Introduction
Malignant peripheral nerve sheath tumors (MPNSTs) are rare, highly aggressive soft tissue sarcomas that develop from peripheral nerve cells including Schwann cells.1 The estimated incidence of MPNSTs in the general population is 0.001% with a greatly increased incidence in individuals with neurofibromatosis type 1 (NF-1).2 The estimated incidence of MPNST in the NF-1 population is between 2 and 29%, with MPNSTs as the most common cause of death in NF-1 patients.3,4 Despite the overall rarity of MPNSTs, they represent 5–10% of all soft tissue sarcomas diagnosed.5 The median age at diagnosis for sporadic MPNST is between 30–60 years and between 20–40 years for NF-1 associated MPNST.6 These tumors present clinically as a rapidly enlarging mass that can be painful. Frequently, there are associated neurological symptoms such as weakness and paresthesias. Tumors most commonly arise from nerve roots and bundles in the extremities and pelvis, particularly the sciatic nerve. At the time of presentation, most tumors are greater than 5 cm in size and up to 50% of patients have metastatic disease, most commonly involving the lungs.7
The mainstay of localized MPNST management is complete surgical resection. Ducatman et al. showed significantly improved overall survival in patients who had a gross total resection compared to subtotal resection. This same study also found that patients with tumors >5 cm and patients with associated NF-1 had lower survival rates.8 Adjuvant radiation therapy has been recommended in tumors >5 cm or in patients with marginal excision. Although adjuvant radiation has shown improved local control rates, a survival benefit has not been shown.9 Chemotherapy has been explored as a treatment option for MPNST. A prospective randomized phase II trial of ifosfamide, doxorubicin, and etoposide in MPNST showed a response rate in 44% of sporadic MPNST and 18% in NF-1 associated MPNST.10
In the setting of refractory or metastatic disease, a phase II clinical trial for MPNST with Erlotinib and various other phase II clinical trials for sarcomas with agents including Sorafenib, Imatinib, Dasatinib, and Alisertib have all failed to demonstrate efficacy. However, they did show the overall poor prognosis of unresectable MPNST with a median progression-free survival less than 2 months and overall survival less than 5 months.11–16
Case
The case study patient presented at the age of 40 with one month of cough refractory to antibiotics and steroids. A computed tomography (CT) scan of the chest revealed a 7.2 cm mass in the right upper lobe that extended to the right hilum. A positron emission tomography (PET) scan at that time revealed no other disease. Thoracotomy with right upper lobectomy and hilar lymph node sampling revealed a 7.3 cm high grade sarcoma with negative margins and 0/3 hilar lymph nodes involved. Histological sections showed a somewhat biphasic malignant neoplasm with hypercellular areas composed of fascicles of elongated, spindle cells with ovoid nuclei and eosinophilic to vacuolated cytoplasm. These areas abruptly transitioned to less cellular areas of epithelioid to spindled cells with moderate amounts of eosinophilic cytoplasm. The pathologist noted that in these areas of less cellularity some of the cells had neural features. Immunohistochemical staining was positive for S-100, CD99, bcl-2 and negative for GIST, CD34, SOX10 and smooth muscle actin. The Ki-67 was noted to be 40–50%. Pathology review in tumor board judged that the histology was MPNST vs fibrosarcoma. FoundationOne testing revealed that the tumor cells had LMNA-NTRK1 fusion, CDKN2A/B deletion and NCOR splice site 5360–10_5370del21 with a low tumor mutation burden. The patient received adjuvant doxorubicin, ifosfamide and mesna, however, he only completed 2 cycles as he opted to stop on the second day of cycle 3.
