Abstract
The type 2 cannabinoid receptor (CB2R) was initially regarded as a peripheral cannabinoid receptor. However, recent technological advances in gene detection, alongside the availability of transgenic mouse lines, indicate that CB2Rs are expressed in both neurons and glial cells in the brain under physiological and pathological conditions, and are involved in multiple functions at cellular and behavioral levels. Brain CB2Rs are inducible and neuroprotective via up-regulation in response to various insults, but display species differences in gene and receptor structures, CB2R expression, and receptor responses to various CB2R ligands. CB2R transcripts also differ between the brain and spleen. In the brain, CB2A is the major transcript isoform, while CB2A and CB2B transcripts are present at higher levels in the spleen. These new findings regarding brain versus spleen CB2R isoforms may in part explain why early studies failed to detect brain CB2R gene expression. Here, we review evidence supporting the expression and function of brain CB2R from gene and receptor levels to cellular functioning, neural circuitry, and animal behavior.
Keywords: Cannabinoids, THC, VTA dopamine neurons, brain CB2 receptors, neuronal CB2 receptors
INTRODUCTION
Cannabis is derived from a flowering plant which has been used for centuries for medical and recreational purposes. The major psychoactive component in cannabis is delta-9-tetrahydrocannabinol (Δ9-THC; Mechoulam et al., 2014), which acts mainly on inhibitory G-protein-coupled cannabinoid CB1 and CB2 receptors (Demuth and Molleman, 2006; Mackie, 2008). CB1 receptors (CB1Rs) are highly expressed in the central nervous system and functionally modulate presynaptic neurotransmitter release (Devane et al., 1988; Herkenham et al., 1990; Howlett, 1998; Matsuda et al., 1990), while CB2Rs were previously believed to be confined to the periphery, particularly the spleen and immune system (Griffin et al., 1997; Munro et al., 1993), and therefore regarded as peripheral cannabinoid receptors (Buckley, 2008). However, recent evidence derived from studies in both rodents and primates suggests that CB2R is also expressed in the brain and in neurons (den Boon et al., 2012; Foster et al., 2016; Gong et al., 2006; Lanciego et al., 2011; Liu et al., 2009; Stempel et al., 2016; Van Sickle et al., 2005; Xi et al., 2011; Zhang et al., 2014). Emerging evidence for brain CB2R expression is largely a result of technological advances in genetics and molecular techniques, allowing greater sensitivity and specificity for CB2R detection (Foster et al., 2016; Liu et al., 2009; Stempel et al., 2016; Van Sickle et al., 2005; Xi et al., 2011; Zhang et al., 2014; Zimmer, 2015). In this review, we discuss evidence that has accumulated over the past decade supporting the expression and function of CB2R in the brain and in neurons, spanning from genetic manipulations to cellular functioning, neural circuitry, and animal behavior, and describe the recent technological advances that have contributed to this discovery.
CB2R GENES, TRANSCRIPTS, AND RECEPTORS
CB2 gene and receptor structures
The CB2R was cloned in 1993 from human leukemia cells (Munro et al., 1993). CB2R has seven transmembrane domains and 44% amino acid sequence homology to the CB1R (Demuth and Molleman, 2006; Pertwee, 1997). While CB2 RNA has been detected in the brain across species, there nonetheless exist significant species differences between human, rat and mouse CB2R genes (Cnr2). The human CB2R gene spans about four times (90 kb) the size of the mouse (23 kb) and the rat (20 kb; Liu et al., 2009; Zhang et al., 2015). Figure 1 (A, B) shows mouse CB2R (mCB2R) gene structure and transcripts, illustrating three exons with two separate promoters in the CB2R gene, which encode two transcripts (mCB2A and mCB2B) using exon 1 or exon 2 as different 5′UTR sequences. The mCB2A transcript contains exon 1 and exon 3, and the mCB2B transcript contains exon 2 and exon 3. In contrast, the rat CB2 (rCB2R) gene includes 3 exons that can be spliced to four rCB2R transcripts (mRNA) isoforms - CB2A, CB2B, CB2C and CB2D, and each displays different expression in the brain and peripheral tissues (Liu et al., 2009; Zhang et al., 2015). The CB2R-encoding regions are located entirely on exon 3 in both rats and mice.
Figure 1.
Quantitative mouse brain CB2 mRNA assays by RT-PCR. (A) Mouse CB2 gene structure; (B) Structures of two CB2 transcripts (CB2A, CB2B), illustrating CB2-encoding sequence on exon 3, the gene-deleted region in Zimmer strain CB2-KO mice, and the binding sites of three Taqman probes used to detect CB2 expression in the brain and spleen; (C) Mouse CB2A over CB2B ratio in the brain and spleen, illustrating that brain CB2A level is much higher (20-30-fold) than brain CB2B mRNA, while in spleen, CB2A is only ~3-fold higher than CB2B; (D) CB1 over CB2A ratio in the brain and spleen, illustrating that brain CB2A mRNA level is 100-300-fold lower than that of CB1 in the brain; (E) The CB2A probe that targets the upstream 5′UTR from the gene-deleted region detected similar levels of CB2 mRNA in WT, CB1-KO and CB2-KO mice; (F) The CB2-KO probe that targets the gene-deleted region detected CB2 mRNA only in WT or CB1-KO mice, but not in CB2-KO mice. UTR: untranslated region.
NM_009924.2 and AK036658.1 are the GenBank cDNA codes.
Like other G-protein coupled receptors, mCB2Rs and rCB2Rs contain an extracellular N-terminus, 7 transmembrane domains (TMs), 3 extracellular and 3 intracellular loops, and an intracellular C-terminus (Zhang et al., 2015). Table 1 shows the CB2R amino acid sequences in mice, rats and humans. Notably, mCB2Rs are 13 amino acid residues shorter than rCB2R or hCB2Rs (347 versus 360) at the C-terminus due to a premature stop codon in the mCB2R gene (Liu et al., 2009; Zhang et al., 2015). The rCB2Rs and mCB2Rs share 93% amino-acid homology (not counting the deleted C-terminal 13 amino acids in mCB2Rs). Human CB2R shares similar amino-acid homologies with mouse (82%) and rat (81%) (Table 1) (Zhang et al., 2015), but there are more similarities in amino acid sequences between human and rat (97%) than human and mouse CB2R (82%) (Pertwee, 1997).
Table 1.
Species Differences in Cannabinoid Receptor 2 Amino Acid Sequences and Antibody Binding Sites.
| Abcam rCB2-Ab | TM1 | ||
| Rat CB2 | MAGCRELELTNGSNGGLEFNPMKEYMILSDAQQIAVAVLCTLMGLLSALENVAVLYLILS | 60 | |
| Mouse CB2 | MEGCRETEVTNGSNGGLEFNPMKEYMILSSGQQIAVAVLCTLMGLLSALENMAVLYIILS | 60 | |
| Human CB2 | MEECWVTEIANGSKDGLDSNPMKDYMILSGPQKTAVAVLCTLLGLLSALENVAVLYLILS | 60 | |
| Cayman hCB2-Ab | |||
| TM2 | TM3 | ||
| Rat CB2 | SQRLRRKPSYLFIGSLAGADFLASVIFACNFVIFHVFHGVDSRNIFLLKIGSVTMTFTAS | 120 | |
| Mouse CB2 | SRRVRRKPSYLFISSLAGADFLASVIFACNFVIFHVFHGVDSNAIFLLKIGSVTMTFTAS | 120 | |
| Human CB2 | SHQLRRKPSYLFIGSLAGADFLASVVFACSFVNFHVFHGVDSKAVFLLKIGSVTMTFTAS | 120 | |
| TM4 | |||
| Rat CB2 | VGSLLLTAVDRYLCLCYPPTYKALVTRGRALVALGVMWVLSALISYLPLMGWTCCPSPCS | 180 | |
| Mouse CB2 | VGSLLVTAVDRYLCLCYPPTYKALVTRGRALVALCVMWVLSALISYLPLMGWTCCPSPCS | 180 | |
| Human CB2 | VGSLLLTAIDRYLCLRYPPSYKALLTRGRALVTLGIMWVLSALVSYLPLMGWTCCPRPCS | 180 | |
| TM5 | Alomone rCB2-Ab | ||
| Rat CB2 | ELFPLIPNDYLLGWLLFIAILFSGIIYTYGYVLWKAHQHVASLAEHQDRQVPGIARMRLD | 240 | |
| Mouse CB2 | ELFPLIPNDYLLGWLLFIAILFSGIIYTYGYVLWKAHRHVATLAEHQDRQVPGIARMRLD | 240 | |
| Human CB2 | ELFPLIPNDYLLSWLLFIAFLFSGIIYTYGHVLWKAHQHVASLSGHQDRQVPGMARMRLD | 240 | |
| TM6 | TM7 | ||
| Rat CB2 | VRLAKTLGLVMAVLLICWFPALALMGHSLVTTLSDKVKEAFAFCSMLCLVNSMINPIIYA | 300 | |
| Mouse CB2 | VRLAKTLGLVLAVLLICWFPALALMGHSLVTTLSDQVKEAFAFCSMLCLVNSMVNPIIYA | 300 | |
| Human CB2 | VRLAKTLGLVLAVLLICWFPVLALMAHSLATTLSDQVKKAFAFCSMLCLINSMVNPVIYA | 300 | |
| Mackie rCB2-Ab | |||
| Rat CB2 | LRSGEIRSAAQHCLTGWKKYLQGLGSEGKEEAPKSSVTETEAEVKTTTGPGSRTPGCSNC | 360 | |
| Mouse CB2 | LRSGEIRSAAQHCLIGWKKYLQGLGPEGKEEGPRSSVTETEADVKTT------------------------ | 347 | |
| Human CB2 | LRSGEIRSSAHHCLAHWKKCVRGLGSEAKEEAPRSSVTETEADGKITPWPDSRDLDLSDC | 360 | |
| NIDA-5633 mCB2-Ab | |||
CB2 mRNA expression in the brain
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) findings
Early RT-qPCR assays failed to detect CB2 mRNA in the brain (Galiegue et al., 1995; Schatz et al., 1997). More recently, the use of isoform-specific probes, which are in a species- and tissue-specific manner, enabled detection of brain CB2R mRNA expression (Liu et al., 2009). Human CB2A was found primarily in the testis and in the brain (including the amygdala, caudate/putamen, NAc, cortex, hippocampus and cerebellum), while the human CB2B isoform was expressed primarily in the spleen and leukocytes (Liu et al., 2009). Mouse CB2A and CB2B were detected predominantly in the spleen and at lower levels in the PFC and striatum, although CB2A showed higher expression in the mouse brain than CB2B (Liu et al., 2009). Mice and rats also show some species differences in CB2R splicing. For example, CB2A and CB2B were found previously in mice and rats, whereas CB2C and CB2D isoforms were only detected in rats, and the mouse brain expresses more CB2R overall than rat (Zhang et al., 2015). While the distribution of CB2R on DA- and non-DA expressing neurons appears equivalent in mice, rats show significantly less CB2R on DA neurons relative to non-DA neurons, which may contribute to behavioral differences in the response to cannabinoid ligands (see further discussion in Zhang et al., 2015).
