Abstract
Background:
The prevalence of binge drinking (BD) has been on the rise in recent years. It is associated with a range of neurocognitive deficits among adolescents and young emerging adults who are especially vulnerable to alcohol use. Attention is an essential dimension of executive functioning and attentional disturbances may be associated with hazardous drinking. The aim of the study was to examine the oscillatory neural dynamics of attentional control during visual target detection in emerging young adults as a function of BD.
Method:
Fifty-one first-year university students (18±0.6 years) were assigned to light drinking (LD, N=26), and BD (N=25) groups based on their alcohol consumption patterns. High-density magnetoencephalography (MEG) signal was combined with structural magnetic resonance imaging (MRI) in an anatomically-constrained MEG model to estimate event-related source power in theta (4–7 Hz) frequency band. Phase-locked co-oscillations were further estimated between the principally activated regions during task performance.
Results:
Overall, the greatest event-related theta power was elicited by targets in the right inferior frontal cortex (rIFC) and it correlated with performance accuracy and selective attention scores. BDs exhibited lower theta power and dysregulated oscillatory synchrony to targets in the rIFC which correlated with higher levels of alcohol consumption.
Conclusions:
These results confirm that a highly interactive network in the rIFC subserves attentional control, revealing the importance of theta oscillations and neural synchrony for attentional capture and contextual maintenance. Attenuation of theta power and synchronous interactions in BDs may indicate early stages of suboptimal integrative processing in young, highly functioning BDs.
Keywords: Binge drinking, alcohol, magnetoencephalography, theta oscillations, attention
1. INTRODUCTION
Binge drinking (BD) is highly prevalent among adolescents and young adults in most Western countries (Eurobarometer, 2010; Johnston, 2011;SAMHSA, 2013) and is associated with a wide range of negative social and health consequences (Hingson et al., 2017; Hingson and White, 2013). This pattern of consumption is characterized by intake of intoxicating quantities of alcohol in a short time interval interspersed with periods of abstinence (Courtney and Polich, 2009).BD is usually defined as imbibing five/four or more drinks for men/women within a two-hour interval, which is likely to bring blood alcohol concentration (BAC) to 0.08% or above (National Institute of Alcohol and Alcoholism, 2004). However, these levels are commonly exceeded by emerging young adults who engage in bouts of much higher levels of alcohol intake (Naimi et al., 2010; Patrick and Terry-McElrath, 2017; Terry-McElrath and Patrick, 2016).
Adolescence emerging into adulthood is a protracted but critical period of neurodevelopment characterized by profound changes (Sherman et al., 2014; Simmonds et al., 2014; Walhovd et al., 2012) which mainly involve prefrontal cortex (PFC) and other higher-order association areas (Gogtay et al., 2004; Lebel and Beaulieu, 2011) and contribute to refinement of cognitive efficiency (Casey et al., 2005). As it continues to mature well into mid 20’s, the adolescent brain is more sensitive to the impact of ethanol exposure, with the frontal lobes being particularly vulnerable (Cservenka and Brumback, 2017; Hermens et al., 2013; Jacobus and Tapert, 2013; Silveri et al., 2016; Squeglia et al., 2014). Extensive evidence obtained from rodent models indicates that BD during adolescence is a high risk factor for the development of alcohol-use disorders (AUD) and enduring neural changes (Crabbe et al., 2011; Crews et al., 2016).
Several studies have reported that BDs differ from light drinkers (LDs) on EEG measures in the absence of behavioral differences (Folgueira-Ares et al., 2017; López-Caneda et al., 2012, 2013). However, other studies report that BDs tend to have lower scores than light-drinkers (LDs) on tasks probing high-level cognitive abilities such as memory and executive functions (for reviews, see Stephens and Duka, 2008; Jacobus and Tapert, 2013; Petit et al., 2014). Attention is a key component of executive functions and is involved in minimizing distraction and in optimal allocation of cognitive resources to task-relevant stimuli (Diamond, 2013). Impairments of attention are related to increased impulsivity and may contribute to engaging in hazardous drinking (Crews and Boettiger, 2009; de Wit, 2009). Despite its importance for response inhibition and cognitive control, attention has been scarcely explored in BD population. Lannoy et al. (2017) used the Attention Network Task (Fan et al., 2005) and reported impairments of alerting and executive control dimensions of attention in BDs. Using the same task, Maurage et al. (2014) showed that alcohol-dependent individuals presented attentional deficits specifically in the executive control of attention compared with social drinkers. These results suggest that attentional disturbances might have a key role in the development of AUD in the context of broader cognitive impairments (Muller-Oehring and Schulte, 2014; Oscar-Berman and Marinković, 2007; Sullivan and Pfefferbaum, 2005). Convergence of neuroimaging and human lesions studies has indicated that the right-dominant fronto-parietal network is crucially involved in attentional processes (Klinke et al., 2017; Vossel et al., 2014). Functional MRI (fMRI) studies have confirmed engagement of a right-lateralized network during target detection with a nexus in the right inferior prefrontal cortex (Gitelman et al., 1999; Nobre et al., 1997; Shulman et al., 2010; Vossel et al., 2014).
A previous EEG study has reported alterations in event-related oscillations (ERO) in BDs during the same equiprobable Go/NoGo task as the one employed in the present study (López-Caneda et al., 2017). This study found lower delta and theta power during target detection in BDs. They proposed that this power decrease in low frequencies may reflect impairments in the neural circuit involved in both the activation and inhibition of a response. Moreover, ERO studies in individuals with AUD have reported attenuated power in slow frequencies (delta and/or theta) in alcoholics during Go/NoGo task (Colrain et al., 2011) and equiprobable Go/NoGo task (Kamarajan et al., 2004; Pandey et al., 2016; Porjesz et al., 2005) which has been interpreted as a deficiency in inhibitory and attentional processing.
Event-related theta (ERT) is sensitive to cognitive effort as it increases in response to higher demands of cognitive control (Brier et al., 2010; Cavanagh and Frank, 2014; Hanslmayr et al., 2008; Jensen and Tesche, 2002; Rosen et al., 2016). Furthermore, ERT is strongly modulated by alcohol. Acute alcohol intoxication attenuates ERT especially on trials evoking cognitive interference (Beaton et al., 2017; Kovacevic et al., 2012; Marinkovic et al., 2012; Rosen et al., 2016), indicating that alcohol primarily affects executive functions.
Theta oscillations have also been implicated in integrating task-relevant representations with top-down effects across long-range neural envelopes (Halgren et al., 2015; Hasselmo and Stern, 2014; Wang et al., 2005). Phase-locking values (PLV) are a measure of phase consistency between two signals (Lachaux et al., 1999) and are suitable for investigating co-oscillations between different brain areas at a level of an interactive dynamic network (Varela et al., 2001). It has been shown that PLVs between the lateral and medial prefrontal cortical areas are sensitive to cognitive conflict (Beaton et al., 2017) and that acute intoxication dysregulates those interactions in humans and rodents (Beaton et al., 2017; Ehlers et al., 2012). There is a paucity of studies assessing task-induced functional connectivity in BDs with temporally sensitive methods. However, altered functional connectivity during resting-state has been reported in BDs compared to LDs (Correas et al., 2015; Correas et al., 2016).
The aim of the present study was to examine neural dynamics of the attentional circuitry during target detection in adolescents emerging into adulthood as a function of BD. To this purpose we employed an anatomically constrained MEG method (aMEG) which is a multimodal approach that combines whole-head MEG recordings and high-resolution MRI images within a distributed source model (Dale et al., 2000; Lin et al., 2004; Marinkovic et al., 2003). It allows for the estimation of where the oscillatory changes are occurring, and it provides a highly precise insight into the temporal sequence (“when”) of the involved neural components. Maps of event-related power estimates and the associated time courses of source estimates were analyzed across time and cortical space in time-frequency domain (Kovacevic et al., 2012; Lin et al., 2004; Marinkovic et al., 2012; Rosen et al., 2016). Additionally, co-oscillations between the principally activated cortical regions provided insight into attentional function in real time and at the level of an interactive system in BD and LD groups (Beaton et al., 2017).
