Abstract
The default-mode network (DMN) is affected by advancing age, where particularly long-range connectivity has been consistently reported to be reduced as compared to young individuals. We examined whether there were any differences in the effects of intermittent theta-burst stimulation (iTBS) in DMN connectivity between younger and older adults, its associations with cognition and brain integrity, as well as with long-term cognitive status. Twenty-four younger and 27 cognitively normal older adults were randomly assigned to receive real or sham iTBS over the left inferior parietal lobule between two resting-state functional magnetic resonance imaging (rs-fMRI) acquisitions. Three years later, those older adults who had received real iTBS underwent a cognitive follow-up assessment. Among the younger adults, functional connectivity increased following iTBS in distal DMN areas from the stimulation site. In contrast, older adults exhibited increases in connectivity following iTBS in proximal DMN regions. Moreover, older adults with functional responses to iTBS resembling those of the younger participants exhibited greater brain integrity and higher cognitive performance at baseline and at the 3-year follow-up, along with less cognitive decline. Finally, we observed that ‘young-like’ functional responses to iTBS were also related to the educational background attained amongst older adults. The present study reveals that functional responses of the DMN to iTBS are modulated by age. Furthermore, combining iTBS and rs-fMRI in older adults may allow characterizing distinctive cognitive profiles in aging and its progression, probably reflecting network plasticity systems that may entail a neurobiological substrate of cognitive reserve.
Keywords: Aging, cognitive reserve, default-mode network, intermittent theta-burst stimulation, resting-state functional magnetic resonance imaging
1. Introduction
Resting-state functional connectivity (rs-FC), studied through functional magnetic resonance imaging (fMRI), has revealed that the brain is organized into distinct networks (Smith et al., 2009) that are relevant to cognition (Bressler & Menon, 2010). Advancing age affects rs-FC (Damoiseaux, 2017; Ferreira & Busatto, 2013; Grady et al., 2016; Jockwitz et al., 2017; Nashiro et al., 2017; Sala-Llonch et al., 2014, 2015; Schultz et al., 2017; Siman-Tov et al., 2017; Staffaroni et al., 2018; Tomasi & Volkow, 2012; Vidal-Piñeiro et al., 2014; Viviano et al., 2017), and most studies have focused on its impact on the default-mode network (DMN; i.e., Sala-Llonch et al., 2015; Siman-Tov et al., 2017). The most important and robust effect of aging on the DMN seems to involve the coupling of the medial frontal and posterior midline structures (Andrews-Hanna et al., 2007; Mevel et al., 2013; Tomasi & Volkow, 2012; Vidal-Piñeiro et al., 2014). Moreover, functional connectivity between cortical and subcortical nodes of the DMN, like the hippocampal areas, is also affected in aging, possibly underlying deficient mnemonic processing (Salami et al., 2014). Indeed, functional adjustments in these components seems to appear at very early stages of Alzheimer’s disease (AD; Chen et al., 2016).
Within the DMN, the changes in connectivity between medial frontal and posterior midline structures are consistent with the hypothesis that in the elderly, resting-state connectivity shows decreases in long-range functional connectivity (Andrews-Hanna et al., 2007; Cao et al., 2014; Ferreira & Busatto, 2013; Sala-Llonch et al., 2014; Tomasi & Volkow, 2012; Vidal-Piñeiro et al., 2014), but increases in the strength of short-distance functional connections (Cao et al., 2014; Sala-Llonch et al., 2014). This age-related neurophysiological process appears to reverse functional brain maturation, characterized by the weakening of short-range connections and the integration of distant regions into functional networks (Dosenbach et al., 2011; Fair et al., 2009; Supekar et al., 2009). The balance between long and short distance connectivity is relevant for individual cognitive profiles in healthy aging, since weakening of long-distance connections correlates with lower memory performance and reduced gray (GM) and white matter (WM) integrity (Andrews-Hanna et al., 2007; Vidal-Piñeiro et al., 2014), while the strengthening of short-distance connections has been associated with poorer memory performance (Sala-Llonch et al., 2014). Importantly, in AD, rs-FC disruption also affects long-distance connections to hub nodes, subsequently leading to loss of network efficiency (Liu et al., 2014). Furthermore, the weakening of long-distance connections is linked to higher cognitive impairment along the AD continuum (Liu et al., 2014).
Non-invasive brain stimulation (NIBS), such as repetitive transcranial magnetic stimulation (rTMS; for review see Rossini et al., 2015), can be used to induce and assess local and network plasticity in humans across the lifespan (Freitas et al., 2011; 2013; Pascual-Leone et al., 2011). Patterned rTMS protocols, like theta-burst stimulation (TBS; Huang et al., 2005), can modulate cortical excitability by inducing long-term potentiation (LTP)- and long-term depression (LTD)-like plasticity (Huang et al., 2005). Proxy metrics revealing plasticity mechanisms and network dynamics in response to TMS can be obtained coupling TMS with fMRI or electroencephalography (EEG; Fox et al., 2012; Pascual-Leone et al., 2011; Shafi et al., 2012). TMS can precisely modulate resting-state networks, such as the DMN, by targeting its accessible nodes including the dorsolateral prefrontal cortex (Van Der Werf et al., 2010), the inferior parietal lobule (IPL; Eldaief et al., 2011) and the lateral cerebellum (Halko et al., 2014). Although these studies reached different conclusions, probably due to applying different rTMS protocols (Di Lazzaro & Rothwell, 2014), intermittent TBS (iTBS) seems to increase the DMN connectivity when is applied to one of its hubs in young subjects (Halko et al., 2014). However, the expected effects of iTBS over the IPL amongst young individuals cannot be precisely predicted, as iTBS has been only used previously to target a cerebellar DMN node (Halko et al., 2014). Furthermore, differences in the effects of iTBS in DMN connectivity between younger and older subjects and its association with their cognitive status and brain integrity are unknown to date.
In the present study, we investigated the effects of iTBS in DMN connectivity in younger and older participants. We hypothesized that older adults who presented similar functional responses to iTBS as the younger subjects would display higher and sustained levels of cognitive performance over time. If so, TMS-induced modulation of the DMN might be a useful surrogate marker to help distinguish between older adults who will maintain cognitive function from those who are likely to show decline.
2. Materials and methods
2.1. Participants
A total of 51 healthy, right-handed adults divided into two age groups were recruited from the general population. Twenty-four younger adults, aged ≤ 30 years (age (mean ± SD), 23.42 ± 1.6 years; age range: 20 – 27 years; 19 females), and 27 older adults, aged ≥ 60 years (age (mean ± SD), 68.15 ± 4.6 years; age range: 60 – 79 years; 22 females), naive to stimulation, participated in this study after giving informed consent, in accordance with the Declaration of Helsinki (1964, last revision 2013). All study procedures were approved by the local Institutional Review Board (IRB 00003099). None of the participants reported a diagnosis of a neurological or psychiatric disorder or any TMS contraindication (Rossi et al., 2009). Inclusion criteria for the older subjects included a normal cognitive profile with mini-mental state examination (MMSE; Folstein et al., 1975) scores of ≥ 24 and performance scores not more than 1.5 standard deviation (SD) below normative data (adjusted for age, gender and years of education) on any of the administered neuropsychological tests (i.e., they did not fulfill the criteria for mild cognitive impairment (MCI); Petersen & Morris, 2005. See section 2.3 for details of administered neuropsychological tests).
2.2. Experimental design
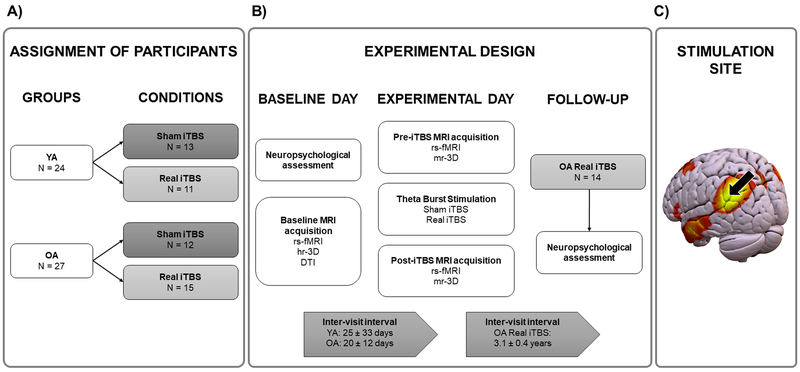
Participants were randomly assigned to receive sham (N younger adults = 13, N older adults = 12) or real iTBS (N younger adults = 11, N older adults = 15; Figure 1A). A simple randomization procedure was used (Altman & Bland, 1999; Kang et al., 2008). On the first visit (baseline day), all participants underwent a resting-state fMRI (rs-fMRI), a high-resolution anatomical MRI, and a diffusion tensor imaging (DTI) scan. Participants returned for a second visit after an inter-visit interval that was not significantly different between the groups or conditions (the mean interval between the visits ± SD was 25 ± 33 days for the younger adults and 20 ± 12 days for the older adults; all p values > 0.05). During the second visit (the experimental day), iTBS was delivered over the left IPL (lIPL) using neuronavigation that was guided by the results of the baseline rsfMRI scan (Figure 1C) between two rs-fMRI scans (experimental design is summarized in Figure 1B). Both rs-fMRI sessions included the acquisition of a medium-resolution anatomical image for co-registration purposes. No significant differences were found between the groups or conditions in the interval between the end of iTBS and the beginning of post-iTBS rs-fMRI (mean ± SD, 33 ± 3 min for the younger adults and 34 ± 5 min for the older adults; all p values > 0.05). Head-motion changes between the pre- and post-iTBS rs-fMRI scans did not differ between the groups or conditions (all p values > 0.05; see Supplementary Material (SM) for further information). Further, conditions did not differ for major factors that may affect plasticity and the response to iTBS (i.e., age and education; all p values > 0.05).
Figure 1.
Study protocol. A) Assignment of participants. Distribution of the participants between groups (younger vs. older adults) and conditions (sham vs. real iTBS). B) Experimental design. Timeline of the procedures and scans acquired on the baseline day, the experimental day and the follow-up. C) The DMN, with an arrow pointing to the stimulation site over the lIPL. Abbreviations: YA, younger adults; OA, older adults; iTBS, intermittent theta-burst stimulation; rs-fMRI, resting-state functional magnetic resonance imaging; hr-3D, high-resolution three-dimensional; DTI, diffusion tensor imaging; mr-3D, medium-resolution three-dimensional.
Additionally, the 15 elderly subjects who had been randomized to receive real iTBS were invited to participate in a follow-up (FU) cognitive assessment. Fourteen of these 15 participants (age (mean ± SD), 71.21 ± 4.7 years; age range: 63 – 77; all female) underwent a cognitive FU three years later (mean ± SD, 3.1 ± 0.4 years; range: 2.4 – 3.8 years). One subject was not followed up as he was undergoing treatment for a non-neurological condition.
