Abstract
Deficits in social engagement emerge in autism during the infant and toddler period and may be related to emotion regulation and stress response systems. This study examined patterns of growth in autonomic functioning related to autism diagnosis and addresses the hypothesis that there are differences in autonomic functioning related to autism in infancy. Heart rate (HR) and respiratory sinus arrhythmia (RSA) were measured at 8 time points from 1 to 72 months of age in infants later diagnosed with autism (n=12) and a non-autistic comparison group (n=106). Multilevel models were used to describe the developmental course of HR and RSA and to test the effect of autism diagnosis on growth trajectories. Both groups showed an expected age-related decrease in HR and increase in RSA. Groups did not differ in the rate of decrease of HR over time. However, infants with autism demonstrated a smaller linear increase in RSA, indicating slower growth in RSA over time in comparison to controls. These results suggest that differences in physiological regulation may develop with age in autism. The slowed RSA growth in autism was most evident after 18 months of age, at a time when behavioral symptoms become prominent. This is consistent with the view that RSA is a marker of functional status in autism rather than a cause of social deficits in autism.
Introduction
Potential markers of risk for autism in infancy include diminished social responses (Chawarska, Macari, & Shic, 2013), visual attention to social information (Jones & Klin, 2013) atypical cry and vocal development (Esposito & Venuti, 2010; Patten et al., 2014; Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011; Sheinkopf, Iverson, Rinaldi, & Lester, 2012; English, Tenenbaum, Levine, Lester, & Sheinkopf, 2018), and delays in early motor development (Iverson & Wozniak, 2007). Deficits in social communication and social attention associated with autism emerge as infants approach 12 months of age and become more prominent during the toddler and preschool period (Jones & Klin, 2013; Landa & Garrett-Mayer, 2006; Ozonoff et al., 2010). Physiologic systems regulating arousal and attention are thought to play a role in the development of social behaviors in autism and may be useful as biomarkers of risk or functional status in this population.
One such physiologic measure of regulation is respiratory sinus arrhythmia (RSA). RSA is an index of central nervous system regulation of heart rate and is a measure of the autonomic response system. RSA is mediated by phasic inhibitory input to the heart from brainstem nuclei via the vagus, resulting in oscillations in heart rate related to respiration. Higher RSA amplitude has been proposed to be an indicator of the integrity of a neural system supporting social engagement (Beauchaine, 2001; Porges, 2001; Porges et al., 2013). However, diminished RSA has also been linked to stress response and early adversity and experience (Conradt, Abar, et al., 2013). Thus, diminished RSA in autism may be thought of as either impairing social engagement or as secondary to the overall level of disability present in autism. Longitudinal studies during early development will help to differentiate these possible models.
Some studies have found that RSA is diminished in autism and correlated with social and communication functioning (Cohen, Masyn, Mastergeorge, & Hessl, 2013; Vaughan Van Hecke et al., 2009). However, other studies have failed to find differences in RSA associated with autism (Levine et al., 2012; Sheinkopf, Neal-Beevers, Levine, Miller-Loncar, & Lester, 2013), or instead have found highly variable physiologic responses (Toichi & Kamio, 2003). There is evidence that higher RSA is associated with better language skills in children with autism (Patriquin, Lorenzi, Scarpa, & Bell, 2013; Watson, Baranek, Roberts, David, & Perryman, 2010), and that changes in RSA in response to a social challenge is associated with social adaptive functioning in young children with autism (Sheinkopf et al., 2013). A relevant study of typically developing children found that RSA trajectories in infancy and early childhood correlated with measures of social engagement (Patriquin, Scarpa, Friedman, & Porges, 2013).
In typically developing children, the amplitude of RSA increases over the course of infancy and approaches adult levels by 6 years of age (Bornstein & Suess, 2000; Porges, Doussard-Roosevelt, Portales, & Suess, 1994). One recent study found that 4-month-old infants later diagnosed with autism had an altered RSA response to a social stressor as compared to non-autistic infants (McCormick et al., 2018). This study found lower RSA during a mildly stressful social challenge, specifically a face-to-face interaction with a novel adult. This preliminary finding suggests that differences in regulatory capacity may be evidenced in early infancy if measured in the appropriate context. However, the early trajectory of RSA in children with autism is not known, and mapping the early developmental course of RSA would improve our understanding of which aspects of autism are primary to the disorder and which are secondary outcomes or effects of autism over time. One possibility is that diminished RSA is present early in development as a precursor of later developing symptoms. Alternatively, RSA may be an index of functional status that is secondary to or an effect of stress and coping processes associated with autism.