2. MATERIALS AND METHODS
2.1. Participants
Fifty-one first-year students of the Complutense University of Madrid (Madrid, Spain) participated in the study. They were assigned to a binge drinking (BD) (N=25, 13 females) or to a light drinking (LD) group (N=26, 14 females) based on a questionnaire and a semi-structured interview inquiring about their alcohol and other drug use. Participants provided a record of their daily alcohol consumption indicating the type(s) and the quantity of the beverage(s) they consumed in the past month as well as the length of time (in hours) it took them to imbibe these beverages (Table 1). Their estimated Blood Alcohol Concentration (est-BAC) was calculated for each drinking episode based on the information they provided for the past month, as well as their gender and weight, according to the following algorithm (Windmark, 1981):
where G corresponds to the highest level of alcohol (grams) consumed on one occasion in the previous month; W is the body weight (Kg); bw or body water is a constant related to the water content of the human body with a value of 0.68/0.55 for male/female; mr is the metabolism rate with a value of 0.15/0.18 for male/female. We considered the est-BAC as a rough index of alcohol intoxication for each subject. Participants reaching an est-BAC of 0.08% or above at least once during the previous month were classified as BDs. In contrast, the control group consisted of students who did not achieve that BAC in the past month. It is important to note that the legal drinking age in Spain is 18 years of age.
Table 1.
Demographic, drinking, personality and neuropsychological variables.
| LDs | BDs | U values | |
|---|---|---|---|
| N (females) | 26 (14) | 25 (11) | 164(chi2) |
| Age | 18±0.8 | 18±0.4 | 650 |
| Est BAC | 0.017±0.02 | 0.17±0.072 | 702** |
| Length of drinking (mos) | 4.55±3.71 | 20.67±10.45 | 205.5** |
| Age of drinking onset | 16.6±1.01 | 14.92±1.08 | 83* |
| Drinking days past mo. | 1.5±1.8 | 7.82±2.2 | 184.5** |
| Drinks on a regular day | 1.1±0.5 | 4±1.7 | 267.5** |
| Drinks on a heavy day | 2.4±1.1 | 5.8±2.6 | 248.5** |
| Drunk days past mo. | 0 | 4.84±2.56 | 688.5** |
| Total AUDIT score | 1.7±1.3 | 7.7±1.9 | 648** |
| Sens. Seeking –Tot. | 16.29±3.5 | 21.48±3.53 | 257** |
| Thrill & Adventure | 5.76±2.66 | 7.58±2.35 | 278 |
| Disinhibition | 2.48±1.22 | 5.17±1.58 | 322** |
| Experience Seeking | 5.56±1.58 | 5.43±1.77 | 175 |
| Boredom Suscept | 2.54±1.81 | 3.17±1.19 | 222 |
| Barrat Impuls. - Tot. | 38.87±10.6 | 44.38±11.5 | 241 |
| Emotional | 14.42±3.81 | 12.84±3.95 | 261.5 |
| Motor | 14.77±6.55 | 12.96±5.53 | 320 |
| Non-planning | 15±4.53 | 14.2±4.95 | 242 |
| Attention D2 - Total | 498.23±64.1 | 468.84±75.26 | 157 |
| IGT – total earnings | 12.89±21.85 | 11.15±20.5 | 216 |
| IGT – total losses | 19.03±17.25 | 21.6±29.3 | 204 |
| Sleeping hours previous night | 7.2±0.98 | 7.04±1.17 | 257.5 |
Mean±SD are listed for all variables. Group comparisons were calculated with a nonparametric Mann-Whitney U test except for age which was tested with chi2. LDs: light drinkers; BDs: binge drinkers; estBAC: the highest estimated Blood Alcohol Concentration reached in the past month; AUDIT: Alcohol Use Disorders Identification Test; IGT: Iowa Gambling Task.
Significance level is indicated as follows:
p < 0.05
p < 0.01
p < 0.001
Participants completed personality tests related to impulsivity variables including Barrat Impulsivity Scale (BIS) (Patton et al., 1995) and Sensation Seeking Scale (SSS) (Zuckerman and Link, 1968). Their selective and sustained attention was measured with the D2 attentional test (Brickenkamp and Seisdedos, 2009), a neuropsychological test in which participants are asked to cross out any “d” letter. Also, their sensitivity to reward was assessed by means of the Iowa Gambling Task (IGT) (Bechara et al., 1994).
Prospective participants with family history of alcohol abuse were excluded from the study as well as individuals reporting personal history of psychiatric disorders based on DSM-IV-TR criteria (American Psychiatric Association, 2000) and/or psychopathological traits as assessed by the Symptom Checklist-90 Revised questionnaire (Derogatis and Melisaratos, 1983). Subjects reported no current medical condition and were medication free at the time of the study. They also completed the Alcohol Use Disorders Identification Test (AUDIT) (Guillamón et al., 1999) and subjects who scored 20 or above were excluded for having AUD. Tobacco smoking was a controlled variable. According to the Syndrome Nicotine Dependence Scale (Becoña et al., 2011), four participants fulfilled the criterion of nicotine dependence, three BDs and one LD.
Participants were asked to refrain from alcohol consumption for at least 24 hours prior to data acquisition. All of them provided written informed consent for the study and were monetarily reimbursed for their participation. The study was approved by the Ethics Committee of the Complutense University of Madrid. Principles of Declaration of Helsinki were followed.
2.2. Task
Participants performed a target detection task with equiprobable target (Go) and nontarget (NoGo) stimuli which consisted of blue/green squares/circles. Participants were instructed to fixate on a cross in the center of the screen and to press a button as fast as possible to targets (green circles and blue squares) and to withhold responding to non-target (blue circles and green squares). The stimuli were presented for 100 ms with a stimulus onset asynchrony (SOA) of 1,400 ± 200 ms within a visual angle of 3.4 degrees. They were presented in random order in two blocks of 200–225 stimuli (Figure 1). Half of subjects pressed the button with the left and half with the right hand in a counterbalanced manner.
Figure 1.
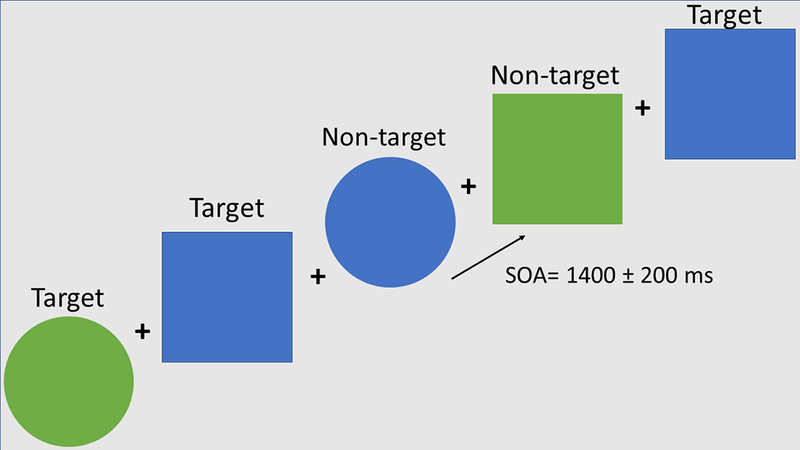
Schematic diagram of the task. The stimuli were presented for 100 ms every 1400±200 ms.
2.3. Data acquisition and analysis
2.3.1. MRI
Structural MRI images were used to define the model for the volume conductor and the solution space for MEG source localization analysis. Images were acquired with a General Electric 1.5 Tesla using an eight-channel head coil. The imaging protocol consisted of: 3D T1-weighted high-resolution images using a Fast-Spoiled Gradient Echo sequence [TR/TE/TI=11.2/4.2/450 ms; flip-angle= 12°; FoV= 250 mm; acquisition matrix= 256 × 256; slice thickness=1 mm]. Structural images were used to reconstruct each person´s cortical surface with FreeSurfer software. Inner skull surface was used for a boundary element model of the volume conductor in the forward calculations. The solution space was approximated by ∼5000 free-rotation dipoles along the gray-white matter surface in the cortex, with spacing between dipole locations ∼7 mm (Kovacevic et al., 2012).