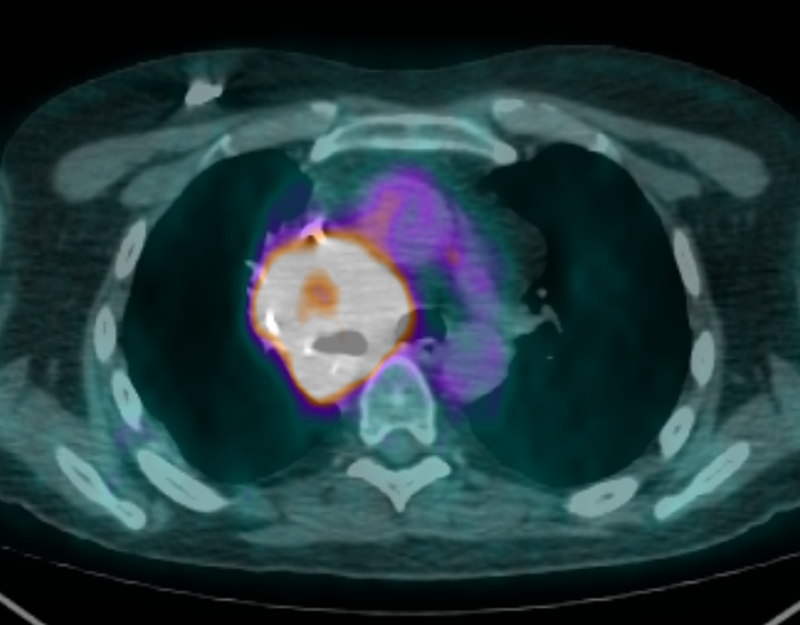
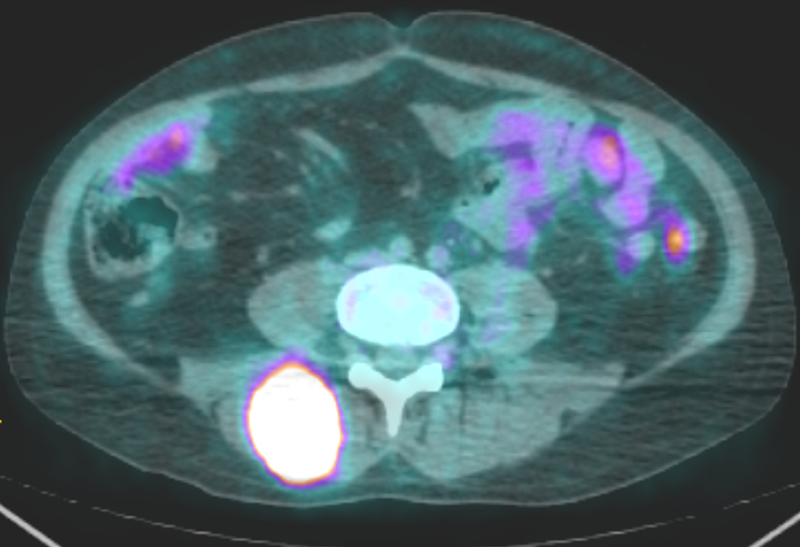
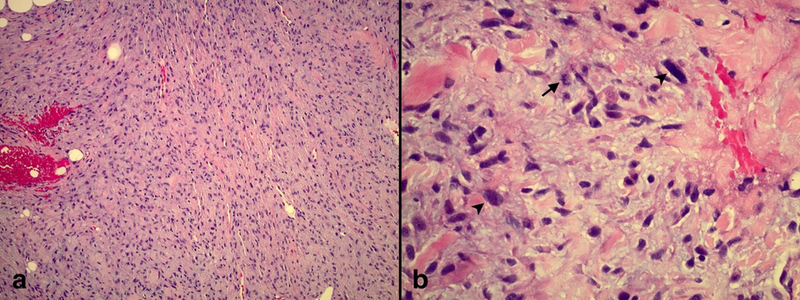
Six months later the patient underwent urgent chest radiation to treat a large right hilar mass compressing the right pulmonary artery. Treatment was with 60 Gy over 15 fractions (Fx) of volumetric-modulated arc therapy (VMAT). Less than one month following completion of this radiation therapy to the mediastinum, the patient presented with low back pain. Magnetic resonance imaging (MRI) of the lumbar spine with contrast revealed a heterogeneous avidly enhancing mass in the right posterior paraspinal (erector spinae) musculature at the L4-L5 vertebral level measuring 4.1 × 3.9 × 6 cm. PET/CT scan displayed an intensely fluorodeoxyglucose (FDG) avid hilar mass that was treated with radiation previously (Fig 1), no further FDG avidity of the original resected right upper lobe lesion, a new intensely FDG avid lesion on the right posterior scalp near the vertex and an intensely FDG avid mass in the right spinalis muscle (Fig 2). A punch biopsy of the scalp lesion showed malignant proliferation into the dermis and subcutaneous tissue (Fig 3). The pathologist confirmed that the findings were consistent with MPNST. The right paraspinal lesion was treated with stereotactic body radiation therapy (SBRT) to 40 Gy over 5 Fx and his scalp lesion was treated with electrons to 35 Gy in 5 Fx. One-week later doxorubicin and olaratumab were started but he only completed one cycle due to severe toxicities.
