Abstract
Background:
Brain and other central nervous system (CNS) cancers are the leading cause of US pediatric cancer mortality. Incidence trends can provide etiological insight. We report trends in incidence rates of pediatric malignant CNS cancers and pilocytic astrocytoma (non-malignant but historically registered) in the US.
Methods:
Age-standardized incidence rates and annual percent changes (APCs) in rates during 1998–2013 were calculated for children aged 0–19, stratified by subtype, age, sex, and for gliomas, histology and location. We estimated the absolute change in number of cases diagnosed US-wide during 2013 compared to the expected number of cases had 1998 rates remained stable.
Results:
Rates of all pediatric malignant CNS cancer combined (n=18,612) did not change (APC:0.16, 95% confidence interval [CI]: −0.21,0.53). There were statistically-significant changes in several sub-types, however: glioma incidence (n=10,664) increased by 0.77%/year (95% CI:0.29,1.26), embryonal cancer rates (n=5,423) decreased by 0.88%/year (95% CI: −1.33, −0.43) and pilocytic astrocytoma rates (n=6,858) increased by 0.89%/year (95% CI:0.21,1.58). Of the 1,171 malignant tumors and 450 pilocytic astrocytomas diagnosed in US children in 2013, we estimated 120 excess gliomas, 94 excess pilocytic astrocytomas, and 72 fewer embryonal CNS tumors than would be expected had 1998 rates remained stable.
Conclusions:
The gradual changes in incidence we observed for specific types of pediatric CNS cancers are likely due to a combination of changes in classification and diagnosis and true changes in CNS cancer.
Impact:
Continued surveillance of pediatric CNS tumors should remain a priority given their significant contribution to pediatric cancer deaths.
Keywords: pediatric cancer, central nervous system tumors, neuro-oncology, glioma, incidence trends
INTRODUCTION
Among US children, cancer is the second leading cause of death. In 2018, it is expected over 10,000 children aged 0 to 14 will be diagnosed with cancer and that just over 1,100 will die from the disease(1). Central nervous system (CNS) tumors are the second leading cause of childhood cancer and as of 2016 became the leading cause of cancer death in children.(2,3) Although this was attributed to a decrease in mortality from leukemia, it is critical to determine if incidence of CNS tumors in children is changing. Studies in the 1980s and 1990s reported increasing rates of childhood CNS tumors, since largely attributed to innovations in diagnosis, namely the increasing use of magnetic resonance imaging (MRI).(4–6)
The etiology of pediatric CNS tumors is largely unknown. Genetic predisposition syndromes, ionizing radiation (such as computed tomography [CT] scans and radiotherapy), and high birth weight are known risk factors for pediatric CNS cancers, but account for a small percentage of cases.(7,8) Ionizing radiation is most strongly associated with the risk of non-malignant brain tumors, namely meningiomas and schwannomas.(9) Radiofrequency electromagnetic fields (RF-EMF) were classified as possibly carcinogenic by the International Agency for Research on Cancer (IARC) based on findings from some of the epidemiological studies, and experimental data.(10) Because RF-EMF is radiation (albeit non-ionizing), and because children are especially sensitive to cranial irradiation, there is concern that rising RF-EMF exposure will result in increasing incidence of pediatric CNS cancers.(11–13) Careful surveillance of trends is, therefore, critical especially in children.
Reports on trends in pediatric CNS cancer in the post-MRI era have had mixed findings, typically reporting small increases or stable rates.(6,14–16) There are many histologic subtypes of pediatric CNS cancers with distinct biologies and potentially distinct time trends. Existing studies have generally reported trends for either all pediatric CNS cancers combined or a single subtype.(17,18) Reporting on all CNS cancers combined can mask important subtype-specific changes over time, while reporting on a single subtype alone may be misleading due to the heterogeneity of CNS cancers and potential shifts in diagnosis and reporting patterns from one subtype to another.
We have aimed to take an approach that balances these two challenges and in so doing, to provide a comprehensive overview of recent (1998–2013) incidence rates and trends for the common histologic subtypes of pediatric malignant CNS tumors and pilocytic astrocytoma (the most common non-malignant pediatric CNS tumor) using clinically relevant categorizations and stratifying by age, sex and race/ethnicity.
MATERIALS AND METHODS
Data were obtained from 29 cancer registries belonging to the North American Association of Central Cancer Registries (NAACCR). NAACCR harmonizes data for registries participating in the Centers for Disease Control and Prevention National Program of Cancer Registries (NPCR) and the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program. Our analyses were restricted to 1998 to 2013 and 29 registries receiving NAACCR certification for at least 14 of the 15 years studied. Certification indicates meeting NAACCR’s standards for completeness, accuracy, and timeliness(19). The selected registries cover approximately 60% of the US population. A listing of included registries is in the Supplementary Material.
We included all malignant tumors and pilocytic astrocytomas diagnosed among persons aged 0–19 years and classified as CNS or miscellaneous intracranial or intraspinal neoplasms according to the International Classification of Childhood Cancer, 3rd Edition (ICCC-3).(20) This excludes germ cell tumors, neuroblastoma and lymphomas of the CNS.
To optimize clinical relevance and ensure sufficient stability in rates, we aggregated specific histologies of malignant tumors according to a scheme like one previously used by the Central Brain Tumor Registry of the United States (CBTRUS)(21), employing ICD-O-3 histology codes. NAACCR provided ICD-O-3 histology codes for the full span of our study, despite ICD-O-3’s implementation in 2001, by converting earlier ICD-O-2 codes to ICD-O-3 with the express aim of minimizing the impact of the change on trends over time. The major groups included gliomas, embryonal tumors and ependymal tumors. Gliomas were subclassified as low-grade, high-grade, or other; embryonal tumors were subclassified as medulloblastomas, primitive neuro-ectodermal tumors (PNET), or other. Glioma grade was determined based on World Health Organization (WHO) histologic grade.(22)
Our justification for this approach, rather than standard classification schemes for pediatric cancer (i.e., ICCC-3(23)) or CNS cancer (i.e., the WHO Classification of Tumors of the CNS(22)) and the details of our groupings can be found in the Supplemental Methods and Supplementary Table 1. Gliomas were stratified by location and histology according to the International Classification of Diseases for Oncology (third edition, ICD-O-3). Rates for pilocytic astrocytoma and glioma were stratified by method of diagnostic confirmation (microscopic vs. radiographic).
Up until 2000, pilocytic astrocytoma was registered as a malignant cancer (behavior code 3). In ICD-O-3, the code changed to borderline behavior (1)(24). Despite the change, US registrars were instructed to continue to record the cancers as behavior code 3 for consistency(25). We included all cancers with a behavior code of 3 in our analyses, making pilocytic astrocytoma an exception.
