Abstract
The goal of this study was to develop a molecular biomarker for the detection of protein aggregation involved in Alzheimer’s disease (AD) by exploiting the features of the water saturation transfer spectrum (Z-spectrum), the CEST signal of which is sensitive to the molecular configuration of proteins. A radial-sampling steady-state sequence based ultrashort echo time (UTE) readout was implemented to image the Z-spectrum in the mouse brain, especially the contributions from mobile proteins at the frequency offsets for the composite protein amide proton (+3.6ppm) and aliphatic proton (−3.6 ppm) signals. Using a relatively weak radiofrequency (RF) saturation amplitude, contributions due to strong magnetization transfer contrast (MTC) from solid-like macromolecules and direct water saturation (DS) were minimized. For practical measure of the changes in the mobile protein configuration, we defined a saturation transfer difference (ΔST) by subtracting the Z-spectral signals at ±3.6 ppm from a control signal at 8 ppm. Phantom studies of glutamate solution, protein (egg white) and hair conditioner show the capability of the proposed scheme to minimize the contributions from amine protons, DS, and MTC, respectively. The ST signal at ±3.6 ppm of the cross-linked bovine serum albumin (BSA) solutions demonstrated that the ΔST signal can be used to monitor the aggregation process of the mobile proteins. High-resolution ΔST images of AD mouse brains at ±3.6 ppm of mouse brains showed significantly reduced ΔST(−3.6) signal compared to the age-matched wild-type (WT) mice. Thus, this signal has potential to serve as a molecular biomarker for monitoring protein aggregation in AD.
Keywords: Chemical Exchange Saturation Transfer (CEST), Magnetization Transfer Contrast (MTC), Alzheimer’s Disease (AD), Saturation Transfer (ST), Radial Acquisition, UTE-CEST
Introduction
Alzheimer’s disease (AD) is a neurological disorder that is pathologically characterized by the accumulation of neuritic plaques comprised of an amyloid-beta (Aβ) peptide core, surrounded by dystrophic neurites, astrocytes and microglia (Hardy and Selkoe, 2002) as well as neurofibrillary tangles (NFT) composed of hyperphosphorylated tau peptide (Ballatore et al., 2007; Irvine et al., 2008; Lee and Trojanowski, 1999; Li et al., 2016; Rojas and Boxer, 2016). Early diagnosis of AD would have a significant impact on its management and may allow for early therapeutic interventions (Baxter et al., 2007; Schenk et al., 1999; Walker and Rosen, 2006). The detection of the aggregation process of proteins involved in AD is a potential strategy for early diagnosis of the disease and is also important for dynamically monitoring the effects of new therapeutic agents on the clearance of such protein aggregates or aggregate precursors. Magnetic resonance imaging (MRI), as a non-invasive imaging technique, has been used for detailed visualization of the medial temporal lobe (MTL) structures as a core diagnostic feature of AD (Burton et al., 2009; Scheltens et al., 1992) but has limited molecular imaging capability because of its low detection sensitivity. Recently, significant progress has been made to visualize Aβ plaques by MRI in transgenic mouse models using T2 or T2* contrast owing to the accumulation of iron in amyloid plaques (Chamberlain et al., 2009; Faber et al., 2007; Jack et al., 2004; Vanhoutte et al., 2005; Wadghiri et al., 2003; Zhang et al., 2004). However, such methods often only work in later stages of AD (Chamberlain et al., 2009; Vanhoutte et al., 2005; Zhang et al., 2004). More recent studies have employed quantitative magnetic susceptibility mapping (QSM) to show co-localization of iron and amyloid in mild cognitive impairment (MCI) (van Bergen et al., 2016). Here, we aim to develop a novel molecular biomarker for very early detection of AD using chemical exchange saturation transfer (CEST) MRI (Chen and Pagel, 2015; Liu et al., 2013; van Zijl and Yadav, 2011; Ward et al., 2000), which has emerged as a novel molecular imaging technique for detecting low-concentration metabolites and proteins (Zhou et al., 2003a; Zhou et al., 2003b) through the water signal.
The saturation transfer (ST) spectrum (Z-spectrum) (van Zijl et al., 2018) of water has composite components from amide protons (at a frequency offset of +3.6ppm) and aliphatic protons (relayed nuclear Overhauser enhancement or rNOE signals around −3.6 ppm) of mobile proteins (Jones et al., 2013; Ling et al., 2008; van Zijl and Yadav, 2011; Xu et al., 2014; Zaiss et al., 2017). Importantly, the line-shape of the Z-spectrum is sensitive to the molecular size and configuration of proteins (broadening with increasing size and aggregation) (Goerke et al., 2015a; Zaiss et al., 2013) and, hence, has great potential for monitoring protein aggregation in AD patients. Before the formation of plaques and tangles, both Aβ and tau are relatively small molecules with rapid internal motions and molecular tumbling. These motions average out several molecular orientation-based interactions between the protons in these molecules, such as through-space dipole-dipole interaction, chemical shift anisotropy and quadrupolar interaction, the main line broadening factors in the MR spectrum. This leads to an MR spectrum and a corresponding well-defined Z-spectrum for small peptides in which the resonances of the different contributing protons can be distinguished. Once the Aβ and tau monomers form oligomers and subsequent plaques and tangles, the above interactions will increase owing to the slower internal and tumbling motions, significantly broadening the spectral line-shape (Cavanagh et al., 2006; Zaiss et al., 2013). This phenomenon has been used in solutions to monitor protein folding as well as protein aggregation of Aβ peptide and Huntingtin protein (Goerke et al., 2015a; Zaiss et al., 2013). However, it has yet to be verified and implemented in vivo, where many confounding factors are present. A major challenge for in vivo CEST MRI is to minimize the interferences from direct water saturation (DS) and conventional magnetization transfer contrast (MTC) induced by solid-like macromolecules in normal tissues (Helms et al., 2004; Hua et al., 2007; Levesque and Pike, 2009; Ng et al., 2009; Portnoy and Stanisz, 2007; Tozer et al., 2003). In addition, high-resolution MRI is expected to be necessary for early detection of the initial burden of small plaques and tangles. In the current study, we implemented a two-dimensional ultrashort echo time (UTE) based steady-state CEST scheme (UTE-CEST) and applied this technique to monitor protein aggregation in an AD transgenic mouse model (APPswe;PS1ΔE9 mouse). A radial acquisition scheme has been shown to have great potential for suppressing image artifacts due to motion and susceptibility differences at tissue interfaces (Du et al., 2014; Fan et al., 2018; Ma et al., 2018b; Wilhelm et al., 2012) and a similar radial MT technique with short echo time has been applied to study tissues with extremely short T2 components such as Achilles tendon and cortical bone (Du et al., 2009; Ma et al., 2018a). Recently, such a scheme was also implemented for quantitative susceptibility mapping (QSM) of brain (Lu et al., 2018; Wei et al., 2018). In this paper, the method was optimized on phantoms for MTC (hair conditioner), mobile protein (egg white and cross-linked bovine serum albumin (BSA)) and glutamate (Glu) solutions, in order to verify that this technique is sensitive enough to detect protein aggregation in AD mice by measuring the differences in Z-spectral intensities between 8 ppm reference frequency and the protein main frequencies of the composite amide (3.6 ppm) and rNOE (−3.6 ppm) signals, and comparing the differences between an AD mouse model and its healthy littermates.
Materials and methods
UTE-CEST Sequence
The pulse sequence was composed of a train of interleaved Gaussian saturation pulses and 2D-UTE modules that implement radial acquisition as illustrated in Fig. 1A, which resembles the steady-state pulsed CEST and MTC techniques (Dixon et al., 2010; Du et al., 2009; Jones et al., 2013). Compared to its Cartesian counterpart, a radial acquisition scheme is inherently less sensitive to motion artifacts (Glover and Lee, 1995; Glover and Pauly, 1992). This advantage originates from: (i) over-sampling of the low-frequency region in k-space resulting in an averaging of artifacts, and (ii) the fact that artifacts are manifested in the form of streaks perpendicular to the direction of motion (Glover and Pauly, 1992a), rather than discrete ghosts along the phase-encoding direction. Adopting the self-gating property of the radial acquisition scheme can further enhance the resistance to motion artifacts (Larson et al., 2004; Seo et al., 2017). Secondly, the radical acquisition can achieve short TE, which leads higher signal-to-noise by detecting all water components with a wide range of T2 values in brain, including bound water in myelin sheets (Wilhelm et al., 2012). Compared to the other gradient echo based sequences with long TE, brain images with short TE usually show weak contrast across the brain due to the short TE and low readout flip angles applied that lead to T1 or proton density weighted contrast. This allows better visualization of the pure CEST contrast due to the RF preparation scheme for CEST MRI.
Fig 1.
(A) Timing diagram of the UTE-CEST sequence. τp is the duration of the saturation pulses, while τmix is the mixing time for saturation transfer between successive saturation pules. (B-E) Flowchart of the drift correction for the ΔST map: (B) typical ST signal intensities as a function of time for four interleaved labeling (offset ±3.6 ppm) and control (offset 8 ppm) images. The first data points before reaching steady state for labeling and control images are indicated by red crosses (x); (C) ST signal after drift correction. (D & E) ΔST maps with and without drift correction.