Figure 1:
An axial PET scan showing an intensely FDG avid mass in the right hilum. This mass had previously undergone radiation therapy of 60 Gy over 15 Fx less than one month prior.
Figure 2:
An axial PET scan showing an intensely FDG avid right posterior paraspinal mass at the L4-L5 vertebral level measuring 4.1 × 3.9 × 6 cm.
Figure 3.
Punch biopsy of scalp lesion. a) Spindled cells arranged in sheets and fascicles within a myxoid stroma with focal invasion into the dermal fat (H&E, 10X magnification). b) Tumor cells have pale eosinophilic cytoplasm and wavy, tapered nuclei with nuclear atypia (arrowhead) and mitoses (arrow) (H&E, 40X magnification).
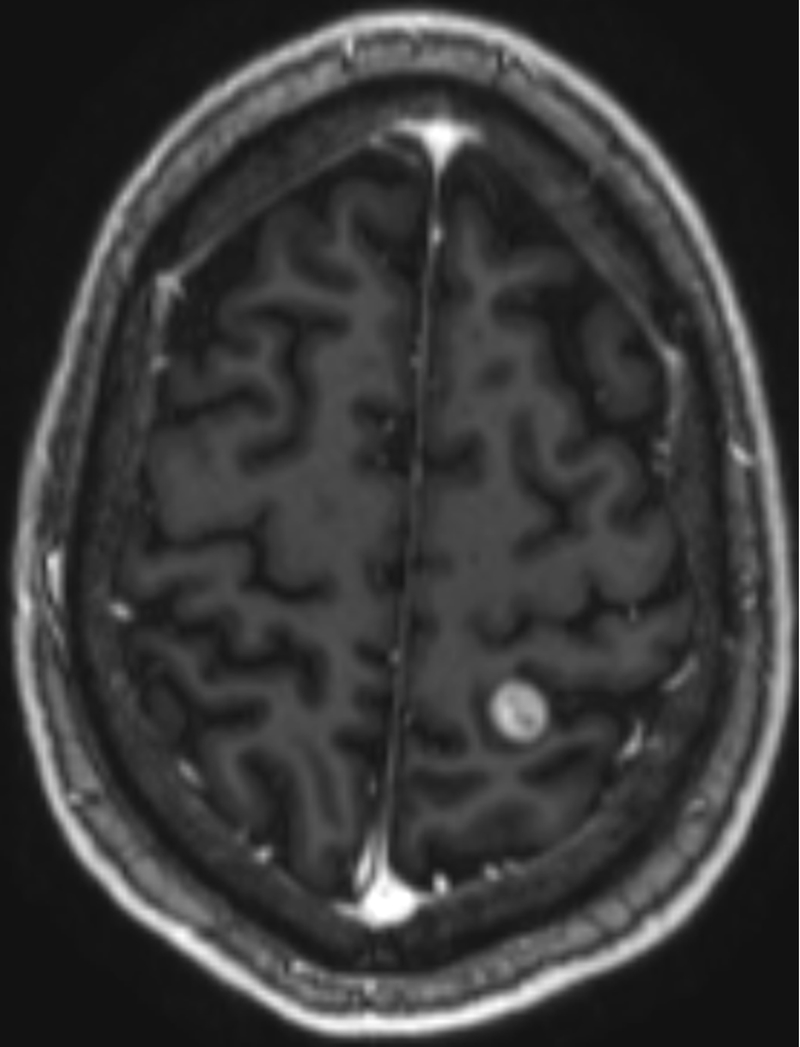
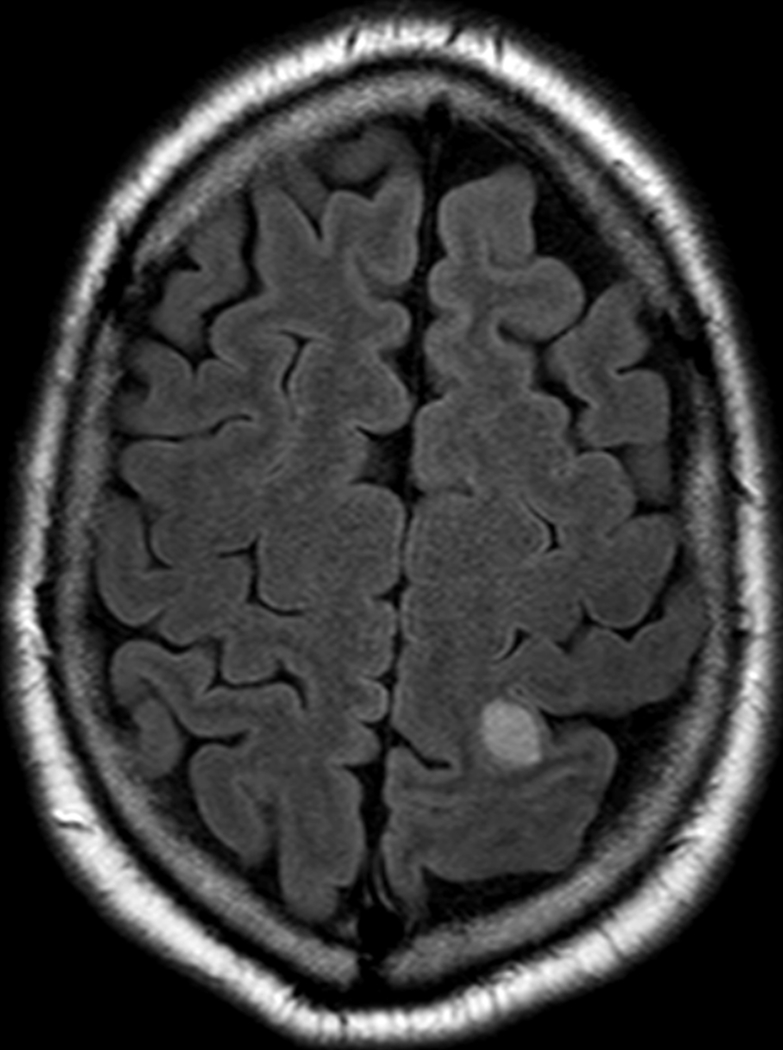
Two months after completion of this radiation to the paraspinal and scalp vertex lesion, the patient noted a new enlarging lesion at a different location of his scalp along the right frontoparietal region. A brain MRI confirmed a new metastasis to the calvarium and an incidentally noted new T2 and FLAIR hyperintense, T1 post-contrast enhancing lesion within the left high parietal lobe measuring 1.2 cm, consistent with brain metastasis (Fig 4, Fig 5). This intraparenchymal lesion received LINAC-based stereotactic radiosurgery (SRS) therapy to 27 Gy in 1 Fx.
Figure 4:
An axial T1 post-contrast brain MRI showing a 1.2 cm contrast-enhancing lesion in the left parietal lobe.
Figure 5:
An axial T2 FLAIR brain MRI showing a 1.2 cm T2 and FLAIR hyperintense metastasis in the left parietal lobe.
Due to the rapid progression of his metastatic disease, within two weeks of SRS therapy he was enrolled onto a clinical trial targeting the NTRK1 fusion found in his tumor.