2.3. Neuropsychological evaluation
The older adults were assessed using a battery of neuropsychological tests covering the major cognitive domains that are affected by aging, following procedures previously undertaken by our group (Vaqué-Alcázar et al., 2017; Vidal-Piñeiro et al., 2014). The battery included (1) a screening test for dementia, using the MMSE, and an evaluation of: (2) premorbid cognition and intelligence quotient (IQ), using the vocabulary subtest of the Wechsler Adult Intelligence Scale-III (WAIS-III) and National Adult Reading Test (NART); (3) verbal memory, using the Free and Cued Selective Reminding Test (SRT); (4) executive functions, using the phonemic fluency task and Trail Making Test B (TMTB); (5) language, using the semantic fluency task and Boston Naming Test (BNT); and (6) speed of processing, using the Symbol Digit Modalities Test (SDMT). Neuropsychological assessment at baseline was completed for all the older participants, except for one subject who missed the vocabulary subtest of the WAIS-III.
In addition to the neuropsychological assessment conducted at the baseline, fourteen of the 15 older adults who had received real iTBS were re-assessed using the same set of neuropsychological tests in the 3-year follow-up visit. The premorbid cognition tests (i.e., vocabulary subtest of the WAIS-III and the NART) were not re-evaluated because these are resistant to age-related cognitive effects. One subject missed the SRT at the follow-up visit. For additional details about demographics and neuropsychological data at baseline and follow-up see Table S1.
2.4. Transcranial magnetic stimulation
All participants underwent a single session of iTBS, using previously described protocols (Huang, et al., 2005). The iTBS protocol was performed with a MagPro X100 Stimulator (MagVenture A/S, Denmark) and a standard figure-eight coil (outer diameter: 75 mm). The bursts consisted of 3 pulses at 50 Hz repeated every 200 ms for 2 s. The pulse trains were separated by an 8-s interstimulus interval for a total of 20 repetitions (600 pulses over 190 s). The intensity of stimulation was set at 80% of the individual active motor threshold (AMT) in the younger adults and at 90% in the older adults. This adjustment was made to avoid stimulation intensities that might not have been high enough to reach the brain of older subjects due to age-related cortical atrophy (Barker, 1991; Kozel et al., 2000; McConnell et al., 2001; Mosimann et al., 2002; Padberg et al., 2002; see also SM). For the sham condition, a sham coil was used, which mimicked the clicking sound. The TMS coil was held tangentially to the scalp and oriented at a 45° angle relative to the mid-sagittal axis; thus, it was perpendicular to the dorsal part of the anterior occipital sulcus (Rademacher et al., 1992). iTBS was applied in a room adjacent to the MRI scanner and was neuronavigated with an MRI-guided frameless stereotactic system (eXimia Navigated Brain Stimulation, Nexstim Plc, Finland).
The stimulation target was individually determined from the baseline rs-fMRI scan as the coordinates within the lIPL that showed the highest correlation with the other main DMN nodes. This was performed as described in previous studies (Eldaief et al., 2011; Vidal-Piñeiro et al., 2015; see SM for additional information).
2.5. Classification of the subjects based on their functional response to iTBS
In addition to examining how different responses to iTBS in the older participants correlated with cognition and brain integrity, older adults who had received real iTBS were subdivided in two different ways. Initially, we conducted a ‘hypothesis-driven’ classification, separating older subjects based on the iTBS-induced changes in their rs-FC. Older adults were classified as ‘young-like’ responders if their functional responses to iTBS were equal or greater than 0 (i.e., had any positive value) in the fMRI cluster where younger subjects exhibited a greater increase in their rs-FC after iTBS compared to sham (Figure 3C). Otherwise, they were classified as ‘non-young-like’ responders. Following this approach, 7 older participants were classified as ‘young-like’ responders and 8 as ‘non-young-like’ responders (Figure S1A; see SM for further information). No differences in head motion or stimulation intensity were found between these subgroups (all p values > 0.05). In addition, a ‘data-driven’ classification was performed using a complete-linkage cluster analysis of all the subjects who had received real iTBS, regardless of age, as done previously to classify rTMS (Gangitano et al., 2002) and continuous TBS (cTBS; Jannati et al., 2017) responses in healthy adults. Two main clusters were found: one contained 9 out of the 11 younger adults (81.8%) and 4 out of the 15 older adults (26.7%), whereas the other cluster contained 2 out of the 11 younger adults (18.2%) and 11 out of the 15 older adults (73.3%; Figure S1B; see SM for further details and control analyses). Interestingly, the 4 older adults who were clustered together with most of the younger adults were previously classified in the ‘hypothesis-driven’ classification as ‘young-like’ responders. These 4 subjects did not display any significant differences in head motion or stimulation intensity compared to the other older adults who had received real iTBS (all p values > 0.05).
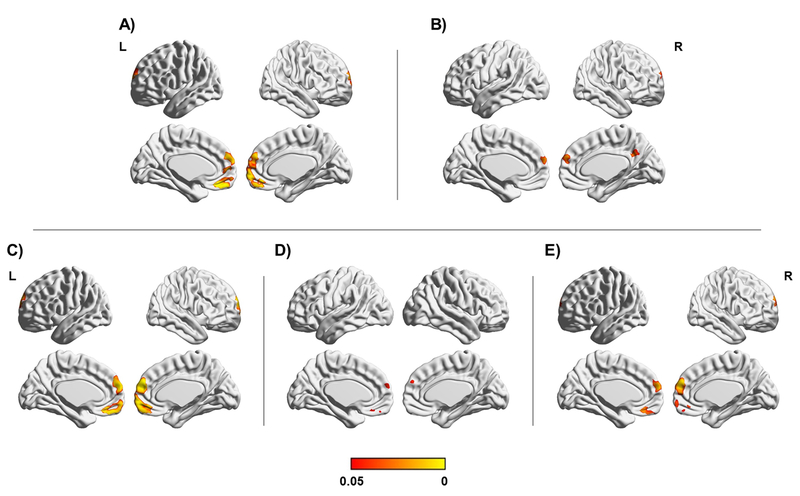
Figure 3.
Seed-to-DMN analyses after iTBS. Significant results at p < 0.05 (FWE-corrected) are shown on the standard MNI map. A) Interaction between group and condition. B) Main effect of condition. C) Younger adults: sham < real iTBS. D) Real iTBS condition: younger > older adults. E) Younger adults in real iTBS condition: post- > pre-iTBS. Color bar indicates FWE-corrected p values.
2.6. MRI acquisition
All participants were scanned with a Siemens Magnetom Trio Tim Syngo 3 Tesla system at the Unitat d’Imatge per Ressonància Magnètica IDIBAPS (Institut d’Investigacions Biomèdiques August Pi i Sunyer) at Hospital Clínic i Provincial de Barcelona, Barcelona, Spain. The imaging sequences were acquired with the following parameters.
2.6.1. Rs-fMRI
Three 5-min rs-fMRI datasets were acquired (T2*-weighted GE-EPI sequence; repetition time [TR] = 2,000 ms; echo time [TE] = 16 ms; 40 slices per volume; slice thickness = 3.0 mm; voxel size = 1.7 × 1.7 × 3.0 mm; field of view [FOV] = 220 mm; 150 volumes) for each subject, one on the baseline day and two during the experimental day before and after iTBS.
2.6.2. Structural MRI
Three 3D structural datasets were acquired for each subject. One high-resolution (hr-3D) sequence (T1-weighted magnetization-prepared rapid gradient-echo [T1-weighted MPRAGE]; sagittal plane acquisition; TR = 2,300 ms; TE = 2.98 ms; slice thickness = 1.0 mm; voxel size = 1.0 × 1.0 × 1.0 mm; FOV = 256 mm; 240 slices) was obtained on the baseline day, while two medium-resolution (mr-3D) datasets (T1-weighted MPRAGE; sagittal plane acquisition; TR = 1,390 ms; TE = 2.86 ms; slice thickness = 1.25 mm; voxel size = 1.3 × 1.3 × 1.3; FOV = 240 mm; 144 slices) were acquired during the experimental day before and after iTBS.
2.6.3. Diffusion MRI
Diffusion-weighted images were sensitized in 30 non-collinear directions with a b value of 1,000 s/mm2 in an echo-planar imaging sequence (TR = 7,700 ms; TE = 89 ms; section thickness = 2.0 mm; voxel size = 2.0 × 2.0 × 2.0 mm; FOV = 250 mm).
All images were inspected visually before analysis to ensure that they did not contain MRI artifacts or excessive movement.
2.7. Image analyses
The FMRIB Software Library (FSL; version 5.0.10; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and the Analysis of Functional NeuroImages (AFNI; https://afni.nimh.nih.gov/) were used for preprocessing and analyzing neuroimaging data.
2.7.1. Functional connectivity analyses
Resting-state functional connectivity analyses were conducted to explore the functional connectivity between the stimulation site (Stim; lIPL) and the DMN by means of seed-to-DMN and DMN-centered seed-to-seed procedures.
2.7.1.1. Functional preprocessing
Rs-fMRI data preprocessing included the removal of the first five volumes, motion correction, skull stripping, spatial smoothing (Full Width at Half Maximum (FWHM) = 7 mm), grand mean scaling and filtering with both high-pass and low-pass filters (0.1- and 0.01-Hz thresholds, respectively). The data were then regressed with six rigid-body realignment motion parameters, mean WM, and mean cerebrospinal fluid (CSF) signal. No global signal regression was used. Registration to Montreal Neurological Institute (MNI) standard space was performed through a two-step linear transformation. Moreover, as head movement may affect rs-fMRI results (Power et al., 2012; 2015; Van Dijk et al., 2012), in-scanner head motion was calculated for every subject for additional control analyses (see SM for further information).
2.7.1.2. Seed-to-DMN
The concatenated fMRI dataset containing both pre- and post-iTBS rs-fMRI acquisitions from the entire sample obtained on the experimental day was decomposed through independent component analysis (ICA) into 15 components using the Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC; version 3.14) part of the FSL (Beckmann et al., 2005; Jenkinson et al., 2012; Smith et al., 2004). The DMN component was identified using spatial correlations with previously defined maps (Smith et al., 2009). Then, a mask covering the most important regions of this network was created (thresholded at 50%; Figure 2A). Furthermore, a hippocampal component was selected and masked for additional analyses (Figure S2; see SM for further details). To conduct the seed-to-DMN analysis, a spherical seed with a 6-mm radius was placed over the stimulation site, following the individualized coordinates used to guide the stimulation (see section 2.4). Subsequently, functional connectivity between this individualized region of interest (ROI) and the DMN mask was analyzed, using the seed-based correlation analysis (SBCA) of the FSL (Johnen et al., 2015; O’Reilly et al., 2010; Oldehinkel et al., 2016).