The duration of the Gaussian saturation pulses was set to 30 ms with a peak B1 of 0.9 μT unless specified, and the repetition time (TR) was 40 ms. The excitation pulse for the 2D-UTE radial readout was a 0.3 ms Gaussian pulse with a peak B1 of 7 μT, which results in a flip angle of 15 degrees. Here, a Gaussian pulse was chosen due to its small bandwidth factor (2740 Hz for a 1 ms pulse). The effective echo time (TE) was 0.3 ms. The acquisition bandwidth was 100 kHz and gradient ramp time was 100 μs. After each saturation pulse, one radial spoke was collected. Each radial spoke was sampled from the center of k-space. In the current study, single slice CEST images were collected. The nominal Cartesian matrix size was set to 96×96 with a resolution of 0.17 ×0.17 mm2 and a slice thickness of 1 mm. For half-echo acquisition mode, the number of spokes is equal to the number of nominal Cartesian phase encoding lines multiplied by π, i.e., 96*π ≈ 302. The number of sampling points is equal to half of nominal Cartesian sampling points plus 6 ramp sampling points, i.e. 96/2+6 = 54. The total saturation time for each offset is 12 s. The width of the saturation pulse should be large enough to achieve good selectivity and minimize direct water saturation when using low B1. However, a long pulse width increases the TR and prolongs the total experimental time. In practice, we found that a 30 ms pulse width was enough to avoid the direct water saturation at the offsets of ±3.6 ppm for a peak B1 of 0.9 μT. In vivo, although the utilization of low saturation amplitude can also significantly suppress the MTC background in the Z-spectrum, there is a small remaining effect. As demonstrated in a previous study (Chen et al., 2017), this remaining effect may cause a vertical shift of the whole Z-spectrum between mice. The problem was addressed by collecting control images at the offset of 8 ppm, while the labeling ST images were acquired with the saturation offsets at ±3.6 ppm. Evidence for this being suitable came from MTC phantoms at this amplitude showing approximately constant MTC between 8 ppm and ±3.6 ppm (see phantom results) (Yadav et al., 2017). The observed saturation transfer difference (ΔST) signals obtained by subtracting the signal intensities at 8 ppm (Z(8)) and ±3.6 ppm (Z(±3.6)), i.e.,
| (1) |
thus have a high degree of MTC background suppression, allowing us to focus on changes in the mobile proteins and peptides. As a comparison, the conventional ST signal at (±3.6) is defined by
| (2) |
A peak B1 of 0.9 μT (30 ms width), corresponding to a 180-degree flip angle for the amide and aliphatic protons, theoretically can generate the highest amide and aliphatic CEST signals because the spins are inverted (Knutsson et al., 2018; Sun et al., 2008; van Zijl and Sehgal, 2016; Zu et al., 2012). Here, the flip angle for a Gaussian shaped pulse is calculated by , where tp is the pulse width and γ the proton gyromagnetic ratio. The signal-to-noise ratio (SNR) of the ΔST maps can be increased by dynamically acquiring successive labeling (offsets ±3.6 ppm) and control (offset 8 ppm) images, calculating the difference between labeling and control images and adding them up. However, this scheme is not suitable for UTE-CEST and may lead to an underestimated value of the ΔST map because the magnetization cannot reach steady state in the successive labeling and control experiments. In the current study, an improved strategy was proposed inspired by the commonly used idea in fMRI (Kwong et al., 1992), i.e., a train of labeling images is interleaved with a train of control images as shown in Fig. 1B. The number of replications for labeling and control images was set to four. As shown in Fig. 1B, the first point of each labeling or control image is inaccurate owing to violation of the steady state. After the first data point, the ST signal approximately reaches a steady-state condition. In order to minimize the steady-state error of the ΔST maps, such first points were discarded (red crosses in Fig. 1B). In practice, this multi-label scheme may suffer from heating effects due to gradient switching and radio frequency, leading to a slow B0-based drift in the time series data (Jones et al., 2013). This low-frequency drift in the time series data is quite similar to that in fMRI (Smith et al., 1999). The true signal can be separated from this drift by applying a high-pass frequency filter to the time series data (see the simulation in the supplemental material) (Kwong et al., 1992; Turner et al., 1998). The high-pass filter was accomplished by ‘spm_filter’, a built-in function of SPM (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). There are three parameters to configure the function, i.e. the observation interval in seconds, time of observation data and cut-off period. In this study, the observation interval was set equal to the repetition time (12 s). The time of observation data was increased linearly with the step size of the observation interval. The whole acquisition time was used as the cut-off period, which is equal to the observation interval (repetition time) multiplied by the repetition number. The ΔST maps are obtained by calculating the average difference between the labeling and control images.
Image Reconstruction
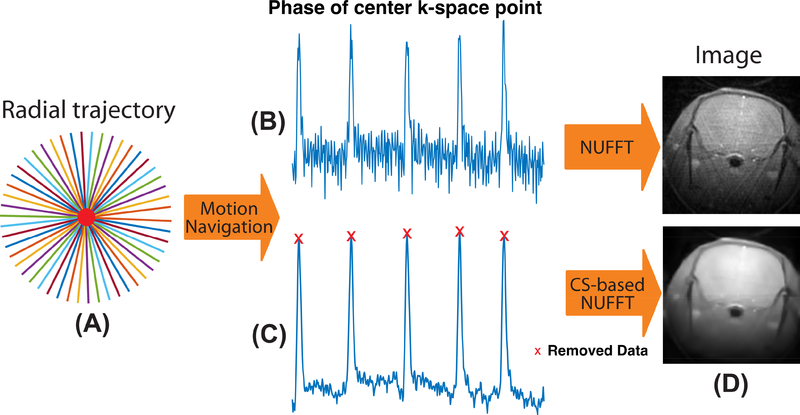
Another advantage of radial acquisition is the ability for self-gating, allowing motion synchronization using the acquired MRI signals (Bonanno et al., 2018; Larson et al., 2004; Seo et al., 2017; Yang et al., 2016). The center of the k-space is repeatedly sampled in each radial spoke, of which the phase information can be used as a navigation to detect the data degraded by bulk motion. As shown in Fig. 2, motion can lead to obvious jumps in the phase navigation signal. The degraded signals acquired during motion were discarded to avoid potential motion artifacts. Non-uniform fast Fourier transform (NUFFT) is a commonly used method to reconstruct non-Cartesian MRI data (Fessler and Sutton, 2003), which consists of the following steps: density compensation, convolution and resampling, inverse FFT and apodization. The density compensation is aimed at reducing the effects of non-uniform sampling density, which can be done by multiplying the acquired radial data with the inverse density sampling function. The convolution and resampling are used to transform the non-Cartesian data into Cartesian form. The weighted radial data are convolved with a Kaiser-Bessel kernel, resampled onto a Cartesian grid. The resampled data are transformed into the image space using inverse FFT and apodization operation. The kernel size was equal to the nominal Cartesian matrix size. The kernel width and overgrid factor were set to 2 and 1.5, respectively.
Fig 2.
Flowchart of image reconstruction for the radial trajectory. (A) in UTE MRI. (B) The center of k-space point is repeatedly sampled in each spoke, of which the phase can be used for the motion navigation. (C) The navigation signal is denoised and spokes affected by motion are removed. (D) The images reconstructed by NUFFT and CS-based NUFFT.
The discarding of degraded spokes may lead to an under-sampled k-space and induce undesirable aliasing artifacts in the image. Compressed sensing (CS) has the ability to retrieve an aliasing free image from undersampled k-space data by exploiting the sparsity of images in the specific transform domain (Block et al., 2007; Lustig et al., 2007). In this study, CS with total variation and wavelet transforms were used to enhance the image quality. A discrete form of the CS-based Non-Uniform FFT (NUFFT) reconstruction can be described as:
| (3) |
where Fn stands for the NUFFT operation; x is the reconstructed image; y is the acquired data after motion navigation and density compensation; Ψ refers to the wavelet transform and ∇ stands for the finite difference transform. ∥·∥1 stands for the l1 norm that enhances the sparsity coefficients in the transform domain and ∥·∥2 stands for the l2 norm that keeps the fidelity between the reconstructed image and acquired data. The regularization parameters λ1 and λ2 control the tradeoff between sparsity and data fidelity. In practice, setting the λ1 and λ2 to 1×10−3 can always obtain a desirable result.
Animals
Eight animals with an age of one year were used for the current study, in which four of them were APPswe;PS1ΔE9 mice (Jankowsky et al., 2003) (APP mice), while another four were age-matched C57BL/6J mice (WT mice). The APP transgenic mice were bred at Johns Hopkins University and co-expressed the Swedish variant of the amyloid precursor protein (APPswe) and the exon-9 deleted variant of presenilin1 (PS1ΔE9). The onset of neurotic plaques in biogenic APP mice typically starts at four to six months of age (Jankowsky et al., 2003; Li et al., 2016). The image slice was located by collecting one sagittal image on the mouse brain using the RARE sequence and the position of the axial image slice was set to −1.4 mm with respect to the anterior commissure (AC).
