Abstract
Background
There have been few studies of sufficient size to address the relationship between glioma risk and the use of aspirin or non-steroidal anti-inflammatory drugs (NSAIDs), and results have been conflicting. The purpose of this study was to examine the associations between glioma and aspirin/NSAID use, and to aggregate these findings with prior published studies using meta-analysis.
Methods
The Glioma International Case-Control Study (GICC) consists of 4,533 glioma cases and 4,171 controls recruited from 2010–2013. Interviews were conducted using a standardized questionnaire to obtain information on aspirin/NSAID use. We examined history of regular use for ≥6 months and duration-response. Restricted maximum likelihood meta-regression models were used to aggregate site-specific estimates, and to combine GICC estimates with previously published studies.
Results
A history of daily aspirin use for ≥6 months was associated with a 38% lower glioma risk, compared to not having a history of daily use (adjusted meta-OR=0.62, 95% CI=0.54–0.70).There was a significant duration-response trend (p=1.67×10−17), with lower ORs for increasing duration of aspirin use. Duration-response trends were not observed for NSAID use. In the meta-analysis aggregating GICC data with five previous studies, there was a marginally significant association between use of aspirin and glioma (mOR=0.84, 95% CI=0.70–1.02), but no association for NSAID use.
Conclusion
Our study suggests that aspirin may be associated with a reduced risk of glioma.
Impact
These results imply that aspirin use may be associated with decreased glioma risk. Further research examining the association between aspirin use and glioma risk is warranted.
Keywords: Aspirin, Non-steroidal anti-inflammatory drugs, Glioblastoma, Glioma Risk
Introduction
Several studies have evaluated associations between various cancers and the use of non-steroidal anti-inflammatory drugs (NSAIDs) (1). With the exception of gastrointestinal tract cancers, these findings have been inconsistent (2,3). With regard to glioma, many studies have reported lack of association with overall NSAID use but relatively few studies have examined aspirin use separately from other NSAIDs, despite the fact that these drugs have different mechanisms of action.
NSAIDs inhibit prostaglandin production via suppression of the cyclooxygenase (COX) enzyme. While most other NSAIDs are reversible competitive COX inhibitors, aspirin acts through a non-competitive mechanism, irreversibly acetylating a residue in the COX active site, resulting in inhibition of the enzyme. These differences in mechanism between aspirin and non-aspirin NSAIDs (NA-NSAIDs) underlie the reason why aspirin is protective against cardiovascular disease, whereas other NSAIDs tend not to have a similar impact. Although there are credible hypotheses on how NSAID/aspirin use could potentially reduce cancer risk, neither an association nor a biological mechanism has been definitively established. Examining the impact of aspirin separately from NA-NSAIDs is important, as these drugs may have heterogeneous effects on cancer risk.
Regular aspirin use is more common than regular exposure to NA-NSAIDs, as low-dose aspirin is recommended for primary prevention of cardiovascular disease among older adults in the United States (U.S) (U.S. Preventive Services Task Force. Final Recommendation Statement: Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Preventive Medication: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer). Aspirin is not recommended for cardioprevention in Europe (4), which could lead to a lower prevalence of exposure in this population compared to the U.S. Using data from the Glioma International Case-Control Study (GICC) from 14 study sites across five countries, we examined the role of aspirin and NA-NSAIDs use on glioma risk. We investigated duration-response relationships and associations by glioma grade. Although our study represents one of the largest international studies of these associations to date, we also sought to contextualize our findings in the broader literature by conducting a meta-analysis with results from previously published studies.
Methods
Study Population
The GICC is a consortium with 14 recruitment sites in five countries; detailed information has been published previously (5). Cases were between 18 and 80 years old at diagnosis, with a histologically-confirmed, supra-tentorial glioma [fibrillary astrocytoma (International Classification of Disease, Oncology 3rd edition (ICD-O-3) code: 9420/3), protoplasmic astrocytoma (ICD-O-3 code: 9410/3), gemistocytic astrocytoma (ICD-O-3 code: 9411/3), oligodendroglioma (ICD-O-3 code: 9450/3), oligoastrocytoma (ICD-O-3 code: 9382/3), anaplastic astrocytoma (ICD-O-3 code: 9401/3), anaplastic oligodendroglioma (ICD-O-3 code: 9451/3), anaplastic oligoastrocytoma (ICD-O-3 code: 9382/3), gliosarcoma (ICD-O-3 code: 9442/3), or glioblastoma (ICD-O-3 code: 9440/3)]. All cases were recruited within one year of diagnosis.
