Abstract
As the leading infectious cause of death worldwide and the primary proximal cause of death in those living with HIV, tuberculosis remains a global concern. Existing tuberculosis control strategies that rely on passive case-finding appear insufficient to achieve targets for reductions in tuberculosis incidence and mortality. Active case-finding strategies aim to detect infectious individuals earlier in their infectious period to reduce onward transmission and improve treatment outcomes. Empirical studies of active case-finding have produced mixed results and determining how to direct active screening to those most at risk remains a topic of intense research. Our systematic review of the literature evaluating the impact of geographically-targeted tuberculosis screening interventions found three studies in low tuberculosis incidence settings, but none conducted in high tuberculosis incidence countries. We enumerate open questions related to the use of spatially targeted approaches for active screening for tuberculosis in countries where tuberculosis incidence is highest.
Introduction:
The current annual rate of decline in global tuberculosis incidence is estimated to be 1.9%.1 This is far short of the 4% annual decline needed to meet the World Health Organization (WHO) End TB Strategy’s 2020 milestone of a 20% reduction compared with 20151 and the more ambitious targets leading to 90% reduction by 2035. Increased investment in the development of new tuberculosis diagnostics over the past two decades has brought new products to market,2,3 but these tools have not yet demonstrated substantial epidemiological impact.4,5 Accordingly, finding best approaches for employing existing tools to reduce the incidence of tuberculosis remains a public health priority.
In high tuberculosis burden countries and among those living with HIV, most incident tuberculosis arises as a result of recent transmission.6,7 Thus, efforts to control tuberculosis in these settings depend on reducing transmission, which theoretically could be achieved through earlier detection and treatment of individuals with active, infectious tuberculosis. Identifying practical approaches for detecting infectious individuals earlier in their course of disease has proven challenging. A recent systematic review failed to find conclusive evidence of the effectiveness of untargeted active case-finding (compared to passive detection).8 Accordingly, current WHO guidance documents9,10 provide a “strong recommendation” that active screening be focused on individuals with HIV infection, household contacts of a tuberculosis case, and workers exposed to silica, and do not support the adoption of active case-finding in the general population. Such active screening may include combinations of symptom interviews, chest radiography, sputum smear or sputum rapid molecular testing, and is usually performed outside of traditional health care facilities (e.g. mobile vans, homes, shelters).
In addition to using individual-level risk factors to target screening, strategies that target active screening within geographically-restricted populations (e.g. neighborhoods, subdistricts) may also be an effective and practical case-finding approach. As the quality and spatial resolution of surveillance systems have improved,11 marked spatial heterogeneity in the incidence and prevalence of tuberculosis has been documented in a wide variety of settings,12–16 frequently approaching 10-fold variation of incidence within a single country. Such patterns have motivated enthusiasm for spatially targeted interventions.17–19 Currently, the WHO “conditionally” recommends that “systematic screening for active tuberculosis…be considered for geographically defined populations with extremely high levels of undetected tuberculosis (1% prevalence or higher)”.9 In most high-burden regions, recent prevalence surveys are not available; in such settings methods (e.g. capture-recapture studies20) for identifying geographic areas where tuberculosis is underdiagnosed are still being validated.
In this manuscript, we consider the rationale and evidence to support spatially targeted tuberculosis screening to reduce tuberculosis transmission. Spatial targeting concentrates screening within geographic “hotspots” of tuberculosis incidence that arise as a result of several different mechanisms (Table 1). We review evidence relevant to high burden countries, many of which also have high prevalence of HIV, as these settings are where more effective strategies are needed most. While screening household contacts of individuals diagnosed with tuberculosis might be considered a very local form of spatial targeting, contact tracing has a large body of literature that has been reviewed by others21,22 and is beyond the scope of this review. In addition to considering the empirical and theoretical basis for spatially targeted screening, we also highlight current gaps in knowledge that must be addressed before the impact and attractiveness of such approaches can be reliably anticipated.
