Abstract
Rationale and Objectives
Adenosine signaling through adenosine A2A receptors (A2AR) is known to influence cocaine-induced behaviors. These studies sought to elucidate how two A2AR antagonists distinguished by their antagonist effects at presynaptic and postsynaptic A2AR influence cocaine-induced locomotion and cocaine seeking.
Methods
Sprague-Dawley rats were used to assess the differential effects of SCH 442416 and istradefylline that antagonize presynaptic and postsynaptic A2AR, respectively. We evaluated the effects of these antagonists on both basal and cocaine-induced locomotion in cocaine-naïve rats and rats that received 7 daily cocaine treatments. The effects of SCH 442416 or istradefylline on cocaine seeking was measured in animals extinguished from cocaine self-administration. We assessed the effects of the A2AR antagonists to induce cocaine seeking when administered alone and their effects on cocaine seeking induced by a cocaine priming injection. Lastly, we evaluated the effects of the antagonists on sucrose seeking in animals extinguished from sucrose self-administration.
Results
Neither istradefylline nor SCH 442416 significantly altered basal locomotion. Istradefylline enhanced acute cocaine-induced locomotion but had no effect on the expression of locomotor sensitization. SCH 44216 had no effect on acute cocaine-induced locomotion but inhibited the expression of locomotor sensitization. Istradefylline was sufficient to induce cocaine seeking and augmented both cocaine-induced seeking and sucrose seeking. SCH 442416 inhibited cocaine-induced seeking, but had no effect on sucrose seeking and did not induce cocaine seeking when administered alone.
Conclusions
These findings demonstrate differential effects of two A2AR antagonists distinguished by their effects at pre- and postsynaptic A2AR on cocaine-induced behaviors.
Keywords: Purinergic Receptors, Psychostimulant, Relapse, Behavioral Sensitization, KW 6002
Introduction
Cocaine addiction affects millions of people around the world, yet no accepted pharmacological treatment exists. Finding neurobiological targets for potential pharmacological intervention could provide a means by which people could more effectively recover from addiction. One such target for treatment may lie within the striatum, a component of the basal ganglia circuitry that is affected by cocaine administration (Kalivas 2009; Gerfen and Surmeier 2011). Here, multiple neurotransmitter systems interact during chronic cocaine administration and are thought to underlie many behavioral characteristics of addiction (Self et al. 2004; Kalivas 2009). A more detailed understanding of these neurotransmitter systems and their target receptors may help uncover new treatment approaches.
Striatal neurons integrate several neurotransmitter systems through the cellular co-localization and synaptic localization of receptor subtypes. One particularly interesting neurotransmitter is adenosine, a nucleoside produced by the breakdown of adenosine triphosphate (ATP) (Dunwiddie and Masino 2001; Schiffmann et al. 2007). Adenosine has the capacity to modulate the functioning of other neurotransmitters, such as dopamine and glutamate, that are known to be involved in the acute and chronic effects of cocaine (Ferré et al. 2007). The modulatory functions of adenosine are largely attributed to the synaptic co-localization of adenosine receptor subtypes with other receptors within the striatum. The two primary adenosine receptor subtypes found in the striatum are the Gi/o protein-coupled adenosine A1 receptor (A1R), and the Gs/olf protein-coupled adenosine A2A receptor (A2AR) (Dunwiddie and Masino 2001). There is substantial evidence demonstrating that A2ARs play a role in cocaine-induced locomotion, reward, and relapse (Knapp et al. 2001; Poleszak and Malec 2002a, b; Filip et al. 2006; Bachtell and Self 2009; O’Neill et al. 2012; Wydra et al. 2015a). The recent development of pharmacological tools to examine different A2AR subpopulations that are expressed at pre- and postsynaptic sites is particularly interesting since these tools may allow for more specific modulation of dopamine and/or glutamate neurotransmission (Rosin et al. 2003; Ciruela et al. 2006; Quiroz et al. 2009; Farrar et al. 2010; Orru et al. 2011a, b).
A2ARs are co-expressed with A1Rs on presynaptic glutamate terminals where they bi-directionally affect glutamate release (Ciruela et al. 2006; Quiroz et al. 2009). Under basal conditions, stimulation of the higher affinity A1R inhibits glutamate release (Barrie and Nicholls 1993; Ciruela et al. 2006). However, in the presence of increased cellular activity and higher phasic adenosine release that may occur during cocaine administration, the lower affinity A2AR is stimulated causing glutamate release into the synapse (Popoli et al. 1995; Marchi et al. 2002; Rodrigues et al. 2005; Ciruela et al. 2006; Shen et al. 2013; Matsumoto et al. 2014). Increased glutamate neurotransmission in the nucleus accumbens (NAc) is associated with increased cocaine seeking and locomotor activation (Schmidt and Pierce 2010; Steketee and Kalivas 2011). The facilitation of glutamate transmission produced by presynaptic A2AR stimulation would therefore be expected to enhance cocaine-mediated behaviors, while blockade of presynaptic A2AR may reduce cocaine seeking and cocaine-induced locomotion.
Stimulation of A2ARs is largely associated with a reduction in cocaine-mediated behaviors (Knapp et al. 2001; Poleszak and Malec 2002a, b; Bachtell and Self 2009; O’Neill et al. 2012; Wydra et al. 2015a). These effects are attributed to the postsynaptic expression of A2ARs on striatopallidal (indirect pathway) medium spiny neurons (MSNs) in the striatum (Svenningsson et al. 1999; Ferre et al. 2008; Azdad et al. 2009; Higley and Sabatini 2010). Activation of postsynaptic A2ARs on these neurons counteracts the inhibitory cellular effects of the Gi/o protein-coupled D2R stimulation via a heteromeric receptor complex with adenylyl cyclase 5 (Hakansson et al. 2006; Harper et al. 2006; Ferre et al. 2008; Farrar et al. 2010; Ferré et al. 2018). Vesicular release of dopamine is accompanied by the release of ATP that can be catabolized to adenosine within a few hundred milliseconds that subsequently acts on adenosine receptors (Poelchen et al. 2001; Ho et al. 2015). Initial D2R stimulation is subsequently counteracted by the latent adenosine signal to restore D2R-induced cellular effects (Chen et al. 2001; Ferre et al. 2008; Ferré et al. 2018). This process is best observed behaviorally following the application of an A2AR antagonist which results in increased locomotor activity due to its permissive effect on D2R activity (Chen et al. 2001; Orru et al. 2011a, b; Ferré 2016). Stimulation of A2ARs with agonists generally inhibits D2R-induced behaviors (Chen et al. 2001; Bachtell and Self 2009; O’Neill et al. 2012; Hobson et al. 2012). Because pre- and postsynaptic expression of A2ARs may have unique functional roles, preferential antagonism of pre- versus postsynaptic A2ARs may result in distinct behavioral effects when administered alone or when combined with cocaine.
