Abstract
Objective:
To determine the incidence of AL amyloidosis in a strictly defined geographic area from 1990 to 2015.
Patients and Methods:
We searched our computerized database for the records of all Olmsted County residents with a diagnosis of AL (immunoglobulin light chain) amyloidosis from January 1, 1990 to December 31, 2015. In addition, records of all residents with a mention of amyloidosis were obtained from the Rochester Epidemiology Project which contains the medical records of the Mayo Clinic and the Olmsted Medical Group. The diagnosis of AL amyloidosis was determined by mass spectrometry, immunohistochemistry or positive Congo red staining.
Results:
Thirty-five patients were identified as having AL amyloidosis from January 1, 1990 to December 31, 2015. The median age at diagnosis was 76 years (range 38–90 years) with males accounting for 54%. The incidence rate of AL amyloidosis from 1990–2015 adjusted for age and sex was 1.2/100,000 person-years (95% CI 0.8, 1.6). Rates were similar across the decades 1990–1999, 2000–2009 and 2010–2015 at 1.1, 0.9 and 1.6/100,000 person-years with no suggestion of an increasing rate over the 26 years. There was a trend of an increasing incidence over time from 1950–2015 in Olmsted County but it was not statistically significant (P=.15). Applying the rate of 1.2/100,000 person-years to the U.S. population of 321 million in 2015, one would expect 3,852 new cases of AL amyloidosis in the United States each year.
Conclusion:
The incidence of AL amyloidosis in Olmsted County, Minnesota, has not changed significantly over the past 66 years.
INTRODUCTION
AL amyloidosis (immunoglobulin light chain) is an uncommon disease that is characterized by the presence of amyloid fibrils that are deposited mainly in the heart, kidneys, gastrointestinal tract, peripheral nerves and blood vessels of virtually all organs. The amyloid fibril is composed of a fragment of an immunoglobulin light chain accompanied by chaperone proteins, including serum amyloid P component, apolipoprotein A-IV and apolipoprotein E. The source of the monoclonal light chains is a plasma cell proliferative process which may fulfill the criteria of multiple myeloma.1–4
Few studies on the epidemiology of AL amyloidosis have been reported.5–10 Furthermore, amyloidosis is not included in the Surveillance, Epidemiology and End Results database (SEER) which provides national estimates of cancer in the U.S.11 The lack of epidemiologic data is due to the infrequency of AL amyloidosis and the diagnostic difficulties in distinguishing AL from other types of systemic amyloidosis. In view of the paucity of epidemiologic studies in AL amyloidosis, we are updating our experience with AL amyloidosis in Olmsted County, MN since 1990 and comparing it to AL over the period of 1950–1989.
METHODS
Patients
After the study was approved by the Mayo Clinic and Olmsted Medical Group Institutional Review Boards, we searched our Dysproteinemia computerized database for the records of all Olmsted County residents with a diagnosis of amyloidosis, including AL (immunoglobulin light chain, primary), AA (serum amyloid A, secondary), ATTR (transthyretin, wild type), age-related (senile), ATTR transthyretin variants (hereditary) as well as all other types of amyloidosis.12 In addition, records of all patients with a mention of amyloidosis were obtained from the Rochester Epidemiology Project which includes the medical records of the Mayo Clinic and the Olmsted Medical Group from January 1, 1990 to December 31, 2015.13 Only those patients who had lived in Olmsted County for at least one year before the diagnosis of amyloidosis were considered to be residents. Patients known to have moved to Olmsted County to facilitate the diagnosis and treatment of symptoms of amyloidosis were excluded. To ensure that no cases of amyloidosis were overlooked, we obtained all available death certificates and autopsy reports for Olmsted County residents with a diagnosis of amyloidosis. All patients were sent a letter of inquiry or contacted by telephone if they had not visited the Mayo Clinic in the preceding year.
Among the 416 records of the Rochester Epidemiology Project with a mention of amyloidosis, a positive report was found in 2 of 53 patients with pathology data. A review of a 10% random sample of the remaining 363 patients revealed no cases of AL amyloidosis.