Table 1:
Mechanisms producing spatially aggregated areas of high tuberculosis incidence
| Mechanism | Supporting evidence | Real-world example |
|---|---|---|
| Local transmission | • Spatially aggregated risk of TST/IGRA conversion • Spatial aggregation of genotypic cluster |
Lima, Peru 23 Concentration of genetically related Mtb isolates in a high-incidence area suggests local transmission as a cause of the hotspot |
| Concentration of risk factor for progression to active disease | • Co-localization of determinant of tuberculosis disease and tuberculosis incidence hotspot | Urumqi, China 24 Overlapping high incidence areas of tuberculosis and tuberculosis / HIV coinfection |
| Migration of individuals from areas with higher risks of infection and disease | • Higher tuberculosis incidence in areas where migrants cluster | Shandong province, China 25 Areas of high incidence along major transportation routes |
TST = Tuberculin skin test
IGRA = Interferon gamma release assay
Mtb = Mycobacterium tuberculosis
Premise of spatially targeted tuberculosis interventions:
In many high incidence settings, a non-trivial fraction of individuals with active tuberculosis will go undiagnosed and untreated. Those who do receive diagnosis often experience substantial delays of months or years during which they may transmit infection to others.26,27 Finding individuals with undiagnosed active tuberculosis and offering them treatment seems an attractive approach for reducing prevalence and interrupting transmission. However tuberculosis prevalence only reaches levels above 1% in the highest burden countries.1 This stands in contrast to pathogens like HIV and malaria, for which prevalence of infection in adults reaches more than 10% in some countries.28,29 The low prevalence of tuberculosis challenges case-finding efforts, and may cause health officials in some settings to de-prioritize tuberculosis activities relative to diseases with higher prevalence.
One approach to address challenges arising from low prevalence of tuberculosis is to focus screening on sub-populations with disproportionately high rates of active disease. The theoretical benefits of such targeted approaches are clear. By identifying sub-populations with higher prevalence of disease, fewer people need to be screened to identify each case, leading to fewer false positive diagnoses and unnecessary treatments.30 Pragmatically, targeted interventions may produce local economies of scale, in contrast to geographically diffuse, untargeted interventions.
Promise of spatially targeted screening:
The potential advantages of strategies that account for marked spatial heterogeneities in tuberculosis burden over strategies that ignore such variability are intuitively compelling. With similar rationale, other infectious disease control programs such as immunization campaigns have implemented geographically targeted strategies.31 But is there empirical evidence to support active screening for tuberculosis?
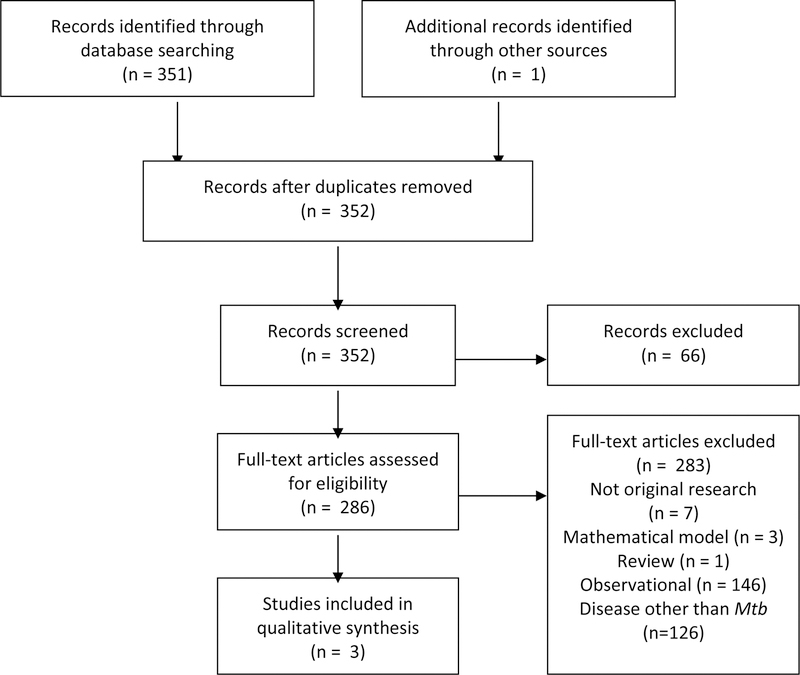
We conducted a literature search for studies in high burden settings that used spatial analyses of tuberculosis to guide subsequent screening interventions to lower tuberculosis transmission or prevalence (Box 1). Articles were required to include a spatial analysis of tuberculosis burden (eg incidence, prevalence, proportion of drug resistance, proportion of recently transmitted cases) and include an intervention that was targeted based on the spatial data. We found only three studies that described a geographically-targeted screening intervention, each of which was conducted in low incidence areas32–34 within the United States. We did not identify any such studies conducted in high tuberculosis incidence settings. While spatially targeted screening may yet prove an effective strategy, there is currently little evidence of impact in areas of high tuberculosis burden.
