Abstract
Traumatic Brain Injury (TBI) affects approximately 2.5 million people in the United States, of which 80% are considered to be mild (mTBI). Previous studies have shown that cerebral glucose uptake and metabolism are altered after brain trauma and functional metabolic deficits observed following mTBI are associated with changes in cognitive performance. Imaging of glucose uptake using [18F] Fluorodeoxyglucose (FDG) based Positron Emission Tomography (PET) with anesthesia during the uptake period demonstrated limited variability in results, but may have depressed uptake. Anesthesia has been found to interfere with blood glucose levels, and hence, FDG uptake. Conversely, forced cognitive testing during uptake may increase glucose demand in targeted regions, such as hippocampus, allowing for better differentiation of outcomes. Therefore, the objective of this study was to investigate the influence of a directed cognitive function task during the FDG uptake period on uptake measurements both in naïve rats and at 2 days after mild lateral fluid percussion (mLFP) TBI. Adult male Sprague Dawley rats underwent FDG uptake with either cognitive testing with the Novel Object Recognition (NOR) test or No Novel Object (NNO), followed by PET scans at baseline (prior to injury) and at 2days post mLFP. At baseline, FDG uptake in the right hippocampus was elevated in rats completing the NOR in comparison to the NNO (control group). Further, the NNO group rats demonstrated a greater fold change in the FDG uptake between baseline and post injury scans than the NOR group. Overall, these data suggest that cognitive activity during FDG uptake affects the regional uptake pattern in the brain, increasing uptake at baseline and suppressing the effects of injury.
Keywords: Fluorodeoxyglucose, Positron Emission Tomography, mild Traumatic Brain Injury, Novel Object Recognition
1. Introduction
Traumatic brain injury (TBI) is the signature injury of the recent United States military conflicts (Greer et al., 2016), and occurs frequently in the civilian population as well, in sports, motor vehicle accidents, falls, etc. The most common TBI’s are mild (mTBI) (Bazarian et al., 2005; Langlois et al., 2006). TBI, even mTBI, often results in cognitive deficits which can adversely affect the quality of life of patients. These neuropsychological deficits are often associated with complex cerebral metabolic alterations (Arciniegas et al., 2005). Several studies have shown the effect of cerebral glucose metabolism after TBI using positron emission tomography (PET) (Bergsneider et al., 2001; Moore et al., 2000a; Yoshino et al., 1991). 2-dexy-2[18F]fluoro-D-glucose (FDG) PET imaging allows for the quantitative evaluation of functional and molecular processes in vivo, and can be used to investigate longitudinal changes during recovery or with treatment intervention.
FDG PET imaging has shown that brain energy metabolism after a TBI typically follows a period of hyperactivity lasting approximately 30 minutes (in rats) to a few hours (in humans) followed by a period of metabolic depression lasting for 5–10 days (in rats) to 30 days (in humans) (Hovda et al., 1995; Selwyn et al., 2016). In animal models, long lasting cognitive deficits are observed not only after severe brain injury but also after moderate and even mild injury, which do not involve appreciable neuronal cell loss (Moore et al., 2000b; Yoshino et al., 1991).
TBI not only causes damage at the site of impact but initiates cellular and molecular processes that leads to delayed or secondary neuronal injury in the surrounding tissues. We have shown that the sub-cortical hippocampus is particularly sensitive to damage after TBI (Brabazon et al., 2017; Selwyn et al., 2016). The hippocampus demonstrates significant alterations in glucose uptake after injury (Brabazon et al., 2017; Cho et al., 2010; Selwyn et al., 2013; Selwyn et al., 2016). Hippocampal damage is often associated with impairment in learning and memory, and tasks that evoke learning and memory function can induce glucose uptake or blood flow in the hippocampus (Newman et al., 2011; Xie et al., 2016). In particular, object recognition and recollection tasks have been shown to induce activity in the hippocampus (Cohen and Stackman, 2015; Gomes et al., 2016). The novel object recognition (NOR) task uses spatial novelty and delayed non-matching to evaluate time spent with a novel object versus no-object as a measure of spatial memory, which is hippocampal dependent (Antunes and Biala, 2012; Bevins and Besheer, 2006).
Previous studies of FDG PET imaging in rodents following TBI involves both anesthetized and awake (conscious) periods during the FDG uptake (Park et al., 2017; Selwyn et al., 2013). The conscious animal uptake shows a three-fold variation in regional brain uptake that is dependent on the environmental conditions (activity, sleep, temperature, etc.), whereas anesthetized animals show less than two-fold variation (Byrnes et al., 2014). However, a detailed analysis of the effect of performance of a standardized cognitive task during glucose metabolism assessment after mTBI has not been conducted yet. In this study, we therefore aimed to evaluate FDG uptake in both ipsilateral (left) and contralateral (right) brain with and without cognitive testing during FDG uptake. We hypothesize that cognitive testing during uptake would enhance FDG uptake into the hippocampus in naïve rats. Further, our previous studies demonstrated reduced hippocampal FDG uptake at sub-acute time-points after mild LFP (Selwyn et al., 2016); we therefore hypothesized that cognitive testing during this period would also increase uptake. Sub-goals of our work were to establish the potential effect of injury and cognitive testing on hippocampal laterality and the effect of testing on other brain regions. We now describe our finding of significant alterations in cerebral glucose metabolism in the contralateral (right) hemisphere following mTBI with and without NOR during FDG uptake.
2. Methods
2.1. Animals
Adult male Sprague Dawley rats (n=9; 300–440 grams, Taconic, Germantown, NY) were used in this study. The rats were given free access to food and water and a 12 hour light / 12 hour dark cycle. All animal procedures were approved by the Uniformed Services University Institutional Animal Care and Use Committee and complied fully with the principles set forth in the “Guide for the Care and Use of Laboratory Animals” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council (DHEW pub. No. (NIH) 85–23, 2985).