2.3.2. MEG
High-density MEG signals were recorded from 204 channels (102 pairs of planar gradiometers) with a whole-head Neuromag Vectorview system (Elekta) in a magnetically and electrically shielded room. The signals were recorded continuously with a 1000 Hz sampling rate and filtered online with a band pass filter 0.1–330 Hz. The position of magnetic coils attached to the skull, the main fiduciary points, as well as a large array of random points spread across the scalp were digitized with 3Space Isotrak II system for subsequent precise co-registration with structural MRI images. Trials with incorrect responses were excluded from the analysis. The number of included trials was equated across the target and nontarget task conditions for each subject. The data were analyzed with a time-frequency analysis stream that uses MATLAB routines (Beaton et al., 2017; Kovacevic et al., 2012; Marinkovic et al., 2012; Rosen et al., 2016) that incorporates publicly available packages including Fieldtrip (Oostenveld et al., 2011), EEGLab (Delorme and Makeig, 2004) and MNE (Gramfort et al., 2014). Continuous data were bandpass filtered from 0.1 to 100 Hz and epoched from −600 to 1000 ms relative to stimulus onset for the stimulus-locked analysis. For each epoch, the data were downsampled by a factor of 4 to 250 Hz. Epoched data were then passed through automatic threshold rejection to remove trials that were contaminated with artifacts. Independent component analysis was used to remove eye-blinks and heart beat artifacts (Delorme and Makeig, 2004). Complex power spectrum was calculated for each trial using convolution with complex Morlet wavelets (Lachaux et al., 1999) in 1 Hz increments from 4 to 7 Hz for theta band. The first and last 300 ms of each epoch were discarded to remove edge artifacts resulting from wavelet analysis. Wavelet results were visually inspected across all epochs for any additional artifacts. We used an anatomically constrained MEG (aMEG) source modeling which comprises cortically constrained minimum norm estimates of the event-related theta power (Dale et al., 2000; Lin et al., 2004). To estimate the noise covariance for calculation of the inverse and to prevent biasing the inverse solution, empty room data were detrended and band-pass filtered between 3 and 40 Hz. The signal-to-noise ratio equaling 5 (Lin et al., 2004; Marinkovic, 2003) was used for scaling of the noise covariance matrix in calculation of the inverse operator. The identity matrix was used for noise-sensitivity normalization of the source-space solution. The noise-sensitivity normalized estimates of total source power were obtained at each location on the cortical surface at each frequency. Source power estimates were calculated based on cortically constrained minimum norm estimates (Kovacevic et al., 2012; Lin et al., 2004; Marinkovic et al., 2012). For each subject, a map of total source power for theta was estimated by averaging across theta frequency (4–7 Hz) and across all trials for each condition. ERT power was baseline-corrected by subtracting the mean theta source power estimate in the 300 ms prestimulus period and expressed as percent signal change from baseline. Uncorrected theta power in the baseline did not differ between groups (F (1,50) = 0.02, p = 0.86) or conditions (F (1,50) = 0.7, p = 0.37) confirming that the stimulus-evoked changes were not affected by possible differences in the baseline. Intersubject averages were created by morphing each subject´s reconstructed surface onto an average representation after aligning their cortical sulcal-gyral patterns (Fischl et al., 1999) and averaging individual source power estimates.
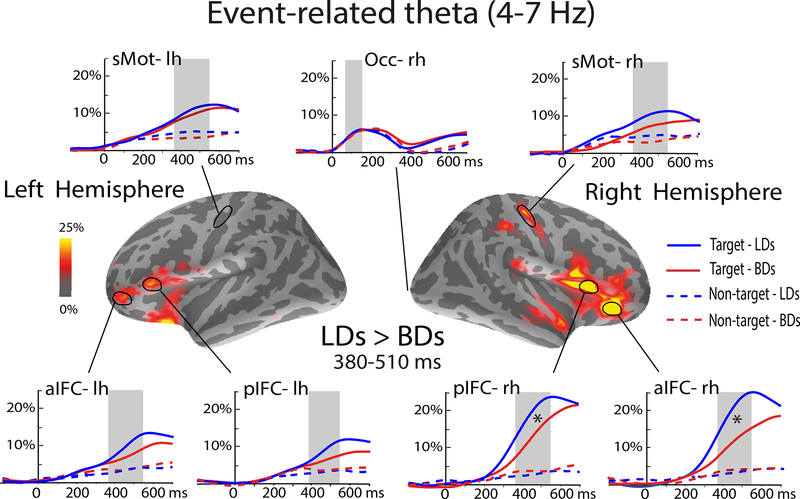
Region-of-interest (ROI) analysis was conducted to further examine possible interactions of task condition, group, and gender on event-related changes in theta power. Unbiased ROIs were selected based on the overall group average across all subjects for each task condition and comprised dipole locations along cortical surface with most notable source power. The same set of group-based ROIs was used for all subjects in a manner blind to their individual activations by applying an automatic spherical morphing procedure (Fischl et al., 1999). Early visual activity was assessed in the occipital region (Occ) while the anterior (aIFC) and posterior inferior frontal cortices (pIFC) and sensorimotor cortices (sMOT) were examined bilaterally as shown in Figure 2. Additionally, co-oscillatory interactions between cortical ROIs were estimated by calculating phase-locking values (PLV) between pairs of frontal ROIs in each hemisphere (Figure 4) (Beaton et al., 2017; Lachaux et al., 1999).
Figure 2.
Group-average difference maps (LD minus BD) of event-related theta source power. Time courses show theta power estimated to the anterior (aIFC) and posterior (pIFC) inferior frontal cortices, the sensorimotor (sMOT) areas and the occipital cortex (Occ) expressed as percent change from baseline. Targets evoked greater theta activity overall which was especially prominent in the rIFC. Theta power in the rIFC was reduced in BD compared to LD participants. Light-shaded vertical bars indicate the analyzed time windows: 50–150 ms (Occ) and the 380–510 ms for the anterior ROIs.
Figure 4.
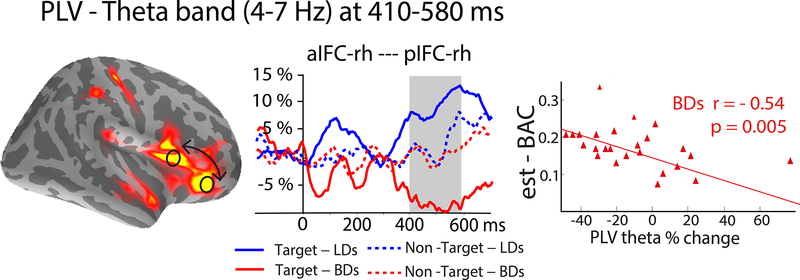
Group-average maps of event-related theta source estimates and time courses of phase-locking values (PLV) calculated between the anterior and posterior inferior frontal cortices (aIFC and pIFC). The PLVs are expressed as percent change from baseline. The aIFC and pIFC of the right IFC showed increased oscillatory synchrony in light drinkers (LDs) form 410–580 ms where PLV decreased in binge drinkers (BDs) and correlated negatively with alcohol consumption (estimated blood alcohol concentration, est-BAC).
A nonparametric cluster-based permutation test (CBPT) (Maris and Oostenveld, 2007), was used to test for significant group differences in event-related theta power, as well as in PLVs. At each time step, group differences were tested by means of a 1-way ANOVA test which provided F values used in the CBPT. Time clusters consisted of ≥25 contiguous time points yielding 100ms time windows which systematically showed group difference with a significance threshold p < = 0.05. Cluster-statistics were assessed through the sum of all F-values corresponding to the time steps comprised in cluster. This procedure was repeated 5000 times after shuffling the original group’s labels which created a null distribution for each comparison. The cluster-statistics obtained from the original data set were compared with those in the randomized data, while keeping the maximum statistic at each repetition. The resulting CBPT p-value represented the proportion of the permutation distribution with cluster-statistic value of the original data. Only the time-cluster that survived the CBPT at p < 0.05 was used to define time windows for the subsequent analyses.
No significant effects of Gender were observed in any of the analyses. Nonparametric Spearman’s rho coefficient was used to compute correlations.
3. RESULTS
3.1. Behavioral Performance
Behavioral performance was assessed by measuring accuracy in detecting targets and non-targets and reactions times to targets. Behavioral results are summarized in Table 2. BDs showed a marginal tendency to respond less accurately and with longer RTs in comparison to LDs.
Table 2.