The mCB2A transcript (mRNA) is the predominant isoform in the mouse brain (Figure 1C), expressing at 20-30-fold higher levels than mCB2B, while in spleen mCB2A and mCB2B levels are not as prominent (mCB2A ~3-fold higher than mCB2B (Zhang et al., 2014). When CB2A mRNA levels in brain and spleen are compared directly, spleen CB2A is about 50-100-fold higher than that in the brain (Figure 1 E). However, using riboprobes that recognize the encoding sequences on both CB2A and CB2B isoforms, CB2R mRNA has been consistently detected in the cortex, hippocampus, and globus pallidus of non-human primates (Lanciego et al., 2011; Sierra et al., 2015), indicating conservation of brain CB2R across species. Relative to CB1 mRNA (Figure 1 D), neural CB2 mRNA is low under normal physiological conditions (about 100~300-fold lower than CB1 mRNA in the brain), but is upregulated under pathological conditions (see discussion below; Yu et al., 2015).
In situ hybridization (ISH) findings.
One key technique in classical (unamplified) ISH involves hybridization to mRNA with oligonucleotide and RNA probes (both radio-labelled and hapten-labelled), allowing localization of gene expression in tissue sections or cells. Early ISH studies to label mRNA targeted CB1R and CX5 (later known as CB2R). In these early studies, high density CB2R was detected in the marginal zone of the spleen, whereas CB1R was detected in the brain and many other peripheral tissues (Lynn and Herkenham, 1994; Munro et al., 1993). In following years, Northern blot experiments also failed to identify CB2 RNA in the brain, instead showing that the CB1 gene was expressed in the central nervous system and CB2R was expressed at low levels in the brain but high levels in peripheral immune tissues (Galiegue et al., 1995; Schatz et al., 1997). CB2R expression in the periphery (spleen and tonsils) was deemed to be equivalent to CB1R in the brain (Galiegue et al., 1995).
RNAscope ISH findings.
RNAscope is a novel method of detecting low level gene expression within intact brain sections that is 2000-fold more sensitive than classical ISH (Wang et al., 2012). This advanced technique uses unique probe designs that selectively amplify target-gene signals without background noise from non-specific hybridization. RNAscope ISH has consistently detected CB2 mRNA expression in the brain, including midbrain dopamine (DA) neurons of the rat and mouse ventral tegmental area (VTA; Zhang et al., 2015; Zhang et al., 2014; Zhang et al., 2017) and hippocampus (Li and Kim, 2015; Stempel et al., 2016). Figure 2 shows CB2R distributions in mouse brain using RNAscope, illustrating that CB2 mRNA in the prefrontal cortex (PFC), hippocampus, midbrain and cerebellum, with much lower levels in the dorsal striatum (DS) and nucleus accumbens (NAc). Double-label RNAscope ISH, combined with immunohistochemistry (IHC) assays, have further indicated that CB2R genes and receptors are expressed in VTA DA neurons (Zhang et al., 2015; Zhang et al., 2014; Zhang et al., 2017). In the hippocampus, RNAscope ISH has shown CB2 mRNA colocalized with NeuN+ and VgluT2+ glutamatergic neurons (Li and Kim, 2015).
Figure 2.
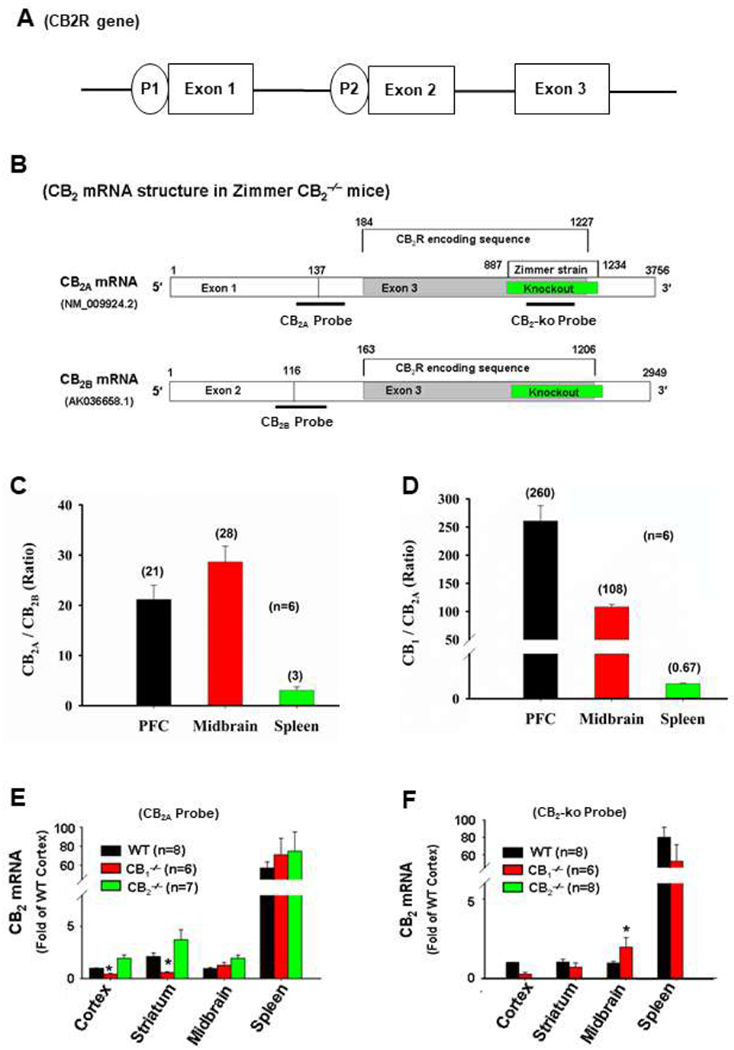
CB2 mRNA distributions in the brain as detected using RNAscope ISH assays. The CB2 mRNA was detected in multiple brain regions, including PFC, hippocampus, midbrain and cerebellum in WT (left) and CB1-KO mice (middle), but not in CB2-KO mice (right). PFC: Prefrontal cortex; Hippo: Hippocampus; DS: Dorsal striatum; NAc: Nucleus accumbens; Cereb: Cerebellum.
Efforts towards identifying cell-type specificity of brain CB2 expression have been advanced by the development of fluorescence-activated cell sorting (FACS), which allows isolation of fluorescently labeled neural sub-populations, followed by RT-PCR for CB2 mRNA detection. These techniques revealed CB2 mRNA expression in NeuN+ neuronal cells in the hippocampus (Stempel et al., 2016). FACS assays also detected significant CB2 mRNA in NeuN-negative (glial) cells (including microglia) in normal subjects, consistent with the findings from RNAscope assays (Li and Kim, 2015) and transgenic CB2-reporter mouse lines (Lopez et al., 2018; Schmole et al., 2015; see discussion below).
Findings from CB2 transgenic mice
Partial germline CB2-KO mice.
The development of CB2-KO mice has provided new advantages in validating CB2R signal specificity in the brain. While the ideal negative control is complete deletion of CB2R, full germline CB2-KO mice are not currently available and the most commonly used CB2-KO mice (the Zimmer and Deltagen strains) involve partial deletion of the CB2R gene. The Zimmer CB2-KO strain has a C-terminal 131 amino acid deletion (Buckley, 2008; Buckley et al., 2000). This truncation eliminates part of the intracellular and extracellular 3rd loops, trans-membrane regions 6 and 7, and the intracellular C-terminus region (Zhang et al., 2015; Zhang et al., 2014; Zhang et al., 2017; Zhang et al., 2018). The Deltagen strain CB2-KO has an N-terminal 112 amino acid deletion (The Jackson Laboratory, Cnr2tm1Dgen/J) (Li and Kim, 2016a, b). This truncation causes loss of part of the extracellular N-terminal (from amino acid residues 26 to 137), trans-membrane regions 1-3 and intracellular loops 1 and 2 (Zhang et al., 2018). Partial knockout strains require matching of probe primers to the deleted portion of the gene. When using the CB2A probe, which targets an upstream undeleted region, we detected similar levels of CB2 mRNA between WT and CB2-KO Zimmer mice (Figure 1 E) (Zhang et al., 2014; Zhang et al., 2017). However, when using a CB2-KO probe that targets the gene deleted region in the Zimmer strain, we detected CB2 mRNA in WT, but not in CB2-KO mice (Figure 1 F; Zhang et al., 2014; Zhang et al., 2017).
Conditional CB2-KO mice.
To date, at least two CB2-floxed mouse lines have been generated using Cre-Lox technology, in which two loxP sites were inserted into the CB2R gene flanking the entire coding region of exon 3 and the upstream splicing acceptor site, resulting in complete deletion of the CB2R protein encoding region upon recombination. The CB2-floxed mice were then crossed with synapsin-Cre or DAT-Cre mice, in which Cre recombinase expression is under control of the synapsin (neuron-specific) or DA transporter (DAT; DA neuron-specific) gene promoters (Liu et al., 2017; Stempel et al., 2016). FACS, combined with qPCR, showed CB2R mRNA expression in NeuN+ cells (neurons) in wild type mice, whereas levels in neurons were reduced by 70% in synapsin-CB2-KO mice and completely abolished in constitutive CB2-KO mice (Stempel et al., 2016). Moreover, double-label RNAscope ISH assays revealed co-localization of CB2 mRNA and the neuronal marker NeuN (Rbfox3) mRNA in most hippocampal neurons in the CA3 region of WT mice, but not in Syn-CB2R KO mice (Stempel et al., 2016), and CB2 mRNA and TH mRNA colocalization in VTA DA neurons in WT, but not in DAT-CB2-KO mice (Liu et al., 2017).
CB2-reporter mouse lines.