Amyloid Diagnosis and Typing
In all 35 patients identified, the diagnosis of amyloidosis was based on the presence of amyloid in tissue sections prepared from paraffin blocks from biopsy or autopsy tissues. All diagnostic specimens contained amyloid deposits that exhibited apple-green birefringence when stained with alkaline Congo red and viewed under polarized light. Paraffin-embedded tissue blocks were available from 29 patients for determination of the amyloid type by laser microdissection followed by tandem mass spectrometry using a previously published method.14 The unlabeled immunoperoxidase method was used with antisera against purified amyloid fibril proteins including Aκ and Aλ light chains, AA (protein A), ATTR (prealbumin (transthyretin)) and AB (β2 microglobulin) with suitable controls to classify the type of amyloid in 2 patients.
For the four patients with positive Congo red staining but no tissue available for typing, the presentation was classic. The first of the four patients had a positive renal biopsy (amyloid in glomeruli and arteries), had an IgG lambda of 4.0 g/dL with 50% monoclonal bone marrow plasma cells, urine M-protein 1.9 g/24 h., hemoglobin 9.0 g with a creatinine 1.6 mg/dL. The second patient, who had 2 positive fat aspirates, had hoarseness, a carpal tunnel syndrome, nephrotic syndrome, and a bone marrow containing 34% plasma cells. The third patient had a positive Congo red stain and periorbital purpura, left ventricular diastolic dysfunction, atrial fibrillation as well as congestive heart failure. After a year of non-specific abdominal pain, the fourth patient had a liver biopsy which was positive for amyloidosis; at autopsy, he was found to have a plasma cell proliferative process and amyloidosis in the kidney glomeruli as well.
Epidemiology
The age-, sex-, and calendar year- specific incidence rates of AL amyloidosis were based on data from Olmsted County, MN, using the number of cases of AL amyloidosis in each age/sex calendar-year group as the numerator and the corresponding decennial census population counts for Olmsted County, MN, as the denominator.5 Age- and sex-adjusted incidence rates were calculated by direct standardization to the 2010 United States population. Ninety-five percent (95%) confidence intervals for the incidence rates were calculated based on the Poisson distribution. Temporal trends in the incidence rates were examined using Poisson regression.
RESULTS
Thirty-five patients were identified as having AL amyloidosis from January 1, 1990 to December 31, 2015. The median age at diagnosis in the 35 patients was 76 years (range 38–90 years) with males accounting for 54%. All patients were white and mostly of northern European extraction. Congestive heart failure was present at diagnosis in 10 patients (29%) and occurred in 9 additional patients (total 54%) during follow-up. Nephrotic syndrome was present in 9 patients (26%) at diagnosis. The diagnosis was made at autopsy in one patient who had Alzheimer disease so confirmation of the clinically suspected amyloidosis was not confirmed until autopsy.
Immunofixation revealed a monoclonal protein in the serum of 33 patients (94%). The bone marrow plasma cell level ranged from 2–97% (median 18%); 41% had 10% or fewer plasma cells. The subcutaneous fat biopsy was positive in 15 (68%) of the 22 patients in whom it was performed at diagnosis. The bone marrow was positive for amyloid in 19 (70%) and negative in 8 (30%) at diagnosis. Only one patient had both a negative fat and bone marrow biopsy for amyloid at diagnosis.
The incidence rate of AL amyloidosis from 1990–2015 adjusted for age and sex was 1.2/100,000 person-years (95% CI, 0.8, 1.6) (Table 1A). Rates were similar across the decades 1990–1999, 2000–2009 and 2010–2015 at 1.1 (0.4, 1.8), 0.9 (0.4, 1.5) and 1.6 (0.8, 2.5)/100,000 person-years with no suggestion of an increasing rate over the 26 years (Table 1A). Over the entire period from 1950 to 2015, there was an increasing trend in the incidence of AL amyloidosis by age (P<.001) and males had a higher incidence than females over all ages, (P=.01) (Figure 1, Table 1B). There was also a trend of an increasing incidence over time across the 66 year period from 1950–2015, although not statistically significant, P=.15 (Table 2 and Figure 2).
Table 1A.