MRI Experiments
All MRI experiments were performed on a horizontal bore 11.7 T Bruker Biospec system (Bruker, Ettlingen, Germany) equipped with actively shielded gradients, with a maximum strength of 74 Gauss/cm. For the in vivo animal MRI, a 72 mm quadrature volume resonator was used as a transmitter and a 2×2 mouse phased array coil for acquisition. For phantoms, a 23 mm volume coil was used for both transmit and receive. All coils were from Bruker. The institutional animal care and use committee approved this study. All animals were anesthetized using 2% isoflurane in medical air, followed by 1% to 1.5% isoflurane for maintenance during the MRI scan. The mouse head was positioned using a bite bar and two ear pins. During the MRI scan, mice were placed on a water-heated animal bed equipped with temperature and respiratory controls. Respiratory rate was monitored via a pressure sensor (SAII, Stony Brook, NY, USA) and maintained at 40–60 breaths per minute. The B0 field over the mouse brain was adjusted using field-mapping and second-order shimming.
In vitro Experiments
Two sets of phantoms (consisting of groups of 5 mm NMR tubes) were used to demonstrate the sensitivity of the UTE-CEST method for detecting protein aggregation. Egg white and hair conditioner (Suave, Estee Lauder) were selected to represent mobile protein and MTC pools, respectively. The hair conditioner contains a lamellar structure similar to membranes in neural tissues (Malyarenko et al., 2014; Varma et al., 2015). Previous studies suggest that, at low B1, the metabolite contribution in the Z-spectrum of brain is far smaller than those from protein and lipids (Chen et al., 2017; Zu et al., 2017), and we therefore assumed the metabolite interference to the ΔST(3.6) signal to be negligible. A glutamate (Glu, 50 mM) phantom was used to represent amine protons in metabolites and mobile proteins that have the potential to interfere with the background of the ΔST(3.6) signal. Except for the hair conditioner, phantoms were prepared in phosphate buffered saline (PBS) and titrated to pH 7.3±0.1.
The second series of phantoms were BSA solutions cross-linked at different temperatures to mimic protein aggregation in the brain. Tubes with BSA solution (10%w/w, pH 7.3) were incubated in heated water (60±1°C, 65±1°C, 70±1°C and 80±1°C) for ten minutes followed by placing the phantom in room temperature air for slow cooling. Once treated by this heat shock (HS), there are many types of components contained in the BSA solutions ranging from monomeric BSA to dimers, trimers and higher order polymers (Aoki et al., 1973). Hence, they can be used as a model to verify the ability of the protein ΔST(±3.6) signals for detecting protein aggregation.
In vivo UTE-CEST Experiments
First, full UTE-CEST Z-spectra were acquired on WT mice to verify that the saturation amplitude of 0.9 μT was sufficiently low to minimize the contributions of MTC and DS to the ΔST(±3.6) maps at 11.7 T. The saturation offset was swept from −15 ppm to 15 ppm with peak saturation amplitudes of 0.9 μT, 1.2 μT and 1.5 μT, which resulted in a total experimental time of 12 minutes for each saturation amplitude. The radial readout and the duration of the saturation pulses were identical for all experiments. The UTE-CEST protocol for recording ΔST(±3.6) maps at 0.9 μT was used to study the age-matched APP and WT mouse groups (1 year) to verify that the ΔST(±3.6) signal is capable of detecting protein aggregation in AD mouse brain. The ΔST(−3.6) map was recorded by averaging 16 images with interleaved offsets of −3.6 ppm and 8 ppm. Each offset was repeated four times. A similar acquisition scheme was also implemented for the ΔST(3.6) map. The total experimental time for the ΔST(−3.6) or ΔST(3.6) maps was 3.5 minutes.
Quantitative Magnetization Transfer (qMT) Experiments
The MT ratio (MTR) has been used as an overall measure of demyelination in brain, and its change has been measured for several neurologic conditions, such as mild cognitive impairment and dementia of Alzheimer’s type (Bozzali et al., 2001; Kabani et al., 2002; Mascalchi et al., 2013; Ridha et al., 2007). In order to examine possible contribution of MTC effects to the ΔST(±3.6) signal in the WT and APP mice, quantitative magnetization transfer (qMT) experiments were also performed on all APP and WT mice using the on-resonance variable delay multiple pulse (onVDMP) technique (van Gelderen et al., 2017; Xu et al., 2017; Xu et al., 2014). The qMT parametric maps were obtained by fitting the onVDMP buildup curves recorded using four binomial pulses (2 ms, 93.6 μT) with five mixing times (0, 25, 50, 150 and 250 ms). The total experimental time for the qMT experiment was 2 minutes. A two-pool model was assumed including the water and the macromolecular protons, respectively. Then, two parametric maps, the macromolecular pool to water exchange rate for the MTC pool (km) and the fractional concentration of the macromolecules relative to that of the water protons (fm), were calculated.
T1/T2 Relaxation Time Measurements
In order to examine the possible contribution of T1 and T2 relaxation times to the difference we observed in the ΔST(±3.6) signal between WT and APP mice, T1 and T2 measurements were also performed on all mice. T1 maps were acquired using the same geometry and spatial resolution as CEST MRI using a RAREVTR sequence (RARE with variable TR =0.5, 1, 1.5, 2, 3.5, 5, 8 s), while the T2 map was obtained by a multi-slice multi-echo (MSME) MRI with geometry identical to that of the T1 map measurement and an echo space of 10 ms.
Data Analysis
All MRI Images were processed using custom-written MATLAB scripts (MathWorks, www.mathworks.com). When processing the radial images, Savitzky-Golay filtering was used to smooth the phase navigation signal, which was implemented using the Matlab built-in function “sgolayfilt” with polynomial order of two and frame size of seven. A baseline correction method named BEADS (Baseline Estimation And Denoising with Sparsity) was used to remove baseline drift in the phase navigation signal (Ning et al., 2014). A local peak searching algorithm was used to find the peaks and corresponding radial spokes, which is accomplished by the Matlab built-in function “findpeaks”. The degraded radial spokes were discarded to avoid potential motion artifacts. In the qMT study, a Matlab script from the literature was used for calculating the parametric maps (Xu et al., 2017). A two-sample t test was performed between the WT and AD mice groups using the MATLAB build-in function “ttest” and was considered statistically significant for p<0.05 and highly significant when p<0.001.
Results
Phantom Experiments
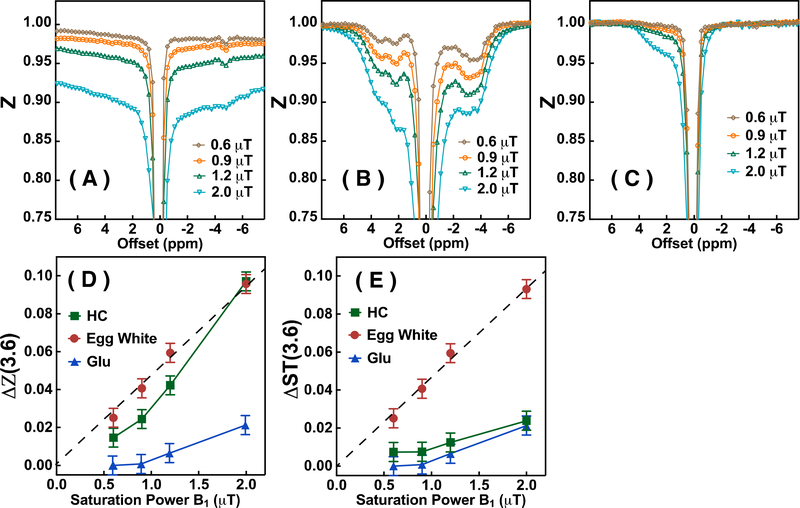
The Z-spectra (Ssat/S0) of hair conditioner (HC), egg white and Glu solution phantoms acquired with various saturation amplitudes (B1) are plotted in Figs. 3A-C, respectively. Broad Z-spectra are observed for the HC phantom (Fig. 3A) with the signal intensity at larger offsets (outside the solution proton spectral region between −5 and 5 ppm) still not unity. The HC Z-spectra are asymmetric with respect to the water offset, an effect that becomes more apparent at higher B1 due to the quadratic increase in MTC effect with B1. In egg white, the model for mobile proteins and peptides (Fig. 3B), the CEST signal represented by the water signal reduction due to saturation transfer ΔZ (Eq. 2), is observed only over the solution proton spectral range from −5 ppm to 5 ppm. The same phenomenon is observed in the Glu solution, with a broad resonance within the range −1 ppm to 3.6 ppm (Fig. 3C). The saturation amplitude dependent water signal reductions (Eq. 2) at 3.6 ppm, ΔZ(3.6), for HC, egg white and Glu solution are plotted in Fig. 3D. It can be seen that the ΔZ(3.6) for the egg white is linearly dependent on the saturation amplitude in the B1 range from 0 to 2 μT, while the ΔZ(3.6) of HC is not. The ΔZ(3.6) of the Glu solution is negligible at saturation amplitudes below 1.0 μT. Therefore, the contamination from the amine protons in the mobile protein Δ3.6 signal can be minimized by applying saturation amplitudes lower than 0.9 μT. When subtracting the Z-spectral signals at 8 ppm and 3.6 ppm to obtain ΔST(3.6), the values (Fig. 3E) are identical to the ΔZ(3.6) in Fig. 3D for egg white and Glu, because of the negligible saturation for these two phantoms at 8 ppm. On the other hand, the ΔST(3.6) signal of HC is significantly smaller (0.7% at 0.9 μT, Fig. 3E) compared to the ΔZ(3.6) signal (2.4% at 0.9 μT, Fig. 3D), which indicates that the proposed method, i.e., subtracting the signal between 8 ppm and ±3.6 ppm of the Z-spectrum, can suppress the main part of the MTC contributions.
