth, our results represent a single site and may not be generalizable to other NCI Cancer Centers or other rural cancer care delivery systems. Finally, our analyses are specific to one phase of care and do not address the significant challenges of coordinating across phases of care (e.g., active treatment to disease management/survivorship).
In conclusion, our findings suggest cancer care coordination during active treatment, measured indirectly using administrative data and network analysis, is in part explained by patient travel burden and geographic dispersion of care. Future work using this approach to evaluate breast cancer care coordination for a larger patient cohort and across phases of care is warranted.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (NIH) National Institute of General Medical Sciences award P30CA023108 (E. Moen), NIH National Cancer Institute award R21CA212687 (T. Onega), and NIH National Institute on Aging award P01AG019783 (E. Moen and J. O’Malley).
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. National Academies Press; 2015;:1–413. [PubMed] [Google Scholar]
- 2.Ganz PA. Institute of Medicine report on delivery of high-quality cancer care. Journal of Oncology Practice. American Society of Clinical Oncology; Alexandria, VA; 2014;10:193–5. [DOI] [PubMed] [Google Scholar]
- 3.WALSH J, YOUNG JM, HARRISON JD, BUTOW PN, SOLOMON MJ, MASYA L, et al. What is important in cancer care coordination? A qualitative investigation. European Journal of Cancer Care. Blackwell Publishing Ltd; 2011;20:220–7. [DOI] [PubMed] [Google Scholar]
- 4.Walsh J, Harrison JD, Young JM, Butow PN, Solomon MJ, Masya L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Services Research. BioMed Central; 2010;10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. Oncologist. AlphaMed Press; 2015;20:1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer. Wiley Subscription Services, Inc., A Wiley Company; 2008;112:909–18. [DOI] [PubMed] [Google Scholar]
- 7.Schroen AT, Brenin DR, Kelly MD, Knaus WA, Slingluff CL. Impact of patient distance to radiation therapy on mastectomy use in early-stage breast cancer patients. Journal of Clinical Oncology. 2005;23:7074–80. [DOI] [PubMed] [Google Scholar]
- 8.Celaya MO, Rees JR, Gibson JJ, Riddle BL, Greenberg ER. Travel distance and season of diagnosis affect treatment choices for women with early-stage breast cancer in a predominantly rural population (United States). Cancer Causes Control. 2006;17:851–6. [DOI] [PubMed] [Google Scholar]
- 9.Gumpertz ML, Pickle LW, Miller BA, Bell BS. Geographic patterns of advanced breast cancer in Los Angeles: associations with biological and sociodemographic factors (United States). Cancer Causes Control. Third Kluwer Academic Publishers; 2006;17:325–39. [DOI] [PubMed] [Google Scholar]
- 10.Lin CC, Bruinooge SS, Kirkwood MK, Olsen C, Jemal A, Bajorin D, et al. Association Between Geographic Access to Cancer Care, Insurance, and Receipt of Chemotherapy: Geographic Distribution of Oncologists and Travel Distance. J Clin Oncol 2015;33:3177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack CE, Weissman GE, Lemke KW, Hussey PS, Weiner JP. Patient Sharing Among Physicians and Costs of Care: A Network Analytic Approach to Care Coordination Using Claims Data. Journal of General Internal Medicine. Springer-Verlag; 2012;28:459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack CE, Weissman G, Bekelman J, Liao K, Armstrong K. Physician Social Networks and Variation in Prostate Cancer Treatment in Three Cities. Health Services Research. 2011;47:380–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandl KD, Olson KL, Mines D, Liu C, Tian F. Provider collaboration: cohesion, constellations, and shared patients. Journal of General Internal Medicine. Springer US; 2014;29:1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong M-S, Olson KL, Chadwick L, Liu C, Mandl KD. The Impact of Provider Networks on the Co-Prescriptions of Interacting Drugs: A Claims-Based Analysis. Drug Saf Springer International Publishing; 2016;:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollack CE, Frick KD, Herbert RJ, Blackford AL, Neville BA, Wolff AC, et al. It’s who you know: patient-sharing, quality, and costs of cancer survivorship care. Journal of Cancer Survivorship. Springer US; 2014;8:156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman ME, Strogatz SH, Watts DJ. Random graphs with arbitrary degree distributions and their applications. Phys Rev E Stat Nonlin Soft Matter Phys American Physical Society; 2001;64:026118. [DOI] [PubMed] [Google Scholar]
- 17.Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature. Nature Publishing Group; 1998;393:440–2. [DOI] [PubMed] [Google Scholar]
- 18.Csárdi G, Nepusz T. The igraph software package for complex network research. Interjournal. Complex Systems:1695. [Google Scholar]
- 19.R Development Core Team. R: A language and environment for statistical computing. http://www.R-project.org Vienna, Austria; 2008. [Google Scholar]
- 20.Kamada T, Kawai S. An algorithm for drawing general undirected graphs. Information Processing Letters. Elsevier; 1989;31:7–15. [Google Scholar]
- 21.Birkmeyer JD. Regionalization of High-Risk Surgery and Implications for Patient Travel Times. JAMA. 2003;290:2703. [DOI] [PubMed] [Google Scholar]
- 22.Ghomrawi HMK, Funk RJ, Parks ML, Owen-Smith J, Hollingsworth JM. Physician referral patterns and racial disparities in total hip replacement: A network analysis approach. Uddin S, editor. PLoS ONE. Public Library of Science; 2018;13:e0193014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnett ML, Landon BE, O’Malley AJ, Keating NL, Christakis NA. Mapping Physician Networks with Self-Reported and Administrative Data. Health Services Research. 2011;46:1592–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.