Abstract
Adherence to pain self-management strategies is associated with favorable psychobehavioral outcomes among individuals with chronic pain. Substantive adherence to treatments teaching these adaptive skills proves challenging, resulting in poor individual and societal outcomes. Evidence demonstrates motivation for behavior change as a key predictor of treatment adherence. Despite behavioral techniques that target motivation, however, nonadherence persists as a barrier to positive clinical outcomes in chronic pain. Understanding the neurobiological mechanisms underlying treatment motivation might highlight novel avenues for augmentative therapies. The purpose of this review is to present theory and evidence that the mesocorticolimbic system (i.e., brain circuitry associated with reward processing and motivation) contributes to treatment motivation among chronic pain patients, ultimately influencing adherence. We review evidence for motivation as a key adherence determinant, detail neuroimaging findings relating mesocorticolimbic circuitry and motivation, and discuss data supporting mesocorticolimbic dysfunction among chronic pain patients. We propose a neurobehavioral model for adherence to pain self-management interventions, listing testable hypotheses. Finally, we discuss potential research and intervention implications from the proposed model.
Keywords: mesocorticolimbic system, chronic pain, Motivational Model for Pain Self-Management, treatment adherence, behavioral pain management
Acute pain is an important signal of potential bodily harm that triggers protective cognitive, emotional, and behavioral reactions1. Over time, an individual learns to implement responses that minimize the deleterious effects of acute pain, such as avoiding touching a hot stove. In the context of pain that is chronic and does not act as a warning signal, however, these learned responses can actually become maladaptive habits that inadvertently exacerbate symptoms2. For example, avoiding exercise due to fear of aggravated pain can contribute to muscle weakness and increased pain intensity3. For this reason, chronic pain management has increasingly centered on interventions that replace maladaptive learned responses with adaptive strategies.
Self-management interventions are nonpharmacological approaches to pain management that teach adaptive strategies through a combination of medical education, behavioral adaptations, interpersonal problem-solving, and/or emotion management4. Pain self-management involves a patient’s active and daily implementation of adaptive health practices that help control distressing symptoms5. Understandably, adopting and consistently practicing positive health behaviors to replace ingrained pain responses can be incredibly challenging6. Estimates suggest that over half of patients engaged in self-management interventions are considered nonadherent7. Given that the Center for Disease Control now recommends nonpharmacologic therapies as one of the first lines of chronic pain treatment8,9, establishing factors that influence adherence to pain self-management practices is imperative to improve the efficaciousness of such therapies.
The central premise of this review is that dysfunction of the mesocorticolimbic system (i.e., brain circuitry associated with hedonic appraisal, reinforcement, and motivated behavior) is associated with attenuated motivation in chronic pain patients, potentially resulting in difficulty adhering to symptom self-management strategies. To support this thesis, we discuss evidence showing that 1) motivation is a strong predictor of adherence to self-management strategies10–14, 2) motivation is subserved by the mesocorticolimbic system15–19, and 3) mesocorticolimbic dysfunction is present in some individuals with chronic pain20–25.
Although research in this area is somewhat nascent, compelling evidence for mesocorticolimbic dysfunction in chronic pain patients suggests that these individuals might have poor response to pain self-management interventions (via low motivation and adherence), as well as current strategies to improve motivation for treatment (via neural deficiencies limiting motivated behaviors; e.g., motivational interviewing). We appreciate that treatment motivation and adherence are complex phenomena and encourage future research to explore additional biopsychosocial factors that might further predict adherence rates (e.g., social support, socioeconomic limitations); however, the scope of this review will be limited to discussion of the above-described factors.
Using the theoretical framework and evidence from the Motivational Model for Pain Self-Management26 (MMPSM), we posit that mesocorticolimbic circuitry presents a target neurobiological mechanism for treatment motivation, ultimately impacting adherence to pain self-management strategies. There are numerous frameworks of health behavior change27–30, with a common thread that patients’ attitudes, beliefs, motivational state, and demographic characteristics shape adherence to self-management strategies. Despite our understanding of the psychosocial components of short- and long-term engagement in these skills, the problem of nonadherence widely persists. We propose that a neurobiologically-informed framework more robustly captures the problem of nonadherence by highlighting mechanisms underlying certain psychological phenomena discussed in these models31.
Importantly, the impetus for the present review is not to propose a biomarker of treatment motivation in individuals with chronic pain. Previous studies have provided neuroimaging evidence of questionnaires/tasks related to mesocorticolimbic function32–36, which can help clinicians feasibly probe dysfunction of this system (i.e., patients potentially at-risk for poor treatment motivation/engagement). Because the US National Pain Strategy calls for improvements in pain self-management programs37, the rationale for this review is to encourage research efforts establishing a mechanistic link between mesocorticolimbic dysfunction and motivation for engagement in and adherence to pain self-management strategies. This evidence will support the development of novel, augmentative therapies that target deficient neural systems underlying motivation in order to promote greater benefit from pain self-management strategies (discussed at the end of this review).