Utilizing pharmacological tools previously shown to preferentially antagonize pre- versus postsynaptic A2ARs (Orru et al. 2011a, b), we determined the differential effects of A2AR antagonists on cocaine-induced behaviors in rats. Specifically, we investigated how the putative presynaptic antagonist, SCH 442416, and postsynaptic antagonist, istradefylline, affected cocaine-induced locomotion and cocaine seeking. We hypothesized that administration of the presynaptic A2AR antagonist would blunt cocaine-induced locomotion and cocaine seeking, while administration of the postsynaptic A2AR antagonist would induce locomotor activity and enhance cocaine seeking. Ultimately, these results shed light on specific roles of the synaptic localization of A2ARs and may lead to novel pharmacotherapeutic approaches.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (Envigo, Indianapolis, USA) weighing 300 +/− 30g were housed individually with ad libitum access to water and food throughout the experiments. Rats were housed under 12:12 light/dark cycle conditions. Animals were allowed at least 7 days after arrival to habituate to the new setting before starting experiments. All procedures complied with the Guide for Care and Use of Animals as adopted and promulgated by the U.S. National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at the University of Colorado Boulder.
Drugs
The selective A2AR antagonists, SCH 442416 (2-(2-Furanyl)-7-[3-(4-methoxyphenyl)propyl]-7Hpyrazolo[4,3-e][1,2,4]triazolo[1,5-c]p yrimidin-5-amine) and istradefylline, also known as KW 6002 (8-[(E)-2-(3,4-dimethoxyphenyl)etheyl]-1,3-diethyl-7-methylpurine-2,6-dione), were purchased from SigmaAldrich (St. Louis, MO, USA). Cocaine hydrochloride was also purchased from Sigma-Aldrich (St. Louis, MO, USA). Both A2AR antagonist drugs were dissolved in 50% DMSO and 50% sterile-filtered physiological (0.9%) saline. Cocaine hydrochloride was dissolved in sterile-filtered physiological (0.9%) saline.
Locomotor testing apparatus and general procedure
Rats were individually placed into plexiglass chambers sized 16×16×15 inches (San Diego Instruments, San Diego, USA) with pairs of infrared beams spaced one inch apart to record horizontal locomotion. All tests occurred in a dark room during the light phase of the light/dark cycle. Rats were habituated to the test room in their home cages for 1 hour prior to each locomotor test. Prior to testing, animals were habituated to the chambers for 2 hours the day prior to experimental testing before returning to their home cages. On test days, animals were habituated to the chambers for 1 hour before receiving intraperitoneal (ip) treatments.
Chronic cocaine administration procedure
Following the first day of habituation, animals were tested in the locomotor boxes as described above. Chronic cocaine (15 mg/kg) or saline was administered with seven daily ip injections. Locomotor testing was conducted on days 1, 7, and 14. Drug administration on days 2 through 6 occurred in home cages. Only animals meeting criterion for locomotor sensitization (day 7 cocaine-induced activity > 1.25 times greater than day 1 cocaine-induced locomotor activity) were subsequently challenged and tested on day 14 after a 7 day drug-free period. The effects of an A2AR antagonist pretreatment, SCH 442416 and istradefylline, were tested on day 14 with either a saline or cocaine (15 mg/kg) challenge (see details below).
Cocaine self-administration and extinction procedures
Self-administration procedures were performed in operant conditioning chambers (Med-Associates, St. Albans, VT, USA) equipped with two retractable levers (an active and an inactive lever), stimulus light cues above the levers, a sound-attenuating fan in each chamber, and an infusion pump system. Animals were initially trained on a fixed ratio 1 (FR1) schedule of reinforcement where pressing the active lever resulted in sucrose pellet delivery. Following adequate training to respond for a sucrose reward (100 sucrose pellets in one session), rats underwent surgery in which an intravenous catheter was implanted into the jugular vein under halothane anesthesia (1–2.5%). Following recovery from surgery, animals were allowed to administer intravenous cocaine (0.5 mg/kg/100 μl injection) over 15 2-h sessions using an FR1 schedule of reinforcement. During these sessions, pressing the drug-paired lever resulted in a 5-s infusion of cocaine and the presentation of the stimulus cue light, while pressing the inactive lever had no consequence. After drug delivery, the house light remained off, and pressing the drug-paired lever did not result in a drug infusion for 20-s. Following self-administration, rats underwent seven 2-hr extinction training sessions where pressing the drug-paired lever no longer resulted in the administration of cocaine or stimulus light illumination.
Experiment 1: Effects of systemic A2AR antagonist administration on locomotion
Following habituation to the locomotor chambers, rats received ip injections of vehicle, istradefylline (0, 0.1, 1.0, or 3.0 mg/kg), or SCH 442416 (0, 0.1, 1.0, or 3.0 mg/kg). Locomotor activity was recorded for 2 hours immediately following antagonist administration. Animals were repeatedly tested such that the dosing order was counterbalanced, rats were treated once every 48 hours, and all rats received each dose of either istradefylline or SCH 442416.
Experiment 2: Effects of A2AR antagonists on acute cocaine-induced locomotion
Rats received chronic saline for 7 days. After 7 days of home cage withdrawal, rats were administered a pretreatment of either vehicle, istradefylline (1 mg/kg, ip), or SCH 442416 (1 mg/kg, ip) 10 minutes prior to either a saline or cocaine (15 mg/kg, ip) challenge on day 14. Rats were immediately placed in the locomotor chambers, and locomotor activity was monitored for 2 hours.
Experiment 3: Effects of A2AR antagonists on locomotion in cocaine-sensitized rats
Rats received chronic cocaine for 7 days. After 7 days of home cage withdrawal, rats were administered a pretreatment of either vehicle, istradefylline (1 mg/kg, ip), or SCH 442416 (1 mg/kg, ip), 10 minutes prior to a saline or cocaine (15 mg/kg, ip) challenge on day 14. Rats were immediately placed in the locomotor chambers, and locomotor activity was monitored for 2 hours.
Experiment 4: Effects of A2AR antagonists on reinstatement of cocaine seeking
After extinguishing drug-paired lever presses, animals underwent a 2-hr reinstatement test. For these tests, animals were pretreated with vehicle, istradefylline (0.3, 1.0, or 3.0 mg/kg, ip), or SCH 442416 (1.0 or 3.0 mg/kg, ip) 10 minutes prior to a systemic saline challenge before being placed into the operant chamber. The number of active and inactive lever presses was then recorded and analyzed, though neither lever resulted in a drug infusion.
Experiment 5: Effects of A2AR antagonists on cocaine-primed reinstatement of drug seeking
Once rats had completed extinction, they underwent a 2-hr reinstatement test. Here, animals were pretreated with vehicle, istradefylline (0.3 mg/kg, ip), or SCH 442416 (1.0 mg/kg, ip), 10 minutes prior to a cocaine challenge. Rats pretreated with istradefylline were primed with both a subthreshold dose (5 mg/kg, ip) and maximal dose (15 mg/kg, ip) of cocaine to reveal the potential enhancement of cocaine seeking. Rats pretreated with SCH 442416 were primed with only a maximal dose (15 mg/kg, ip) of cocaine to reveal potential inhibitory effects on cocaine seeking. Following the cocaine primes, rats were immediately placed back into operant chambers, and the number of active and inactive lever presses was recorded and analyzed. During the test, neither lever resulted in a drug infusion.