Transgenic reporter mouse lines express a DNA sequence encoding green fluorescent protein (GFP) or enhanced GFP (EGFP) in a target gene (Vacaru et al., 2014). Two published CB2-reporter mouse lines exist: the CB2-GFP BAC transgenic mouse line (Schmole et al., 2015) and the CB2EGFP/f/f mouse line (Lopez et al., 2018). In these reporter mouse lines GFP or EGFP signals were detected in peripheral immune cells and hippocampal microglia, but not in hippocampal neurons in normal, healthy subjects. However, in the BAC clone construct, Clal fragments (74 kb) containing exons 1, 2 and GFP (which replaced the open reading frame of exon 3 of the Cnr2), were injected into fertilized eggs. Since the location of genomic integration, size, and copy numbers are unknown in this mouse line (Schmole et al., 2015) and endogenous Cnr2 may still exist, it remains possible that GFP expression was independent of endogenous CB2R expression. In the second reporter mouse line, the homologous recombination construct, in which internal ribosome entry site (IRES) and EGFP cassettes were inserted into the 3′UTR region downstream of CB2R STOP codon, was integrated into mouse Cnr2 locus (Lopez et al., 2018). In theory, EGFP expression should have been driven by endogenous CB2R expression. However, translation initiation from the transgene IRES site is cap-independent and lacks CB2R structural 5’UTR while endogenous CB2R protein expression is cap-dependent (Leppek et al., 2018), suggesting that CB2-GFP expression may also be independent of endogenous CB2R expression in this mouse line. One explanation as to why GFP was identified in microglia and not in neurons in these reporter lines may therefore be due to non-specific upregulation in gene expression during microglial activation, which fluctuates more frequently than neurons (Holtman et al., 2017; Schmole et al., 2015; Walter et al., 2003).
CB2 RECEPTOR EXPRESSION IN THE BRAIN
Findings from immunoreactivity and immunostaining
Low levels of gene expression in brain do not necessarily mean low levels of receptor expression. Brain opioid receptor mRNA levels are generally low, particularly in the cerebral cortex, olfactory bulb, and spinal cord (Mansour et al., 1995a; Mansour et al., 1994a), but high densities of opioid receptors are expressed in these brain regions (Mansour et al., 1995b; Mansour et al., 1994b). Similarly, brain CB2A mRNA levels are very low (<50-100-fold) compared to that in spleen, while western blot (WB) and IHC assays consistently detect CB2R immunoreactive band(s) or immunostaining in the brain (Ashton et al., 2006; Baek et al., 2008; Brusco et al., 2008a; Brusco et al., 2008b; Garcia-Gutierrez et al., 2018; Gong et al., 2006; Schmidt et al., 2012; Van Sickle et al., 2005; Zhang et al., 2014; Zhang et al., 2017; Zhang et al., 2018). Immunohistochemical studies in post mortem human brains identified CB2R on microglia in the cerebellum (Nunez et al., 2004), while IHC assays (with and without ISH) show CB2-immunostaining in hippocampal glutamate neurons (Li and Kim, 2015, 2017; Stempel et al., 2016), cortical pyramidal neurons (Gong et al., 2006; Garcia-Gutierrez et al., 2018), VTA DA neurons (Aracil-Fernandez et al., 2012; Garcia et al., 2015; Zhang et al., 2014; Zhang et al., 2017; Zhang et al., 2018), and other neurons in the NAc (Aracil-Fernandez et al., 2012), brainstem (Van Sickle et al., 2005) and cerebellum (Gong et al., 2006). Similarly, electron microscopy and immunolabeling identified CB2R in the mouse cortex, hippocampus, and cerebellum (Onaivi et al., 2008b; Onaivi et al., 2006), although the specificity of CB2 antibody signals in early studies were questioned (Ashton et al., 2014; Atwood and Mackie, 2010; Baek et al., 2013; Marchalant et al., 2014; Zhang et al., 2018).
Antibody signal specificity
An ideal antibody should meet specific criteria, including selective immunolabeling, consistent results across assays, the ability to be blocked by an immunizing peptide, and absence of labeling in genetic KO animals (Lorincz and Nusser, 2008; Rhodes and Trimmer, 2006). Several commercially available antibodies (e.g., Cayman, Abcam, and Santa Cruz) for CB2R have failed the KO control test, showing neuronal staining patterns in the hippocampus of both WT mice and CB2-KO mice (Baek et al., 2013). However, the signal specificity of a given CB2 antibody depends upon the antibody epitope and which strain of partial CB2-KO mice is used as a control (Zhang et al., 2018). When using both the Zimmer and Deltagen partial CB2-KO strains as controls, most antibodies tested exhibit a degree (50~70%) of mCB2 specificity when the appropriate partial CB2-KO strain was used (Zhang et al., 2018). Going forward, full CB2-KO mice and monoclonal CB2R antibodies will be critical for highly specific labeling of brain CB2R protein (Zhang et al., 2018).
CB2R IMPACT ON NEURONAL FUNCTION
Electrophysiological studies on brain CB2Rs
Several lines of electrophysiological evidence indicate that activation of brain CB2Rs alters neuronal activity and excitability. Systemic and local administration of JWH133, a highly selective CB2R agonist, significantly inhibits VTA DA neurons both in vivo and ex vivo (Zhang et al., 2014; Zhang et al., 2017). Specifically, in perforated and cell-attached patch clamp recordings from either single neurons or brain slices, JWH133 dose-dependently reduced VTA DA neuron firing in wild type mice, an effect that was blocked by application of the CB2R antagonist, AM630, and absent in CB2-KO mice (Zhang et al., 2014). In living anesthetized mice, systemic administration of JWH133 reduced both phasic and tonic VTA DA neuron firing (Zhang et al., 2014). In contrast, VTA GABA neuron firing was unaffected by JWH133, suggesting CB2Rs may be confined to DA cells in the VTA (Zhang et al., 2014). Similar effects have been observed in rats (Zhang et al., 2017), indicating cross-species conservation of CB2R functionality in the brain.
Multiple intracellular signaling mechanisms are recruited by CB2R activation, which modulates neuronal activity and plasticity in a cell type-specific manner. When endocannabinoids, such as anandamide and 2-arachidonoylglycerol (2-AG), or exogenous CB2R ligands bind to CB2R, a Gαi/o-mediated signaling cascade is activated. This signaling results in inhibition of adenyl cyclase, activation of intracellular (including the PI3K-Akt pathway) and extracellular signal-regulated (ERK) kinases, and ultimately suppression of neuronal activity (Demuth and Molleman, 2006; Ibsen et al., 2017). CB2R also interacts with the MAP kinase pathway (Martinez-Pinilla et al., 2017). Compared to CB1R, CB2R has higher affinity for Gαi than Gαo, as shown by in situ studies (Glass and Northup, 1999; Ibsen et al., 2017). Like CB1 and other Gi-coupled receptors, CB2R activation triggers G-protein-coupled inwardly-rectifying potassium channels (GIRKs) in cortical neurons (Stumpf et al., 2018). However, unlike other Gi-coupled receptors, JWH133 (a CB2R agonist) did not alter GIRK activation in VTA DA neurons, but instead enhanced M-type potassium (KCNQ7.4) currents, leading to inhibition of neuronal firing and hyperpolarization of the cell (Ma et al., 2018).
CB2Rs also display region- and cell type-specific modulation of neuronal activity. In the rat medial PFC, activation of CB2Rs induces IP3R activation and opening of calcium-dependent chloride channels on pyramidal cells, as measured by voltage and current clamp experiments (den Boon et al., 2014; den Boon et al., 2012). Application of JWH133 to the medial PFC reduced neuronal firing rates by 45%, an effect blocked by the CB2R antagonist SCH 356036 (den Boon et al., 2014). Similarly, in the hippocampus activation of CB2R via endogenous cannabinoid release or pharmacological ligands result in long-lasting hyperpolarization of CA3 and CA2 pyramidal cells through altered activity of the sodium-bicarbonate co-transporter (Stempel et al., 2016). In contrast, chronic administration of a CB2 receptor agonist (JWH133 or GP1a) in cultures of rodent hippocampal slices for 7-10 days significantly increased quantal glutamate release and spine density via ERK signaling (Tang et al., 2015). Chronic intraperitoneal injections of JWH133 also increased excitatory synaptic transmission in mice (Kim and Li, 2015). Accordingly, CB2-KO mice show reduced excitatory synaptic transmission, long-term potentiation, and dendritic spine density in the hippocampus (Li and Kim, 2016b). In the medial entorhinal area of the rat, the endogenous cannabinoid 2-AG suppresses GABAergic inhibition, an effect mimicked by the CB2R agonist JWH133, but blocked by the CB2R antagonist AM630 (Morgan et al., 2009). Furthermore, JTE-907, a CB2R inverse agonist structurally unrelated to AM-630, increased GABAergic neurotransmission at picomolar concentrations (Morgan et al., 2009). Beyond the cortex and VTA, activation of CB2 receptors inhibits spontaneous and evoked neuronal responses to noxious stimuli in the dorsal root ganglia, spinal cord, and thalamus (Jhaveri et al., 2008; Nackley et al., 2004; Sagar et al., 2005).
Neurochemical studies on brain CB2Rs
Microdialysis and voltammetry studies have revealed additional insights into the effects of CB2R signaling on neurotransmitter release. Both systemic and local administration of JWH133 into the NAc dose-dependently reduced extracellular DA levels, an effect that was blocked by co-administration of AM630 and absent in CB2-KO mice (Xi et al., 2011; Zhang et al., 2017). These findings confirm electrophysiological reports that CB2R activation attenuates VTA DA neuron firing. Using in vivo voltammetry assays to record endogenous DA release from dopaminergic terminals in the NAc, CB2R activation was found to inhibit presynaptic DA release and gives rise to the antipsychotic action produced by positive allosteric modulators of muscarinic M4 acetylcholine receptors (Foster et al., 2016). In the hippocampus, local infusion of Δ9-THC or JWH133 dose-dependently reduces glutamate or GABA release by activation of CB2Rs (Ando et al., 2012; Zheng et al., 2015), providing further evidence that CB2R activation has physiological implications in multiple regions of the brain.
