Abstract
Background
Recurrent preschool wheezing is a heterogeneous disorder with significant morbidity, yet little is known about phenotypic determinants and their impact on clinical outcomes.
Objective
Latent class analysis (LCA) was used to identify latent classes of recurrent preschool wheeze and their association with future exacerbations and inhaled corticosteroid (ICS) treatment response.
Methods
Data from five clinical trials of 1,708 children age 12–71 months with recurrent wheezing were merged. LCA was performed on 10 demographic, exposure and sensitization variables to determine the optimal number of latent classes. The primary outcome was the annualized rate of wheezing exacerbations requiring systemic corticosteroids during the study intervention period; the secondary outcome was the time to first exacerbation. Exploratory analyses examined the effect of daily ICS treatment on exacerbation outcomes.
Results
Four latent classes of recurrent wheezing were identified; these were not distinguished by current symptoms or historical exacerbations but differed with regard to allergen sensitization and/or exposures. Annualized exacerbation rates (mean ± SEM/year) were 0.65 ± 0.06 for class 1 (“minimal sensitization”), 0.93 ± 0.10 for class 2 (“sensitization with indoor pet exposure”), 0.60 ± 0.07 for class 3 (“sensitization with tobacco smoke exposure”), and 0.81 ± 0.10 for class 4 (“multiple sensitization and eczema”) (p < 0.001). In a research setting of high adherence, daily ICS treatment improved exacerbation rates in classes 2 and 4 but not the other groups.
Conclusion
Sensitization and exposure assessments are useful in the prediction of future exacerbation and may identify children most likely to respond favorably to daily ICS treatment.
Keywords: Asthma in children, Asthma exacerbation, Wheeze, Preschool child, Phenotype, Inhaled corticosteroid, Sensitization, Latent class analysis, Type 2 inflammation
Introduction
Wheezing is a troubling symptom in preschool children that has tripled in prevalence over the past 30 years.1 At present, nearly 50% of all preschool children experience one episode of wheezing before 6 years of age; up to 40% of these children have recurrent wheezing episodes during early life.2 Although there is variability among affected children with regard to wheezing pathobiology3–6 and the severity, frequency and persistence of wheezing in later childhood,7-14 all children with recurrent wheezing experience morbidity. Compared to older children with persistent asthma, preschool children with recurrent wheezing have nearly twice the rate of outpatient physician visits and emergency department visits for wheezing exacerbations and more than five times the rate of hospitalization.15 Missed days from school or work16 and impaired caregiver functional status17 are also significant concerns that drive the growing economic burden of wheezing in preschool children, which was estimated at nearly $3 billion in 2013.18
Although there are mandates for “personalized” treatment approaches for these young children to reduce respiratory morbidity,19 progress has been slow. Knowledge from older children cannot be easily extrapolated to younger children and thus the evidence base for pharmacotherapy in preschool children with recurrent wheezing is quite limited.18, 20 Furthemore, although it is recognized that preschool children with recurrent wheezing are a heterogeneous group,3, 5, 6, 21 phenotypic characterizations of preschool children are quite limited in comparison to adults and there are few existing longitudinal studies of preschool children to aid in prediction of those children at highest risk for poor outcomes such as exacerbation.22, 23 Historical inconsistencies in the definition of “exacerbation”24 and variable prescription of (and adherence to) asthma controller medications such as inhaled corticosteroids (ICS) and leukotriene receptor antagonists (LTRA) also complicate assessment of longitudinal outcomes. Early identification of preschool children with recurrent wheezing who are at high risk for poor outcomes (i.e., exacerbations) is therefore one of the primary challenges faced by those who provide care to these children. As a result, the clinical course of preschool children with recurrent wheezing remains an enigma that is difficult to predict; there is also limited evidence to guide pharmacotherapy18, 20 and a sizeable knowledge gap.23, 25
Given these challenges, we applied latent class analysis (LCA) to a large dataset of well-characterized and medication adherent preschool children with recurrent wheeze enrolled in multi-center clinical trials sponsored by the National Heart, Lung and Blood Institute’s AsthmaNet and Childhood Asthma Research and Education (CARE) Network. LCA is a statistical method that is useful for identification of unobservable “class” memberships among participants with multivariate categorical data. The purposes of this study were to: 1) identify latent classes of preschool wheeze, and 2) determine the clinical relevance of the resultant latent classes in the prediction of future exacerbations and response to ICS therapy. We hypothesized that latent classes with Type-2 inflammatory features would have the highest exacerbation probability and the greatest response to ICS treatment.
Methods
Baseline and intervention period data from 3 CARE Network clinical trials and 2 AsthmaNet clinical trials involving 1,708 preschool participants ages 12–71 months with recurrent wheezing were merged. All studies were overseen by a common Quality Control Committee and Data Coordinating Center (Pennsylvania State University) and utilized similar intake questionnaires. Paper case report forms were entered electronically and mailed to the Data Coordinating Center for review and accuracy upon completion.
Details of the included studies (i.e., Prevention of Early Asthma in Kids (PEAK, NCT00272441),26 Acute Intermittent Management Strategies (AIMS, NCT00319488),27 Maintenance and Intermittent Inhaled Corticosteroids in Wheezing Toddlers (MIST, NCT00675584),28 Azithromycin for Preventing the Development of Upper Respiratory Tract Illnesses into Lower Respiratory Tract Symptoms (APRIL, NCT01272635),29 and Individualized Therapy for Asthma in Toddlers (INFANT, NCT01606306))30 were published previously and are listed in Table 1. Exclusion criteria for each of the studies included premature birth, other significant respiratory conditions, gastroesophageal reflux, recent antibiotic or systemic corticosteroid use within the previous 2–4 weeks, or a life-threatening wheezing episode. Written informed consent was obtained from all caregivers.
Table 1.
Features of the included studies.
| Study feature | PEAK | AIMS | MIST | APRIL | INFANT |
|---|---|---|---|---|---|
| Years conducted | 2001–2005 | 2004 | 2008–2010 | 2011–2015 | 2013–2015 |
| Participants enrolled | 285 | 238 | 278 | 607 | 300 |
| Age of participants | 24–48 months | 12–59 months | 12–53 months | 12–71 months | 12–59 months |
| Positive Modified Asthma Predictive Index1 required | Yes | No | Yes | No | No |
| Additional requirements in the past year | None | ≥2 clinically significant wheezing exacerbations2 | ≥1 clinically significant wheezing exacerbation2 | ≥2 clinically significant wheezing exacerbations2 | Uncontrolled asthma3 |
| Study design | Parallel arm | Parallel arm | Parallel arm | Parallel arm | Cross-over |
| Run-in period | 4 weeks | 2 weeks | 2 weeks | 2–4 weeks | 2–8 weeks |
| Run-in medication4 | Placebo | No medication | Placebo | No medication | Placebo or openlabel ICS or LTRA |
| Treatment arm duration | 104 weeks | 52 weeks | 52 weeks | 52–78 weeks | 16 weeks |
| Treatment arm interventions | Daily ICS Placebo | Intermittent ICS5 Intermittent LTRA5 Placebo | Daily ICS Intermittent ICS5 | Azithromycin5 Placebo | Daily ICS Daily LTRA As-needed ICS |
Defined as frequent wheezing (at least 4 episodes in the previous year) and either 1 major risk factor (parental history of asthma, personal history of atopic dermatitis, or aeroallergen sensitization) or 2 of 3 minor risk factors (peripheral blood eosinophilia ≥4%, 527 wheezing without colds, or allergic sensitization to foods)
Defined as a wheezing episode necessitating an urgent care visit, hospitalization, or systemic corticosteroids
Defined as symptoms >2 days per week (previous 2 weeks), nighttime awakening from asthma at least once (previous 4 weeks), ≥4 wheezing episodes within the past year, or ≥2 exacerbations requiring systemic corticosteroids in the preceding 6 months.
Open-label albuterol sulfate was permitted during the run-in for each study
Administered only during respiratory tract illnesses
Participant characterization.
Each clinical center maintained staff and site certification and utilized the same manual of procedures for participant characterization. At the baseline visit of each trial, caregivers completed questionnaires to elicit data on demographics, family history, child allergy and respiratory symptoms, and treatment of symptoms including medications and healthcare utilization. Episode-free days (EFDs), also referred to as Asthma Control Days in some studies, were obtained during the run-in period from caregiver-completed diaries and were defined as full calendar days without use of albuterol, daytime or nighttime respiratory symptoms, or unscheduled healthcare visits for respiratory symptoms. Compliance with the diaries was used to estimate adherence and willingness to participate in the study; participants with unacceptable adherence (<75–80%) were ineligible for randomization.
Peripheral blood eosinophils were quantified from whole blood by means of an automated assay at each clinical site. Total serum IgE was quantified centrally (St. Louis Children’s Hospital, St. Louis, Mo; Advanced Diagnostic Laboratories, National Jewish Health, Denver, CO). Skin testing (PEAK, AIMS, MIST trials) to 8 common aeroallergens (house dust mite mixture [Dermatophagoides pteronyssinus and Dermatophagoides farinae], cockroach [American and German], dog [mixed breeds], cat [standardized], mold [mix no.1], grass [standardized Southern mix]. Tree [eastern 8 tree mix], and weed [national mix] and 3 foods [cow’s milk, chicken and whole egg, and peanut; Greer Laboratories, Lenoir, NC) was performed using the Multi-test II (Lincoln Diagnostics, Decatur, IL) prick technique. Tests were considered positive if the prick resulted in a wheal with a mean diameter (mean of maximum and 90° midpoint diameters) that was at least 3 mm greater than that produced by the saline control.31, 32 Specific IgE levels (ImmunoCAP; APRIL, INFANT) were performed for a nationally representative panel of 11 aeroallergens (cat dander [ImmunoCAP test code E1], dog dander [E5], mold mix [Mx1], German cockroach [i6], grass mix [gx2], tree mix [Tx4, Tx6], (9) weed mix [Wx1], giant ragweed [W3], Dermatophagoides pteronyssinus [D2], Dermatophagoides farinae [D2]) and 3 foods (milk [f2], egg, [f1], peanut [f13]) at a central laboratory (Advanced Diagnostic Laboratories, National Jewish Health, Denver, CO). Tests with levels >0.34 IU/mL were considered positive.