The current study examined RSA longitudinally in a sample of infants observed from the neonatal period and later diagnosed with autism. Subjects were ascertained from a longitudinal cohort that was large enough to yield a sample of infants with autism, but not selected on the basis of risk for autism specifically. Most research on autism in infancy uses an infant sibling design, based on recruiting infant siblings of children with autism. While such enriched risk samples have the advantage of increasing the likelihood of studying infants who have a later diagnosis, results from such studies may not be generalizable to the more general population of children with autism. This study employed a case control methodology to identify an unbiased comparison sample from the longitudinal cohort, controlling for pre- and perinatal risks present in the sample. Longitudinal growth analyses were used to model the developmental course of RSA in these two groups. Developmental changes in heart rate (HR) were also examined as it can be expected that as RSA increases developmentally (Bornstein & Suess, 2000), HR should decrease over the same time frame (Flemming et al, 2011).
Methods
Participants
Infants were selected from a sample of 1,388 infants from the Maternal Lifestyle Study (MLS), a multi-site investigation of the effects of prenatal exposure to cocaine and other substances (Lester et al., 2001; Lester et al., 2003). The study had approval from the institutional review board at each site. Prenatal cocaine exposure was identified by maternal report or positive meconium assay. History of prenatal exposure to other substances (tobacco, alcohol, marijuana) was determined by maternal interview (opiate exposure was exclusionary for this analysis). Details on the sampling criteria and cohort matching have been previously reported (Lester et al., 2003).
Children with possible autism diagnoses were identified using information collected from study-specific medical history interview forms that were used to ask caregivers about basic health history at each study visit, and by having study site personnel review study records for notes that indicated potential autism diagnoses. There were 19 children with potential diagnoses of autism. Diagnostic confirmation was performed at age 9 years or later. Diagnostic confirmation was defined as having scores that exceeded standard thresholds for autism or autism spectrum on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1989), and overall clinical impression by an experienced clinician (author 1 or 2). Diagnosis was confirmed for 13 of 19 children. Of the children with unconfirmed diagnoses, 4 could not be contacted and 2 had below threshold scores on the ADOS and clinician impressions that autism was not present.
A case-control comparison sample was developed by first identifying potential controls from the overall MLS sample who were matched on the presence or absence of cocaine exposure, presence or absence of other substance exposure, and birth weight (in 3 groups: <1500g, 1500-2499g, and ≥2500g). Each autistic case had a variable number of potential non-autistic matches, with some cases competing for controls with similar characteristics. The number of controls to be selected as matches was limited by the cases with the fewest available controls. Based on this approach, nine (9) controls per autism case were randomly selected from the group of potential controls. Children born less than 36 weeks gestational age (obstetrician best estimate) were excluded from these analyses, including one child with autism born at 28 weeks. Two additional controls were excluded due inconsistent data on birth weight and gestational age. The final sample included 12 children with autism and 106 comparison participants. Characteristics of the groups are presented in Table 1.
Table 1:
Subject Characteristics
| Autistic (n=12) | non-Autistic (n=106) | p | |
|---|---|---|---|
| Gender (n) | .001 | ||
| Male | 12 | 58 | |
| Female | 0 | 48 | |
| Race/Ethnicity (n) | .001 | ||
| Black | 4 | 86 | |
| White | 6 | 17 | |
| Hispanic | 2 | 3 | |
| SES – Hollingshead (n) | ns | ||
| Mid/High (levels I – III) | 7 | 49 | |
| Low (levels IV– V) | 5 | 56 | |
| Prenatal Drug Exposure, n (%) | |||
| Cocaine | 3 (25%) | 35 (33%) | ns |
| Other drugs/substances | 11 (91%) | 79 (75%) | ns |
| Gestational Age, weeks: mean (SD) | 38.8 (1.9) | 38.4 (1.7) | ns |
| Birth weight, grams: mean (SD) | 3191.5 (722.9) | 3033.2 (645.5) | ns |
| APGAR, 5 minutes: median | 9 | 9 | ns |
Because the MLS was not initially designed as a study of autism, and because of variation in later functional levels in the children with autism, standardized assessments of cognitive and language abilities were not available at all ages in the children with autism. The Bayley Scales of Infant Development (Bayley, 1993) were administered at 12, 24, and 36 months, and the Wechsler Preschool and Primary Scales of Intelligence, revised (Wechsler, 1989) at 48 and 54 months of age. Composite scores from both measures have a mean of 100 and a standard deviation of 15. Overall developmental ability level was estimated from the composite score from the latest available age at which an individual was assessed. For the autism group, the mean cognitive score was 72.6 (SD = 24.6; min = 50; max = 127), with 5 of 12 (42%) having scores ≥ 80. The latest available cognitive scores for the autism group were as follows: 12 month Bayley scales for 2 children, 36 month Bayley scales for 1 child, 48 month WPPSI scales for 3 children and 54 months WPPSI scores for 6 children (one child did not have cognitive scores at any time point). For the non-autistic comparison group, the mean cognitive score at 54 months was 84.0 (n = 79; SD = 13.4; min = 60; max = 120), with 64% having scores ≥ 80.