Box 1: Search strategy and selection criteria.
We searched multiple databases for articles published through August 1st, 2017. PubMed was searched with the terms: (“Tuberculosis”[Mesh]) AND (((“Spatial Analysis”[Mesh]) OR “Geographic Information Systems”[Mesh])), Embase was searched with the terms: (geographic information system/ OR exp spatial analysis/) AND exp tuberculosis/), Scopus was searched with the terms: (“Geographic Information System*” OR “Global Positioning System*” OR “Spatial Analys*”) AND (Tuberculosis OR “Koch’s disease” OR “Koch disease” OR “kochs disease”), and Web of Science was searched with the terms: (“Geographic Information System*” OR “Global Positioning System*” OR “Spatial Analys*”) AND (Tuberculosis OR “Koch’s disease” OR “Koch disease” OR “kochs disease”). Articles resulting from these searches and relevant references cited in those articles were reviewed. 352 abstracts were reviewed, and original peer reviewed publications published in English, Spanish and Portuguese were included.
Interpretation: There were no studies of tuberculosis screening strategies in high burden countries that utilized spatial information to target interventions. There were three studies from low incidence areas.
In the absence of direct empirical evidence of the impact of targeted screening, modelling has provided some insight into factors that could theoretically modify the efficacy of spatially targeted screening. Transmission dynamic models provide a useful framework for estimating the direct impact of spatially targeted screening on those screened and the indirect impact on transmission. For example, Dowdy et al. created a tuberculosis model of Rio de Janeiro, where there exists substantial spatial heterogeneity in tuberculosis notifications. They estimated the degree to which three hotspots of tuberculosis incidence contributed to transmission in the city as a whole. Next, they modeled case-finding interventions targeted at the hotspots35 and found that benefits of such targeting were dependent on the amount of social mixing between individuals in the hotspot and surrounding areas, which is not typically well measured. In one scenario within which a hotspot comprised 6% of the city’s population, reducing transmission within the hotspot had a similar impact on long-term overall city TB rates as halving the time to treatment among the remaining 94% of the city’s population. Under most modeled assumptions about hotspot-to-community population mixing, focusing screening efforts within the hotspots appeared to be an attractive approach for achieving better tuberculosis control in the city overall.
Open problems in spatially targeted screening:
Despite a reasonable theoretical premise in support of spatially targeted tuberculosis screening, the lack of empirical evidence of epidemiologic impact suggests the need for caution. Recently, a community randomized trial36 of spatially targeted approaches for malaria control (which was justified in part on promising transmission modeling studies37) failed to show an impact on prevalence. The authors concluded that there was no significant impact outside the targeted hotspots because “transmission may not primarily occur from hotspots to the surrounding areas”. While the mode of malaria transmission is distinct from tuberculosis, this example provides an important reminder that we must actually test these spatially targeted tuberculosis strategies to determine their impact. Empiric studies will also inform our understanding of epidemiological mechanisms driving local heterogeneities. In the absence of ambitious randomized studies of spatially targeted tuberculosis screening strategies that can provide definitive evidence of impact, there remain several tractable questions that, if addressed, will help to identify settings in which spatially targeted approaches may be most attractive.
1). When can we achieve better tuberculosis control within targeted areas?
Is there evidence that increased screening efforts within a tuberculosis incidence hotspot will reduce disease burden in the hotpot itself? Surprisingly there are few studies that assess the community level effects of tuberculosis screening; a recent systematic review found only five such studies.8 The authors concluded that the evidence for screening’s impact on tuberculosis incidence or prevalence was “weak”. Active screening does increase the number of individuals with tuberculosis found. However, we do not know the proportion of transmission that can be averted through earlier detection and treatment9 since people diagnosed during earlier, less symptomatic stages of disease may also be less infectious and may be less likely to initiate and complete therapy.38
Recent studies of active case-finding in high burden settings illustrate current uncertainty about the effectiveness of community level screening. In Zimbabwe, a cluster randomized trial of active case-finding (DETECTB) determined that screening using mobile vans resulted in an increase in case notifications, leading to a 40% reduction in the prevalence of active tuberculosis by the end of six rounds of screening over two years.39 By contrast, a cluster randomized controlled trial in South Africa and Zambia (ZAMSTAR) found no statistically significant impact of two active case-finding strategies (community-level enhanced tuberculosis case-finding (ECF) and household level tuberculosis–HIV care) on the prevalence of active tuberculosis.40 These studies differed in numerous ways, both in terms of epidemiological setting and the specific approaches used for active case-finding, which makes it difficult to understand what drove these different outcomes. Nevertheless, unless screening interventions can reliably produce detectable reductions in tuberculosis prevalence in high burden settings, targeting screening to areas of especially high incidence is unlikely to be successful.