CB2R INVOLVEMENT IN BEHAVIOR
Cannabis action
Cannabinoid-induced tetrad:
In animals, cannabinoid agonists such as Δ9-THC produce a characteristic combination of tetrad symptoms – hypothermia, analgesia, hypoactivity, and catalepsy (Metna-Laurent et al., 2017; Wiley and Martin, 2003; Zimmer et al., 1999), known as the cannabinoid-induced tetrad. Pharmacological blockade of CB1R by SR141716A or AM251, or genetic CB1R deletion, suppressed the Δ9-THC-induced tetrad (Metna-Laurent et al., 2017; Zimmer et al., 1999), indicating tetrad behaviors mediated by activation of brain CB1R. However, following recent findings of functional CB2R and GPR55 (also called CB3R) in the brain (Henstridge et al., 2011; Ryberg et al., 2007; Van Sickle et al., 2005), we have begun to explore potential involvement of both CB1R and CB2R in the cannabinoid-induced tetrad using CB2-KO mice and GPR55-KO mice as controls. As anticipated, Δ9-THC and WIN55212-2 produced dose-dependent analgesia, hypothermia, catalepsy and rotarod impairment in WT, but not CB1-KO mice. However, deletion of CB2Rs in CB2-KO mice selectively blocked Δ9-THC- and WIN55212-2-induced analgesia and catalepsy (Wang et al., 2018). To determine whether these effects are mediated by activation of CB2Rs in the brain or periphery, Δ9-THC was then locally microinjected into the lateral cerebral ventricles. Intracerebral ventricular microinjection of Δ9-THC produced the full tetrad in WT, but not CB1-KO mice. Genetic deletion of CB2Rs also blocked intracranial Δ9-THC-induced analgesia and catalepsy, suggesting neuronal CB2R involvement. Consistent with these findings, Liu and colleagues (2017) also recently reported that selective deletion of CB2R from DA neurons increased basal level of locomotor behavior and altered WIN-55212-2-induced analgesia (tail flick) and catalepsy. Surprisingly, the tetrad produced by both Δ9-THC and WIN55212-2 was enhanced significantly in GPR55-KO mice compared to WT, suggesting that activation of GPR55 produces an inhibitory effect on the cannabinoid-induced tetrad. Together, these findings suggest that 1) brain CB1Rs plays dominant role in mediating cannabinoid-induced tetrad effects; 2) brain CB2Rs also play an important role in mediating cannabinoid-induced analgesia and catalepsy; and 3) brain GPR55 acts as an inhibitory brake to CB1R or CB2R effects in the cannabinoid-induced tetrad.
Cannabinoid reward versus aversion:
Cannabis is well known for its ability to produce euphoria, pleasure, and relaxation (Fattore et al., 2008; Maldonado et al., 2006; Parsons and Hurd, 2015). However, not all users enjoy cannabis and some experience dysphoria, anxiety, and depression (D’Souza et al, 2004; Raft et al, 1977). Similar paradoxical effects of Δ9-THC have been found in non-human primates. For example, Δ9-THC is self-administered by squirrel monkeys (Justinova et al, 2003; Tanda et al, 2000), but not self-administered by rhesus monkeys (John et al, 2017; Mansbach et al, 1994). In rodents, Δ9-THC or other cannabinoid compounds can be rewarding, ineffective or aversive, as assessed by intravenous self-administration, conditioned place preference, and electrical brain-stimulation reward (Panagis et al, 2008; Vlachou and Panagis, 2014).
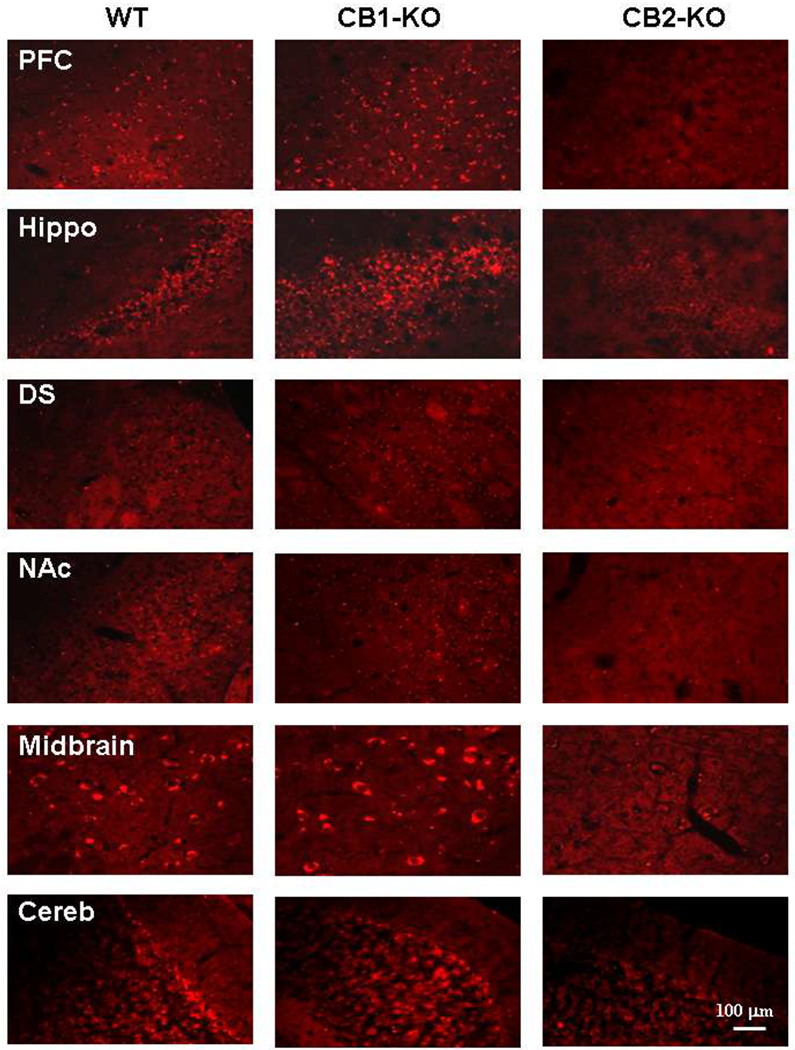
Since CB1R is highly expressed in the central nervous system and CB2R is expressed predominantly in peripheral tissues, the rewarding effects of cannabinoids were assumed to be mediated by activation of CB1R, and not CB2R (Fratta and Fattore, 2013; Mackie, 2005). This hypothesis is supported by electrophysiological evidence that activation of CB1Rs on GABAergic neurons may increase VTA DA neuron activity via disinhibition (Lupica and Riegel, 2005; Lupica et al, 2004; Lupica and Hoffman, 2018; Szabo et al, 2002). In vivo microdialysis studies also show that Δ9-THC increases DA release in the NAc of rats (Chen et al, 1991; Tanda et al, 1997). However, there is no direct behavioral evidence in vivo demonstrating whether a CB1R-dependent mechanism underlies cannabis reward. In contrast to this view, we have recently reported that activation of CB1R in glutamatergic neurons by Δ9-THC produces aversive effects (Han et al., 2017).
With the recent finding that functional CB2R is expressed in VTA DA neurons (Foster et al., 2016; Zhang et al., 2014; Zhang et al., 2017), we hypothesized that distinct CB1R and CB2R mechanisms mediate cannabis reward versus aversion, respectively (Figure 3). To test this hypothesis, we used the electrical intracranial self-stimulation (ICSS) paradigm to evaluate the effects of various cannabinoids on brain stimulation reward (BSR). At low doses, Δ9-THC and WIN55,212-2 produced mild enhancement of BSR, but inhibition at higher doses, indicating biphasic effects. Pretreatment with a CB1R antagonist (AM251) attenuated the low dose-enhanced BSR, while a CB2R antagonist (AM630) attenuated the high dose-inhibited BSR (Spiller et al., 2018). To confirm these opposing effects, rats were treated with selective CB1R and CB2R agonists, which produced significant BSR enhancement and inhibition, respectively (Spiller et al., 2018). CB1R activation therefore produces reinforcing effects, whereas CB2R activation is aversive. The subjective effects of cannabis may depend on the balance of opposing CB1R and CB2R effects. Cannabis may be either rewarding or aversive in humans dependent upon individual differences in neural CB1 and CB2 receptor expression.
Figure 3.
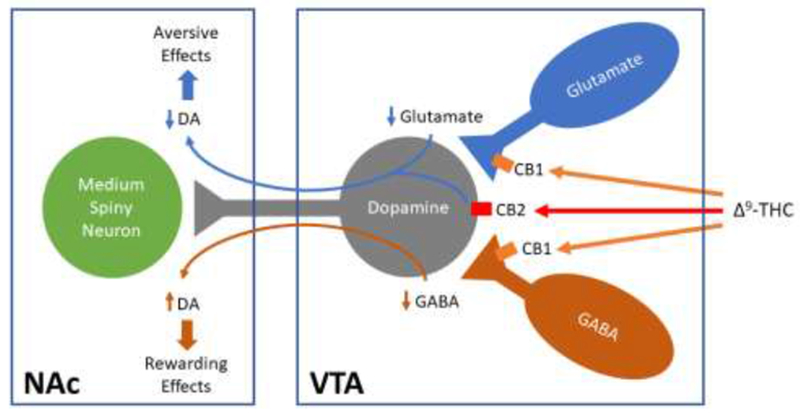
Diagram showing how Δ9-THC modulates the mesolimbic DA system. Δ9-THC may produce rewarding effects by binding to CB1R on GABAergic interneurons in the VTA, thereby reducing GABA-mediated inhibition of VTA DA and increasing DA release in the NAc. Conversely, Δ9-THC may produce aversive effects by activating CB1R on glutamatergic neurons in the VTA, or CB2R on DA neurons, thereby inhibiting VTA DA release to the NAc. The subjective effects of Δ9-THC may thus depend on the balance of opposing CB1R and CB2R effects and individual differences in neural CB1 and CB2 receptor expression. VTA: Ventral tegmental area. NAc: Nucleus accumbens, DA: Dopamine.
Feeding
CB1Rs and CB2Rs play crucial roles in regulating energy metabolism (Li et al., 2011). Genetic ablation of CB1R results in reduced body weight in mice (Zimmer et al., 1999), while genetic deletion of CB2R results in increased food intake and obesity with age (Agudo et al., 2010). CB2R agonists reduce food intake in lean mice (Ishiguro et al., 2010) and improve both body weight and obesity-associated inflammation in diet-induced obese mice (Verty et al., 2015). Moreover, CB2 genetic ablation results in adiposity (Schmitz K et al., 2016). A common CB2 variant, Q63R, causing reduced CB2R function, has been associated with eating disorders in humans (Ishiguro et al., 2010). However, the mechanisms underlying CB2R modulation of body weight and obesity are poorly understood. Since CB2Rs are also expressed in organs controlling metabolism such as the liver, adipose tissue, skeletal muscle and the endocrine pancreas (Deveaux et al., 2009; Rossi et al., 2011), it is generally believed that peripheral CB2Rs may be involved. However, overexpression of CB2Rs in the brain induces hyperglycaemia and a lean phenotype in adult mice (Romero-Zerbo et al., 2012), suggesting that brain CB2Rs are also involved in food intake and energy metabolism. Taken together, these findings suggest that CB2Rs may represent a new pharmacological target for the treatment of binge-eating and obesity.