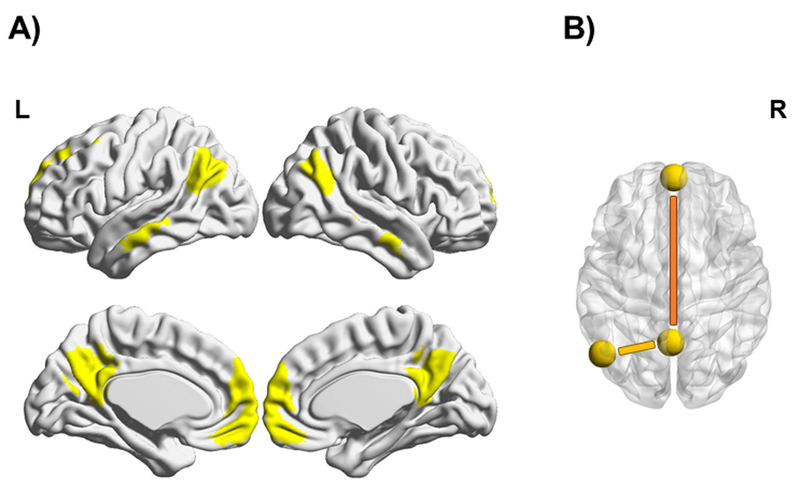
Figure 2.
A) The DMN mask covering the most important regions of this network is shown in yellow on the standard MNI map. B) The two functional couplings used for analyzing long-range and local connectivity within the DMN. The long-range DMN connectivity measure (mPFC-to-PCC) is shown in orange, while the local DMN connectivity measure (Stim-to-PCC) is shown in yellow. The yellow spheres represent the selected DMN seeds.
2.7.1.3. Long-range and local connectivity in the DMN
Since long- and short-range functional connections are differentially affected in aging (Sala-Llonch et al., 2014; Vidal-Piñeiro et al., 2014), two seed-to-seed functional couplings involving DMN connectivity were considered in a similar manner as previously used by our group (Vidal-Piñeiro et al., 2014; 2015). First, to obtain a long-range DMN connectivity measure, two spherical seeds with a 6-mm radius each were placed over the peak voxels of the core DMN areas (Figure 2B, in orange): medial prefrontal cortex [mPFC; x, y, z = 0, 58, - 6] and posterior cingulate cortex [PCC; x, y, z = - 2, - 58, 32]. Second, a measure reflecting local DMN connectivity was derived from the functional connectivity metrics between the individualized stimulation site (see section 2.4) and the PCC ROI (Figure 2B, in yellow), which was the main DMN node and the nearest hub to the lIPL.
2.7.2. Structural connectivity analyses
Diffusion MRI images were analyzed with the FMRIB’s Diffusion Toolbox (FDT) software from the FSL. Before analysis, we applied eddy current and motion correction and brain extraction (Smith, 2002). Then, individual fractional anisotropy (FA) maps were obtained with a diffusion tensor model fit (DTIFIT) and used for group analysis performed with the tract-based spatial statistics (TBSS) protocol (Smith et al., 2006). TBSS was applied to perform a nonlinear registration (FNIRT) of the FA images to the MNI standard space, generating a mean FA skeleton that represented the center of all the tracts common to the entire group. The aligned FA image for each subject was then projected onto the skeleton by filling the skeleton with FA values from the nearest relevant tract center. One subject was excluded from DTI analyses due to poor-quality data.
2.8. Statistical analyses
The first level of analysis was performed with imaging data. These analyses were conducted within different general linear model (GLM) matrices (randomized with 5,000 iterations). To investigate the effects of iTBS on rs-FC between the stimulation site and DMN, a univariate analysis of variance (ANOVA) was conducted with group (younger vs. older adults) and condition (sham vs. real iTBS) as between-subject factors and time (post- vs. pre-iTBS) as a within-subject factor. Following this ANOVA, two pairwise analyses were conducted. First, independent-samples t-tests were conducted to compare the iTBS-induced change in Stim-to-DMN rs-FC between the conditions in each group and amongst the groups in each condition. Second, paired-samples t-tests were conducted to assess differences in Stim-to-DMN rs-FC over time in each group and condition. Additionally, direct comparisons with independent-samples t-tests were conducted to explore differences in connectivity between the younger and older groups before stimulation. GLM matrices with head motion and stimulation intensity as covariates were created when statistical models included between-group comparisons that exhibited differences in these factors. Finally, time series of the different seeds (i.e., stimulation site, mPFC, and PCC) were extracted from the preprocessed and regressed images to conduct further non-imaging analyses.
For imaging data analyses, statistical significance was set at p ≤ 0.05 (corrected for family-wise error (FWE)). The threshold-free cluster enhancement (TFCE) method (Smith & Nichols, 2009) was used to define the clusters. Neuroimaging results were considered significant only when they persisted after controlling for relevant covariates in each model. BrainNet Viewer (Xia et al., 2013; http://www.nitrc.org/projects/bnv/) and Surf Ice (https://www.nitrc.org/projects/surfice/) were used for visualizing the neuroimaging data.
The second level of analysis was carried out with non-imaging data. These analyses were performed using IBM SPSS Statistics (version 23.0; IBM Corp., Armonk, NY, USA), MATLAB (version 7.12.0.635 R2011a; The MathWorks Inc., Natick, MA, USA), STATA (version 14.0; StataCorp, College Station, TX, USA) and GraphPad Prism (version 6.00 for Windows, GraphPad Software, La Jolla, CA, USA).
To obtain an rs-FC measure for each seed-to-seed coupling in each subject, previously obtained time series were correlated using Pearson’s correlation coefficients. To assess iTBS-induced modulation in both DMN seed-to-seed connections, two univariate ANOVAs were conducted with group (younger vs. older adults) and condition (sham vs. real iTBS) as between-subject factors and time (post- vs. pre-iTBS) as a within-subject factor. These statistical models were additionally controlled for head motion and stimulation intensity as they included between-group comparisons exhibiting differences in these factors, and the results were only considered when persisted significant after controlling for these covariates. Furthermore, four univariate ANOVAs with condition (sham vs. real iTBS) as a between-subject factor and time (post- vs. pre-iTBS) as a within-subject factor were conducted to assess the iTBS-induced modulation of functional connectivity for each group in both DMN seed-to-seed couplings. Following these ANOVAs, two different pairwise analyses were performed. First, independent-samples t-tests were used to explore differences in the changes in rs-FC between the two conditions in each group in both DMN seed-to-seed connections. Paired-samples t-tests were used to detect differences in the changes in rs-FC over time within each group and condition in both DMN seed-to-seed couplings. Furthermore, pre-iTBS long-range and local DMN connectivity measures adjusted for head motion were compared between the two groups using independent-samples t-tests. Finally, differences in the means and variability of local connectivity were explored before and after stimulation between the two conditions in the older group using independent-samples t-test and Levene’s test, respectively.
Relationships between the iTBS-induced modulation of functional connectivity and cognition, years of education and brain integrity were assessed for the older subjects. The iTBS-induced change in functional connectivity was obtained from the neuroimaging cluster where younger subjects exhibited a greater increase in Stim-to-DMN connectivity after real iTBS compared to sham (Figure 3C). In addition, the iTBS-induced change in the local DMN seed-to-seed connectivity was assessed. Individual results from the neuropsychological assessments and MRI analyses were extracted to obtain the following: scores in each cognitive test (both at baseline and at FU along with a subtraction between them to measure age-related cognitive change), the rs-FC in both DMN seed-to-seed measures (i.e., long-range and local couplings) and the rs-FC within the hippocampal network (HN) before stimulation adjusted for head motion, and the mean FA from the skeleton mask. Data from both sets of analyses were normalized to z scores and their relationships were assessed using Pearson’s partial correlations, adjusted for age (at baseline or at FU) and the time interval between stimulation and the post-iTBS rs-fMRI scan. Furthermore, ANOVAs with both DMN seed-to-seed measures (i.e., long-range and local connections) and the rs-FC within the HN before stimulation adjusted for head motion, and the baseline FA integrity, were performed considering three subgroups (i.e., younger adults, as well as ‘young-like’ responders and ‘non-young-like’ responders among the older adults). All post-hoc pairwise comparisons were subjected to Bonferroni corrections. Finally, additional analyses were conducted to examine whether the type of functional response to iTBS could be predicted by the pre-iTBS DMN rs-FC (considering the long-range and local measures) or the pre-iTBS HN rs-FC. Specifically, three logistic regression analyses were performed on all the subjects who received real iTBS, with Response (1 for younger adults / older adults with ‘young-like’ responses and 0 for older adults with ‘non-young-like’ responses) as dependent variable, the pre-iTBS long-range DMN rs-FC, the pre-iTBS local DMN rs-FC, or the pre-iTBS rs-FC within the HN as a predictor, and controlling for head motion as a covariate. Standard sensitivity and specificity analyses, including receiver operating characteristic (ROC) curves, were performed for the logistic regression analyses. Lastly, the neuropsychological estimates obtained at baseline and FU in the older adults who had received real iTBS were entered into univariate ANOVAs, with the type of response in the older adults (‘young-like’ vs. ‘non-young-like’) as a between-subject factor and cognitive change (follow-up vs. baseline cognitive scores) as a within-subject factor. Following these ANOVAs, pairwise analyses as detailed before were performed.
Data distribution considerations and sanity check analyses are detailed in SM. All statistical analyses were two-tailed and α was set at 0.05.
3. Results
3.1. Functional connectivity patterns after iTBS
3.1.1. Seed-to-DMN
There was an interaction between group and condition in the anterior regions of the DMN (Figure 3A). A main effect of condition was observed within the posteromedial cortex and frontal areas (Figure 3B). Pairwise comparisons revealed that functional connectivity in the anterior DMN regions was greater after real iTBS compared to sham among the younger participants (Figure 3C). Moreover, functional connectivity in frontal areas was higher after real iTBS in the younger participants than in the older participants (Figure 3D). Post-hoc pairwise comparisons demonstrated that among the younger participants who had received real iTBS, rs-FC was increased after stimulation in the anterior regions of the DMN compared to the rs-FC before stimulation (Figure 3E; results are summarized in Table S2). No significant effects were detected for the sham condition and there were no significant differences between the groups before stimulation.