Other non-malignant brain tumors were excluded because they have only been continuously recorded since 2004, when cancer registries began routinely collecting these cases in response to the 2002 Benign Brain Tumor Cancer Registries Amendment Act, which recognized the disruption to normal function caused by these tumors and the importance of their surveillance. Quality and completeness of registration for non-malignant tumors are likely to have increased since 2004 and therefore trends were not considered reliable.
Statistical Methods
Incidence rates were calculated using SEER*Stat (Version 8.3.4), age-standardized to the 2000 US standard population by 5-year age groups and expressed per million person-years. Analysis of trends was conducted using the JoinPoint regression program (Version 4.5.0.1). Joinpoint models a best-fit regression line through the observed data.(26) The annual percent change (APC) has been reported as a summary measure without inflection points (or joinpoints) in order to provide an interpretable summary and prevent over-interpretation of potentially unstable estimates at the tails. Annual rates of each subtype by age are displayed graphically so readers can make interpretations based on the the shape of the curves.
Age-standardized and age-specific (0–4, 5–9, 10–14, and 15–19 years) rates and trends were stratified by subtype (see above), sex, and race/ethnicity (Latino, non-Latino white [White], non-Latino black [Black], non-Latino other). Narrower race/ethnicity categories recorded in registries (Asian/Pacific Islander and American Indian/Alaska Native) could not be used due to small numbers.
We compared the expected case counts in 2013 under the current trends to the counts expected had 1998 rates remained stable until 2013. We estimated this by applying 1998 and 2013 rates to age-specific 2013 US census population counts. Joinpoint models produce fitted rates for each year along the best-fit regression line. Modeled rates from the Joinpoint models for 1998 and 2013, rather than the observed rates were used to reduce the effect of instability (noise) on the single-year rates.
The analysis was approved by the NAACCR Institutional Review Board.
RESULTS
Between 1998 and 2013, 18,612 malignant CNS tumors and 6,856 pilocytic astrocytomas were diagnosed among children aged 0–19 in 29 U.S. cancer registries. Age-standardized incidence rates were 22.81 (95% confidence interval [CI]: 22.49–23.14) and 8.41 (95%CI:8.21,8.61) per million person-years, respectively (Table 1).
Table 1.
Age-standardizeda incidence rates (ASR) per million person-years and annual percent change (APC) by subtype, age, sex, and race/ethnicity, 1998–2013.
| Malignant CNS cancer | Pilocytic astrocytoma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | ASR (95%CI) | APC (95% CI) | n (%) | ASR (95% CI) | APC (95% CI) | |||||
| TOTAL | 18,612 (100) | 22.81 (22.49, 23.14) | 0.16 (−0.21, 0.53) | 6,856 (100) | 8.41 (8.21, 8.61) | 0.89 (0.21, 1.58) | ||||
| AGE, years | 0 to 4 | 6,307 (33.9) | 32.11 (31.32, 32.91) | 0.35 (−0.35, 1.06) | 1,949 (28.4) | 9.93 (9.49, 10.38) | 1.06 (−0.12, 2.26) | |||
| 5 to 9 | 4,969 (26.7) | 24.77 (24.09, 25.47) | −0.02 (−0.70, 0.66) | 1,903 (27.8) | 9.49 (9.06, 9.92) | 0.45 (−0.77, 1.69) | ||||
| 10 to 14 | 3,884 (20.9) | 18.61 (18.03, 19.2) | 0.13 (−0.56, 0.83) | 1,769 (25.8) | 8.47 (8.08, 8.88) | 0.76 (−0.16, 1.70) | ||||
| 15 to 19 | 3,452 (18.5) | 16.20 (15.67, 16.75) | 0.15 (−0.66, 0.97) | 1,235 (18.0) | 5.80 (5.48, 6.13) | 1.54 (0.42, 2.67) | ||||
| SEX | Male | 10,242 (55.0) | 24.52 (24.04, 25.00) | 0.06 (−0.46, 0.57) | 3,533 (51.5) | 8.46 (8.18, 8.74) | 1.22 (0.44, 2.01) | |||
| Female | 8,370 (45.0) | 21.03 (20.58, 21.48) | 0.30 (−0.26, 0.86) | 3,323 (48.5) | 8.35 (8.07, 8.64) | 0.54 (−0.31, 1.40) | ||||
| ETHNICITY/ RACEb | Latino | 3,330 (17.9) | 19.04 (18.40, 19.70) | −1.06 (−1.61, −0.52) | 1,056 (15.4) | 6.08 (5.72, 6.46) | 0.33 (−0.74, 1.42) | |||
| White | 12,018 (64.6) | 25.58 (25.12, 26.04) | 0.49 (0.07, 0.92) | 4,697 (68.5) | 9.95 (9.67, 10.24) | 1.13 (0.38, 1.89) | ||||
| Black | 2,139 (11.5) | 18.17 (17.41, 18.96) | 0.65 (−0.28, 1.60) | 716 (10.4) | 6.08 (5.64, 6.54) | 2.72 (0.56, 4.94) | ||||
| Other | 884 (4.7) | 16.54 (15.47, 17.67) | 1.08 (−0.38, 2.57) | 261 (3.8) | 4.89 (4.31, 5.52) | 0.32 (−1.32, 1.99) | ||||
| Unknown | 241 (1.3) | 126 (1.8) | ||||||||
Age-standardized to the 2000 US standard population by 5-year age groups and expressed per million person-years. CI: Confidence Interval
Race/ethnicity categories are mutually-exclusive. Unknown race/ethnicity cancers do not have incidence rates or trends given a lack of denominator (population size) estimate for this group.
There was no significant change in incidence for all malignant CNS cancers combined (APC:0.16, 95%CI:−0.21,0.53, Table 1). In stratified analyses, CNS cancer rates significantly decreased among Latinos (APC:−1.06, 95%CI:−1.61,−0.52), and increased significantly among Whites (APC:0.49, 95%CI:0.07,0.92, Table 2).
Table 2.