Phenotype analyses.
All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC). Data were used from the total sample of 1,708 participants at the baseline and randomization visits. Blood eosinophil and IgE data were missing in <2% of participants; these data were considered missing completely at random and therefore multiple imputation was performed to retain these participants in the analyses. Other self-reported variables with missing responses (<0.1% of responses) or responses recorded as “don’t know” (<3% of responses) were recoded as “no.”
To limit the number of parameters in the model, variables were selected based on clinical relevance and consistency across the five studies. LCA was performed using the PROC LCA procedure33 (SAS software, version 9.4, SAS Institute, Cary, NC) on 10 variables to determine the optimal number of latent classes, including five dichotomous variables and five categorical variables. Dichotomous variables included: 1) sex, 2) parent with asthma (ever in lifetime), 3) tobacco smoke exposure (defined as any smoker in any household in which the participant regularly spends time), 4) eczema (ever), and 5) indoor pet ownership (defined as a cat or dog inside the home). Categorical variables included: 1) race/ethnicity (non-Hispanic black/non-Hispanic white/Hispanic/other), 2) aeroallergen sensitization (none, 1–2 positive tests, 3 or more positive tests), 3) food sensitization (none, 1–2 positive tests, 3 positive tests), 4) blood eosinophil percentage quartile, and 5) serum IgE quartile. Conditional probabilities (i.e., the probability of selected characteristics within a class) and posterior probabilities (i.e., the probability of latent class membership for each participant) were calculated. Wheeze models of 1 to 10 latent classes were repeatedly fitted with the number of latent classes in a stepwise fashion. Models were freely estimated and no parameter restrictions were specified. Best fit was assessed with comparison of the bootstrapped p-values for the likelihood ratio test and the Bayesian information criterion (BIC) test. Each participant was assigned to the phenotype with the highest membership probability.
Outcomes.
The primary outcome was the annualized rate of wheezing exacerbations during the study intervention period; the secondary outcome was the time to first exacerbation. The definition of exacerbation was consistent with that proposed by a National Institutes of Health Working Group24 and was defined as respiratory symptoms resulting in treatment with systemic corticosteroids (prednisolone). Exploratory outcomes focused on the effect of ICS treatment on the exacerbation rate and time to first exacerbation within the phenotype groups.
Intervention period data were collected over a 14-year period (PEAK 2001–2004; AIMS 2004; MIST 2008–2010; APRIL 2011–2015; INFANT 2013–15). For each study, irrespective of treatment allocation, caregivers received a written action plan that detailed instructions for administration of open-label albuterol sulfate (90 mcg/actuation) when a pre-specified threshold of symptoms was met. The action plan was reviewed and reinforced at each clinic visit. Children whose symptoms did not resolve or who required albuterol treatments for more than 24 hours received a 4-day burst of open-label oral prednisolone (2 mg/kg/day for 2 days followed by 1 mg/kg/day for 2 days) as specified in the action plan. Physician discretion for prednisolone administration was also permitted provided that a specific reason for the initiation was documented. Two courses of systemic corticosteroids had to be separated by at least one week to count as two exacerbations.
Outcome analyses.
The annualized rate of exacerbations (primary outcome) and the time to first exacerbation (secondary outcome) were assessed in the placebo arms of the PEAK, AIMS, and APRIL studies (N = 489). Phenotype groups were compared with respect to the frequency of exacerbations using a log-linear model with a negative binomial distribution and an offset for each participant of time followed in the study.34 Proportional-hazards regression models were used to analyze time to first exacerbation. Exploratory analyses focused on daily ICS treatment effects in placebo and ICS-treated participants in the PEAK trial. Generalized linear models were used to compare the rate of exacerbations between ICS and placebo treatment arms within each phenotype group and proportional-hazards regression models were used to analyze time to first exacerbation. All analyses utilized a 0.05 significance level without adjustment for multiple testing.
Results
The sample used for phenotype identification consisted of 1,708 children with recurrent wheeze (mean age 33.8 months, 62.5% male). Features of the combined sample, with stratification by study, are shown in Table E1. Overall, the combined sample was quite heterogeneous with regard to healthcare utilization, exposures, and sensitization patterns. Respiratory symptoms and associated albuterol use during the run-in periods were also variable.
Table E1.
Features of the participants in the included studies. Data represent the number of participants (%), the mean ± SD, or the median (35th, 75th percentile). EFDs = episode free days.
| Feature | PEAK N = 285 | AIMS N = 238 | MIST N = 278 | APRIL N = 607 | INFANT N = 300 |
|---|---|---|---|---|---|
| Age at enrollment (months) | 36.0 ± 7.0 | 29.5 ± 12.8 | 34.9 ± 11.2 | 41.5 ± 16.5 | 39.9 ± 13.2 |
| Males | 177 (62.1) | 154 (64.7) | 192 (69.1) | 365 (60.1) | 179 (59.7) |
| Race/ethnicity | |||||
| Non-Hispanic White | 152 (53.3) | 133 (55.9) | 113 (40.6) | 215 (35.4) | 96 (32.0) |
| Non-Hispanic Black | 38 (13.3) | 24 (10.1) | 39 (14.0) | 142 (23.4) | 93 (31.0) |
| Hispanic | 55 (19.3) | 59 (24.8) | 84 (30.2) | 183 (30.1) | 72 (24.0) |
| Other | 40 (14.0) | 22 (9.2) | 42 (15.1) | 67 (11.0) | 39 (13.0) |
| Current tobacco smoke exposure | 111 (38.9) | 24 (10.1) | 122 (43.9) | 240 (39.5) | 110 (36.7) |
| Current cat or dog in the home | 129 (45.3) | 107 (45.0) | 129 (46.4) | 280 (46.1) | 139 (46.3) |
| Emergency department or urgent care visit (past year) | 133 (46.7) | 96 (40.3) | 170 (61.2) | 582 (95.9) | 261 (87.0) |
| Hospitalization (past year) | 20 (7.0) | 19 (8.0) | 53 (19.1) | 82 (13.5) | 65 (21.7) |
| Eczema (ever) | 148 (51.9) | 89 (37.4) | 146 (52.5) | 328 (54.0) | 160 (53.3) |
| Parent with asthma (ever) | 184 (64.6) | 106 (44.5) | 171 (61.5) | 314 (51.7) | 178 (59.3) |
| Blood eosinophils (%)1 | 3.2 (2.0, 5.6) | 3.5 (2.0, 5.6) | 3.1 (2.0, 6.0) | 3.0 (2.0, 5.6) | 3.5 (2.0, 6.0) |
| Quartile 1 | 93 (33.3) | 69 (30.0) | 96 (36.9) | 194 (34.2) | 76 (29.3) |
| Quartile 2 | 44 (15.8) | 43 (18.7) | 35 (13.5) | 112 (19.7) | 43 (16.6) |
| Quartile 3 | 86 (30.8) | 68 (29.6) | 70 (26.9) | 150 (26.4) | 80 (30.9) |
| Quartile 4 | 56 (20.1) | 50 (21.7) | 59 (22.7) | 112 (19.7) | 60 (23.2) |
| Total serum IgE (IU/mL) | 44.0 (14.0, 112.0) | 46.7 (10.0, 138.0) | 58.0 (21.0, 186.0) | 51.1 (14.6, 170.3) | 70.0 (22.0, 208.0) |
| Quartile 1 | 72 (27.2) | 71 (32.4) | 56 (21.5) | 144 (26.2) | 54 (18.7) |
| Quartile 2 | 74 (27.9) | 46 (21.0) | 72 (27.6) | 140 (25.5) | 66 (22.8) |
| Quartile 3 | 68 (25.7) | 54 (24.7) | 60 (23.0) | 123 (22.4) | 83 (28.7) |
| Quartile 4 | 51 (19.2) | 48 (21.9) | 73 (28.0) | 143 (26.0) | 86 (29.8) |
| Positive aeroallergen tests2 | |||||
| None | 116 (40.7) | 127 (53.4) | 117 (42.1) | 374 (61.6) | 174 (58.0) |
| 1–2 | 65 (22.8) | 58 (24.4) | 64 (23.0) | 133 (21.9) | 73 (24.3) |
| 3 or more | 104 (36.5) | 53 (22.3) | 97 (34.9) | 100 (16.5) | 53 (17.7) |
| % of positive aeroallergens | 16.9 ± 19.9 | 11.1 ± 16.2 | 18.5 ± 24.0 | 12.0 ± 21.0 | 14.4 ± 23.9 |
| Positive food allergen tests2 | |||||
| None | 185 (64.9) | 178 (74.8) | 183 (65.8) | 366 (60.3) | 170 (56.7) |
| 1–2 | 65 (22.8) | 41 (17.2) | 42 (15.1) | 103 (17.0) | 51 (17.0) |
| 3 | 35 (12.3) | 19 (8.0) | 53 (19.1) | 138 (22.7) | 79 (26.3) |
| % of positive foods | 17.2 ± 27.2 | 11.8 ± 23.0 | 21.5 ± 33.9 | 24.2 ± 33.7 | 29.3 ± 36.4 |
| EFDs (average number/week during study run-in period) | 5.1 ± 1.7 | 5.8 ± 1.3 | 4.8 ± 2.1 | 5.4 ± 1.7 | 6.0 ± 1.2 |
| Albuterol inhalations (average number/week during study run-in period) | 1.0 ± 1.3 | 0.8 ± 2.0 | 0.5 ± 0.8 | 0.1 ± 0.1 | 1.7 ± 2.9 |
N = 1423; Absolute eosinophil counts were not available from the PEAK study. Quartiles were derived from eosinophil percentages.