Eligible controls were between 18 and 80 years old. Four sites recruited clinic-based controls (Duke, University of California San Francisco, University of Southern California and Mayo Clinic Rochester), seven sites recruited visitors accompanying patients (Brigham and Women’s Hospital, Case Western Reserve University, Columbia, Memorial Sloan-Kettering Cancer Center, MD Anderson Cancer Center, NorthShore and United Kingdom [U.K.]), and three sites used population-based controls (Denmark, Sweden and Israel). Clinic-based controls, hospital visitor controls, and population controls were recruited through the clinic’s daily patient lists, in the ward/waiting areas of the hospitals, or through the country’s population registries. All sites received Institutional Review Board or ethical board approval to conduct the study, and written informed consent was obtained from all participants.
Data Collection
A common study protocol and questionnaire were used at all sites. The risk factor questionnaire collected information on demographics, past medical/medication history, and occupational history. Questionnaires were administered in-person (48% cases and 60.4% controls), by phone (26.3% cases and 15.5% controls), or through mailed self-administered forms (20.2% cases and 21.2% controls)., Participants were asked whether they used NA-NSAID and/or aspirin at least one year prior to their brain tumor diagnosis or enrollment. If so, the participants were asked if they had taken these medications for a total of ≥6 months. Frequency of use (daily, weekly, or monthly) and the cumulative duration of use (how many years/months total these medications were used at the reported frequencies) were recorded. Self-reported information on usual dose, brand used most frequently, and reason for use was also collected. Because of the high level of variability, analyses were not conducted by brand.
Regular NA-NSAID use was defined as the use at least once per week for ≥6 months. Because many individuals take aspirin for cardioprevention, the sample size could support analyses of daily aspirin use, whereas at least weekly NA-NSAID use was examined as an exposure of interest because daily use was less common (<10% of participants used NA-NSAIDs daily).
Statistical Analysis
The overall analysis plan (including sensitivity analyses) and population demographics by study site have been published previously (5). We compared cases and controls on relevant characteristics, overall, by study site, by control types (visitor, clinic, or population-based), and by tumor grade (high-grade: grade IV; lower-grade: grade II/III) among cases. Exposures included: daily aspirin use for a total of ≥6 months (cumulative), weekly NA-NSAID use for a total of ≥6 months (cumulative), reason for use, and duration of use (<6 months or not reported; ≥6 months to ≤2 years; >2 years to ≤5 years; >5 years to ≤10 years or >10 years).
Analyses were conducted both in the overall study population and stratified by tumor grade. Site-specific odds ratios (ORs), along with corresponding 95% Wald confidence intervals (95% CIs) were calculated using unconditional logistic regression adjusted for age and sex. Sites with less than five cases or controls in the exposed or unexposed groups were not included in the meta-analyses.
Meta-analysis ORs (Meta-ORs) were calculated using two-stage random-effects restricted maximum likelihood (REML) modeling to aggregate estimates across study sites. The I2 statistic assessed the percentage of variability in the effect estimates due to heterogeneity. The τ2 statistic and the heterogeneity test p-value were calculated to evaluate the inter-site variance. Forest plots were generated as a visual representation of the site-specific estimates. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC) and R version 3.1.2.
Due to small sample sizes, analyses of specific drug types were performed by pooling the data from sites. The analyses of regular use of aspirin combined all aspirin-containing products, because only small numbers of participants used brands that combined salicylic acid with other ingredients.
Sensitivity analyses included running models with and without proxy responses to ensure that there were no meaningful discrepancies in ORs. After we observed that cases were more likely than controls to have used aspirin/NA-NSAIDs for headache, we also performed sensitivity analyses excluding those participants who took aspirin/NA-NSAIDs for headache. We also performed separate meta-analyses to examine potential differences between sites with different control types. Most analyses examined at least weekly use of NA-NSAIDs (daily and weekly combined); therefore, daily use was also examined separately as a sensitivity analysis.
Meta-Analysis with Previous Studies
Articles reporting previous association analyses between glioma and aspirin or NA-NSAIDs were identified through literature search, using PubMed (Figure S1). Keywords used to identify these studies included: (“Aspirin”[Mesh] OR “Anti-Inflammatory Agents, Non-Steroidal”[Mesh] OR “Analgesics”[Mesh]) AND (“Brain Neoplasms”[Mesh] OR “Glioma”[Mesh] OR “Glioblastoma”[Mesh]). Additional studies were identified by reviewing the references of studies identified via PubMed. Case-control or cohort studies that investigated the relationship between NSAIDs and glioma were included in the meta-analysis. Articles that reported on overlapping patient populations or did not specifically examine glioma were excluded.
We reviewed all included studies and abstracted the following information: author’s names, publication year, country, study design, number of participants, exposure definition, tumor type (i.e., glioma or glioblastoma), covariates, and risk estimates with corresponding 95% CIs. Studies examining NSAIDs varied in the formulations included in this category, and in one case (6), these drugs included aspirin.