3.1.2. Long-range and local connectivity in the DMN
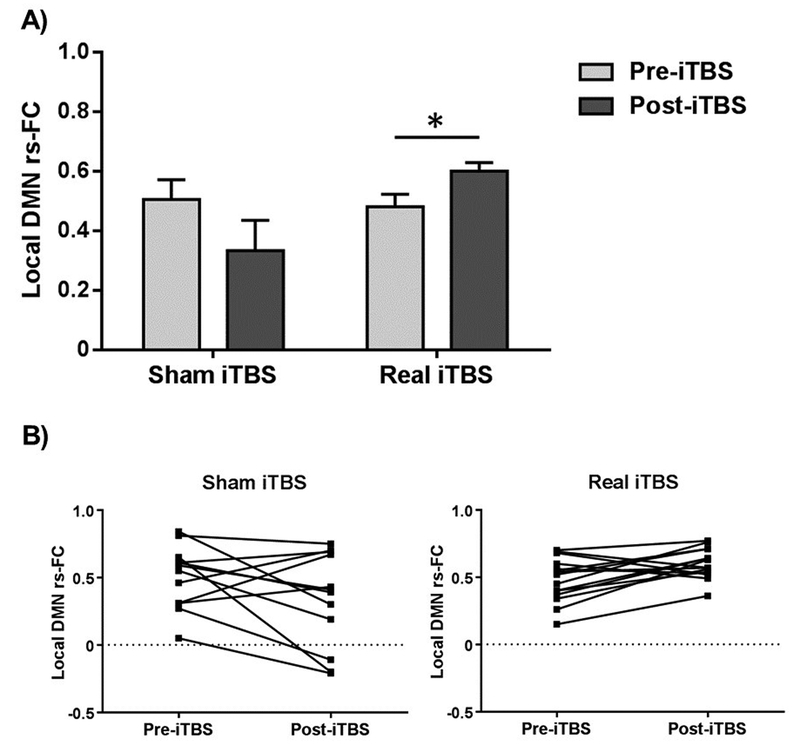
No significant interactions or main effects were found regarding long-range DMN rs-FC (all p values > 0.05). For local DMN rs-FC, a main effect of condition was observed (F(1,47) = 10.633, p = 0.002, ηp2 = 0.184). Analyses within each group were subsequently conducted separately, revealing a significant interaction between condition and time for local DMN rs-FC in the older participants (F(1,25) = 8.392, p = 0.008, ηp2 = 0.251; Figure 4A). Pairwise analyses revealed a significant difference in the change in local connectivity after iTBS in the older adults between both conditions (t = − 2.694, p = 0.017). Further pairwise comparisons indicated a significant difference in the change in local DMN rs-FC after real iTBS in the older adults (t = 2.814, p = 0.014), while no significant changes were observed in the older adults who had received sham iTBS (t = − 1.725, p = 0.113). Before stimulation, no differences were detected in local DMN rs-FC (p > 0.05), but there was an expected greater long-range DMN rs-FC adjusted for head motion in the younger participants compared to the older subjects (t = 3.255, p = 0.002; Figure S3). Additional analyses were conducted amongst the older adults who had received real iTBS regarding local DMN rs-FC to explore if the observed findings reflected a ‘mean group change’ or were associated with increases or decreases in the within-group inter-individual variability. Before stimulation, there were no significant differences in the means (t = 0.316, p = 0.755) or inter-individual variability (F = 1.870, p = 0.184) for local DMN rs-FC between the two conditions. After stimulation, both the means and variances significantly differed between the conditions (mean difference: t = − 2.522, p = 0.026; variability difference: F = 13.730, p = 0.001), indicating small inter-individual variability after real iTBS (SD = 0.112) and large variability after sham iTBS (SD = 0.353). These findings imply that functional variability was reduced due to real iTBS compared to sham iTBS (Figure 4B). No significant effects of iTBS in local DMN rs-FC were found in the younger group (p > 0.05; Figure S4).
Figure 4.
Local DMN connectivity in older adults. A) A significant interaction between condition and time was found for the local DMN rs-FC among the older adults. Post-hoc pairwise analyses revealed that real iTBS increased local DMN rs-FC amongst the older adults, while no significant changes were found in the older adults who had received sham iTBS. B) Inter-individual variability in local DMN rs-FC before and after stimulation among the older adults is shown separately for sham and real iTBS conditions. Data in A) are represented as mean with standard error of the mean (SEM). * Significant differences (p < 0.05). Abbreviations: iTBS, intermittent theta-burst stimulation; DMN, default-mode network; rs-FC, resting-state functional connectivity.
3.2. Relationships between iTBS responses and cognition or brain integrity in older adults at baseline
3.2.1. Cognitive performance
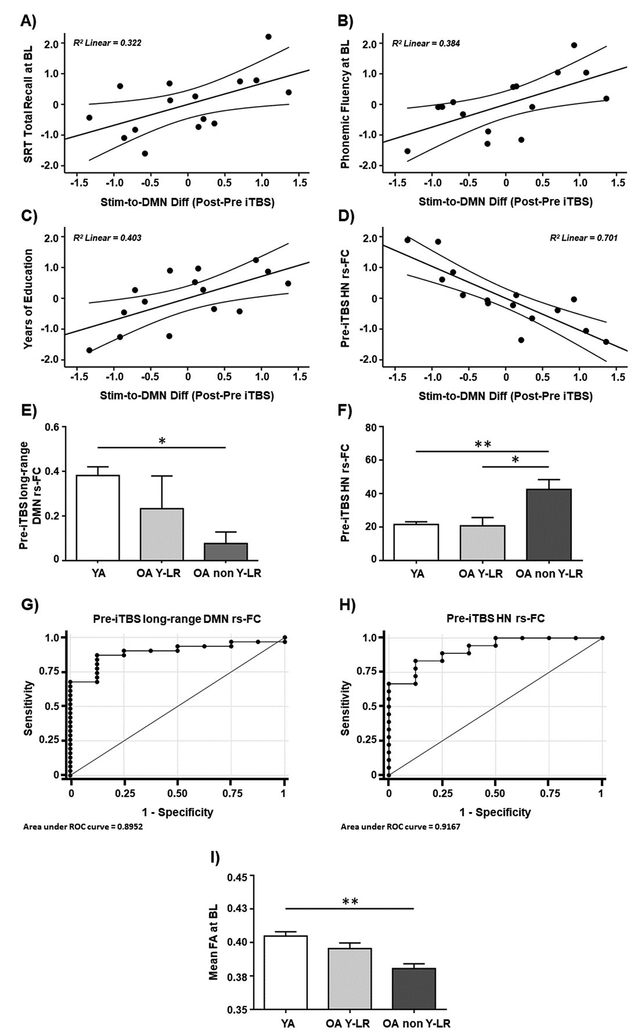
Amongst older adults who had received real iTBS, higher functional responses within the same fMRI cluster where younger adults showed increased rs-FC after iTBS (compared to sham; Figure 3C) were positively correlated with verbal memory (SRT total recall: r = 0.567, p = 0.043; Figure 5A) and executive function (phonemic fluency: r = 0.620, p = 0.024; Figure 5B). In addition, there was an association in the older adults in real iTBS condition between the increase of connectivity within the stated fMRI cluster (Figure 3C) and the years of educational attainment, a common proxy of cognitive reserve (CR; r = 0.635; p = 0.020; Figure 5C). Regarding seed-to-seed analyses, the degree of iTBS-induced change in local DMN rs-FC showed a negative trend with memory performance (SRT total recall: r = − 0.513, p = 0.073). All the sanity checks conducted verified that none of these associations were present in the sham condition and were not due to greater intrinsic connectivity before stimulation (all p values > 0.05).
Figure 5.
Scatter plots showing the relationships between iTBS-induced changes in the DMN rs-FC observed in the main experimental day and baseline A) SRT total recall, B) phonemic fluency, C) years of education, and D) pre-iTBS HN rs-FC. Bar charts showing significant interactions and Bonferroni-corrected post-hoc analyses between the three characterized subgroups with regards to E) pre-iTBS long-range DMN rs-FC, F) pre-iTBS HN rs-FC, and I) mean FA at baseline. The ROC curves for predicting a younger / ‘young-like’ response to iTBS based on G) pre-iTBS long-range DMN rs-FC, and H) pre-iTBS HN rs-FC. Data in A), B), C), and D) are presented with adjusted z scores. Data in E), F), and I) are represented as mean with SEM. Data in (I) are presented adjusted to two decimals. * Significant differences (p < 0.05), ** Significant differences (p < 0.001). Abbreviations: Stim, stimulation site; DMN, default-mode network; Diff; difference; iTBS, intermittent theta-burst stimulation; SRT, Selective Reminding Test; BL, baseline; HN, hippocampal network; rs-FC, resting-state functional connectivity; YA, younger adults; OA YL-R, older adults with ‘young-like’ responses; OA non YL-R, older adults with ‘non-young-like’ responses; ROC, receiver operating characteristic; FA, fractional anisotropy.
3.2.2. Functional connectivity
Older adults who had received real iTBS and showed greater connectivity within the same fMRI cluster where younger adults displayed increased DMN connectivity after real iTBS (compared to sham; Figure 3C) presented lower pre-iTBS HN rs-FC (r = − 0.837, p < 0.001; Figure 5D). The sanity checks confirmed that this association was not present in the sham condition and was not due to higher functional connectivity before stimulation (all p values > 0.05).
Moreover, pre-iTBS long-range DMN rs-FC differed significantly amongst the three subgroups (i.e., younger adults, as well as ‘young-like’ responders and ‘non-young-like’ responders among the older adults; F(2,36) = 5.631; p = 0.007, ηp2= 0.238; Figure 5E). Bonferroni-corrected post-hoc analyses revealed that the ‘non-young-like’ responders had significantly lower long-range DMN rs-FC than the younger adults (p = 0.007), while no significant difference between the ‘young-like’ responders and the younger subjects was found (p > 0.05). Moreover, a significant interaction was found between the specified subgroups as regards pre-iTBS HN rs-FC (F(2,36) = 11.503; p < 0.001, ηp2= 0.390; Figure 5F). Bonferroni-corrected post-hoc comparisons indicated higher hippocampal rs-FC in the ‘non-young-like’ responders compared to the ‘young-like’ responders (p = 0.002) and the younger adults (p < 0.001). There was no significant difference between the ‘young-like’ responders and the younger adults (p > 0.05).
Finally, it was found that higher pre-iTBS long-range DMN rs-FC predicted a significantly higher likelihood of a young / ‘young-like’ response to iTBS (, p = 0.013; Figure 5G). The area under the ROC curve based on this analysis was 0.90, with 87.10% sensitivity, 87.5% specificity, and 87.18% of cases correctly classified. Moreover, a significant effect of pre-iTBS HN rs-FC was found (, p = 0.022; Figure 5H). The area under the ROC curve based on this analysis was 0.92, with 83.33% sensitivity, 87.5% specificity, and 84.6% of cases correctly classified. The significant, negative coefficient indicated that stronger pre-iTBS connectivity within the HN predicted a lower likelihood of a younger / ‘young-like’ response to iTBS. The effect of head motion was not significant in any of the logistic regression analyses (all p values > 0.05).
3.2.3. Structural connectivity
A significant interaction was found between WM integrity (as measured by whole-brain FA) and the three subgroups specified in the preceding section (F(2,35) = 9.555; p < 0.001, ηp2 = 0.353; Figure 5I). Bonferroni-corrected post-hoc analyses revealed that the ‘non-young-like’ responders had significantly lower FA values than the younger adults (p < 0.001). Interestingly, FA integrity between the ‘young-like’ responders and the younger adults did not differ significantly (p > 0.05).