Skin tests were considered positive if the prick resulted in a wheal with a mean diameter (mean of maximum and 90° midpoint diameters) that was at least 3 mm greater than that produced by the saline control. Specific IgE tests were considered positive if values were >0.34
Given the exploratory nature of these analyses, three, four and five-class solutions were evaluated; the four-class solution was chosen as the best fit for phenotype identification as it had the lowest BIC value with minimal loss of entropy (Table E2). The four-class solution yielded a high class membership probability for the majority of participants (Figure E1) and provided further subdivision to class 2 as identified in the 3-class solution (Table E3), resulting in a more even distribution of participants between groups. The item response probabilities associated with the 4-class solution are provided in Table E4 and the descriptive features of children posteriorly assigned to each of the four resultant phenotype groups are shown in Table 2.
Table E2.
Measures of latent class analysis model fit.
| Latent classes | AIC | BIC | Adjusted BIC | Entropy | Log-likelihood |
|---|---|---|---|---|---|
| 3 | 5948.00 | 6252.81 | 6074.90 | 0.65 | −14804.28 |
| 4 | 5759.17 | 6167.40 | 5929.14 | 0.67 | −14690.87 |
| 5 | 5726.21 | 6237.86 | 5939.24 | 0.68 | −14655.39 |
AIC = Akaike information criterion
BIC = Bayesian information criterion (BIC)
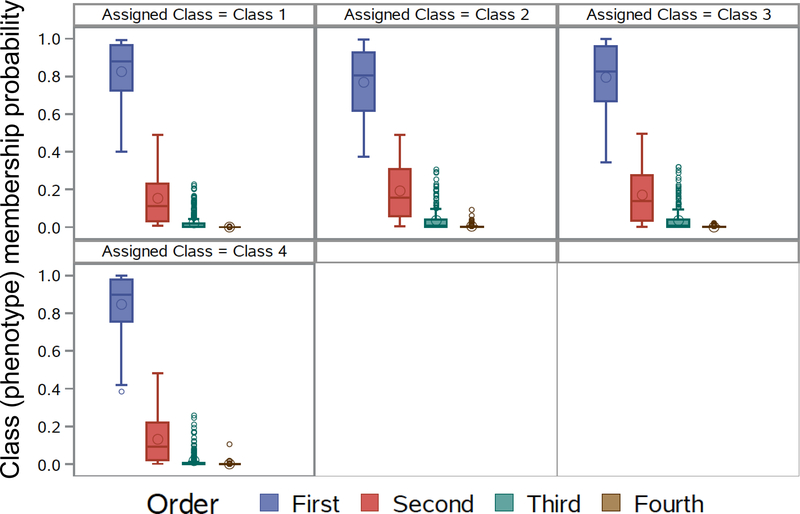
Figure E1.
Class (i.e., phenotype) membership probability for all participants for the 4-class model. Results demonstrate that for each of the 4 latent classes, the probability of assignment to that latent class was >0.80 on average for each participant.
Table E3.
Distribution of participants in the three-class versus four-class model. Numbers reflect the number of participants within each assigned class (i.e., phenotype group).
| Four Class Model | ||||||
|---|---|---|---|---|---|---|
| Class 1 | Class 2 | Class 3 | Class 4 | Total | ||
| Three Class Model | Class 1 | 490 | 24 | 97 | 0 | 608 |
| Class 2 | 4 | 371 | 355 | 43 | 773 | |
| Class 3 | 0 | 14 | 0 | 310 | 324 | |
| Total | 493 | 403 | 454 | 358 | 1708 | |
Table E4.
Features, shown as probabilities, of the four latent classes of recurrent wheeze. Class membership probabilities are presented as gamma estimates (standard error). Other data are presented as item response probabilities (i.e., Rho estimates) with standard errors in parentheses.
| Feature | Minimal sensitization N = 494 | Sensitization with indoor pets N = 409 | Sensitization with tobacco smoke exposure N = 452 | Multiple sensitization with eczema N = 353 |
|---|---|---|---|---|
| Latent class | 1 | 2 | 3 | 4 |
| Class membership probability | 0.28 (0.03) | 0.26 (0.03) | 0.26 (0.02) | 0.20 (0.02) |
| Male | 0.53 (0.03) | 0.75 (0.03) | 0.56 (0.03) | 0.70 (0.03) |
| Race/ethnicity | ||||
| Non-Hispanic White | 0.61 (0.04) | 0.66 (0.05) | 0.00 (0.00) | 0.36 (0.03) |
| Non-Hispanic Black | 0.04 (0.03) | 0.00 (0.01) | 0.52 (0.04) | 0.23 (0.03) |
| Hispanic | 0.27 (0.03) | 0.26 (0.04) | 0.27 (0.04) | 0.26 (0.03) |
| Other | 0.09 (0.02) | 0.07 (0.02) | 0.21 (0.03) | 0.19 (0.02) |
| Parent with asthma | 0.59 (0.03) | 0.50 (0.03) | 0.63 (0.03) | 0.60 (0.03) |
| Eczema | 0.38 (0.03) | 0.45 (0.03) | 0.56 (0.03) | 0.73 (0.03) |
| Current tobacco smoke exposure | 0.24 (0.03) | 0.26 (0.03) | 0.61 (0.03) | 0.33 (0.03) |
| Current cat or dog in the home | 0.57 (0.03) | 0.61 (0.04) | 0.20 (0.03) | 0.30 (0.03) |
| Positive aeroallergen tests | ||||
| None | 0.87 (0.03) | 0.42 (0.05) | 0.65 (0.04) | 0.04 (0.02) |
| 1–2 | 0.09 (0.02) | 0.37 (0.03) | 0.23 (0.03) | 0.26 (0.03) |
| 3 or more | 0.04 (0.01) | 0.22 (0.03) | 0.12 (0.02) | 0.70 (0.04) |
| Positive food allergen tests | ||||
| None | 0.95 (0.02) | 0.62 (0.04) | 0.68 (0.03) | 0.14 (0.03) |
| 1–2 | 0.05 (0.02) | 0.26 (0.03) | 0.20 (0.02) | 0.22 (0.03) |
| 3 | 0.00 (0.01) | 0.25 (0.03) | 0.12 (0.02) | 0.64 (0.04) |
| Blood eosinophil (%) quartile | ||||
| Lowest quartile | 0.58 (0.03) | 0.28 (0.04) | 0.35 (0.03) | 0.04 (0.02) |
| Second quartile | 0.20 (0.02) | 0.15 (0.03) | 0.26 (0.03) | 0.05 (0.02) |
| Third quartile | 0.18 (0.02) | 0.35 (0.03) | 0.30 (0.03) | 0.33 (0.03) |
| Highest quartile | 0.04 (0.01) | 0.22 (0.04) | 0.09 (0.02) | 0.58 (0.03) |
| Total serum IgE (IU/mL) quartile | ||||
| Lowest quartile | 0.66 (0.04) | 0.08 (0.03) | 0.18 (0.04) | 0.00 (0.00) |
| Second quartile | 0.28 (0.03) | 0.31 (0.04) | 0.37 (0.03) | 0.00 (0.00) |
| Third quartile | 0.05 (0.03) | 0.39 (0.04) | 0.31 (0.03) | 0.24 (0.03) |
| Highest quartile | 0.01 (0.02) | 0.22 (0.04) | 0.14 (0.03) | 0.76 (0.03) |
Table 2.
Features of the four latent classes of recurrent wheeze. Posterior probabilities of class membership were assigned to each participant. Data represent the number of participants (%), the mean ± standard deviation, or the median (25th, 75th percentile).