One month following SRS, a brain MRI showed the left high parietal brain lesion was decreased in size from 1.2 cm previously to 0.8 cm. No new suspicious enhancing foci were appreciated. Two months after SRS he received another brain MRI which showed further reduction in the lesion size to 0.6 cm. During cycle 6 of treatment, 5 months after SRS, he received a follow up brain MRI which showed complete resolution of his scalp and brain lesions.
It has been 24 months since initial diagnosis and lung resection, 11 months since brain SRS therapy and 10 months since enrollment in the clinical trial and appears to have stable intrathoracic and paraspinal disease on systemic therapy, with complete resolution of the brain metastases following radiotherapy and his target lesions on systemic treatment.
This case report includes a retrospective review of a single patient’s medical record and is de-identified, which appropriately protects the privacy rights of human subjects and has been carried out in accordance with the Code of Ethics of the World Medical Association.
Discussion
Brain metastasis from MPNSTs is an exceedingly rare occurrence. A single institution case report found that of 179 patients with MPNSTs treated, only one patient had MPNST metastasis to the brain, resulting in an incidence of 0.5% of MPNSTs diagnosed.17 A literature review by Shweikeh et al. in 2014 found only 21 documented cases of MPNST brain metastases.18–37 Since that time, one additional report of MPNST brain metastasis has been published.17 Of the 22 previously reported cases, nine lesions received no treatment, six were managed with surgical resection, one patient was treated with whole brain radiation therapy, one case did not report a treatment modality, and the remainder received multimodality therapy. Two of the patients who received multimodality therapy received gamma knife radiosurgery (GKRS) as part of their treatment.
The first documented case of MPNST brain metastasis treated with GKRS was an 83year-old man with a left frontal lobe lesion which was initially removed with microsurgical resection (Table 1).36 Seven months following resection the tumor recurred to a volume of 15.2 cm3. This was treated with GKRS of 20 Gy to the 50% isodose line. Nine months following treatment a 97% reduction in volume was noted, but three new metastases were seen. One 3.1 cm3 left occipital, one 0.07 cm3 left temporal, and one 0.25 cm3 right cerebellopontine angle tumor. These three tumors were also treated with GKRS of 20 Gy to the 45% isodose line. Three months following treatment the left occipital tumor decreased to 0.25 cm3 and the other two metastases were no longer visualized. The patient had a Karnofsky Performance Status (KPS) of 70 and survived for 14 months after initial GKRS.
Table 1:
Comparison of two patients with MPNST brain metastases treated with GKRS and one patient treated with LINAC-based SRS.
| Age (Gender) | KPS | Treatment modality / dose | Surgery Before SRS | WBRT | Systemic Therapy | Number Lesions Treated | Volume pre-treatment | Volume post-treatment | Survival after first SRS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Current Case | 40 (M) | 100 | LINAC SRS / 27 Gy to 86% isodose line | No | No | Clinical trial drug post treatment | 1 | 1.4 cm3 | 0 | Alive 11 mo after SRS |
| van Eck and Horstmann, 200643 | 83 (M) | 70 | GKRS / 20 Gy, 45 −50% isodose line | Yes | No | No | 4 | 15.2 cm3 3.1 cm3 0.07 cm3 0.25 cm3 | 0.33 cm3 0.25 cm3 0 0 | Deceased 14 mo after SRS |
| Flannery et al, 201031 | 34 (F) | 70 | GKRS / Not reported | Yes (x2) | Yes | No | 1 | Not reported | Not reported | Deceased 9 mo after SRS |
The second case treated with GKRS was from a study evaluating GKRS treatment of 21 patients with sarcoma metastasis to the brain. One patient in the study had MPNST metastasis (Table 1). The patient was a 34-year-old female with neurofibromatosis type 2 (NF-2) who developed brain metastasis 3 years after MPNST diagnosis.24 She initially underwent two resections of her brain lesion as well as whole brain radiotherapy. These treatment methods failed and GKRS was administered. The study did not specify the dose or isodose line for this patient’s tumor specifically, but the median dose in the study was 16 Gy and the most common isodose line treated was 50%. She had a KPS of 70 at the time of treatment and survived for nine months following administration of GKRS.