Fig 3.
The Z-spectra of (A) hair conditioner (HC), (B) egg white and (C) Glu solution acquired by UTE-CEST MRI with different saturation amplitudes (0.6 μT, 0.9 μT, 1.2 μT and 2.0 μT). TR=40 ms, TE=0.3 ms and matrix size 96×96 were used for the experiments. (D) The water signal reduction at 3.6 ppm (ΔZ(3.6) for HC, egg white and Glu solution as a function of saturation amplitude. (E) The ΔST(3.6) signal of egg white, HC and Glu solution obtained by taking the signal difference between offsets 3.6 ppm and 8 ppm plotted as a function of saturation amplitude. The dashed line is a linear least square fitted curve for the ΔST(3.6) signal (Eq.1) of egg white (y = 0.047x).
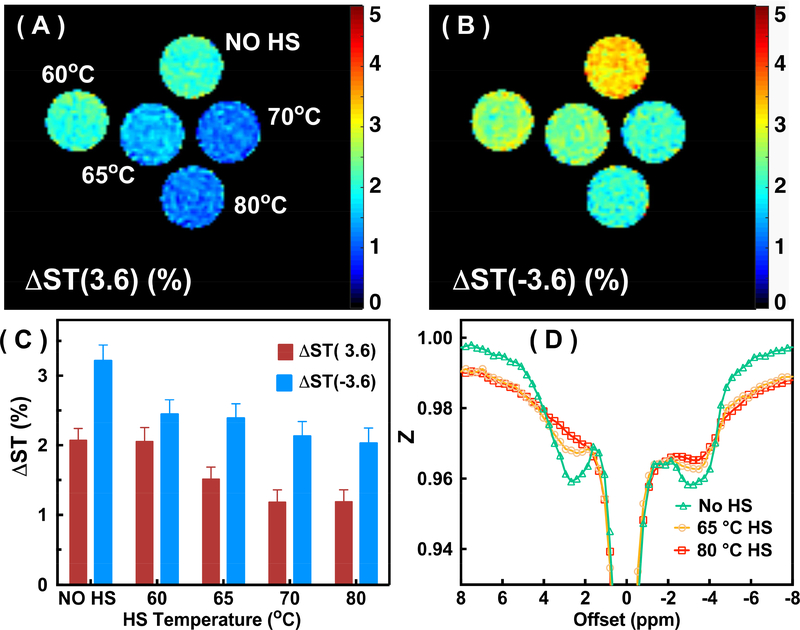
The ΔST(±3.6) maps and mean values for the BSA phantoms before and after HS at different temperatures are illustrated in Figs. 4A-C. After the BSA protein was cross-linked, both the ΔST(3.6) and ΔST(−3.6) signals dropped significantly. The ΔST(3.6) values dropped from 2.1±0.08% (no HS) to 1.2±0.08% (HS at 80°C) (P<0.001), while the ΔST(−3.6) values dropped from 3.2±0.1% (no HS) to 2.0±0.1% (HS at 80°C) (P<0.001). The decrease of the ΔST(±3.6) values as a function of HS temperature indicates that the ratio between cross-linked protein and the monomeric BSA increases as a function of the HS temperature. The ΔST(±3.6) shows only a small difference between 70ºC (ΔST(3.6) = 1.2%; ΔST(−3.6) = 2.1%) and 80ºC (ΔST(3.6) = 1.2%; ΔST(−3.6) = 2.0%), which suggests that the majority of protein has already been cross-linked at 70ºC. The typical full Z-spectra obtained by UTE-CEST MRI for the BSA phantoms before and after HS are plotted in Fig. 4D. We can see that all saturation effects for signals between −5 ppm and 5 ppm decrease substantially after the HS. At the same time, there is an increase in broad MTC-based signal saturations at −8 and 8 ppm induced by cross-linking the protein. These combined factors cause the significant reduction of ΔST(±3.6)when cross-linking the protein.
Fig 4.
The ΔST(3.6) (A) and ΔST(−3.6) (B) maps for the BSA solutions without heat shock (HS) (No HS) and after HS treatment at 60°C, 65°C, 70°C and 80°C. The maps were recorded using the UTE-CEST method with the parameters given in Fig. 3, and the ΔST values were calculated by subtracting the signals at 8 ppm and ±3.6 ppm of the Z-spectrum. (C) The ΔST(±3.6) values for the different tubes. (D) Z-spectra of the BSA solution before and after HS treatments (at 65°C and 80°C). Notice the signal reduction in the proton spectral range for mobile components and the broadening of the background signal.
In vivo Experiments
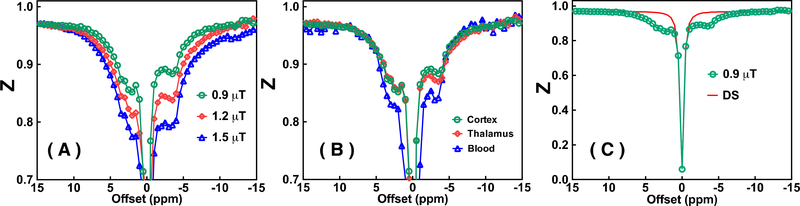
The number of discarded spokes is related to the animal physical condition during the experiment. In our in vivo UTE-CEST experiments, it was 68 ± 17. The sampling rate after removing degraded spokes was above 70%. The signal drift due to B0 shifts during the UTE-CEST scan was found to vary significantly across mice (0–0.8% intensity change) and could be corrected well by the proposed drift correction method. Fig. 5A shows some Z-spectra recorded for the WT mice using the UTE-CEST with different saturation amplitudes, while the Z-spectra of three regions of interest (cortex, thalamus and blood vessels) are plotted in Fig. 5B. The Z value differences between ±8 ppm (Z(8ppm)- Z(−8ppm)) are 0.4% (1.2 μT) and 1.6% (1.5 μT), respectively. However, at the low saturation amplitude of 0.9 μT, the Z values at ±8 ppm differ only about 0.09% suggesting that the MTC effect in the mouse brain is approximately symmetric for positive and negative offsets larger than 8 ppm or smaller than −8 ppm at this amplitude. The regional Z-spectra for the cortex, thalamus, and blood are shown in Fig. 5B. It can be seen that the MTC background (−15 ppm to −8 ppm and 8 ppm to 15 ppm) as well as the ΔZ(3.6) values are similar in the cortex and thalamus while the ΔZ(−3.6) values differed: ΔZ(−3.6) =7.7±0.5% and 9.1±0.6%, respectively. It can be seen that the ΔZ values at 3.6 ppm, 2 ppm and −3.6 ppm for blood are significantly higher than those for other tissues but have a similar shape. The observed signal in blood is known to come mainly from mobile proteins (albumin and hemoglobin) (Shah et al., 2017; Zheng et al., 2014), lending qualitative support to a large part of the in vivo signal in tissue being due to mobile proteins. In order to estimate the water signal contribution, i.e., the DS, and the water frequency, a Lorentzian line-shape was fitted to the Z-spectral points measured in the three chemical shift ranges −1 ppm to 1 ppm, 8 ppm to 15 ppm, and −15ppm to −8 ppm (Fig. 5C). The contribution to the ΔST(±3.6) from DS was found to be 0.53% for the saturation amplitude of 0.9 μT.
Fig 5.
(A) The full Z-spectra of mouse brain (cortex) recorded with peak saturation amplitudes (0.9 μT, 1.2 μT and 1.5 μT). (B) Typical Z-spectra of cortex, thalamus and blood (anterior cerebral artery, ACA) brain regions obtained with a peak saturation amplitude of 0.9 μT. (C) The Z-spectrum of cortex recorded with saturation amplitude of 0.9 μT, as well as water direct saturation (DS, red line) fitted using a Lorentzian line shape.