| Feature | Combined sample N = 1708 | Minimal sensitization N = 494 | Sensitization with indoor pets N = 409 | Sensitization with tobacco smoke exposure N = 452 | Multiple sensitization with eczema N = 353 |
|---|---|---|---|---|---|
| Latent class | 1 | 2 | 3 | 4 | |
| Age at enrollment (months) | 37.6 ± 14.0 | 35.1 ± 13.8 | 37.9 ± 13.7 | 37.1 ± 14.3 | 41.1 ± 13.4 |
| Age <24 months | 271 (15.9) | 92 (18.6) | 57 (13.9) | 87 (19.2) | 35 (9.9) |
| Male | 1067 (62.5) | 260 (52.6) | 314 (76.8) | 252 (55.8) | 241 (68.3) |
| Race/ethnicity | |||||
| Non-Hispanic White | 709 (41.5) | 301 (60.9) 8 | 283 (69.2) | -- | 125 (35.4) |
| Non-Hispanic Black | 336 (19.7) | (1.6) | -- | 245 (54.2) | 83 (23.5) |
| Hispanic | 453 (26.5) | 143 (28.9) | 104 (25.4) | 107 (23.7) | 99 (28.0) |
| Other | 210 (12.3) | 42 (8.5) | 22 (5.4) | 100 (22.1) | 46 (13.0) |
| Parent with asthma | 953 (55.8) | 288 (58.3) | 192 (46.9) | 277 (61.3) | 196 (55.5) |
| Eczema (ever) | 871 (51.0) | 184 (37.2) | 166 (40.6) | 260 (57.5) | 261 (73.9) |
| Current tobacco smoke exposure | 607 (35.5) | 108 (21.9) | 96 (23.5) | 287 (63.5) | 116 (32.9) |
| Current cat or dog in the home | 737 (43.1) | 291 (58.9) | 262 (64.1) | 81 (17.9) | 103 9.2) |
| Blood eosinophils | |||||
| Absolute count (per microL)1 | 248.4 (148.0, 480.0) | 165.7 (104.0, 236.5) | 292.0 (164.0, 492.0) | 208.0 (139.2, 330.0) | 598.5 (377.3, 783.0) |
| % of differential | 3.1 (2.0, 6.0) | 2.0 (1.4, 3.0) | 4.0 (2.0, 6.0) | 3.0 (2.0, 4.0) | 7.0 (5.0, 10.0) |
| Quartile 1 | 528 (33.1) | 268 (59.2) | 110 (28.4) 54 | 143 (34.1) | 7 (2.1) |
| Quartile 2 | 277 (17.4) | 95 (21.0) | (14.0) | 114 (27.2) | 14 (4.2) |
| Quartile 3 | 454 (28.4) | 75 (16.6) 15 | 134 (34.6) 89 | 128 (30.5) | 117 (34.7) |
| Quartile 4 | 337 (21.1) | (3.3) | (23.0) | 34 (8.1) | 199 (59.1) |
| Eosinophils ≥4% | 702 (44.0) | 71 (15.7) | 197 (50.9) | 137 (32.7%) | 297 (88.1) |
| Total serum IgE | |||||
| IU/mL | 53.9 (15.0, 162.1) | 10.0 (4.7, 18.4) | 86.0 (38.1, 149.0) | 48.0 (20.6, 99.6) | 321.5 (168.0, 670.0) |
| Lowest quartile | 397 (25.1) | 312 (70.6) | 13 (3.4) | 72 (17.0) | -- |
| Second quartile | 398 (25.1) | 122 (27.6) | 112 (29.2) | 164 (38.7) | -- |
| Third quartile | 388 (24.5) | 8 (1.8) | 173 (45.2) | 131 (30.9) | 76 (22.7) |
| Highest quartile | 401 (25.3) | -- | 85 (22.2) | 57 (13.4) | 259 (77.3) |
| Positive aeroallergen tests2 | |||||
| None | 908 (53.2) | 446 (90.3) | 155 (37.9) | 300 (66.4) | 7 (2.0) |
| 1–2 | 393 (23.0) | 34 (6.9) | 169 (41.3) | 101 (22.3) | 89 (25.2) |
| 3 or more | 407 (23.8) | 14 (2.8) | 85 (20.8) | 51 (11.3) | 257 (72.8) |
| % of positive aeroallergens | 14.2 +/− 21.4 | 1.9 +/− 7.8 | 12.7 +/− 14.1 | 7.4 +/− 14.4 | 41.2 +/− 24.9 |
| Indoor allergen sensitization3 | 693 (40.6) | 47 (9.5) | 199 (48.7) | 128 (28.3) | 319 (90.4) |
| Outdoor allergen sensitization4 | 429 (25.1) | 37 (7.5) | 99 (24.2) | 77 (17.0) | 216 (61.2) |
| Mold sensitization | 179 (10.8) | 11 (2.3) | 37 (9.2) | 25 (5.8) | 106 (30.3) |
| Positive food allergen tests2 | |||||
| None | 1082 (63.3) | 474 (96.0) | 254 (62.1) | 310 (68.6) | 44 (12.5) |
| 1–2 | 302 (17.7) | 19 (3.8) | 113 (27.6) | 92 (20.4) | 78 (22.1) |
| 3 | 324 (19) | 1 (0.2) | 42 (10.3) | 50 (11.1) | 231 (65.4) |
| % of positive foods | 21.6 +/− 32.3 | 1.5 +/− 7.2 | 17.1 +/− 25.1 | 15.2 +/− 24.7 | 62.2 +/− 33.6 |
N = 1423; Absolute eosinophil counts were not available from the PEAK study. Quartiles were derived from eosinophil percentages.
Skin tests were considered positive if the prick resulted in a wheal with a mean diameter (mean of maximum and 90° midpoint diameters) that was at least 3 mm greater than that produced by the saline control. Specific IgE tests were considered positive if values 541 were >0.34 IU
Defined as sensitization to dust mites, cockroach, cats or dogs
Defined as sensitization to grasses, trees, or weeds
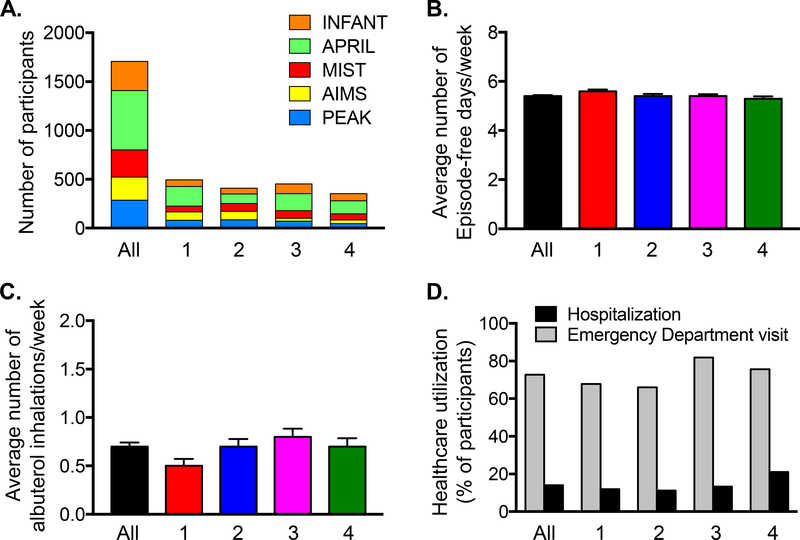
Individual studies were evenly distributed among the phenotype groups (Figure 1A). Groups were not markedly different with regard to current symptom presentation as reflected by EFDs and albuterol inhalations during the run-in periods (Figure 1B,C) or self-reported healthcare utilization for wheezing exacerbations in the prior year (Figure 1D). However, notable differences in atopy, exposures and race were observed (Table 2). To simplify discussion, each class was assigned a summary label. Key features of the resultant latent classes are discussed below.
Figure 1.
(A) Distribution of studies, (B) episode-free days and (C) albuterol inhalations during the study run-in periods (mean ± SEM), and (D) prior year healthcare utilization for wheezing or asthma symptoms in all participants (N=1708) and each latent class (1 = minimal sensitization [N=494], 2 = sensitization with indoor pets [N=409], 3 = sensitization with tobacco smoke exposure [N=452], 4 = multiple sensitization with eczema [N=353]).
Latent class 1 (class membership probability = 0.28).
Approximately 30% of preschool children with recurrent wheeze were classified in this group, termed “minimal sensitization.” Children in this latent class were predominantly non-Hispanic White (61%) and were fairly proportionate with regard to sex (53% male). These children also had a high prevalence of indoor pet ownership (59%) but the lowest prevalence of eczema, the fewest blood eosinophils, and the lowest serum IgE levels. The majority (>90%) of children in this group had no aeroallergen sensitization and no food sensitization.
Latent class 2 (class membership probability = 0.26).
This group, termed “sensitization with indoor pet exposure,” was identified in approximately 25% of participants. Children with this phenotype were predominantly non-Hispanic White (69%) and male (77%) with the lowest parental history of asthma (47%). These children also had the highest prevalence of pet ownership (64%), elevated blood eosinophils (51% with eosinophils ≥4%), and elevated serum IgE levels. The majority of children in this latent class had sensitization to at least one aeroallergen (62%). Sensitization patterns were mostly confined to indoor allergens (49%), with lesser sensitization to outdoor allergens (24%) and minimal sensitization to mold (9.2%). Only one third of children in this latent class (38%) had food sensitization.
Latent class 3 (class membership probability = 0.26).
Approximately 25% of children with recurrent wheeze were classified in this group, termed “sensitization with tobacco smoke exposure.” Children in this class were exclusively non-White (100%) with a slightly higher proportion of males (56%) and the highest prevalence of parental asthma (61%). This group also had the highest prevalence of tobacco smoke exposure (64%) and some atopic features including eczema (58%), but only modest elevations in blood eosinophils (33% with blood eosinophils ≥4%) and serum IgE levels. Furthermore, only 34% and 31% of children in this latent class had sensitization to aeroallergens and foods, respectively. Sensitization patterns were mostly confined to indoor allergens (28%), with less sensitization to outdoor allergens (17%) and mold (6%). Indoor pet exposure was also lowest in this group.
Latent class 4 (class membership probability = 0.20).
This latent class was termed “multiple sensitization with eczema” and was identified in approximately 20% of children. This class had fairly proportionate racial and ethnic representation (35% non-Hispanic White, 24% non-Hispanic Black, 28% Hispanic) but was older (90% ≥24 months) with a higher proportion of males (68%). The distribution of parental asthma was relatively proportionate (56%). Children in this latent class had the highest reported eczema (74%), the highest blood eosinophils (88% with blood eosinophils ≥4%), and the highest IgE levels. Ninety-eight percent of children also had aeroallergen sensitization and 73% were sensitized to 3 or more allergens. Sensitization patterns also differed from the other classes, with 90%, 61% and 30% of children in this group sensitized to indoor allergens, outdoor allergens, and mold, respectively. 87% of children in this latent class also had food sensitization and 65% were sensitized to all three foods evaluated.
Exacerbation outcomes.