Behavioral data for the LD and BD groups (mean±SD).
| Behavioral Performance | LDs | BDs | t-test (49) | P-values |
|---|---|---|---|---|
| Reaction Time (ms) | 509.08 ± 73.61 | 531.46 ± 74.32 | 1.03 | p = 0.1 |
| % correct Responses (target) | 94.59 ± 4.5 | 92.01 ± 5.41 | 1.84 | p = 0.09 |
| % correct Non-Responses (non-target) | 88.66 ± 7.8 | 86.76 ± 9.14 | 0.79 | p = 0.45 |
3.2. MEG: Event-related theta
The aMEG method provided insight into spatio-temporal stages of the ERT power during target detection. As shown in Figure 2, there is an early theta increase estimated to the occipital cortex across both groups and conditions. However, subsequent theta increase was observed only to the target stimuli. In contrast, nontargets did not elicit appreciable ERT increase relative to the baseline. The greatest theta activity overall was observed in the right IFC peaking after ~450 ms. Both the right aIFC and pIFC were also sensitive to group differences with BDs showing attenuated theta to targets as compared to LDs (Table 3) during the 380–510 ms time window.
Table 3.
MEG Theta Band Results.
| ROIs | Condition, F(1,50) | Group, F(1,50) | Condition × Group, F(1,50) | Target BD vs LD, F(1,50) | NonTarget BD vs LD, F(1,50) |
|---|---|---|---|---|---|
| Right alFC | 41.65*** | 4.1* | 4.74* | 4.74* | 0.006 |
| Right plFC | 41.06*** | 4.12* | 4.18* | 4.34* | 0.004 |
| Right sMot | 21.25*** | 3.8 | 0.7 | 4.02 | 2.3 |
| Left alFC | 30.1*** | 0.5 | 1.82 | 1.33 | 0.5 |
| Left plFC | 28.68*** | 1.94 | 0.6 | 0.75 | 0.15 |
| Left sMot | 20.54*** | 0.7 | 0.003 | 0.14 | 1.27 |
| Occipital | 0.97 | 0.31 | 0.001 | 0.02 | 0.03 |
ANOVA results for the Group and Condition main effects and interactions, as well as Group comparisons for the target and non-target conditions. Significance level is indicated as follows:
p < 0.05
p < 0.01
p < 0.001.
Results are expressed as F-values (1,50).
3.2.1.1. ERT correlates with alcohol intake, performance, and attention
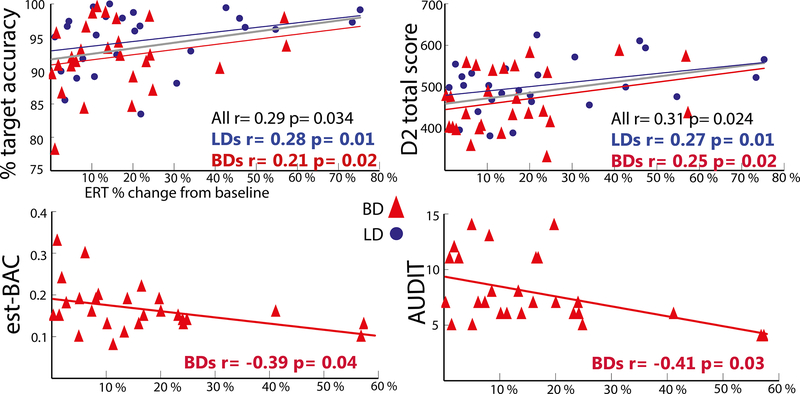
As shown in Figure 3, ERT power to targets in the right aIFC was positively correlated with response accuracy to targets (r=0.29, p<0.05) and with the total D2 attentional test score (r=0.31, p<0.05), indicating that right prefrontal theta power underlies successful target detection and higher attentional ability. In contrast, target-induced theta power estimated to the right aIFC was negatively correlated with the est-BAC (r=−0.39, p<0.05) and AUDIT scores (r=−0.41, p>0.03) in BDs. Correlations with the pIFC were very similar in pattern and significance indicating the association between lower theta power in the rIFC and higher levels of alcohol consumption which may underlie behavioral impairments.
Figure 3.
Scatter plots of the correlations between target theta power in the right aIFC and the performance accuracy, D2 attentional test scores, the estimated BAC and the AUDIT scores.
3.2.2. Co-oscillations in theta band (4–7 Hz) estimated with PLVs
To investigate functional interactions among the ROIs in real time, we estimated the degree of synchronous, in-phase co-oscillations by calculating the PLV in theta frequency band (Figure 4). The PLV between the right aIFC and pIFC areas to targets increased only in LDs F(1,50)= 9.65, p<0.01. Overall, synchronous co-oscillations were greater in LDs compared to BD as indicated by a main effect of group: F(1,50)= 6.7, p<0.05, and were greater for targets only, Group x Condition interaction: F(1,50) = 6.84, p<0.05. The synchrony was maximal in the time interval encompassing response execution (300–580 ms), indicating involvement of the ventrolateral prefrontal network. In addition, the PLV declined in BDs and was negatively correlated with the est-BAC (r=−0.54, p<0.01), suggesting that hazardous drinking levels are associated with dysregulation of network interactions.
4. DISCUSSION
The present study used a multimodal approach to examine theta band oscillatory activity and functional connectivity during target detection in adolescents emerging into adulthood as a function of binge drinking. The main findings can be summarized as follows: 1) the greatest event-related theta (ERT) was evoked by targets in the right inferior frontal cortex (rIFC); 2) It correlated with performance accuracy and selective attention scores, confirming the importance of rIFC in target detection, decision making, and response mapping. 3) Lower ERT to targets was observed in BDs compared to LDs in the rIFC. 4) Furthermore, ERT correlated negatively with the est-BAC and the AUDIT score in the BD group, lending support to the idea that lower attentional function in BDs is associated with heavy alcohol consumption. 5) Theta band co-oscillations between the right aIFC and pIFC were dysregulated in BDs indicating decreased connectivity of the prefrontal network during attentional engagement, response selection, and execution. The lower neural synchrony in BDs was associated with alcohol intake.
The IFC has been characterized as a critical substrate of cognitive control which refers to the ability to flexibly and strategically respond to contextual demands in agreement with past experiences and current goals (Aron et al., 2014; Brass et al., 2005; Chambers et al., 2009; Hampshire et al., 2010; Levy and Wagner, 2011; Munakata et al., 2011; Wiecki and Frank, 2013). Cognitive control is multiply determined by different underlying cognitive processes with specific contributions (Badre, 2008; Brass et al., 2005; Chikazoe, 2010; Criaud and Boulinguez, 2013; Koechlin et al., 2003; Levy and Wagner, 2011). It has been well established that the rIFC is engaged by tasks eliciting suppression of prepotent responses (Aron et al., 2014; Chikazoe, 2010; Garavan et al., 1999; Swick et al., 2011; Wessel and Aron, 2017). However, inherent to those paradigms are non-inhibitory confounds such as attention to salient, infrequent stimuli. Indeed, there is extensive evidence of the rIFC recruitment during attentional tasks that impose no or minimal inhibitory requirements (Erika-Florence et al., 2014; Hampshire et al., 2010; Hampshire and Sharp, 2015). Therefore, rIFC does not seem to solely specialize for motor inhibition but is activated by salient cues capturing attention (Hampshire et al., 2009, 2010). Studies using attentional manipulations confirm that the rIFC is an essential hub of the ventral attentional network (Chica et al., 2013; Corbetta et al., 2008; Corbetta and Shulman, 2002). Moreover, the ventral prefrontal network is engaged during deliberate target detection (Hampshire et al., 2009; Indovina and Macaluso, 2007; Shulman et al., 2010; Vossel et al., 2014) which relies on attentional control, response mapping, and maintenance of task sets (Dosenbach et al., 2008). Results of the present study are aligned with such evidence as much greater ERT was elicited by target stimuli in the rIFC due to its salience. In comparison, equiprobable nontarget stimuli elicited virtually no theta in any of the frontal areas (Figure 2) subsequent to evoking equivalent early activity in the visual cortex. This finding is consistent with previous reports of greater theta to task-relevant stimuli with contributions of memory retrieval, attention, working memory, decision making, and continuous monitoring (Cavanagh and Frank, 2014; Cheyne et al., 2012; Halgren et al., 2015; Kovacevic et al., 2012; Nyhus and Curran, 2010; Sauseng et al., 2010; Womelsdorf et al., 2010). Furthermore, theta power scales with task difficulty and cognitive demands confirming its essential contributions to cognitive control (Beaton et al., 2017; Ishii et al., 2014; Kovacevic et al., 2012; Rosen et al., 2016). In the present study, ERT to targets in the rIFC correlated with better response accuracy and higher scores on attentional test (Figure 3) which supports the involvement of theta in attentional processing and responding to task-relevant stimuli.