Age-standardizeda incidence rates (ASR) of pediatric malignant brain and CNS cancers per million person-years and annual percent change (APC) by subtype, age, sex, and race/ethnicity, 1998–2013.
| n (%) | ASR (95% CI) | APC (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gliomas | |||||||||
| TOTAL | 10,664 (100) | 13.05 (12.81, 13.31) | 0.77 (0.29, 1.26) | ||||||
| AGE, years | 0 to 4 | 2,906 (27.3) | 14.80 (14.26, 15.34) | 0.55 (−0.08, 1.18) | |||||
| 5 to 9 | 2,919 (27.4) | 14.55 (14.03, 15.09) | 0.73 (−0.40, 1.87) | ||||||
| 10 to 14 | 2,471 (23.2) | 11.84 (11.37, 12.31) | 1.09 (0.30, 1.89) | ||||||
| 15 to 19 | 2,368 (22.3) | 11.12 (10.67, 11.57) | 0.75 (−0.31, 1.83) | ||||||
| SEX | Male | 5,638 (52.9) | 13.47 (13.12, 13.82) | 0.57 (−0.08, 1.23) | |||||
| Female | 5,026 (47.1) | 12.62 (12.27, 12.97) | 1.00 (0.30, 1.71) | ||||||
| ETHNICITY/ RACEb | Latino | 1,712 (16.1) | 9.94 (9.47, 10.42) | −0.44 (−1.70, 0.84) | |||||
| White | 7,057 (66.2) | 14.94 (14.59, 15.29) | 1.31 (0.64, 1.98) | ||||||
| Black | 1,251 (11.7) | 10.61 (10.03, 11.21) | 0.94 (−0.15, 2.03) | ||||||
| Other | 473 (4.4) | 8.86 (8.08, 9.70) | 1.08 (−0.68, 2.87) | ||||||
| Unknown | 171 (1.6) | ||||||||
| Embryonal | |||||||||
| TOTAL | 5,423 (100) | 6.67 (6.49, 6.85) | −0.88 (−1.33, −0.43) | ||||||
| AGE, years | 0 to 4 | 2,250 (41.5) | 11.45 (10.99, 11.94) | 0.14 (−1.03, 1.33) | |||||
| 5 to 9 | 1,560 (28.8) | 7.78 (7.40, 8.17) | −1.11 (−2.08, −0.12) | ||||||
| 10 to 14 | 968 (17.8) | 4.64 (4.35, 4.94) | −2.13 (−3.80, −0.42) | ||||||
| 15 to 19 | 645 (11.9) | 3.03 (2.80, 3.27) | −1.98 (−4.02, 0.10) | ||||||
| SEX | Male | 3,218 (59.3) | 7.74 (7.47, 8.01) | −0.86 (−1.36, −0.36) | |||||
| Female | 2,205 (40.7) | 5.55 (5.32, 5.79) | −0.88 (−1.96, 0.21) | ||||||
| ETHNICITY/ RACEb | Latino | 1,104 (20.4) | 6.21 (5.85, 6.59) | −2.55 (−3.54, −1.56) | |||||
| White | 3,402 (62.7) | 7.31 (7.07, 7.56) | −0.66 (−1.33, 0.01) | ||||||
| Black | 590 (10.9) | 5.03 (4.64, 5.46) | 0.30 (−0.66, 1.27) | ||||||
| Other | 280 (5.2) | 5.24 (4.65, 5.89) | 0.03 (−2.40, 2.52) | ||||||
| Unknown | 47 (0.9) | ||||||||
| Ependymal | |||||||||
| TOTAL | 1,881 (100) | 2.30 (2.20, 2.41) | 0.63 (−0.89, 2.16) | ||||||
| AGE, years | 0 to 4 | 839 (44.6) | 4.27 (3.99, 4.57) | 0.97 (−1.18, 3.18) | |||||
| 5 to 9 | 393 (20.9) | 1.96 (1.77, 2.16) | −0.15 (−2.51, 2.27) | ||||||
| 10 to 14 | 328 (17.4) | 1.57 (1.41, 1.75) | −0.26 (−3.05, 2.61) | ||||||
| 15 to 19 | 321 (17.1) | 1.51 (1.35, 1.68) | 1.89 (−1.53, 5.43) | ||||||
| SEX | Male | 1,034 (55.0) | 2.47 (2.32, 2.63) | 1.16 (−0.54, 2.89) | |||||
| Female | 847 (45.0) | 2.13 (1.99, 2.27) | −0.01 (−1.78, 1.79) | ||||||
| ETHNICITY/ RACEb | Latino | 399 (21.2) | 2.26 (2.04, 2.49) | 1.32 (−1.09, 3.79) | |||||
| White | 1,158 (61.6) | 2.47 (2.33, 2.62) | 0.25 (−1.48, 2.01) | ||||||
| Black | 211 (11.2) | 1.80 (1.56, 2.06) | 1.11 (−3.16, 5.56) | ||||||
| Other | 101 (5.4) | 1.88 (1.53, 2.28) | 4.05 (−0.23, 8.52) | ||||||
| Unknown | 12 (0.6) | ||||||||
| Other | |||||||||
| TOTAL | 644 (100) | 0.79 (0.73, 0.85) | −2.79 (−5.01, −0.51) | ||||||
| AGE, years | 0 to 4 | 312 (48.4) | 1.59 (1.42, 1.77) | −2.00 (−4.28, 0.35) | |||||
| 5 to 9 | 97 (15.1) | 0.48 (0.39, 0.59) | −4.07 (−8.45, 0.53) | ||||||
| 10 to 14 | 117 (18.2) | 0.56 (0.46, 0.67) | −1.65 (−5.65, 2.52) | ||||||
| 15 to 19 | 118 (18.3) | 0.55 (0.46, 0.66) | −4.50 (−9.01, 0.23) | ||||||
| SEX | Male | 352 (54.7) | 0.84 (0.75, 0.93) | −3.27 (−5.69, −0.8) | |||||
| Female | 292 (45.3) | 0.73 (0.65, 0.82) | −2.16 (−5.07, 0.84) | ||||||
| ETHNICITY/ RACEb | Latino | 115 (17.9) | 0.63 (0.52, 0.76) | −4.32 (−8.77, 0.34) | |||||
| White | 401 (62.3) | 0.86 (0.78, 0.95) | −3.16 (−6.22, 0.01) | ||||||
| Black | 87 (13.5) | 0.74 (0.59, 0.91) | −1.65 (−5.61, 2.48) | ||||||
| Other | 30 (4.7) | 0.56 (0.38, 0.79) | |||||||
| Unknown | 11 (1.7) | ||||||||
Age-standardized to the 2000 US standard population by 5-year age groups and expressed per million person-years
Race/ethnicity categories are mutually-exclusive; Unknown race/ethnicity cancers do not have incidence rates or trends given a lack of denominator (population size) estimate for this group.