Experiment 6: Effects of A2AR antagonists on reinstatement of sucrose seeking
Sucrose self-administration procedures occurred in operant boxes as described above. Food restricted rats were trained to lever press for 45 mg banana-flavored sucrose pellet (Bio-Serv, Flemington, NJ) into a food magazine located between the retractable levers on an FR1 schedule of reinforcement. Correct lever responses also resulted in the presentation of a stimulus cue light (5-s) followed by a 20-s time-out period when active lever pressing no longer resulted in sucrose pellet delivery. After 10 sessions and stabilization of sucrose self-administration, lever pressing was extinguished in seven 120-min sessions. Animals then underwent a reinstatement protocol similar to that described above. Animals were pre-treated with vehicle, istradefylline (1.0 mg/kg, ip), or SCH 442416 (1.0 mg/kg, ip) 10-min prior to being placed into the chambers for a 120-min reinstatement session. During the reinstatement session, 5 sucrose pellets were delivered noncontingently every 120-s during the first 10-min of the session. Throughout the session, responses on the previously sucrose-paired lever resulted in the presentation of stimulus cue light but not sucrose pellets. Active and inactive lever presses were recorded and analyzed. Responses on the inactive lever had no programmed consequences.
Statistical analyses
Total number of beam breaks per session was used to measure overall locomotion. For Experiments 1–3, the effect of the A2AR antagonists on beam breaks was analyzed using a between subjects one-way ANOVA with either antagonist dose or antagonist drug as the between subjects variable. Total active and inactive lever presses were used to evaluate the effects of the antagonists on cocaine seeking. In experiment 4, the effect of A2AR antagonist pretreatment dose on reinstatement of lever pressing was analyzed using a between subjects 2 × 4 factorial ANOVA with lever (active and inactive) and istradefylline (0.0, 0.3, 1.0, and 3.0 mg/kg) as the variables. A separate 2 × 3 factorial ANOVA with lever (active x inactive) and SCH 442416 (0.0, 1.0, and 3.0 mg/kg) was also utilized.
Experiment 5 was analyzed with between subjects 2 × 2 factorial ANOVAs with lever (active and inactive) and cocaine dose (5 or 15 mg/kg) to evaluate istradefylline, or lever (active and inactive) and treatment (vehicle, cocaine, or SCH 442416) to evaluate SCH 442416. A between subjects one-way ANOVA was used to analyze Experiment 6 with pretreatment drug as the between subjects variable to assess the effects of antagonist pretreatment on reinstatement of sucrose seeking. Significant main effects and interactive effects were subsequently analyzed by a one-way ANOVA and/or Bonferroni’s test for multiple comparisons. Significance level was set at p < 0.05.
Results
The A2AR antagonist, istradefylline, increases locomotor activity
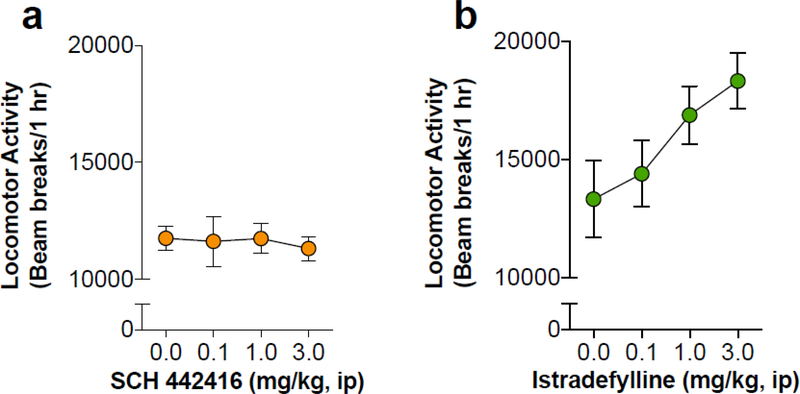
Experiment 1 investigated the effects of various doses of the A2AR antagonists on locomotor activity in male rats (Fig. 1). We found that administration of the postsynaptic A2AR antagonist, istradefylline, increased locomotion, although this did not reach statistical significance (F3,31 = 2.77, p = 0.06) (Fig. 1A). The presynaptic antagonist, SCH 442416, did not significantly alter locomotor activity at any of the selected doses compared to vehicle-treated rats (Fig. 1B). Given these and previous findings, the dose of 1.0 mg/kg was chosen for both A2AR antagonists in all subsequent studies (Harper et al. 2006; Orru et al. 2011a, b).
Figure 1.
Dose-dependent effects of the two adenosine A2AR antagonists on locomotion in habituated rats. A) Systemic administration of SCH 442416 had no effect on locomotor activity at any of the tested doses. B) Systemic administration of istradefylline dose-dependently increased locomotor activity, although no statistical significance was detected.
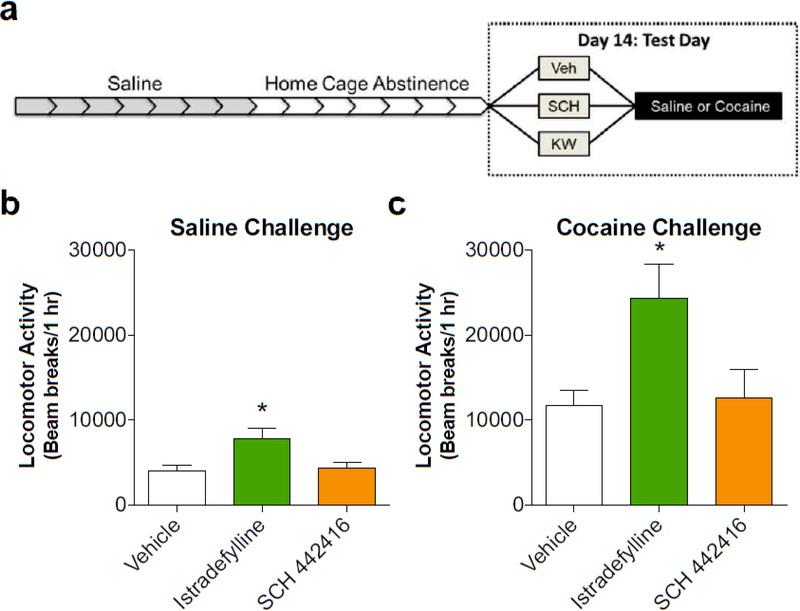
The A2AR antagonist, istradefylline, increases cocaine-induced locomotion
Experiment 2 investigated the effects of A2AR antagonism on locomotor activity in drug-naïve animals to understand how the antagonists affected acute cocaine-induced locomotion. We first tested the effects of the A2AR antagonists on animals having experienced a protocol in which they received repeated saline administration (Fig. 2A). There was a significant effect of antagonist pretreatment where animals receiving istradefylline treatments had significantly higher locomotor activity than vehicle- or SCH 442416-treated animals (F2,37 = 6.37, p = 0.0042) (Fig. 2B). We next wanted to characterize how these antagonists affected cocaine-induced locomotion (Fig. 2C). Here, animals pretreated with istradefylline had elevated locomotor activity compared to vehicle- and SCH 442416-pretreated animals (F2,21 = 6.22, p = 0.0076) (Fig. 2C). These results suggest that postsynaptic A2AR antagonism enhances locomotor activity while presynaptic A2AR antagonism is without effect, substantiating findings from Experiment 1 and supporting previous findings (Harper et al. 2006; Orru et al. 2011a, b).
