Abstract
Rationale:
Alcohol use disorder (AUD) has been associated with greater discounting of delayed monetary rewards, but it is unclear whether this association is primarily related to alcohol consumption or is secondary to the presence of psychiatric comorbidities. It is also unclear if steeper rates of discounting are associated with greater AUD severity.
Objective:
We sought to determine whether the presence of comorbid psychiatric disorders affected the relationship between AUD and delay discounting. We also examined whether more severe AUD was associated with greater delay discounting.
Methods:
In this cross-sectional study, 793 adults completed a delay discounting task. Subjects were divided into four groups based on diagnosis: current AUD with psychiatric comorbidities (N = 226), current AUD without psychiatric comorbidities (N = 203), past AUD (N = 69), and healthy controls (N = 295). In those with AUD, we investigated the relationship between delay discounting and alcohol dependence symptom count and recent drinking history. We also compared individuals seeking treatment to non-treatment seeking individuals. Psychiatric comorbidities examined included mood disorders, anxiety disorders, and substance use disorders.
Results:
After adjusting for age, sex, income, and education, individuals with current AUD showed significantly higher rates of delay discounting than healthy controls and individuals with a past diagnosis of AUD (partial η2 = 0.017). The presence of comorbid psychiatric diagnoses was not associated with steeper discounting. Among those with AUD, there was no evidence for a continuous relationship between delay discounting and AUD severity or alcohol consumption. Finally, non-treatment seekers with AUD had steeper delay discounting than treatment seekers.
Conclusions:
Individuals with AUD show steeper delay discounting than healthy adults, but the effect is small and there is no added effect from comorbid psychopathology or increased AUD severity. This suggests that steeper delay discounting may have a more limited effect on human alcohol use than previously supposed.
Keywords: Alcoholism, Delay Discounting, Decision Making, Impulsivity, Substance-Related Disorders, Anxiety Disorders, Depression
Introduction
Evidence from many species and contexts shows that individuals place less value on delayed reinforcers relative to immediate ones (Ainslie 1974; Green et al. 1994; Wilhelm and Mitchell 2009; Woolverton et al. 2007; Wright et al. 2012). This process is called delay discounting, and it is considered a form of choice impulsivity (Swann et al. 2002). Individuals differ in the rate at which they discount delayed outcomes (Shamosh et al. 2008) and substantial evidence suggests that individuals with alcohol use disorder show steeper rates of delay discounting relative to healthy controls (Bjork et al. 2004; Bobova et al. 2009; MacKillop et al. 2011; MacKillop et al. 2010). Further, healthy young adults with steeper delay discounting are more likely to achieve binge-level of consumption during a two-hour alcohol self-administration session (Gowin et al. 2017). Consequently, it is theorized that steep discounting of delayed outcomes may contribute to the development and maintenance of problematic alcohol and substance use (Bickel et al. 2012). For example, an individual with alcohol use disorder may choose the immediate effects of intoxication rather than the delayed benefits associated with moderation or abstinence, such as improved health (Griswold et al. 2018; Smyth et al. 2015), productivity (Sacks et al. 2012), and interpersonal relationships (Billings and Moos 1983; Kelly et al. 2000).
The theory that steep delay discounting underlies problematic alcohol use is well-supported by evidence, yet a number of questions remain. For example, alcohol use disorder is frequently comorbid with other psychiatric disorders, such as anxiety disorders (Grant et al. 2015), and there is evidence that depression (Yoon et al. 2007), anxiety (Rounds et al. 2007), and other substance use disorders (Bickel et al. 1999) are also associated with steep delay discounting. Meta-analysis has shown that, relative to healthy adults, substance use disorders also share common neural circuitry abnormalities with anxiety and depression during cognitive control tasks (McTeague et al. 2017). The regions affected, such as the insula, anterior cingulate, and lateral prefrontal cortex, have also been linked to decision making during delay discounting (Kishinevsky et al. 2012; Wittmann et al. 2007), suggesting that shared neural processing deficits that affect delay discounting could be common to multiple psychiatric disorders. However, most studies have not assessed the role of these disorders to determine whether steeper delay discounting is specifically related to alcohol use or is due to comorbid disorders. Thus, it remains unclear to what extent differences in delay discounting that have been attributed to problematic alcohol use are actually due to these comorbidities.
There is also some evidence that greater delay discounting is associated with more severe alcohol use problems (Vuchinich and Simpson 1998). For example, meta-analytic evidence has shown that groups meeting clinical diagnoses for alcohol use disorder show greater disparities with comparison groups relative to heavy drinkers without a diagnosis of an alcohol use disorder (MacKillop et al. 2011). However, the studies in this analysis had sample sizes that ranged from 9 to 121 with a median of 24 (MacKillop et al. 2011), so the estimates of effect size are likely imprecise. Further, some studies included in the meta-analysis did not adjust for age, income, and education (Bjork et al. 2004; Field et al. 2007), but these factors are likely to contribute to differences in delay discounting behavior (Reimers et al. 2009). Additional meta-analytic evidence has shown that individuals with steeper rates of delay discounting have more severe alcohol use disorders and greater consumption, but the effect size of this relationship is small, and there was also evidence of publication bias and some evidence of a small decline in the magnitude of effect size over time (Amlung et al. 2017). Given these factors, the true effect size remains unknown. Further, it remains unclear whether delay discounting is progressively steeper in individuals with higher levels of alcohol consumption, or whether delay discounting is steeper in those with AUD but fails to increase with greater levels of consumption.