3.3. Associations between iTBS responses and cognition in older adults at follow-up
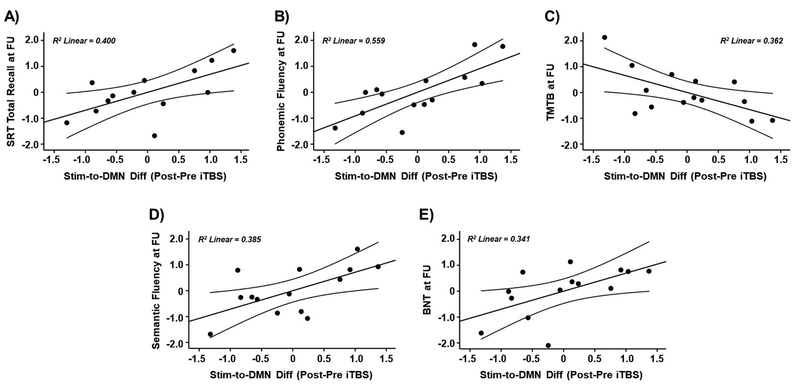
At follow-up, amongst older adults who had received real iTBS, higher functional responses within the same fMRI cluster where younger adults showed increased rs-FC after iTBS (compared to sham; Figure 3C) were correlated with better verbal memory (SRT total recall: r = 0.632, p = 0.037; Figure 6A), greater executive function (phonemic fluency: r = 0.748, p = 0.005; Figure 6B, and TMTB: r = − 0.602, p = 0.039; Figure 6C), and better performance in the language domain (semantic fluency: r = 0.620, p = 0.031; Figure 6D, and BNT: r = 0.584, p = 0.046; Figure 6E). The sanity checks verified that these associations were not related to higher connectivity before stimulation in the main experimental day (all p values > 0.05).
Figure 6.
Scatter plots showing the relationships between iTBS-induced changes in the DMN rs-FC observed in the main experimental day and follow-up cognitive scores in A) SRT total recall, B) phonemic fluency, C) TMTB, D) semantic fluency, and E) BNT. Data in A), B), C), D), and E) are presented with adjusted z scores. Abbreviations: Stim, stimulation site; DMN, default-mode network; Diff; difference; iTBS, intermittent theta-burst stimulation; SRT, Selective Reminding Test; FU, follow-up; TMTB, Trail Making Test B; BNT, Boston Naming Test.
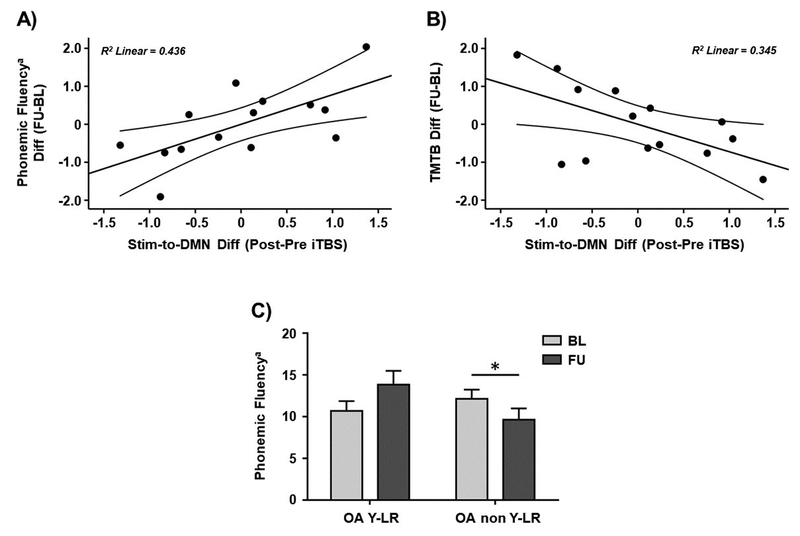
Finally, we investigated whether responses to iTBS were associated with age-related changes in cognitive performance. The latter was measured as the difference in the cognitive test performance between the follow-up visit and baseline. Significant results with executive function were found. Specifically, a significant positive correlation was found between the functional responses of the older adults with ‘young-like’ responses and age-related changes in phonemic fluency (letter M; r = 0.660, p = 0.019; Figure 7A). Furthermore, a significant negative association was observed for age-related changes in TMTB (r = − 0.587, p = 0.045; Figure 7B). The sanity checks confirmed that these associations were not related to greater intrinsic connectivity before stimulation in the main experimental day (all p values > 0.05). Moreover, a significant interaction was found between the older adults with ‘young-like’ responses and the older adults with ‘non-young-like’ responses in phonemic fluency at both visits (letter M; F(1,12) = 15.215, p = 0.002, ηp2 = 0.559; Figure 7C). Subsequent pairwise comparisons demonstrated a significant difference in the change in phonemic fluency between these two subgroups (t = 3.901, p = 0.002). Further pairwise analyses indicated that phonemic fluency was significantly lower at follow-up compared to baseline among the ‘non-young-like’ responders (t = − 3.307, p = 0.013), whereas ‘young-like’ responders performed marginally better at follow-up (t = 2.342; p = 0.066).
Figure 7.
Scatter plots showing the relationships between iTBS-induced changes in the DMN rs-FC observed in the main experimental day and the difference in A) phonemic fluency and B) TMTB scores between the follow-up and baseline. C) Bar chart showing significant interactions and pairwise post-hoc comparisons between the older adults with ‘young-like’ and ‘non-young-like’ responses with regards to phonemic fluency at both timepoints. Data in A) and B) are presented with adjusted z scores. Data in C) are represented as mean with SEM. a Phonemic fluency for letter M. * Significant differences (p < 0.05). Abbreviations: Stim, stimulation site; DMN, default-mode network; Diff; difference; iTBS, intermittent theta-burst stimulation; FU, follow-up; BL, baseline; TMTB, Trail Making Test B; OA YL-R, older adults with ‘young-like’ responses; OA non YL-R, older adults with ‘non-young-like’ responses.
4. Discussion
In the present study, we investigated age-related responses to iTBS in DMN connectivity. We observed that younger adults exhibited clear increases in functional connectivity in distal DMN areas from the stimulated lIPL region, while the older adults showed increased functional connectivity in proximal DMN regions (i.e., between the lIPL and PCC). Notably, older adults with ‘young-like’ responses to iTBS (i.e., increases in long-range connectivity between the lIPL and anterior DMN regions) had preserved brain integrity, more years of education, and better cognitive performance at baseline and the 3-year follow-up, along with greater maintenance of cognitive function, than those with ‘non-young-like’ responses. These associations were not observed in the fMRI data analyses before stimulation, indicating that iTBS-induced responses in functional connectivity represent a closer surrogate marker to general measures of brain integrity and clinical phenotype in our subjects.
Previous studies have used rTMS to investigate specific modulations of the DMN (Eldaief et al., 2011; Halko et al., 2014; Van Der Werf et al., 2010). However, the present study is the first to characterize age-related functional differences in the effects of iTBS when the lIPL is targeted. Our findings are consistent with those of Halko and colleagues (2014), who demonstrated that functional connectivity increases within the cortical hubs of the DMN when a cerebellar node of the DMN is stimulated with iTBS. Comparing our results with those of rTMS studies conducted with non-patterned rTMS protocols (i.e., Eldaief et al., 2011; Van Der Werf et al., 2010) is less straightforward because conventional and patterned rTMS protocols are likely to involve different mechanisms of action modulating different neuronal populations (Di Lazzaro & Rothwell, 2014). Nevertheless, the converging evidence from Halko and colleagues (2014) and the present study suggests that applying iTBS to distinct DMN nodes modulates this network by increasing its functional connectivity. Furthermore, these effects appear to remain consistent across the ages, although the changes are primarily evident in short-distance connections in the older subjects. The nature of these age-related differences in the iTBS-induced modulation of DMN connectivity may be linked to the descriptive neuroimaging observations indicating that the dissimilar changes in connectivity in aging depend on the connection path length (Cao et al., 2014; Ferreira & Busatto, 2013; Sala-Llonch et al., 2014; Tomasi & Volkow, 2012). Not all the parts of a particular network display the same changes with age (Shaw et al., 2015), with long-range couplings appearing to be preferentially reduced during aging (Cao et al., 2014; Ferreira & Busatto, 2013; Sala-Llonch et al., 2014; Tomasi & Volkow, 2012), a fact that seems to particularly apply to the DMN (Andrews-Hanna et al., 2007; Ferreira & Busatto, 2013; Tomasi & Volkow, 2012; Vidal-Piñeiro et al., 2014). At the same time, the strength of short-range connections is increased in aging (Cao et al., 2014; Sala-Llonch et al., 2014), which can, in turn, boost between-network connectivity (Chan et al., 2014; Grady et al., 2016; Spreng et al., 2016).
We observed that the older participants who showed a functional response in distal DMN regions displayed superior memory and executive function, whereas older adults with localized responses exhibited a marginally negative association with memory. Furthermore, older adults who displayed greater changes in long-range functional connectivity in response to iTBS maintained higher functioning in the memory, executive and language domains at the 3-year follow-up. The finding that some older adults maintained or slightly improved their cognitive function at follow-up (while others showed an expected cognitive decline) could be related to practice effects, defined as an improvement in cognitive test performance due to repeated exposure to the test materials (Duff et al., 2012; McCaffrey & Westervelt, 1995). Critically, practice effects has been suggested as an index of long-term cognitive function and as a diagnostic and prognostic marker in the AD continuum (Cooper et al., 2001; 2004; Darby et al., 2002; Duff et al., 2007; 2008, 2017; Howieson et al., 2008; Machulda et al., 2013). Finally, a young-like response was characteristic of older adults with higher estimates of education, which is the most commonly used proxy of CR (Stern, 2009). CR is a relevant concept in aging because it helps explaining inter-individual differences in the adaptability and susceptibility to cope with age-related brain changes, pathology or insult (Stern et al., 2018). While a number of neuroimaging studies have been conducted in the field reflecting greater brain integrity and efficiency of functional brain networks amongst elders with high CR estimates, the biological pathways through which reserve operates are not currently well understood. In previous studies, we proposed that CR could reflect an index of brain plasticity in aging (Bartrés-Faz & Arenaza-Urquijo, 2011; Pascual-Leone et al. 2011). Present results reinforce these previous interpretations and provide new evidence of the neurophysiological mechanisms subtending CR.