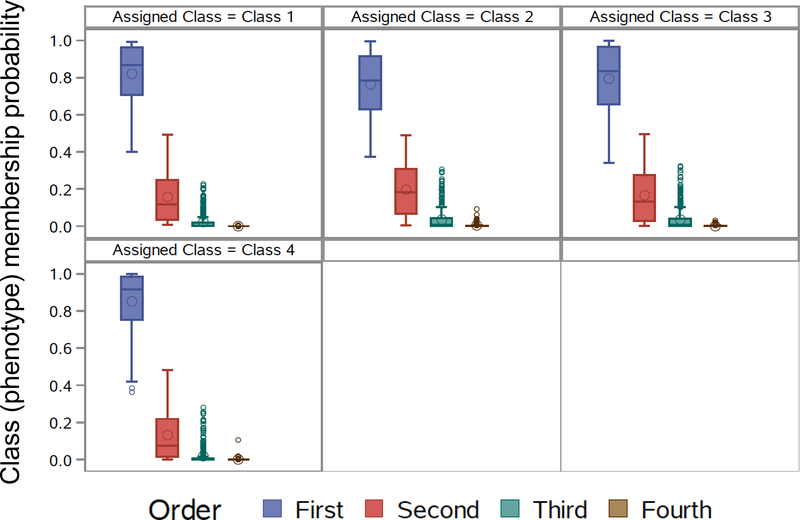
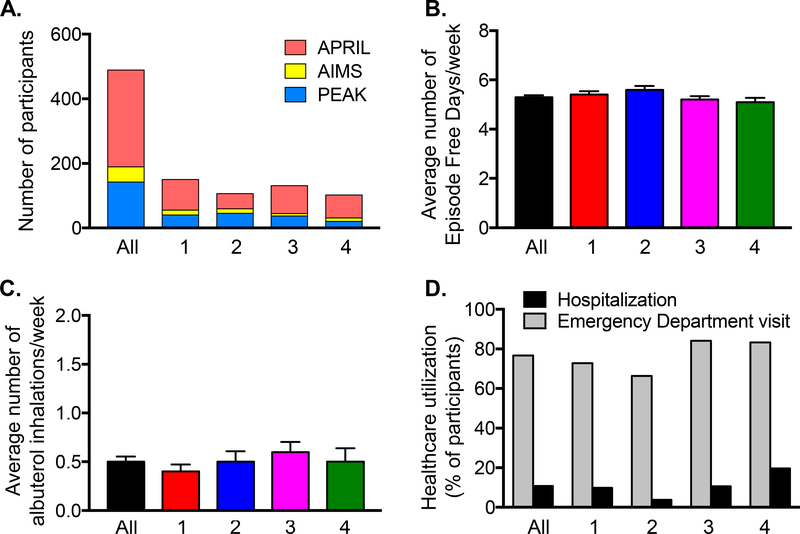
To determine whether the identified latent classes were clinically meaningful with regard to future exacerbations, the rate of exacerbations (primary outcome) and time to first exacerbation (secondary outcome) were examined in placebo-treated participants in the PEAK, AIMS and APRIL studies (N = 489) to eliminate the potential confounding effects of asthma controller medications such as ICS and LTRA. Model performance with regard to class (i.e., group) membership probability was also high in this subset (Figure E2). Features of the participants included in outcome assessment are shown in Table E5 and Figure E3 and were similar to those of the larger sample used for latent class identification.
Figure E2.
Class (i.e., phenotype) membership probability for participants included in outcome analysis, utilizing the 4-class model. Results demonstrate that for each of the 4 latent classes, the probability of assignment to that latent class was >0.80 on average for each participant.
Table E5.
Features of placebo-treated participants in the PEAK, AIMS and APRIL studies included in primary outcome (exacerbation) analysis. Posterior probabilities of class membership were assigned to each participant. Data represent the number of participants (%), the mean ± standard deviation, or the median (25th, 75th percentile).
| Feature | Combined sample N = 489 | Minimal sensitization N = 151 | Sensitization with indoor pets N = 104 | Sensitization with tobacco smoke exposure N = 132 | Multiple sensitization with eczema N = 102 |
|---|---|---|---|---|---|
| Latent class | 1 | 2 | 3 | 4 | |
| Study | |||||
| PEAK | 142 (29) | 41 (27.2) | 44 (42.3) | 38 (28.8) | 19 (18.6) |
| AIMS | 47 (9.6) | 15 (9.9) | 14 (13.5) | 7 (5.3) | 11 (10.8) |
| APRIL | 300 (61.3) | 95 (62.9) | 46 (44.2) | 87 (65.9) | 72 (70.6) |
| Age at enrollment (months) | 38.5 ± 14.5 | 37.2 ± 15.0 | 37.8 ± 13.7 | 37.8 ± 14.4 | 42.1 ± 14.1 |
| Male | 294 (60.1) | 82 (54.3) | 79 (76.0) | 70 (53.0) | 63 (61.8) |
| Race/ethnicity | |||||
| Non-Hispanic White | 213 (43.6) | 93 (61.6) | 77 (74.0) | -- | 43 (42.2) |
| Non-Hispanic Black | 98 (20) | 2 (1.3) | -- | 70 (53.0) | 26 (25.5) |
| Hispanic | 130 (26.6) | 46 (30.%) | 24 (23.1) | 35 (26.5) | 25 (24.5) |
| Other | 48 (9.8) | 10 (6.6) | 3 (2.9) | 27 (20.5) | 8 (7.8) |
| Parent with asthma | 271 (55.4) | 89 (58.9) | 50 (48.1) | 83 (62.9) | 49 (48.0) |
| Eczema (ever) | 259 (53) | 56 (37.1) | 49 (47.1) | 82 (62.1) | 72 (70.6) |
| Current tobacco smoke exposure | 179 (36.6) | 37 (24.5) | 22 (21.2) | 85 (64.4) | 35 (34.3) |
| Current dog or cat in the home | 204 (41.7) | 86 (57.0) | 67 (64.4) | 22 (16.7) | 29 (28.4) |
| Blood eosinophils | |||||
| % of differential | 3.0 (2.0, 5.5) | 2.0 (1.3, 2.9) | 4.0 (2.0, 5.9) | 2.6 (2.0, 4.0) | 7.0 (5.0, 10.0) |
| Absolute count (per microL)1 | 235.0 (141.5, 432.6) | 155.5 (102.0, 228.6) | 298.2 (199.8, 420.0) | 200.7 (136.4, 280.8) | 603.0 (370.0, 834.0) |
| Total serum IgE (kU/L) | 48.4 (14.4, 140.4) | 10.2 (4.1, 19.0) | 92.0 (43.7, 156.6) | 48.4 (19.5, 98.7) | 301.5 (133.5, 551.2) |
| % Positive aeroallergen tests | 13.8 ± 21.4 | 1.7 ± 8.5 | 13.6 ± 14.1 | 6.4 ± 13.7 | 41.1 ± 24.7 |
| % Positive food allergen tests | 23.1 ± 32.7 | 1.1 ± 6.1 | 20.5 ± 27.2 | 16.2 ± 24.9 | 65.7 ± 29.8 |
N = 347; Absolute eosinophil counts were not available from the PEAK study
Figure E3.
(A) Distribution of studies, (B) episode-free days and (C) albuterol inhalations during the study run-in periods (mean ± SEM), and (D) healthcare utilization during the previous year in participants selected for outcome assessment (All, N = 489) and in the identified latent classes (1 = minimal sensitization, 2 = sensitization with indoor pets, 3 = sensitization with tobacco smoke exposure, 4 = multiple sensitization with eczema).
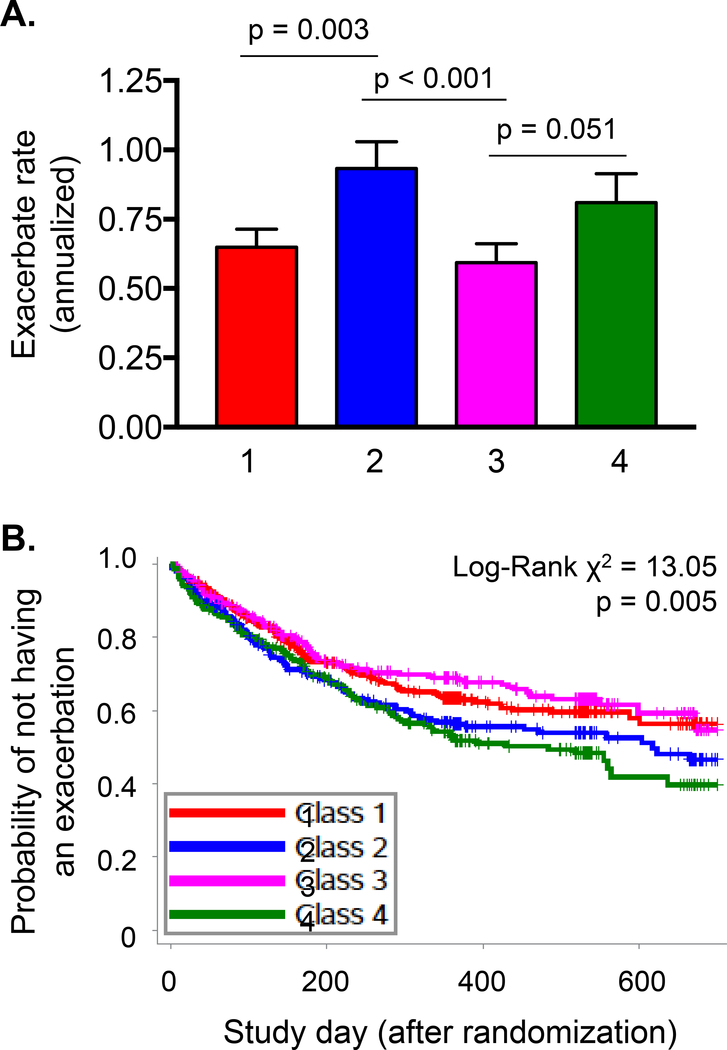
The annualized rate of exacerbations (mean ± SEM/year, 95% confidence interval) for each of the latent classes was as follows: class 1 (minimal sensitization), 0.65 ± 0.06 (0.53, 0.79); class 2 (sensitization with indoor pets), 0.93 ± 0.10 (0.76, 1.15); class 3 (sensitization with indoor tobacco smoke exposure), 0.60 ± 0.07 (0.47, 0.74); class 4 (multiple sensitization and eczema), 0.81 ± 0.10 (0.63, 1.04) (Figure 2A). Over two years, the probability of exacerbation was greatest in children with sensitization and indoor pet exposure (latent class 2) and children with multiple sensitization and eczema (latent class 4) (Figure 2B).
Figure 2.
(A) Annualized rate (mean ± SEM) and (B) probability of exacerbation in placebo-treated children with minimal sensitization (latent class 1, N=151), sensitization with indoor pets (latent class 2, N=104), sensitization with tobacco smoke exposure (latent class 3, N=132), and multiple sensitization with eczema (latent class 4, N=102) in the PEAK, AIMS, and APRIL studies. Numbers correspond to latent class groups.
ICS treatment effects.