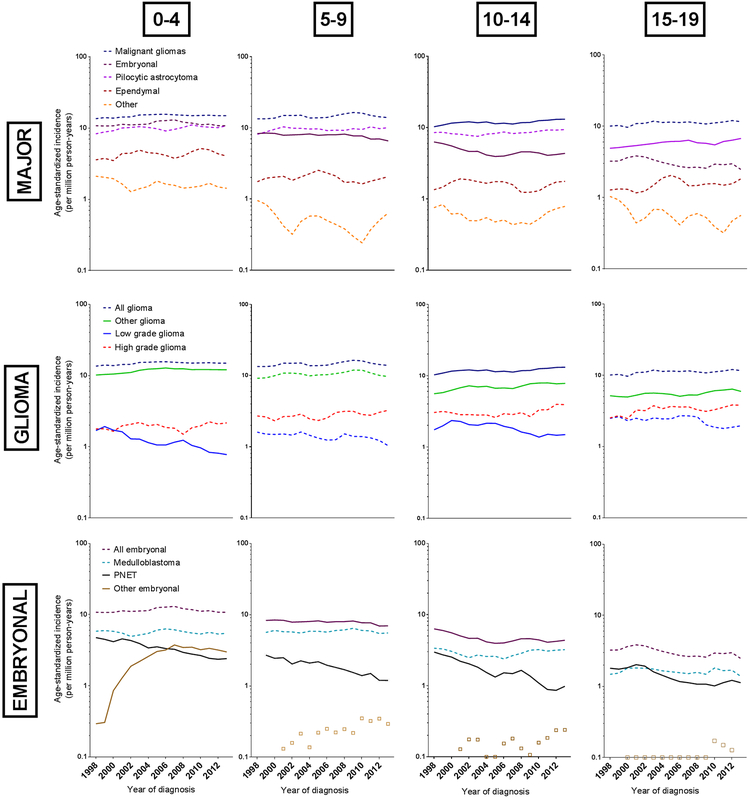
Subtype-specific trends are illustrated in Figure 1 and Tables 3 and 4. Gliomas accounted for 57% (n=10,664) of all malignant CNS tumors. The incidence rate of glioma increased by 0.77% per year (95%CI:0.29,1.26), equivalent to 120 excess cases US-wide in 2013 compared to 1998 estimated rates. Increases appeared to be consistent across age, sex, and race/ethnicity, groups other than Latinos though only reached statistical significance among 10–14-year-olds (APC:1.09, 95%CI:0.30,1.89), females (APC:1.00, 95% CI:0.30,1.71), and Whites (APC:1.31, 95%CI:0.64,1.98). As shown in Figure 1 and Table 4, low grade gliomas (12.9% of gliomas) decreased significantly (APC:−2.85, 95%CI:−4.23,−1.46), while rates of high grade (21.3%) and other (65.8%) increased (APCother:1.25, 95%CI:0.68,1.83, APChigh grade:1.55, 95%CI:0.18,2.95).
Figure 1.
Age-specific incidence rate of brain/CNS cancers per million person-years, by subtype and age at diagnosis, 3-year moving average, 1998–2013. From left to right, the columns of graphs show incidence rates for children ages 0–4, 5–9, 10–14 and 15–19 years of age. From top to bottom, the rows of graphs show incidence rates for all major subtypes, glioma subtypes and embryonal cancer subtypes. Solid lines indicate significant trends. Lines omitted when there were two few cases for a trend analysis. Colour legend: dark blue: malignant glioma; purple: embryonal cancer; fuchsia: pilocytic astrocytoma; orange: other; green: other glioma; red: high grade glioma; royal blue: low grade glioma; teal: medulloblastoma; black: PNET; brown: other embryonal.
Table 3.
Age-standardized incidence rate (ASR) and annual percent change (APC) for brain and CNS cancers by subtype and age at diagnosis, 1998–2013.
| Cancer types | n | ASR | 95%CI | APC | 95%CI | |
|---|---|---|---|---|---|---|
| All ages | ||||||
| Glioma | 10,664 | 13.05 | (12.81, 13.31) | 0.77 | (0.29, 1.26) | |
| Low grade glioma | 1,374 | 1.67 | (1.58, 1.76) | −2.85 | (−4.23, −1.46) | |
| High grade glioma | 2,274 | 2.77 | (2.66, 2.89) | 1.55 | (0.18, 2.95) | |
| Other glioma | 7,016 | 8.61 | (8.41, 8.82) | 1.25 | (0.68, 1.83) | |
| Embryonal | 5,423 | 6.67 | (6.49, 6.85) | −0.88 | (−1.33, −0.43) | |
| Medulloblastoma | 3,201 | 3.95 | (3.81, 4.09) | −0.05 | (−0.79, 0.69) | |
| PNET | 1,678 | 2.05 | (1.96, 2.16) | −5.67 | (−6.54, −4.8) | |
| Other embryonal | 544 | 0.67 | (0.61, 0.73) | 6.99 | (1.49, 12.77) | |
| 0–4 years | ||||||
| Glioma | 2,906 | 14.8 | (14.26, 15.34) | 0.55 | (−0.08, 1.18) | |
| Low grade glioma | 234 | 1.19 | (1.04, 1.35) | −5.75 | (−8.57, −2.84) | |
| High grade glioma | 376 | 1.91 | (1.72, 2.12) | 0.66 | (−1.53, 2.9) | |
| Other glioma | 2,296 | 11.69 | (11.22, 12.18) | 1.2 | (0.41, 2) | |
| Embryonal | 2,250 | 11.45 | (10.99, 11.94) | 0.14 | (−1.03, 1.33) | |
| Medulloblastoma | 1,096 | 5.58 | (5.26, 5.92) | −0.27 | (−1.57, 1.06) | |
| PNET | 679 | 3.46 | (3.2, 3.73) | −5.33 | (−7.29, −3.33) | |
| Other embryonal | 475 | 2.42 | (2.2, 2.64) | 6.93 | (1.25, 12.93) | |
| 5–9 years | ||||||
| Glioma | 2,919 | 14.55 | (14.03, 15.09) | 0.73 | (−0.4, 1.87) | |
| Low grade glioma | 271 | 1.35 | (1.19, 1.52) | −1.67 | (−4.96, 1.74) | |
| High grade glioma | 556 | 2.77 | (2.55, 3.01) | 1.7 | (−0.63, 4.08) | |
| Other glioma | 2,092 | 10.43 | (9.99, 10.88) | 0.84 | (−0.38, 2.07) | |
| Embryonal | 1,560 | 7.78 | (7.4, 8.17) | −1.11 | (−2.08, −0.12) | |
| Medulloblastoma | 1,161 | 5.79 | (5.46, 6.13) | −0.05 | (−1.19, 1.11) | |
| PNET | 362 | 1.8 | (1.62, 2) | −5.39 | (−8, −2.71) | |
| Other embryonal | 37 | 0.18 | (0.13, 0.25) | |||
| 10–14 years | ||||||
| Glioma | 2,471 | 11.84 | (11.37, 12.31) | 1.09 | (0.3, 1.89) | |
| Low grade glioma | 379 | 1.82 | (1.64, 2.01) | −2.93 | (−5.2, −0.59) | |
| High grade glioma | 638 | 3.06 | (2.82, 3.3) | 1.78 | (−0.46, 4.08) | |
| Other glioma | 1,454 | 6.97 | (6.61, 7.33) | 1.86 | (0.86, 2.86) | |
| Embryonal | 968 | 4.64 | (4.35, 4.94) | −2.13 | (−3.8, −0.42) | |
| Medulloblastoma | 602 | 2.88 | (2.66, 3.12) | 0.51 | (−1.39, 2.45) | |