The following key differences exist between these prior two reports and our current case: 1) our patient was treated with SRS from a linear accelerator (LINAC), 2) did not have surgical resection and, 3) was administered systemic therapy following SRS.
1) Although both GKRS and LINAC-based SRS deliver high dose radiation to tumors, there are differences in dose planning and distribution. In the reported cases of GKRS for MPNST brain metastasis, a dose of 20 Gy to either the 45 or 50% isodose line was used. This radiation plan results in 40 to 44.4 Gy reaching the center of the tumor volume. This is significantly higher than our LINAC plan which used 27 Gy to the 86% isodose line with resultant 31.4 Gy to the lesion’s center. Prior to our case, concern may have existed about the efficacy and durability of this lower central dose. It appears that this LINAC based planned was effective despite dose and modality differences.
2) Although surgical resection has been thought of as initial treatment for MPNST brain metastasis, there are situations where surgery is not possible or advisable. Such situations include patients who are not healthy enough for surgery due to comorbid conditions or when the tumor is located too close to eloquent areas of the brain. Furthermore, the establishment of surgery as initial therapy for MPNST brain tumor has largely occurred in the absence of data regarding SRS for these lesions. Interestingly, the patient in the report by van Eck and Horstmann had tumor recurrence seven months following initial surgical resection. However, once GKRS was instituted as salvage therapy there appeared to be a durable, near complete response of the target tumor, during his remaining 14 months of life. He did have three additional metastases develop which were also treated and well controlled with GKRS.36 As with our study, upfront treatment with SRS and no surgical resection may prove to be a noninvasive viable option for durable local control.
3) Our patient began systemic therapy with a targeted agent two weeks following SRS to his brain metastasis. There was initial concern that this could confound the results of his complete tumor response as it would be difficult to delineate what was the result of radiation therapy vs what was occurring in response to systemic therapy.
As mentioned previously, the median progression free survival of unresectable brain MPNST is two months and overall survival only five months.18–23 Our patient with unresected brain MPNST continues to survive 11 months after SRS with no evidence of intracranial recurrence or progression. This suggests that there may be improved progression free survival or an overall survival benefit to be discovered with future studies.
Limitations of this study include its retrospective nature, sample size of one, and origination from a single institution.
Conclusion
Although further research is needed, LINAC-based SRS for MPNST brain metastasis appears to be a safe, viable option for therapy even in the absence of primary surgical resection. To our knowledge there is no other documented case of MPNST brain metastasis treated with LINAC-based stereotactic radiosurgery.
Acknowledgments
We acknowledge the Translational Pathology Shared Resource supported by NCI/NIH Cancer Center Support Grant 5P30 CA68485–19 and the Vanderbilt Mouse Metabolic Phenotyping Center Grant 2 U24 DK059637–16. This work was supported by the National Institutes of Health grant number 5K12CA090625–18 to ANK from the Vanderbilt Clinical Oncology Research Career Development Program.