Binge drinkers exhibited lower theta power to targets compared to LDs in the rIFC (Figure 2, Table 3). Higher levels of alcohol consumption were associated with lower theta in BDs (Figure 3), suggesting that it may potentially serve as an index of reduced cognitive capacity in young, highly functioning individuals as a function of their drinking levels. Group differences in task performance eluded statistical significance although BDs showed a marginal tendency to respond with longer reaction times and lower accuracy (Table 2). On a behavioral level, these results are in line with the attentional deficits observed in BDs (Lannoy et al., 2017) and in AUD individuals (Maurage et al., 2014; Muller-Oehring and Schulte, 2014; Oscar-Berman and Marinković, 2007; Tedstone and Coyle, 2004), suggesting that impaired attentional control might contribute to alcohol-related problems. Indeed, it has been well established that alcoholics manifest reduced ERT power during cognitive tasks (Campanella et al., 2009; Kamarajan and Porjesz, 2015; Pandey et al., 2012; Porjesz et al., 2005; Rangaswamy and Porjesz, 2014). Previous EEG studies have examined ERT with equiprobable Go/NoGo tasks in individuals with AUD and reported decreased theta power to both conditions (Kamarajan et al., 2004; Pandey et al., 2016). Similarly, a recent EEG study that used the same paradigm in a shared sample as the current study (López-Caneda et al., 2017) reported reduced power in delta and theta bands in BDs to both target and non-target stimuli. These findings were interpreted as reflecting dysfunctional response execution and response inhibition. They were partially replicated in our study inasmuch as we found lower ERT in BDs. However, our results clearly indicate much greater neural engagement during target (Go) trials suggesting that the observed activity was primarily elicited by attention-inducing, target-relevant processes. In contrast, theta activity to task irrelevant NoGo trials was barely above baseline levels indicating negligible contributions of response inhibition. Furthermore, our method has permitted a more refined spatial specificity indicating selective sensitivity of the rIFC to higher levels of alcohol consumption with lower ERT to target trials in BDs. Additional studies are needed to explore these discrepancies, but they may be due to different data analysis methods and may reflect better spatial sensitivity of the MEG signal.
In a series of aMEG studies using different executive tasks and alcohol challenge in social drinkers, we have observed attenuated ERT in the prefrontal cortex during alcohol intoxication especially under higher cognitive load (Beaton et al., 2017; Kovacevic et al., 2012; Marinkovic et al., 2012; Rosen et al., 2016). Lower theta has also been reported in high-risk offspring of individuals with AUD (Kamarajan and Porjesz, 2015). Taken together, lower task-induced theta oscillations have been proposed as an endophenotype for susceptibility to AUD (Andrew and Fein, 2010; Hodgkinson et al., 2010; Porjesz et al., 2005; Rangaswamy and Porjesz, 2008; Salvatore et al., 2015).
Target processing relies on sustained selective attention. Imaging studies confirm that the rIFC may be involved in evaluating significance of salient events across sensory modalities (Downar et al., 2002). Its sensitivity to visual salience (Vossel et al., 2011) is supported by evidence from single-cell recordings in monkeys indicating that the IFC receives detailed visual information from the ventral visual pathway (Sakagami and Pan, 2007; Sakagami and Tsutsui, 1999; Scalaidhe et al., 1999). Functional specialization and material-specific sensitivity of micro-domains in the IFC has been shown with human intracranial recordings (Marinkovic et al., 2000). Attentional processing is essential not only for detecting relevant stimuli but also for managing distraction in a broader context of goal-dependent constraints. Altered behavioral and brain-based indices of distractibility have been observed in abstinent alcoholics (Ahveninen et al., 2000) while acute alcohol intoxication selectively impairs attention to novelty (Marinkovic et al., 2001). Increased impulsivity and vulnerability to distraction are strongly implicated in alcohol and drug addiction (Evenden, 1999). Inability to exert inhibitory control over drinking may result from an interaction of genetic predisposition and drug-induced deficits (Begleiter and Porjesz, 1999; Dick et al., 2010). IFC has been implicated in top-down attentional control and emotional reappraisal (Goldin et al., 2008; Green et al., 2011) and it may subserve the integration of attentional control, decision making, and top-down regulative influences, and their degradation in addiction (Goldstein and Volkow, 2002; Koob and Volkow, 2010).
Drawing on excellent temporal resolution of the MEG signal, the present study examined co-oscillations between the two principal activated areas in the rIFC in real time (Figure 4). We calculated PLVs in theta band to quantify consistency of the oscillatory phase between the aIFC and pIFC (Lachaux et al., 1999). Co-oscillations are known to transiently increase at the time of active interactions between two ROIs of interest (Beaton et al., 2017; Klopp et al., 2000; Tallon-Baudry and Bertrand, 1999). Theta oscillations are particularly suitable to investigate cross-cortical integration as they are primarily generated in superficial cortical layers (Halgren et al., 2015, 2018) and are sensitive to episodic encoding and retrieval (Anderson et al., 2010; Hanslmayr and Staudigl, 2014; Hasselmo and Stern, 2014; Klimesch et al., 2010; Womelsdorf et al., 2010). As shown in Figure 4, co-oscillations between the aIFC and pIFC were negligible on nontarget trials in both groups. In contrast, they were significantly greater to target stimuli in LDs during the time of attentional selection, response preparation, and execution. This suggests that the aIFC and pIFC were co-active during attentional control and motor response in LDs. Given their functional synchronization, the two areas appear to be linked in an interactive IFC network which is consistent with other evidence showing a high degree of interconnectivity in the IFC (Goulas et al., 2012). However, due to potential confounds due to field spread (Schoffelen and Gross, 2009), these findings should be considered with caution. The converging findings suggest that the IFC may not be organized into separable, specialized modules based on their specific functional profiles but that they are flexibly engaged and integrated by adaptive contextual maintenance (Reynolds et al., 2012). Studies investigating the functional organization of the IFC by manipulating hierarchical levels of rule representation have confirmed a lack of spatial differentiation across different representational levels (Pischedda et al., 2017). On that view, the contributions of the IFC to cognitive function are represented in spatially overlapping areas that are fluidly engaged to conform to task rules. Other studies have also suggested that the IFC and the neighboring anterior insular cortex represent a core hub for rule processing and maintenance of task sets, integrating different dimensions of cognitive control (Dosenbach et al., 2007; Menon and Uddin, 2010).
Our results indicate that the oscillatory synchrony within the IFC was dysregulated in BDs and their PLV decrease was associated with alcohol intake (Figure 4). Previous studies reported that alcohol intoxication abolished PLV during a task evoking cognitive control (Beaton et al., 2017; Ehlers et al., 2012). Altered connectivity in BDs has also been reported during wakeful resting-state as reflected in PLV measured with MEG (Correas et al., 2015). Similarly, resting-state functional connectivity MRI studies in individuals with AUD have found lower connectivity in the executive control (Kim et al., 2017; Müller-Oehring et al., 2015; Weiland et al., 2014) and reward networks (Camchong et al., 2013; Wang et al., 2016). Thus, the attenuated co-oscillations to targets observed in the current study may indicate suboptimal integrative processing that could underlie cognitive deficits in BDs.
5. CONCLUSIONS AND LIMITATIONS
Taken together, these results confirm essential contributions of the rIFC to attentional control in a target detection task which relies on sustained and selective attention, response selection and execution, and performance monitoring. Lower ERT to target stimuli was observed in the rIFC in adolescents emerging into adulthood as a function of BD. It was associated with higher alcohol intake and worse performance accuracy and scores on a test of attention further indicating an impairment in attentional control in BDs. Heavy consumption was further associated with deficient co-oscillations in the rIFC that may underlie impaired cognitive functions in BDs. These indices may assist in devising interventions aimed at reducing drinking levels in young emerging adults.
Given the cross-sectional nature of this type of observational cohort studies, it is not possible to make causal inferences about the main factors underlying the loss of cognitive control. Although the observed correlations between the neural indices and alcohol intake suggest that binge drinking is associated with decrease cognitive control, it cannot be excluded that these differences precede alcohol consumption.
Acknowledgement
We thank Pablo Cuesta and Burke Rosen for their technical assistance.
Funding
This study was supported by the projects SPI/2010/134 and SPI/2010/051 from the Spanish Ministry of Health and Social Politics (National Plan of Drugs), and the National Institutes of Health, (R01-AA016624). Eduardo López-Caneda was supported by the Postdoctoral Fellowship of the Portuguese Foundation for Science and Technology SFRH/BPD/109750/2015.