2.2. PET/CT Imaging, Reconstruction and Analysis
To monitor brain activity in defined brain regions in live anesthetized rats, we used PET/computed tomography (CT) imaging and the VivoQuant (inviCRO, Boston, MA) atlas-based quantification approach (Fig. 1A & B).
Fig. 1.
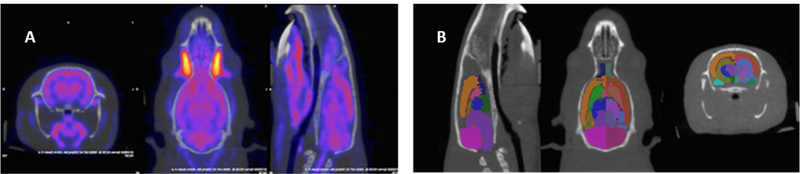
Co-registered PET/CT images showing atlas-based analysis using inviCRO VivoQuant software, v3.0.
A. Co-registered PET CT images cropped in the region surrounding the brain using automatic registration tool from VivoQuant software. B. Image showing 26 regions rat brain atlas (ROI’s) registered to the CT data (which is registered to PET, hence CT, atlas and PET all co-registered).
In this approach we used [18F] FDG uptake to monitor glucose uptake as an indicator of regional activity of 13 brain regions which includes basal ganglia, thalamus, amygdala, cerebellum, cortex, hypothalamus, midbrain, corpus callosum, olfactory, hippocampus, septal area, ventricles and white matter. Images were acquired at two time points: 1) baseline, (2–5 days prior to injury and 2) two days post injury. Animals were anesthetized (isoflurane, 4% for induction and 1–2% for maintenance, in oxygen at 2L/min) during the tail vein injection of FDG (1.67 ± 0.25 mCi ) and image acquisition. After injection, the animals were returned to a separate box for conscious uptake and either cognitive test (NOR; n=5) or non-directed exploration (No Novel Object, NNO; n=4) during uptake. The total uptake time before PET imaging was 45 minutes (30 minutes awake and 15 minutes anesthetized). The images were acquired using Siemens Inveon preclinical scanner (Erlangen, Germany). The PET scans were acquired for 30 minutes followed by a brief CT acquisition for anatomical localization and attenuation and scatter correction (Fig. 2A & B).
Fig. 2.
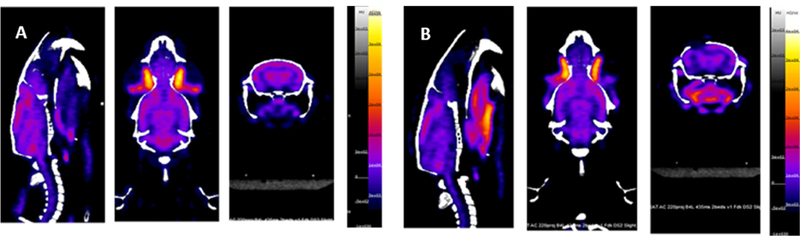
Representative PET/CT images of rat head acquired with Siemens Inveon preclinical scanner.
A. Baseline PET/CT image, prior to injury. B. PET/CT image acquired two days post injury. Note the craniectomy location on the left.
The PET images were acquired in list mode for 30 minutes with a coincidence timing window of 3.438 ns and the energy window set to 350–650 keV. The images were reconstructed as a single frame with 3D Ordered Subsets Expectation Maximization (OSEM) followed by Maximum A Posteriori (MAP) (2 OSEM iterations, 18 MAP iterations, requested resolution: 0.8mm) and corrected for attenuation and scatter (Siemens Inveon Workplace software, v2.1). The PET image dimensions were 256 × 256 × 159 with a voxel size of 0.39 × 0.39 × 0.08 mm.
The CT was acquired using a two bed CT at 500 μA and 80 kVp. The images were reconstructed using the Feldkamp algorithm, downsampled by a factor of 2, and beam hardening and Hounsfield Unit corrections were applied. The CT image dimensions were 384 × 384 × 425 with a voxel size of 0.22 mm3 isotropic.
The reconstructed PET and CT images were processed and analyzed using VivoQuant Software version 3.0 (inviCRO, Boston, MA). The PET data were registered to the 26 regions (left and right hemisphere) rat brain atlas (Brabazon et al., 2017). The Standardized Uptake Value (SUV), which is the activity concentration in a specific region normalized to the total injected activity and body weight, was calculated for each of the 26 regions and then normalized with the SUV of the entire atlas (SUVw, whole brain normalization). The Relative Uptake Value (RUV) (Byrnes et al., 2014; Selwyn et al., 2013; Selwyn et al., 2016). The final value obtained from either of the methods above is a semi quantitative value for the each of the 26 brain regions for each animal. To choose a region in the brain as reference tissue for RUV, one of the criteria is for it to be stable during the course of the experiment (Byrnes et al., 2014). The uptake concentration in cerebellum, both left and right remained unchanged at baseline scans in both NOR and NNO group, however, at 2 days post injury in NNO group there is a significant change in the uptake compared to baseline (Fig. 3A & B). Based on this data, we decided to continue our analysis using SUVw.
Fig. 3.
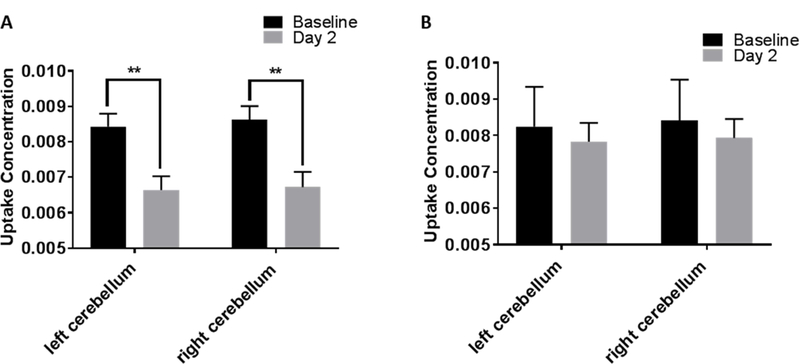
Plot showing uptake concentration in left and right cerebellum at baseline and 2 days post injury in NNO (n = 4) and NOR (n = 5) groups. A. In the NNO group, both left and right cerebellum shows change at 2 days post-injury compared to baseline. B. In the NOR group, no change was found in either left or right cerebellum at 2 days post-injury compared to baseline. (**p=0.001; Two-way RM ANOVA; bars = mean +/− SEM).