Further, we also observed that ‘young-like’ responses over the DMN can be predicted by the mPFC-to-PCC connection. Although other studies have explored how certain functional connectivity features can predict the response to iTBS (Cárdenas-Morales et al., 2014; Nettekoven et al., 2015), this particular result indicates that whether the main long-range connectivity within the DMN is preserved, this network maintains its capability to exhibit large-scale modulatory responses to stimulation. Moreover, HN connectivity also predicted the type of functional response to iTBS within the DMN. Subjects who exhibited greater long-distance responses to iTBS within the DMN showed lower HN rs-FC before stimulation. The relationship between the DMN and HN has been demonstrated to break down in aging, leading to deficient mnemonic processing (Salami et al., 2014). Specifically, it has been found that age-related decrements in the connectivity of cortical DMN nodes are accompanied by increased connectivity between bilateral hippocampi and decreased DMN-to-HN connectivity. Moreover, greater HN connectivity at rest restricts the degree to which the hippocampus interacts with other brain regions during memory tasks, resulting in memory deficits (Salami et al., 2014). Importantly, in the AD model, the first events preceding detectable CSF aβ and tau abnormalities were reported to be increases in the HN connectivity along with decreases within the DMN rs-FC (Chen et al., 2016).
Regarding brain structural integrity, we found that the older adults with ‘young-like’ responses exhibited higher FA integrity. Aging is associated with decreases in WM and GM integrity (Burzynska et al., 2010; Driscoll et al., 2009; Fjell et al., 2009; 2014; Westlye et al., 2010). Moreover, age-related changes in functional and structural connectivity are closely aligned (Betzel et al., 2014; Fjell et al., 2016; Zimmermann et al., 2016), while rs-FC and GM associations in aging remain relatively unclear (Damoiseaux et al., 2016; Fjell et al., 2016; Persson et al., 2014).
Current results are in line with previous findings indicating that stronger long-distance connections are characteristic of older adults with preserved cognition and brain structure (Andrews-Hanna et al., 2007; Vidal-Piñeiro et al., 2014), whereas age-related functional clustering is associated with poorer cognitive performance (Sala-Llonch et al., 2014). Nonetheless, it is important to note that in our study, the presented associations between DMN functioning and cognitive and brain integrity measures were only observed when analyzing the brain induced responses to iTBS, but not before stimulation. This suggests that distinct biological underpinnings underlie these evoked connectivity changes, and due to the characteristics of the TBS protocol, they likely reflect the engagement of plasticity-like brain mechanisms (Huang et al., 2005). Therefore, in the form they were measured here, these responses appear to be more sensitive to identify meaningful associations between the expression of brain networks and cognitive status in reduced samples of participants, as compared to baseline fMRI assessments (i.e., before iTBS). In a similar line, measuring connectivity responses to iTBS could be used as an approach to enrich samples of participants for future interventional initiatives (i.e., cognitive training or pharmacological interventions) where the modulation of synaptic plasticity is thought to represent the underlying therapeutic mechanism. Modulating brain activity with NIBS techniques can yield a unique insight into network dynamics in health and disease (Eldaief et al., 2011; Fox et al., 2012; Halko et al., 2014; Pascual-Leone et al., 2011; Shafi et al., 2012, 2014, 2015). Ultimately, disrupting functional connectivity with TMS-fMRI may lead to a “brain flexibility” index (Santarnecchi et al., 2015; Santarnecchi & Rossi, 2016), a dynamic rather than a static marker that can be used to unravel the physiological basis of different cognitive profiles in a multilayered and accurate manner.
Finally, it is worth noting that large inter-individual variability has been observed in aging in cognitive as well as brain structural and functional phenotypes (Dickie et al., 2013; Hedden & Gabrieli, 2004; Hultsch et al., 2002; Mowinckel et al., 2012; Ylikoski et al., 1999). Some older subjects maintain cognitive function during their lifespan, while others present different levels of cognitive decline (Nyberg et al., 2012; Yaffe et al., 2009). Similarly, inter-individual variability in response to different rTMS protocols has also been described (Hamada et al., 2013; Jannati et al., 2017; López-Alonso et al., 2014; Müller-Dahlhaus et al., 2008; Nettekoven et al., 2015; Schilberg et al., 2017), with potential factors contributing to such variability, including differences in the activated intracortical networks (Hamada et al., 2013), functional connectivity within the targeted network (Nettekoven et al., 2015), differences in cortical excitability (Jannati et al., 2017), and single-nucleotide polymorphisms (SNPs) in the genes that can influence neuroplasticity, such as brain-derived neurotrophic factor (BDNF; Antal et al., 2010; Cheeran et al., 2008; Cirillo et al., 2012; Di Lazzaro et al., 2015; Jannati et al., 2017; Lee et al., 2013). On the basis of these inter-individual differences, affecting both aging and the impact of NIBS, it is worth to consider that a better neurophysiological response to iTBS could have been observed in the present investigation priming iTBS with cTBS (although this approach has been shown to be ineffective for motor cortex stimulation in older adults; Opie et al., 2017), or through a co-stimulation approach of iTBS with gamma transcranial alternating current stimulation (ɣ-tACS; Guerra et al., 2018). Further, the uncertainty remains about how our subjects would respond to other TBS protocols, like cTBS (i.e., Hamada et al., 2013), as well as to other TMS or even transcranial direct current stimulation (tDCS) protocols (i.e., López-Alonso et al., 2014). In any case, here we showed that variability in iTBS responses is not disadvantageous, but may in fact help identify individual factors that influence naturally-occurring plasticity, thereby revealing the brain physiology underlying cognitive processes (Polanía et al., 2018) and cognitive reserve (Bartrés-Faz & Arenaza-Urquijo, 2011; Pascual-Leone et al., 2011).
The present study suffers from several limitations. The first constraint was the sample sizes, particularly the one addressed in the follow-up investigation. The second limitation was that our sample was mainly composed by women, raising the possibility that our results could be derived from a gender effect. Third, although older participants underwent a comprehensive cognitive assessment, the cognitive profile of the younger subjects was not formally evaluated. Furthermore, absence of neurological or psychiatric condition was self-reported, lacking a detailed clinical exploration. Finally, subjective memory complaints were not formally assessed in aged subjects, and further studies are needed to explore how this factor can interfere with the modulation of brain networks in aging.
5. Conclusions
The present investigation revealed that iTBS effects over DMN when the lIPL is targeted are modulated by age. Furthermore, the combined iTBS-fMRI approach may offer individual assessments of large-scale network plasticity across the lifespan, which probably reflects a neurobiological substrate of cognitive reserve (Bartrés-Faz & Arenaza-Urquijo, 2011; Pascual-Leone et al., 2011). Lastly, when integrated with other factors, these plasticity measures can contribute to the construction of a brain health index (i.e., genetics and diet; Freitas et al., 2013).
Supplementary Material
Highlights.
iTBS exerts distinctive effects in DMN connectivity in younger and older adults.
iTBS-fMRI metrics can identify meaningful associations between brain networks functioning and cognition in aging.
The iTBS-fMRI approach can allow to distinguish different cognitive trajectories in aging.
iTBS-fMRI measures could reflect a network plasticity mechanism of cognitive reserve.
Acknowledgments
This work was supported by two research grants from the Spanish Ministry of Economy and Competitiveness (MINECO/FEDER) to D.B.-F (PSI2012–38257 and PSI2015–64227-R). K.A.-P. was supported by a predoctoral fellowship from the Spanish Ministry of Education, Culture and Sport (MECD; reference number, FPU14/02728). L.V.A. was supported by a predoctoral fellowship associated with the MINECO/FEDER PSI2015–64227-R grant (reference number, BES-2016–077620). A.J. was supported by postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada (NSERC PDF 454617) and the Canadian Institutes of Health Research (CIHR 41791). E.S. was supported by the Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA; via 2014–13121700007), Beth Israel Deaconess Medical Center (BIDMC; via the Chief Academic Officer (CAO) grant 2017) and the Defense Advanced Research Projects Agency (DARPA; via HR001117S0030). A.P.-L. was supported by the Sidney R. Baer Jr. Foundation, the National Institutes of Health (NIH R01 MH100186, R01 NS073601, R01 HD069776, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616), Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758), DARPA (via HR001117S0030) and the Football Players Health Study at Harvard University.
Footnotes
Conflict of Interest
A.P.-L. serves on the scientific advisory boards for Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Constant Therapy, NovaVision, Cognito and Neosync, and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. E.S. serves on the scientific advisory boards for EBNeuro Ltd and Neuroelectrics, and is listed as an inventor on issued and pending patents on the integration of non-invasive brain stimulation with neuroimaging data for therapeutic applications in neurodegenerative disorders. The remaining authors declare no competing interests.