Figure 2.
Locomotor activity was significantly enhanced by the postsynaptic A2AR antagonist, istradefylline, but not the presynaptic A2AR antagonist, SCH 442416. A) Timeline of experiment depicting that rats received 7 daily injections of saline (0.9%, i.p.), followed by 7 days in home cages without treatment. Locomotion was tested on Day 14 following both an A2AR antagonist pretreatment (Vehicle, 1.0 mg/kg istradefylline, or 1.0 mg/kg SCH 442416) and challenge (Saline or 15 mg/kg, i.p. cocaine). B) Rats receiving a pretreatment of istradefylline showed heightened locomotor activity compared to vehicle-treated animals when challenged with saline on Day 14. C) A pretreatment of istradefylline increased cocaine-induced locomotor activity compared to vehicle- and SCH 442416pretreated animals. * indicates significance between both Vehicle and SCH 442416 (p < 0.05)
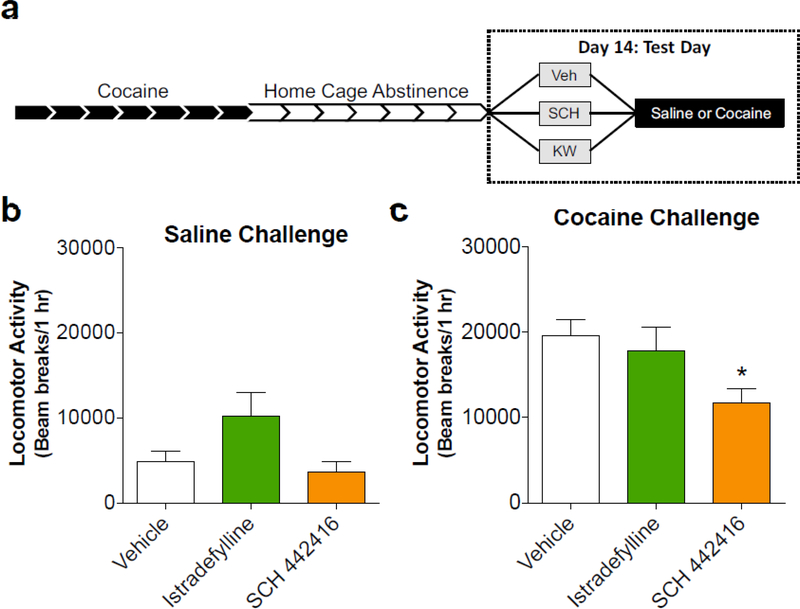
The A2AR antagonist, SCH 442416, decreases expression of cocaine sensitization
After studying the effects of the A2AR antagonists on acute cocaine treatments, Experiment 3 assessed the effects of these antagonists following repeated cocaine administration. Rats that reached the criterion for locomotor sensitization were counterbalanced and assigned to a pretreatment group (vehicle, istradefylline, or SCH 442416) and either the saline or cocaine challenge treatment group. On day 14 of testing, rats received either a vehicle, istradefylline, or SCH 442416 injection prior to the saline/cocaine challenge (Fig. 3A). Prior to a saline challenge, there was a main effect of antagonist pretreatment (F2,15 = 3.951, p = 0.042), although a post hoc test did not reveal a significant difference between individual pretreatment groups (Fig. 3B). Interestingly, the A2AR antagonists had a much different locomotor activity profile prior to a cocaine challenge in rats chronically treated with cocaine. We observed a main effect of antagonist pretreatment (Fig. 3C, F2,27 = 4.26, p = 0.025) with the pretreatment of SCH 442416 significantly reducing locomotor activity compared to vehicle-pretreated animals.
Figure 3.
The presynaptic A2AR antagonist, SCH 442416, prevents expression of cocaine-sensitized locomotion, while the postsynaptic A2AR antagonist, istradefylline, increases locomotion in cocaine-sensitized animals prior to a saline challenge. A) Timeline of experiment depicting that rats received 7 daily injections of cocaine (15 mg/kg, i.p.), followed by 7 days in home cages without treatment. Locomotion was tested on Day 14 following both an A2AR antagonist pretreatment (vehicle, 1.0 mg/kg istradefylline, or 1.0 mg/kg SCH 442416) and challenge (saline or 15 mg/kg, i.p. cocaine). B) Cocaine-sensitized rats that were challenged with saline on Day 14 displayed an increase in locomotor activity following a pretreatment of istradefylline, although this did not reach statistical significance. C) Cocaine-sensitized rats that were challenged with cocaine following a pretreatment of SCH 442416 displayed overall decreased locomotor activity compared to vehicle-pretreated rats. * indicates significance from vehicle pretreatment (p < 0.05)
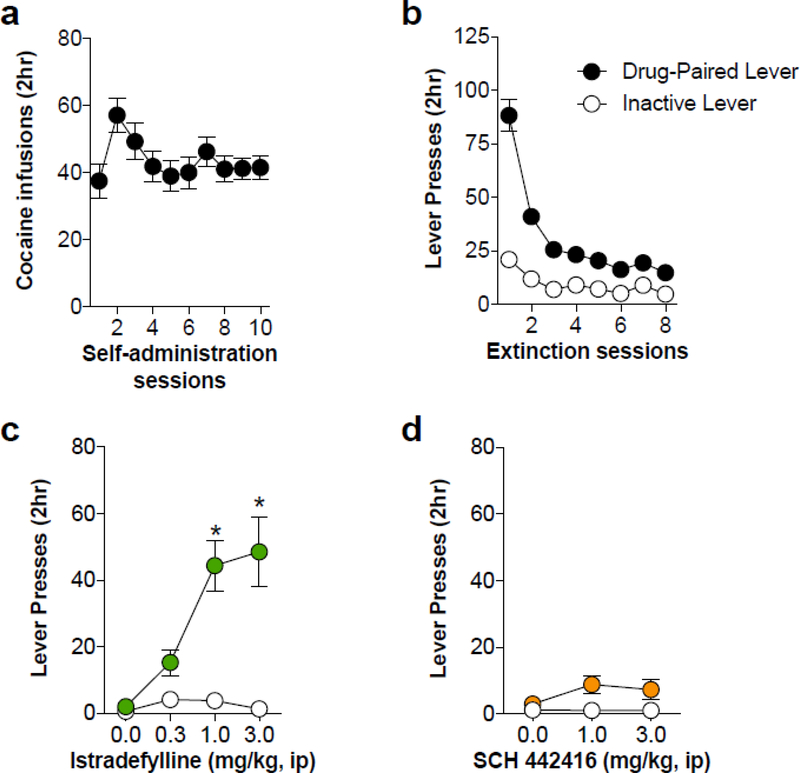
Differential effects of A2AR antagonists on cocaine seeking
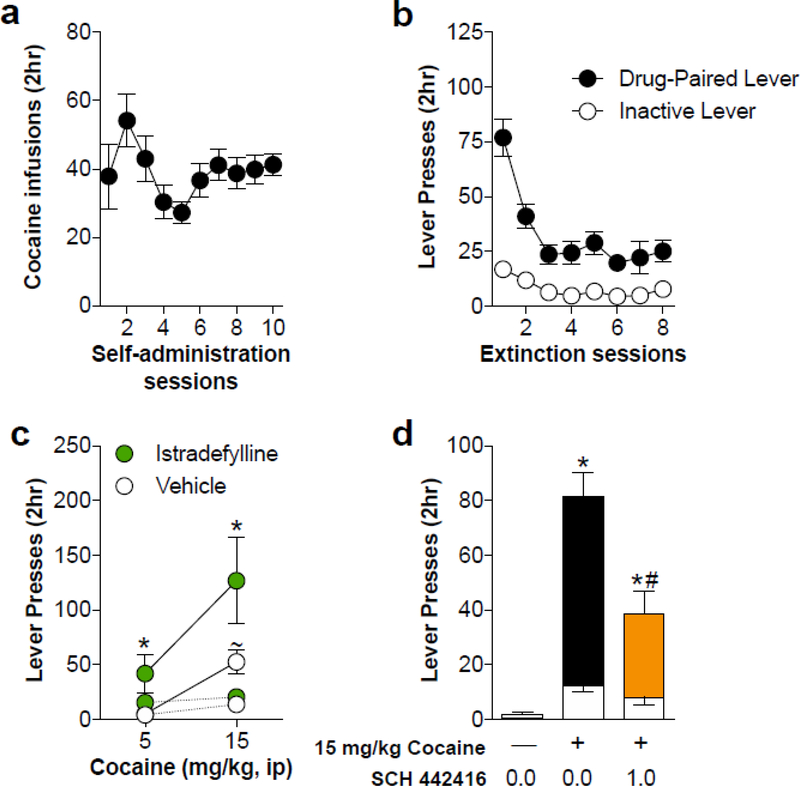
To augment the above results utilizing non-contingent drug delivery, we used a cocaine self-administration paradigm. Animals quickly learned to self-administer cocaine on an FR1 schedule and responded consistently after 10 days of testing (Fig. 4A). Prior to reinstatement testing, lever responding was extinguished over 8 sessions resulting in a progressive decrease in responding at the formerly cocaine-paired lever (Fig. 4B). To evaluate the ability of the A2AR antagonists to reinstate non-reinforced lever responding, a dose-response curve was generated for both istradefylline and SCH 442416. Istradefylline significantly increased responding on the previously drug-paired lever (F3, 162 = 9.685, p < 0.0001) with the higher doses of (1.0 and 3.0 mg/kg, ip) having the most robust effects (Fig 4C). Conversely, SCH 442416 was ineffective at reinstating lever responding at any of the doses tested (Fig. 4D). These results are consistent with our observations in locomotor testing following experimenter-administered A2AR antagonist treatments.