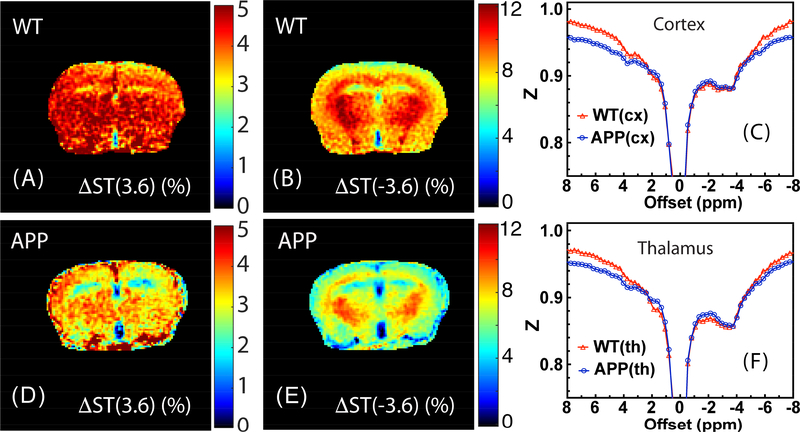
Fig. 6 shows typical ΔST(±3.6) maps for the WT and APP mice as well as representative Z-spectra from the cortex (cx) and thalamus (th). The ΔST(3.6) maps show contrast between white matter (WM) and grey matter (GM) in the brains of both WT and APP mice, which is somewhat enhanced in the APP mice. The ΔST(−3.6) maps of WT and APP mice both display an obvious contrast between WM and GM, but again enhanced in APP. The ΔST(±3.6) maps of WT mice have overall higher intensity in comparison with those of the APP mice. The Z-spectra of the APP and WT mice show clear differences for both cortex and thalamus, with the Z-spectral intensity decreasing over the −4 ppm to 2 ppm range. When comparing to the signal at 8 ppm there is a reduction over the −5 to 5 ppm range, which is reflected in the maps. Notice that there are global differences in intensity across Z-spectra between WT and APP mice that appear similar to the BSA phantom data before and after heat shock. However, at low saturation amplitude, the relatively small saturation effects (ΔZ(8 ppm)) can be easily affected by experimental factors, such as small (a few percent) differences in the chosen ROI and slice between animals and B1 variations (see supplemental material) (Chen et al., 2017). Such effects can be on the same order of magnitude as the change in Z(8ppm) between the two types of mice. By taking the difference between the Z-spectrum intensities at 8 ppm and ±3.6 ppm, most of these undesirable effects can be subtracted out, and a more robust and reliable result can be obtained.
Fig 6.
Representative ΔST(3.6) maps (A, D) and ΔST(−3.6) (B, E) maps for the WT (A, B) and APP (D, E) mice obtained with a saturation amplitude of 0.9 μT. The maps were recorded with 16 averages by interleaving the labeling (±3.6 ppm) and control (8 ppm) offsets as described in the main text. The in-plane resolution is 0.17×0.17 mm2 with a slice thickness of 1 mm. The averaged Z-spectra (n=2) for WT and APP mice recorded with a peak B1 of 0.9 μT for (C) cortex and (F) thalamus. Notice the broadening leading to lower intensity at the high frequency offsets as well as a reduction in saturation in the middle of the spectrum.
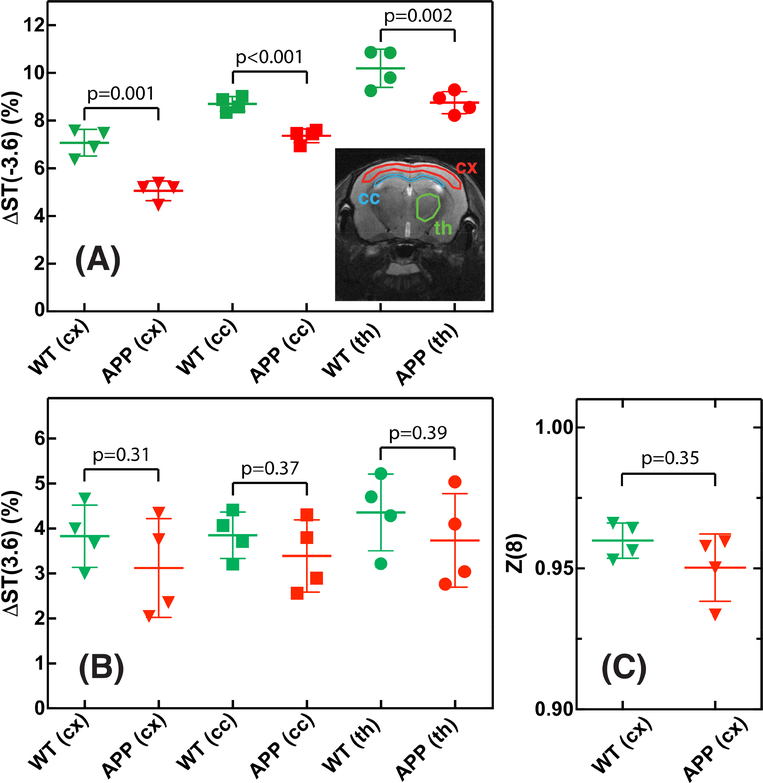
The regional ΔST(±3.6) values as well as Z(8) values for the APP and WT mice are plotted in Fig. 7. The ΔST(3.6) values showed a large variation (range of 2–3%) for both WT and APP mice in all three brain regions studied: cx, corpus callosum (cc) and th. The average ΔST(3.6) values for these three regions were found to be 3.8±0.8% (cx), 3.8±0.6% (cc) and 4.3±1.1% (th) for the WT mice and 3.1±1% (cx), 3.4±0.8% (cc) and 3.7±1% (th) for the APP mice, showing a trend of reduction but no significant effect across these three regions (p=0.3–0.4). The ΔST(−3.6) values, on the other hand, were markedly different between the three brain regions with the highest values found in the thalamus (8–11%) and the lowest values (4–8%) in the cortex. Different from ΔST(3.6), the ΔST(−3.6) values from APP mice were significantly lower than the corresponding values from WT mice for all brain regions (p≤0.002). The Z(8 ppm) was found to be 0.96 ±0.006 and 0.95±0.012 for WT and APP mice respectively, which was not significant (p = 0.35).
Fig 7.
(A,B) Comparison of ΔST(±3.6) values between WT and APP mice for cortex (cx), corpus callosum (cc) and thalamus (th) in four mice. The image shows the location of the typical ROIs for the three brain regions. (C) Comparison of Z(8) values between WT and APP mice (n=4) for the cortex region.
qMT for both WT and APP mice
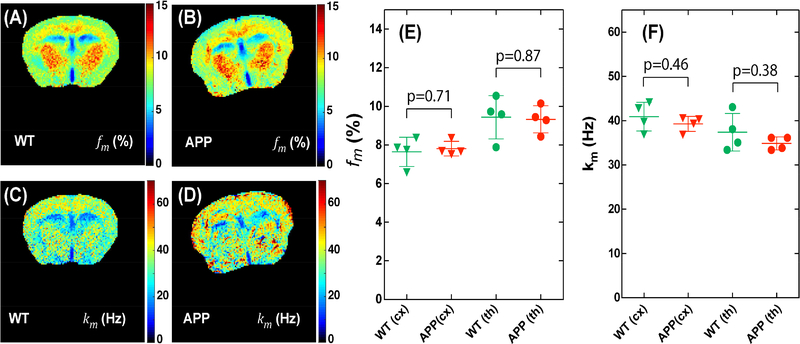
Typical macromolecular fraction (fm) and exchange rate (km) maps and the corresponding regional values for WT and APP mice are plotted in Fig. 8. The macromolecular fraction maps of cortex and thalamus displayed a strong contrast between white and gray matter, similar to the ΔST(−3.6) maps measured by UTE-CEST method (p=0.0025). However, there was no clear difference between the WT and APP mice as demonstrated by the regional values of the fraction map (Fig. 8E) (p=0.71). The exchange rate maps (Figs. 8C,D) also did not show clear regional differences between the two types mice (Fig. 8F).
Fig 8.
Comparison of maps of macromolecular fraction fm (A,B) and exchange rate km (C,D) for WT (A,C) and APP (B,D) mice. The in-plane resolution is 0.17×0.17 mm2 with a slice thickness of 1 mm. The corresponding regional values of the macromolecular fraction (E) and exchange rate (F) for cortex (cx) and thalamus (th) in all mice studied.
T1/T2 for WT and APP mice
The T1 values were found to be 1.8±0.12 s and 1.7±0.12 s for the cortex of the WT and APP mice, respectively, while the T2 values were 35.2±0.2 ms (WT) and 35.6±0.5 ms (APP). Similar to the qMT study, no clear differences were observed between WT and APP mice (p=0.27 and 0.24 for the T1 and T2 values, respectively).
Discussion
We studied the saturation transfer difference signals between 8 ppm and ±3.6 ppm to assess protein aggregation occurring in AD. A UTE-CEST scheme with weak saturation amplitude was applied to minimize contamination by DS and MTC effects. A radial acquisition combined with retrospective motion correction and compressed sensing reconstruction was used to suppress the motion artifacts induced by bulk motion and other temporal fluctuations. This allowed us to acquire high-resolution ΔST images at ±3.6 ppm for mouse brains, which showed significantly reduced ΔST(−3.6) signal for the APP mice compared to the age-matched WT mice.