Footnotes
Declaration of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
6. REFERENCES
- Ahveninen J, Jääskeläinen IP, Pekkonen E, et al. (2000) Increased distractibility by task-irrelevant sound changes in abstinent alcoholics. Alcoholism, clinical and experimental research 24(12): 1850–4. [PubMed] [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson KL, Rajagovindan R, Ghacibeh GA, et al. (2010) Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cerebral cortex (New York, N.Y. : 1991) 20(7): 1604–12. DOI: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- Andrew C and Fein G (2010) Induced theta oscillations as biomarkers for alcoholism. Clinical Neurophysiology 121(3): 350–358. DOI: 10.1016/j.clinph.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW and Poldrack RA (2014) Inhibition and the right inferior frontal cortex: one decade on. Trends in Cognitive Sciences 18(4): 177–185. DOI: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Badre D (2008) Cognitive control, hierarchy, and the rostro–caudal organization of the frontal lobes. Trends in Cognitive Sciences 12(5): 193–200. DOI: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Beaton LE, Azma S and Marinkovic K (2017) When the brain changes its mind: Oscillatory dynamics of conflict processing and response switching in a flanker task during alcohol challange. PloS one. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, et al. (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50(1–3): 7–15. [DOI] [PubMed] [Google Scholar]
- Becoña E, Fernández del Río E, López A, et al. (2011) [The Short Nicotine Dependence Syndrome Scale (NDSS-S) in Spanish smokers]. Psicothema 23(1): 126–32. [PubMed] [Google Scholar]
- Begleiter H and Porjesz B (1999) What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism, clinical and experimental research 23(7): 1125–35. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann BU, et al. (2005) The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences 9(7): 314–316. DOI: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R and Seisdedos N (2009) D2, test de atención [D2 Attention test] (3rd ed.). TEA, Madrid, Spain. [Google Scholar]
- Brier MR, Ferree TC, Maguire MJ, et al. (2010) Frontal theta and alpha power and coherence changes are modulated by semantic complexity in Go/NoGo tasks. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 78(3): 215–24. DOI: 10.1016/j.ijpsycho.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Camchong J, Stenger VA and Fein G (2013) Resting-state synchrony in short-term versus long-term abstinent alcoholics. Alcoholism, clinical and experimental research 37(5): 794–803. DOI: 10.1111/acer.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella S, Petit G, Maurage P, et al. (2009) Chronic alcoholism: insights from neurophysiology. Neurophysiologie clinique = Clinical neurophysiology 39(4–5): 191–207. DOI: 10.1016/j.neucli.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, et al. (2005) Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences 9(3): 104–110. DOI: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF and Frank MJ (2014) Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences 18(8): 414–421. DOI: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Garavan H and Bellgrove M a (2009) Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience and biobehavioral reviews 33(5): 631–46. DOI: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Cheyne DO, Ferrari P and Cheyne JA (2012) Intended actions and unexpected outcomes: automatic and controlled processing in a rapid motor task. Frontiers in Human Neuroscience 6: 237 DOI: 10.3389/fnhum.2012.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB, Bartolomeo P and Lupiáñez J (2013) Two cognitive and neural systems for endogenous and exogenous spatial attention. Behavioural brain research 237: 107–23. DOI: 10.1016/j.bbr.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Chikazoe J (2010) Localizing performance of go/no-go tasks to prefrontal cortical subregions. Current Opinion in Psychiatry 23(3): 267–272. DOI: 10.1097/YCO.0b013e3283387a9f. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Sullivan E V, Ford JM, et al. (2011) Frontally mediated inhibitory processing and white matter microstructure: age and alcoholism effects. Psychopharmacology 213(4): 669–79. DOI: 10.1007/s00213-010-2073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M and Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nature reviews. Neuroscience 3(3): 201–15. DOI: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G and Shulman GL (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58(3): 306–24. DOI: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas A, Rodriguez Holguín S, Cuesta P, et al. (2015) Exploratory Analysis of Power Spectrum and Functional Connectivity During Resting State in Young Binge Drinkers: A MEG Study. International Journal of Neural Systems 25(03): 1550008 DOI: 10.1142/S0129065715500082. [DOI] [PubMed] [Google Scholar]
- Correas A, Cuesta P, López-Caneda E, et al. (2016) Functional and structural brain connectivity of young binge drinkers: A follow-up study. Scientific Reports 6 DOI: 10.1038/srep31293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE and Polich J (2009) Binge Drinking in Young Adults: Data, Definitions and Determinants. Psychol bull 135(1): 142–156. DOI: 10.1037/a0014414.Binge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA and Koob GF (2011) Preclinical studies of alcohol binge drinking. Annals of the New York Academy of Sciences 1216(1): 24–40. DOI: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT and Boettiger CA (2009) Impulsivity, frontal lobes and risk for addiction Pharmacology, biochemistry, and behavior 93(3). Elsevier Inc.: 237–47. DOI: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, et al. (2016) Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacological reviews 68(4): 1074–1109. DOI: 10.1124/pr.115.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M and Boulinguez P (2013) Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience and biobehavioral reviews 37(1): 11–23. DOI: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Cservenka A and Brumback T (2017) The Burden of Binge and Heavy Drinking on the Brain: Effects on Adolescent and Young Adult Neural Structure and Function. Frontiers in psychology 8: 1111 DOI: 10.3389/fpsyg.2017.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, et al. (2000) Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 26(1): 55–67. [DOI] [PubMed] [Google Scholar]
- de Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction biology 14(1): 22–31. DOI: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A and Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods 134(1): 9–21. DOI: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Derogatis LR and Melisaratos N (1983) The Brief Symptom Inventory: an introductory report. Psychological medicine 13(3): 595–605. [PubMed] [Google Scholar]
- Diamond A (2013) Executive functions. Annual review of psychology 64(1): 135–68. DOI: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, et al. (2010) Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology 15(2): 217–226. DOI: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, et al. (2007) Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences 104(26): 11073–11078. DOI: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair D a, Cohen AL, et al. (2008) A dual-networks architecture of top-down control. Trends in cognitive sciences 12(3): 99–105. DOI: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, et al. (2002) A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of neurophysiology 87(1): 615–20. DOI: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN and Havstad J (2012) Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain research 1450: 67–79. DOI: 10.1016/j.brainres.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erika-Florence M, Leech R and Hampshire A (2014) A functional network perspective on response inhibition and attentional control. Nature Communications 5: 4073 DOI: 10.1038/ncomms5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eurobarometer (2010) EU citizens´attitudes towards alcohol. Special Eurobarometer 331/Wave 72.3.