Figure 4.
Postsynaptic A2AR antagonism with istradefylline dose-dependently reinstates cocaine seeking. A) Average number of cocaine infusions during each 2-hr cocaine self-administration session on an FR1 schedule. B) Lever responding was extinguished over 8 consecutive 2-hr sessions during which lever responding had no programmed consequences. C) Administration of istradefylline dose-dependently increased previously drug-paired lever responses with the higher doses (1.0 and 3.0 mg/kg, ip), demonstrating robust cocaine seeking. D) Administration of SCH 442416 had no effect on cocaine seeking at any of the tested doses. * indicates significance from vehicle (p < 0.05)
We next tested the effects of the A2AR antagonists in combination with a cocaine priming injection. A separate cohort of animals was trained to self-administer cocaine (Fig. 5A) and was extinguished (Fig. 5B) prior to reinstatement testing. Administration of istradefylline at a dose that was insufficient to reinstate cocaine seeking alone (0.3 mg/kg, ip) was tested on two doses of cocaine (5 or 15 mg/kg, ip). We found a significant main effect of pretreatment with istradefylline (F1,30 = 6.91, p = 0.013) and a significant main effect of cocaine dose (F1, 30 = 9.79, p = 0.0039) (Fig. 5C). Interactive effects were not significant. Administration of SCH 442416 prior to a cocaine priming injection significantly reduced cocaine-induced reinstatement of cocaine seeking (Fig. 5D; F2,42 = 22.45, p < 0.0001). These findings are congruent with the locomotor studies and demonstrate that istradefylline enhances cocaine seeking, while SCH 442416 reduces cocaine seeking.
Figure 5.
Presynaptic A2AR antagonism with SCH 442416 reduces cocaine-induced seeking, while postsynaptic antagonism with istradefylline enhances cocaine-induced seeking. A) Average number of cocaine infusions during each 2-hr self-administration session on an FR1 schedule. B) Lever responding was extinguished over 8 consecutive 2-hr sessions during which lever responding had no programmed consequences. C) Administration of istradefylline at a dose (0.3 mg/kg, i.p.) ineffective at inducing cocaine seeking on its own enhanced cocaine seeking when administered prior to a low (5 mg/kg, i.p.) and high (10 mg/kg, i.p.) priming dose of cocaine. Solid lines indicate drug-paired lever responding while dashed lines indicate inactive lever responding. D) Cocaine seeking resulting from a cocaine challenge (15 mg/kg, i.p.) was significantly reduced following a pretreatment of SCH 442416. Solid bars indicate drug-paired lever responses and white overlayed bars indicate inactive lever responses. * indicates significance from vehicle pretreatment (p < 0.05), ~ indicates significance from 5 mg/kg cocaine (p < 0.05), # indicates significance between vehicle-cocaine group
Effects of A2AR antagonists on reinstatement of sucrose seeking
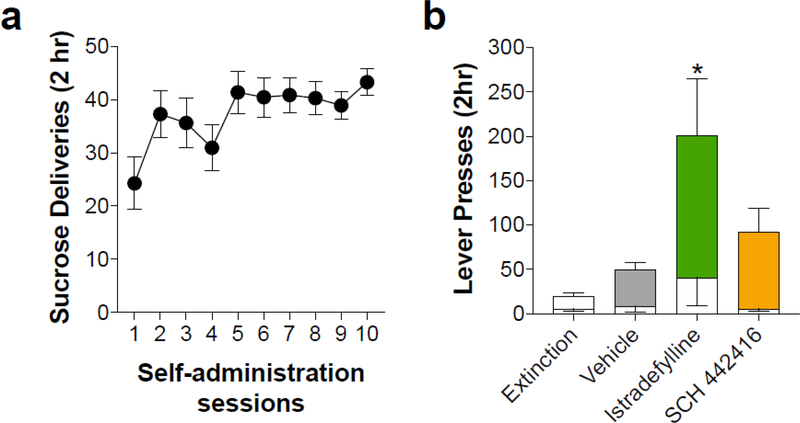
In order to evaluate the potential motor suppressant and/or motivational effects of the adenosine A2AR antagonists, we conducted an experiment using a sucrose seeking paradigm. Thus, we investigated the effects of istradefylline and SCH 442416 on sucrose seeking in rats that were extinguished from sucrose pellet self-administration. Animals learned to self-administer sucrose pellets during ten 120-min sessions (Fig. 6A) and sucrose-paired lever pressing was then extinguished. As shown in Fig. 6B, all rats displayed sucrose seeking compared with the final day of extinction responding (F3,32 = 8.37, p < 0.001). Interestingly, rats treated with istradefylline showed enhanced sucrose seeking compared with vehicle, suggesting that the enhancement in cocaine seeking observed with istradefylline generalizes to seeking of natural rewards. Rats treated with SCH 442416 displayed a trend toward enhanced sucrose seeking, although this did not reach statistical significance. These findings suggest that the suppressive effects of SCH 442416 on cocaine locomotor sensitization and cocaine seeking are not due to a generalized suppression of activity or motivation.