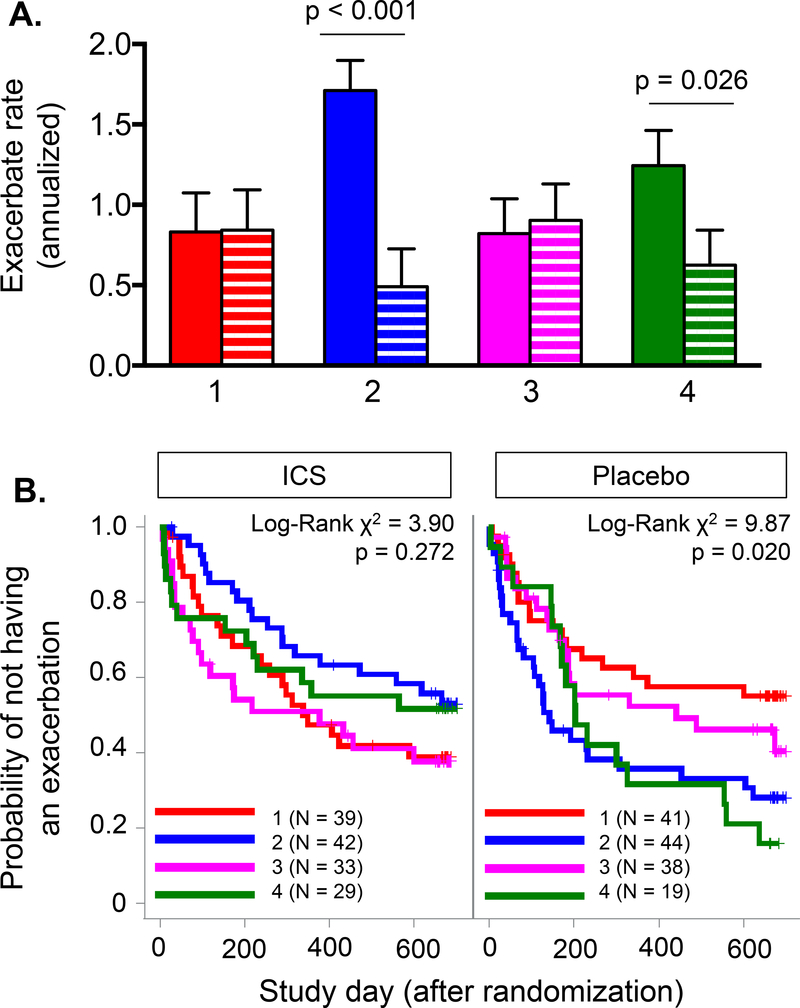
To determine the potential impact of daily ICS treatment on exacerbation rates, an exploratory analysis was performed on participants in the PEAK study (both placebo and ICS treatment arms) (N = 285). Results are presented in Figure 3. Daily ICS treatment was associated with a significantly lower exacerbation rate in children with sensitization and indoor pet exposure (latent class 2) and children with multiple sensitization and eczema (latent class 4), but not in children with minimal sensitization (latent class 1) or children with sensitization and indoor tobacco smoke exposure (latent class 3). Exacerbation rates did not differ between latent classes after daily ICS treatment (Figure 3A). Likewise, daily ICS treatment also lowered the exacerbation probability in children with sensitization and indoor pet exposure (latent class 2; Log-rank X2 = 9.226; p = 0.002) and children with multiple sensitization and eczema (latent class 4; Log-rank X2 = 4.710; p = 0.030) (Figure 3B).
Figure 3.
(A) Annualized rate (mean ± SEM) and (B) probability of exacerbation in the PEAK study placebo (solid bar) and daily inhaled corticosteroid (ICS, hatched bar) treatment arms. Numbers correspond to latent class groups (1 = minimal sensitization, 2 = sensitization with indoor pets, 3 = sensitization with tobacco smoke exposure, 4 = multiple sensitization with eczema).
Discussion
LCA is a subset of structural equation modeling with foundations in the social sciences that is useful for identifying unobservable “class” membership among participants with multivariate categorical data. Unlike clustering methods which have no objective criteria for judging the suitability of solutions,35 LCA is model-based and allows comparisons to be statistically tested.36 Our results obtained by LCA support prior reports that have highlighted the importance of allergic sensitization in preschool children with recurrent wheezing.3, 5–14, 21, 37 While those studies identified eczema,7, 10 atopic dermatitis,14 aeroallergen sensitization5, 7-9, 11, 13 and/or food sensitization5, 7, 9, 11 as key risk factors, the objective of those reports differed and focused primarily on the identification of wheezing trajectories from infancy to later childhood. Here, we focused on a disease population similar to that which is encountered in asthma specialist settings. Our results extend the literature with a unique focus on exacerbations and ICS treatment responsiveness, which have not been previously studied in preschool children in a highly supervised, medication-adherent research setting.
Using LCA, we identified four latent classes of recurrent wheezing in preschool children associated with varying degrees of allergic sensitization and exposures. These classes were not distinguished by current symptoms or historical exacerbation occurrence or severity (as reflected by healthcare utilization) at the time of study enrollment, but instead differed in longitudinal exacerbation outcomes and ICS treatment responsiveness. However, we recognize that our approach, applied to a heterogeneous longitudinal dataset, is exploratory and hypothesis generating. Nonetheless, the latent classes that we identified are plausible and clinically relevant. Our latent classes 2 and 4 had the greatest magnitude of sensitization and Type-2 inflammation as assessed by systemic biomarkers, and also the greatest exacerbation rate. Similarly, other studies have noted that the timing of sensitization (i.e., <12 months versus ≥12 months), the pattern of sensitization (i.e., multiple versus single allergen sensitization), and the specific allergens to which are child is sensitized (i.e., cats/dogs versus foods) are more important than sensitization alone in the determination of future asthma risk.38, 39
Despite greater exacerbation rates in latent classes 2 and 4 in the present study, in a setting of high adherence, daily low-dose ICS treatment significantly lowered exacerbation rates in these groups. This finding could be attributed to higher baseline exacerbation rates in these groups, with more room for improvement with ICS initiation. However, the findings are also consistent with prior studies of ICS in older children with elevated Type-2 inflammatory biomarkers,44–46 since blood eosinophils were similarly elevated in these children. The results are also consistent with a prior sub-analysis of the PEAK study47 that noted differences in EFDs, oral corticosteroid use, emergency department/urgent care visits and supplementary controller medication use in children with and without sensitization to at least one aeroallergen treated with ICS versus placebo. The present study extends that prior analysis by considering multiple variables simultaneously (as opposed to single variables) in latent class determination.
It is also important to note that exacerbations treated with systemic corticosteroids still occurred in each of the identified latent classes after ICS initiation. This observation suggests that some exacerbations may result from other triggers independent of Type-2 inflammation that are not suppressed by low-dose ICS, such as respiratory infections and neutrophilic-predominant patterns of inflammation. However, the fact that exacerbation rates were lower in the latent class of children with tobacco smoke exposure was unexpected and warrants further study since tobacco smoke exposure has been identified as a significant risk factor for recurrent wheezing in young children less than 3 years with lower respiratory viral infections.48 Although the children in this latent were quite symptomatic as reflected by EFDs and albuterol use at enrollment, it is possible that the underlying mechanisms associated with wheezing in response to nicotine or other components of tobacco smoke are different and convey a different risk with regard to future exacerbation. Prior studies suggest that prenatal49 and early-life50 tobacco smoke exposure may impact early lung development and promote wheezing through airway fibroblast-mediated neurogenic inflammation and structural changes in airway caliber.51 These observations might explain why ICS treatment in the present analysis did not impact exacerbations in this latent class, and why tobacco smoke exposure in asthma patients has been previously associated with a poorer response to ICS independent of sensitization.52 Alternatively, the lack of response to ICS in this latent class (and the latent class of children with minimal sensitization) in the present study may also be due to lower baseline exacerbation rates and limited room for improvement.
Strengths of the present analyses include the large and heterogeneous sample size and comprehensive characterization of enrolled participants. However, generalization to the larger population of preschool children with wheeze is a potential concern. Duijts et al.12 previously observed that wheezing after 18 months was more strongly associated with wheezing persistence in later childhood. Therefore, given the age range of our participants (~3 years on average) and the relatively small proportion of children less than 24 months included in our analysis (15.9%), younger children with transient wheeze patterns may not have been adequately represented. Furthermore, given the inclusion criteria of the clinical trials selected for our analysis, all participants were required to have more than one prior wheezing episode and therefore were at higher risk for future asthma development. This criterion minimized inclusion of children with isolated bronchiolitis but likely did capture some children with episodic wheezing associated with respiratory viral infection since more than 50% of the included participants had no evidence of aeroallergen or food sensitization.
Another important strength of the present analyses was the prospective and standardized assessment of exacerbation in the context of highly supervised daily ICS (or placebo) use. Many prior observational studies in this age group utilized inconsistent definitions of exacerbation and did not account for the impact of asthma medications such as ICS on self-reported symptoms.24 Our results (in a highly adherent population) highlight the potentially confounding effects of ICS on phenotype-outcome associations and argue for more rigorous assessment of ICS adherence in future studies, given that real-world adherence to these medications is typically poor, with <40% of patients taking these medications daily as prescribed.53
The multi-center design of the studies included in our latent class analysis was another strength. Compared to other single-center studies, our analysis had good geographic representation across the United States with a relatively high-prevalence of underrepresented minorities. However, because the included studies were primarily performed at large academic medical centers, our results may not generalize to less populated areas with differing access to healthcare. This is an important limitation since urban-rural differences in preschool wheeze phenotypes have been previously reported.37 We were also unable to directly compare household measures of socioeconomic status in the present study, so it is unclear if the racial disparities noted in our latent classes were attributable to modifiable factors such as economic hardships and other environmental variables such as indoor allergen exposure that impact asthma disease manifestation and asthma-related healthcare utilization.54-57 However, the fact that more nonHispanic Black children were represented in latent class 3 (sensitization with tobacco smoke exposure), does support a prior study demonstrating nearly two-fold higher odds of secondhand smoke exposure in Black and Puerto Rican/Hispanic children compared to non-Hispanic White children.58 In that same study, secondhand smoke exposure prevalence was also three times higher in publicly-insured children versus privately-insured children.58
In conclusion, we identified four latent classes of recurrent wheezing in preschool children with differing exacerbation rates and responses to daily ICS treatment. However, each of the latent classes experienced some exacerbation burden and these groups were relatively indistinguishable with regard to current symptoms and historical exacerbations at study entry. Therefore, although sensitization was identified as an important risk factor for exacerbation outcomes, more studies are needed to determine how this risk factor leads to overt disease, how sensitization impacts anti-viral and other innate immune defenses, and how sensitization might be prevented. Studies are also needed to determine whether these latent classes correspond to clinically useful phenotypes for the purpose of individualized pharmacotherapy.