- Evenden JL (1999) Varieties of impulsivity. Psychopharmacology 146(4): 348–61. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, et al. (2005) The activation of attentional networks. NeuroImage 26(2): 471–9. DOI: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, et al. (1999) High-resolution intersubject averaging and a coordinate system for the cortical surface. Human brain mapping 8(4): 272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgueira-Ares R, Cadaveira F, Rodríguez Holguín S, et al. (2017) Electrophysiological Anomalies in Face-Name Memory Encoding in Young Binge Drinkers. Frontiers in psychiatry 8: 216 DOI: 10.3389/fpsyt.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ and Stein E a (1999) Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America 96(14): 8301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, et al. (1999) A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain : a journal of neurology 122 ( Pt 6): 1093–106. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America 101(21): 8174–9. DOI: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, et al. (2008) The Neural Bases of Emotion Regulation: Reappraisal and Suppression of Negative Emotion. Biological Psychiatry 63(6): 577–586. DOI: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ and Volkow ND (2002) Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. American Journal of Psychiatry 159(10): 1642–1652. DOI: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas A, Uylings HBM and Stiers P (2012) Unravelling the Intrinsic Functional Organization of the Human Lateral Frontal Cortex: A Parcellation Scheme Based on Resting State fMRI. Journal of Neuroscience 32(30): 10238–10252. DOI: 10.1523/JNEUROSCI.5852-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, et al. (2014) MNE software for processing MEG and EEG data. NeuroImage 86: 446–60. DOI: 10.1016/j.neuroimage.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JJ, Doesburg SM, Ward LM, et al. (2011) Electrical Neuroimaging of Voluntary Audiospatial Attention: Evidence for a Supramodal Attention Control Network. Journal of Neuroscience 31(10): 3560–3564. DOI: 10.1523/JNEUROSCI.5758-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamón M, Solé A and Farran J (1999) Test para la identificacion de trastornos por uso de alcohol (AUDIT): Traducción y validación del AUDIT al catalán y el castellano. Adicciones 11: 337–347. [Google Scholar]
- Halgren E, Kaestner E, Marinkovic K, et al. (2015) Laminar profile of spontaneous and evoked theta: Rhythmic modulation of cortical processing during word integration. Neuropsychologia 76: 108–24. DOI: 10.1016/j.neuropsychologia.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren M, Fabó D, Ulbert I, et al. (2018) Superficial Slow Rhythms Integrate Cortical Processing in Humans. Scientific Reports 8(1): 2055 DOI: 10.1038/s41598-018-20662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A and Sharp D (2015) Inferior PFC Subregions Have Broad Cognitive Roles. Trends in Cognitive Sciences 19(12): 712–713. DOI: 10.1016/j.tics.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, et al. (2009) Selective tuning of the right inferior frontal gyrus during target detection. Cognitive, affective & behavioral neuroscience 9(1): 103–12. DOI: 10.3758/CABN.9.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, et al. (2010) The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage 50(3): 1313–1319. DOI: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S and Staudigl T (2014) How brain oscillations form memories — A processing based perspective on oscillatory subsequent memory effects. NeuroImage 85: 648–655. DOI: 10.1016/j.neuroimage.2013.05.121. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Pastötter B, Bäuml K-H, et al. (2008) The electrophysiological dynamics of interference during the Stroop task. Journal of cognitive neuroscience 20(2): 215–25. DOI: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME and Stern CE (2014) Theta rhythm and the encoding and retrieval of space and time. NeuroImage 85: 656–666. DOI: 10.1016/j.neuroimage.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens DF, Lagopoulos J, Tobias-Webb J, et al. (2013) Pathways to alcohol-induced brain impairment in young people: A review. Cortex 49(1): 3–17. [DOI] [PubMed] [Google Scholar]
- Hingson RW and White A (2013) Trends in Extreme Binge Drinking Among US High School Seniors. JAMA Pediatrics 167(11): 996 DOI: 10.1001/jamapediatrics.2013.3083. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W and White AM (2017) Drinking Beyond the Binge Threshold: Predictors, Consequences, and Changes in the U.S. American journal of preventive medicine 52(6): 717–727. DOI: 10.1016/j.amepre.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Enoch M-A, Srivastava V, et al. (2010) Genome-wide association identifies candidate genes that influence the human electroencephalogram. Proceedings of the National Academy of Sciences of the United States of America 107(19): 8695–700. DOI: 10.1073/pnas.0908134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I and Macaluso E (2007) Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cerebral cortex (New York, N.Y. : 1991) 17(7): 1701–11. DOI: 10.1093/cercor/bhl081. [DOI] [PubMed] [Google Scholar]
- Ishii R, Canuet L, Ishihara T, et al. (2014) Frontal midline theta rhythm and gamma power changes during focused attention on mental calculation: an MEG beamformer analysis. Frontiers in human neuroscience 8: 406 DOI: 10.3389/fnhum.2014.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J and Tapert SF (2013) Neurotoxic effects of alcohol in adolescence. Annual review of clinical psychology 9: 703–21. DOI: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O and Tesche CD (2002) Frontal theta activity in humans increases with memory load in a working memory task. The European journal of neuroscience 15(8): 1395–9. [DOI] [PubMed] [Google Scholar]
- Johnston L (2011) Monitoring the Future: National Results on Adolescent Drug Use: Overview of Key Findings.
- Kamarajan C and Porjesz B (2015) Advances in Electrophysiological Research. Alcohol research : current reviews 37(1): 53–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, et al. (2004) The role of brain oscillations as functional correlates of cognitive systems : a study of frontal inhibitory control in alcoholism. 51: 155–180. DOI: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Im S, Lee J, et al. (2017) Disrupted Control Network Connectivity in Abstinent Patients with Alcohol Dependence. Psychiatry Investigation 14(3): 325 DOI: 10.4306/pi.2017.14.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R and Sauseng P (2010) Oscillatory mechanisms of process binding in memory. Neuroscience & Biobehavioral Reviews 34(7): 1002–1014. DOI: 10.1016/j.neubiorev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Klinke ME, Hjaltason H, Tryggvadóttir GBFR, et al. (2017) Hemispatial neglect following right hemisphere stroke: clinical course and sensitivity of diagnostic tasks. Topics in stroke rehabilitation: 1–11. DOI: 10.1080/10749357.2017.1394632. [DOI] [PubMed] [Google Scholar]
- Klopp J, Marinkovic K, Chauvel P, et al. (2000) Early widespread cortical distribution of coherent fusiform face selective activity. Human brain mapping 11(4): 286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C and Kouneiher F (2003) The Architecture of Cognitive Control in the Human Prefrontal Cortex. Science 302(5648): 1181–1185. DOI: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Koob GF and Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35(1). American College of Neuropsychopharmacology: 217–38. DOI: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, et al. (2012) Theta oscillations are sensitive to both early and late conflict processing stages: Effects of alcohol intoxication. PLoS ONE 7(8): 1–11. DOI: 10.1371/journal.pone.0043957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, et al. (1999) Measuring phase synchrony in brain signals. Human brain mapping 8(4): 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S, Heeren A, Moyaerts N, et al. (2017) Differential impairments across attentional networks in binge drinking. Psychopharmacology 234(7): 1059–1068. DOI: 10.1007/s00213-017-4538-4. [DOI] [PubMed] [Google Scholar]
- Lebel C and Beaulieu C (2011) Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience 31(30): 10937–47. DOI: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ and Wagner AD (2011) Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences 1224(1): 40–62. DOI: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F-H, Witzel T, Hämäläinen MS, et al. (2004) Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage 23(2): 582–95. DOI: 10.1016/j.neuroimage.2004.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Caneda E, Cadaveira F, Crego A, et al. (2012) Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction (Abingdon, England) 107(10): 1796–808. DOI: 10.1111/j.1360-0443.2012.03908.x. [DOI] [PubMed] [Google Scholar]
- López-Caneda E, Cadaveira F, Crego A, et al. (2013) Effects of a persistent binge drinking pattern of alcohol consumption in young people: a follow-up study using event-related potentials. Alcohol and alcoholism (Oxford, Oxfordshire) 48(4): 464–71. DOI: 10.1093/alcalc/agt046. [DOI] [PubMed] [Google Scholar]
- López-Caneda E, Rodríguez Holguín S, Correas Á, et al. (2017) Binge drinking affects brain oscillations linked to motor inhibition and execution. Journal of Psychopharmacology 31(7). DOI: 10.1177/0269881116689258. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Trebon P, Chauvel P, et al. (2000) Localised face processing by the human prefrontal cortex: face-selective intracerebral potentials and post-lesion deficits. Cognitive neuropsychology 17(1): 187–99. DOI: 10.1080/026432900380562. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E and Maltzman I (2001) Arousal-related P3a to novel auditory stimuli is abolished by a moderately low alcohol dose. Alcohol and alcoholism (Oxford, Oxfordshire) 36(6): 529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, et al. (2003) Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron 38(3): 487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rosen BQ, Cox B, et al. (2012) Event-Related Theta Power during Lexical-Semantic Retrieval and Decision Conflict is Modulated by Alcohol Intoxication: Anatomically Constrained MEG. Frontiers in Psychology 3: 121 DOI: 10.3389/fpsyg.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E and Oostenveld R (2007) Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods 164(1): 177–190. [DOI] [PubMed] [Google Scholar]
- Maurage P, de Timary P, Billieux J, et al. (2014) Attentional alterations in alcohol dependence are underpinned by specific executive control deficits. Alcoholism, clinical and experimental research 38(7): 2105–12. DOI: 10.1111/acer.12444. [DOI] [PubMed] [Google Scholar]
- Menon V and Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function 214(5–6): 655–667. DOI: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM and Schulte T (2014) Alcohol and the Nervous System, Cognition emotion and attention In: Sullivan E V and Pfefferbaum A (eds) Handbook of Clinical Neurology, pp. 341–349. [DOI] [PubMed] [Google Scholar]
- Müller-Oehring EM, Jung Y-C, Pfefferbaum A, et al. (2015) The Resting Brain of Alcoholics. Cerebral cortex (New York, N.Y. : 1991) 25(11): 4155–68. DOI: 10.1093/cercor/bhu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, et al. (2011) A unified framework for inhibitory control. Trends in cognitive sciences 15(10): 453–9. DOI: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi TS, Nelson DE and Brewer RD (2010) The Intensity of Binge Alcohol Consumption Among U.S. Adults. American Journal of Preventive Medicine 38(2): 201–207. DOI: 10.1016/j.amepre.2009.09.039. [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol and Alcoholism (2004) NIAAA council approves definition of binge drinking. NIAAA newsletter, 3 p. 3. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (n.d.) Drinking Levels Defined | National Institute on Alcohol Abuse and Alcoholism (NIAAA). Available at: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed 18 December 2017).