Adherence to Pain Self-Management Strategies and Clinical Outcomes
Self-management interventions vary in the use of specific strategies but share common features. The Stanford model of self-management emphasizes positive patient-provider rapport in order to 1) promote the patient’s role in problem-solving barriers to skills practice, 2) empower him/her to decide when strategies should be implemented, 3) teach how resources can be effectively used, and 4) make an action plan for accomplishing intervention goals4. Similarly, cognitive-behavioral therapies are self-management interventions that teach cognitive and behavioral strategies to reduce emotional distress associated with pain and improve quality of life38,39. A common theme among these interventions is the goal to teach transferable skills that improve the patient’s self-efficacy in managing symptoms long-term40. Further, they fundamentally assume that patients will adhere to strategies discussed during intervention sessions in order to develop long-term lifestyle changes.
The term “adherence” implies that the patient is an active, volitional participant in his/her healthcare decision-making and takes the initiative to implement positive health behaviors. This term differs from “compliance,” which implies a passive involvement in care characterized by following providers’ orders41. Not surprisingly, adherence to prescribed treatment regimens requires motivation and effort, especially when the treatment involves alteration of lifelong habits.
Adherence to pain self-management interventions is less frequently studied than medication compliance. However, estimates suggest that adherence rates for nonpharmacological modalities are generally lower than those for medications42. For example, in a large study of over 500 chronic pain patients completing a 3-week pain self-management intervention, only 30% of patients endorsed regularly using all instructed strategies43. Premature drop-out rates for cognitive-behavioral pain interventions range from approximately 27%44 to 60%7,45, and failure of long-term maintenance is estimated upwards of 39%46. Finally, findings from home physical exercise programs similarly demonstrate moderate adherence, with poor long-term maintenance of home exercise practice47,48.
Nonadherence consistently serves as a barrier to positive pain treatment outcomes49–51. Low engagement specifically leads to poorer health outcomes52, adds wasteful healthcare costs53, and negatively impacts patient/provider rapport54. Alternatively, strong adherence predicts favorable clinical outcomes, such as reduced emotional distress and improved quality of life12,43,54–59. For these reasons, the World Health Organization cites adherence as a critical, modifiable component of health system effectiveness, with poor individual health outcomes and high societal healthcare costs as the main consequences of poor long-term adherence60.
Nonadherence can occur for multiple reasons specific to the patient or the environment (e.g., provider traits, sociodemographic barriers). Examples of patient factors include low expectations for treatment efficacy, perceived barriers to treatment, personality factors, and lack of support27,28,54,61,62 (for an in-depth review of such components, please refer to Mathes et al.63). These variables operate in tandem to yield an individual’s readiness for change, or motivation for treatment. In this regard, motivation refers to the likelihood that a person will initiate therapy, actively participate in treatment, and maintain changes implemented over the course of therapy64. Motivation is one of the most important predictors of adherence to pain management skills12,65,66, as well as adherence to other positive health behaviors10,11,13,67. It is also predictive of favorable clinical outcomes following treatment14,68.
The Motivational Model for Pain Self-Management
The MMPSM proposes that adherence to pain self-management strategies is a result of an individual’s motivation to engage in treatment26. Examples of self-management strategies described in the MMPSM include exercise, activity pacing, relaxation exercises, avoiding catastrophizing, and assertiveness in interpersonal relationships. In this framework, motivation is malleable and influenced by a variety of factors that play into 1) perceived importance of engaging in or refraining from treatment and 2) the individual’s beliefs that adherence to behavior change is possible. First, perceived importance results from patients’ mental cost/benefit analysis of engaging in pain self-management strategies, learning history with previous behavior change attempts, and appreciation of contingencies surrounding strategy engagement. Second, perceived self-efficacy can be influenced by experience in successfully applying self-management skills, modeling of others who sufficiently implement similar strategies, verbal persuasion in the form of self-talk and encouragement from others, and perceived barriers to engaging in self-management strategies.
Evidence demonstrates the applicability of the Motivational Model for Pain Self-Management in describing motivation and adherence to behavioral pain treatments in individuals with chronic pain from multiple sclerosis69 and spinal cord injury70. Other research groups have also found associations among perceived importance of treatment, perceived self-efficacy in engaging in strategies, treatment motivation, and adherence to pain self-management strategies61,65,71. Importantly, a community-based pilot study demonstrated that addressing individuals’ beliefs about the importance of self-management strategies, expectations for engagement in these strategies, and self-efficacy for applying skills prior to intervention resulted in significantly higher rates of adherence to treatment72. These findings collectively highlight the value of the MMPSM in explaining adherence to pain self-management strategies and suggest that some factors, such as self-efficacy, both promote adherence and contribute to pain-related treatment outcomes.