2.3. Novel Object Recognition (NOR) Test
The Novel Object Recognition (NOR) test was similar to that originally described by Ennaceur and Delacour (Ennaceur and Delacour, 1988). Each rat was placed in a large opaque box (20” × 20”) after FDG injection to acclimate for 10 minutes. The rat was then removed from the box to the home cage for 5 minutes. Two identical objects (Lego® Duplo ® blocks) were placed on one side of the box, with sufficient space for the rats to survey all sides of the objects. The objects were affixed to the box floor with tape. The rat was returned to the opaque box and the video recorder was turned on, pointing towards the box with a view of the rat head and 2 blocks. The rats was allowed to explore the objects for 5 minutes. The rat was then placed back in the home cage for 5 minutes. In the second testing phase, one of the objects (object on the left) was replaced with a novel object of a different material, shape and color (shot glass). The rat was returned to the opaque box and the video recorder was turned on for another 5 minute exploration. Before each phase of NOR test, the object and the box were cleaned with 70% ethanol.
Cumulative time spent by the rats at each of the objects, including time spent sniffing or climbing objects, during the first and second phase test was measured by a blinded observer reviewing the video tapes. Percent time spent with novel object was calculated.
Control rats, (NNO) spent the entire uptake time after recovery from anesthesia in the same opaque box without any objects.
2.4. Mild Lateral Fluid Percussion Injury (LFP)
Mild Lateral fluid percussion (LFP) injury was performed as previously described (Selwyn et al., 2013) with some modifications. Briefly, the rats were anesthetized with isoflurane (4% induction, 2% maintenance), temperature was measured rectally and maintained at 36.5° - 37.5°C. A 5 mm craniotomy was performed over the left parietal cortex midway between lambda and bregma and a 2.5 mm from the vertex. The fluid percussion device (VCU Health System Custom Design and Fabrication Model 01-B) was attached via a short piece to a plastic female luer lock that was cemented over the craniotomy. An impact with an estimated pressure of 1.5 atm was achieved by dropping a pendulum onto the water tube from an angle of 10°or 15°. No significant difference in any outcome measures was noted between rats receiving 10°or 15° impacts, so these groups have been combined in all reporting.
2.5. Statistics
Quantitative data are presented as mean +/− standard error of the mean. PET values and functional data were obtained by an investigator blinded to the groups. Data were analyzed using unpaired t-test, two-way ANOVA with Sidak’s multiple comparisons post-test, repeated measures (RM) ANOVA or one-way ANOVA, as appropriate. All statistical tests were performed using the GraphPad Prism Program, version 7.01 for windows (GraphPad Software, San Diego, CA). A p value < 0.05 was considered statistically significant.
3. Results
3.1. Injury impairs function on the NOR test
NOR test was performed in the directed exploration group (n=5) at baseline (2–3 days prior to mLFP) and 2 days post injury. Prior to injury, rats spent an average of 53.6 +/− 5% of the time with the novel object. Following injury, this dropped to an average of 18.4 +/− 14% [*p=0.04, unpaired t-test] which is expected for this type of test (Zhao et al., 2012).
3.2. NOR does not affect global FDG uptake
To evaluate the effect of cognitive testing on the entire brain uptake, we calculated whole brain SUV in both, uninjured [baseline, Fig. 4A] and injured [2 days post injury, Fig. 4B] rats in NOR and NNO groups.
Fig. 4.
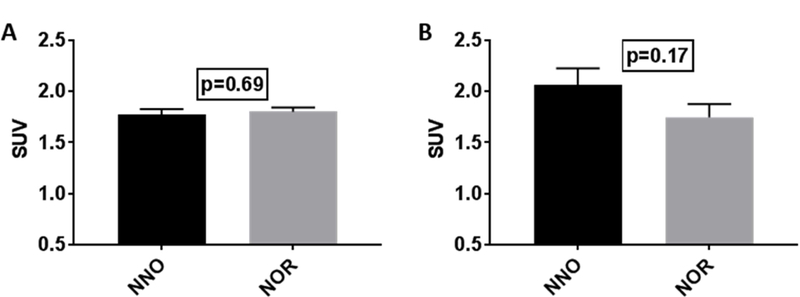
Whole Brain (WB) Standardized Uptake Value (SUV) plotted for the two groups (NNO; n=4 and NOR; n=5) at baseline and two days post injury.
A. No significant change in the WB uptake at baseline between the two group, NNO and NOR. B. No significant change in the WB uptake at 2 days post injury between the two groups, NNO and NOR. (Unpaired t-test, two-tailed, bars= mean +/− SEM).
3.3. The data shows whole brain SUV remains unchanged by NOR or by injury. (p>0.05, unpaired t-test, two-tailed).NOR alters hippocampal FDG uptake
As NOR is suggested to be related to hippocampal function (Cohen and Stackman, 2015; Gomes et al., 2016), we first investigated FDG uptake in the ipsilateral (left) and contralateral (right) hippocampus at baseline and 2 days post injury [Fig. 5]. At baseline, SUVw within the right hippocampus shows higher FDG uptake in NOR group compared to NNO group [*p=0.007, unpaired t-test, two-tailed; Fig. 5A]. The left hippocampus remains unchanged [p=0.08, unpaired t-test two-tailed; Fig. 5B].
Fig. 5.