Meta-ORs were calculated using REML modeling to aggregate estimates across studies. Heterogeneity among studies was assessed using Cochran’s Q and I2 statistics. For the purposes of meta-analysis, hazard ratios and risk ratios from cohort studies were considered equivalent to ORs. Funnel plots and corresponding rank correlation tests and regression asymmetry tests were used to assess publication bias in prior studies
Results
Table 1 presents the population characteristics of the GICC study by case-control status and glioma grade. Age and sex were similarly distributed between cases and controls. Grade IV cases had an older age at diagnosis than grade II/III cases and were more likely to be male. Approximately 11% of cases and of controls reported experiencing migraines.
Table 1.
Demographic characteristics of the Glioma International Case Control Study by glioma grade.
| Cases | Controls | P-value | Grade IV Cases^ | Grade II and III Cases^ | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||
| Sex | 0.0099 | ||||||||
| Male | 2679 | 59.1 | 2351 | 56.37 | 1728 | 62.29 | 916 | 54.30 | |
| Female | 1854 | 40.9 | 1820 | 43.63 | 1046 | 37.71 | 771 | 45.70 | |
| Diagnosis/enrollment age | 0.0327 | ||||||||
| 18–29 years | 308 | 6.79 | 294 | 7.05 | 62 | 2.24 | 228 | 13.52 | |
| 30–39 years | 521 | 11.49 | 473 | 11.34 | 108 | 3.89 | 398 | 23.59 | |
| 40–49 years | 813 | 17.94 | 680 | 16.30 | 417 | 15.03 | 384 | 22.76 | |
| 50–59 years | 1150 | 25.37 | 1079 | 25.87 | 796 | 28.7 | 338 | 20.04 | |
| 60–69 years | 1239 | 27.33 | 1098 | 26.32 | 993 | 35.8 | 238 | 14.11 | |
| 70–80 years | 502 | 11.07 | 547 | 13.11 | 398 | 14.35 | 101 | 5.99 | |
| Education* | < 0.0001 | ||||||||
| High School or less | 1126 | 27.67 | 911 | 22.47 | 716 | 28.66 | 392 | 25.99 | |
| Some college | 1104 | 27.13 | 1293 | 31.89 | 651 | 26.06 | 433 | 28.71 | |
| Bachelor’s degree | 1020 | 25.06 | 955 | 23.55 | 595 | 23.82 | 409 | 27.12 | |
| Advanced degree | 808 | 19.85 | 892 | 22.00 | 530 | 21.22 | 268 | 17.77 | |
| Missing | 12 | 0.29 | 4 | 0.10 | 6 | 0.24 | 6 | 0.40 | |
| Race/ethnicity | < 0.0001 | ||||||||
| Non-Hispanic white | 4163 | 91.84 | 3691 | 88.49 | 2577 | 92.9 | 1522 | 90.22 | |
| Non-Hispanic black | 71 | 1.57 | 139 | 3.33 | 41 | 1.48 | 26 | 1.54 | |
| Asian | 84 | 1.85 | 87 | 2.09 | 35 | 1.26 | 48 | 2.85 | |
| Hispanicy | 162 | 3.57 | 224 | 5.37 | 93 | 3.35 | 67 | 3.97 | |
| Other | 38 | 0.84 | 26 | 0.62 | 22 | 0.79 | 15 | 0.89 | |
| Missing | 15 | 0.33 | 4 | 0.10 | 6 | 0.22 | 9 | 0.53 | |
| Migraines | 0.5964 | ||||||||
| Yes | 478 | 10.54 | 465 | 11.15 | 262 | 9.44 | 203 | 12.03 | |
| No | 3982 | 87.85 | 3634 | 87.13 | 2470 | 89.04 | 1455 | 86.25 | |
| Missing | 73 | 1.61 | 71 | 1.70 | 42 | 1.51 | 29 | 1.72 | |
Sum of grade II & III and grade IV cases does not equal total number of cases, due to cases with unclassified tumors
Education information was not collected by one study site (The Institute of Cancer Research, London, UK)
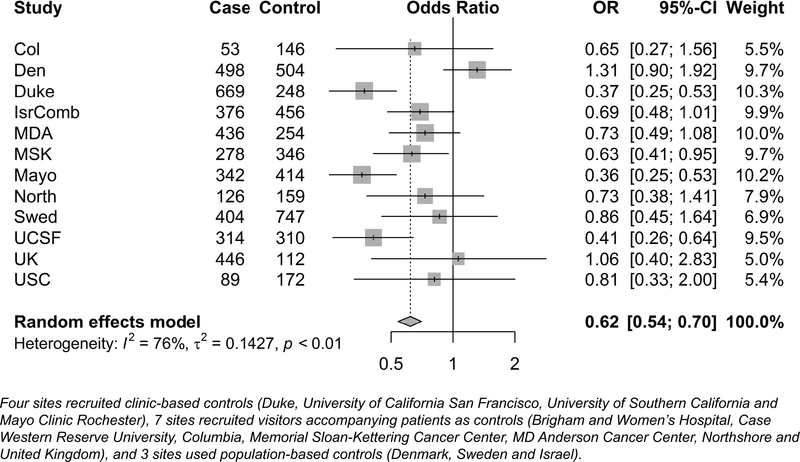
History of aspirin use.