Drug addiction
Cocaine addiction:
Growing evidence suggests that cannabinoids or medical marijuana may be useful for the treatment for drug addiction (Gonzalez-Cuevas et al., 2018). Prolonged or acute exposure to drugs of abuse, including cocaine and morphine, increases brain CB2R mRNA expression by up to five-fold in key reward-related regions such as the VTA, NAc, PFC, and striatum (Figure 4; Bystrowska et al., 2018; Onaivi et al., 2008a; Zhang et al., 2017), suggesting that CB2R ligands may have therapeutic potential for cocaine abuse (Zhang et al., 2017b). Accordingly, systemic and local administration of CB2R agonists (JWH133 or GW405833) into the VTA or NAc dose-dependently reduced intravenous cocaine self-administration in WT and CB1-KO mice, an effect that was blocked by the CB2R antagonist AM630 and absent in CB2-KO mice (Xi et al., 2011; Zhang et al., 2015; Zhang et al., 2014). JWH133 also blocked cocaine-induced hyperactivity, conditioned place preferences, and extracellular NAc DA in mice (Canesco-Alba et al., 2018; Delis et al., 2017; Xi et al., 2011). Although JWH133 was less effective in attenuating cocaine self-administration in rats (Zhang et al., 2015), systemic administration of beta-caryophyllene (BCP), a dietary CB2R agonist extracted from cannabis or other plants, dose-dependently reduced cocaine intake in both rats and mice (Zhang et al., 2017b). Similarly, overexpression of CB2Rs in the mouse brain decreased locomotor responses to cocaine and reduced mouse cocaine self-administration (Aracil-Fernandez et al., 2012). In contrast, selective deletion of CB2R from DA neurons increased acute psychostimulant hyperactivity and augmented cocaine-conditioned place preferences, possibly due to enhanced NAc DA response to psychostimulants (Canesco-Alba et al., 2018; Liu et al., 2017). In other studies, CB2R antagonists and inverse agonists reduced cocaine-primed reinstatement to drug seeking, but were not effective in attenuating ongoing cocaine self-administration in rats (Adamczyk et al., 2012).
Figure 4:
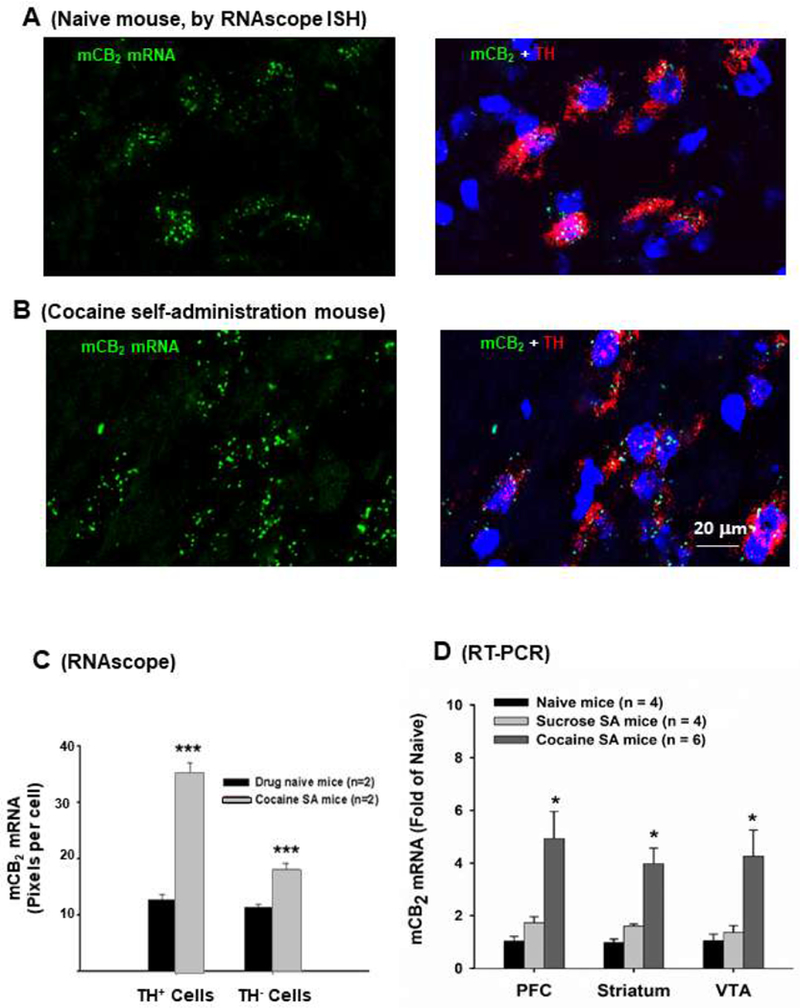
Effects of cocaine self-administration (SA) on CB2 mRNA expression in the brain and in VTA DA neurons in mice. (A) CB2 mRNA expression in naive mouse, illustrating CB2 mRNA expression in tyrosine hydroxylase (TH)-positive dopamine neurons as measured by RNAscope ISH; (B) CB2 mRNA expression in mice after 4-6 weeks of cocaine SA, illustrating that prolonged cocaine self-administration (1 mg/kg/infusion, 3 hrs per daily session, maximal 50 infusions per session) significantly up-regulates CB2 expression in VTA DA neurons; (C) Quantitative results in RNAscope assays, illustrating that cocaine SA significantly increased CB2 mRNA expression in VTA dopamine neurons; (D) Quantitative RT-PCR results, illustrating that cocaine, but not sucrose, self-administration significantly up-regulated CB2 mRNA expression in the prefrontal cortex (PFC), striatum and VTA-containing midbrain. *p<0.05. ***p<0.001, compared to naive control mice.
Alcohol and nicotine addiction:
CB2Rs also appear to play a role in alcohol and nicotine abuse. CB2-KO mice drink more alcohol and develop more robust alcohol conditioned place preferences than WT controls (Ortega-Alvaro et al., 2015; Powers et al., 2015). The CB2R agonist BCP reduces alcohol intake and acquisition of alcohol conditioned place preferences in mice (Al Mansouri et al., 2014). However, a recent report suggest that selective deletion of CB2 from DA neurons attenuated alcohol-conditioned place preferences and blocked chronic stress-induced increase in alcohol consumption (Liu et al., 2017), indicating further research is needed to understand the role of CB2 in alcohol reward. CB2-KO mice also do not show conditioned place preferences to nicotine, and CB2R antagonists block nicotine conditioned place preferences (Canesco-Alba et al., 2018; Ignatowska-Jankowska et al., 2013; Navarrete et al., 2013).
Opioid addiction:
Although few studies to date have examined the impact of CB2R ligands on opioid abuse, CB2R does interact with mu opioid receptors to attenuate chronic pain. Co-administration of a CB2R agonist alongside morphine produces synergistic increases in the anti-nociceptive effects of morphine (Grenald et al., 2017). Conversely, CB2R antagonism reduces the analgesic effects of morphine, but also reduces morphine tolerance (Altun et al., 2015). These findings on brain CB2R involvement in drug reward and addiction suggest that brain CB2Rs may constitute new therapeutic targets in medication development for the treatment of substance use disorders.
CB2R INVOLVEMENT IN NEUROPSYCHIATRIC DISORDERS
CB2R is inducible
Although brain CB2R levels are low in healthy subjects under basal conditions, many studies suggest that brain CB2Rs are inducible or up-regulated in response to various insults (Atwood and Mackie, 2010; Mechoulam and Parker, 2013), including chronic pain (Beltramo et al., 2006; Luongo et al., 2010), ischemia-induced hypoxia (Ashton and Glass, 2007), drug addiction (Bystrowska et al., 2018; Onaivi et al., 2008b; Zhang et al., 2017; Zhang et al., 2003), Alzheimer’s disease, HIV-induced encephalitis and multiple sclerosis (Benito et al., 2008; Lopez et al., 2018; Schmole et al., 2015). Brain CB2R upregulation may be a common neuroprotective response to various central nervous system insults. Below we briefly review evidence supporting CB2R involvement in various cognitive functions and neuropsychiatric conditions.
Learning and memory
CB2R signaling plays an important regulatory role in learning and memory. Deletion of CB2R in mice disrupts consolidation of aversive memories of a foot-shock and reduces hippocampal synapse proliferation and expression of stress- and growth factor-related genes, such as in brain-derived neurotrophic factor (BDNF) and glucocorticoid receptors (NR3C1) (Garcia-Gutierrez et al., 2013). Systemic administration of AM630, a CB2R antagonist, impairs aversive memory consolidation, whereas JWH133, the CB2R agonist, enhances aversive memory consolidation as measured by performance on an inhibitory avoidance test (Garcia-Gutierrez et al., 2013). Similarly, in rats cannabidiol disrupts consolidation of fear memories via activity at CB1R and CB2R in the dorsal hippocampus (Ratano et al., 2017; Stern et al., 2017). More specifically, using the inhibitory avoidance task alongside pharmacological manipulations (i.e., inhibition of monoacylglcerol lipase to increase 2-AG levels, with and without CB1R/CB2R antagonists), Ratano and colleagues reported 2-AG enhanced memory consolidation specifically through CB2R and not CB1R (Ratano et al., 2018). Moreover, fear conditioning increased CB2R, but not CB1R, gene expression in the hippocampus, which correlated with anxiety-related behaviors such as freezing and escape latencies (Robertson et al., 2017).
Recent technological advances in genome editing techniques allow for additional insights into cell-type specific effects of CB2R activation. Genetic deletion of CB2Rs impaired hippocampus-dependent, long-term contextual fear memory, but had no effect on hippocampus-independent, cued fear memory (Li and Kim, 2016a). Overexpression of CB2Rs using Crispr-Cas9 in hippocampal pyramidal neurons reduced anxiety, whereas deletion of CB2Rs in pyramidal neurons enhanced spatial working memory. In contrast, overexpression of CB2Rs in hippocampal microglia enhanced, and reduction of microglial CB2R expression reduced, the expression of contextual fear memories (Li and Kim, 2017).