The water direct saturation in Z-spectra has been well studied previously and can be calculated with measured R1 and R2 values according to R1ρ theory (Chen et al., 2017; Jin et al., 2011; Mulkern and Williams, 1993; Zaiss and Bachert, 2013a, b). Using R1 = 0.5 s‒1 and R2 = 25 s‒1 (de Graaf et al., 2006), the DS contribution to the ΔST signal at ±3.6 ppm can be estimated to be 0.4 % for a UTE-CEST sequence with a peak saturation amplitude of 0.9 μT. This is consistent with the value determined by fitting the Z-spectrum of the WT mice (0.53%, Fig. 5C) and is much lower than the ΔST(±3.6) signal of the mouse brain (3–10%). Therefore, the DS contribution to ΔST(±3.6) signal could be neglected in the current study. It should be noted that the DS contribution will be significantly increased when implementing the current method on low field scanners (see supplemental material). Then, either substantially lower saturation amplitudes are suggested to avoid the DS contamination, or a correction has to be applied. The residual MTC signal in ΔST(±3.6) can be estimated by assuming the MTC changes to be linear with saturation offset between ±3.6 ppm to ±15 ppm, when using low saturation amplitudes (Desmond et al., 2014). The Z-spectra between 8 ppm and 15 ppm were used to fit such a linear function and the residual MTC contrasts at ±3.6 ppm were calculated to be around 0.66±0.22%, which is far smaller than the ΔST(±3.6) values (around 4–10%). The MTC contrast at negative frequencies can in principle be better approximated by acquiring additional UTE-CEST images with saturation offsets between −8 ppm and −15 ppm. However, this comes with a price of prolonging the total scan time by 50%.
The major source of the ΔST(−3.6) signal for the brain at lower saturation amplitudes comes from the aliphatic protons of the mobile protein and mobile lipids. Several studies by MRS and amide proton transfer (APT) based CEST MRI have shown that the protein concentration is homogenous across the brain (Snoussi et al., 2015; Xu et al., 2014; Xu et al., 2016). The contrast between the cortex and thalamus for the ΔST(−3.6) signal in the mouse brain (Fig. 6) may arise from MTC (Goerke et al., 2015b). The MTC in the mouse brain has been separated into two different components, namely fast-exchanging MTC (fast-MTC) and slow-exchanging MTC (Chen et al., 2018). The fast-MTC is symmetric with respect to the water frequency; hence, it can be well suppressed by the current MTC suppression method. On the other hand, the slow-MTC shows a maximum at around −3.6 ppm and cannot be suppressed completely by the current scheme. The qMT studies on both mice show that the macromolecular fractions for both WT and APP mice are indistinguishable, which indicates this baseline macromolecular fraction is not sensitive to the protein aggregation. In contrast, the ΔST(−3.6) signal showed a much higher sensitivity in detecting differences between WT and APP, which we attribute to sensitivity in changes in mobile protein signals due to protein aggregation.
The ΔST(3.6 ppm) signal contains three major components: the amide protons from mobile proteins, aromatic protons (Zaiss et al., 2017) and the amine protons from mobile proteins and metabolites such as glutamine and glutamate. Different from the ΔST(−3.6) signal, the ΔST(3.6) showed much higher variation across mice as evidenced by the coefficient of variation (CoV) for the two signals: 3.5% (ΔST(−3.6)) vs 17% (ΔST(3.6)) (Fig. 7). As such, ΔST(−3.6) should be a better biomarker in detecting protein aggregation than the ΔST(3.6) signal not only because of its much higher intensity, but also because of the smaller variation of the signal. However, ΔST(3.6) may still provide some extra information about the protein aggregation process as it does not include the lipid signal. ΔST(3.6) and ΔST(−3.6) have different sensitivity to B0 inhomogeneity. As we can see from Fig. 5B and Figs. 6 C&F, due to the broad and strong rNOE peak, the variation of ST between −3 ppm and −4 ppm is relatively small. On our 11.7T, the B0 inhomogeneity over the mouse brain is usually less than 0.3 ppm after shimming. Therefore, a B0 correction should not significantly affect ΔST(−3.6). However, the variation of ST between 3 ppm and 4 ppm is much larger, which means B0 inhomogeneity has the potential to cause large changes in the ΔST(3.6) signal. As B1 can also impact the Z(8) and ΔST(−3.6) values (Fig. S3), it needs to be consistent among mice when performing experiments. The protein aggregation process in AD mouse is a complicated process, which may not be well mimicked by cross-linked BSA. Further studies using Aβ and tau peptide aggregations or other better phantoms should be useful to further assess the origin of the ΔST(±3.6) change.
On lower field scanners, the constant MTC background approximation between ±3.6 ppm and ±8 ppm is challenged by the increasing water direct saturation. To address this concern, simulations of DS contamination as a function of B1 for different field scanners were performed (see Supplementary Material). From the simulations, the proposed method should work quite well at 3T and 7T. Assuming similar DS for APP and WT mice, it should also be possible at 1.5T, where the effect could be analyzed using a full Z-spectrum and fitting out the DS (Jones et al., 2013). Another concern when transferring the method to clinical scanners is that traditional radial acquisition is relatively time-consuming compared to fast readout methods such as EPI and gradient and spin-echo (GRASE) acquisitions. This issue can be solved by using more advanced techniques, such as parallel imaging, golden angle radial acquisition and compressed sensing (Bonanno et al., 2018; Haris et al., 2017). In addition, the proposed method doesn’t require the acquisition of a full Z-spectrum and there is no need to wait for full recovery of the saturation signal between successive images. Therefore, it’s possible to further shorten the total acquisition time to a clinically acceptable time.
Conclusion
The UTE-CEST technique proposed provides a simple way of acquiring high-resolution saturation transfer difference signals from mobile proteins in tissue. When comparing WT and APP mice, we found a significant decrease in ΔST(−3.6) signal, which we attributed to the effect of protein aggregation involved in AD, based on comparison with phantom experiments. The ΔST signal may potentially be a marker for studying protein aggregation process in other diseases such as Huntington’s disease and cirrhosis in the liver. However, further large-scale studies are still needed to determine the exact contributions to the ΔST signal decrease in AD.
Supplementary Material
Highlights:
A low-B1 radial-sampling steady-state MRI acquisition scheme was developed to acquire high-resolution saturation transfer difference (ΔST) maps.
Low B1 was used to focus on the chemical exchange saturation transfer (CEST) effects of mobile proteins and minimize the effects of direct water saturation and the semi-solid magnetization transfer contrast (MTC) effects in normal tissue.
ΔST maps at offset frequencies of ±3.6 ppm from water reflected changes in protein aggregation, providing a novel imaging biomarker.
Detection of protein aggregation associated with Alzheimer’s disease using a mouse model by a two-dimensional UTE-CEST sequence.
Acknowledgments
This work was supported by R01EB015032, P41EB015909, R03NS109664, P50AG05146 and DOD CDMRP AZ170028. Lin Chen thanks the China Scholarship Council (201506310130) for financial support.
Grant support from NIH: R01EB015032, P41EB015909, R03NS109664, P50AG05146 and DOD CDMRP AZ170028.