Here, we gathered the largest sample to date of healthy controls, individuals with a past diagnosis of alcohol use disorder, and individuals with a current diagnosis of alcohol use disorder, both with and without comorbid psychopathology, to perform a multi-item, monetary delay discounting task in the laboratory. We examined the hypothesis that individuals with AUD would have steeper rates of delay discounting relative to controls, and that individuals with comorbid anxiety, depression, or substance use disorders would have steeper rates of discounting relative to individuals with AUD without comorbidity. These analyses will help clarify whether steeper delay discounting is a specific risk factor for alcoholism or whether it is also associated with the presence of related comorbidities. We also hypothesized that steeper delay discounting would be associated with both greater quantities of alcohol consumption and greater severity of alcohol use disorder. Finally, we aimed to provide a more reliable estimate of the magnitude of the effect size of differences in delay discounting due to associated with AUD.
Methods
Participants
Participants (N = 839) were recruited to participate in one of three NIH Institutional Review Board approved screening and evaluation protocols at the National Institutes of Health Clinical Center in Bethesda, Maryland. The purpose of these protocols is to characterize healthy adults and individuals with alcohol use disorder by collecting a common set of measures for all participants at our institute and to determine eligibility to participate in other studies. The protocol into which the participant was screened depended on which was active and whether the participant was seeking treatment. Exclusion criteria for the screening studies were pregnancy, being less than 18 years of age, being unable to provide informed consent, or being a prisoner. Individuals seeking inpatient treatment for alcohol-related problems were admitted for an approximately 4-week hospitalization and completed assessments during this period. Individuals who were not seeking treatment were enrolled upon arrival for a screening visit in the clinic and completed assessments during that visit or during a follow-up outpatient visit.
Participants were divided into four groups based on alcohol use disorder diagnosis and presence of psychiatric comorbidities. First, healthy controls (N = 295) were not nicotine dependent and did not meet criteria for current or past alcohol use abuse or dependence or other Axis I diagnoses. Second, individuals with past alcohol use disorder (N = 69) met criteria for a past but not current alcohol abuse or dependence. Due to the relatively small size, this group contained individuals with and without comorbid psychopathology, though only 7 individuals had any diagnosis (see Table 1). A third group contained individuals with current alcohol abuse or dependence but without any comorbid diagnoses (N = 203). The fourth group (N = 226) contained subjects with a current diagnosis of alcohol abuse or dependence and a current comorbid diagnosis of any of the following disorders: depression, anxiety, cannabis abuse or dependence, cocaine abuse or dependence, or nicotine dependence.
Table 1.
Sample Characteristics
| Control (N = 295) |
Past AUD (N = 69)1 |
AUD Without Comorbidity (N = 203) |
AUD With Comorbidities (N = 226) |
||
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | Test Statistic | |
| Sex (male) | 149 (50.5) | 39 (56.5) | 160 (78.8) | 144 (63.7) | χ2(3) = 42.08 |
| Treatment Seeking | 0 (0.0) | 0 (0.0) | 90 (44.3) | 161 (71.2) | χ2(3) = 347.38 |
| Cannabis Abuse or Dependence | 0 (0.0) | 0 (0.0) | 0 (0.0) | 37 (16.4) | χ2(3) = 97.37 |
| Cocaine Abuse or Dependence | 0 (0.0) | 0 (0.0) | 0 (0.0) | 18 (8.0) | χ2(3) = 46.21 |
| Depression Diagnosis2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 59 (26.1) | χ2(3) = 159.92 |
| Anxiety Diagnosis2 | 0 (0.0) | 7 (10.1) | 0 (0.0) | 93 (41.2) | χ2(3) = 239.29 |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| Age3 | 29.80 (17.0) | 33.40 (16.0) | 47.10 (19.0) | 47.80 (17.0) | H(3) = 144.31 |
| Years of Education4 | 16 | 16 | 13–15 | 12 | H(3) = 142.42 |
| Household Income4 | $40000–$49999 | $30000–$39999 | $20000–$29999 | $10000–$19999 | H(3) = 79.68 |
| FTND Score5,6 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4.0 (5.0) | H(3) = 386.11 |
| MADRS Score7,8 | 0.0(1.0) | 0.0 (4.0) | 2.0 (5.0) | 5.0 (8.0) | H(3) = 199.83 |
| STAI Score7,9 | 27.0(9.0) | 32.0(14.0) | 38.0 (16.0) | 51.0(18.0) | H(3) = 268.32 |
Bold font indicates p < 0.001.
Of these, 29 had past dependence and 40 had past abuse
See Methods for a list of diagnoses included
Pairwise comparisons showed that both Controls and Past AUD were significantly different than both current AUD groups, but Controls and Past AUD did not differ, and the current AD groups did not differ.
Pairwise comparisons showed that all groups were significantly different from each other except for Controls and Past AUD individuals.
Pairwise comparisons showed that Controls were significantly different from the other three groups, and current AUD with comorbidities were significantly different from the other three groups. Past AUD did not differ from current AUD without comorbidity.
The FTND is the Fagerström Test for Nicotine Dependence.
Pairwise comparisons indicated that all groups were significantly different from each other
The MADRS, or Montgomery Asberg Depression Rating Scale, was used to assess depressive symptoms. For this measure, the number of participants were as follows: Control = 244, Past AUD = 62, AUD without comorbidity = 184, and AUD with comorbidity = 212.
The STAI-T, or State-Trait Anxiety Inventory-Trait version, was used to assess anxiety symptoms. For this measure, the number of participants were as follows: Control = 278, Past AUD = 66, AUD without comorbidity = 151, and AUD with comorbidity = 161.