As highlighted in Table 1, hotspots of tuberculosis incidence may not necessarily arise as a result of local transmission. Understanding the degree to which local transmission is responsible for the local concentration of incident tuberculosis is important for estimating the potential epidemiological impact of spatially targeted active screening. There are a growing number of investigations that bring together spatial and pathogen genetic analysis to offer new insights into the importance of local transmission on the generation and maintenance of tuberculosis incidence hotspots (Table 2). The increased resolution of whole genome sequencing in determining clusters of recent tuberculosis transmission will help to increase the power of these studies.41
Table 2:
Examples of use of spatial and pathogen genetic data to understand local transmission
| Study population and setting | Spatial data | Pathogen genetic data | Conclusion |
|---|---|---|---|
| All people with culture positive tuberculosis in a mid-sized city in Brazil42 | Household location | IS6110 RFLP | Most transmission observed outside the household but within locations less than 2000 meters away |
| All people diagnosed with tuberculosis in 12 of the 43 districts of metropolitan Lima, Peru23 | Household location | 24-loci MIRU-VNTR | Location was an equally strong risk factor for MDR-TB as history of prior tuberculosis treatment |
| All people with culture positive tuberculosis in two urban communities in South Africa43 | Household location | IS6110 RFLP | Within a very high tuberculosis incidence community there was extensive transmission outside the household but within the community |
| All people diagnosed with tuberculosis in a rural town in eastern Uganda44 | Household, healthcare, work and social locations | Spoligotyping | Transmission likely at healthcare and social venues |
| Most people diagnosed with tuberculosis in 14 Inuit communities in Canada45 | Community location | Whole Genome Sequencing | Transmission more common within each community than between communities with limited contact |
| All people with culture positive tuberculosis in a suburb of Shanghai46 | Household location | 12-loci MIRU-VNTR and Whole Genome Sequencing for strains within MIRU clusters | Spatial proximity positively associated with genomic similarity among strains within a MIRU cluster, consistent with local transmission |
RFLP = Restriction Fragment Length Polymorphism
MIRU-VNTR = Mycobacterial Interspersed Repetitive Units - Variable Numbers of Tandem Repeats
Spoligotyping = Spacer oligonucleotide typing
Spatially targeted interventions may still be effective even in incidence hotspots not driven by transmission, such as those arising through migration or aggregation of vulnerable hosts. The intervention strategies employed may differ depending on the mechanism driving the hotspot. For example, an incidence hotspot resulting from a concentration of risk factors for progression to active disease (such as malnutrition or HIV) may benefit more from detection and treatment of latent tuberculosis infection than an incidence hotspot resulting from local transmission.
2). Will the benefits of better local control achieved through targeted screening have a spillover effect for the surrounding areas?
If targeted screening in high burden areas is successful in lowering incidence locally, do benefits in the hotspot also accrue to individuals living outside the hotspot? Transmission dynamic models suggest35 that the attractiveness of targeted screening increases when there is a spillover of transmission from hotspots to surrounding areas. Increasingly, pathogen genotyping23,47 and social network and mobility data48 have been used to understand the transmission of disease across spatial gradients.
Several recent studies have documented how hotspots may serve as transmission sources for tuberculosis in the wider community. For example, using spatial and pathogen genetic data from Lima, Peru, Zelner et al. identified patterns of disease that were consistent with transmission of multidrug-resistant (MDR) tuberculosis from a hotspot to surrounding areas of the city.23 In Brazil, Sacchi et al. found that epidemiological and pathogen genotypic links suggest increased tuberculosis transmission within prisons, which extends into the communities surrounding the prison.47 Such data provide evidence that spillover from hotspots to the broader community occurs, but the extent to which this happens on a population level will influence the impact of spatially targeted case detection on tuberculosis incidence in the community. Understanding the genetic relatedness of tuberculosis isolates from within geographic hotspots and between those hotspots and lower burden areas may inform better estimates of the probable impact of targeted interventions, and new approaches for characterizing transmission events based on whole genome sequencing data may yield additional insights into these patterns.49
3). Is spatially targeted screening practical?