There were 1,641 participants who took aspirin daily for ≥6 months: 764 (19.0%) cases and 877 (22.7%) controls (Table 2). Figure 1 presents the association between history of regular aspirin use and glioma risk across all sites. The overall mOR was 0.62 (95% CI=0.54–0.70). Of the 12 sites included, ten site-specific ORs were in the inverse direction, and four were statistically significant. The two ORs in adverse direction were not statistically significant. We found decreasing ORs with increasing duration of regular aspirin use (ptrend=1.67×10−17). Results stratified by grade were similar to each other and to the overall effect estimates (ORGradeIV=0.57, 95% CI=0.49–0.66; ORGradeII/III=0.61, 95% CI=0.50–0.74).
Table 2.
Duration of daily aspirin use and risk of glioma
| Duration group | Cases (n=4031) | Controls (n=3868) | OR1 | 95% Confidence Intervals | P-value2 | ||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| No daily use | 3267 | 81.05 | 2991 | 77.33 | 1 | ||
| Missing duration or <6 months3 | 108 | 2.68 | 72 | 1.86 | 1.17 | 0.85–1.62 | 0.3300 |
| >=6 months – ≤2 years | 163 | 4.04 | 156 | 4.03 | 0.73 | 0.57–0.93 | 0.0100 |
| >2 years–≤5 years | 193 | 4.79 | 221 | 5.71 | 0.58 | 0.46–0.72 | 8.33×10−7 |
| >5 years–≤10 years | 171 | 4.24 | 220 | 5.69 | 0.52 | 0.41–0.65 | 1.45×10−8 |
| >10 years | 129 | 3.2 | 208 | 5.38 | 0.46 | 0.36–0.59 | 5.91×10−10 |
Adjusted for sex, age, race, education, and study centers
Ptrend=1.67×10−17
A total of 133 participants were missing duration of use: 85 cases and 48 controls
Figure 1.
Forest plot representing the meta-analysis of aspirin use and glioma risk by study sites.
The three major indications for aspirin use were headaches, muscle/joint aches, and cardioprevention. Cases were more likely to have taken aspirin for headache. We conducted a sensitivity analysis excluding those who took aspirin for headaches. The relationship between aspirin use and glioma remained unchanged (mORSensitivity=0.60, 95% CI=0.47–0.76).
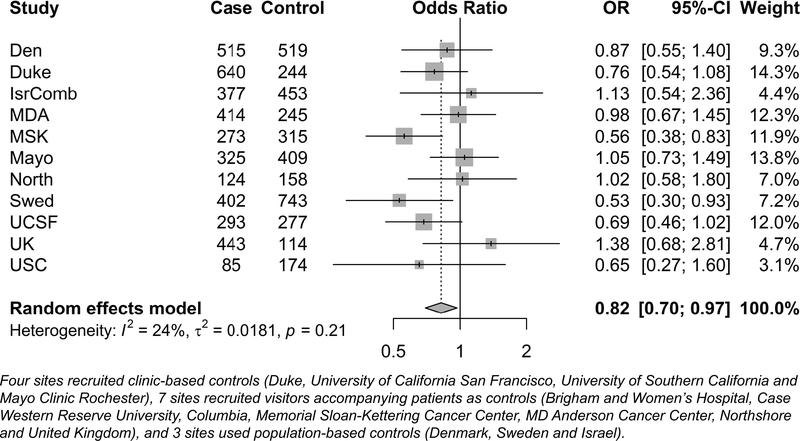
History of non-aspirin NSAID use.
Among cases, 542 (13.9%) reported history of regular NA-NSAID use, compared to 533 (14.6%) among controls (Table 3). The mOR was borderline statistically significant (mOR=0.82, 95% CI=0.70–0.97; Figure 2). Of 11 study sites, six had ORs in the inverse direction, but only two were statistically significant. There were significantly protective ORs for regular use between six months and two years, and between five to ten years, but there was no discernible pattern (ptrend>0.05).
Table 3.
Duration of weekly or daily NA-NSAIDs use and risk of glioma
| Duration group | Case (n=3891) | Control (n=3651) | OR1 | 95% Confidence Intervals | P2 | ||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| No weekly or daily use | 3349 | 91.61 | 3118 | 88.91 | 1 | ||
| Missing duration or <6 months3 | 140 | 1.96 | 97 | 1.90 | 1.12 | 0.84–1.49 | 0.430 |
| >=6 months–≤2 years | 100 | 1.83 | 120 | 2.75 | 0.70 | 0.52–0.93 | 0.010 |
| >2 years–≤5 years | 107 | 1.7 | 97 | 2.15 | 0.95 | 0.71–1.27 | 0.720 |
| >5 years–≤10 years | 95 | 1.48 | 119 | 2.53 | 0.62 | 0.46–0.83 | 0.001 |
| >10 years | 100 | 1.42 | 100 | 1.76 | 0.80 | 0.60–1.08 | 0.150 |
Adjusted for sex, age, race, education, and study centers
Ptrend>0.05
A total of 166 participants were missing duration of use: 110 cases and 56 controls
Figure 2.