Procedures
A monetary delay discounting task was used to measure choice impulsivity (Gowin et al. 2017). Individuals in treatment typically completed this task 2–3 weeks after admission and were, therefore, not in acute withdrawal. During this task, participants were presented with 66 sets of choices between hypothetical amounts of money. Each set was a choice between a smaller, immediate reward or a larger reward of $100 to be received after a delay (e.g. $80 now or $100 in 14 days). The time delay varied between 0, 7, 14, 20, 25, and 30 days, and the immediate monetary amounts varied between $0, $10, $20, $30, $40, $50, $60, $70, $80, $90, and $100. These rewards and delays were randomly presented (i.e. order of presentation was not dependent on the participant’s choices). The degree of discounting was indexed by a constant, k, calculated from a hyperbolic equation developed by Mazur (1987) using MATLAB software. As values of k were not normally distributed, we used the natural log transformed value of k, ln (k), as our primary measure. A higher value of ln (k) indicates a steeper discounting of delayed rewards. Always opting for $100 in the future instead of a lower value now (e.g. $90 now) resulted in a ln (k) value of −6.03, but it was possible to achieve a lower value of ln (k) by selecting $100 in the future instead of $100 now; we reasoned that such a choice was likely due to inattention rather than preference, so participants with a value lower than −6.03 were given the value of −6.03 (N=31; 12 controls [4.1%], 7 past AUD [10.1%], 3 AUD without comorbidities [1.5%], 9 AUD with comorbidities [4.0%]). In addition, as the hyperbolic function does not always fit an individual’s data, the area under the curve was also examined, but the raw values were lost for 58 participants, so only 735 participants could be analyzed using the area under the curve. Analyses were conducted using both the area under the curve and ln (k), but since results were nearly identical, area under the curve analyses are presented in supplemental materials and only ln (k) is reported in the Results section.
Participants completed a Structured Clinical Interview for DSM-IV-TR Axis I disorders (SCID-IV; (First et al. 2002). Primary outcomes of interest were diagnoses of current or past alcohol abuse or dependence, current depressive disorders (i.e. dysthymia, major depressive disorder), current anxiety disorders (i.e. panic disorder, agoraphobia, specific phobia, obsessive compulsive disorder, post-traumatic stress disorder, social anxiety disorder, and generalized anxiety disorder), current cannabis abuse and dependence, and current cocaine abuse and dependence. We only included cannabis and cocaine for comorbid substance use disorders because there were too few individuals with diagnoses of any other substance use disorder (e.g. opioid use disorder). Nine participants in the alcohol use disorder with comorbidities group also met criteria for a current opioid use or sedative use disorder, but these additional diagnoses were not considered in the analyses. Some participants did not complete the SCIDIV during their visit and were excluded (N = 24). Healthy controls were excluded if they met criteria for any Axis I diagnosis (N = 14). Participants with a current or lifetime diagnosis of bipolar disorder or a psychotic disorder (N = 6) were also excluded, as there were too few to conduct meaningful analysis. Individuals seeking treatment for alcohol problems were assessed for alcohol dependence, but the abuse section of the SCID-IV was skipped. Thus, analyses of alcohol use disorder severity examined the number of alcohol dependence symptoms endorsed, from zero to seven. Thirty-one participants met criteria for alcohol dependence but their symptom counts were not recorded, and these individuals were excluded from the analysis of alcohol dependence severity.
The Fagerström Test for Nicotine Dependence (FTND) was used to determine tobacco use (Heatherton et al. 1991), and a cut-off score of 4 or higher was used as a marker of tobacco dependence and included as a comorbid disorder. Non-smokers were scored as 0 for continuous analyses. Those with scores below 4 but above 0 were included in the analyses as non-dependent smokers and categorized into their appropriate group.
Participants completed a demographics questionnaire that included sex, race, age, years of education, and household income. All participants provided sex, race, and age, but a small number of participants chose not to identify their education or income (N = 6). Years of education was divided into five groups: (1) less than 12 years of education and did not graduate high school, (2) completed 12 years education and graduated high school, (3) completed some university (>12 years, <16 years) but did not receive a bachelor’s degree, (4) graduated university but did not attend graduate school, or (5) completed university and attended graduate school. Household income was split into nine brackets of those whose annual household income was less than $5,000, $5,999-$9,999, $10,000-$19,999, $20,000-$29,999, $30,000-$39,999, $40,000-$49,999, $50,000-$74,999, $75,000-$100,000, and greater than $100,000.
Participants completed the 90-day Timeline Followback (TLFB) interview, a calendar-based method of retrospectively self-reporting daily alcohol consumption (Sobell and Sobell 1992). The primary variable used from this assessment was the total number of drinks in the past 90 days. We used the US standard drink definition of 14g of pure alcohol, which roughly equates to a 12 oz. beer, a 5 oz. glass of wine, or a 1.5 oz. shot of a distilled spirit (NIAAA 2004).
To assess depression as a continuous measure for linear regression analyses, we used the Montgomery Asberg Depression Rating Scale (MADRS; (Svanborg and Asberg 1994), collected as part of the Comprehensive Psychopathological Rating Scale (CPRS; (Asberg et al. 1978). Individuals in treatment completed this questionnaire weekly during the inpatient study, but we used the day 23 measure, as it was closest in time to when they completed the delay discounting task. For participants with missing values for this questionnaire (N = 201), the sample mean was imputed for analysis. Analyses was also conducted by excluding participants with missing values to assess whether imputation altered results.
To assess anxiety as a continuous measure for linear regression analyses, we used the State-Trait Anxiety Inventory (STAI) trait version (y2; (Spielberger 1983). Individuals in treatment completed this measure at baseline. For participants with missing values for this questionnaire (N = 201), the sample mean was imputed for analysis. Analyses were also conducted by excluding participants with missing values.
Analytic Approach
To examine whether psychiatric comorbidities were associated with steeper discounting, we employed a one-way analysis of variance (ANOVA) to compare the delay discounting values of the four groups (controls, past AUD, AUD with comorbidity, and AUD without comorbidity). Both ln (k) and area under the curve were examined to ensure that individuals with large values for the root mean square of the error for the hyperbolic function did not bias results. Next, we conducted an analysis of covariance (ANCOVA) to examine potential covariates of group differences. Since the groups differed in terms of age, sex, income, and education (see Table 1), these factors were included as covariates in the model. Post-hoc comparisons were conducted with Tukey’s Honestly Significant Difference test.