Abbreviations List
- CT
Computed Tomography
- GKRS
Gamma Knife Radiosurgery
- FDG
Fluorodeoxyglucose
- FX
Fractions
- H&E
Hematoxylin and Eosin stain
- KPS
Karnofsky Performance Status
- LINAC
Linear Accelerator
- MPNST
Malignant Peripheral Nerve Sheath Tumor
- MRI
Magnetic Resonance Imaging
- NF-1
Neurofibromatosis type 1
- NF-2
Neurofibromatosis type 2
- PET
Positron Emission Tomography
- SBRT
Stereotactic Body Radiation Therapy
- SRS
Stereotactic Radiosurgery
- TRK
Tropomyosin Receptor Kinase
- VMAT
Volumetric-Modulated Arc Therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None
References
- 1.Gupta G, Maniker A. Malignant peripheral nerve sheath tumors. Neurosurg Focus 2007;22(6):E12. [DOI] [PubMed] [Google Scholar]
- 2.Mrugala MM, Batchelor TT, Plotkin SR. Peripheral and cranial nerve sheath tumors. Curr Opin Neurol 2005;18(5):604–610. [DOI] [PubMed] [Google Scholar]
- 3.Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors. Cancer 2006;107(5):1065–1074. [DOI] [PubMed] [Google Scholar]
- 4.Evans DGR. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 2002;39(5):311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang JH, Zhang J, Zager EL. Diagnosis and treatment options for nerve sheath tumors. Expert Rev Neurother 2005;5(4):515–523. [DOI] [PubMed] [Google Scholar]
- 6.Widemann BC. Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep 2009;11(4):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farid M, Demicco EG, Garcia R, et al. Malignant Peripheral Nerve Sheath Tumors. Oncologist 2014;19(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986;57(10):2006–2021. [DOI] [PubMed] [Google Scholar]
- 9.Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys 1998;42(2):351–360. [DOI] [PubMed] [Google Scholar]
- 10.Widemann BC, Reinke DK, Helman LJ, et al. SARC006: Phase II trial of chemotherapy in sporadic and neurofibromatosis type 1 (NF1)-associated high-grade malignant peripheral nerve sheath tumors (MPNSTs). J Clin Oncol 2013. [Google Scholar]
- 11.Maki RG, D’Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM, Takimoto CH, Kraft AS, Elias AD, Brockstein B, Blachère NE, Edgar MA, Schwartz LH, Qin LX, Antonescu CR, Schwartz GK. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 2009;27(19):31333140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chugh R, Wathen JK, Maki RG, et al. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol 2009;27(19):3148–3153. [DOI] [PubMed] [Google Scholar]
- 13.Schuetze S, Wathen K, Choy E, et al. Results of a Sarcoma Alliance for Research through Collaboration (SARC) phase II trial of dasatinib in previously treated, high-grade, advanced sarcoma. J Clin Oncol 2010. [Google Scholar]
- 14.Dickson MA, Mahoney MR, Tap WD, et al. Phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma. Ann Oncol 2016;27(10):1855–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albritton K, Rankin C, Coffin CM, et al. Phase II trial of erlotinib in metastatic or unresectable malignant peripheral nerve sheath tumor (MPNST). J Clin Oncol 2006;249518. [Google Scholar]
- 16.Kim A, Stewart DR, Reilly KM, Viskochil D, Miettinen MM, Widemann BC. Malignant Peripheral Nerve Sheath Tumors State of the Science: Leveraging Clinical and Biological Insights into Effective Therapies. Sarcoma 2017;2017:7429697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puffer RC, Graffeo CS, Mallory GW, Jentoft ME, Spinner RJ. Brain Metastasis From Malignant Peripheral Nerve Sheath Tumors. World Neurosurg 2016;92:580.e1–e580.e4. [DOI] [PubMed] [Google Scholar]
- 18.Shweikeh F, Bukavina L, Saeed K, et al. Brain metastasis in bone and soft tissue cancers: a review of incidence, interventions, and outcomes. Sarcoma 2014;2014:475175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Q, Xing B, Huang X, Wang R, Li Y, Yang Z. Primary malignant peripheral nerve sheath tumor of the cauda equina with metastasis to the brain in a child: case report and literature review. Spine J 2012;12(4):e7–e13. [DOI] [PubMed] [Google Scholar]