ECG Acquisition and Processing
Electrocardiogram (ECG) recordings were collected at multiple time points beginning at 1 month of age (assessment ages were corrected for prematurity up to age 24 months). ECG was recorded via three electrodes placed on the chest and abdomen. The ECG signal was sampled at 1kHz and stored on a computer for later analysis. Interbeat intervals were defined by detection of R-waves to the nearest millisecond.
A series of automated algorithms were used to detect R-R (inter-beat) intervals outside of expected values. Missed or spurious R-waves were flagged and corrected by linear interpolation. Heart rate (HR) and RSA were derived from the R-R time-series. A 21-point moving polynomial was applied to remove low frequency trends in the HR signal. A bandpass filter extracted the variance in heart period within the frequency band of spontaneous respiration in infants (0.24-1.04 Hz) by removing periodicities in the ECG signal that are outside the frequency range of the respiratory cycle. RSA was computed as the natural logarithm of heart period variance and reported in units of milliseconds (ms) squared, ln(ms)2 using Porges’ algorithm (Porges, 1985, 1986). RSA was calculated in 30 second overlapping windows and then averaged within each observational period at each age of measurement. The RSA data for an individual was used as long as there was a 30-s segment with less than 20% of the segment identified with artifact (Jennings et al., 1981). Small amounts of artifact can be expected to have a minimal effect on measures of heart rate variability such as RSA (Berntson et al., 1997). The ECG acquisition and analysis process used in this study have been previously reported (Conradt et al., 2013; Sheinkopf et al., 2008).
ECG was acquired at 1, 12, 18, 24, 36, 48, 60, and 72 months of age. At 1 month, RSA and HR were assessed during the orientation portion of a newborn infant examination, the NICU Network Neurobehavioral Scales (NNNS; Lester & Tronick, 2004). This section of the NNNS was selected because it offered a contiguous period of time (about 6 minutes) when infants were in a calm but awake state. At the other ages, RSA and HR were assessed during 3 minutes of quiet toy play while children sat on a parent’s lap or in a high chair or chair as appropriate for the child’s age. The recording conditions (procedures) for ECG acquisition were consistent across children within each time point. These observations were not designed to be highly stressful and thus were useful as a means to assess time related trends in HR and RSA across the first 6 years of life. For the non-autistic children estimates of RSA across this age range were similar to those reported by other studies using the same algorithm for calculating RSA (Bornstein & Suess, 2000; Calkins & Keane, 2004; Patriquin, Lorenzi, et al., 2013; Porges et al., 1994).
Analytic Strategy
Multilevel models were fit in SAS 9.4 with the PROC MIXED procedure using a full information maximum likelihood estimator to account for missing data over time. Missing data was due to a child missing a specific age visit or technical or administration problems resulting in missing or unusable physiology data. Heart rate and RSA were available for the follow number of participants at each age point: 116 (1 month); 68 (21 months); 57 (18 months); 46 (24 months); 63 (36 months); 65 (48 months); 57 (60 months); 63 (72 months). Three models were tested to examine change in RSA and HR across this developmental period: no growth, linear slope, and quadratic growth. For the linear and quadratic models, time was entered as age at assessment. Group (ASD) and biological sex (female) were included in the model as main effects. In the linear and quadratic models, interactions between group (ASD) and slope were included to examine group differences in growth over time. In the case of significant interactions between group and slope, the model was re-run with time centered at each assessment (1 month, 12 months, 18 months, 2 years, 3 years, 4 years, 5 years, 6 years) to explore the main effect of group at each age point. Model fit was evaluated with akaike information criteria (AIC), bayesian information criteria (BIC), and chi-square log-likelihood deviance tests.