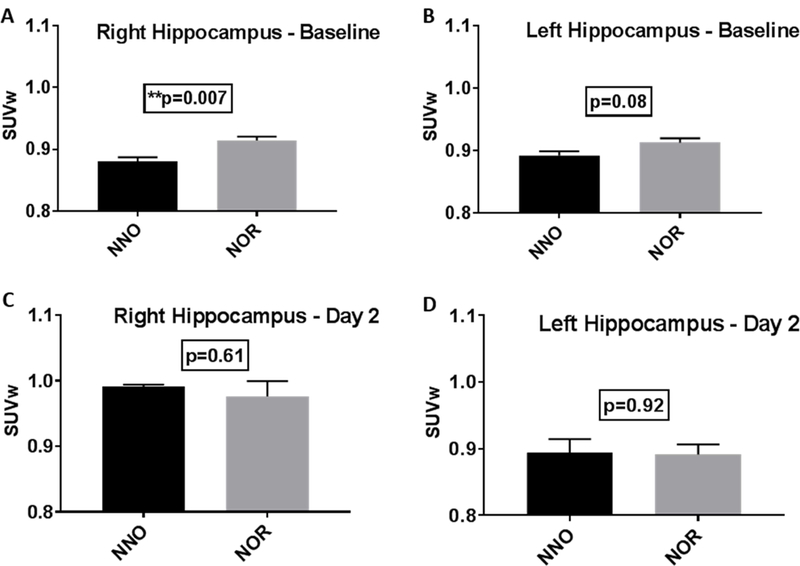
Standardized Uptake Value normalized to the Whole Brain (SUVw) in the hippocampus of the NNO and NOR groups at baseline and day 2 post injury.
A. The right hippocampus shows increased uptake in the NOR group compared to the NNO group at baseline (**p=0.007). B. The left hippocampus showed no statistical difference between the two groups at baseline. C. The right hippocampus shows no statistical difference between the two groups at 2 days post injury. D. The left hippocampus at 2 days post injury also shows no statistical difference between the two groups. (unpaired t-test, two-tailed, bars= mean +/− SEM)
At 2 days post injury, no significant difference was seen in the FDG uptake in contralateral (right) [p=0.61, unpaired t-test two-tailed; Fig. 5C] or ipsilateral (left) hippocampus [p=0.92, unpaired t-test two-tailed; Fig. 5D] between NOR and NNO groups.
We also compared FDG uptake changes over time (from baseline to 2 days post injury) within each group (NOR and NNO) and between ipsilateral (left) and contralateral (right) hippocampus. The ipsilateral (left) hippocampus showed no significant change in response to injury in both the groups, [Fig. 6A & B]
Fig. 6.
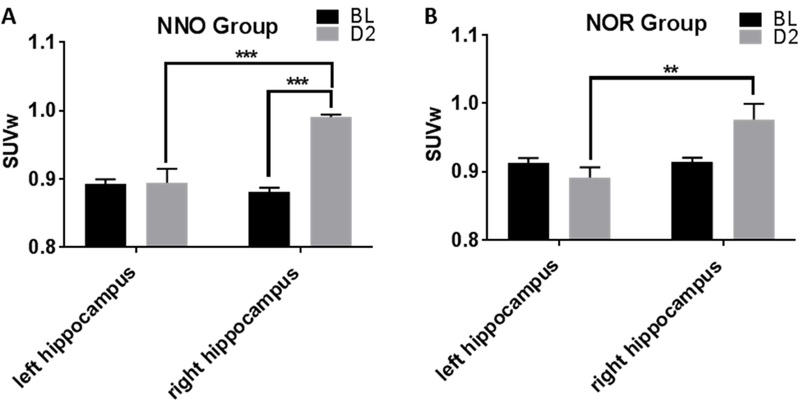
SUVw in the left and right hippocampus over time showed more difference without cognitive testing. A. In the NNO group, the right (contra) hippocampus shows increased uptake compared to left (ipsilateral) after injury (***p=0.0001). The right hippocampus also shows increased uptake post injury compared to its baseline (***p=0.0003). B. In the NOR group, the right (contra) hippocampus also showed increased uptake compared to left (ipsilateral) after injury (**p=0.002), however the difference between baseline and day 2 was not observed (Two-way RM ANOVA, bars = mean +/− SEM).
The contralateral (right) hippocampus showed increased FDG uptake at 2 days post injury compared to baseline only in the NNO group [***p=0.0003, two-way RM ANOVA with Sidak’s multiple comparisons post hoc test; Fig. 6A]; no significant change between baseline and 2 days post-injury was noted in the NOR group [p>0.05, two-way RM ANOVA with Sidak’s multiple comparisons post hoc test; Fig. 6B]. A significant increase in FDG uptake was also seen in the contralateral (right) hippocampus compared to ipsilateral (left) at day 2 in both NNO group [***p=0.0001, two-way RM ANOVA with Sidak’s multiple comparisons post hoc test; Fig. 6A] and NOR group [**p=0.002, two-way RM ANOVA with Sidak’s multiple comparisons post hoc test; Fig. 6B]. However, the NNO group showed a greater fold change (1.125 fold) from baseline to day 2 than the NOR group (1.05; p = 0.06, one-tailed t-test).
3.4. NOR testing reduces FDG uptake in injured hemisphere
Quantification of FDG uptake between baseline and day 2 post injury in the 13 regions of the ipsilateral (left) cerebral hemisphere of the NNO group showed that mLFP led to a significant reduction in uptake in basal ganglia (****p<0.0001), thalamus (****p<0.0001), cortex (****p<0.0001), and corpus callosum (**p=0.008), as well as significant increase in the hypothalamus (*p=0.01) and midbrain (*p=0.02, two-way RM ANOVA with Sidak’s multiple comparisons post hoc test; Fig. 7A). In contrast, addition of the NOR test during uptake resulted in significant reduction in uptake in only the basal ganglia (*p=0.02), thalamus (*p=0.03), cortex (****p<0.0001) and corpus callosum (****p<0.0001); the alteration in hypothalamus and midbrain were not observed in this group [Fig. 7B]. Overall, NOR addition seemed to lead to an increase in variability within the regions, reducing the ability of differences to reach statistical significance in comparison to the NNO group.
Fig. 7.