Highlights Box.
What is already known about this topic? (word count = 34)
Preschool children with recurrent wheezing are a heterogenous group. Consequently, the specific factors that contribute to recurrent wheezing exacerbations are unclear; there is also limited evidence to direct pharmacotherapy and a sizeable knowledge gap.
What does this article add to our knowledge? (word count = 35)
Latent class analysis identified four groups with differing sensitization patterns, exposures and annualized exacerbation rates. In a research setting of high adherence, daily inhaled corticosteroid (ICS) treatment improved exacerbation rates only in children with predominant Type-2 inflammatory features.
How does this study impact current management guidelines? (word count = 22)
Sensitization is a useful predictor of future exacerbation in preschool children, but exacerbations are common in all groups and may result from other triggers independent of Type-2 inflammation that are not suppressed by low-dose ICS.
Acknowledgments
Funding sources:
Analysis supported by:
• Children’s Healthcare of Atlanta Pediatric Research Alliance, Center for Clinical Outcomes 50 Research and Public Health
• The National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378
• The AsthmaNet Data Coordinating Center U10 HL098115
Financial and Organizational Disclosures:
Anne M. Fitzpatrick, Ph.D.
Nothing to disclose.
Leonard B. Bacharier, M.D.
Dr. Bacharier reports personal fees from GlaxoSmithKline, personal fees from Genentech/Novartis, personal fees from Merck, personal fees from DBV Technologies, personal fees from Teva, personal fees from Boehringer Ingelheim, personal fees from Sanofi/Regeneron, personal fees from Vectura, personal fees from Circassia, personal fees from AstraZeneca, outside the submitted work.
Theresa W. Guilbert, M.D.
Dr. Guilbert reports personal fees from American Board of Pediatrics; Pediatric Pulmonary Subboard, personal fees from GSK, personal fees from Regeneron Pharmaceuticals, personal fees from Merck, personal fees from Novartis/Regeneron, personal fees from Aviragen, personal fees from GSK/Regneron, grants and personal fees from Sanofi/Regeneron, grants from NIH, other from UpToDate outside the submitted work.
Daniel J. Jackson, M.D.
Dr. Jackson reports personal fees from Vectura Group, Boehringer Ingelheim, and GlaxoSmithKline and consulting fees from Novartis, outside the submitted work.
Stanley J. Szefler, M.D.
Dr. Szefler reports consultancy fees from Merck, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Aerocrine, Novartis, Astra Zeneca, Daiichi Sankyo, Roche, and Teva; grants from GlaxoSmithKline, outside the submitted work.
Avraham Beigelman, M.D.
Nothing to disclose.
Michael D. Cabana, M.D.
Dr. Cabana reports personal fees from Merck, personal fees from ThemoFisher, personal fees from Genentech, personal fees from Novartis, outside the submitted work.
Ronina Covar, M.D.
Dr. Covar reports grants Roche, grants from Astra Zeneca, non-financial support from GSK, outside the submitted work.
Fernando Holguin, M.D.
Nothing to disclose.
Robert F. Lemanske Jr., M.D.
Dr. Lemanske reports grants from Pharmaxis, personal fees from Elsevier, personal fees from UpToDate, outside the submitted work.
Fernando D. Martinez, M.D.
Dr. Martinez reports grants from NIH/Office of the Director, grants from Johnson & Johnson, personal fees from Copeval, personal fees from Commense, Inc, outside the submitted work.
Wayne Morgan, M.D.
Dr. Morgan reports grants from the Cystic Fibrosis Foundation, personal fees from Cystic Fibrosis Foundation, personal fees from Genentech, personal fees from American Thoracic Society, personal fees from American College of Chest Physicians outside the submitted work.
Wanda Phipatanakul, M.D. M.S.
Nothing to disclose.
Jacqueline A. Pongracic, M.D.
Dr. Pongracic reports grants from Northwestern University, during the conduct of the study; other from Boehringer-Ingelheim, other from GSK, other from TEVA, other from Merck, outside the submitted work.
Robert S. Zeiger, M.D. Ph.D.
Dr. Zeiger reports grants from NHLBI, during the conduct of the study; grants from Aerocrine, grants from Genentech, grants from MedImmune/AstraZeneca, grants from Merck, grants from GlaxoSmithKline, grants from ALK Pharma, personal fees from AstraZeneca, personal fees from Genentech, personal fees from Novartis, personal fees from TEVA, personal fees from GlaxoSmithKline, personal fees from Theravance BioPharma, personal fees from Regeneron Pharmaceuticals, personal fees from Patara Pharma, outside the submitted work.
David T. Mauger, Ph.D.
Dr. Mauger reports non-financial support from Merck, non-financial support from Boerhinger-Ingleim, non-financial support from GSK, non-financial support from TEVA, non-financial support from Vifor, outside the submitted work.
We would like to acknowledge the following individuals:
AsthmaNet Pediatric Investigators:
Leonard B. Bacharier, MD; Sachin Baxi, MD; Avraham Beigelman, MD; Mindy Benson, MSN, PN; Kathryn Blake, PharmD; Susan Boehmer, MA; Carey-Ann Burnham, PhD; Michael Cabana, MD; Mario Castro, MD, MPH; James Chmiel, MD, MPH; Ronina Covar, MD; Cori Daines, MD; Michael Daines, MD; Anne Fitzpatrick, PhD; Jonathan Gaffin, MD, MMSc; Deborah Ann Gentile, MD; W. Adam Gower, MD; Theresa Guilbert, MD; Fernando Holguin, MD; Elliot Israel, MD; Daniel Jackson, MD; H. William Kelly, Pharm D; Harsha Vardhan Kumar, MD; Jason Lang, MD, MPH; Stephen Lazarus, MD; John Lima, PharmD; Robert Lemanske Jr, MD; Ngoc Ly, MD; Fernando Martinez, MD; Jyothi Marbin, MD; David Mauger, PhD; Kelley Meade, MD; Wayne Morgan, MD; James Moy, MD; Ross Myers, MD; Tod Olin, MD; Ian Paul, MD, MSc; Stephen Peters, MD; Wanda Phipatanakul, MD, MS; Jacqueline Pongracic, MD; Hengameh Raissy, PharmD; Rachel Robison, MD; Kristie Ross, MD; Christine Sorkness, PharmD; William Sheehan, MD; Stanley Szefler, MD; W. Gerald Teague, MD; Shannon Thyne, MD.
CARE Network Pediatric Investigators:
Leonard Bacharier, MD; Elizabeth Bade, MD; Avraham Beigelman, MD; Jessica Beiler, MPH; Gordon Bloomberg, MD; Susan Boehmer, MA; Mark Brown, MD; Vernon M. Chinchilli, PhD; Sandra Christiansen, MD; James Corry, MD; Ronina Covar, MD; Timothy Craig, DO; Loretta Doty; Noah Friedman, MD; James Gern, MD; Gavin Graff, MD; Theresa W. Guilbert, MD; Gregory Heldt, MD; Hal Hoffman, MD; Alfredo Jalowayski, PhD; Michael Kaplan, MD; H. William Kelly, PharmD; Marzena Krawiec, MD; Gary Larsen, MD; Robert Lemanske, Jr. MD; Andrew Liu, MD; Jonathan Malka-Rais, MD; John Mark, MD; Fernando Martinez, MD; David Mauger, PhD; Elizabeth Mauger, PhD; Michael Mellon, MD; Wayne Morgan, MD; Mark Moss, MD; Ian Paul, MD; Brenda R. Phillips, MS; Hengameh Raissy, PharmD; Michael Schatz, MD, MS; Christine Sorkness, PharmD; Joseph Spahn, MD; Robert Strunk, MD; Stanley Szefler, MD; Lynn Taussig, MD; Robert Zeiger, MD, PhD.
Others:
We would also like to acknowledge the hundreds of study coordinators, personnel and referring physicians for the AsthmaNet and CARE Network studies, as well as the participants and their families.
Abbreviations
- AIMS
Acute Intermittent Management Strategies
- APRIL
Azithromycin for Preventing the Development of Upper Respiratory Tract Illnesses into Lower Respiratory Tract Symptoms
- BIC
Bayesian information criterion
- CARE
Childhood Asthma Research and Education
- EFD
Episode-free day
- ICS
Inhaled corticosteroid
- INFANT
Individualized Therapy for Asthma in Toddlers
- LCA
Latent class analysis
- LTRA
Leukotriene receptor antagonist
- MIST
Maintenance and Intermittent Inhaled Corticosteroids in Wheezing Toddlers
- PEAK
Prevention of Early Asthma in Kids
Footnotes
Study registrations:
APRIL ClinicalTrials.gov number, NCT01272635
INFANT ClinicalTrials.gov number, NCT01606306
PEAK ClinicalTrials.gov number, NCT00272441
AIMS ClinicalTrials.gov number, NCT00319488
MIST ClinicalTrials.gov number, NCT00675584 61
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.2015. National Health Interview Survey (NHIS) Data, Table 3–1 and Table 4–1. Available: https://www.cdc.gov/asthma/most_recent_data.htm Last accessed: August 25, 2017.