Annual incidence of AL Amyloidosis per 100,000 persons-years in Olmsted County, MN, 1990–2015 by decadea
| Age (yrs) | 1990–1999 | 2000–2009 | 2010–2015 | Total | Overall | |||
|---|---|---|---|---|---|---|---|---|
| No. | Rate | No. | Rate | |||||
| 30–49 | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 1 | 0.04 |
| 50–59 | 0 | 0.0 | 2 | 1.2 | 1 | 0.8 | 3 | 0.8 |
| 60–69 | 1 | 1.4 | 3 | 3.0 | 3 | 4.0 | 7 | 2.8 |
| 70–79 | 4 | 7.9 | 4 | 6.2 | 6 | 13.2 | 14 | 8.7 |
| ≥ 80 | 4 | 11.6 | 2 | 4.3 | 4 | 12.2 | 10 | 8.8 |
| Total | 10 | 11 | 14 | 35 | ||||
| Adjusted rate (95% CI)b | 1.1 (0.4, 1.8) | 0.9 (0.4, 1.5) | 1.6 (0.8, 2.5) | 1.2 (0.8, 1.6) | ||||
Results are expressed as: number of cases (rate per 100,000)
Age- and sex-adjusted to the 2010 U.S. population
Figure 1:
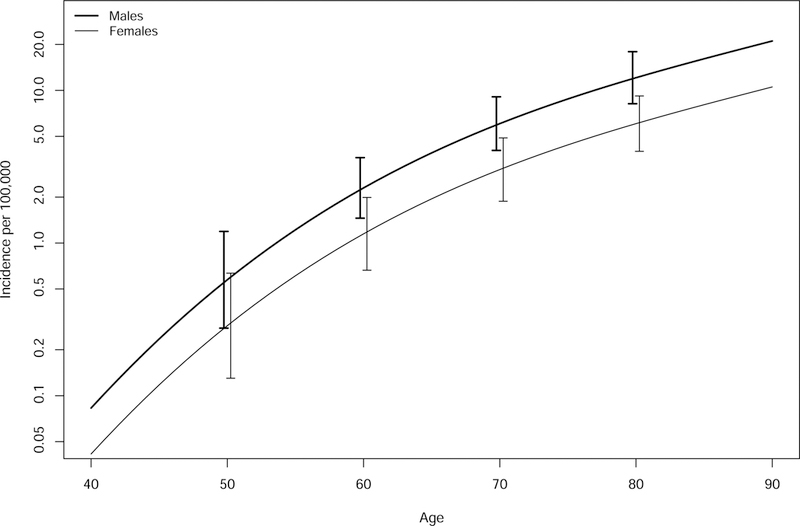
Incidence of AL amyloidosis by age and gender 1950–2015.
Table 1B.
Incidence of AL amyloidosis per 100,000 person-years in Olmsted County, MN, 1990–2015, by age and gendera
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| No. | Rate | No. | Rate | No. | Rate | |
| Age (yr) | ||||||
| 30–49 | 0 | 0.0 | 1 | 0.1 | 1 | 0.04 |
| 50–59 | 2 | 1.0 | 1 | 0.5 | 3 | 0.8 |
| 60–69 | 5 | 4.2 | 2 | 1.5 | 7 | 2.8 |
| 70–79 | 8 | 11.2 | 6 | 6.7 | 14 | 8.7 |
| ≥ 80 | 4 | 10.6 | 6 | 8.0 | 10 | 8.8 |
| Total | 19 | 16 | 35 | |||
| Adjusted rate (95% CI) | 1.5 (0.8−2.2)b | 0.9 (0.5−1.4)b | 1.2 (0.8, 1.6)c | |||
Results are expressed as: number of cases (rate per 100,000)
Age-adjusted to the 2010 U.S. population
Age- and sex-adjusted to the 2010 U.S. population
Table 2.