To determine whether severity of AUD was associated with steeper discounting, we used three approaches. First, we hypothesized that treatment-seeking individuals with AUD would have more severe problems than non-treatment-seekers, and treatment-seekers would also show steeper discounting. To confirm that treatment-seekers had more severe disorders than non-treatment seekers, we compared alcohol dependence symptom counts between the two groups using a Mann-Whitney test. We then conducted a t-test of both ln(k) values and areas under the curve between these two groups.
Second, we examined an index of AUD severity by conducting an ordinal logistic regression among individuals with a current diagnosis of an alcohol use disorder who had symptom counts for alcohol dependence (N=381). The dependent variable was number of DSM-IV alcohol dependence symptoms met. Since participants with alcohol abuse were included in the sample, we used a category of 2 or fewer symptoms, and we also had categories for 3, 4, 5, 6, and 7 symptoms. The primary independent variable was delay discounting, indexed by ln (k). Age was included as a covariate. Years of education was included as a categorical variable. Income was included as a categorical variable, but, to conserve degrees of freedom, the number of categories was reduced to three: low (<$30,000), middle ($30,000–$74,999) and high (≥$75,000). The model also included binary variables for sex, presence of a comorbid substance use diagnosis (i.e. cannabis abuse or dependence or cocaine abuse or dependence, or nicotine dependence as defined by FTND score greater than or equal to 4) and presence of a comorbid anxiety or depression diagnosis.
Third, we examined quantity of alcohol consumption as an index of heavier drinking using linear regression analyses. The total number of drinks from the 90-day Timeline Followback served as the dependent variable. One analysis was conducted separately for a sub-sample restricted to controls and a second analysis was conducted for a sub-sample restricted to individuals with a current diagnosis of AUD. Number of drinks was natural log transformed since the distribution was significantly positively skewed, and this affected the diagnostics of the linear regression model. Since 58 controls consumed zero drinks, one drink was added to the total drinks prior to the log transformation for controls only. The primary independent variable of interest was delay discounting, with analyses run independently with ln (k) and the area under the curve. Other variables included continuous metrics of anxiety and depression as assessed by the STAI and MADRS, respectively, as well as income, years of education, sex, and age. The model restricted to AUD individuals also included a continuous metric of nicotine dependence severity as assessed by the FTND, and a binary variable indicating presence of a comorbid substance use diagnosis (i.e. cannabis abuse or dependence or cocaine abuse or dependence). The model restricted to AUD individuals was also conducted with a binary variable for comorbid anxiety or depression diagnosis since a large portion of the sample was missing MADRS and STAI measurements.
All statistical analyses were conducted using SPSS version 25 (IBM Corporation), except the ordinal regression, which was conducted with R, version 3.5.1.
Results
Group characteristics and differences
The control group was younger, had a larger percentage of females, and had higher income and education levels relative to both current AUD groups (all p < 0.05, see Table 1). The past AUD group was intermediate between controls and current AUD groups on each of these measures except education, where they had the same median education level as controls. The current AUD group with psychiatric comorbidity contained a larger percentage of individuals seeking treatment relative to the current AUD group without comorbidity (p < 0.05, Table 1). The current AUD group with comorbidities had a higher median number of dependence symptoms (median = 7, IQR = 2) than the current AUD group without comorbidities (median = 5, IQR = 3), and the distributions were significantly different between groups (U381 = 24,468.0, p < 0.001). Treatment seekers (median = 7, IQR = 1) reported significantly more symptoms of dependence than non-treatment seekers (median = 4, IQR = 3), and this difference was significant (U381 = 30,828.0, p < 0.001).
Diagnostic Group and Delay Discounting
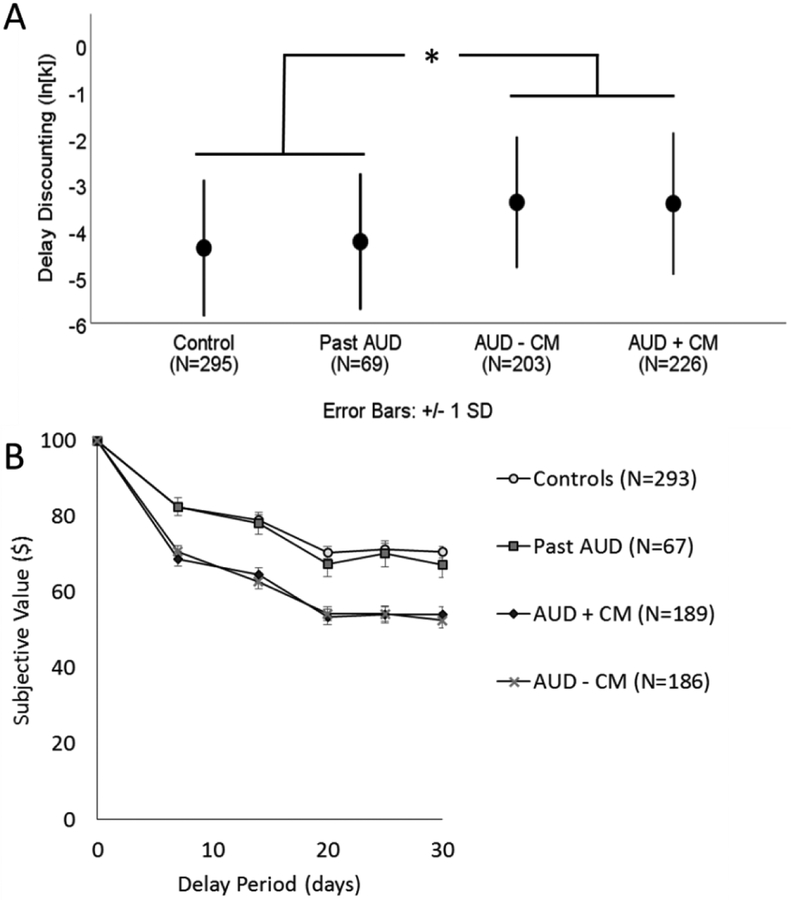
A one-way ANOVA revealed a main effect of diagnostic group on delay discounting as indexed by both ln(k) (F(3, 789) = 27.16, p < 0.001, partial η2 = 0.094; Figure 1). Healthy controls had significantly lower values of ln (k) relative to both current AUD groups (p < 0.001 for both tests). However, individuals with past AUD did not differ significantly from healthy controls (p = 0.505), and the two current AUD groups did not differ from each other (p = 0.983). The effect size between healthy controls and AUD with and without comorbidities was approximately the same (compared to AUD with comorbidities: Cohen’s d = 0.65; compared to AUD without comorbidity: Cohen’s d = 0.67).