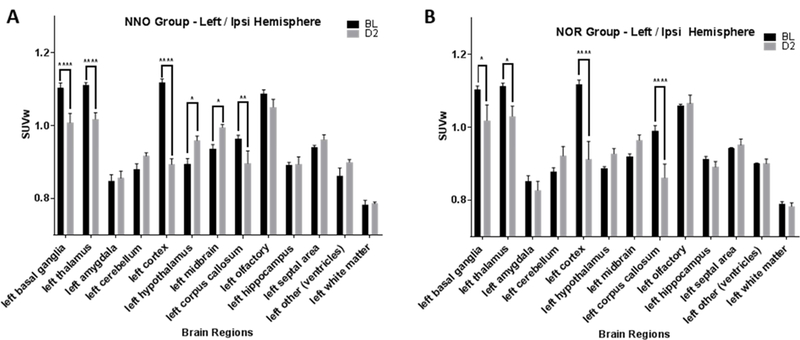
SUVw plotted for the 13 left (ipsilateral) brain regions assessed at baseline and day 2 post injury in the NNO and NOR groups. A. In the NNO group, the left hemisphere shows changes in 6 brain regions post injury compared to baseline. B. In the NOR group, the left (ipsilateral) hemisphere shows changes only in 4 brain regions post injury compared to baseline. (*p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001, Two-way RM ANOVA; bars = mean +/− SEM).
3.5. NOR exhibit less regional variations in uninjured hemisphere
With the differential effect of the NOR task on the activity of various ipsilateral (left) brain regions, we next examined the effect of this task on the activity of these regions in the contralateral (right) hemisphere. Contrary to the reduced activity of basal ganglia, thalamus, corpus callosum and cortex in the ipsilateral hemisphere (NNO group), all of these regions, (basal ganglia ***p=0.0001, thalamus ***p=0.0004 and corpus callosum ****p<0.0001, two-way RM ANOVA with Sidak’s multiple comparisons post hoc test) except for cortex (p=0.92) in the contralateral (right) hemisphere, showed a compensatory increase in activity as compared to their respective baseline activity, [Fig. 8A]. On the other hand, cognitive testing (NOR group) following mLFP injury, shows no increase in the activity in these regions in the contralateral hemisphere except amygdala (*p=0.01), hypothalamus (**p=0.001) and olfactory (***p=0.0009; Fig. 8B). Again, NOR addition led to increase in variability in addition to a blunting of effect, which reduced the ability of differences to reach statistical significance in comparison to the NNO group.
Fig. 8.
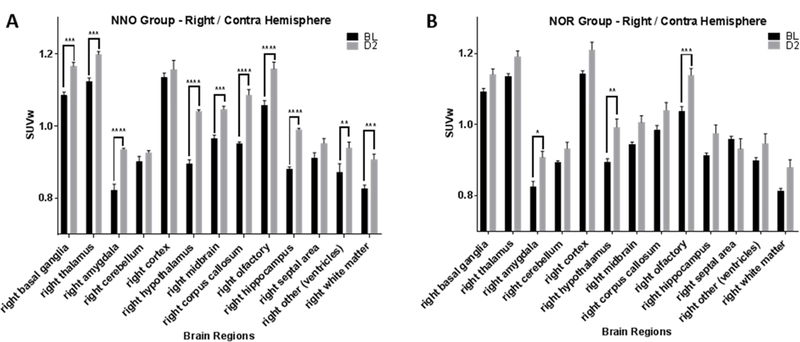
SUVw plotted for the 13 right (contralateral) brain regions at baseline and day 2 post injury in the NNO and NOR groups. A. In the NNO group, the right hemisphere shows changes in 10 brain regions post injury compared to baseline. B. In the NOR group, the right hemisphere shows changes only in 3 brain regions post injury compared to baseline. (*p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001, Two-way RM ANOVA; bars = mean +/− SEM.)
4. Discussion
In preclinical PET studies, anesthetics are commonly used during imaging to immobilize the animals, but anesthesia itself can influence the biodistribution of FDG in the brain. In general FDG uptake in the brain is reduced by isoflurane anesthesia (Selwyn et al., 2013; Toyama et al., 2004). However, while isoflurane anesthesia reduces FDG uptake in olfactory bulb, cortex, thalamus and basal ganglia, it increases in midbrain, hypothalamus, hippocampus and cerebellum (Park et al., 2017). In contrast to FDG uptake under anesthesia, FDG uptake in the brain of awake animals varies greatly due to the animal’s activity (Byrnes et al 2014). Use of anesthesia to control factors such as activity, sleep, temperature reduces the variability of FDG uptake due to these factors.
To examine the effect of cognitive task in both, naïve and injured rats we needed the animals to be fully awake during FDG uptake. However, to image the animals in the PET scanner they needed to be anesthetized after 30 minutes of awake uptake. This need could be avoided in principle by the use of miniaturized PET scanners mounted directly onto the head of the animals, avoiding the need for anesthesia while also avoiding motion between the brain and the scanner. However, these scanners have lower sensitivity than the fixed scanner used in our study and mounting the scanners may induce undue stress in the animal due to inhibition of their natural movement (Spangler-Bickell et al., 2016).
The results from this study indicate that performance of the NOR during uptake plays a significant role in glucose (FDG) uptake, and potentially metabolism. At baseline scans, there is an increase in the uptake of FDG in the right hippocampus in the NOR group compared to the NNO group. However, at 2 days post injury, the right (contralateral) hippocampus shows significantly higher uptake in both the NNO and NOR groups compared to left (ipsilateral), however, the NNO group showed a greater increase; implying that injury induced changes were reduced by NOR testing.
Our laboratory previously demonstrated that mLFP TBI reduced cerebral glucose uptake in both ipsilateral and contralateral region-of-interest (ROI) with a peak depression at 24 hours post injury. The reduced FDG uptake in the ROI’s returned to baseline level by day 9 post injury (Selwyn et al., 2013). Our current work evaluated uptake in awake, functioning rats, while this previous work utilized anesthesia during FDG uptake. Since anesthesia (isoflurane) plays a significant role in blood glucose level and metabolism (Behdad et al., 2014; Byrnes et al., 2014; Hildebrandt et al., 2008; Park et al., 2017), this could have altered our results in comparison to the previous study. Further analysis was performed at day 2 post injury in the current study, while our previous study used 3 hours, 24 hours and 5 days post injury scans. Since the regional brain uptake is dependent upon the time of injury (Byrnes et al., 2014), this could have also caused differences in our results.