Annual incidence of AL amyloidosis in Olmsted County, MN, by decadea
| Age (yrs) | 1950–1959 | 1960–1969 | 1970–1979 | 1980–1989 | 1990–1999 | 2000–2009 | 2010–2015 | Overall |
|---|---|---|---|---|---|---|---|---|
| 30–49 | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.04) |
| 50–59 | 1 (1.9) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.2) | 1 (0.8) | 5 (0.7) |
| 60–69 | 0 (0.0) | 1 (2.1) | 0 (0.0) | 2 (3.3) | 1 (1.4) | 3 (3.0) | 3 (4.0) | 10 (2.2) |
| 70–79 | 0 (0.0) | 1 (3.3) | 2 (5.6) | 5 (12.0) | 4 (7.9) | 4 (6.2) | 6 (13.2) | 22 (7.5) |
| ≥80 | 0 (0.0) | 1 (7.6) | 2 (10.8) | 4 (15.8) | 4 (11.6) | 2 (4.3) | 4 (12.2) | 17 (9.5) |
| Total | 2 | 4 | 4 | 11 | 10 | 11 | 14 | 56 |
| Adjusted rate (95%CI)b | 0.4 (0, 1.0) | 0.9 (0.0, 1.7) | 0.8 (0.0, 1.5) | 1.6 (0.6, 2.6) | 1.1 (0.4, 1.8) | 0.9 (0.4, 1.5) | 1.6 (0.8, 2.5) | 1.1 (0.8, 1.4) |
Results are expressed as: number of cases (rate per 100,000). Data shown include 1950–1989 as per prior Olmsted publication.5
Age- and sex-adjusted to the 2010 U.S. population
Figure 2:
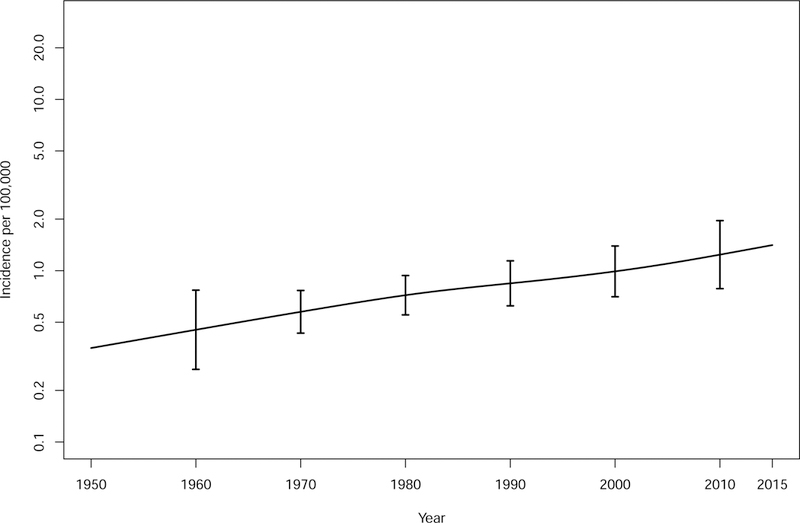
Incidence of AL amyloidosis from 1950–2015.
Applying the rate of 1.2/100,000 person-years to the U.S. population of 321 million in 2015 and assuming no geographic variation, one would expect 3,852 new cases of AL in the United States each year.
DISCUSSION
Within Olmsted County, Minnesota, the incidence rates of AL amyloidosis have remained relatively stable over a period of 66 years. We had previously reported that in Olmsted County, the incidence was stable at a rate of 0.89/100,000 person-years from 1950–1989.5 After reviewing all patients with a diagnosis of AL amyloidosis between 1990 and 2015, a time frame in which amyloid typing was possible, the overall incidence remained stable at 1.2 (0.8, 1.6) per 100,000. Also unchanged was the increasing risk with advancing age.
Our results are in line with the other 5 publications in which authors attempt to establish the incidence of AL amyloidosis even though the methodologies differ (Table 3).6–10 Only one is a true population based study.9 Duhamel et al performed a retrospective population based study to estimate incidence in the Limosin region of France from 2012 to 2016.9 Over the 5-year period, they found a crude yearly incidence of 1.25 (95% CI, 0.56–1.94) cases per 100,000 inhabitants. How the amyloidosis typing was performed was not specified. Pinney et al.6 reported an incidence rate of AL amyloidosis of a minimum of 0.3 cases/100,000 in England in 2008 by extrapolating from referral rates to the National Amyloidosis Centre, death certificate reports of amyloidosis, and the distribution of types of systemic amyloidosis cases seen at the National Amyloidosis Centre. They also estimated that the total number of deaths from any form of systemic amyloidosis in England in 2008 was 0.9 per 1000 deaths. Hemminki et al took a different approach to derive an annual incidence rate of AL amyloidosis in Sweden from 2001 to 2018 by using myeloma statistics and amyloid hospital discharge diagnoses.7 Their estimate for AL amyloidosis was 0.3/100, 000 and for all non-hereditary amyloidosis, 0.83/100,000. Aguirre et al. reported a rate of 0.61/100,000 (95% CI, 0.26–0.97) adjusted for the population of Buenos Aires (2010 census) based on 12 persons with AL amyloidosis8. Finally, Quock et al estimated the incidence of AL amyloidosis by using US claims data from 2007 to 2015 and derived an age and gender adjusted annual incidence of 1.08 to 1.52 cases per 100,000 person years with no statistically significant increase.10
Table 3.