Forest plots representing the meta-analysis of non-aspirin NSAIDs and glioma risk by study sites.
While there was a significant association between regular NA-NSAID use and glioblastoma (ORGradeIV=0.73, 95% CI=0.61–0.86), there was no association with lower-grade glioma (ORGradeII/III=1.01, 95% CI=0.84–1.21).
Sensitivity analyses.
Neither questionnaire administration type nor the removal of proxy responses meaningfully altered the meta-regression results for NA-NSAIDs or aspirin. Differences in the association for regular aspirin use were found by control type, with associations being attenuated among population-based and visitor controls (Clinic-based OR=0.40, 95% CI=0.33–0.50; Visitor-based OR=0.64, 95% CI=0.50–0.81; Population-based OR=0.97, 95% CI=0.77–1.24). Three of the four non-U.S. sites used population-based controls; thus, the OR restricted to non-U.S. sites was similar to the population-based OR.
We repeated key analyses adjusting for educational level and results were not changed. We restricted the analysis to adults who were >50 years of age, and found that OR for regular aspirin use remained very similar to the overall mOR (ORadjusted=0.62; 95% CI=0.54–0.71).
Results of Meta-Analysis with Previous Studies
Figure S1 provides an overview of the literature review and selection process. A total of seven previous studies on NSAIDs/aspirin and glioma were selected, and study characteristics are reported in Table S1.
Aspirin Use.
Data were available on regular use versus never use of aspirin from five previous studies (7–12). When these results were combined with GICC, there was a marginally significant association between use of aspirin and glioma (mOR=0.84, 95% CI=0.70–1.02, Q=36.6, I2=84.0%, Figure S2A-B). When analysis was limited to studies conducted in the U.S. only (7–9), there was a significant inverse association between aspirin use and glioma (mOR=0.63, 95% CI=0.49–0.80, Q=46.0, I2=76.5%, Figure S3A-B).
Data on long-term aspirin use was available from GICC and four prior studies (8–11), though definition of long-term exposure varied by study (all ≥5 years of exposure). The mOR for long-term use was 0.64 (95% CI=0.47–0.85, Q=17.0, I2=74.8%, Figure S4A-B). Association between long-term aspirin use and glioblastoma was also significant, with mOR of 0.55 (95% CI=0.39–0.78, Q=9.0, I2=67.5%, Figure S5A-B).
NSAID Use.
Data were available on regular use versus never use of NSAIDs from six previous studies (8–10,12,13). When these results were combined with GICC, there was no significant association between use of NSAIDs and glioma (mOR=0.89, 95% CI=0.77–1.03, Q=34.1, I2=82.9%, Figure S6A-B). When the analysis was limited to the five studies (including GICC) that examined only NA-NSAIDs (8–10,12), the association was null (mOR=0.97, 95% CI=0.87–1.08, Q=8.4, I2=58.6%, Figure S7A-B).
Discussion
The date on the roles of aspirin and NA-NSAID use in glioma. We found that a history of daily aspirin use for at least six months was significantly associated with a reduced risk of glioma. Duration-response analyses suggested that longer durations of regular use were associated with stronger protective effects (ptrend=1.67×10−17). Results aggregated with previously published studies yielded relatively consistent findings for regular aspirin use, though the mOR was of borderline statistical significance. Although the mOR for regular use of NA-NSAIDs was also in the inverse direction and marginally significant, no clear trends were detectable with increasing duration of use, and aggregation with previous studies yielded null results. This study contributes new information on the associations between glioma and aspirin/NSAIDs, and our original findings are placed into the broader context of the existing literature through meta-analysis with previous studies. Additional research on the association between aspirin use and gliomagenesis is warranted, as a stronger understanding of this relationship could help clarify the role of inflammation in brain tumorigenesis.
Several studies have examined the role of NSAIDs in glioma risk, but there are relatively fewer studies that have investigated the role of aspirin, specifically. We found an adjusted mOR of 0.62 (95% CI=0.54–0.70) for the association between glioma and regular aspirin use. Similar to our study, a recent case-control study by Egan et al. reported an OR of 0.69 (95% CI=0.56–0.87) for a history of regular aspirin use (defined as use at least twice a week for 12 consecutive months) for indications other than headache, as well as a significant duration-response relationship (8). An older case-control study by Ferris et al. (11) reported an OR of 0.68 (95% CI=0.49–0.96) for regular aspirin use (>6 months of at least twice weekly use) and glioma. The most recent meta-analysis of eight studies by Zhang, et al., found a meta-RR of 0.86 (95% CI=0.76–0.95) for the association between aspirin use and glioma (14). Some studies overlapped between this meta-analysis and those presented here, our results (mOR=0.84, 95% CI=0.70–1.02), which include our findings and Egan et al., are very similar to that of Zhang et al. (14). They also found a 10% decrease in CNS tumor risk for every three aspirin prescriptions (RR=0.90 per three prescriptions; 95% CI=0.85–0.95), but did not report a glioma-specific estimate.