References
- Altman DG, & Bland JM (1999). How to randomise. BMJ (Clinical Research Ed.), 319(7211), 703–704. 10.1136/bmj.319.7211.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, & Buckner RL (2007). Disruption of Large-Scale Brain Systems in Advanced Aging. Neuron, 56(5), 924–935. 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Chaieb L, Moliadze V, Monte-silva K, Poreisz C, Thirugnanasambandam N, … Paulus W (2010). Brain-derived neurotrophic factor ( BDNF ) gene polymorphisms shape cortical plasticity in humans. Brain Stimulation, 3(4), 230–237. 10.1016/j.brs.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Barker A (1991). An Introduction to the Basic Principles of Magnetic Nerve Stimulation. Journal of Clinical Neurophysiology, 8(1), 26–37. [DOI] [PubMed] [Google Scholar]
- Bartrés-Faz D, & Arenaza-Urquijo EM (2011). Structural and functional imaging correlates of cognitive and brain reserve hypotheses in healthy and pathological aging. Brain Topography, 24(3–4), 340–357. 10.1007/s10548-011-0195-9 [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, & Smith SM (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1457), 1001–1013. 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo XN, & Sporns O (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage, 102(P2), 345–357. 10.1016/j.neuroimage.2014.07.067 [DOI] [PubMed] [Google Scholar]
- Bressler SL, & Menon V (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. 10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li SC, Lindenberger U, & Heekeren HR (2010). Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. NeuroImage, 49(3), 2104–2112. 10.1016/j.neuroimage.2009.09.041 [DOI] [PubMed] [Google Scholar]
- Cao M, Wang JH, Dai ZJ, Cao XY, Jiang LL, Fan FM, … He Y (2014). Topological organization of the human brain functional connectome across the lifespan. Developmental Cognitive Neuroscience, 7(16), 76–93. 10.1016/j.dcn.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas-Morales L, Volz LJ, Michely J, Rehme AK, Pool EM, Nettekoven C, … Grefkes C (2014). Network connectivity and individual responses to brain stimulation in the human motor system. Cerebral Cortex, 24(7), 1697–1707. 10.1093/cercor/bht023 [DOI] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, & Wig GS (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences, 111(46), E4997–E5006. 10.1073/pnas.1415122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, … Tor R (2008). A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. The Journal of Physiology, 586(23), 5717–5725. 10.1113/jphysiol.2008.159905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shu H, Chen G, Ward BD, Antuono PG, Zhang Z, & Li SJ (2016). Staging Alzheimer’s Disease Risk by Sequencing Brain Function and Structure, Cerebrospinal Fluid, and Cognition Biomarkers. Journal of Alzheimer’s Disease, 54(3), 983–993. 10.3233/JAD-160537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J, Hughes J, Ridding M, Thomas PQ, & Semmler JG (2012). Differential modulation of motor cortex excitability in BDNF Met allele carriers following experimentally induced and use-dependent plasticity. European Journal of Neuroscience, 36(5), 2640–2649. 10.1111/j.1460-9568.2012.08177.x [DOI] [PubMed] [Google Scholar]
- Cooper DB, Epker M, Lacritz L, Weine M, Rosenberg RN, Honig L, & Cullum CM (2001). Effects of practice on category fluency in Alzheimer’s disease. The Clinical Neuropsychologist, 15(1), 125–128. 10.1076/clin.15.1.125.1914 [DOI] [PubMed] [Google Scholar]
- Cooper DB, Lacritz LH, Weiner MF, Rosenberg RN, & Cullum CM (2004). Category fluency in mild cognitive impairment: Reduced effect of practice in test-retest conditions. Alzheimer Disease and Associated Disorders, 18(3), 120–122. 10.1097/01.wad.0000127442.15689.92 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Viviano RP, Yuan P, & Raz N (2016). Differential effect of age on posterior and anterior hippocampal functional connectivity. NeuroImage, 133, 468–476. 10.1016/j.neuroimage.2016.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS (2017). Effects of aging on functional and structural brain connectivity. NeuroImage, 160, 32–40 10.1016/j.neuroimage.2017.01.077 [DOI] [PubMed] [Google Scholar]
- Darby D, Maruff P, Collie A, & McStephen M (2002). Mild cognitive impairment can be dtected by multiple assessments in a single day. Neurology, 59(7), 1042–1046. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, & Rothwell JC (2014). Corticospinal activity evoked and modulated by noninvasive stimulation of the intact human motor cortex. The Journal of Physiology, 592(19), 4115–4128. 10.1113/jphysiol.2014.274316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pellegrino G, Di Pino G, Corbetto M, Ranieri F, Brunelli N, … Capone F (2015). Val66met bdnf gene polymorphism influences human motor cortex plasticity in acute stroke. Brain Stimulation, 8(1), 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie DA, Job DE, Gonzalez DR, Shenkin SD, Trevor S, Murray AD, & Wardlaw JM (2013). Variance in brain volume with advancing age: implications for defining the limits of normality, PLoS One, 8(12), e84093 10.1371/journal.pone.0084093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair D. a, Power D, Church J. a, … Schlaggar BL (2011). Prediction of Individua Brain Maturity Using fMRI. Science, 329(5997), 1358–1361. 10.1126/science.1194144.Prediction [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM (2009). Longitudinal pattern of regional brain volume change differentiates normal aging fromMCI. Neurology, 72(22), 1906–1913. 10.1212/WNL.0b013e3181a82634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Beglinger LJ, Schultz SK, Moser DJ, McCaffrey RJ, Haase RF, … Paulsen JS (2007). Practice effects in the prediction of long-term cognitive outcome in three patient samples: a novel prognostic index. Archives of Clinical Neuropsychology : The Official Journal of the National Academy of Neuropsychologists, 22(1), 15–24. 10.1016/j.acn.2006.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Beglinger LJ, Van Der Heiden S, Moser DJ, Schultz SK, & Paulsen JS (2008). Short-term practice effects in amnestic mild cognitive impairment: implications for diagnosis and treatment. International Psychogeriatrics, 20(5), 986–999. 10.1017/S1041610208007254.Short-term [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Callister C, Dennett K, & Tometich D (2012). Practice effects: A unique cognitive variable. Clinical Neuropsychologist, 26(7), 1117–1127. 10.1080/13854046.2012.722685 [DOI] [PubMed] [Google Scholar]
- Duff K, Hammers DB, Dalley BCA, Suhrie KR, Atkinson TJ, Rasmussen KM, … Hoffman JM (2017). Short-Term Practice Effects and Amyloid Deposition: Providing Information Above and Beyond Baseline Cognition. The Journal of Prevention of Alzheimer’s Disease, 4(2), 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, & Pascual-Leone A (2011). Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proceedings of the National Academy of Sciences, 108(52), 21229–21234. 10.1073/pnas.1113103109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, … Petersen SE (2009). Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology, 5(5), e1000381 10.1371/journal.pcbi.1000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, & Busatto GF (2013). Resting-state functional connectivity in normal brain aging. Neuroscience and Biobehavioral Reviews, 37(3), 384–400. 10.1016/j.neubiorev.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, … Dale AM (2009). One-Year Brain Atrophy Evident in Healthy Aging. Journal of Neuroscience, 29(48), 15223–15231. 10.1523/JNEUROSCI.3252-09.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, & Walhovd KB (2014). What is normal in normal aging? Effects of Aging, Amyloid and Alzheimer’s Disease on the Cerebral Cortex and the Hippocampus. Progress Neurobiology, 117, 20–40. 10.1016/j.pneurobio.2014.02.004.What [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Sneve MH, Storsve AB, Grydeland H, Yendiki A, & Walhovd KB (2016). Brain Events Underlying Episodic Memory Changes in Aging: A Longitudinal Investigation of Structural and Functional Connectivity, Cerebral Cortex, 26(3), 1272–1286. 10.1093/cercor/bhv102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, & Pascual-Leone A (2012). Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). NeuroImage, 62(4), 2232–2243. 10.1016/j.neuroimage.2012.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, … Pascual-Leone A (2011). Changes in cortical plasticity across the lifespan. Front Aging Neurosci, 3(April), 5 10.3389/fnagi.2011.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Farzan F, & Pascual-Leone A (2013). Assessing brain plasticity across the lifespan with transcranial magnetic stimulation: Why, how, and what is the ultimate goal? Frontiers in Neuroscience, 7(42). 10.3389/fnins.2013.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangitano M, Valero-Cabré A, Tormos JM, Mottaghy FM, Romero JR, & Pascual-Leone A (2002). Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clinical Neurophysiology, 113(8), 1249–1257. 10.1016/S1388-2457(02)00109-8 [DOI] [PubMed] [Google Scholar]
- Grady C, Sarraf S, Saverino C, & Campbell K (2016). Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiology of Aging, 41, 159–172. 10.1016/j.neurobiolaging.2016.02.020 [DOI] [PubMed] [Google Scholar]
- Guerra A, Suppa A, Bologna M, D’Onofrio V, Bianchini E, Brown P, … Berardelli A (2018). Boosting the LTP-like plasticity effect of intermittent theta-burst stimulation using gamma transcranial alternating current stimulation. Brain Stimulation, 11(4), 734–742. 10.1016/j.brs.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, & Pascual-Leone A (2014). Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. The Journal of Neuroscience, 34(36), 12049–12056. 10.1523/JNEUROSCI.1776-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, & Rothwell JC (2013). The role of interneuron networks in driving human motor cortical plasticity. Cerebral Cortex, 23(7), 1593–1605. 10.1093/cercor/bhs147 [DOI] [PubMed] [Google Scholar]
- Hedden T, & Gabrieli JDE (2004). Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience, 5(2), 87–96. 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- Howieson DB, Carlson NE, Moore MM, Wasserman D, Abendroth CD, Payne-Murphy J, & Kaye JA (2008). Trajectory of mild cognitive impairment onset. Journal of the International Neuropsychological Society, 14(2), 192–198. 10.1017/S1355617708080375 [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, & Rothwell JC (2005). Theta burst stimulation of the human motor cortex. Neuron, 45(2), 201–206. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS, & Dixon RA (2002). Variability in reaction time performance of younger and older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 57(2), 101–115. 10.1093/geronb/57.2.P101 [DOI] [PubMed] [Google Scholar]
- Jannati A, Block G, Oberman LM, Rotenberg A, & Pascual-Leone A (2017). Interindividual variability in response to continuous theta-burst stimulation (cTBS) in healthy adults. Clinical Neurophysiology, 128(11), 2268–2278. 10.1016/j.clinph.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, & Smith SM (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jockwitz C, Caspers S, Lux S, Eickhoff SB, Jütten K, Lenzen S, … Amunts K (2017). Influence of age and cognitive performance on resting-state brain networks of older adults in a population-based cohort. Cortex, 89, 28–44. 10.1016/j.cortex.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Johnen VM, Neubert FX, Buch ER, Verhagen LM, O’Reilly J, Mars RB, & Rushworth MFS (2015). Causal manipulation of functional connectivity in a specific neural pathway during behaviour and at rest. Elife, 4, e04585 10.7554/eLife.04585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Ragan BG, & Park J-H (2008). Issues in Outcomes Research: An Overview of Randomization Techniques for Clinical Trials. Journal of Athletic Training, 43(2), 215–221. 10.4085/1062-6050-43.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel FA, Nahas Z, DeBrux C, Molloy M, Lorberbaum JP, Bohning D, … George MS (2000). How Coil–Cortex Distance Relates to Age, Motor Threshold, and Antidepressant Response to Repetitive Transcranial Magnetic Stimulation. The Journal of Neuropsychiatry and Clinical Neurosciences, 12(3), 376–384. 10.1176/jnp.12.3.376 [DOI] [PubMed] [Google Scholar]
- Lee M, Kim SE, Kim WS, Lee J, Yoo HK, Park K, … Lee HW (2013). Interaction of motor training and intermittent theta burst stimulation in modulating motor cortical plasticity: influence of BDNF Val66Met polymorphism. PLoS One, 8(2), e57690 10.1371/journal.pone.0057690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yu C, Zhang X, Liu J, Duan Y, Alexanderbloch AF, … Bullmore E (2014). Impaired long distance functional connectivity and weighted network architecture in Alzheimer’s disease. Cerebral Cortex, 24(6), 1422–1435. 10.1093/cercor/bhs410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Alonso V, Cheeran B, Río-Rodríguez D, & Fernández-Del-Olmo M (2014). Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimulation, 7(3), 372–380. 10.1016/j.brs.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Machulda MM, Pankratz VS, Christianson TJ, Ivnik RJ, Mielke MM, Roberts RO, … Petersen RC (2013). Practice Effects and Longitudinal Cognitive Change in Normal Aging vs. Incident Mild Cognitive Impairment and Dementia in The Mayo Clinic Study of Aging. The Clinical Neuropsychologist, 27(8), 1247–1264. 10.1080/13854046.2013.836567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey RJ, & Westervelt HJ (1995). Issues associated with repeated neuropsychological assessments. Neuropsychology Review, 5(3), 203–221. 10.1007/BF02214762 [DOI] [PubMed] [Google Scholar]
- McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, & George MS (2001). The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: A replication in healthy adults comparing two methods of assessing the distance to cortex. Biological Psychiatry, 49(5), 454–459. 10.1016/S0006-3223(00)01039-8 [DOI] [PubMed] [Google Scholar]
- Mevel K, Landeau B, Fouquet M, La Joie R, Villain N, Mézenge F, … Chételat G (2013). Age effect on the default mode network, inner thoughts, and cognitive abilities. Neurobiology of Aging, 34(4), 1292–1301. 10.1016/j.neurobiolaging.2012.08.018 [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Marré SC, Schmitt W, Hess CW, Fisch HU, & Schlaepfer TE (2002). Antidepressant Effects of Repetitive Transcranial Magnetic Stimulation in the Elderly: Correlation Between Effect Size and Coil-Cortex Distance. Archives of General Psychiatry, 59(6), 560–561. [DOI] [PubMed] [Google Scholar]
- Mowinckel AM, Espeseth T, & Westlye LT (2012). Network-specific effects of age and in-scanner subject motion: A resting-state fMRI study of 238 healthy adults. NeuroImage, 63(3), 1364–1373. 10.1016/j.neuroimage.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus JFM, Orekhov Y, Liu Y, & Ziemann U (2008). Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Experimental Brain Research, 187(3), 467–475. 10.1007/s00221-008-1319-7 [DOI] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Braskie MN, & Mather M (2017). Resting-state networks associated with cognitive processing show more age-related decline than those associated with emotional processing. Neurobiology of Aging, 54, 152–162. 10.1016/j.neurobiolaging.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Leimbach M, Pool EM, Rehme AK, Eickhoff SB, … Grefkes C (2015). Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. NeuroImage, 118, 209–218. 10.1016/j.neuroimage.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, & Bäckman L (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16(5), 292–305. 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, & Johansen-Berg H (2010). Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cerebral Cortex, 20(4), 953–965. 10.1093/cercor/bhp157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel M, Beckmann CF, Franke B, Hartman CA, Hoekstra PJ, Oosterlaan J, … Mennes M (2016). Functional connectivity in cortico-subcortical brain networks underlying reward processing in attention-deficit/hyperactivity disorder. NeuroImage: Clinical, 12, 796–805. 10.1016/j.nicl.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie GM, Vosnakis E, Ridding MC, Ziemann U, & Semmler JG (2017). Priming theta burst stimulation enhances motor cortex plasticity in young but not old adults. Brain Stimulation, 10(2), 298–304. 10.1016/j.brs.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Padberg F, Zwanzger P, Keck ME, Kathmann N, Mikhaiel P, Ella R, … Moller HJ (2002). Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology, 27(4), 638–645. 10.1016/S0893-133X(02)00338-X [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, … Rotenberg A (2011). Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topography, 24(3–4), 302–315. 10.1007/s10548-011-0196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Pudas S, Nilsson L, & Nyberg L (2014). Neurobiology of Aging Longitudinal assessment of default-mode brain function in aging. Neurobiology of Aging, 35(9), 2107–2117. 10.1016/j.neurobiolaging.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Petersen RC, & Morris JC (2005). Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology, 62(7), 1160–1163; discussion 1167. 10.1001/archneur.62.7.1160 [DOI] [PubMed] [Google Scholar]
- Polanía R, Nitsche MA, & Ruff CC (2018). Studying and modifying brain function with non-invasive brain stimulation. Nature Neuroscience, 21(2), 174–187. 10.1038/s41593-017-0054-4 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, & Petersen SE (2015). Recent progress and outstanding issued in motion correction resting state fmri. NeuroImage, 105, 536–551. 10.1016/j.neuroimage.2014.10.044.Recent [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, & Caviness VS (1992). Human cerebral cortex: localization, parcellation, and morphometry with magnetic resonance imaging. Journal of Cognitive Neuroscience, 4(4), 352–74. 10.1162/jocn.1992.4.4.352 [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Avanzini G, Bestmann S, … Ziemann U (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, … Ziemann U (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application: An updated report from an I.F.C.N. Committee. Clinical Neurophysiology, 126(6), 1071–1107. 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Junqué C, Arenaza-Urquijo EM, Vidal-Piñeiro D, Valls-Pedret C, Palacios EM, … Bartrés-Faz D (2014). Changes in whole-brain functional networks and memory performance in aging. Neurobiology of Aging, 35(10), 2193–2202. 10.1016/j.neurobiolaging.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, & Junqué C (2015). Reorganization of brain networks in aging: a review of functional connectivity studies. Frontiers in Psychology, 6(663). 10.3389/fpsyg.2015.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Pudas S, & Nyberg L (2014). Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. Proceedings of the National Academy of Sciences of the United States of America, 111(49), 17654–17659. 10.1073/pnas.1410233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Rossi S, & Rossi A (2015). The smarter, the stronger: Intelligence level correlates with brain resilience to systematic insults. Cortex, 64, 293–309. 10.1016/j.cortex.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Santarnecchi E, & Rossi S (2016). Advances in the Neuroscience of Intelligence: From Brain Connectivity to Brain Perturbation. Spanish Journal of Psychology, 19, E94. [DOI] [PubMed] [Google Scholar]
- Schilberg L, Schuhmann T, & Sack AT (2017). Interindividual Variability and Intraindividual Reliability of Intermittent Theta Burst Stimulation-induced Neuroplasticity Mechanisms in the Healthy Brain. Journal of Cognitive Neuroscience, 29(6), 1022–1032. 10.1162/jocn_a_01100 [DOI] [PubMed] [Google Scholar]
- Schultz AP, Chhatwal JP, Hedden T, Mormino EC, Hanseeuw BJ, Sepulcre J, … Sperling RA (2017). Phases of Hyperconnectivity and Hypoconnectivity in the Default Mode and Salience Networks Track with Amyloid and Tau in Clinically Normal Individuals. The Journal of Neuroscience, 37(16), 4323–4331. 10.1523/JNEUROSCI.3263-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi MM, Westover MB, Fox MD, & Pascual-Leone A (2012). Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. European Journal of Neuroscience, 35(6), 805–825. 10.1111/j.1460-9568.2012.08035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi MM, Westover MB, Oberman L, Cash SS, & Pascual-Leone A (2014). Modulation of EEG functional connectivity networks in subjects undergoing repetitive transcranial magnetic stimulation. Brain Topography, 27(1), 172–191. 10.1007/s10548-013-0277-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi MM, Vernet M, Klooster D, Chu CJ, Boric K, Barnard ME, … Chang BS (2015). Physiological consequences of abnormal connectivity in a developmental epilepsy. Annals of Neurology, 77(3), 487–503. 10.1002/ana.24343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw EE, Schultz AP, Sperling RA, & Hedden T (2015). Functional Connectivity in Multiple Cortical Networks Is Associated with Performance Across Cognitive Domains in Older Adults. Brain Connectivity, 5(8), 505–516. 10.1089/brain.2014.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman-Tov T, Bosak N, Sprecher E, Paz R, Eran A, Aharon-Peretz J, & Kahn I (2017). Early Age-Related Functional Connectivity Decline in High-Order Cognitive Networks. Frontiers in Aging Neuroscience, 8(330). 10.3389/fnagi.2016.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23 (Suppl. 1), S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, … Behrens TEJ (2006). Tract based spatial statistics: voxelwise analysis of multi-subjects diffusion data. NeuroImage, 31(4), 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, … Beckmann CF (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040–13045. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, & Nichols TE (2009). Threshold-free cluster enhancement : Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Viviano JD, & Schacter DL (2016). Neurobiology of Aging Attenuated anticorrelation between the default and dorsal attention networks with aging : evidence from task and rest. Neurobiology of Aging, 45, 149–160. 10.1016/j.neurobiolaging.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni AM, Brown JA, Casaletto KB, Elahi FM, Deng J, Neuhaus J, … Kramer JH (2018). The Longitudinal Trajectory of Default Mode Network Connectivity in Healthy Older Adults Varies As a Function of Age and Is Associated with Changes in Episodic Memory and Processing Speed. The Journal of Neuroscience, 38(11), 2809–2817. 10.1523/JNEUROSCI.3067-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, … Vuoksimaa E (2018). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & Dementia, pii: S1552-5260(18)33491-5 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, & Menon V (2009). Development of large-scale functional brain networks in children. PLoS Biology, 7(7), e1000157 10.1371/journal.pbio.1000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, & Volkow ND (2012). Aging and functional brain networks. Molecular Psychiatry, 17(5), 549–558. 10.1038/mp.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Sanz-Arigita EJ, Menning S, & Van Den Heuvel OA (2010). Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neuroscience, 11(145). 10.1186/1471-2202-11-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk KRA, Sabuncu MR, & Buckner RL (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438. 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaqué-Alcázar L, Sala-Llonch R, Valls-Pedret C, Vidal-Piñeiro D, Fernández-Cabello S, Bargalló N, … Bartrés-Faz D (2017). Differential age-related gray and white matter impact mediates educational influence on elders’ cognition. Brain Imaging and Behavior, 11(2), 318–332. 10.1007/s11682-016-9584-8 [DOI] [PubMed] [Google Scholar]
- Vidal-Piñeiro D, VallsPedret C, FernándezCabello S, ArenazaUrquijo EM, SalaLlonch R, Solana E, … Bartrés-Faz D (2014). Decreased Default Mode Network connectivity correlates with age-associated structural and cognitive changes. Frontiers in Aging Neuroscience, 6(256). 10.3389/fnagi.2014.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Piñeiro D, Martín-Trias P, Falcón C, Bargalló N, Clemente IC, Valls-Solé J, … Bartrés-Faz D (2015). Neurochemical modulation in posteromedial default-mode network cortex induced by transcranial magnetic stimulation. Brain Stimulation, 8(5), 937–944. 10.1016/j.brs.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Viviano RP, Raz N, Yuan P, & Damoiseaux JS (2017). Associations between dynamic functional connectivity and age, metabolic risk, and cognitive performance. Neurobiology of Aging, 59, 135–143. 10.1016/j.neurobiolaging.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, … Fjell AM (2010). Life-Span Changes of the Human Brain White Matter : Diffusion Tensor Imaging ( DTI ) and Volumetry. Cerebral Cortex, 20(9), 2055–2068. 10.1093/cercor/bhp280 [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, & He Y (2013). BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE, 8(7), e68910 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Fiocco a J., Lindquist K, Vittinghoff E, Simonsick EM, Newman a B., … Harris TB (2009). Predictors of maintaining cognitive function in older adults. Neurology, 72, 2029–2035. 10.1212/WNL.0b013e3181a92c36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski R, Ylikoski A, Keskivaara P, Tilvis R, Sulkava R, & Erkinjuntti T (1999). Heterogeneity of cognitive profiles in aging: Successful aging, normal aging, and individuals at risk for cognitive decline. European Journal of Neurology, 6(6), 645–652. 10.1046/j.1468-1331.1999.660645.x [DOI] [PubMed] [Google Scholar]
- Zimmermann J, Ritter P, Shen K, Rothmeier S, Schirner M, & McIntosh AR (2016). Structural architecture supports functional organization in the human aging brain at a regionwise and network level. Human Brain Mapping, 37(7), 2645–2661. 10.1002/hbm.23200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.