Epidemiology Studies in AL amyloidosis
| Reference | Location | Design | Age/ gender | Yearly incidence (per 100,000 person-years) |
|---|---|---|---|---|
| Current study | Olmsted county | 1990–2015: Population-based study; primarily mass spectrometry typing | Median age: 76 years; males 54% | 1.2 (95%CI 0.8, 1.6)a |
| Duhamel9 | Limosin region, France | 2012 to 2016: Population-based study; no mention of amyloid typing methodology | Median age, 72.5 years; 70% men | 1.25 (95% CI, 0.56−1.94)b |
| Quock10 | USA | 2007 to 2015: US claims data | Mean age 64 years; 54% male | 1.08 −1.52a |
| Pinney6 | England | 2008: Extrapolated through death certificates and referral rates to and amyloidosis types at the NAC | Peaked at age 60–69; | 0.3c |
| Hemminki7 | Sweden | 2001 to 2018: Extrapolated through myeloma statistics and amyloid hospital discharge diagnoses | No data | 0.32c |
| Aguirre8 | Argentina | 2006 to 2015: Extrapolated through registrants in the Medical Care Program in Buenos Aires | No data | 0.61 (95% CI, 0.26−0.97a,d |
NAC, National Amyloidosis Centre
Age and sex adjusted
Crude estimate
Adjusted for age only
Adjusted to the Buenos Aires Census (2010 census)
It is notable that the estimated incidence derived from the two studies which were population-based (present study and Limoges study) were quite comparable. The U.S. claims-based study estimated a similar incidence in a more diverse population.10 A flaw in their design, however, was in how they differentiated AL amyloidosis from other forms of amyloidosis based on what “AL defining therapies” the patients received; they included doxycycline, which is not uncommonly used in patients with ATTR cardiomyopathy. Where evaluated, all studies showed a higher incidence with advancing age and with the male sex.
Strengths of our study are the relatively stable population base, the quality of ascertainment of cases, our ability to review the individual cases, and most importantly the method of amyloid typing. In Olmsted County, MN, medical care for the population of Rochester and surrounding Olmsted County is provided by the Mayo Clinic and the Olmsted Medical Group. Virtually all medical, surgical and pathologic diagnoses of significant illnesses among Olmsted County residents at any local healthcare facility have been compiled in a centralized records-linkage system. Information in this central file is supplemented by a periodic search of the records of the medical facilities in the surrounding counties where on infrequent occasions, Olmsted County residents might have been seen for medical care. Information from death certificates and autopsy reports for all Olmsted County residents is also entered into the comprehensive database for the defined population. In terms of the quality of the amyloid classification, of the 35 cases, 29 were typed by mass spectrometry, 2 by immunochemistry and the remaining 4 by Congo red staining and typical clinical and laboratory features.