Spatially targeted screening strategies will be possible only if surveillance systems can reliably identify areas where tuberculosis incidence is most intense, distinguishing true variability in incidence from variability in population density, tuberculosis detection, or reporting. Improvements in tuberculosis surveillance systems are needed to improve effectiveness of interventions,50 yet many high burden countries have struggled to implement high-quality tuberculosis surveillance systems.51 If quality of surveillance is poor or variable within a country, routine tuberculosis notification may not adequately identify true spatial clusters of tuberculosis, especially as those most affected by tuberculosis often have the least access to tuberculosis services.52 Furthermore, even with increasingly cheap and accessible tools for production, processing, and analysis of spatial data,11 local expertise may need to be developed to assure data quality and reliable analysis and interpretation.
In addition, the costs of targeted screening interventions are uncertain. Hotspot identification may require improvements in routine surveillance or periodic surveys. Furthermore, targeted screening may decrease costs by focusing efforts on high-yield areas; alternatively, targeting could increase costs if it is expensive to identify and operate in hotspots due to transient populations, weak infrastructure and few local health care facilities from which to operate. Encouragingly, recent studies in challenging high burden settings such as Cambodia and South Africa have shown specific non-geographically targeted active case-finding strategies to be cost effective.53,54 A more generalized understanding of the costs associated with spatially targeted interventions will be crucial to understanding whether these approaches are a good investment.
Lastly, healthcare systems must be robust enough to properly manage additional cases discovered through active screening. Without capacity to definitively diagnose and treat individuals who screen positive for tuberculosis, resources expended on finding these additional cases will be wasted.
4). When is spatially targeted screening culturally appropriate?
Will the use of targeted strategies be acceptable to community members? Targeting of screening to specific communities may expose subpopulations to stigma and related social and economic costs.55 Alternatively, such targeting may galvanize communities at risk to take actions that could facilitate better disease control, as Moonan et al. found when targeting high risk neighborhoods in Texas.32
Active case-finding measures that reach out into the community will require substantial local support to succeed. Accordingly, we must better understand how targeting of case-finding to specific geographic areas is likely to be viewed and what approaches are most likely to be acceptable to target populations.
Beyond the desire to optimize tuberculosis screening to reap the most benefit from available resources, spatially targeted screening may address issues of equity. Tuberculosis is often associated with barriers to care, including poverty, malnutrition and poor housing, as well as other risk factors such as HIV;52 many of these factors are often spatially concentrated.14,56,57 By preferentially delivering screening services to areas where the burden of tuberculosis is highest, the needs of disadvantaged populations may be better addressed.
Conclusion
Analysis of surveillance data has revealed steep gradients in tuberculosis incidence across geography in many settings, including those with high HIV prevalence. While empirical evidence is sparse, models suggest that disease control strategies that preferentially target tuberculosis incidence hotspots could improve local control and may create indirect reductions in prevalence for the surrounding community by reducing transmission spillover. At this time, there are important questions to be answered about how effective active screening needs to be to lower levels of tuberculosis transmission in hotspots, and the degree to which the benefit of local control will spill over into surrounding areas. The feasibility of spatially targeted screening under programmatic conditions remains to be determined.
While we remain optimistic about the role of active case-finding and spatially targeted screening to accelerate tuberculosis control in high burden countries, we also recognize the need to invest in research to determine where, when, and how such strategies should be utilized. Ultimately, interventional trials of spatially targeted screening would be the definitive way to address many of the questions we raise here. Over the shorter timeframe, investing in smaller studies to use spatial and pathogen genetic data to understand local transmission dynamics and to estimate the costs and community acceptability of such interventions would move the field forward. We believe that spatially targeting screening can be an important component of new strategies to accelerate reductions in tuberculosis incidence and modest investments in research could rapidly improve our ability to identify areas where such targeting will be most effective.