Results
Heart Rate
Results indicated that the model with a random intercept and fixed linear and quadratic terms provided the best for the data. The interaction between diagnostic group and the linear and quadratic slope terms reached significance, therefore group differences were tested at each time point by recentering age. Main effect of group reached significance when the model was centered at 18 months and subsequent assessment points up to age 5. Results are reported from the model centered at the 18-month time point. Model parameters are reported in Table 2. The significant main effect of diagnostic group (β = 6.42, p < .05) revealed that on average the ASD group had a higher heart rate, 132.70 CI [ 129.89, 135.52] than the control group, 126.28 CI [125.27, 127.29]. The model revealed a significant main effect of sex (β = 4.38, p < .01). On average, females demonstrated higher heart rate, 130.66 CI [129.28, 132.04] compared to males 126.28 CI [125.27, 127.29]. The significant negative linear slope (β=−1.29, p <.001) and positive quadratic slope (β= .01, p <.001) indicate that for controls HR declines across this developmental period but that decline gradually decreases. The significant linear (β = 0.38, p < .01) and quadratic (β = −0.01, p <.05) interaction terms with diagnostic group indicate the infants with an ASD outcome have a more attenuated decrease in HR compared to controls and essentially no plateauing during this developmental period.
Table 2.
Parameter estimates of HR and RSA at the 18 month time point
| HR | RSA | |
|---|---|---|
| Fixed effects | ||
| Intercept (controls) | 126.28**** | 4.10**** |
| age | −1.29**** | 0.07**** |
| Age*age | 0.01**** | −0.001**** |
| ASD | 6.42* | −0.76** |
| sex | 4.38** | −0.10 |
| Age*ASD | 0.38** | −0.04*** |
| Age*age*ASD | −0.01* | 0.001** |
| Random effects | ||
| Within-person | 108.15*** | 0.82*** |
| Intercept | 23.90*** | 0.31*** |
Note.
p<.05
p<.01
p<.001
p<.0001
Respiratory Sinus Arrhythmia
Results indicated that the model with a random intercept fixed linear and quadratic terms provided the best fit for the data. The interaction between diagnostic group and the linear and quadratic slope terms reached significance, therefore group differences were tested at each time point by recentering age. Main effect of group reached significance when the model was centered at 18 months and subsequent assessment points up to age 60 months. The group difference was not statistically significant at 72 months. Results are reported from the model centered at the 18-month time point. Model parameters are reported in Table 2. At this time point, children in the ASD group demonstrated lower RSA, 2.98 CI [2.71, 3.25], than controls, 4.50 CI [4.28, 4.72], The significant linear (β = 0.07, p <.0001) and quadratic (β = −0.0008, p <.0001) terms indicate that in the control group RSA increases and then gradually flattens. The significant linear (β = −0.04, p <.001) and quadratic (β = 0.001, p < .01) interaction terms with group indicate that participants with ASD have a slower increase in RSA compared to controls and essentially no plateauing across this developmental period. These results are depicted in Figure 1.
Figure 1:
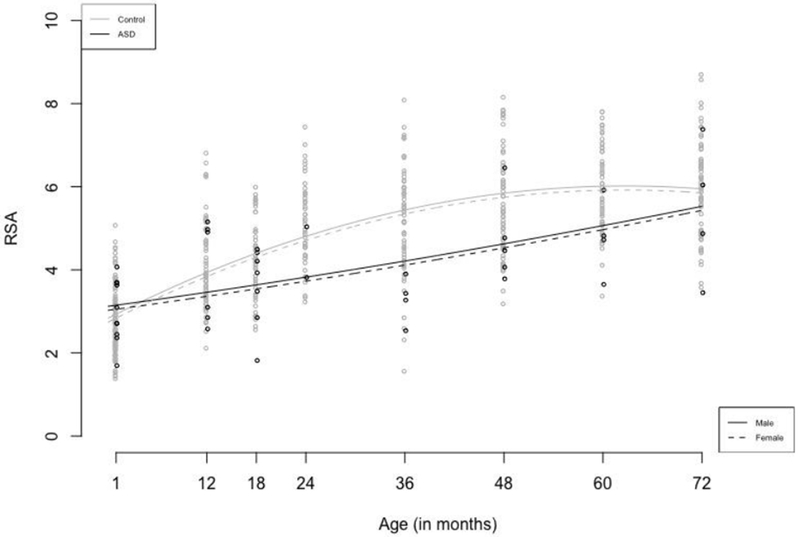
Growth in RSA for the infants with later autism diagnoses in comparison to non-autistic infants from 1 to 72 months of age.
Model results indicated that the main effect of sex did not reach significance. In addition, we evaluated the potential impact of cognitive ability at outcome as a potential confound to the earlier RSA differences. We tested whether RSA was correlated with IQ at 54 months within the control group. None of the correlation coefficients reached statistical significance, and only one of the tests had a p value <0.10 (the correlation between RSA at 24 months outcome IQ was r = −0.25, p = .08).