Hippocampal dysfunction and / or smaller volume has been associated with memory deficits in older adults and patients with Alzheimer’s disease (Wicking et al., 2014). Further, hippocampus in patients with mild cognitive impairment (MCI) have also shown reduced metabolic rate of glucose (MRglc) in hippocampus (Li et al., 2008). We and others have shown that, similar to these neurodegenerative conditions, TBI results in reduced glucose uptake in the hippocampus (Brabazon et al., 2016). While our current study failed to show significant reductions in glucose uptake in the ipsilateral hippocampus at 2 days post-injury, a trend towards reduction was observed. It is possible that the current injury level (using a 10–15 degree angle for pendulum release during LFP) was slightly less than that of our previous work (using a 22 degree angle). Alterations and improvements in the fluid percussion device, including replacement of conduction tubing, required reduction of the angle to achieve similar pressures, but slight differences in pressure and tubing may have resulted in slightly different injury severities.
We hypothesized that functional testing (NOR) during the uptake period could trigger certain brain regions differently than when under anesthesia or just conscious (awake) uptake. We demonstrate that at baseline, uptake in the right hippocampus is elevated with cognitive testing. Functional activation of cells within the brain, by optogenetics or by other modes of stimulation, have previously been shown to induce increased cellular metabolism (Takata et al., 2018; Wieraszko, 1982). In fact, previous studies on classical conditioning have shown that the hippocampus alters it glucose uptake patterns in response to conditioning signals (Barrett et al., 2003; Gonzalez-Lima and Scheich, 1986). In human subjects, FDG-PET alterations have long been associated with function; cognitive testing during uptake has been used to distinguish locations of impaired glucose metabolism or correlate anatomical regions with specific memory functions (Gardener et al., 2016; Xie et al., 2016). Therefore, it is possible that the cognitive testing is leading to elevated function of normal hippocampal neurons, resulting in elevated glucose uptake.
Conversely, we demonstrated that cognitive testing during uptake following injury led to an overall reduction in uptake in multiple regions in comparison to the NNO group, suggesting that cognitive testing reduced injury-induced changes in glucose uptake. The mechanism behind this effect is unclear. However, studies have shown that neuronal activity can lead to reduced inflammation (Almeida et al., 2015), which can in turn lead to reduced glucose uptake (Brabazon et al., 2016). New learning paradigms have also been shown to increase neurogenesis and alter neuroinflammatory effects (Ryan and Kelly, 2016), which can also alter glucose metabolic responses. While the mechanism of this effect is not known, it is also not clear if this is a beneficial or negative effect. There are several studies suggesting that cognitive activity after concussion may not necessarily help patients in recovery (Meehan and Bachur, 2015). Brown et al (Brown et al., 2014) have concluded that cognitive activity after concussion is associated with longer symptom duration and recommended limiting extensive cognitive activity after injury. However, the duration of time for limiting the motor or cognitive activity after concussion or mTBI is a gray area and depends on several factors including the severity of concussion and the level of difficulty of the task. In our study, we imaged animals 2 days post mild injury and challenged them with the NOR test (cognitive task). Both NOR and NNO animals showed increased FDG activity in contralateral hippocampus at 2 days post injury, however, the NOR group showed less uptake in the ipsilateral hippocampus compared to baseline, implying less blood flow or glucose uptake. This may suggest agreement with detrimental effects of acute cognitive challenge after TBI. However, assessment of cognitive abilities between groups after the 2 day time point has not yet been completed.
Previous work has shown that several structures in the brain show laterality, in both gross anatomical structure and function (Diamond et al., 1982). This laterality in the hippocampus is present at the molecular level and plays a role in intrahippocampal signaling and possibly in learning and memory function (Kohl et al., 2011; Shimbo et al., 2018). Further injury on the left versus right side of the brain leading to hippocampal damage has resulted in mild differences in learning and memory function. Specifically, damage to the right hippocampus resulted in significant delays in memory acquisition in the Morris Water Maze test, a test of spatial memory (Schurman et al., 2017). In the current study, the mLFP injury was performed on the left side of the head and the novel object was always placed on the right side of the box. It is also possible that this could have biased our result of increased uptake on the right (contralateral) side of the brain. Future study is needed to further explore this phenomenon.
Previously, the uptake concentration in ipsilateral and contralateral ROI’s was normalized to a reference region (cerebellum). This reference tissue ratio was then normalized to the animal’s baseline reference tissue ratio (Selwyn et al., 2013). In our current study we normalized the uptake concentration by calculating the Standardized Uptake Value (SUV), which takes into account the total injected activity and body weight of the animal. This regional SUV was then normalized to the whole brain (SUVw), to further reduce the variability due to differences in the injected activity and uptake. We could not use the previous method of reference tissue (cerebellum) normalization as in this study the cerebellum was found to change with injury (Fig. 1). For tissue to be used as reference tissue, it has to stable and not affected by the experimental paradigms (novel object, surgery).
The finite spatial resolution of PET scanners may lead to partial volume effects (PVE). PVE is caused by the smoothening of PET images such that some of the radioactivity from a region of higher concentration can get mis-attributed to an adjacent region of lower activity. This would contribute to noise in the measured activity concentration and thus to misinterpretation of the PET data. This has been noted in brain scans where the radioactivity concentration values are differently variated in different regions-of-interest (ROI) (Harri et al., 2007) (Lehnert et al., 2012). The PVE start to diminish if the dimensions of the selected ROIs are over 2–3 times greater than the Full Width Half Maximum (FWHM) of the spatial resolution of the scanner. The intrinsic resolution of the Siemens Inveon preclinical scanner is 1.4 mm at the center of the PET field of view. As the volume of the ROI (each left or right hippocampus) is approximately 67 mm3, the error due to PVE is significantly minimized in our analyses. In addition to the size of the ROI we also used the iterative method of the PET reconstruction algorithm (OSEM3D/MAP), which further reduces the impact of PVE. These simple correction methods involving standardized acquisition, processing and analysis of the PET data substantially enhance the reliability of our quantification despite the known biases that can be introduced by PVE (Soret et al., 2007).