Figure 1:
Systematic review study selection flow diagram
Key Points:
-Modelled analyses show that spatially targeted strategies for tuberculosis screening could help reduce local disease transmission as well as have beneficial effects outside the targeted areas, but empiric evidence in high burden settings is lacking.
-Questions remaining about the effectiveness of targeted strategies include 1) the effectiveness of active tuberculosis screening for reducing transmission in high burden settings, 2) the degree to which hotspots of tuberculosis disease produce spillover risk for surrounding communities, 3) the challenges and costs of implementing spatial targeting in programmatic settings, and 4) the community acceptability and ethical ramifications of targeting populations for additional screening.
-Due to differences in local transmission dynamics, lessons learned about the effectiveness of spatially targeted strategies from low tuberculosis incidence settings may not be directly applicable to high incidence settings.
Acknowledgements
The authors wish to thank Mark Gentry and Alexandria Brackett for their assistance with the literature search.
Funding Source
This publication and workshop have been funded in whole or in part with Federal Funds from the Division of AIDS, National Institute of Allergy and Infectious Disease (NIAID), US National Institutes of Health (NIH), Department of Health and Human Services under contract number HHSN272201600001G Research Support Services for the Division of AIDS. Funding support was also provided in part by NIH grants R01AI112438 to TC, TW009338 and T32AI007517 to PGTC, and T32 AI007433 to AB. The South African Medical Research Council co-hosted the event. The Bill and Melinda Gates foundation provided funding support for some participants travel to the workshop. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Declaration of Interests
We declare that we have no conflicts of interest.
References
- 1.World Health Organization. Global Tuberculosis Report 2017 Geneva: World Health Organization, 2017. [Google Scholar]
- 2.Boehme CC, Nabeta P, Hillemann D, et al. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance. N Engl J Med 2010; 363: 1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathavitharana RR, Cudahy PGT, Schumacher SG, Steingart KR, Pai M, Denkinger CM. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. Eur Respir J 2017; 49: 1601075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet Lond Engl 2014; 383: 424–35. [DOI] [PubMed] [Google Scholar]
- 5.Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health 2015; 3: e450–e457. [DOI] [PubMed] [Google Scholar]
- 6.Glynn JR, Crampin AC, Yates MD, et al. The Importance of Recent Infection with Mycobacterium tuberculosis in an Area with High HIV Prevalence: A Long-Term Molecular Epidemiological Study in Northern Malawi. J Infect Dis 2005; 192: 480–7. [DOI] [PubMed] [Google Scholar]
- 7.Houben RMGJ, Glynn JR. A systematic review and meta-analysis of molecular epidemiological studies of tuberculosis: development of a new tool to aid interpretation. Trop Med Int Health 2009; 14: 892–909. [DOI] [PubMed] [Google Scholar]
- 8.Kranzer K, Afnan-Holmes H, Tomlin K, et al. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review [State of the art series. Case finding/screening. Number 2 in the series]. Int J Tuberc Lung Dis 2013; 17: 432–46. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization, editor. Systematic screening for active tuberculosis: principles and recommendations Geneva, Switzerland: World Health Organization, 2013. [PubMed] [Google Scholar]
- 10.World Health Organization. Systematic screening for active tuberculosis: an operational guide 2015. http://apps.who.int/iris/bitstream/10665/181164/1/9789241549172_eng.pdf?ua=1 (accessed Sept 26, 2017).