Alzheimer’s disease
In addition to modulating learning and memory in healthy subjects, CB2R has been implicated in several neuropsychiatric disorders involving memory deficits. For example, post-mortem analysis of human brains with Alzheimer’s disease (AD) show CB2R upregulation in the frontal cortex, which correlated with amyloid beta levels and plaque scores, two primary markers of disease severity (Solas et al., 2013). Similarly, an AD mouse model (a CB2R-reporter line crossed with mice expressing five familial AD mutations), showed significantly increased CB2-EGFP levels in the central nervous system at 3 months of age, coincident with inflammation and amyloid plaques in the cortex, hippocampus, brain stem and thalamus (Lopez et al., 2018). In another mouse model of AD, chronic CB2R activation using a selective agonist reduced microglial inflammation in the hippocampus, increased clearance of amyloid plaques, improved hippocampal plasticity and glutamatergic signaling, and enhanced memory performance in the Morris water maze (Wu et al., 2017). Similarly, the CB2R agonist 1-phenylisatin improved learning and memory on an attentional set shifting task and reduced amyloid beta plaque load in the hippocampus in another mouse model of AD (Jayant et al., 2016).
Neuroinflammation
Consistent with a role for CB2R in memory disorders, mouse models of post-operative cognitive dysfunction have identified increased CB2R expression in the hippocampus and prefrontal cortex, accompanied by memory loss, following surgery. Surprisingly, these effects were reversed with JWH133 treatment (a CB2R agonist) but exacerbated by AM630 treatment (a CB2R antagonist) (Sun et al., 2017). In animal models of traumatic brain injury (TBI), administration of a CB2R inverse agonist attenuated neuronal death in the cortex, striatum and amygdala (Bu et al., 2016), and reversed TBI-induced electrophysiological changes in hippocampal and prefrontal cortex oscillatory activity (Liu et al., 2017b). In a rat model of vascular dementia, the CB2R agonist BCP improved learning and memory on the Morris water maze, increased cerebral blood flow, and upregulated CB2R expression in the hippocampus (Lou et al., 2017). Similarly, another CB2R agonist, 1-phenylisatin, improved learning and memory and reversed mitochondrial and acetylcholinesterase deficits in a different rodent model of vascular dementia (Jayant et al., 2016).
Epilepsy
CB2R also regulates seizure activity in animal models of epilepsy. The CB2R agonist BCP decreases seizure frequency and spread in mice undergoing maximal electroshock seizure and kainic-acid induced neurotoxicity tests, respectively (Tchekalarova et al., 2018). BCP also reduced oxidative stress resulting from seizure activity and improved memory performance in the Morris water maze (Tchekalarova et al., 2018). These findings suggest that CB2Rs may be a valuable medication target for treating memory loss, cognitive deficits, and inflammation in neuropsychiatric conditions ranging from dementia to epilepsy.
Mood disorders
CB2R also appears to be involved in the neurobiology of mood disorders. Transgenic mice overexpressing CB2Rs exhibited an endophenotype resistant to acute and chronic anxiogenic- and depression-like stimuli (Garcia-Gutierrez and Manzanares, 2011; Garcia-Gutierrez et al., 2010). These striking behavioral features were associated with pronounced alterations in brain regions related to stress, anxiety, and depression, including the hippocampus and amygdala. In contrast, CB2-KO mice displayed high vulnerability to stressful stimuli (Ortega-Alvaro et al., 2011). Chronic administration of AM630, a selective CB2R antagonist, resulted in anxiolytic- and antidepressant-like effects (Garcia-Gutierrez et al., 2011; Garcia-Gutierrez et al., 2010), while administration of JWH133, a selective CB2R agonist, produced the opposite behavioral and neurochemical effects (Garcia-Gutierrez et al., 2010). In addition, an association between CB2R polymorphisms and depression was detected in a Japanese population (Onaivi et al., 2008a), and alterations in cortical CB2R (and GPR55) gene and protein expression are associated with suicide incidents (Garcia-Gutierrez et al., 2018). Taken together, these findings suggest an important role of CB2R in regulating anxiety and depression.
CONCLUSIONS
CB2R was initially assumed to express exclusively in the periphery. However, recent technological advances in the sensitivity and specificity of RNA and protein detection, alongside progress in genome editing techniques, have generated converging evidence across species supporting the presence of functional CB2R in the brain. Today, corroboration for brain CB2R is available at every level in biology, from genes to protein expression and from functional effects at the neuronal level to behavior and neuropsychiatric disease. Ongoing work will develop increasingly specific antibodies to identify CB2R protein expression, as well as full CB2-KO mouse lines in which more commercially-available antibodies can be validated. Future research will also identify more selective CB2R agonists and antagonists that have promise as pharmacological tools and pharmacotherapeutics. Given the broad involvement of CB2Rs in behavior, understanding brain CB2R signaling may provide fresh insights into interventions and treatments for disorders ranging from dementia to epilepsy and drug addiction.
Highlights.
Cannabinoid CB2R was initially assumed to be exclusively in the periphery
Technological innovations have revealed functional CB2R expression in neurons and glial cells
Species differences exist in CB2R genes, receptor expression, and function
Region-specific CB2R transcripts are found in the brain and periphery
Brain CB2Rs are inducible and neuroprotective against various insults, involved in cannabis action, drug addiction, and other psychiatric disorders
Acknowledgments
We thank Dr. Qing-Rong Liu at the National Institute on Ageing Intermural Research Program for his critical reading and modifications on this article. This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA; Z1A DA000620-02), National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflicts of Interest
The authors have no personal or financial conflicts of interest.
References
- Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegalinski E, 2012. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res 1444, 45–54. [DOI] [PubMed] [Google Scholar]
- Agudo J, Martin M, Roca C, Molas M, Bura AS, Zimmer A, Bosch F, Maldonado R, 2010. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia 53, 2629–2640. [DOI] [PubMed] [Google Scholar]
- Al Mansouri S, Ojha S, Al Maamari E, Al Ameri M, Nurulain SM, Bahi A, 2014. The cannabinoid receptor 2 agonist, beta-caryophyllene, reduced voluntary alcohol intake and attenuated ethanol-induced place preference and sensitivity in mice. Pharmacol Biochem Behav 124, 260–268. [DOI] [PubMed] [Google Scholar]
- Altun A, Yildirim K, Ozdemir E, Bagcivan I, Gursoy S, Durmus N, 2015. Attenuation of morphine antinociceptive tolerance by cannabinoid CB1 and CB2 receptor antagonists. J Physiol Sci 65, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando RD, Biro J, Csolle C, Ledent C, Sperlagh B, 2012. The inhibitory action of exo- and endocannabinoids on [(3)H]GABA release are mediated by both CB(1)and CB(2)receptors in the mouse hippocampus. Neurochem Int 60, 145–152. [DOI] [PubMed] [Google Scholar]
- Aracil-Fernandez A, Trigo JM, Garcia-Gutierrez MS, Ortega-Alvaro A, Ternianov A, Navarro D, Robledo P, Berbel P, Maldonado R, Manzanares J, 2012. Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB(2) receptors. Neuropsychopharmacology 37, 1749–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Friberg D, Darlington CL, Smith PF, 2006. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett 396, 113–116. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Glass M, 2007. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol 5, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Zheng Y, Darlington C, Baek JH, Smith PF, 2014. Cannabinoid CB2 receptor immunolabelling in the healthy brain--still a live possibility. Naunyn Schmiedebergs Arch Pharmacol 387, 301. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K, 2010. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek JH, Darlington CL, Smith PF, Ashton JC, 2013. Antibody testing for brain immunohistochemistry: brain immunolabeling for the cannabinoid CB(2) receptor. J Neurosci Methods 216, 87–95. [DOI] [PubMed] [Google Scholar]
- Baek JH, Zheng Y, Darlington CL, Smith PF, 2008. Cannabinoid CB2 receptor expression in the rat brainstem cochlear and vestibular nuclei. Acta Otolaryngol 128, 961–967. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A, 2006. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci 23, 1530–1538. [DOI] [PubMed] [Google Scholar]
- Benito C, Tolon RM, Pazos MR, Nunez E, Castillo AI, Romero J, 2008. Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol 153, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, Onaivi ES, 2008a. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse 62, 944–949. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro PA, Saez T, Onaivi ES, 2008b. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann N Y Acad Sci 1139, 450–457. [DOI] [PubMed] [Google Scholar]
- Bu W, Ren H, Deng Y, Del Mar N, Guley NM, Moore BM, Honig MG, Reiner A, 2016. Mild Traumatic Brain Injury Produces Neuron Loss That Can Be Rescued by Modulating Microglial Activation Using a CB2 Receptor Inverse Agonist. Front Neurosci 10, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NE, 2008. The peripheral cannabinoid receptor knockout mice: an update. Br J Pharmacol 153, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A, 2000. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol 396, 141–149. [DOI] [PubMed] [Google Scholar]
- Bystrowska B, Frankowska M, Smaga I, Pomierny-Chamiolo L, Filip M, 2018. Effects of Cocaine Self-Administration and Its Extinction on the Rat Brain Cannabinoid CB1 and CB2 Receptors. Neurotox Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canseco-Alba A, Schanz N, Sanabria B, Zhao J, Lin Z, Liu Q-R, Onaivai ES, 2018. Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behav Brain Res, Available online November 30, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Lowinson JH, Gardner EL, 1991. Strain-specific facilitation of dopamine efflux by delta 9-tetrahydrocannabinol in the nucleus accumbens of rat: an in vivo microdialysis study. Neurosci Lett 129, 136–180. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH, 2004. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29, 1558–1572. [DOI] [PubMed] [Google Scholar]
- Delis F, Polissidis A, Poulia N, Justinova Z, Nomikos GG, Goldberg SR, Antoniou K, 2017. Attenuation of Cocaine-Induced Conditioned Place Preference and Motor Activity via Cannabinoid CB2 Receptor Agonism and CB1 Receptor Antagonism in Rats. Int J Neuropsychopharmacol 20, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth DG, Molleman A, 2006. Cannabinoid signalling. Life Sci 78, 549–563. [DOI] [PubMed] [Google Scholar]
- den Boon FS, Chameau P, Houthuijs K, Bolijn S, Mastrangelo N, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR, 2014. Endocannabinoids produced upon action potential firing evoke a C1(−) current via type-2 cannabinoid receptors in the medial prefrontal cortex. Pflugers Arch 466, 2257–2268. [DOI] [PubMed] [Google Scholar]