Some studies have not detected an inverse association between aspirin use and glioma (10, 11, 13, 16). An older meta-analysis of seven studies yielded null results (mOR=1.01; 95% CI=0.85–1.21) (15). The NIH-AARP study (12), which is a prospective cohort study, also did not observe an association, but this study lacked data on duration of use, meaning that participants who used aspirin regularly for only a few weeks could be lumped in the same exposure category as those who used it regularly for several years, potentially attenuating the association. Results from the Women’s Health Study, where patients were randomized to a 100 mg dose of aspirin or placebo administered every other day, did not support an association between low-dose aspirin use and brain cancer risk (16). This study did not differentiate between gliomas and other types of brain tumors, and the results were based on only 31 incident brain tumors (17 in the aspirin group and 14 in the placebo group). Similarly, a case-control by Gaist et al. (10) reported an OR of 0.80 (95% CI: 0.53–1.21) for long-term use of low-dose aspirin, but this was based on only 31 total exposed cases (1.5% exposure prevalence in the full study population). Such studies highlight the importance of large, adequately-powered case-control studies when investigating rare conditions, like glioma.
Previous studies on NSAID use and glioma have also yielded mixed results. Two relatively recent matched case-control studies that used prescription data found no associations between risk of glioma and COX-2 inhibitor/NSAID use, which was evaluated differently in each study (number of prescriptions versus defined daily dose) (10,17). However, these two studies used potentially overlapping study populations from the U.K. Clinical Practice Research Datalink. Another case-control study also reported no association between NA-NSAID use and glioma among 2,688 cases and 18,848 population-based controls, using data from the Danish national registries (9). Similarly, a meta-analysis of 10 studies yielded null results (15). One of the first studies on NSAIDs and glioblastoma risk found a strong inverse association with a history of using at least 600 total pills (7) and reported similar results when examining specific NSAIDs separately (i.e., ibuprofen and aspirin). While this previous study was based on a relatively small number of cases (n=236), our study also found an association aggregated with four previous studies (mOR=0.97, 95% CI=0.87–1.08), cumulatively imply between NA-NSAID use and glioblastoma risk. The borderline significant mOR and the lack of duration-response trends in the GICC study, in combination with the mOR from our results that a strong association is unlikely.
In general, direct comparisons of our findings to the previous literature are challenging because of substantial heterogeneity in the definitions of long-term exposure to aspirin/NSAIDs across studies (e.g., by number of years but not accounting for daily use, or by high vs. low defined daily dose). Therefore, some of the inconsistencies between previous studies on aspirin/NSAIDs and glioma may be partly attributable to the wide variety of exposure assessment strategies and analysis units. Studies using self-reported exposure to aspirin/NSAIDs may be subject to reverse causation, in that cases may be using NSAIDS more regularly in order to alleviate headaches or other brain tumor symptoms. However, such bias due to reverse causation would drive the OR towards a positive, not an inverse, association. Accounting for indication of use or omitting data from less than a year prior to diagnosis may reduce such bias. Another consideration when using self-reported data is the potential for poor recall among cases due to cognitive deficits. While prescription data (e.g., data used by Gaist, et al. or Bannon, et al.) are generally considered more accurate than self-report, such data are still not a direct measure of medication ingestion, and do not adequately reflect use of medications that can be purchased without a prescription. In some European countries, including the U.K., a substantial proportion of NSAID sales are over-the-counter (OTC), and studies using data exclusively from prescription registries would, therefore, be subject to exposure misclassification (11). Future studies should consider combining electronic health record and interview data to obtain a more complete picture of aspirin/NSAID use. Given the relatively short median survival time of glioma, such a strategy may present challenges for retrospective or case-control studies. Although prospective studies may be able to incorporate a more comprehensive approach to exposure assessment, glioma is a rare outcome, and prospective studies are likely to be underpowered.
The possibility of an inverse relationship between aspirin/NSAIDs and gliomagenesis is supported by in vitro studies (13,18–21), and several possible mechanisms have been proposed. One possibility is inhibition of COX-2, which has been shown to be upregulated in most high-grade gliomas (19). Inhibition of COX-2 by NSAIDs prohibits the production of prostaglandin E2 (PGE2). PGE2 induces the differentiation and expansion of myeloid-derived suppressor cells (MDSCs), which can attenuate antitumor immunosurveillance. It has been hypothesized that blocking the COX-2 pathway may suppress gliomagenesis by inhibiting MDSCs (21). There are also COX-2 independent pathways through which aspirin/NSAIDs may potentially confer a protective effect. NSAIDs may also inhibit the growth of glioblastoma cells by up-regulating the cell cycle inhibitor p21 and a major prostaglandin catabolic enzyme (15-hydroxyprostaglandin dehydrogenase) (20). One other potential mechanism is gentisic acid, a metabolite of aspirin which blocks binding of fibroblastic growth factor and has been found to inhibit growth of glioblastoma in vivo (22). Since the findings of most epidemiologic studies on aspirin/NSAIDs remain mixed, additional in vitro and in vivo studies are warranted.