Discussion
Infants later diagnosed with autism were matched to a longitudinal comparison group and measures of heart rate (HR) and respiratory sinus arrhythmia (RSA) were examined over time. Growth curve models revealed that the autistic group had diminished growth in RSA, with lower RSA in the autistic group by the 18 month assessment point as compared to the non-autistic children. This result suggests that disruptions in physiologic systems regulating engagement with the environment emerge, or at least magnify with development in autism.
These findings are unique in part because they are based on prospective, longitudinal observations of infants later diagnosed with autism. Roberts et al. (2012) reported that lower RSA was associated with increased autism symptoms in a group of infants with Fragile X syndrome beginning at 22 months of age. The age at which RSA growth in the ASD group diverged from the comparison group in the current results is consistent with the age range studied by Roberts et al. where relations between autism symptoms and RSA were found. The current results are also consistent with findings from a non-clinical cohort by Patriquin et al. (2013) who reported that a two-group model explained individual differences in RSA over a similar time frame as the present study. Patriquin et al. identified a subgroup of infants with diminished RSA growth in the 24 to 48 month age range coinciding with parental ratings of diminished social functioning. The current study adds to this body of evidence by showing that developmental differences in RSA, a measure of autonomic regulatory capacity, emerge longitudinally in infants later diagnosed with autism.
Slower RSA growth resulted in the group differences reaching statistical significance by 18 months of age, and group differences remained significant out to the 60-month time point. This finding is additional evidence that RSA is a marker of functional status in autism at specific ages (Patriquin, Scarpa, et al., 2013; Roberts et al., 2012; Sheinkopf et al., 2013; Vaughan Van Hecke et al., 2009). However, it is possible that the lack of initial differences in RSA in early infancy is the result of developmental floor effects on RSA early in life and/or due to low statistical power due the small sample size of the autistic group in this study. An alternative explanation is that differences in RSA (and perhaps other measures of stress, coping, and regulatory processes) are not present early in life and could be secondary to the social communication and developmental impairments that become especially prominent in the toddler and preschool period. The current findings are consistent with this latter explanation, but the presence of smaller but meaningful differences in RSA earlier in infancy cannot be ruled out.
While the autism group had lower RSA values from 18 to 60 months, there was not a significant group difference at 72 months. However, visual inspection of the data in Figure 1 indicates that there was a significant amount of variability in the autism group. Certainly other studies have reported a high degree of variability in autonomic cardiorespiratory responses in individuals with autism (Levine et al, 2012; Sheinkopf et al. 2013, Toichi & Kamio, 2003). There is strong interest in the nature of heterogeneity in course and outcome of individuals with autism, and biologically based measures, including indices of autonomic responses, have the potential to help parse this heterogeneity into clinically meaningful subgroups (Masi, DeMayo, Glozier & Guastell, 2017). While speculative, it is possible that the individual differences seen at the end of the observational period in this study may be reflective of underlying subgroups. Clearly, however, much larger sample sizes will be needed to determine the presence or absence, and clinical significance of such subgroups.
A unique aspect of this study is the use of a non-infant-sibling design to study autism in infancy. Studies of infants later diagnosed with autism have primarily relied upon observations of infants who have an older sibling with this condition, and prior review papers have nicely summarized this important work (Jones, Gliga, Bedford, Charman, & Johnson, 2014; Rogers, 2009; Zwaigenbaum et al., 2006). Such infant sibling studies have the advantage of increased efficiency of identifying babies who go on to have a diagnosis. However, the drawback to the infant sibling approach is that there is unknown generalizability of findings to the wider population of individuals with autism. Both enriched risk cohort designs (such as infant sibling studies) and research on less selected samples are needed to provide a full view of the range of developmental patterns associated with later diagnoses. By leveraging a large longitudinal cohort of infants, this study provides one model for such research.
Limitations of this study are acknowledged. First, the size of autism group was relatively small, and there was missing data on infants at various time points. The latent modeling method addressed this problem of missing data. The small sample size limits statistical power to detect differences at specific ages. The limited sample size also precludes the identification of subgroups of children with autism. Lower RSA associated with autism have been reported only inconsistently in prior research (Benevides & Lane, 2015), and this may in part be a function of variable underlying characteristics in children with autism. Nonetheless, the shape of the growth curve in RSA was statistically different in the infants with autism than in the controls, indicating that there is likely to be an interaction between age and diagnosis on developmental changes in regulatory processes across infancy. Describing the nature and timing of departures from typical developmental processes can be expected to inform efforts to improve screening and treatment development (Jones & Klin, 2009). Second, participants were drawn from a high-risk cohort designed to investigate the developmental effects of prenatal drug exposure. Prior reports based on this cohort have documented effects of prenatal drug exposure on RSA at one month of age (Conradt, Sheinkopf, et al., 2013), and combined effects of prenatal substance exposure and environmental adversity on RSA growth between 3 and 6 years of age (Conradt, Abar, et al., 2013). In the present study, the case-control method matched infants on the presence of pre- and perinatal risks reducing the likelihood that our findings were confounded influences of prenatal substance exposure, birth weight, or prematurity.