- 2.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995; 332:133–8. [DOI] [PubMed] [Google Scholar]
- 3.Havstad S, Johnson CC, Kim H, Levin AM, Zoratti EM, Joseph CL, et al. Atopic phenotypes identified with latent class analyses at age 2 years. J Allergy Clin Immunol 2014; 134:722–7 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hose AJ, Depner M, Illi S, Lau S, Keil T, Wahn U, et al. Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol 2017; 139:1935–45 e12. [DOI] [PubMed] [Google Scholar]
- 5.Just J, Gouvis-Echraghi R, Couderc R, Guillemot-Lambert N, Saint-Pierre P. Novel severe wheezy young children phenotypes: boys atopic multiple-trigger and girls nonatopic uncontrolled wheeze. J Allergy Clin Immunol 2012; 130:103–10 e8. [DOI] [PubMed] [Google Scholar]
- 6.Simpson A, Tan VY, Winn J, Svensen M, Bishop CM, Heckerman DE, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med 2010; 181:1200–6. [DOI] [PubMed] [Google Scholar]
- 7.Lodge CJ, Zaloumis S, Lowe AJ, Gurrin LC, Matheson MC, Axelrad C, et al. Early-life risk factors for childhood wheeze phenotypes in a high-risk birth cohort. J Pediatr 2014; 164:289–94 e1–2. [DOI] [PubMed] [Google Scholar]
- 8.Spycher BD, Silverman M, Brooke AM, Minder CE, Kuehni CE. Distinguishing phenotypes of childhood wheeze and cough using latent class analysis. Eur Respir J 2008; 31:974–81. [DOI] [PubMed] [Google Scholar]
- 9.Savenije OE, Granell R, Caudri D, Koppelman GH, Smit HA, Wijga A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol 2011; 127:1505–12 e14. [DOI] [PubMed] [Google Scholar]
- 10.Panico L, Stuart B, Bartley M, Kelly Y. Asthma trajectories in early childhood: identifying modifiable factors. PLoS One 2014; 9:e111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depner M, Fuchs O, Genuneit J, Karvonen AM, Hyvarinen A, Kaulek V, et al. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med 2014; 189:129–38. [DOI] [PubMed] [Google Scholar]
- 12.Duijts L, Granell R, Sterne JA, Henderson AJ. Childhood wheezing phenotypes influence asthma, lung function and exhaled nitric oxide fraction in adolescence. Eur Respir J 2016; 47:510–9. [DOI] [PubMed] [Google Scholar]
- 13.Spycher BD, Silverman M, Pescatore AM, Beardsmore CS, Kuehni CE. Comparison of phenotypes of childhood wheeze and cough in 2 independent cohorts. J Allergy Clin Immunol 2013; 132:1058–67. [DOI] [PubMed] [Google Scholar]
- 14.Tse SM, Rifas-Shiman SL, Coull BA, Litonjua AA, Oken E, Gold DR. Sex-specific risk factors for childhood wheeze and longitudinal phenotypes of wheeze. J Allergy Clin Immunol 2016; 138:1561–8 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3 2012:1–58. [PubMed] [Google Scholar]
- 16.Bacharier LB, Phillips BR, Bloomberg GR, Zeiger RS, Paul IM, Krawiec M, et al. Severe intermittent wheezing in preschool children: a distinct phenotype. J Allergy Clin Immunol 2007; 119:604–10. [DOI] [PubMed] [Google Scholar]
- 17.Jensen ME, Mendelson MJ, Desplats E, Zhang X, Platt R, Ducharme FM. Caregiver’s functional status during a young child’s asthma exacerbation: A validated instrument. J Allergy Clin Immunol 2016; 137:782–8 e6. [DOI] [PubMed] [Google Scholar]
- 18.Bui AL, Dieleman JL, Hamavid H, Birger M, Chapin A, Duber HC, et al. Spending on Children’s Personal Health Care in the United States, 1996–2013. JAMA Pediatr 2017; 171:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Opina MT, Moore WC. Phenotype-Driven Therapeutics in Severe Asthma. Curr Allergy Asthma Rep 2017; 17:10. [DOI] [PubMed] [Google Scholar]
- 20.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120:S94–138. [DOI] [PubMed] [Google Scholar]
- 21.Hose AJ, Depner M, Illi S, Lau S, Keil T, Wahn U, et al. Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol 2016. [DOI] [PubMed] [Google Scholar]
- 22.Levy BD, Noel PJ, Freemer MM, Cloutier MM, Georas SN, Jarjour NN, et al. Future Research Directions in Asthma. An NHLBI Working Group Report. Am J Respir Crit Care Med 2015; 192:1366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szefler SJ, Chmiel JF, Fitzpatrick AM, Giacoia G, Green TP, Jackson DJ, et al. Asthma across the ages: knowledge gaps in childhood asthma. J Allergy Clin Immunol 2014; 133:3–13; quiz 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr., Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012; 129:S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Benedictis FM, Bush A. Infantile wheeze: rethinking dogma. Arch Dis Child 2017; 102:371–5. [DOI] [PubMed] [Google Scholar]
- 26.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Longterm inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006; 354:1985–97. [DOI] [PubMed] [Google Scholar]
- 27.Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF Jr., et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol 2008; 122:1127–35 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF, Jr, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med 2011; 365:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA 2015; 314:2034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol 2016; 138:1608–18 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol 2004; 114:1282–7. [DOI] [PubMed] [Google Scholar]
- 32.Guilbert TW, Morgan WJ, Krawiec M, Lemanske RF, Jr, Sorkness C, Szefler SJ, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials 2004; 25:286310. [DOI] [PubMed] [Google Scholar]
- 33.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: A SAS Procedure for Latent Class Analysis. Struct Equ Modeling 2007; 14:671–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilbe J Negative binomial regression. 2. New York: Cambridge University Press; 2007. [Google Scholar]
- 35.Schreiber JB, Pekarik AJ. Technical note: Using latent class analysis versus K-means or hierarchical clustering to understand museum visitors. Curator: The Museum Journal 2014; 57:45–59. [Google Scholar]
- 36.Collins LM, Lanza ST. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. Hoboken, NJ: John Wiley & Sons, Inc; 2010. [Google Scholar]
- 37.Kutzora S, Weber A, Heinze S, Hendrowarsito L, Nennstiel-Ratzel U, von Mutius E, et al. Asthmatic/wheezing phenotypes in preschool children: Influential factors, health care and urban-rural differences. Int J Hyg Environ Health 2017. [DOI] [PubMed] [Google Scholar]
- 38.Stoltz DJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE, et al. Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy 2013; 43:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol 2017; 139:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gergen PJ, Mitchell HE, Calatroni A, Sever ML, Cohn RD, Salo PM, et al. Sensitization and Exposure to Pets: The Effect on Asthma Morbidity in the US Population. J Allergy Clin Immunol Pract 2018; 6:101–7 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood RA, Chapman MD, Adkinson NF Jr., Eggleston PA The effect of cat removal on allergen content in household-dust samples. J Allergy Clin Immunol 1989; 83:730–4. [DOI] [PubMed] [Google Scholar]
- 42.Shirai T, Matsui T, Suzuki K, Chida K. Effect of pet removal on pet allergic asthma. Chest 2005; 127:1565–71. [DOI] [PubMed] [Google Scholar]
- 43.Portnoy J, Kennedy K, Sublett J, Phipatanakul W, Matsui E, Barnes C, et al. Environmental assessment and exposure control: a practice parameter--furry animals. Ann Allergy Asthma Immunol 2012; 108:223 e1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 2005; 115:233–42. [DOI] [PubMed] [Google Scholar]
- 45.Knuffman JE, Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Martinez FD, et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol 2009; 123:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol 2007; 119:64–72. [DOI] [PubMed] [Google Scholar]
- 47.Bacharier LB, Guilbert TW, Zeiger RS, Strunk RC, Morgan WJ, Lemanske RF, Jr, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol 2009; 123:1077–82, 82 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicolai A, Frassanito A, Nenna R, Cangiano G, Petrarca L, Papoff P, et al. Risk Factors for Virus-induced Acute Respiratory Tract Infections in Children Younger Than 3 Years and Recurrent Wheezing at 36 Months Follow-Up After Discharge. Pediatr Infect Dis J 2017; 36:179–83. [DOI] [PubMed] [Google Scholar]
- 49.Stein RT, Holberg CJ, Sherrill D, Wright AL, Morgan WJ, Taussig L, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children’s Respiratory Study. Am J Epidemiol 1999; 149:1030–7. [DOI] [PubMed] [Google Scholar]
- 50.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. Combined Impact of Smoking and Early-Life Exposures on Adult Lung Function Trajectories. Am J Respir Crit Care Med 2017; 196:1021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wongtrakool C, Grooms K, Bijli KM, Crothers K, Fitzpatrick AM, Hart CM. Nicotine stimulates nerve growth factor in lung fibroblasts through an NFkappaB-dependent mechanism. PLoS One 2014; 9:e109602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng X, Guan W, Zheng J, Ye P, Liu S, Zhou J, et al. Smoking influences response to inhaled corticosteroids in patients with asthma: a meta-analysis. Curr Med Res Opin 2012; 28:1791–8. [DOI] [PubMed] [Google Scholar]
- 53.Andrews AL, Bundy DG, Simpson KN, Teufel RJ 2nd, Harvey J, Simpson AN. Inhaled Corticosteroid Claims and Outpatient Visits After Hospitalization for Asthma Among Commercially Insured Children . Acad Pediatr 2017; 17:212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beck AF, Huang B, Auger KA, Ryan PH, Chen C, Kahn RS. Explaining Racial Disparities in Child Asthma Readmission Using a Causal Inference Approach. JAMA Pediatr 2016; 170:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck AF, Huang B, Simmons JM, Moncrief T, Sauers HS, Chen C, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics 2014; 133:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silber JH, Rosenbaum PR, Calhoun SR, Reiter JG, Hill AS, Guevara JP, et al. Racial Disparities in Medicaid Asthma Hospitalizations. Pediatrics 2017; 139. [DOI] [PubMed] [Google Scholar]
- 57.Taylor-Robinson DC, Pearce A, Whitehead M, Smyth R, Law C. Social inequalities in wheezing in children: findings from the UK Millennium Cohort Study. Eur Respir J 2016; 47:818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollenbach JP, Schifano ED, Hammel C, Cloutier MM. Exposure to secondhand smoke and asthma severity among children in Connecticut. PLoS One 2017; 12:e0174541. [DOI] [PMC free article] [PubMed] [Google Scholar]