Abbreviations:
- ST
saturation transfer
- DS
direct saturation
- CEST
Chemical Exchange Saturation Transfer
- MTC
Magnetization Transfer Contrast
- UTE
Ultrashort echo time
- HS
heat shock
- AC
anterior commissure
- BSA
bovine serum albumin
- AD:
Alzheimer’s disease
- NFT
neurofibrillary tangles
- RARE
rapid imaging with refocused echoes
- VDMP
Variable Delay Multiple Pulse
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki K, Sato K, Nagaoka S, Kamada M, Hiramatsu K, 1973. Heat denaturation of bovine serum albumin in alkaline pH region. Biochim. Biophys. Acta 328, 323–333. [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ, 2007. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurol 8, 663–672. [DOI] [PubMed] [Google Scholar]
- Baxter EW, Conway KA, Kennis L, Bischoff F, Mercken MH, Winter HL, Reynolds CH, Tounge BA, Luo C, Scott MK, Huang Y, Braeken M, Pieters SM, Berthelot DJ, Masure S, Bruinzeel WD, Jordan AD, Parker MH, Boyd RE, Qu J, Alexander RS, Brenneman DE, Reitz AB, 2007. 2-Amino-3,4-dihydroquinazolines as inhibitors of BACE-1 (beta-site APP cleaving enzyme): Use of structure based design to convert a micromolar hit into a nanomolar lead. J. Med. Chem 50, 4261–4264. [DOI] [PubMed] [Google Scholar]
- Block KT, Uecker M, Frahm J, 2007. Undersampled radial MRI with multiple coils. Iterative image reconstruction using a total variation constraint. Magn. Reson. Med 57, 1086–1098. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Hays AG, Weiss RG, Schar M, 2018. Self-gated golden angle spiral cine MRI for coronary endothelial function assessment. Magn. Reson. Med 80, 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Franceschi M, Falini A, Pontesilli S, Cercignani M, Magnani G, Scotti G, Comi G, Filippi M, 2001. Quantification of tissue damage in AD using diffusion tensor and magnetization transfer MRI. Neurology 57, 1135–1137. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, Kalaria RN, O’Brien JT, 2009. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 132, 195–203. [DOI] [PubMed] [Google Scholar]
- Cavanagh J, Fairbrother WJ, Palmer AG, Skelton NJ, Rance M, 2006. Protein NMR Spectroscopy Principles and Practice. Academic Press. [Google Scholar]
- Chamberlain R, Reyes D, Curran GL, Marjanska M, Wengenack TM, Poduslo JF, Garwood M, Jack CR Jr., 2009. Comparison of amyloid plaque contrast generated by T2-weighted, T2*-weighted, and susceptibility-weighted imaging methods in transgenic mouse models of Alzheimer’s disease. Magn. Reson. Med 61, 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xu X, Zeng H, Chan K, Yadav NN, Cai S, Schunke KJ, Faraday N, van Zijl PCM, Xu J, 2018. Separating Fast and Slow Exchange Transfer and Magnetization Transfer Using Off-resonance Variable Delay Multiple Pulse (VDMP) MRI. Magn. Reson. Med 80, 1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zeng H, Xu X, Yadav NN, Cai S, Puts NA, Barker PB, Li T, Weiss RG, van Zijl PCM, Xu J, 2017. Investigation of the contribution of total creatine to the CEST Z-spectrum of brain using a knockout mouse model. NMR Biomed. 30, e3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LQ, Pagel MD, 2015. Evaluating pH in the Extracellular Tumor Microenvironment Using CEST MRI and Other Imaging Methods. Adv. Radiol 2015, 206405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf RA, Brown PB, McIntyre S, Nixon TW, Behar KL, Rothman DL, 2006. High magnetic field water and metabolite proton T1 and T2 relaxation in rat brain in vivo. Magn. Reson. Med 56, 386–394. [DOI] [PubMed] [Google Scholar]
- Desmond KL, Moosvi F, Stanisz GJ, 2014. Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7 T. Magn. Reson. Med 71, 1841–1853. [DOI] [PubMed] [Google Scholar]
- Dixon WT, Hancu I, Ratnakar SJ, Sherry AD, Lenkinski RE, Alsop DC, 2010. A multislice gradient echo pulse sequence for CEST imaging. Magn. Reson. Med 63, 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Ma G, Li S, Carl M, Szeverenyi NM, VandenBerg S, Corey-Bloom J, Bydder GM, 2014. Ultrashort echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. Neuroimage 87, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Takahashi AM, Bydder M, Chung CB, Bydder GM, 2009. Ultrashort TE imaging with off-resonance saturation contrast (UTE-OSC). Magn. Reson. Med 62, 527–531. [DOI] [PubMed] [Google Scholar]
- Faber C, Zahneisen B, Tippmann F, Schroeder A, Fahrenholz F, 2007. Gradient-echo and CRAZED imaging for minute detection of Alzheimer plaques in an APPV717I x ADAM10-dn mouse model. Magn. Reson. Med 57, 696–703. [DOI] [PubMed] [Google Scholar]
- Fan SJ, Ma Y, Zhu Y, Searleman A, Szeverenyi NM, Bydder GM, Du J, 2018. Yet more evidence that myelin protons can be directly imaged with UTE sequences on a clinical 3T scanner: Bicomponent T2* analysis of native and deuterated ovine brain specimens. Magn. Reson. Med 80, 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler JA, Sutton BP, 2003. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Trans. Signal Process 51, 560–574. [Google Scholar]
- Glover GH, Lee AT, 1995. Motion artifacts in fMRI: comparison of 2DFT with PR and spiral scan methods. Magn. Reson. Med 33, 624–635. [DOI] [PubMed] [Google Scholar]
- Glover GH, Pauly JM, 1992. Projection reconstruction techniques for reduction of motion effects in MRI. Magn. Reson. Med 28, 275–289. [DOI] [PubMed] [Google Scholar]
- Goerke S, Zaiss M, Kunz P, Klika KD, Windschuh JD, Mogk A, Bukau B, Ladd ME, Bachert P, 2015a. Signature of protein unfolding in chemical exchange saturation transfer imaging. NMR Biomed. 28, 906–913. [DOI] [PubMed] [Google Scholar]
- Goerke S, Zaiss M, Longo D, Garello F, Gregorio ED, Breitling J, Ladd ME, Bachert P, 2015b. CEST Signals of Lipids. 25th ISMRM ISMRM, Honolulu, p. 0201. [Google Scholar]
- Hardy J, Selkoe DJ, 2002. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Haris K, Hedstrom E, Bidhult S, Testud F, Maglaveras N, Heiberg E, Hansson SR, Arheden H, Aletras AH, 2017. Self-gated fetal cardiac MRI with tiny golden angle iGRASP: A feasibility study. J Magn Reson Imaging 46, 207–217. [DOI] [PubMed] [Google Scholar]
- Helms G, Dathe H, Hagberg GE, 2004. Pulsed saturation of the standard two-pool model for magnetization transfer. Part II: The transition to steady state. Concepts Magn. Reson 21A, 50–62. [Google Scholar]
- Hua J, Jones CK, Blakeley J, Smith SA, van Zijl PC, Zhou J, 2007. Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain. Magn. Reson. Med 58, 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM, 2008. Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol. Med 14, 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Garwood M, Wengenack TM, Borowski B, Curran GL, Lin J, Adriany G, Grohn OH, Grimm R, Poduslo JF, 2004. In vivo visualization of Alzheimer’s amyloid plaques by magnetic resonance imaging in transgenic mice without a contrast agent. Magn. Reson. Med 52, 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Xu G, Fromholt D, Gonzales V, Borchelt DR, 2003. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol 62, 1220–1227. [DOI] [PubMed] [Google Scholar]
- Jin T, Autio J, Obata T, Kim S-G, 2011. Spin-locking versus chemical exchange saturation transfer MRI for investigating chemical exchange process between water and labile metabolite protons. Magn. Reson. Med 65, 1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Huang A, Xu J, Edden RA, Schar M, Hua J, Oskolkov N, Zaca D, Zhou J, McMahon MT, Pillai JJ, van Zijl PC, 2013. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. NeuroImage 77C, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabani NJ, Sled JG, Shuper A, Chertkow H, 2002. Regional magnetization transfer ratio changes in mild cognitive impairment. Magn. Reson. Med 47, 143–148. [DOI] [PubMed] [Google Scholar]
- Knutsson L, Xu J, Ahlgren A, van Zijl PCM, 2018. CEST, ASL, and magnetization transfer contrast: How similar pulse sequences detect different phenomena. Magn. Reson. Med 80, 1320–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. , 1992. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. USA 89, 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP, 2004. Self-gated cardiac cine MRI. Magn. Reson. Med 51, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Trojanowski JQ, 1999. Neurodegenerative tauopathies: human disease and transgenic mouse models. Neuron 24, 507–510. [DOI] [PubMed] [Google Scholar]
- Levesque IR, Pike GB, 2009. Characterizing healthy and diseased white matter using quantitative magnetization transfer and multicomponent T(2) relaxometry: A unified view via a four-pool model. Magn. Reson. Med 62, 1487–1496. [DOI] [PubMed] [Google Scholar]
- Li T, Braunstein KE, Zhang J, Lau A, Sibener L, Deeble C, Wong PC, 2016. The neuritic plaque facilitates pathological conversion of tau in an Alzheimer’s disease mouse model. Nat. Commun 7, 12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Regatte RR, Navon G, Jerschow A, 2008. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc. Natl. Acad. Sci. USA 105, 2266–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Song X, Chan KW, McMahon MT, 2013. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 26, 810–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Ma Y, Chang EY, He Q, Searleman A, von Drygalski A, Du J, 2018. Simultaneous quantitative susceptibility mapping (QSM) and R2* for high iron concentration quantification with 3D ultrashort echo time sequences: An echo dependence study. Magn. Reson. Med 79, 2315–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig M, Donoho D, Pauly JM, 2007. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 58, 1182–1195. [DOI] [PubMed] [Google Scholar]