| PNET | 344 | 1.65 | (1.48, 1.83) | −7.51 | (−10.04, −4.9) | |
| Other embryonal | 22 | 0.11 | (0.07, 0.16) | |||
| 15–19 years | ||||||
| Glioma | 2,368 | 11.12 | (10.67, 11.57) | 0.75 | (−0.31, 1.83) | |
| Low grade glioma | 490 | 2.3 | (2.1, 2.51) | −2.19 | (−4.52, 0.2) | |
| High grade glioma | 704 | 3.3 | (3.07, 3.56) | 1.72 | (−0.42, 3.9) | |
| Other glioma | 1,174 | 5.51 | (5.2, 5.84) | 1.4 | (0.09, 2.73) | |
| Embryonal | 645 | 3.03 | (2.8, 3.27) | −1.98 | (−4.02, 0.1) | |
| Medulloblastoma | 342 | 1.61 | (1.44, 1.78) | −0.12 | (−2.3, 2.1) | |
| PNET | 293 | 1.38 | (1.22, 1.54) | −4.48 | (−7.32, −1.55) | |
| Other embryonala | ||||||
CI: Confidence interval; PNET: Primitive neuroectodermal tumor.
Fewer than 16 cells, trends and rates ommitted.
Table 4.
Age standardized incidence rates (ASR) per million person-years and annual percent change (APC) of glioma by topography and histology, 1998–2013.
| n | ASR | 95% CI | APC | 95% CI | |
|---|---|---|---|---|---|
| Topography | |||||
| Brain stem | 2,658 | 3.28 | (3.16, 3.41) | 0.89 | (0.20, 1.58) |
| Optic nerve | 1,392 | 1.72 | (1.63, 1.81) | 3.92 | (1.76, 6.13) |
| Temporal lobe | 1,263 | 1.53 | (1.45, 1.62) | 0.01 | (−0.88, 0.91) |
| Cerebrum | 1,129 | 1.38 | (1.3, 1.47) | 0.74 | (−0.69, 2.18) |
| Frontal lobe | 1,069 | 1.29 | (1.22, 1.37) | 2.58 | (0.82, 4.37) |
| Brain, NOS | 779 | 0.95 | (0.89, 1.02) | −1.4 | (−3.18, 0.41) |
| Cerebellum, NOS | 530 | 0.65 | (0.59, 0.71) | 0.7 | (−1.97, 3.45) |
| Parietal lobe | 503 | 0.61 | (0.56, 0.67) | −2.59 | (−4.71, −0.43) |
| Overlapping lesions | 487 | 0.59 | (0.54, 0.65) | −1.2 | (−3.07, 0.71) |
| Other | 854 | 1.04 | (0.97, 1.12) | −0.38 | (−1.82, 1.09) |
| Histology | |||||
| Low grade gliomas (major histologies) | |||||
| Oligodendroglioma, NOS | 580 | 0.42 | (0.65, 0.76) | −5.16 | (−6.58, −3.71) |
| Fibrillary astrocytoma | 398 | 0.93 | (0.44, 0.54) | −4.13 | (−7.17, −1.00) |
| Pleomorphic xanthoastrocytoma | 347 | 0.70 | (0.38, 0.47) | 3.45 | (0.99, 5.98) |
| High grade gliomas (major histologies) | |||||
| Astrocytoma, anaplastic | 761 | 1.38 | (0.86, 1.00) | 0.72 | (−1.12, 2.59) |
| Other gliomas (major histologies) | |||||
| Glioma, malignant | 4,589 | 5.65 | (5.49, 5.82) | 3.13 | (2.19, 4.08) |
| Astrocytoma, NOS | 2,096 | 2.56 | (2.45, 2.67) | −2.4 | (−3.38, −1.42) |
| All other glioma histologies combined | |||||
| Other | 762 | 1.41 | (0.86, 0.99) | −1.97 | (−4.64, 0.78) |
Forty-three percent (n=4,589) of gliomas were coded “glioma, malignant”, without specific classification and are therefore categorized as “other” rather than “low-” or “high-grade” (Table 4). Of these, 72% (n=3,323) were in the brain stem or optic nerve, where biopsies and surgery are rarely performed. Overall, the incidence of “glioma, malignant” increased 3.13%/year (95%CI:2.19,4.08), but the increase appears to have been driven primarily by glioma of the optic nerve (APC:3.92, 95%CI:1.76,6.13). Of the specific glioma histologies, significant increases were observed for glioblastoma (APC:3.01, 95%CI:0.97,5.08) and pleomorphic xanthoastrocytoma (APC:3.45, 95%CI:0.99,6.98). Significant decreases were observed for astrocytoma NOS (APC:−2.40, 95%CI:−3.38,−1.42), oligodendroglioma NOS (APC:−5.16, 95%CI:−6.58,−3.71), and fibrillary astrocytoma (APC:−4.13, 95%CI:−7.17,−1.00, Table 4 and Supplemental Figure 1).
Embryonal cancers accounted for 29% (n=5,423) of all malignant CNS tumors with incidence rates decreasing significantly (APC:−0.88, 95%CI:−1.33,−0.43, Table 2) between 1998 and 2013, equivalent to 72 fewer cases in 2013, compared to expected based on 1998 rates. The decreasing trend was consistent by sex, but was not observed for 0–4-year-olds (APC=0.14, 95%CI:−1.03,1.33). Rates of medulloblastoma, the most common embryonal tumor, remained stable (APC:0.05, 95% CI:−0.79,0.69, Figure 1 and Table 3) while rates of primitive neuroectodermal tumors (PNETs) decreased significantly (APC:−5.67, 95%CI:−6.54,−4.80), and rates of other embryonal tumors (which include atypical teratoid/rhabdoid tumors [ATRT]) increased significantly (APC:6.99, 95%CI:1.49, 12.77).