- 11.Caprarelli G, Fletcher S. A brief review of spatial analysis concepts and tools used for mapping, containment and risk modelling of infectious diseases and other illnesses. Parasitology 2014; 141: 581–601. [DOI] [PubMed] [Google Scholar]
- 12.Worrell MC, Kramer M, Yamin A, Ray SM, Goswami ND. Use of Activity Space in a Tuberculosis Outbreak: Bringing Homeless Persons into Spatial Analyses. Open Forum Infect Dis 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S, Cook AR, Wah W, et al. Spatial dynamics of TB within a highly urbanised Asian metropolis using point patterns. Sci Rep 2017; 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alene KA, Viney K, McBryde ES, Clements ACA. Spatial patterns of multidrug resistant tuberculosis and relationships to socioeconomic, demographic and household factors in northwest Ethiopia. PLoS ONE 2017; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazdani-Charati J, Siamian H, Kazemnejad A, Mohammad V. Spatial Clustering of Tuberculosis Incidence in the North of Iran. Glob J Health Sci 2014; 6: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munch Z, Van Lill SWP, Booysen CN, Zietsman HL, Enarson DA, Beyers N. Tuberculosis transmission patterns in a high-incidence area: a spatial analysis. Int J Tuberc Lung Dis 2003; 7: 271–7. [PubMed] [Google Scholar]
- 17.Cohen T, Manjourides J, Hedt-Gauthier B. Linking Surveillance with Action against Drug-Resistant Tuberculosis. Am J Respir Crit Care Med 2012; 186: 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theron G, Jenkins HE, Cobelens F, et al. Data for action: Collecting and using local data to more effectively fight tuberculosis. Lancet Lond Engl 2015; 386: 2324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowdy DW, Azman AS, Kendall EA, Mathema B. Transforming the Fight Against Tuberculosis: Targeting Catalysts of Transmission. Clin Infect Dis 2014; 59: 1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassili A, Al-Hammadi A, Al-Absi A, et al. Estimating the tuberculosis burden in resource-limited countries: a capture-recapture study in Yemen. Int J Tuberc Lung Dis 2013; 17: 456–61. [DOI] [PubMed] [Google Scholar]
- 21.Fox GJ, Dobler CC, Marks GB. Active case finding in contacts of people with tuberculosis. Cochrane Database Syst Rev 2011; CD008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox GJ, Nhung NV, Sy DN, et al. Household-Contact Investigation for Detection of Tuberculosis in Vietnam. N Engl J Med 2018; 378: 221–9. [DOI] [PubMed] [Google Scholar]
- 23.Zelner JL, Murray MB, Becerra MC, et al. Identifying Hotspots of Multidrug-Resistant Tuberculosis Transmission Using Spatial and Molecular Genetic Data. J Infect Dis 2016; 213: 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Zhang WS, Alayi A, Yan C, Zhang WW, Cao MQ. The Characteristics of TB Epidemic and TB/HIV Co-Infection Epidemic: A 2007–2013 Retrospective Study in Urumqi, Xinjiang Province, China. Plos One 2016; 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge E, Lai PC, Zhang X, et al. Regional transport and its association with tuberculosis in the Shandong province of China, 2009–2011. J Transp Geogr 2015; 46: 232243. [Google Scholar]
- 26.Subbaraman R, Nathavitharana RR, Satyanarayana S, et al. The Tuberculosis Cascade of Care in India’s Public Sector: A Systematic Review and Meta-analysis. PLOS Med 2016; 13: e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naidoo P, Theron G, Rangaka MX, et al. The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges. J Infect Dis 2017; 216: S702–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fettig J, Swaminathan M, Murrill CS, Kaplan JE. Global Epidemiology of HIV. Infect Dis Clin North Am 2014; 28: 323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. World malaria report: 2016 Geneva: World Health Organization, 2016. [Google Scholar]
- 30.Shapiro AE, Chakravorty R, Akande T, Lönnroth K, Golub JE. A systematic review of the number needed to screen to detect a case of active tuberculosis in different risk groups. World Health Organ 2013. http://www.who.int/entity/tb/Review3NNS_case_active_TB_riskgroups.pdf?ua=1&ua=1. [Google Scholar]
- 31.Vandelaer J, Bilous J, Nshimirimana D. Reaching Every District (RED) approach: a way to improve immunization performance. Bull World Health Organ 2008; 86: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moonan PK, Oppong J, Sahbazian B, et al. What is the outcome of targeted tuberculosis screening based on universal genotyping and location? Am J Respir Crit Care Med 2006; 174: 599–604. [DOI] [PubMed] [Google Scholar]