- Ma YJ, Chang EY, Carl M, Du J, 2018a. Quantitative magnetization transfer ultrashort echo time imaging using a time-efficient 3D multispoke Cones sequence. Magn. Reson. Med 79, 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Zhu Y, Lu X, Carl M, Chang EY, Du J, 2018b. Short T2 imaging using a 3D double adiabatic inversion recovery prepared ultrashort echo time cones (3D DIR-UTE-Cones) sequence. Magn. Reson. Med 79, 2555–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyarenko DI, Zimmermann EM, Adler J, Swanson SD, 2014. Magnetization transfer in lamellar liquid crystals. Magn. Reson. Med 72, 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascalchi M, Ginestroni A, Bessi V, Toschi N, Padiglioni S, Ciulli S, Tessa C, Giannelli M, Bracco L, Diciotti S, 2013. Regional analysis of the magnetization transfer ratio of the brain in mild Alzheimer disease and amnestic mild cognitive impairment. Am. J. Neuroradiol 34, 2098–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkern RV, Williams ML, 1993. The general solution to the Bloch equation with constant rf and relaxation terms: application to saturation and slice selection. Med. Phys 20, 5–13. [DOI] [PubMed] [Google Scholar]
- Ng M-C, Hua J, Hu Y, Luk KD, Lam EY, 2009. Magnetization transfer (MT) asymmetry around the water resonance in human cervical spinal cord. J. Magn. Reson 29, 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning XR, Selesnick IW, Duval L, 2014. Chromatogram baseline estimation and denoising using sparsity (BEADS). Chemometrics and Intelligent Laboratory Systems 139, 156–167. [Google Scholar]
- Portnoy S, Stanisz GJ, 2007. Modeling pulsed magnetization transfer. Magn. Reson. Med 58, 144–155. [DOI] [PubMed] [Google Scholar]
- Ridha BH, Symms MR, Tozer DJ, Stockton KC, Frost C, Siddique MM, Lewis EB, MacManus DG, Boulby PA, Barker GJ, Rossor MN, Fox NC, Tofts PS, 2007. Magnetization transfer ratio in Alzheimer disease: comparison with volumetric measurements. Am. J. Neuroradiol 28, 965–970. [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Boxer AL, 2016. Neurodegenerative disease in 2015: Targeting tauopathies for therapeutic translation. Nat. Rev. Neurol 12, 74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Valk J, 1992. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry 55, 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P, 1999. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177. [DOI] [PubMed] [Google Scholar]
- Seo H, Kim D, Oh C, Park H, 2017. Self-gated cardiac cine imaging using phase information. Magn. Reson. Med 77, 1216–1222. [DOI] [PubMed] [Google Scholar]
- Shah SM, Mougin OE, Carradus AJ, Geades N, Dury R, Morley W, Gowland PA, 2017. The z-spectrum from human blood at 7T. Neuroimage 167, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Lewis BK, Ruttimann UE, Ye FQ, Sinnwell TM, Yang Y, Duyn JH, Frank JA, 1999. Investigation of low frequency drift in fMRI signal. NeuroImage 9, 526–533. [DOI] [PubMed] [Google Scholar]
- Snoussi K, Gillen JS, Horska A, Puts NA, Pradhan S, Edden RA, Barker PB, 2015. Comparison of brain gray and white matter macromolecule resonances at 3 and 7 Tesla. Magn. Reson. Med 74, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun PZ, Benner T, Kumar A, Sorensen AG, 2008. Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn. Reson. Med 60, 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer D, Ramani A, Barker GJ, Davies GR, Miller DH, Tofts PS, 2003. Quantitative magnetization transfer mapping of bound protons in multiple sclerosis. Magn. Reson. Med 50, 83–91. [DOI] [PubMed] [Google Scholar]
- Turner R, Howseman A, Rees GE, Josephs O, Friston K, 1998. Functional magnetic resonance imaging of the human brain: data acquisition and analysis. Exp. Brain Res 123, 5–12. [DOI] [PubMed] [Google Scholar]
- van Bergen JM, Li X, Hua J, Schreiner SJ, Steininger SC, Quevenco FC, Wyss M, Gietl AF, Treyer V, Leh SE, Buck F, Nitsch RM, Pruessmann KP, van Zijl PC, Hock C, Unschuld PG, 2016. Colocalization of cerebral iron with Amyloid beta in Mild Cognitive Impairment. Sci. Rep 6, 35514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen P, Jiang X, Duyn JH, 2017. Rapid measurement of brain macromolecular proton fraction with transient saturation transfer MRI. Magn. Reson. Med 77, 2174–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl PCM, Lam WW, Xu J, Knutsson L, Stanisz GJ, 2018. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. Neuroimage 168, 222–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl PCM, Sehgal AA, 2016. Proton Chemical Exchange Saturation Transfer (CEST) MRS and MRI. eMagRes 5, 1307–1332. [Google Scholar]
- van Zijl PCM, Yadav NN, 2011. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med 65, 927–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte G, Dewachter I, Borghgraef P, Van Leuven F, Van der Linden A, 2005. Noninvasive in vivo MRI detection of neuritic plaques associated with iron in APP[V717I] transgenic mice, a model for Alzheimer’s disease. Magn. Reson. Med 53, 607–613. [DOI] [PubMed] [Google Scholar]
- Varma G, Duhamel G, de Bazelaire C, Alsop DC, 2015. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn. Reson. Med 73, 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadghiri YZ, Sigurdsson EM, Sadowski M, Elliott JI, Li Y, Scholtzova H, Tang CY, Aguinaldo G, Pappolla M, Duff K, Wisniewski T, Turnbull DH, 2003. Detection of Alzheimer’s amyloid in transgenic mice using magnetic resonance microimaging. Magn. Reson. Med 50, 293–302. [DOI] [PubMed] [Google Scholar]
- Walker LC, Rosen RF, 2006. Alzheimer therapeutics-what after the cholinesterase inhibitors? Age Ageing 35, 332–335. [DOI] [PubMed] [Google Scholar]
- Ward KM, Aletras AH, Balaban RS, 2000. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J. Magn. Reson 143, 79–87. [DOI] [PubMed] [Google Scholar]
- Wei H, Cao P, Bischof A, Henry RG, Larson PEZ, Liu C, 2018. MRI gradient-echo phase contrast of the brain at ultra-short TE with off-resonance saturation. Neuroimage 175, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm MJ, Ong HH, Wehrli SL, Li C, Tsai PH, Hackney DB, Wehrli FW, 2012. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc. Natl. Acad. Sci. USA 109, 9605–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chan KW, Xu X, Yadav N, Liu G, van Zijl PC, 2017. On-resonance variable delay multipulse scheme for imaging of fast-exchanging protons and semisolid macromolecules. Magn. Reson. Med 77, 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yadav NN, Bar-Shir A, Jones CK, Chan KW, Zhang J, Walczak P, McMahon MT, van Zijl PC, 2014. Variable delay multi-pulse train for fast chemical exchange saturation transfer and relayed-nuclear overhauser enhancement MRI. Magn. Reson. Med 71, 1798–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yadav NN, Zeng HF, Jones CK, Zhou JY, van Zijl PCM, Xu JD, 2016. Magnetization Transfer Contrast-Suppressed Imaging of Amide Proton Transfer and Relayed Nuclear Overhauser Enhancement Chemical Exchange Saturation Transfer Effects in the Human Brain at 7T. Magn. Reson. Med 75, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav NN, Yang X, Li Y, Li W, Liu G, van Zijl PCM, 2017. Detection of dynamic substrate binding using MRI. Sci. Rep 7, 10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Sharif B, Pang J, Kali A, Bi X, Cokic I, Li D, Dharmakumar R, 2016. Free-breathing, motion-corrected, highly efficient whole heart T2 mapping at 3T with hybrid radial-cartesian trajectory. Magn. Reson. Med 75, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss M, Bachert P, 2013a. Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: a review of theoretical approaches and methods. Phys. Med. Biol 58, R221–269. [DOI] [PubMed] [Google Scholar]
- Zaiss M, Bachert P, 2013b. Exchange-dependent relaxation in the rotating frame for slow and intermediate exchange -- modeling off-resonant spin-lock and chemical exchange saturation transfer. NMR Biomed. 26, 507–518. [DOI] [PubMed] [Google Scholar]
- Zaiss M, Kunz P, Goerke S, Radbruch A, Bachert P, 2013. MR imaging of protein folding in vitro employing nuclear-Overhauser-mediated saturation transfer. NMR Biomed. 26, 1815–1822. [DOI] [PubMed] [Google Scholar]
- Zaiss M, Windschuh J, Goerke S, Paech D, Meissner JE, Burth S, Kickingereder P, Wick W, Bendszus M, Schlemmer HP, Ladd ME, Bachert P, Radbruch A, 2017. Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn. Reson. Med 77, 196–208. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yarowsky P, Gordon MN, Di Carlo G, Munireddy S, van Zijl PC, Mori S, 2004. Detection of amyloid plaques in mouse models of Alzheimer’s disease by magnetic resonance imaging. Magn. Reson. Med 51, 452–457. [DOI] [PubMed] [Google Scholar]
- Zheng S, van der Bom IM, Zu Z, Lin G, Zhao Y, Gounis MJ, 2014. Chemical exchange saturation transfer effect in blood. Magn. Reson. Med 71, 1082–1092. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PCM, 2003a. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn. Reson. Med 50, 1120–1126. [DOI] [PubMed] [Google Scholar]
- Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC, 2003b. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med 9, 1085–1090. [DOI] [PubMed] [Google Scholar]
- Zu Z, Janve VA, Li K, Does MD, Gore JC, Gochberg DF, 2012. Multi-angle ratiometric approach to measure chemical exchange in amide proton transfer imaging. Magn. Reson. Med 68, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Z, Louie EA, Lin EC, Jiang X, Does MD, Gore JC, Gochberg DF, 2017. Chemical exchange rotation transfer imaging of intermediate-exchanging amines at 2 ppm. NMR Biomed. 30, e3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.