In addition, we evaluated the potential for both cognitive level at outcome and sex to be potential confounding factors. With respect to cognitive levels, we did not find any significant correlations between RSA at any age point and later cognitive ability. While variation in cognitive outcome is unlikely to play a significant role in these specific results, appropriate matching and/or statistical control in future studies will be needed to clarify both the sensitivity and specificity of differences in autonomic functioning in relation to ASD outcomes. We also evaluated the potential impact of sex on our findings. We did not find a sex effect on RSA levels or growth. However, we did find that heart rate was higher in females than males in the control group. An inspection of confidence intervals for the HR model indicated that the ASD group (all males) had higher heart rate than both the overall control group and the males in the control group. Some normative data report higher rate for females , with differences smaller and less consistent in childhood and somewhat larger in adolescence (Semizel, Ozturk, Bostan, Cil, & Ediz, 2008). However, the group difference on heart rate was not an effect of sex, and sex differences did not appear to confound the effects reported in this study. Nonetheless, as noted by Benevides and Lane (2015), prior research on autonomic functioning in ASD has not consistently controlled for sex or gender. Such effects should be controlled for in future work in this area. In addition, larger study samples will allow for tests of sex and gender differences within groups who share an ASD diagnosis.
Conclusion
This is the first study to describe the longitudinal course of autonomic functioning in infants later diagnosed with autism. Specifically, infants with later autism diagnoses had attenuated growth in RSA, a physiologic process related to social and adaptive functioning. The results suggest that differences in physiological regulation may develop with age in autism, with attenuations in RSA emerging during a period where autism symptoms become prominent and have been shown to be associated with reduced RSA in other samples. This is consistent with the view that RSA is a marker of functional status in autism and raises the hypothesis that differences in RSA emerge as a secondary to symptoms and experiences related to autism. Measures of autonomic function may prove useful as biomarkers of functioning and perhaps early treatment effects in infants at risk for autism.
Highlights.
Infants with later autism demonstrated expected decline in resting heart rate
Infants with later autism had slower growth in heart rate variability
Lower heart rate variability in infants with autism was seen by 18 months of age
Acknowledgments
This research was supported by the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network and an inter institute agreement with the National Institute on Drug Abuse (NIDA) through cooperative agreements (U10-DA-024117-01, U10-HD-21385, U10-DA-024128-06, U10-HD-2786, U10-DA-024119-01, U10-HD-27904, U10-DA-024118-01, U10-HD-21397; NICHD contract N01-HD-2-3159), and Bailey’s Team for Autism. This research was partially supported by Institutional Development Award Number U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds Advance Clinical and Translational Research (Advance-CTR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephen J. Sheinkopf, Department of Psychiatry & Human Behavior and Department of Pediatrics, Warren Alpert Medical School of Brown University
Todd P. Levine, Department of Psychiatry & Human Behavior and Department of Pediatrics, Warren Alpert Medical School of Brown University
Carolyn E. B. McCormick, Department of Human Development and Family Studies, Purdue University
Gavino Puggioni, Department of Computer Science and Statistics, University of Rhode Island.
Elisabeth Conradt, Department of Psychology, University of Utah.