- 33.Goswami ND, Hecker EJ, Vickery C, et al. Geographic information system-based screening for TB, HIV, and syphilis (GIS-THIS): a cross-sectional study. PloS One 2012; 7: e46029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cegielski JP, Griffith DE, McGaha PK, et al. Eliminating Tuberculosis One Neighborhood at a Time. Am J Public Health 2013; 103: 1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowdy DW, Golub JE, Chaisson RE, Saraceni V. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci 2012; 109: 9557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bousema T, Stresman G, Baidjoe AY, et al. The Impact of Hotspot-Targeted Interventions on Malaria Transmission in Rachuonyo South District in the Western Kenyan Highlands: A Cluster-Randomized Controlled Trial. PLOS Med 2016; 13: e1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bousema T, Griffin JT, Sauerwein RW, et al. Hitting Hotspots: Spatial Targeting of Malaria for Control and Elimination. PLOS Med 2012; 9: e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassels A, Heineman E, LeClerq S, Gurung PK, Rahut CB. Tuberculosis case-finding in Eastern Nepal. Tubercle 1982; 63: 175–85. [DOI] [PubMed] [Google Scholar]
- 39.Corbett EL, Bandason T, Duong T, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. The Lancet 2010; 376: 1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayles H, Muyoyeta M, Toit ED, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. The Lancet 2013; 382: 1183–94. [DOI] [PubMed] [Google Scholar]
- 41.Meehan CJ, Moris P, Kohl TA, et al. The relationship between transmission time and clustering methods in Mycobacterium tuberculosis epidemiology. bioRxiv 2018; 302232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribeiro FKC, Pan W, Bertolde A, et al. Genotypic and Spatial Analysis of Mycobacterium tuberculosis Transmission in a High-Incidence Urban Setting. Clin Infect Dis 2015; 61: 758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Middelkoop K, Mathema B, Myer L, et al. Transmission of Tuberculosis in a South African Community With a High Prevalence of HIV Infection. J Infect Dis 2015; 211: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chamie G, Wandera B, Marquez C, et al. Identifying locations of recent TB transmission in rural Uganda: a multidisciplinary approach. Trop Med Int Health TM IH 2015; 20: 537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee RS, Radomski N, Proulx J-F, et al. Population genomics of Mycobacterium tuberculosis in the Inuit. Proc Natl Acad Sci 2015; 112: 13609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang C, Lu L, Warren JL, et al. Internal migration and transmission dynamics of tuberculosis in Shanghai, China: an epidemiological, spatial, genomic analysis. Lancet Infect Dis 2018; published online April 19 DOI: 10.1016/S1473-3099(18)30218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacchi FPC, Praça RM, Tatara MB, et al. Prisons as reservoir for community transmission of tuberculosis, Brazil. Emerg Infect Dis 2015; 21: 452–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wesolowski A, Eagle N, Tatem AJ, et al. Quantifying the impact of human mobility on malaria. Science 2012; 338: 267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Didelot X, Fraser C, Gardy J, Colijn C. Genomic Infectious Disease Epidemiology in Partially Sampled and Ongoing Outbreaks. Mol Biol Evol 2017; 34: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization, editor. TB impact measurement: policy and recommendations for how to assess the epidemiological burden of TB and the impact of TB control Geneva: World Health Organization, 2009. [Google Scholar]
- 51.van der Werf MJ, Borgdorff MW. Targets for tuberculosis control: how confident can we be about the data? Bull World Health Organ 2007; 85: 370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. Ethics guidance for the implementation of the end TB strategy Geneva: World Health Organization, 2017. http://apps.who.int/iris/bitstream/10665/254820/1/9789241512114-eng.pdf. [Google Scholar]
- 53.Yadav RP, Nishikiori N, Satha P, Eang MT, Lubell Y. Cost-Effectiveness of a Tuberculosis Active Case Finding Program Targeting Household and Neighborhood Contacts in Cambodia. Am J Trop Med Hyg 2014; 90: 866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kranzer K, Lawn SD, Meyer-Rath G, et al. Feasibility, Yield, and Cost of Active Tuberculosis Case Finding Linked to a Mobile HIV Service in Cape Town, South Africa: A Cross-sectional Study. PLoS Med 2012; 9: e1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Courtwright A, Turner AN. Tuberculosis and Stigmatization: Pathways and Interventions. Public Health Rep 2010; 125: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magalhães MAFM, Medronho RA. Spatial analysis of tuberculosis in Rio de Janeiro in the period from 2005 to 2008 and associated socioeconomic factors using micro data and global spatial regression models. Cienc E Saude Coletiva 2017; 22: 831–9. [DOI] [PubMed] [Google Scholar]
- 57.Souza WV, Ximenes R, Albuquerque MFM, et al. The use of socioeconomic factors in mapping tuberculosis risk areas in a city of northeastern Brazil. Rev Panam Salud Pública 2000; 8: 403–10. [DOI] [PubMed] [Google Scholar]