Figure 1.
Panel A shows the mean of the delay discounting metric, ln(k), for the control group (mean=−4.38, SD = 1.47), individuals who with past alcohol dependence (Past AD, mean= −4.24, SD = 1.47), individuals who with current alcohol dependence but no comorbidity (AUD – CM, mean = −3.41, SD = 1.44), and individuals with current alcohol dependence and comorbidities (AUD + CM, mean = −3.41, SD = 1.52). Error bars represent standard deviations. After adjusting for sex, age, and socioeconomic status, the pairwise comparison between controls and AUD – CM was significant, but no other pairwise comparisons were significant. Panel B depicts the subjective value of $100 after each of the 5 delays by group. The markers represent the mean of the group and the error bars represent one standard deviation. Each individual’s subjective value was determined by the indifference point, the value at which they would equally prefer $100 after the delay or the smaller, immediate amount. The number of participants depicted in Panel B is less than in the final sample because the raw data for decisions was lost for some current and past alcohol dependent participants, although the summary data (k) for these participants was preserved. * indicates p<0.05
After adjusting for age, sex, income, and education using ANCOVA, there remained a main effect of diagnostic group (F(7,781) = 4.46, p = 0.004, partial η2 = 0.017), but the variance explained by group was reduced. Age was not significant, but sex (p = 0.001, partial η2 = 0.013), education (p < 0.001, partial η2 = 0.043), and income (p < 0.001, partial η2 = 0.027) were all significant. Education and income explained more of the variance of delay discounting behavior than diagnostic group. Pairwise comparisons showed that healthy controls had significantly lower levels of delay discounting relative to individuals with AUD without comorbidities (p = 0.005), but the difference with AUD with comorbidities was reduced to trend-level (p = 0.083). Healthy controls did not differ from individuals with past AUD (p = 0.826). No other pairwise comparisons reached significance. When comparing marginal means, effect size between healthy controls and AUD individuals was reduced (compared to AUD without comorbidity: Cohen’s d = 0.32; compared to AUD with comorbidity: Cohen’s d = 0.24).
Treatment-Seeking Status and Delay Discounting
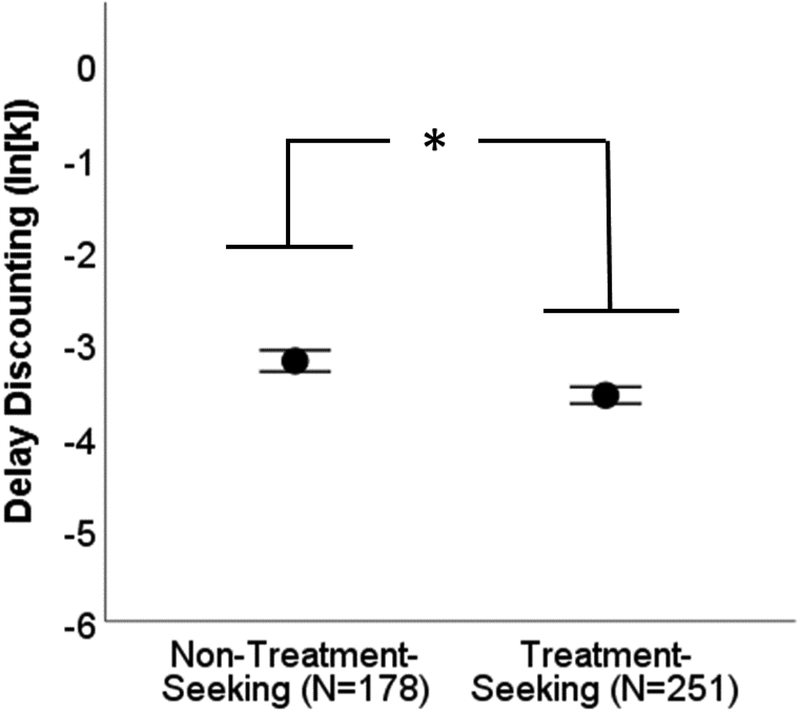
Non-treatment seeking individuals with AUD showed steeper delay discounting as indexed by ln(k) (M = −3.15, SD = 1.55) relative to treatment-seeking individuals (M = −3.56, SD = 1.42), and this effect was significant (t412 = −2.79, p = 0.006, Cohen’s d = 0.27, Figure 2).
Figure 2.
Individuals with alcohol use disorder who were seeking treatment showed significantly lower rates delay discounting relative to individuals with alcohol use disorder who were not seeking treatment. This evidence opposed our hypothesis that individuals with a more severe disorder would show greater discounting. Markers represent the mean and the error bars represent one standard error. * indicates p<0.05.
Alcohol Use Disorder Severity and Delay Discounting
An ordinal regression analysis was conducted among individuals with a current diagnosis of AUD, and severity was indexed by the DSM-IV Alcohol Dependence symptom count (Table 2). The model indicated that ln(k) was not a significant predictor of AUD severity (odds ratio=0.91, 95% CI=0.79–1.04, p = 0.169). Similarly, sex, income, and education were not significant predictors of AUD severity (all p>0.05). Older age was associated with greater AUD severity (odds ratio=1.03, 95% CI=1.02–1.05, p<0.001). Presence of a second substance use disorder (odds ratio=2.49, 95% CI=1.64–3.80, p < 0.001) and presence of an anxiety or depressive disorder (odds ratio=2.78, 95%CI=1.75–4.47, p < 0.001) were both associated with more severe AUD.