Our study has some limitations inherent to multisite consortia. There is a significant amount of site-to-site heterogeneity, and we have provided site-specific ORs, in addition to mORs. Different control types and questionnaire administration methods were used between sites. While an ideal control group would capture members of the underlying source population from which the cases originated, this would not be entirely feasible in the GICC study because glioma cases were recruited from several large referral centers, where the source population includes a combination of both national and international patients. Therefore, the underlying source population for the cases cannot be easily defined or identified. In fact, most of the GICC U.S. sites were tertiary-care and/or referral centers, which serve international patient populations. Additionally, because none of the U.S. sites utilized population-based controls, we are unable to disentangle whether the null OR in non-U.S. sites is attributable entirely to control type or whether it may also partly be explained by the relatively low exposure prevalence in the non-U.S. sites.
To help address some of the concerns related to differing control types, we conducted a series of sensitivity analyses (5) to examine findings by these differences. The association between aspirin and glioma was strongest when comparing cases to clinic-based controls, implying the possibility of bias. However, most site-specific ORs were in the inverse direction, including some with non-clinic controls, and only two site-specific ORs were above 1.0 (not statistically significant). Additionally, when clinic-based controls were excluded, the mOR for aspirin use remained statistically significant and inversely associated (mOR=0.82, 95% CI=0.69–0.96). However, we cannot discount the possibility that our findings may be influenced by selection bias related to control recruitment strategy.
Another common limitation in case-control studies of glioma is the use of proxy respondents. However, the proportion of proxy responses in our study is low (<10%), and their exclusion did not meaningfully impact our findings.
Because of the guidelines related to cardioprevention in the U.S., our sample size could support analyses of daily aspirin use, but we were unable to examine daily NA-NSAID use (<10% of participants used NA-NSAIDs daily). Therefore, regular NA-NSAID use was defined as weekly use for at least 6 months. Being unable to examine daily use of NA-NSAIDs constitutes another limitation of our study.
With regard to the meta-analysis of GICC data with prior studies, the observed association between aspirin use and glioma was stronger when limited to studies conducted in the U.S. (mOR=0.63, 95% CI=0.49–0.80) than when international studies were included. Exposure prevalence of daily or weekly aspirin use is much lower in countries in which aspirin is not routinely recommended for cardioprevention (4). While this can impact statistical power in non-U.S. studies, it also implies that individuals who do take aspirin regularly in these countries are unlikely to be generalizable to the rest of the population. It is possible that the cardiopreventive guidelines in the U.S. differentially increase the probability that controls take aspirin regularly, compared to individuals who have other health complications. Our findings should be interpreted cautiously and in the milieu of current knowledge about potential biologic mechanisms that may underlie such an association.
Conclusions
Although many studies, including ours, do not strongly support the role of NA-NSAIDs in glioma prevention, our findings are relatively consistent with previous studies that report an approximately 30% lower glioma risk associated with a history of aspirin use (8,11). The duration-response trend in aspirin use was also significant providing a strong rationale for further investigation. Future studies can bolster the literature by utilizing more comprehensive exposure assessment strategies and examining each NSAID separately in order to account for differences in COX-2 selectivity or pharmacological properties.
Supplementary Material
Acknowledgments
Financial support: This work was supported by grants from the National Institutes of Health (R01CA207972, R01CA139020, R01CA52689, P50097257, P30CA008748, and P30CA125123). Additional support was provided by the McNair Medical Institute at Baylor College of Medicine (Houston, Texas) and the Population Sciences Biorepository at Baylor College of Medicine. QTO is supported by a Research Training Grant from the Cancer Prevention and Research Institute of Texas (CPRIT; RP160097T).