Ten percent of all malignant CNS tumors were ependymal (n=1,881); incidence rates remained stable (APC:0.63, 95%CI:−0.89,2.16) with no significant trends observed in any demographic group (Table 2). Other malignant CNS cancers (3.4%, n=644) decreased significantly (APC:−2.79, 95%CI:−5.01,−0.51) and consistently across all strata.
Incidence rates of pilocytic astrocytoma increased by 0.89% per year (95%CI:0.21,1.58), equivalent to 94 excess cases. In stratified analyses, rates increased across age, sex and racial/ethnic groups; however, statistically-significant increases were limited to 15–19-year-olds (APC:1.54, 95%CI:0.42,2.67), males (APC:1.22, 95%CI:0.44,2.01), non-Latino whites (APC:1.13, 95%CI:0.38,1.89) and non-Latino blacks (APC:2.72, 95%CI:0.56,4.94).
Exploratory analyses showed an increasing proportion of gliomas, and to a much lesser extent pilocytic astrocytomas, were confirmed radiographically rather than microscopically (Supplemental Figure 2). Beginning around 2006 and until 2013, there was a modest increase in the rate of radiographically-confirmed pilocytic astrocytoma. For gliomas, the incidence of radiographically-confirmed cases increased between 1998 and 2004 and then stabilized. The rate of microscopically-confirmed malignant gliomas decreased modestly and steadily over the entire study period.
DISCUSSION
In this large, population-based study covering 60% of the U.S. population, we did not observe a statistically-significant change in the incidence rates of all malignant CNS cancers combined between 1998 and 2013. We observed a statistically-significant increase in the incidence rate of glioma (0.77%/year) and of pilocytic astrocytoma (0.89%/year) and a statistically-significant decrease in incidence of embryonal cancer (−0.88%/year). We estimated that there were 120 excess cases of glioma, 72 fewer embryonal cancers, and 94 excess cases of pilocytic astrocytoma diagnosed US-wide in 2013 compared to expected had 1998 rates remained stable.
Childhood CNS tumor incidence trends in the US have rarely been reported with the granularity presented here, making comparisons of our findings with others challenging. Consistent with our results, however, CBTRUS (using 52 NAACCR registries) reported a borderline significant increase of 0.6%/year for all malignant CNS tumors among 0–14 year-olds between 2000 and 2014 and of 1%/year among 15–19 year-olds from 2000 to 2008.(14,27) Between 2000 and 2014, they estimated an increase in gliomas among 0–14 year-olds of 1.5% per year.(14) The Centers for Disease Control and Prevention (using 47 NAACCR registries) reported a decrease in embryonal tumors from 2000 to 2010 of 0.6% per year, also consistent with our results.(27) The compatible findings within the US are not surprising given the shared data sources. By restricting to only high-quality registries, we decreased the likelihood of data quality influencing our findings. By including more narrowly-defined histological subgroups, we have shown diverging patterns in subtype-specific incidence rates underlying a higher-level, average trend. Our results are consistent with recent trends in Europe showing, for example, increasing incidence of pilocytic astrocytoma and ATRT and decreasing incidence of PNET and embryonal cancers overall.(28,29)
The trends we report are likely to be the result of a combination of changing classification, reporting, diagnosis, and true changes in CNS cancer incidence. We observed increasing incidence rates of radiographically-confirmed diagnoses of malignant glioma and pilocytic astrocytoma. We hypothesize there are at least three ways for shifting patterns in method of confirmation to influence incidence trends: (1) as imaging becomes more powerful and accessible, an increasing share of diagnoses have been made through imaging rather than biopsy leading to more general diagnosis categories; (2) not previously detectable cancers are now being detected radiographically; (3) more frequent surveillance of patients with tumor predisposition syndromes and diagnoses by radiologists have led to increased reporting to registries by radiologists. The latter two factors could lead to the appearance of increasing incidence in the absence of a true increase in CNS cancer. A detailed analysis of subtype-specific trends in mortality and survival over the same period has not been conducted to our knowledge but may provide insight into whether detection of potentially indolent cancers is contributing to the observed trends.
In addition to changes in method of detection, some sub-type trends are likely explained by the increasing availability and understanding of molecular pathology. For example, we found that ‘other’ embryonal tumors (mostly ATRT) increased by 6.99%/year and PNET incidence decreased 5.67%/year. Up until the late 1990s, PNET and ATRT were difficult to distinguish based on clinical and pathological features and most tumors were classified as PNET. Since then, increasing recognition of the distinct genetic signature of ATRT has increased the frequency of its diagnosis(30,31). Similarly, tumors previously identified as astrocytoma NOS which, after molecular testing show H3F3A mutations, are now being diagnosed and classified as glioma NOS based on evolving understanding of the etiology and prognosis of cancers displaying these mutations.(22,32) This is reflected in our findings; astrocytoma NOS incidence decreased by 2.4%/year and glioma NOS incidence increased by 3.13%/year.
To address the possibility that subtype trends reflect classification shifts rather than true net changes, we reported trends in broader, aggregated groups that are less subject to classification patterns. Even trends in the aggregated groups, however, can be subject to practice changes, if for example, new technology identifies cancers that would not have previously been reported. In the future, large biobanking efforts such as the Children’s Brain Tumor Tissue Consortium,(33) may allow for the retrospective classification of tumors using uniform standards that reflect our present day understanding of etiology. For this to contribute to trends research, however, accurate estimates of the size of the population contributing to the consortium would be needed as rate denominators.
Aside from classification and diagnosis, changes in risk factor prevalence could have contributed to changing incidence of pediatric CNS tumors. Ionizing radiation is the strongest known modifiable risk factor for pediatric CNS tumors.(7) The dose-response relationship is strongest for meningiomas, which are non-malignant and excluded from this analysis, but a significant association has also been found with gliomas.(9) Steeply increasing use of CT scans could contribute to increasing incidence of gliomas among children aged 5 and older (younger children would have insufficient latency).(34) Recent guidelines to identify pediatric patients at emergency departments who may not benefit from CT,(35) and the increasing adoption of the “as low as reasonably achievable (ALARA)” principle for balancing dose with image quality, may decrease current and future dose from pediatric CT scans.(36) Given the uncertain association between RF-EMF and malignant CNS tumors,(37) it is unclear if and to what extent increases in exposure contribute to the glioma trends.