Table 2.
Ordinal Logistic Regression Model of Alcohol Use Disorder Severity as Indicated by Dependence Symptom Count
| Variable | Odds Ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Delay discounting (In (k)) | 0.91 | 0.79, 1.04 | 0.169 |
| Gender (Male) | 1.07 | 0.69, 1.68 | 0.754 |
| Age (years) | 1.03 | 1.02, 1.05 | <0.001 |
| Years of Education (12 years)1 | 1.28 | 0.69, 2.35 | 0.435 |
| Years of Education (13–15 years)1 | 1.65 | 0.85, 3.21 | 0.135 |
| Years of Education (16 years)1 | 1.20 | 0.60, 2.40 | 0.604 |
| Years of Education (17–30 years)1 | 2.02 | 0.85, 4.89 | 0.112 |
| Low Income Category2 | 1.02 | 0.52, 1.99 | 0.959 |
| Middle Income Category2 | 0.77 | 0.39, 1.51 | 0.454 |
| Anxiety or Depressive Diagnosis | 2.78 | 1.75, 4.47 | <0.001 |
| Cocaine, Cannabis, or Nicotine Abuse or Dependence | 2.49 | 1.64, 3.80 | <0.001 |
Reference category is “Less than 12 years of education”
Reference category is “High income (≥$75,000)”
Recent Drinking and Delay Discounting
After examining group differences and the influence of discounting on AUD severity, we examined the influence of delay discounting on recent drinking history after adjusting for additional variables. A linear regression analysis conducted among only individuals with a current diagnosis of AUD (N = 429) explained 15.7% of the variance of total drinks (Table 3). Male sex, less education, higher nicotine dependence severity, and higher anxiety score were significantly associated with a greater number of drinks (all p < 0.05). Delay discounting was not associated with alcohol consumption (b = 0.02, 95% confidence interval: −0.03, 0.07, t = 0.74, p = 0.459, Supplemental Figure 1) and did not explain any of the variance in total drinks (ΔR2 = 0.00%). However, 117 individuals were missing an anxiety score, and 33 were missing a depression score. Seven individuals were missing both scores, so a total of 143 individuals had incomplete data. When the model was conducted by excluding participants with missing values for the continuous measures of depression and anxiety, resulting in a sample of 287 AUD individuals, the model produced consistent results (i.e. no variables became significant or lost significance).
Table 3.
Linear Regression Models of Recent Drinking
| Current Alcohol Dependence Model1 | |||
|---|---|---|---|
| b | 95% Confidence Interval | p-value | |
| Constant | 5.56 | 4.97, 6.15 | <0.001 |
| Delay Discounting (In (k)) | 0.02 | −0.03, 0.07 | 0.459 |
| Male Sex | 0.29 | 0.12, 0.46 | 0.001 |
| Age (years) | 0.00 | 0.00, 0.01 | 0.426 |
| Education | −0.09 | −0.16, −0.02 | 0.017 |
| Income | −0.02 | −0.05, 0.01 | 0.166 |
| Nicotine Dependence Severity2 | 0.06 | 0.03, 0.09 | <0.001 |
| Anxiety (STAI score)3 | 0.01 | 0.00, 0.02 | 0.010 |
| Depression (MADRS score)4 | 0.01 | −0.01, 0.02 | 0.334 |
| Cocaine or Cannabis Use Disorder5 | 0.11 | −0.10, 0.31 | 0.322 |
| Healthy Control Model | |||
| b | 95% Confidence Interval | p-value | |
| Constant | 1.08 | −0.40, 2.57 | 0.153 |
| Delay Discounting (In (k)) | 0.07 | −0.06, 0.19 | 0.322 |
| Male Sex | 0.35 | −0.01, 0.71 | 0.054 |
| Age (years) | −0.05 | −0.06, −0.03 | <0.001 |
| Education | 0.55 | 0.37, 0.74 | <0.001 |
| Income | 0.05 | −0.03, 0.13 | 0.189 |
| Anxiety (STAI score)3 | 0.02 | −0.01, 0.05 | 0.186 |
| Depression (MADRS score)4 | 0.07 | −0.05, 0.18 | 0.263 |
This model imputed missing values for Anxiety and Depression ratings
Nicotine dependence was assessed via Fagerström Test for Nicotine Dependence.
The STAI, or State-Trait Anxiety Inventory, was used to assess anxiety symptoms.
The MADRS, or Montgomery Asberg Depression Rating Scale, was used to assess depressive symptoms.
Cocaine or cannabis diagnosis included current abuse or dependence as assessed by the SCID-IV-TR.
A linear regression model conducted in healthy controls (N = 243) explained 20.6% of the variance of number of drinks (Table 3). However, 17 individuals were missing an anxiety score, and 51 were missing a depression score. Sixteen individuals were missing both scores, so a total of 52 individuals had incomplete data. When the model was conducted by excluding participants with missing values rather than imputing the mean, the model remained significant and no variables changed significance status. After adjusting for covariates, delay discounting was not associated with alcohol consumption (b = 0.07, 95% confidence interval: −0.06, 0.19, t = 0.99, p = 0.322, Supplemental Figure 1). Delay discounting accounted for a negligible proportion of the variance in total drinks (ΔR2 = 0.01%). Younger age and greater education were significantly associated with a greater number of drinks (p < 0.001), but no other variables reached significance.
Discussion
Our results suggest that steep rates of delay discounting among individuals with AUD are not attributable to comorbid psychopathology but are due to AUD itself. Although steep delay discounting is associated with pathologic alcohol use, the effect size when controlling for covariates (d = 0.32) was smaller than the magnitude (d = 0.50) reported by a comprehensive meta-analysis of clinical samples of alcohol users (MacKillop et al. 2011). Surprisingly, among individuals with AUD, increased discounting of delayed rewards was not associated with higher levels of alcohol consumption or more severe alcohol use disorder.