- den Boon FS, Chameau P, Schaafsma-Zhao Q, van Aken W, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR, 2012. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci U S A 109, 3534–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC, 1988. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34, 605–613. [PubMed] [Google Scholar]
- Deveaux V, Cadoudal T, Ichigotani Y, Teixeira-Clerc F, Louvet A, Manin S, Nhieu JT, Belot MP, Zimmer A, Even P, Cani PD, Knauf C, Burcelin R, Bertola A, Le Marchand-Brustel Y, Gual P, Mallat A, Lotersztajn S, 2009. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS One 4, e5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Spano MS, Pistis M, Fratta W, 2008. Neurobiological mechanisms of cannabinoid addiction. Mol Cell Endocrinol 286, S97–S107. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, Conn PJ, 2016. Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release. Neuron 91, 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratta W, Fattore L, 2013. Molecular mechanisms of cannabinoid addiction. Curr Opin Neurobiol 23, 487–492. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P, 1995. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232, 54–61. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J, 2011. Chronic blockade of cannabinoid CB(2) receptors induces anxiolytic-like actions associated to alterations in GABA(A) receptors. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Manzanares J, 2011. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol 25, 111–120. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Navarrete F, Navarro G, Reyes-Resina I, Franco R, Lanciego JL, Giner S, Manzanares J, 2018. Alterations in Gene and Protein Expression of Cannabinoid CB2 and GPR55 Receptors in the Dorsolateral Prefrontal Cortex of Suicide Victims. Neurotherapeutics 15, 796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Ortega-Alvaro A, Busquets-Garcia A, Perez-Ortiz JM, Caltana L, Ricatti MJ, Brusco A, Maldonado R, Manzanares J, 2013. Synaptic plasticity alterations associated with memory impairment induced by deletion of CB2 cannabinoid receptors. Neuropharmacology 73, 388–396. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Perez-Ortiz JM, Gutierrez-Adan A, Manzanares J, 2010. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol 160, 1773–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MC, Cinquina V, Palomo-Garo C, Rabano A, Fernandez-Ruiz J, 2015. Identification of CB(2) receptors in human nigral neurons that degenerate in Parkinson’s disease. Neurosci Lett 587, 1–4. [DOI] [PubMed] [Google Scholar]
- Glass M, Northup JK, 1999. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol 56, 1362–1369. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR, 2006. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res 1071, 10–23. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cuevas G, Martin-Fardon R, Kerr TM, Stouffer DG, Parsons LH, Hammell DC, Banks SL, Stinchcomb AL, Weiss F, 2018. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacology 43, 2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenald SA, Young MA, Wang Y, Ossipov MH, Ibrahim MM, Largent-Milnes TM, Vanderah TW, 2017. Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology 116, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G, Fernando SR, Ross RA, McKay NG, Ashford ML, Shire D, Huffman JW, Yu S, Lainton JA, Pertwee RG, 1997. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol 339, 53–61. [DOI] [PubMed] [Google Scholar]
- Han X, He Y, Bi GH, Zhang HY, Song R, Liu QR, Egan JM, Gardner EL, Li J, Xi ZX, 2017. CB1 Receptor Activation on VgluT2-Expressing Glutamatergic Neurons Underlies Delta(9)-Tetrahydrocannabinol (Delta(9)-THC)-Induced Aversive Effects in Mice. Sci Rep 7, 12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Kargl J, Andradas C, Brown AJ, Irving A, Sanchez C, Waldhoer M, 2011. Minireview: recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol Endocrinol 25, 1835–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC, 1990. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87, 1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman IR, Skola D, Glass CK 2017. Transcriptional control of microglia phenotypes in health and disease. J Clin Invest 127, 3220–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, 1998. The CB1 cannabinoid receptor in the brain. Neurobiol Dis 5, 405–416. [DOI] [PubMed] [Google Scholar]
- Ibsen MS, Connor M, Glass M, 2017. Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res 2, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Muldoon PP, Lichtman AH, Damaj MI, 2013. The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology (Berl) 229, 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Carpio O, Horiuchi Y, Shu A, Higuchi S, Schanz N, Benno R, Arinami T, Onaivi ES, 2010. A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse 64, 92–96. [DOI] [PubMed] [Google Scholar]
- Jayant S, Sharma BM, Bansal R, Sharma B, 2016. Pharmacological benefits of selective modulation of cannabinoid receptor type 2 (CB2) in experimental Alzheimer’s disease. Pharmacol Biochem Behav 140, 39–50. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Elmes SJ, Richardson D, Barrett DA, Kendall DA, Mason R, Chapman V, 2008. Evidence for a novel functional role of cannabinoid CB(2) receptors in the thalamus of neuropathic rats. Eur J Neurosci 27, 1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Martin TJ, Nader MA, 2017. Behavioral Determinants of Cannabinoid Self-Administration in Old World Monkeys. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR, 2003. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 169, 135–140. [DOI] [PubMed] [Google Scholar]
- Kim J, Li Y, 2015. Chronic activation of CB2 cannabinoid receptors in the hippocampus increases excitatory synaptic transmission. J Physiol 593, 871–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Barroso-Chinea P, Rico AJ, Conte-Perales L, Callen L, Roda E, Gomez-Bautista V, Lopez IP, Lluis C, Labandeira-Garcia JL, Franco R, 2011. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol 25, 97–104. [DOI] [PubMed] [Google Scholar]
- Leppek K, Das R, Barna M, 2018. Functional 5’ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat Rev Mol Cell Biol 19, 158–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Jones PM, Persaud SJ, 2011. Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas. Pharmacol Ther 129, 307–320. [DOI] [PubMed] [Google Scholar]
- Li Y, Kim J, 2015. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience 311, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kim J, 2016a. CB2 Cannabinoid Receptor Knockout in Mice Impairs Contextual Long-Term Memory and Enhances Spatial Working Memory. Neural Plast 2016, 9817089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kim J, 2016b. Deletion of CB2 cannabinoid receptors reduces synaptic transmission and long-term potentiation in the mouse hippocampus. Hippocampus 26, 275–281. [DOI] [PubMed] [Google Scholar]
- Li Y, Kim J, 2017. Distinct roles of neuronal and microglial CB2 cannabinoid receptors in the mouse hippocampus. Neuroscience 363, 11–25. [DOI] [PubMed] [Google Scholar]
- Liu QR, Canseco-Alba A, Zhang HY, Tagliaferro P, Chung M, Dennis E, Sanabria B, Schanz N, Escosteguy-Neto JC, Ishiguro H, Lin Z, Sgro S, Leonard CM, Santos-Junior JG, Gardner EL, Egan JM, Lee JW, Xi ZX, Onaivi ES, 2017. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci Rep 7, 17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Pan CH, Hishimoto A, Li CY, Xi ZX, Llorente-Berzal A, Viveros MP, Ishiguro H, Arinami T, Onaivi ES, Uhl GR, 2009. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav 8, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, McAfee SS, Guley NM, Del Mar N, Bu W, Heldt SA, Honig MG, Moore BM 2nd, Reiner A, Heck DH, 2017b. Abnormalities in Dynamic Brain Activity Caused by Mild Traumatic Brain Injury Are Partially Rescued by the Cannabinoid Type-2 Receptor Inverse Agonist SMM-189. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, Vazquez C, Amores M, Ruiz-Perez G, Garcia-Garcia E, Beatka M, Tolon RM, Dittel BN, Hillard CJ, Romero J, 2018. Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J Neuroinflammation 15, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z, 2008. Specificity of immunoreactions: the importance of testing specificity in each method. J Neurosci 28, 9083–9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J, Teng Z, Zhang L, Yang J, Ma L, Wang F, Tian X, An R, Yang M, Zhang Q, Xu L, Dong Z, 2017. beta-Caryophyllene/Hydroxypropyl-beta-Cyclodextrin Inclusion Complex Improves Cognitive Deficits in Rats with Vascular Dementia through the Cannabinoid Receptor Type 2 -Mediated Pathway. Front Pharmacol 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR., Riegel AC., Hoffman AF. 2004. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol 143, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR., Hoffman AF. 2018. Cannabinoid disruption of learning mechanisms involved in reward processing. Learn Mem 25, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo L, Palazzo E, Tambaro S, Giordano C, Gatta L, Scafuro MA, Rossi FS, Lazzari P, Pani L, de Novellis V, Malcangio M, Maione S, 2010. 1-(2′,4′-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyraz ole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol Dis 37, 177–185. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, 2005. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 48, 1105–1116. [DOI] [PubMed] [Google Scholar]
- Lynn AB, Herkenham M, 1994. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J Pharmacol Exp Ther 268, 1612–1623. [PubMed] [Google Scholar]
- Ma Z, Gao F, Larsen B, Gao M, Chen D, Ma XK, Qiu S, Zhou Y, Xie JX, Xi ZX, Wu J (2018). Mechanisms of CB2 receptor-induced reduction of dopamine neuronal excitability in mouse ventral tegmental area. Under review. [DOI] [PMC free article] [PubMed]
- Mackie K, 2005. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol, 299–325. [DOI] [PubMed] [Google Scholar]
- Mackie K, 2008. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol 20 Suppl 1, 10–14. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F, 2006. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29, 225–232. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Nicholson KL, Martin BR, Balster RL, 1994. Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav Pharmacol 5, 219–225. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ, 1995a. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18, 22–29. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ, 1995b. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat 8, 283–305. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ, 1994a. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 350, 412–438. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ, 1994b. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res 643, 245–265. [DOI] [PubMed] [Google Scholar]
- Marchalant Y, Brownjohn PW, Bonnet A, Kleffmann T, Ashton JC, 2014. Validating Antibodies to the Cannabinoid CB2 Receptor: Antibody Sensitivity Is Not Evidence of Antibody Specificity. J Histochem Cytochem 62, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pinilla E, Varani K, Reyes-Resina I, Angelats E, Vincenzi F, Ferreiro-Vera C, Oyarzabal J, Canela EI, Lanciego JL, Nadal X, Navarro G, Borea PA, Franco R, 2017. Binding and Signaling Studies Disclose a Potential Allosteric Site for Cannabidiol in Cannabinoid CB2 Receptors. Front Pharmacol 8, 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI, 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Hanus LO, Pertwee R, Howlett AC, 2014. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci 15, 757–764. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, 2013. The endocannabinoid system and the brain. Annu Rev Psychol 64, 21–47. [DOI] [PubMed] [Google Scholar]