Figure 6.
Postsynaptic A2AR antagonism with istradefylline enhances sucrose seeking. A) Average number of sucrose deliveries during ten daily 2-hr sessions. B) Sucrose seeking on the previously sucrose-paired lever was enhanced compared to extinction responding in all groups. Istradefylline produced a significant enhancement in sucrose seeking compared to vehicle treated animals. Solid bars indicate sucrose-paired lever responses and white overlayed bars indicate inactive lever responses. * indicates significance from vehicle pretreatment (p < 0.05)
Discussion
The goal of the present study was to elucidate the effects of two A2AR antagonists, SCH 442416 and istradefylline, that were previously shown to inhibit pre- and postsynaptic A2AR, respectively, on cocaine-induced behavior (Orru et al. 2011a). We found that SCH 442416 had no effect on locomotion in cocaine naïve animals but significantly decreased cocaine-induced locomotion in cocaine-sensitized animals. Similarly, SCH 442416 did not induce cocaine seeking when administered alone but blunted cocaine seeking when administered prior to a cocaine challenge. Istradefylline, however, modestly increased locomotor activity and induced cocaine seeking when administered alone. Although istradefylline had no effect on locomotion in sensitized rats, istradefylline enhanced cocaine seeking when combined with both a subthreshold and maximal cocaine-priming injection. Finally, we showed that istradefylline also enhanced sucrose seeking, while SCH 442416 had no effect. These results suggest that presynaptic A2AR antagonism may decrease cocaine-induced behavior in animals with a cocaine history, while postsynaptic A2AR antagonism increases behavioral activation and exacerbates cocaine-induced behaviors.
Our results demonstrate distinct qualitative behavioral differences following the administration of two A2AR antagonists. Recent evidence suggests that the A2AR antagonists bind selectively to different subpopulations of A2ARs based on the formation of specific heteromeric receptors (Orru et al. 2011a, b). In vitro binding assays demonstrate that SCH 442416 has lower affinity for A2ARs that are transfected into D2R-expressing cells, while istradefylline has high affinity for A2ARs transfected into D2Rexpressing cells (Orru et al. 2011b). Very different binding characteristics are observed when A2ARs are transfected into A1R-expressing cells, where SCH 442416 demonstrates high-affinity binding, while istradefylline has relatively low-affinity binding. These findings suggest that SCH 442416 binds preferentially to A1R-A2ARs receptor complexes while istradefylline preferentially binds to A2AR-D2R complexes (Orru et al. 2011b). These A2AR heteromeric complexes are important given their synaptic localization where A1R-A2ARs are localized to presynaptic glutamate terminals and A2AR-D2R are localized at postsynaptic sites on indirect pathway MSNs (Ciruela et al. 2006; Ferre et al. 2008; Azdad et al. 2009). While these findings remain speculative, our findings suggest that qualitatively different behavioral effects arise from the administration of these antagonists having differential binding profiles at presynaptic and postsynaptic A2ARs.
There is growing evidence suggesting that the localization of A2AR is an important factor in determining the behavioral effects of A2AR antagonism. A2AR stimulation impairs many behavioral effects of cocaine, including cocaine-induced locomotion, cocaine reward, and cocaine seeking during periods of abstinence, while A2AR blockade generally enhances these effects (Knapp et al. 2001; Poleszak and Malec 2002a, b; Justinova et al. 2003; Filip et al. 2006; Bachtell and Self 2009; O’Neill et al. 2012; Doyle et al. 2012; Hobson et al. 2012; Wydra et al. 2015a, b). Our findings support these observations while also suggesting that the synaptic localization of A2AR may be an important consideration. Similar findings were observed in another study investigating the rewarding properties of the psychoactive cannabinoid compound, tetrahydrocannabidiol (TCH). Here, SCH 442416 reduced the rewarding effects of THC, while istradefylline enhanced the rewarding effects of THC (Justinová et al. 2014). We also find that istradefylline enhances sucrose seeking, while SCH 442416 does not influence sucrose seeking. These findings suggest that blocking postsynaptic A2ARs may increase motivation and/or behavioral activation and that presynaptic A2AR blockade may counteract the effects of drugs of abuse to reduce drug dependence.
The ability of the presynaptic A2AR antagonism to inhibit cocaine-sensitized locomotion and drug seeking may arise from the unique A2AR expression profile in the striatum. A2ARs colocalize with A1Rs on presynaptic glutamate terminals (Hettinger et al. 2001; Rosin et al. 2003; Ciruela et al. 2006; Quiroz et al. 2009; Farrar et al. 2010; Orru et al. 2011a, b). Stimulation of A1Rs and A2ARs has opposing effects on glutamate release, with A1R stimulation inhibiting and A2AR stimulation enhancing glutamate release (Popoli et al. 1995; Karcz-Kubicha et al. 2003; Ciruela et al. 2006; Quiroz et al. 2009; Shen et al. 2013). The inhibitory effects of presynaptic A2AR antagonism on cocaine-induced behavior may be especially relevant when coupled with the underlying cocaine-induced changes in glutamate neurotransmission observed following repeated cocaine exposure. While acute cocaine has little effect on extracellular glutamate levels, cocaine re-exposure produces a large surge in glutamate that is critical for the expression of cocaine sensitization and reinstatement (Pierce et al. 1996; McFarland et al. 2003). Evidence suggests that SCH 442416 blunts striatal glutamate release induced by cortical stimulation in both rats and mice (Orru et al. 2011b; Ferré et al. 2018). Thus, presynaptic A2AR antagonism may also blunt the cocaine-induced glutamate surge in the striatum of animals following exposure to repeated cocaine that is critical for the sensitized locomotor response and cocaine-induced reinstatement (Steketee and Kalivas 2011). Additional support for this notion is derived from our observation that SCH 442416 does not influence acute cocaine-induced locomotion or sucrose seeking, two behaviors not associated with changes in glutamate signaling (Pierce et al. 1996; Bobadilla et al. 2017). The ability of presynaptic A2AR antagonism to inhibit cocaine seeking is also supported by recent work demonstrating that SCH 442416 administered during extinction training produces a lasting reduction in cocaine-induced drug seeking (O’Neill et al. 2014). The lasting effects of SCH 442416 occur in the absence of any overt behavioral suppression during the extinction training when the drug was administered. These effects of presynaptic A2AR antagonism mimic those observed when an A1R agonist is administered during extinction training, suggesting that presynaptic A2AR antagonism with SCH 442416 facilitates A1R activity to reduce glutamate transmission. Future research should be aimed at determining whether SCH 442416 alters enhanced glutamate transmission that is associated with cocaine seeking.