This study did not find that individuals with AUD and psychiatric comorbidity showed steeper delay discounting than individuals with AUD alone. The lack of an additive effect supports recent studies showing that alcohol abusers with and without comorbid nicotine or cocaine dependence showed similar levels of discounting (Moody et al. 2016b) and individuals with substance use disorder without comorbidity did not significantly differ from individuals with a use disorder with comorbid depression or antisocial personality disorder (Moody et al. 2016a). It may also be consistent with evidence that users of different substances show similar rates of delay discounting (Gowin et al. 2018). Another study showed that delay discounting is associated with nicotine dependence, but there was no additional effect of stimulant, opioid, or marijuana use (Amlung and MacKillop 2014). Thus, the aggregate of evidence suggests that having a substance use disorder is associated with greater levels of delay discounting, but once that increase is accounted for, there is no additive effect of additional psychopathology.
This study provided conflicting evidence regarding recent drinking, severity of AUD, and delay discounting. First, individuals with past AUD showed lower rates of delay discounting relative to individuals with current AUD, and past AUD individuals did not differ from controls. This corroborates a previous study that showed that former smokers discounted monetary rewards at similar rates compared with controls, and that both groups discounted delayed rewards less than current smokers (Bickel et al. 1999). By itself, such evidence could suggest a link between degree of substance use pathology and rate of delay discounting, where a greater burden of disorder would be associated with heightened discounting. However, the similar rates of discounting among controls and individuals with past AUD could be due to their similar levels of income and education, which explained more of the variance in discounting that AUD group. Without accounting for these variables, diagnostic group accounted for nearly 9% of the variance in discounting rate, but when accounting for them, diagnostic group only explained about 2% of the variance in delay discounting. It is also possible that the similar rates of discounting between individuals with a past AUD and healthy adults represents survivorship bias, where more cognitively intact individuals with AUD may be better able to maintain goals and sobriety. This executive function could be the same skillset that allows occasional drinkers to resist declines into AUD in the first place.
The relationship between severity of AUD and delay discounting was assessed as a continuous relationship. Using regression analyses, we found no relationship between delay discounting and quantity of consumption or severity of AUD. The scatter plots in Supplemental Figure 1 show substantial heterogeneity in rates of discounting across the spectrum of recent drinking, with no trend for increased discounting among individuals who consumed more alcohol. The evidence from this study therefore suggests a ceiling effect; delay discounting was associated with the presence of AUD but did not continue to increase with greater levels of AUD severity.
To further explore whether severity of AUD was associated with discounting, we compared treatment seekers to non-treatment seekers. Treatment-seeking individuals endorsed more alcohol dependence symptoms than non-treatment seeking individuals, and we hypothesized that this would translate into steeper discounting. Instead, non-treatment seekers showed steeper discounting. This could be because the individuals in treatment did not complete the delay discounting task until they had been treated for three weeks, whereas the non-treatment seekers completed the task on their first day. Treatment seekers may have exhibited steeper discounting if assessed upon admission, when some of them were experiencing withdrawal, since withdrawal has been shown to exacerbate discounting rates in some (Giordano et al. 2002), but not all (Miglin et al. 2017), studies. The differences between treatment and non-treatment seekers could also be due to factors that were not assessed. For example, a common reason that individuals avoid treatment is because they deny having a problem (Grant 1997), and this mindset may coincide with steeper delay discounting.
A major limitation of the current study was our recruitment strategy, as the sample was derived from both community volunteers and treatment-seeking patients with alcohol problems instead of a homogenous population. This recruitment strategy may also have affected the observed relationship between alcohol use and delay discounting. The AUD sample was older, less educated, less wealthy, and had a greater percentage of males relative to the healthy controls, and these demographic differences could affect decision-making regarding subjective value of money across delays. Our analyses controlled for these variables, mitigating some concern, but do not preclude the possibility that demographic differences affected our results.
Another potential limitation is our assessment of delay discounting. Our task employed hypothetical money that is likely less salient than real money. Further, we only tested one monetary value, $100, and a relatively short delay of 30 days. However, hypothetical discounting tasks have shown a strong relationship with discounting of real rewards (Lagorio and Madden 2005), and behavior on one hypothetical task tends to be correlated with behavior on other tasks (Odum 2011). Another limitation is that we did not assess all types of psychopathology, so our findings may not generalize, for example, to individuals with alcohol use disorder and comorbid bipolar disorder.
This study represents the largest sample to date comparing individuals with alcohol use disorder and controls using a multi-item choice task of delay discounting. It thereby allowed for well-powered analyses, finding that alcohol use disorder was associated with delay discounting regardless of the presence of comorbid diagnoses. Nonetheless, the effect size is small. In those with AUD, our findings did not support a continuous association between delay discounting and alcohol consumption or AUD severity, which contradicted our hypothesis. Instead, there was little evidence of an additive influence of additional psychopathology or AUD severity on delay discounting behavior. Thus, although these results support delay discounting as a specific risk factor for alcoholism, they suggest a more limited and circumscribed effect and challenge theories that place steeper delay discounting as a central feature of alcoholism.
Supplementary Material
Acknowledgements:
This study was supported by the NIAAA Division of Intramural Clinical and Biological Research (Z1A AA000466) and a career development award (K99AA024778, PI: Gowin). We are grateful to Melanie Schwandt for compiling the data set and maintaining the database, and to Michael Kerich and Betsy Davis for maintaining the computer systems used for collecting the delay discounting data. We thank the nursing staff and the research assistants who have helped collect the data, and to all the participants for their time and cooperation. We also thank Alexandria Jensen for assistance with the ordinal logistic regression model.