5. Conclusion
We have shown increased FDG uptake in the right hippocampus in naïve rats in the group exposed to novel object recognition task (NOR) compared to group with no directed task (NNO). However, when we compared FDG uptake after 2 days post mild injury, both the groups (NOR and NNO) showed increased uptake in the contralateral hippocampus compared to ipsilateral but the NNO group showed more uptake compared to NOR. The ipsilateral hippocampus showed a trend of lower FDG uptake in the NOR group only (not NNO group) compared to baseline. These data suggest that novel object plays an intriguing role during FDG uptake, both in naïve and 2 days post TBI rats. Future work is required to further investigate the role of novel object on both the time course and the laterality of changes in the FDG uptake.
Acknowledgements
This work was supported by the National Institutes of Health/NINDS (grant number 1 R21 NS095116-01A1) and the Center for Neuroscience and Regenerative Medicine at Uniformed Services University.
Abbreviations:
- mTBI
mild Traumatic Brain Injury
- FDG
Fluorodeoxyglucose
- PET/CT
Positron Emission Tomography / Computed Tomography
- mLFP
mild lateral fluid percussion
- NOR
Novel Object Recognition
- NNO
No Novel Object
- SUVw
Standardized Uptake Value normalized to whole brain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida C, DeMaman A, Kusuda R, Cadetti F, Ravanelli MI, Queiroz AL, Sousa TA, Zanon S, Silveira LR, Lucas G, 2015. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain 156, 504–513. [DOI] [PubMed] [Google Scholar]
- Antunes M, Biala G, 2012. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13, 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciniegas DB, Anderson CA, Topkoff J, McAllister TW, 2005. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 1, 311–327. [PMC free article] [PubMed] [Google Scholar]
- Barrett D, Shumake J, Jones D, Gonzalez-Lima F, 2003. Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response. J Neurosci 23, 5740–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, McClung J, Shah MN, Cheng YT, Flesher W, Kraus J, 2005. Mild traumatic brain injury in the United States, 1998--2000. Brain Inj 19, 85–91. [DOI] [PubMed] [Google Scholar]
- Behdad S, Mortazavizadeh A, Ayatollahi V, Khadiv Z, Khalilzadeh S, 2014. The Effects of Propofol and Isoflurane on Blood Glucose during Abdominal Hysterectomy in Diabetic Patients. Diabetes Metab J 38, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, McArthur DL, Etchepare M, Huang SC, Sehati N, Satz P, Phelps ME, Becker DP, 2001. Metabolic recovery following human traumatic brain injury based on FDG-PET: Time course and relationship to neurological disability. J Head Trauma Rehabil 16, 135–148. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, 2006. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1, 1306–1311. [DOI] [PubMed] [Google Scholar]
- Brabazon F, Wilson CM, Jaiswal S, Reed J, Frey WHN, Byrnes KR, 2017. Intranasal insulin treatment of an experimental model of moderate traumatic brain injury. J Cereb Blood Flow Metab 37, 3203–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabazon F, Wilson CM, Shukla DK, Mathur S, Jaiswal S, Bermudez S, Byrnes KR, Selwyn R, 2016. [18F]FDG-PET Combined with MRI Elucidates the Pathophysiology of Traumatic Brain Injury in Rats. J Neurotrauma [DOI] [PubMed]
- Brown NJ, Mannix RC, O’Brien MJ, Gostine D, Collins MW, Meehan WP 3rd, 2014. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics 133, e299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes KR, Wilson CM, Brabazon F, von Leden R, Jurgens JS, Oakes TR, Selwyn RG, 2014. FDG-PET imaging in mild traumatic brain injury: a critical review. Front Neuroenergetics 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ZH, Son YD, Kim HK, Kim ST, Lee SY, Chi JG, Park CW, Kim YB, 2010. Substructural hippocampal glucose metabolism observed on PET/MRI. J Nucl Med 51, 1545–1548. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW Jr., 2015. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MC, Murphy GM Jr., Akiyama K, Johnson RE, 1982. Morphologic hippocampal asymmetry in male and female rats. Exp Neurol 76, 553–565. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J, 1988. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31, 47–59. [DOI] [PubMed] [Google Scholar]
- Gardener SL, Sohrabi HR, Shen KK, Rainey-Smith SR, Weinborn M, Bates KA, Shah T, Foster JK, Lenzo N, Salvado O, Laske C, Laws SM, Taddei K, Verdile G, Martins RN, 2016. Cerebral Glucose Metabolism is Associated with Verbal but not Visual Memory Performance in Community-Dwelling Older Adults. J Alzheimers Dis 52, 661–672. [DOI] [PubMed] [Google Scholar]
- Gomes CA, Figueiredo P, Mayes A, 2016. Priming for novel object associations: Neural differences from object item priming and equivalent forms of recognition. Hippocampus 26, 472–491. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Scheich H, 1986. Classical conditioning of tone-signaled bradycardia modifies 2-deoxyglucose uptake patterns in cortex, thalamus, habenula, caudate-putamen and hippocampal formation. Brain Res 363, 239–256. [DOI] [PubMed] [Google Scholar]
- Greer N, Sayer N, Kramer M, Koeller E, Velasquez T, 2016. Prevalence and Epidemiology of Combat Blast Injuries from the Military Cohort 2001–2014, Washington (DC). [PubMed] [Google Scholar]