A limitation of our study is the relatively homogenous population; ours is not a true cross section of the United States. Ninety-nine percent of Olmsted County residents in 1970 were white, in 1990 it was 96% and in 2010 86% were white. It is conceivable, but not likely, that AL amyloidosis patients in Olmsted County were missed in part due to the Olmsted County ascertainment methods and to the clinical interest of physicians and the unique diagnostic facilities present at the Mayo Clinic. Not only is a clinical suspicion for the diagnosis required, but also histologic proof of amyloid and its precursor protein are required since there are 36 known amyloid proteins that can cause systemic disease.12
In conclusion, it is challenging to estimate the incidence of AL over time because of changing criteria for diagnosis, alterations in clinical practice and pathologic diagnosis, variation in the method of diagnostic indexing of medical records and a reduced autopsy rate. We report an age- and sex-specific incidence rate of AL of 1.2 per 100,000 person-years in Olmsted County from 1990–2015 which is not statistically different (P=.10) than those from 1950–1989 in the same population (0.89 per 100,000 person-years). There was a higher incidence rate among males than females over the 66 year period. The incidence of AL amyloidosis we report is approximately one-fourth of the reported incidence of multiple myeloma (4.3 per 100,000 person-years) from the same (Olmsted) county.15 However, the total number of new cases diagnosed with AL amyloidosis in the United States each year based on our Olmsted County rates would be 3,852 cases each year. This is much lower than the actual number of new cases of multiple myeloma in the U.S. (30,770 in 2018)16, suggesting that this is likely due to lower recognition and greater difficulty in diagnosing AL amyloidosis. Understanding the discrepancy between the estimates and actual rates of these two diseases requires further study.
Conclusion
The incidence rate of AL amyloidosis from 1990–2015 were similar across the decades with no evidence of an increasing rate. Although there was a trend of increasing incidence over time from 1950–2015, the change was not statistically significant.
Acknowledgments
Financial Support: Supported in part by research grants CA107476 and CA168762 from the National Cancer Institute and Prothena Therapeutics Limited, grant BRI-222091. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AL
amyloidosis (immunoglobulin light chain amyloidosis)
Footnotes
Conflicts of Interest Disclosure: Dr. Dispenzieri has research support from Prothena, Jannsen, Alnylam, Celgene, Takeda, Pfizer and GSK. Dr. Kumar has research grants for clinical trials to the institution: Celgene, Takeda, Janssen, BMS, Sanofi, KITE, Merck, Abbvie, Medimmune, Novartis, Roche-Genentech and Amgen. Consulting with no personal payments: Celgene, Takeda, Janssen, KITE, Merck, Abbvie, Medimmune,Genentech, Oncopeptides and Amgen. IRC chair: Oncopeptides. Honorarium: Dr. Reddys Lab. The other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyle RA, Bayrd ED. “Primary” systemic amyloidosis and myeloma. Discussion of relationship and review of 81 cases. Arch Intern Med 1961;107344–353. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Greipp PR. Amyloidosis (AL). Clinical and laboratory features in 229 cases. Mayo Clinic Proceedings 1983;58(10):665–683. [PubMed] [Google Scholar]
- 3.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Seminars in Hematology 1995;32(1):45–59. [PubMed] [Google Scholar]
- 4.Vrana JA, Theis JD, Dasari S, et al. Clinical diagnosis and typing of systemic amyloidosis in subcutaneous fat aspirates by mass spectrometry-based proteomics. Haematologica 2014;99(7):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. [see comment]. Blood 1992;79(7):1817–1822. [PubMed] [Google Scholar]
- 6.Pinney JH, Smith CJ, Taube JB, et al. Systemic amyloidosis in England: an epidemiological study. British journal of haematology 2013;161(4):525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemminki K, Li X, Forsti A, Sundquist J, Sundquist K. Incidence and survival in non-hereditary amyloidosis in Sweden. BMC Public Health 2012;12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguirre MA, Boietti BR, Nucifora E, et al. Incidence rate of amyloidosis in patients from a medical care program in Buenos Aires, Argentina: a prospective cohort. Amyloid 2016;23(3):184–187. [DOI] [PubMed] [Google Scholar]
- 9.Duhamel S, Mohty D, Magne J, et al. Incidence and Prevalence of Light Chain Amyloidosis: A Population-Based Study. Blood 2017;130(Suppl 1):5577–5577. [Google Scholar]
- 10.Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv 2018;2(10):1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Program https://seer.cancer.gov/.
- 12.Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 2016;23(4):209–213. [DOI] [PubMed] [Google Scholar]
- 13.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd., History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic Proceedings 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 2009;114(24):4957–4959. [DOI] [PubMed] [Google Scholar]
- 15.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ. Incidence of multiple myeloma in Olmsted County, Minnesota - Trend over 6 decades. Cancer 2004;101(11):2667–2674. [DOI] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]