- 20.Cras P, Ceuterick-de Groote C, Van Vyve M, Vercruyssen A, Martin JJ. Malignant pigmented spinal nerve root schwannoma metastasizing in the brain and viscera. Clin Neuropathol 1990;9(6):290–294. [PubMed] [Google Scholar]
- 21.D’agostino AN, Soule EH, Miller RH. SARCOMAS OF THE PERIPHERAL NERVES AND SOMATIC SOFT TISSUES ASSOCIATED WITH MULTIPLE NEUROFIBROMATOSIS (VON RECKLINGHAUSEN’S DISEASE). Cancer 1963;16:1015–1027. [DOI] [PubMed] [Google Scholar]
- 22.DʼAngelo V, Casadei G, Bizzozero L. Cerebral Metastasis from an Epithelioid Malignant Schwannoma: Case Report. Neurosurgery 1991;29(6):906–909. [DOI] [PubMed] [Google Scholar]
- 23.Fenzi F, Moretto G, Zamboni G, Passarin MG, Rizzuto N. Brain metastases from post-radiation malignant peripheral nerve sheath tumour. Ital J Neurol Sci 1995;16(7):495–498. [DOI] [PubMed] [Google Scholar]
- 24.Flannery T, Kano H, Niranjan A, et al. Gamma knife radiosurgery as a therapeutic strategy for intracranial sarcomatous metastases. Int J Radiat Oncol Biol Phys 2010;76(2):513519. [DOI] [PubMed] [Google Scholar]
- 25.Haisa T, Kondo T, Shinoura N, Hara T, Nasu M. Malignant schwannoma metastasizing to the dura mater--case report. Neurol Med Chir 1996;36(7):462–465. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa M, Tanaka H, Watanabe I, Uehara T, Nasu M. Malignant schwannoma and follicular thyroid carcinoma associated with von Recklinghausen’s disease. The Journal of Laryngology & Otology 1984;98(10):1057–1061. [DOI] [PubMed] [Google Scholar]
- 27.Hirose T, Sumitomo M, Kudo E, et al. Malignant peripheral nerve sheath tumor (MPNST) showing perineurial cell differentiation. Am J Surg Pathol 1989;13(7):613–620. [DOI] [PubMed] [Google Scholar]
- 28.Macaulay RA. Neurofibrosarcoma of the radial nerve in von Recklinghausen’s disease with metastatic angiosarcoma. J Neurol Neurosurg Psychiatry 1978;41(5):474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maschke M, Kastrup O, Reinhardt V, Keidel M. Multiple intracerebral metastases of a 17-year-old girl with previously diagnosed neurofibromatosis type I. Clin Neuropathol 1999;18(1):42–44. [PubMed] [Google Scholar]
- 30.Matyja E, Nagańska E, Górski R, Zabek M. Multiple brain metastases from malignant peripheral nerve sheath tumour (MPNST). Folia Neuropathol 2004;42(1):43–48. [PubMed] [Google Scholar]
- 31.Oishi H, Ishii K, Bandou K, Ashida H, Abe M. Malignant schwannoma metastasizing to the parenchyma of the brain--case report. Neurol Med Chir 2000;40(2):116–119. [DOI] [PubMed] [Google Scholar]
- 32.Park S-K, Yi H-J, Paik S-S, Kim Y-J, Ko Y, Oh S-J. Metastasizing malignant peripheral nerve sheath tumor initially presenting as intracerebral hemorrhage. Case report and review of the literature. Surg Neurol 2007;68(1):79–84; discussion 84. [DOI] [PubMed] [Google Scholar]
- 33.Probst-Cousin S, Schaus M, Feldt B, Al-Dandashi N, Gullotta F. Malignant peripheral nerve sheath tumor with extensive miliary metastases: a case report. Gen Diagn Pathol 1997;142(5–6):357–360. [PubMed] [Google Scholar]
- 34.Tilgner J, Müller K, Ghanem N, Lutterbach J, Vesper J. Brain metastases as primary manifestation of a melanocytic malignant peripheral nerve sheath tumor in a 60-year-old man. BMC Neurol 2007;7(1). doi: 10.1186/1471-2377-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valdueza JM, Hagel C, Westphal M, Hänsel M, Herrmann HD. Primary spinal malignant schwannoma: clinical, histological and cytogenetic findings. Neurosurg Rev 1991;14(4):283291. [DOI] [PubMed] [Google Scholar]
- 36.van Eck ATCJ, Horstmann GA. Gamma Knife surgery for multiple brain metastases from a malignant schwannoma of the penis. J Neurosurg 2006;105 Suppl:238–240. [DOI] [PubMed] [Google Scholar]
- 37.White HR Jr. Survival in malignant schwannoma. An 18-year study. Cancer 1971;27(3):720–729. [DOI] [PubMed] [Google Scholar]