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol 2015;26(1):47–57 doi 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Y, Nishihara R, Wu K, Wang M, Ogino S, Willett WC, et al. Population-wide Impact of Long-term Use of Aspirin and the Risk for Cancer. JAMA Oncol 2016. doi 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. Journal of the National Cancer Institute 2007;99(8):608–15 doi 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 4.Miedema MD, Huguelet J, Virani SS. Aspirin for the Primary Prevention of Cardiovascular Disease: In Need of Clarity. Current atherosclerosis reports 2016;18(1):4 doi 10.1007/s11883-015-0555-0. [DOI] [PubMed] [Google Scholar]
- 5.Amirian ES, Armstrong GN, Zhou R, Lau CC, Claus EB, Barnholtz-Sloan JS, et al. The Glioma International Case-Control Study: A Report From the Genetic Epidemiology of Glioma International Consortium. Am J Epidemiol 2016;183(2):85–91 doi 10.1093/aje/kwv235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheurer ME, Amirian ES, Davlin SL, Rice T, Wrensch M, Bondy ML. Effects of antihistamine and anti-inflammatory medication use on risk of specific glioma histologies. International journal of cancer 2011;129(9):2290–6 doi 10.1002/ijc.25883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivak-Sears NR, Schwartzbaum JA, Miike R, Moghadassi M, Wrensch M. Case-control study of use of nonsteroidal antiinflammatory drugs and glioblastoma multiforme. Am J Epidemiol 2004;159(12):1131–9 doi 10.1093/aje/kwh153. [DOI] [PubMed] [Google Scholar]
- 8.Egan KM, Nabors LB, Thompson ZJ, Rozmeski CM, Anic GA, Olson JJ, et al. Analgesic use and the risk of primary adult brain tumor. European journal of epidemiology 2016;31(9):917–25 doi 10.1007/s10654-016-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaist D, Garcia-Rodriguez LA, Sorensen HT, Hallas J, Friis S. Use of low-dose aspirin and non-aspirin nonsteroidal anti-inflammatory drugs and risk of glioma: a case-control study. Br J Cancer 2013;108(5):1189–94 doi 10.1038/bjc.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bannon FJ, O’Rorke MA, Murray LJ, Hughes CM, Gavin AT, Fleming SJ, et al. Non-steroidal anti-inflammatory drug use and brain tumour risk: a case-control study within the Clinical Practice Research Datalink. Cancer Causes Control 2013;24(11):2027–34 doi 10.1007/s10552-013-0279-9. [DOI] [PubMed] [Google Scholar]
- 11.Ferris JS, McCoy L, Neugut AI, Wrensch M, Lai R. HMG CoA reductase inhibitors, NSAIDs and risk of glioma. International journal of cancer 2012;131(6):E1031–7 doi 10.1002/ijc.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daugherty SE, Moore SC, Pfeiffer RM, Inskip PD, Park Y, Hollenbeck A, et al. Nonsteroidal anti-inflammatory drugs and glioma in the NIH-AARP Diet and Health Study cohort. Cancer Prev Res (Phila) 2011;4(12):2027–34 doi 10.1158/1940-6207.CAPR-11-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang N, Chen S, Deng J, Huang Q, Liao P, Wang F, et al. Overexpression of S100A9 in human glioma and in-vitro inhibition by aspirin. Eur J Cancer Prev 2013;22(6):585–95 doi 10.1097/CEJ.0b013e328364f1c9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Yang X, Liu P, Zhou J, Luo J, Wang H, et al. Association between nonsteroidal anti-inflammatory drugs use and risk of central nervous system tumors: a dose-response meta analysis. Oncotarget 2017;8(60):102486–98 doi 10.18632/oncotarget.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Lu Y, Wang J, Xie L, Li T, He Y, et al. Association between nonsteroidal anti-inflammatory drug use and brain tumour risk: a meta-analysis. Br J Clin Pharmacol 2014;78(1):58–68 doi 10.1111/bcp.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med 2013;159(2):77–85 doi 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seliger C, Meier CR, Becker C, Jick SS, Bogdahn U, Hau P, et al. Use of Selective Cyclooxygenase-2 Inhibitors, Other Analgesics, and Risk of Glioma. PLoS One 2016;11(2):e0149293 doi 10.1371/journal.pone.0149293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ming J, Sun B, Li Z, Lin L, Meng X, Han B, et al. Aspirin inhibits the SHH/GLI1 signaling pathway and sensitizes malignant glioma cells to temozolomide therapy. Aging 2017;9(4):1233–47 doi 10.18632/aging.101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joki T, Heese O, Nikas DC, Bello L, Zhang J, Kraeft SK, et al. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res 2000;60(17):4926–31. [PubMed] [Google Scholar]
- 20.Wakimoto N, Wolf I, Yin D, O’Kelly J, Akagi T, Abramovitz L, et al. Nonsteroidal anti-inflammatory drugs suppress glioma via 15-hydroxyprostaglandin dehydrogenase. Cancer Res 2008;68(17):6978–86 doi 10.1158/0008-5472.CAN-07-5675. [DOI] [PubMed] [Google Scholar]
- 21.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res 2011;71(7):2664–74 doi 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altinoz MA, Elmaci I, Cengiz S, Emekli-Alturfan E, Ozpinar A. From epidemiology to treatment: Aspirin’s prevention of brain and breast-cancer and cardioprotection may associate with its metabolite gentisic acid. Chem Biol Interact 2018;291:29–39 doi 10.1016/j.cbi.2018.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.