- Nobre AC, Sebestyen GN, Gitelman DR, et al. (1997) Functional localization of the system for visuospatial attention using positron emission tomography. Brain : a journal of neurology 120 ( Pt 3): 515–33. [DOI] [PubMed] [Google Scholar]
- Nyhus E and Curran T (2010) Functional role of gamma and theta oscillations in episodic memory. Neuroscience & Biobehavioral Reviews 34(7): 1023–1035. DOI: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, et al. (2011) FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational intelligence and neuroscience 2011: 156869 DOI: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M and Marinković K (2007) Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology review 17(3): 239–57. DOI: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Rangaswamy M, et al. (2012) Event-Related Oscillations in Alcoholism Research: A Review. Journal of addiction research & therapy Suppl 7(1). NIH Public Access. DOI: 10.4172/2155-6105.S7-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Manz N, et al. (2016) Delta, theta, and alpha event-related oscillations in alcoholics during Go/NoGo task: Neurocognitive deficits in execution, inhibition, and attention processing. Progress in Neuro-Psychopharmacology and Biological Psychiatry 65(February): 158–171. DOI: 10.1016/j.pnpbp.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME and Terry-McElrath YM (2017) High-intensity drinking by underage young adults in the United States. Addiction (Abingdon, England) 112(1): 82–93. DOI: 10.1111/add.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS and Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology 51(6): 768–74. [DOI] [PubMed] [Google Scholar]
- Petit G, Maurage P, Kornreich C, et al. (2014) Binge drinking in adolescents: a review of neurophysiological and neuroimaging research. Alcohol and alcoholism (Oxford, Oxfordshire) 49(2): 198–206. DOI: 10.1093/alcalc/agt172. [DOI] [PubMed] [Google Scholar]
- Pischedda D, Görgen K, Haynes J-D, et al. (2017) Neural Representations of Hierarchical Rule Sets: The Human Control System Represents Rules Irrespective of the Hierarchical Level to Which They Belong. The Journal of Neuroscience 37(50): 12281–12296. DOI: 10.1523/JNEUROSCI.3088-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, et al. (2005) The utility of neurophysiological markers in the study of alcoholism. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 116(5): 993–1018. DOI: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M and Porjesz B (2008) Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain research 1235: 153–71. DOI: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M and Porjesz B (2014) Understanding alcohol use disorders with neuroelectrophysiology. Handbook of clinical neurology 125: 383–414. DOI: 10.1016/B978-0-444-62619-6.00023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, O’Reilly RC, Cohen JD, et al. (2012) The function and organization of lateral prefrontal cortex: a test of competing hypotheses. Gilbert S (ed.) PloS one 7(2): e30284 DOI: 10.1371/journal.pone.0030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BQ, Padovan N and Marinkovic K (2016) Alcohol Hits You When It Is Hard: Intoxication, Task Difficulty, and Theta Brain Oscillations. Alcoholism: Clinical and Experimental Research 40(4): 743–752. DOI: 10.1111/acer.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M and Pan X (2007) Functional role of the ventrolateral prefrontal cortex in decision making. Current opinion in neurobiology 17(2): 228–33. DOI: 10.1016/j.conb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Sakagami M and Tsutsui K (1999) The hierarchical organization of decision making in the primate prefrontal cortex. Neuroscience research 34(2): 79–89. [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Gottesman II and Dick DM (2015) Endophenotypes for Alcohol Use Disorder: An Update on the Field. Current addiction reports 2(1): 76–90. DOI: 10.1007/s40429-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (2013) Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survery on Drug Use and Health: 13–4795.
- Sauseng P, Griesmayr B, Freunberger R, et al. (2010) Control mechanisms in working memory: a possible function of EEG theta oscillations. Neuroscience and biobehavioral reviews 34(7): 1015–22. DOI: 10.1016/j.neubiorev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Scalaidhe SP, Wilson FA and Goldman-Rakic PS (1999) Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: evidence for intrinsic specialization of neuronal coding. Cerebral cortex (New York, N.Y. : 1991) 9(5): 459–75. [DOI] [PubMed] [Google Scholar]
- Schoffelen J-M and Gross J (2009) Source connectivity analysis with MEG and EEG. Human Brain Mapping 30(6): 1857–1865. DOI: 10.1002/hbm.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, et al. (2014) Development of the default mode and central executive networks across early adolescence: a longitudinal study. Developmental cognitive neuroscience 10: 148–59. DOI: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Pope DLW, Astafiev S V, et al. (2010) Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. The Journal of neuroscience : the official journal of the Society for Neuroscience 30(10): 3640–51. DOI: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Dager AD, Cohen-Gilbert JE, et al. (2016) Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neuroscience and biobehavioral reviews 70: 244–259. DOI: 10.1016/j.neubiorev.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, et al. (2014) Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. NeuroImage 92: 356–68. DOI: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J and Tapert SF (2014) The effect of alcohol use on human adolescent brain structures and systems. In: Handbook of clinical neurology, pp. 501–510. DOI: 10.1016/B978-0-444-62619-6.00028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN and Duka T (2008) Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 363(1507): 3169–79. DOI: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E V and Pfefferbaum A (2005) Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology 180(4): 583–94. DOI: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V and Turken U (2011) Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage 56(3): 1655–65. DOI: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudryand Bertrand (1999) Oscillatory gamma activity in humans and its role in object representation. Trends in cognitive sciences 3(4): 151–162. [DOI] [PubMed] [Google Scholar]
- Tedstone D and Coyle K (2004) Cognitive impairments in sober alcoholics: performance on selective and divided attention tasks. Drug and alcohol dependence 75(3): 277–86. DOI: 10.1016/j.drugalcdep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM and Patrick ME (2016) Intoxication and binge and high-intensity drinking among US young adults in their mid-20s. Substance Abuse 37(4): 597–605. DOI: 10.1080/08897077.2016.1178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, et al. (2001) The brainweb: phase synchronization and large-scale integration. Nature reviews. Neuroscience 2(4): 229–39. DOI: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vossel S, Weidner R and Fink GR (2011) Dynamic Coding of Events within the Inferior Frontal Gyrus in a Probabilistic Selective Attention Task. Journal of Cognitive Neuroscience 23(2): 414–424. DOI: 10.1162/jocn.2010.21441. [DOI] [PubMed] [Google Scholar]
- Vossel S, Geng JJ and Fink GR (2014) Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 20(2): 150–9. DOI: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brown TT, et al. (2012) Long-term influence of normal variation in neonatal characteristics on human brain development. Proceedings of the National Academy of Sciences of the United States of America 109(49): 20089–94. DOI: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, et al. (2005) Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. The Journal of neuroscience : the official journal of the Society for Neuroscience 25(3): 604–13. DOI: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fan Y, Dong Yue, et al. (2016) Alterations in Brain Structure and Functional Connectivity in Alcohol Dependent Patients and Possible Association with Impulsivity. Zuo X-N(ed.) PLOS ONE 11(8): e0161956 DOI: 10.1371/journal.pone.0161956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, et al. (2014) Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcoholism, clinical and experimental research 38(9): 2445–53. DOI: 10.1111/acer.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR and Aron AR (2017) On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron 93(2): 259–280. DOI: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki T V and Frank MJ (2013) A computational model of inhibitory control in frontal cortex and basal ganglia. Psychological review 120(2): 329–55. DOI: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- Windmark EMP (1981) Principles and Applications of Medicolegal Alcohol Determination. Davis(ed.). Biomedical Publications. [Google Scholar]
- Womelsdorf T, Vinck M, Leung LS, et al. (2010) Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Frontiers in human neuroscience 4: 210 DOI: 10.3389/fnhum.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M and Link K (1968) Construct validity for the sensation-seeking scale. Journal of consulting and clinical psychology 32(4): 420–6. [DOI] [PubMed] [Google Scholar]