High birthweight is associated equally with increased risk of gliomas, astrocytomas and embryonal tumors (5% increase in risk per 500 gram increase)(38). However, it is unlikely that high birthweight is driving increases in glioma and astrocytoma, given the US prevalence of high birthweight has decreased over recent decades.(39) Cancer predisposition syndromes, such as neurofibromatosis types 1 and 2, Li-Fraumeni syndrome and Cowden syndrome are estimated to account for approximately 9% of all pediatric CNS cancers,(40,41) and prevalence of these inherited syndromes occurs over generations, making them unlikely drivers of the observed trends over the 15-years of this study. As brain tumor etiology is poorly understood, changes in another unknown exposure could have contributed to the changes in CNS tumor incidence we observed. Further research on the causes of pediatric brain tumors is merited.
The primary strength of this work is the coverage, size and quality of the registries. Despite being the leading cause of childhood cancer death,(2) pediatric CNS cancers are rare. The 29 geographically-dispersed registries yielded sufficient cases to estimate recent trends by narrow subtype, age, sex, and race/ethnicity. The contributing registries have high completeness and standards that minimize misclassification and missingness.(42,43) Unlike previous studies,(14,27,44,45) we restricted to registries that received NAACCR certification to ensure the highest data quality. This is especially critical for CNS cancers, given the rarity of these tumors, the diversity of subtypes, and the varying classification systems.
The data does have inherent limitations. First, as has been outlined, trends in classification, diagnosis, or reporting could be mistaken for true trends in underlying rates. In light of this, we have shown results for all subtypes at specific and aggregated levels, and been cautious in our interpretation. Second, the collection of demographic variables both in cancer registries (numerators) and in the census (denominators) can impact the estimation of rates and trends in subpopulations defined by these variables. For example, NAACCR introduced a Latino identification algorithm in 2003. Prior to that, each registry used its own approach.(46) Additionally, the 2010 census used different methods than the 2000 census to assign race/ethnicity to those reporting ‘some other race’ or multiple races.(47) The decreasing incidence rates of all malignant CNS cancers and gliomas among Latinos were not observed in the other race/ethnic groups and should be interpreted cautiously given the changes in race/ethnicity classification in the registry and the census, and distinct Latinos migration patterns.(48,49)
In sum, we present recent trends in pediatric CNS tumor incidence rates in the US, overall and stratified by subtype, age, sex, and race/ethnicity. Our data demonstrated that the overall incidence rate of malignant CNS tumors has not changed significantly between 1998 and 2013. We observed increasing rates of glioma and pilocytic astrocytoma and decreasing rates of embryonal cancer. Absolute increases were small (equivalent to 94 excess pilocytic astrocytoma and 120 excess glioma cases in a year) and partially offset by decreases in embryonal cancers (72 cases). Continued surveillance of pediatric CNS tumors should remain a priority given their significant contribution to pediatric cancer deaths. The development and promotion of a historically compatible, clinically-relevant, widely accepted classification scheme for pediatric CNS tumors, would improve the feasibility, comparability, and usefulness of trends analyses.
Supplementary Material
ACKNOWLEDGEMENTS
The assistance of Dr. Martha Linet is gratefully acknowledged. These data used in the presented analyses are based on the NAACCR December 2015 data submission. Support for cancer registries is provided by the state, province or territory in which the registry is located. In the U.S., registries also participate in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program or the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) or both.
FINANCIAL SUPPORT: This work was supported by the Intramural program of the National Cancer Institute.
Abbreviations:
- ALARA
as low as reasonably achievable
- APC
annual percent change
- ATRT
atypical teratoid/rhabdoid tumors
- CBTRUS
Central Brain Tumor Registry of the United States
- CI
confidence interval
- CNS
central nervous system
- CT
computed tomography
- IARC
International Agency for Research on Cancer
- ICCC-3
International Classification of Childhood Cancer, 3rd Edition
- ICD-O-3
International Classification of Diseases for Oncology, 3rd Edition
- MRI
magnetic resonance imaging
- NAACCR
North American Association of Central Cancer Registries
- NOS
not otherwise specified
- NPCR
National Program of Cancer Registries
- PNET
primitive neuro-ectodermal tumors
- RF-EMF
radiofrequency electromagnetic fields
- SEER
Surveillance, Epidemiology and End Results
- WHO
World Health Organization
Footnotes
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018. doi 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7–30 doi 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7–30 doi 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Black WC. Increasing incidence of childhood primary malignant brain tumors--enigma or no-brainer? J Natl Cancer Inst 1998;90(17):1249–51. [DOI] [PubMed] [Google Scholar]
- 5.Smith MA, Freidlin B, Gloeckler Ries L, Simon R. Trends in Reported Incidence of Primary Malignant Brain Tumors in Children in the United States. 1998. [DOI] [PubMed] [Google Scholar]
- 6.McKean-Cowdin R, Razavi P, Barrington-Trimis J, Baldwin RT, Asgharzadeh S, Cockburn M, et al. Trends in childhood brain tumor incidence, 1973–2009. J Neurooncol 2013;115(2):153–60 doi 10.1007/s11060-013-1212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin RT, Preston-Martin S. Epidemiology of brain tumors in childhood—a review. Toxicol Appl Pharmacol 2004;199(2):118–31. [DOI] [PubMed] [Google Scholar]
- 8.Crump C, Sundquist J, Sieh W, Winkleby MA, Sundquist K. Perinatal and familial risk factors for brain tumors in childhood through young adulthood. Cancer Res 2014:canres. 2285.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braganza MZ, Kitahara CM, Berrington de Gonzalez A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol 2012;14(11):1316–24 doi 10.1093/neuonc/nos208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kheifets L, Ahlbom A, Crespi C, Feychting M, Johansen C, Monroe J, et al. A pooled analysis of extremely low-frequency magnetic fields and childhood brain tumors. Am J Epidemiol 2010;172(7):752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato Y, Kiyohara K, Kojimahara N, Yamaguchi N. Time trend in incidence of malignant neoplasms of the central nervous system in relation to mobile phone use among young people in Japan . Bioelectromagnetics 2016;37(5):282–9 doi 10.1002/bem.21982. [DOI] [PubMed] [Google Scholar]
- 12.Chapman S, Azizi L, Luo Q, Sitas F. Has the incidence of brain cancer risen in Australia since the introduction of mobile phones 29 years ago? Cancer Epidemiol 2016;42:199–205 doi 10.1016/j.canep.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Little MP, Rajaraman P, Curtis RE, Devesa SS, Inskip PD, Check DP, et al. Mobile phone use and glioma risk: comparison of epidemiological study results with incidence trends in the United States. BMJ 2012;344:e1147 doi 10.1136/bmj.e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 2017;19(suppl_5):v1–v88 doi 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009. Pediatrics 2014;134(4):e945–55 doi 10.1542/peds.2013-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol 2006;8(1):27–37 doi 10.1215/S1522851705000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64(2):83–103 doi 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 18.Khanna V, Achey RL, Ostrom QT, Block-Beach H, Kruchko C, Barnholtz-Sloan JS, et al. Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J Neurooncol 2017. doi 10.1007/s11060-017-2594-6. [DOI] [PubMed] [Google Scholar]
- 19.North American Association of Central Cancer Registries. 2018. 09/05/2018. Certification Criteria. <https://www.naaccr.org/certification-criteria/>. Accessed 2018 09/05/2018.