Linda L. LaGasse, Department of Pediatrics and Department of Psychiatry & Human Behavior, Warren Alpert Medical School of Brown University
Barry M. Lester, Department of Psychiatry & Human Behavior and Department of Pediatrics, Warren Alpert Medical School of Brown University
References
- Bayley N (1993). Bayley Scales of Infant Development, Second Edition San Antonio: Psychological Corporation. [Google Scholar]
- Beauchaine T (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol, 13(2), 183–214. [DOI] [PubMed] [Google Scholar]
- Benevides TW, & Lane SJ (2015). A review of cardiac autonomic measures: considerations for examination of physiological response in children with autism spectrum disorder. J Autism Dev Disord, 45(2), 560–575. doi: 10.1007/s10803-013-1971-z [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … Van der Molen MW (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34, 623–648. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, & Suess PE (2000). Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Dev Psychol, 36(1), 54–65. [PubMed] [Google Scholar]
- Calkins SD, & Keane SP (2004). Cardiac vagal regulation across the preschool period: stability, continuity, and implications for childhood adjustment. Dev Psychobiol, 45(3), 101–112. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol Psychiatry, 74(3), 195–203. doi:S0006-3223(12)01030-X [pii] 10.1016/j.biopsych.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Masyn K, Mastergeorge A, & Hessl D (2013). Psychophysiological Responses to Emotional Stimuli in Children and Adolescents with Autism and Fragile X Syndrome. J Clin Child Adolesc Psychol. doi: 10.1080/15374416.2013.843462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Abar B, Sheinkopf S, Lester B, Lagasse L, Seifer R, … Higgins R (2013). The role of prenatal substance exposure and early adversity on parasympathetic functioning from 3 to 6 years of age. Dev Psychobiol. doi: 10.1002/dev.21155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Sheinkopf SJ, Lester BM, Tronick E, Lagasse LL, Shankaran S, … Hammond JA (2013). Prenatal Substance Exposure: Neurobiologic Organization at 1 Month. J Pediatr, 163, 989–994. doi:S0022-3476(13)00498-8 [pii] 10.1016/j.jpeds.2013.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English MS, Tenenbaum EJ, Levine TP, Lester BM, & Sheinkopf SJ (2018) Perception of cry characteristics in infants with autism spectrum disorder. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-018-3788-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, & Venuti P (2010). Developmental changes in the fundamental frequency (f0) of intants’ cries: a study of children with Autism Spectrum Disorder. Early Child Development and Care, 180(8), 1093–1102. doi: 10.1080/03004430902775633 [DOI] [Google Scholar]
- Fleming S, Thompson M, Stevens R, Heneghan C, Pluddemann A, Maconochie I, Tarassenko L, & Mant D (2011). Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet, 377 (9770), 1011–1018. doi: 10.1016/S0140-6736(10)62226-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, & Wozniak RH (2007). Variation in vocal-motor development in infant siblings of children with autism. J Autism Dev Disord, 37(1), 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Berg WK, Hutcheson JS, Obrist P, Porges S, & Turpin G (1981). Committee report. Publication guidelines for heart rate studies in man. Psychophysiology, 18(3), 226–231. [DOI] [PubMed] [Google Scholar]
- Jones EJ, Gliga T, Bedford R, Charman T, & Johnson MH (2014). Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci Biobehav Rev, 39, 1–33. doi:S0149-7634(13)00298-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, & Klin A (2009). Heterogeneity and homogeneity across the autism spectrum: the role of development. J Am Acad Child Adolesc Psychiatry, 48(5), 471–473. [DOI] [PubMed] [Google Scholar]
- Jones W, & Klin A (2013). Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature, 504, 427–431. doi:nature12715 [pii] 10.1038/nature12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, & Garrett-Mayer E (2006). Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry, 47(6) 629–638. [DOI] [PubMed] [Google Scholar]
- Lester BM, ElSohly M, Wright LL, Smeriglio VL, Verier J, Bauer CR, … Maza PL (2001). The Maternal Lifestyle Study: Drug Use by Meconium Toxicology and Maternal Self-Report. Pediatrics, 107(2) 309–317. [DOI] [PubMed] [Google Scholar]
- Lester BM, Lagasse L, Seifer R, Tronick EZ, Bauer CR, Shankaran S, … Maza PL (2003). The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. J Pediatr, 142(3) 279–285. [DOI] [PubMed] [Google Scholar]
- Lester BM, & Tronick EZ (2004). History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics, 113(3 Pt 2), 634–640. [PubMed] [Google Scholar]
- Levine TP, Sheinkopf SJ, Pescosolido M, Rodino A, Elia G, & Lester BM (2012). Physiologic Arousal to Social Stress in Children with Autism Spectrum Disorders: A Pilot Study. Res Autism Spectr Disord, 6(1), 177–183. doi: 10.1016/j.rasd.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, & Schopler E (1989). Autism Diagnostic Observation Schedule: A standardized observation of communicative and social behavior. J Autism Dev Disord, 19, 185–212. [DOI] [PubMed] [Google Scholar]