Both human and animal studies indicate that cocaine craving and relapse is associated with increased activity of the prefrontal cortex, amygdala, hippocampus, ventral mesencephalon, and thalamus, all of which send glutamate projections to the striatum (Kalivas and Volkow 2011).
Given the diversity of glutamate projections to the striatum that contribute to cocaine relapse, it is difficult to determine which of these glutamate inputs express A2AR and may be important for the observed findings. Presynaptic A2AR expression has been best characterized in the cortical glutamate terminals where its physical presence has been shown with electron microscopy, and its functional importance has been observed on postsynaptic excitatory synaptic transmission, intracellular signaling, and glutamate release in the striatum (Ciruela et al. 2006; Quiroz et al. 2009; Orru et al. 2011a; Ferré et al. 2018). Though other presynaptic populations of A2AR may exist and play a role in our observations, A2AR expressed on cortical glutamatergic terminals have been well-characterized and likely predominate in these effects. Future research using circuit-specific molecular approaches will aid in uncovering specific pathways that mediate these effects.
Unlike SCH 442416, istradefylline enhanced sucrose and cocaine seeking, and induced behavioral activation in cocaine-naïve animals. These observations are congruent with previous studies using A2AR antagonists having either a preferentially postsynaptic or a mixed pre- and postsynaptic binding profile. For example, both systemic and intra-NAc administration of A2AR antagonists having greater activity at postsynaptic A2AR, such as istradefylline, MSX-3 and SCH 58261, augment cocaine and sucrose seeking (O’Neill et al. 2012; Wydra et al. 2015b). It is presumed that postsynaptic A2AR antagonist effects predominate over presynaptic A2AR mechanisms since D2R stimulation is necessary to reveal A2AR antagonist-induced behavioral activation (Harper et al. 2006; Orru et al. 2011a, b; O’Neill et al. 2012; Wydra et al. 2015b). Blockade of A2ARs also prevents behavioral deficits induced by a D2R antagonist and mimics the cellular signaling effects of a D2R agonist (Hakansson et al. 2006; Farrar et al. 2010; Nunes et al. 2010; Orru et al. 2011a). In fact, postsynaptic A2AR antagonists are a therapeutic approach for the motor disturbances observed in Parkinson’s Disease due to their ability offset D2R deficiencies and induce movement without worsening dyskinesias observed after long-term levodopa treatments (Navarro et al. 2016). Istradefylline, in particular, has been approved in Japan as an adjunct therapy with levodopa (Dungo and Deeks 2013). Together, these findings demonstrate that postsynaptic A2AR antagonism disinhibits indirect pathway MSNs resulting in enhanced behavioral outputs including cocaine-induced locomotion, and cocaine and sucrose seeking.
These studies sought to determine how two A2AR antagonists having differential binding profiles affected cocaine seeking and locomotor activation in rats. We show that antagonism of postsynaptic A2ARs induced locomotor activation, cocaine seeking, and sucrose seeking. Blockade of presynaptic A2ARs reversed the expression of cocaine sensitization and inhibited cocaine seeking. These findings suggest that SCH 442416 may prohibit some aspects of neurotransmission that contribute to cocaine-induced behaviors in animals with a history of cocaine administration. Given that A2ARs are most densely expressed in the striatal regions (Svenningsson et al. 1997, 1999), we speculate that the A2AR antagonists are having these differential effects based on their unique expression profiles on presynaptic glutamate terminals and postsynaptic MSNs. These studies represent an initial step in identifying a novel treatment strategies for psychostimulant use disorders. It will be important for future work to more fully characterize the functional activity of the presynaptic A2AR antagonists within specific neurocircuits.
Acknowledgments
Funding:
This work was funded by National Institutes of Health (Grant DA033358).
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Azdad K, Gall D, Woods AS, et al. (2009) Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology 34:972–86. doi: 10.1038/npp.2008.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Self DW (2009) Effects of adenosine A(2A) receptor stimulation on cocaineseeking behavior in rats. Psychopharmacol Berl 206:469–78. doi: 10.1007/s00213-0091624-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrie AP, Nicholls DG (1993) Adenosine A1 receptor inhibition of glutamate exocytosis and protein kinase C-mediated decoupling. J Neurochem 60:1081–1086 [DOI] [PubMed] [Google Scholar]
- Bobadilla A-C, Garcia-Keller C, Heinsbroek JA, et al. (2017) Accumbens Mechanisms for Cued Sucrose Seeking. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 42:2377–2386. doi: 10.1038/npp.2017.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, et al. (2001) The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proc Natl Acad Sci U A 98:1970–5. doi: 10.1073/pnas.98.4.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, et al. (2006) Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci 26:2080–7. doi: 10.1523/JNEUROSCI.3574-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Breslin FJ, Rieger JM, et al. (2012) Time and sex-dependent effects of an adenosine A2A/A1 receptor antagonist on motivation to self-administer cocaine in rats. Pharmacol Biochem Behav 102:257–63. doi: 10.1016/j.pbb.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungo R, Deeks ED (2013) Istradefylline: first global approval. Drugs 73:875–882. doi: 10.1007/s40265-013-0066-7 [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA (2001) The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24:31–55. doi: 10.1146/annurev.neuro.24.1.31 [DOI] [PubMed] [Google Scholar]
- Farrar AM, Segovia KN, Randall PA, et al. (2010) Nucleus accumbens and effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience 166:1056–67. doi: 10.1016/j.neuroscience.2009.12.056 [DOI] [PubMed] [Google Scholar]
- Ferré S (2016) Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology (Berl). doi: 10.1007/s00213-016-4212-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Agnati LF, Ciruela F, et al. (2007) Neurotransmitter receptor heteromers and their integrative role in “local modules”: the striatal spine module. Brain Res Rev 55:55–67. doi: 10.1016/j.brainresrev.2007.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Bonaventura J, Zhu W, et al. (2018) Essential Control of the Function of the Striatopallidal Neuron by Pre-coupled Complexes of Adenosine A2A-Dopamine D2 Receptor Heterotetramers and Adenylyl Cyclase. Front Pharmacol 9:243. doi: 10.3389/fphar.2018.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Quiroz C, Woods AS, et al. (2008) An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des 14:1468–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, et al. (2006) Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res 1077:67–80 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466. doi: 10.1146/annurev-neuro-061010-113641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson K, Galdi S, Hendrick J, et al. (2006) Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem 96:482–8. doi: 10.1111/j.14714159.2005.03558.x [DOI] [PubMed] [Google Scholar]