- 20.National Cancer Institute. 2008 12/11/2017. ICCC Recode ICD-O-3/WHO 2008. <https://seer.cancer.gov/iccc/iccc-who2008.html>. Accessed 2017 12/11/2017.
- 21.Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro Oncol 2015;16 Suppl 10:x1–x36 doi 10.1093/neuonc/nou327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis D, Ohgaki H, Wiestler O, Cavenee W, Cancer IAfRo World Health Organization Histological Classification of Tumours of the Central Nervous System (Revised 4th edition). Lyon: International Agency for Research on Cancer; 2016. [Google Scholar]
- 23.Steliarova‐Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer. Cancer 2005;103(7):1457–67. [DOI] [PubMed] [Google Scholar]
- 24.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Parkin DM, et al. International classification of diseases for oncology. Geneva: World Health Organization; 2000. [Google Scholar]
- 25.National Cancer Institute. Topographic Sites. <https://training.seer.cancer.gov/brain/tumors/abstract-code-stage/topographic.html>. Accessed 2018.
- 26.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19(3):335–51. [DOI] [PubMed] [Google Scholar]
- 27.Gittleman HR, Ostrom QT, Rouse CD, Dowling JA, de Blank PM, Kruchko CA, et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer 2015;121(1):102–12 doi 10.1002/cncr.29015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgakis MK, Karalexi MA, Kalogirou EI, Ryzhov A, Zborovskaya A, Dimitrova N, et al. Incidence, time trends and survival patterns of childhood pilocytic astrocytomas in Southern-Eastern Europe and SEER, US. J Neurooncol 2017;131(1):163–75 doi 10.1007/s11060-016-2284-9. [DOI] [PubMed] [Google Scholar]
- 29.Tulla M, Berthold F, Graf N, Rutkowski S, von Schweinitz D, Spix C, et al. Incidence, Trends, and Survival of Children With Embryonal Tumors. Pediatrics 2015;136(3):e623–32 doi 10.1542/peds.2015-0224. [DOI] [PubMed] [Google Scholar]
- 30.Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg 1996;85(1):56–65 doi 10.3171/jns.1996.85.1.0056. [DOI] [PubMed] [Google Scholar]
- 31.Versteege I, Sévenet N, Lange J, Rousseau-Merck M-F, Ambros P, Handgretinger R, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 1998;394(6689):203. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez FJ, Vizcaino MA, Lin MT. Recent Advances on the Molecular Pathology of Glial Neoplasms in Children and Adults. J Mol Diagn 2016;18(5):620–34 doi 10.1016/j.jmoldx.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felmeister A, Lulla R, Waanders A, Raman P, Santi M, Lilly J, et al. Gene-12. The Children’s Brain Tumor Tissue Consortium (cbttc) Infrastructure Facilitates Collaborative Research In Pediatric Central Nervous System Tumors. Neuro Oncol 2017;19(suppl_4):iv20–iv1. [Google Scholar]
- 34.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol 2005;109(1):93–108. [DOI] [PubMed] [Google Scholar]
- 35.Kuppermann N, Holmes JF, Dayan PS, Hoyle JD, Atabaki SM, Holubkov R, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. The Lancet 2009;374(9696):1160–70 doi 10.1016/s0140-6736(09)61558-0. [DOI] [PubMed] [Google Scholar]
- 36.Frush DP, Frush KS. The ALARA concept in pediatric imaging: building bridges between radiology and emergency medicine: consensus conference on imaging safety and quality for children in the emergency setting, Feb. 23–24, 2008, Orlando, FL - Executive Summary . Pediatr Radiol 2008;38 Suppl 4:S629–32 doi 10.1007/s00247-008-1006-7. [DOI] [PubMed] [Google Scholar]
- 37.Baan R, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, et al. Carcinogenicity of radiofrequency electromagnetic fields. The Lancet Oncology 2011;12(7):624–6 doi 10.1016/s1470-2045(11)70147-4. [DOI] [PubMed] [Google Scholar]
- 38.O’Neill KA, Murphy MF, Bunch KJ, Puumala SE, Carozza SE, Chow EJ, et al. Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases. Int J Epidemiol 2015;44(1):153–68 doi 10.1093/ije/dyu265. [DOI] [PubMed] [Google Scholar]
- 39.Donahue SM, Kleinman KP, Gillman MW, Oken E. Trends in birth weight and gestational length among singleton term births in the United States: 1990–2005. Obstet Gynecol 2010;115(2 Pt 1):357–64 doi 10.1097/AOG.0b013e3181cbd5f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N Engl J Med 2015;373(24):2336–46 doi 10.1056/NEJMoa1508054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hottinger AF, Khakoo Y. Neurooncology of familial cancer syndromes. J Child Neurol 2009;24(12):1526–35. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. 2017 Jan 23, 2018. NPCR Standards. <https://www.cdc.gov/cancer/npcr/standards.htm>. Jan 23, 2018.
- 43.Havener L, Thornton M. Standards for cancer registries volume II: Data standards and data dictionary. Version; 2006.
- 44.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 2013;15 Suppl 2:ii1–56 doi 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro Oncol 2016;18(suppl_5):v1–v75 doi 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howe H NAACCR Hispanic Identification Algorithm. North American Association of Central Cancer Registries Annual Meeting 2003.
- 47.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120(9):1290–314 doi 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribble F, PhD M, Keddie M. Understanding the Hispanic paradox. Ethn Dis 2001;11(3):496–518. [PubMed] [Google Scholar]
- 49.Turra CM, Elo IT. The impact of salmon bias on the Hispanic mortality advantage: New evidence from social security data. Population research and policy review 2008;27(5):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.