- Metna-Laurent M, Mondesir M, Grel A, Vallee M, Piazza PV, 2017. Cannabinoid-Induced Tetrad in Mice. Curr Protoc Neurosci 80, 9 59 51–59 59 10. [DOI] [PubMed] [Google Scholar]
- Morgan NH, Stanford IM, Woodhall GL, 2009. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology 57, 356–368. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M, 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG, 2004. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol 92, 3562–3574. [DOI] [PubMed] [Google Scholar]
- Navarrete F, Rodriguez-Arias M, Martin-Garcia E, Navarro D, Garcia-Gutierrez MS, Aguilar MA, Aracil-Fernandez A, Berbel P, Minarro J, Maldonado R, Manzanares J, 2013. Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology 38, 2515–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, Gonzalez S, Tolon RM, Romero J, 2004. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse 53, 208–213. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR, 2008a. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One 3, e1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Liu QR, Chirwa SS, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR, 2008b. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann N Y Acad Sci 1139, 434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR, 2006. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci 1074, 514–536. [DOI] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J, 2011. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology 36, 1489–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Ternianov A, Aracil-Fernandez A, Navarrete F, Garcia-Gutierrez MS, Manzanares J, 2015. Role of cannabinoid CB2 receptor in the reinforcing actions of ethanol. Addict Biol 20, 43–55. [DOI] [PubMed] [Google Scholar]
- Panagis G, Vlachou S, Nomikos GG, 2008. Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence-producing properties. Curr Drug Abuse Rev 1, 350–374. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL, 2015. Endocannabinoid signalling in reward and addiction. Nature reviews. Neuroscience 16, 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, 1997. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74, 129–180. [DOI] [PubMed] [Google Scholar]
- Powers MS, Breit KR, Chester JA, 2015. Genetic Versus Pharmacological Assessment of the Role of Cannabinoid Type 2 Receptors in Alcohol Reward-Related Behaviors. Alcohol Clin Exp Res 39, 2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft D, Gregg J, Ghia J, Harris L, 1977. Effects of intravenous tetrahydrocannabinol on experimental and surgical pain. Psychological correlates of the analgesic response. Clin Pharmacol Ther 21, 26–33. [DOI] [PubMed] [Google Scholar]
- Ratano P, Palmery M, Trezza V, Campolongo P, 2017. Cannabinoid Modulation of Memory Consolidation in Rats: Beyond the Role of Cannabinoid Receptor Subtype 1. Front Pharmacol 8, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratano P, Petrella C, Forti F, Passeri PP, Morena M, Palmery M, Trezza V, Severini C, Campolongo P, 2018. Pharmacological inhibition of 2-arachidonoilglycerol hydrolysis enhances memory consolidation in rats through CB2 receptor activation and mTOR signaling modulation. Neuropharmacology 138, 210–218. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS, 2006. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci 26, 8017–8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Achua JK, Smith JP, Prince MA, Staton CD, Ronan PJ, Summers TR, Summers CH, 2017. Anxious behavior induces elevated hippocampal Cb2 receptor gene expression. Neuroscience 352, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Zerbo SY, Garcia-Gutierrez MS, Suarez J, Rivera P, Ruz-Maldonado I, Vida M, Rodriguez de Fonseca F, Manzanares J, Bermudez-Silva FJ, 2012. Overexpression of cannabinoid CB2 receptor in the brain induces hyperglycaemia and a lean phenotype in adult mice. J Neuroendocrinol 24, 1106–1119. [DOI] [PubMed] [Google Scholar]
- Rossi F, Bellini G, Nobili B, Maione S, Perrone L, del Giudice EM, 2011. Association of the cannabinoid receptor 2 (CB2) Gln63Arg polymorphism with indices of liver damage in obese children: an alternative way to highlight the CB2 hepatoprotective properties. Hepatology 54, 1102; author reply 1102-1103. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ, 2007. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Kelly S, Millns PJ, O’Shaughnessey CT, Kendall DA, Chapman V, 2005. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur J Neurosci 22, 371–379. [DOI] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE, 1997. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol 142, 278–287. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Schafer F, Striggow V, Frohlich K, Striggow F, 2012. Cannabinoid receptor subtypes 1 and 2 mediate long-lasting neuroprotection and improve motor behavior deficits after transient focal cerebral ischemia. Neuroscience 227, 313–326. [DOI] [PubMed] [Google Scholar]
- Schmole AC, Lundt R, Gennequin B, Schrage H, Beins E, Kramer A, Zimmer T, Limmer A, Zimmer A, Otte DM, 2015. Expression Analysis of CB2-GFP BAC Transgenic Mice. PLoS One 10, e0138986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra S, Luquin N, Rico AJ, Gomez-Bautista V, Roda E, Dopeso-Reyes IG, Vazquez A, Martinez-Pinilla E, Labandeira-Garcia JL, Franco R, Lanciego JL, 2015. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct Funct 220, 2721–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller K, Bi GH, He Y, Gardner EL, Xi ZX. 2018. Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. Br J Pharmacol, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solas M, Francis PT, Franco R, Ramirez MJ, 2013. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol Aging 34, 805–808. [DOI] [PubMed] [Google Scholar]
- Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Racz I, Ponomarenko A, Xi ZX, Zimmer A, Schmitz D, 2016. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 90, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CAJ, da Silva TR, Raymundi AM, de Souza CP, Hiroaki-Sato VA, Kato L, Guimaraes FS, Andreatini R, Takahashi RN, Bertoglio LJ, 2017. Cannabidiol disrupts the consolidation of specific and generalized fear memories via dorsal hippocampus CB1 and CB2 receptors. Neuropharmacology 125, 220–230. [DOI] [PubMed] [Google Scholar]
- Stumpf A, Parthier D, Sammons RP, Stempel AV, Breustedt J, Rost BR, Schmitz D, 2018. Cannabinoid type 2 receptors mediate a cell type-specific self-inhibition in cortical neurons. Neuropharmacology 139, 217–225. [DOI] [PubMed] [Google Scholar]
- Sun L, Dong R, Xu X, Yang X, Peng M, 2017. Activation of cannabinoid receptor type 2 attenuates surgery-induced cognitive impairment in mice through anti-inflammatory activity. J Neuroinflammation 14, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I, 2002. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci 15, 2057–2061. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR, 2000. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci 3, 1073–1074. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G, 1997. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276, 2048–2050. [DOI] [PubMed] [Google Scholar]
- Tang J, Tao Y, Tan L, Yang L, Niu Y, Chen Q, Yang Y, Feng H, Chen Z, Zhu G, 2015. Cannabinoid receptor 2 attenuates microglial accumulation and brain injury following germinal matrix hemorrhage via ERK dephosphorylation in vivo and in vitro. Neuropharmacology 95, 424–433. [DOI] [PubMed] [Google Scholar]
- Tchekalarova J, da Conceicao Machado K, Gomes Junior AL, de Carvalho Melo Cavalcante AA, Momchilova A, Tzoneva R, 2018. Pharmacological characterization of the cannabinoid receptor 2 agonist, beta-caryophyllene on seizure models in mice. Seizure 57, 22–26. [DOI] [PubMed] [Google Scholar]
- Vacaru AM, Vitale J, Nieves J, Baron MH, 2014. Generation of transgenic mouse fluorescent reporter lines for studying hematopoietic development. Methods Mol Biol 1194, 289–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA, 2005. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332. [DOI] [PubMed] [Google Scholar]
- Verty AN, Stefanidis A, McAinch AJ, Hryciw DH, Oldfield B, 2015. Anti-Obesity Effect of the CB2 Receptor Agonist JWH-015 in Diet-Induced Obese Mice. PLoS One 10, e0140592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Panagis G, 2014. Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Curr Pharm Des 20, 2072–2088. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N 2003. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci 15, 1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y, 2012. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Galaj E, Zhang C, Zhan J, Bi GH, Gardner EL, Xi ZX (2018). Distinct receptor mechanisms underlying the tetrad effects of phytocannabinoids and synthetic cannabinoids. Neuropsychopharmacology, under review. [Google Scholar]
- Wiley JL, Martin BR, 2003. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol 471, 185–193. [DOI] [PubMed] [Google Scholar]
- Wu J, Hocevar M, Foss JF, Bie B, Naguib M, 2017. Activation of CB2 receptor system restores cognitive capacity and hippocampal Sox2 expression in a transgenic mouse model of Alzheimer’s disease. Eur J Pharmacol 811, 12–20. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL, 2011. Brain cannabinoid CB receptors modulate cocaine’s actions in mice. Nat Neurosci 14, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SJ, Reiner D, Shen H, Wu KJ, Liu QR, Wang Y, 2015. Time-Dependent Protection of CB2 Receptor Agonist in Stroke. PLoS One 10, e0132487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Bi GH, Li X, Li J, Qu H, Zhang SJ, Li CY, Onaivi ES, Gardner EL, Xi ZX, Liu QR, 2015. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology 40, 1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J, Xi ZX, 2014. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A 111, E5007–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Shen H, Bi GH, Yang HJ, Liu QR, Wu J, Gardner EL, Bonci A, Xi ZX, 2017. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addict Biol 22, 752–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY and Xi ZX (2017). Cannabinoid CB2 Receptor: A New Target for Treatment of Cocaine Addiction In: Preedy Victor R., editors, The Neuroscience of Cocaine: Academic Press, 2017, pp. 689–698. [Google Scholar]
- Zhang HY, Shen H, Jordan CJ, Liu QR, Gardner EL, Bonci A, Xi ZX, 2018. CB2 receptor antibody signal specificity: correlations with the use of partial CB2-knockout mice and anti-rat CB2 receptor antibodies. Acta Pharmacol Sin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D, 2003. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci 17, 2750–2754. [DOI] [PubMed] [Google Scholar]
- Zheng L, Wu X, Dong X, Ding X, Song C, 2015. Effects of Chronic Alcohol Exposure on the Modulation of Ischemia-Induced Glutamate Release via Cannabinoid Receptors in the Dorsal Hippocampus. Alcohol Clin Exp Res 39, 1908–1916. [DOI] [PubMed] [Google Scholar]
- Zimmer A, 2015. Genetic Manipulation of the Endocannabinoid System. Handb Exp Pharmacol 231, 129–183. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI, 1999. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A 96, 5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]