- Masi A, DeMayo MM, Glozier N, & Guastella AJ (2017). An overview of Autism Spectrum Disorder, heterogeneity and treatment options. Neuroscience Bulletin, 33, 183–193. DOI 10.1007/s12264-017-0100-y 10.1007/s12264-017-0100-ywww.springer.com/12264www.springer.com/12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CEB, Sheinkopf SJ, Levine TP, LaGasse LL, Tronick E, & Lester BL (2018). Diminished respiratory sinus arrhythmia response in infants later diagnosed with autism spectrum disorder. Autism Res. doi: 10.1002/aur.1929 [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Young GS (2010). A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry, 49(3), 256–266 e251–252. doi:00004583-201003000-00009 [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, Lorenzi J, Scarpa A, & Bell MA (2013). Developmental trajectories of respiratory sinus arrhythmia: Associations with social responsiveness. Dev Psychobiol., 56(3) 317–326. doi: 10.1002/dev.21100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, Scarpa A, Friedman BH, & Porges SW (2013). Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Dev Psychobiol, 55(2), 101–112. doi: 10.1002/dev.21002 [DOI] [PubMed] [Google Scholar]
- Patten E, Belardi K, Baranek GT, Watson LR, Labban JD, & Oller DK (2014). Vocal patterns in infants with autism spectrum disorder: canonical babbling status and vocalization frequency. J Autism Dev Disord, 44(10), 2413–2428. doi: 10.1007/s10803-014-2047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, & Klin A (2011). Out of the mouths of babes: vocal production in infant siblings of children with ASD. J Child Psychol Psychiatry, 52(5), 588–598. doi: 10.1111/j.1469-7610.2010.02332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (1985). U.S. Patent No. 4510944. U. S. Patent and Trademark Office. [Google Scholar]
- Porges SW (1986). Respiratory sinus arrhythmia: Physiological basis, quantitative methods, and clinical implications In Grossman P, Janssen K, & Vaitl D (Eds.), Cardiorespiratory and cardiosomatic psychophysiology (pp. 101–115). New York: Plenum Press. [Google Scholar]
- Porges SW (2001). The polyvagal theory: Phylogenetic substrates of the social nervous system. International Journal of Psychophysiology, 42, 123–146. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, & Suess PE (1994). Cardiac vagal tone: stability and relation to difficultness in infants and 3-year-olds. Dev Psychobiol, 27, 289–300. [DOI] [PubMed] [Google Scholar]
- Porges SW, Macellaio M, Stanfill SD, McCue K, Lewis GF, Harden ER, … Heilman KJ (2013). Respiratory sinus arrhythmia and auditory processing in autism: modifiable deficits of an integrated social engagement system? International Journal of Psychophysiology, 88(3), 261–270. doi:S0167-8760(12)00669-1 [pii] 10.1016/j.ijpsycho.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen B, Robinson A, & Shinkareva SV (2012). Heart activity and autistic behavior in infants and toddlers with fragile x syndrome. Am J Intellect Dev Disabil, 117(2) 90–102. doi: 10.1352/1944-7558-117.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ (2009). What are infant siblings teaching us about autism in infancy? Autism Res, 2(3), 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semizel E, Oztürk B, Bostan OM, Cil E, & Ediz B (2008). The effect of age and gender on the electrocardiogram in children. Cardiol Young 18, 26–40. [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Iverson JM, Rinaldi ML, & Lester BM (2012). Atypical Cry Acoustics in 6-Month-Old Infants at Risk for Autism Spectrum Disorder. Autism Res, 5(5), 331–339. doi: 10.1002/aur.1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lagasse LL, Lester BM, Liu J, Seifer R, Bauer CR, … Das A (2007). Vagal tone as a resilience factor in children with prenatal cocaine exposure. Dev Psychopathol, 19(3) 649–673. [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Neal-Beevers AR, Levine TP, Miller-Loncar C, & Lester B (2013). Parasympathetic response profiles related to social functioning in young children with autistic disorder. Autism Res Treat, 2013, 868396. doi: 10.1155/2013/868396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toichi M, & Kamio Y (2003). Paradoxical autonomic response to mental tasks in autism. J Autism Dev Disord, 33(4), 417–426. [DOI] [PubMed] [Google Scholar]
- Vaughan Van Hecke A, Lebow J, Bal E, Lamb D, Harden E, Kramer A, Porges SW (2009). Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Dev, 80(4), 1118–1133. doi:CDEV1320 [pii] 10.1111/j.1467-8624.2009.01320.x [DOI] [PubMed] [Google Scholar]
- Watson LR, Baranek GT, Roberts JE, David FJ, & Perryman TY (2010). Behavioral and physiological responses to child-directed speech as predictors of communication outcomes in children with autism spectrum disorders. Journal of Speech Language and Hearing Research, 53(4), 1052–1064. doi:1092-4388_2009_09-0096 [pii] 10.1044/1092-4388(2009/09-0096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1989). Wechsler Preschool and Primary Scales of Intelligence, Revised. San Antonio: Psychological Corporation. [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, … Sigman M (2006). Studying the Emergence of Autism Spectrum Disorders in High-risk Infants: Methodological and Practical Issues. J Autism Dev Disord. [DOI] [PubMed] [Google Scholar]