Role of Funding Source: Nothing declared
Footnotes
Conflict of Interest: No conflict declared
References
- Ainslie GW (1974) Impulse Control in Pigeons. Journal of the Experimental Analysis of Behavior 21: 485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, MacKillop J (2014) Clarifying the Relationship Between Impulsive Delay Discounting and Nicotine Dependence. Psychology of Addictive Behaviors 28: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J (2017) Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction 112: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asberg M, Montgomery SA, Perris C, Schalling D, Sedvall G (1978) A comprehensive psychopathological rating scale. Acta Psychiatr Scand Suppl: 5–27. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM (2012) Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacology & Therapeutics 134: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ (1999) Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology 146: 447–454. [DOI] [PubMed] [Google Scholar]
- Billings AG, Moos RH (1983) Psychosocial processes of recovery among alcoholics and their families: Implications for clinicians and program evaluations. Addictive behaviors 8: 205–218. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C (2004) Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol 34: 133–150. [DOI] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J (2009) Disinhibitory Psychopathology and Delay Discounting in Alcohol Dependence: Personality and Cognitive Correlates. Experimental and Clinical Psychopharmacology 17: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A (2007) Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction 102: 579–586. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JB (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCIDI/PW/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. November [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ (2002) Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology 163: 174–182. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Ramchandani VA, Paulus MP, Lane SD (2018) Differences in decision-making as a function of drug of choice. Pharmacology Biochemistry and Behavior 164: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA (2017) Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. The American journal of psychiatry 174: 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B (1997) Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. Journal of Studies on Alcohol 58: 365–371. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72: 757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J (1994) Discounting of Delayed Rewards - a Life-Span Comparison. Psychological Science 5: 33–36. [Google Scholar]
- Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, Venkateswaran V, Tapp AD, Forouzanfar MH, Salama JS (2018) Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 392: 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Kelly AB, Halford WK, Young RM (2000) Maritally distressed women with alcohol problems: the impact of a short-term alcohol-focused intervention on drinking behaviour and marital satisfaction. Addiction 95: 1537–1549. [DOI] [PubMed] [Google Scholar]
- Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW, Weller RE (2012) fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite 58: 582–592. [DOI] [PubMed] [Google Scholar]
- Lagorio CH, Madden GJ (2005) Delay discounting of real and hypothetical rewards III: Steady-state assessments, forced-choice trials, and all real rewards. Behavioural Processes 69: 173–187. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR (2011) Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology 216: 305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Monti PM, Ray LA, Murphy JG, Rohsenow DJ, McGeary JE, Swift RM, Tidey JW, Gwaltney CJ (2010) Alcohol Demand, Delayed Reward Discounting, and Craving in Relation to Drinking and Alcohol Use Disorders. Journal of Abnormal Psychology 119: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE (1987) An adjusting procedure for studying delayed reinforcement. Commons, ML; Mazur, JE; Nevin, JA: 55–73. [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A (2017) Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. The American journal of psychiatry 174: 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglin R, Kable JW, Bowers ME, Ashare RL (2017) Withdrawal-Related Changes in Delay Discounting Predict Short-Term Smoking Abstinence. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco 19: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody L, Franck C, Bickel WK (2016a) Comorbid depression, antisocial personality, and substance dependence: Relationship with delay discounting. Drug and Alcohol Dependence 160: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody L, Franck C, Hatz L, Bickel WK (2016b) Impulsivity and Polysubstance Use: A Systematic Comparison of Delay Discounting in Mono-, Dual-, and Trisubstance Use. Experimental and Clinical Psychopharmacology 24: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA (2004) NIAAA council approves definition of binge drinking. NIAAA newsletter 3. Odum AL (2011) Delay discounting: Trait variable? Behavioural Processes 87: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers S, Maylor EA, Stewart N, Chater N (2009) Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Personality and Individual Differences 47: 973–978. [Google Scholar]
- Rounds JS, Beck JG, Grant DM (2007) Is the delay discounting paradigm useful in understanding social anxiety? Behaviour Research and Therapy 45: 729–735. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD (2012) 2010 National and State Costs of Excessive Alcohol Consumption. American Journal of Preventive Medicine 49: e73–e79. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, Engle RW, Braver TS, Gray JR (2008) Individual Differences in Delay Discounting Relation to Intelligence, Working Memory, and Anterior Prefrontal Cortex. Psychological Science 19: 904–911. [DOI] [PubMed] [Google Scholar]
- Smyth A, Teo KK, Rangarajan S, O’Donnell M, Zhang XH, Rana P, Leong DP, Dagenais G, Seron P, Rosengren A, Schutte AE, Lopez-Jaramillo P, Oguz A, Chifamba J, Diaz R, Lear S, Avezum A, Kumar R, Mohan V, Szuba A, Wei L, Yang W, Jian B, McKee M, Yusuf S, Investigators P (2015) Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet 386: 1945–1954. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back Measuring alcohol consumption. Springer, pp 41–72 [Google Scholar]
- Spielberger CD (1983) Manual for the State-Trait Anxiety Inventory STAI (form Y)(“self-evaluation questionnaire”).
- Svanborg P, Asberg M (1994) A New Self-Rating Scale for Depression and Anxiety-States Based on the Comprehensive Psychopathological Rating-Scale. Acta Psychiatrica Scandinavica 89: 21–28. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM (2002) Two models of impulsivity: Relationship to personality traits and psychopathology. Biological Psychiatry 51: 988–994. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA (1998) Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology 6: 292–305. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH (2009) Strain differences in delay discounting using inbred rats. Genes Brain and Behavior 8: 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP (2007) Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res 179: 643–53. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Myerson J, Green L (2007) Delay discounting of cocaine by rhesus monkeys. Experimental and Clinical Psychopharmacology 15: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HF, Mills DS, Pollux PMJ (2012) Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris). Physiology & Behavior 105: 676–682. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ (2007) Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology 15: 176–186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.