- Harper LK, Beckett SR, Marsden CA, et al. (2006) Effects of the A 2A adenosine receptor antagonist KW6002 in the nucleus accumbens in vitro and in vivo. Pharmacol Biochem Behav 83:114–21. doi: 10.1016/j.pbb.2005.12.014 [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL (2001) Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol 431:331–46 [DOI] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL (2010) Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13:958–966. doi: 10.1038/nn.2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T, Jobling AI, Greferath U, et al. (2015) Vesicular expression and release of ATP from dopaminergic neurons of the mouse retina and midbrain. Front Cell Neurosci 9:389. doi: 10.3389/fncel.2015.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson BD, Merritt KE, Bachtell RK (2012) Stimulation of adenosine receptors in the nucleus accumbens reverses the expression of cocaine sensitization and cross-sensitization to dopamine D2 receptors in rats. Neuropharmacology 63:1172–81. doi: 10.1016/j.neuropharm.2012.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Segal PN, et al. (2003) Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther 307:977–86. doi: 10.1124/jpet.103.056762 [DOI] [PubMed] [Google Scholar]
- Justinová Z, Redhi GH, Goldberg SR, Ferré S (2014) Differential effects of presynaptic versus postsynaptic adenosine A2A receptor blockade on Δ9-tetrahydrocannabinol (THC) selfadministration in squirrel monkeys. J Neurosci Off J Soc Neurosci 34:6480–6484. doi: 10.1523/JNEUROSCI.5073-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–72. doi: 10.1038/nrn2515 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–986. doi: 10.1038/mp.2011.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, et al. (2003) Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology 28:1281–91. doi: 10.1038/sj.npp.1300167 [DOI] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Cottam N, et al. (2001) Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol Biochem Behav 68:797–803 [DOI] [PubMed] [Google Scholar]
- Marchi M, Raiteri L, Risso F, et al. (2002) Effects of adenosine A1 and A2A receptor activation on the evoked release of glutamate from rat cerebrocortical synaptosomes. Br J Pharmacol 136:434–440. doi: 10.1038/sj.bjp.0704712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto JPP, Almeida MG, Castilho-Martins EA, et al. (2014) Protein kinase A mediates adenosine A2a receptor modulation of neurotransmitter release via synapsin I phosphorylation in cultured cells from medulla oblongata. Neurosci Res 85:1–11. doi: 10.1016/j.neures.2014.05.007 [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Borroto-Escuela DO, Fuxe K, Franco R (2016) Purinergic signaling in Parkinson’s disease. Relevance for treatment. Neuropharmacology 104:161–168. doi: 10.1016/j.neuropharm.2015.07.024 [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Santerre JL, et al. (2010) Differential effects of selective adenosine antagonists on the effort-related impairments induced by dopamine D1 and D2 antagonism. Neuroscience 170:268–80. doi: 10.1016/j.neuroscience.2010.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill CE, Hobson BD, Levis SC, Bachtell RK (2014) Persistent reduction of cocaine seeking by pharmacological manipulation of adenosine A1 and A2A receptors during extinction training in rats. Psychopharmacology (Berl) 231:3179–3188. doi: 10.1007/s00213-0143489-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill CE, LeTendre ML, Bachtell RK (2012) Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology 37:1245–56. doi: 10.1038/npp.2011.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M, Bakesova J, Brugarolas M, et al. (2011a) Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS ONE 6:e16088. doi: 10.1371/journal.pone.0016088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M, Quiroz C, Guitart X, Ferre S (2011b) Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum. Neuropharmacology 61:967–74. doi: 10.1016/j.neuropharm.2011.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW (1996) Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci 16:1550–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchen W, Sieler D, Wirkner K, Illes P (2001) Co-transmitter function of ATP in central catecholaminergic neurons of the rat. Neuroscience 102:593–602 [DOI] [PubMed] [Google Scholar]
- Poleszak E, Malec D (2002a) Adenosine receptor ligands and cocaine in conditioned place preference (CPP) test in rats. Pol J Pharmacol 54:119–26 [PubMed] [Google Scholar]
- Poleszak E, Malec D (2002b) Cocaine-induced hyperactivity is more influenced by adenosine receptor agonists than amphetamine-induced hyperactivity. Pol J Pharmacol 54:359–66 [PubMed] [Google Scholar]
- Popoli P, Betto P, Reggio R, Ricciarello G (1995) Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur J Pharmacol 287:215–7. doi: 00142999(95)00679-6 [pii] [DOI] [PubMed] [Google Scholar]
- Quiroz C, Lujan R, Uchigashima M, et al. (2009) Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. ScientificWorldJournal 9:1321–44. doi: 10.1100/tsw.2009.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, et al. (2005) Co-localization and functional interaction between adenosine A(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem 92:433–41. doi: 10.1111/j.14714159.2004.02887.x [DOI] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J (2003) Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology 61:S12–8 [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, et al. (2007) Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol 83:277–92. doi: 10.1016/j.pneurobio.2007.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC (2010) Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Acad Sci 1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, et al. (2004) Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem 11:648–57. doi: 10.1101/lm.81404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Canas PM, Garcia-Sanz P, et al. (2013) Adenosine A(2)A receptors in striatal glutamatergic terminals and GABAergic neurons oppositely modulate psychostimulant action and DARPP-32 phosphorylation. PLoS One 8:e80902. doi: 10.1371/journal.pone.0080902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW (2011) Drug wanting: behavioral sensitization and relapse to drugseeking behavior. Pharmacol Rev 63:348–365. doi: 10.1124/pr.109.001933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB (1999) Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol 59:355–96. doi: S03010082(99)00011-8 [pii] [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Kull B, et al. (1997) Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience 80:1171–85 [DOI] [PubMed] [Google Scholar]
- Wydra K, Golembiowska K, Suder A, et al. (2015a) On the role of adenosine (A)(2)A receptors in cocaine-induced reward: a pharmacological and neurochemical analysis in rats. Psychopharmacol Berl 232:421–35. doi: 10.1007/s00213-014-3675-2 [DOI] [PubMed] [Google Scholar]
- Wydra K, Suder A, Borroto-Escuela DO, et al. (2015b) On the role of A(2)A and D(2) receptors in control of cocaine and food-seeking behaviors in rats. Psychopharmacol Berl 232:1767–78. doi: 10.1007/s00213-014-3818-5 [DOI] [PMC free article] [PubMed] [Google Scholar]