- Harri M, Mika T, Jussi H, Nevalainen OS, Jarmo H, 2007. Evaluation of partial volume effect correction methods for brain positron emission tomography: Quantification and reproducibility. J Med Phys 32, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt IJ, Su H, Weber WA, 2008. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J 49, 17–26. [DOI] [PubMed] [Google Scholar]
- Hovda DA, Lee SM, Smith ML, von Stuck S, Bergsneider M, Kelly D, Shalmon E, Martin N, Caron M, Mazziotta J, al. E, 1995. The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J Neurotrauma 12, 903–906. [DOI] [PubMed] [Google Scholar]
- Kohl MM, Shipton OA, Deacon RM, Rawlins JN, Deisseroth K, Paulsen O, 2011. Hemisphere-specific optogenetic stimulation reveals left-right asymmetry of hippocampal plasticity. Nat Neurosci 14, 1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM, 2006. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 21, 375–378. [DOI] [PubMed] [Google Scholar]
- Lehnert W, Gregoire MC, Reilhac A, Meikle SR, 2012. Characterisation of partial volume effect and region-based correction in small animal positron emission tomography (PET) of the rat brain. Neuroimage 60, 2144–2157. [DOI] [PubMed] [Google Scholar]
- Li Y, Rinne JO, Mosconi L, Pirraglia E, Rusinek H, DeSanti S, Kemppainen N, Nagren K, Kim BC, Tsui W, de Leon MJ, 2008. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer’s disease. Eur J Nucl Med Mol Imaging 35, 2169–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan WP 3rd, Bachur RG, 2015. The recommendation for rest following acute concussion. Pediatrics 135, 362–363. [DOI] [PubMed] [Google Scholar]
- Moore AH, Osteen CL, Chatziioannou AF, Hovda DA, Cherry SR, 2000a. Quantitative assessment of longitudinal metabolic changes in vivo after traumatic brain injury in the adult rat using FDG-microPET. J Cereb Blood Flow Metab 20, 1492–1501. [DOI] [PubMed] [Google Scholar]
- Moore AH, Osteen CL, Chatziioannou AF, Hovda DA, Cherry SR, 2000b. Quantitative assessment of longitudinal metabolic changes in vivo after traumatic brain injury in the adult rat using FDG-MicroPET. J Cereb Blood Flow Metab 20, 1492–1501. [DOI] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE, 2011. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One 6, e28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TY, Nishida KS, Wilson CM, Jaiswal S, Scott J, Hoy AR, Selwyn RG, Dardzinski BJ, Choi KH, 2017. Effects of isoflurane anesthesia and intravenous morphine self-administration on regional glucose metabolism ([(18) F]FDG-PET) of male Sprague-Dawley rats. Eur J Neurosci 45, 922–931. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Kelly AM, 2016. Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer’s disease. Ageing Res Rev 27, 77–92. [DOI] [PubMed] [Google Scholar]
- Schurman LD, Smith TL, Morales AJ, Lee NN, Reeves TM, Phillips LL, Lichtman AH, 2017. Investigation of left and right lateral fluid percussion injury in C57BL6/J mice: In vivo functional consequences. Neurosci Lett 653, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn R, Hockenbury N, Jaiswal S, Mathur S, Armstrong RC, Byrnes KR, 2013. Mild traumatic brain injury results in depressed cerebral glucose uptake: An (18)FDG PET study. J Neurotrauma 30, 1943–1953. [DOI] [PubMed] [Google Scholar]
- Selwyn RG, Cooney SJ, Khayrullina G, Hockenbury N, Wilson CM, Jaiswal S, Bermudez S, Armstrong RC, Byrnes KR, 2016. Outcome after Repetitive Mild Traumatic Brain Injury Is Temporally Related to Glucose Uptake Profile at Time of Second Injury. J Neurotrauma 33, 1479–1491. [DOI] [PubMed] [Google Scholar]
- Shimbo A, Kosaki Y, Ito I, Watanabe S, 2018. Mice lacking hippocampal left-right asymmetry show non-spatial learning deficits. Behav Brain Res 336, 156–165. [DOI] [PubMed] [Google Scholar]
- Soret M, Bacharach SL, Buvat I, 2007. Partial-volume effect in PET tumor imaging. J Nucl Med 48, 932–945. [DOI] [PubMed] [Google Scholar]
- Spangler-Bickell MG, de Laat B, Fulton R, Bormans G, Nuyts J, 2016. The effect of isoflurane on (18)F-FDG uptake in the rat brain: a fully conscious dynamic PET study using motion compensation. EJNMMI Res 6, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Sugiura Y, Yoshida K, Koizumi M, Hiroshi N, Honda K, Yano R, Komaki Y, Matsui K, Suematsu M, Mimura M, Okano H, Tanaka KF, 2018. Optogenetic astrocyte activation evokes BOLD fMRI response with oxygen consumption without neuronal activity modulation. Glia [DOI] [PubMed]
- Toyama H, Ichise M, Liow JS, Vines DC, Seneca NM, Modell KJ, Seidel J, Green MV, Innis RB, 2004. Evaluation of anesthesia effects on [18F]FDG uptake in mouse brain and heart using small animal PET. Nucl Med Biol 31, 251–256. [DOI] [PubMed] [Google Scholar]
- Wicking M, Nees F, Steiger F, 2014. Neuropsychological measures of hippocampal function. Front Neurol Neurosci 34, 60–70. [DOI] [PubMed] [Google Scholar]
- Wieraszko A, 1982. Changes in the hippocampal slices energy metabolism following stimulation and long-term potentiation of Schaffer collaterals-pyramidal cell synapses tested with the 2-deoxyglucose technique. Brain Res 237, 449–457. [DOI] [PubMed] [Google Scholar]
- Xie L, Dolui S, Das SR, Stockbower GE, Daffner M, Rao H, Yushkevich PA, Detre JA, Wolk DA, 2016. A brain stress test: Cerebral perfusion during memory encoding in mild cognitive impairment. Neuroimage Clin 11, 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP, 1991. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res 561, 106–119. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Loane DJ, Murray MG 2nd, Stoica BA, Faden AI, 2012. Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J Neurotrauma 29, 2475–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]