Mesocorticolimbic Function and Motivated Behaviors
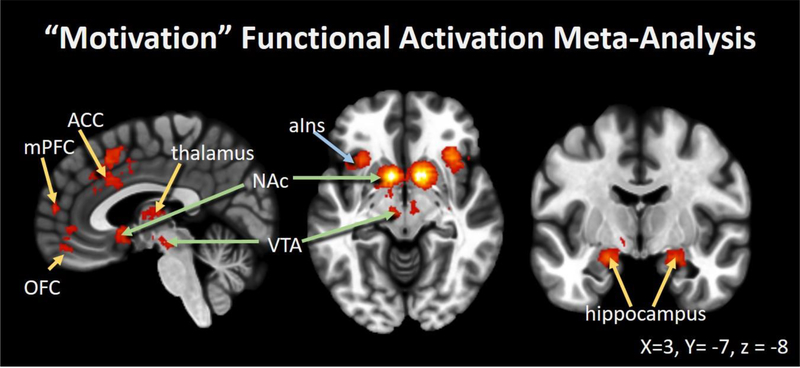
Neuroimaging studies describe the mesolimbic system and its cortical projections (i.e., mesocorticolimbic system) as circuitry that is activated during motivation to approach and avoid appetitive and aversive stimuli, respectively16,73. This system includes neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens (NAc), thalamus, hippocampus, and amygdala. Further, there are dense afferent projections to corticolimbic regions associated with emotion and memory, such as the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and ventromedial prefrontal cortex (vmPFC)74, as well as indirect connections to paralimbic structures (e.g., anterior insula)75,76. Figure 1 depicts consistently activated regions identified through a Neurosynth77 meta-analysis of 135 functional neuroimaging studies that highly load on the key term “motivation.” Most prominently highlighted are mesolimbic regions, including bilateral NAc and VTA, as well as corticolimbic OFC, ACC, and mPFC. Dopaminergic receptors densely populate the NAc, and dopamine is released during the regulation of motivated behavior, attention, and hedonic processes15,76,78.
Figure 1.
Motivation Circuitry: A forward-inference functional activation statistical map of regions consistently activated across 135 neuroimaging studies that load highly on the key term “motivation” (Neurosynth: http://neurosynth.org/). Results indicate that activated areas include mesolimbic (green arrows), corticolimbic (yellow arrows), and paralimbic (blue arrow) structures. This meta-analysis emphasizes mesocorticolimbic circuitry’s involvement in motivated behaviors across studies. Abbreviations: medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), ventral tegmental area (VTA), anterior insula (aIns), and nucleus accumbens (NAc)
Various neuroimaging techniques can be used to quantify mesocorticolimbic function in humans. Functional connectivity (FC), or correlated activity among spatially remote regions79, is increasingly recognized as an informative technique for assessing network-level dysfunction across clinical populations80,81. In this regard, “dysfunction” can refer to hyperconnectivity within a given neural network or regions outside of the network compared to healthy individuals. Hyperconnectivity is thought to represent an increase in communication among brain regions resultant from nonlinear interactions between situational demands, neurological challenge, and resource availability82. Alternatively, hypoconnectivity compared to healthy individuals is thought to represent loss of communication among regions within a neural network83.
In healthy individuals, unique FC patterns among mesocorticolimbic regions have been reported in the context of appetitive and aversive stimuli processing84–86. Initiation of motivated behaviors is associated with connectivity among VTA, NAc, and the dorsolateral PFC87. Stimulus-dependent FC between hippocampus and OFC is related to motivated behaviors for appetitive vs neutral cues88. Further, decision-making based on behaviors requiring greater effort are associated with intranetwork FC among NAc, VTA, ventral pallidum, ACC, and amygdala19. These results support the use of FC in understanding the role of mesocorticolimbic circuitry in motivated behaviors.
Hypoconnectivity among mesocorticolimbic regions has been demonstrated in individuals with low motivation resultant from unipolar and bipolar depression89–91. Additionally, preliminary evidence from the substance abuse literature demonstrates an association between treatment motivation/adherence and mesocorticolimbic activity92 or gray matter volume93,94. Reductions in gray matter volume reported in these studies might result from atrophy via excitotoxicity and inflammation95–97, which could alter functional communication among regions given diminished neural resources. Collectively, these findings suggest that structure and function of mesocorticolimbic regions are associated with motivated behavior, and individuals with mesocorticolimbic dysfunction might have attenuated motivation for treatment adherence.
Mesocorticolimbic Dysfunction in Chronic Pain
The findings reviewed thus far support a mechanistic link between motivation and mesocorticolimbic circuitry. Accordingly, mesocorticolimbic dysfunction is associated with impairments in motivated behaviors. Emerging evidence highlights alterations in mesocorticolimbic function and associated reward processing in individuals with chronic pain. Although specific patterns of mesocorticolimbic response to acute pain and dysfunction in chronic pain are still being established98–101, combined results highlight the vulnerability of this patient population to poor motivation for symptom self-management.
Previous studies demonstrated a higher likelihood of transitioning from subacute to chronic low back pain in individuals with hyperconnectivity between NAc-mPFC102,103 and hippocampus-mPFC104. Once in a chronic pain state, patients show slower rates of extinction for reinforced pain behaviors105, blunted operant learning slope for pain habituation (Becker, Kleinböhl, Baus, & Hölzl, 2011), and poorer reward responsiveness106. It is possible that chronically-taxed mesolimbic structures work less efficiently over time, contributing to poorer motivation and concomitant treatment adherence. These findings are supported by studies showing that baseline dopamine metabolism is reduced in patients with fibromyalgia107–109, burning mouth syndrome110, and chronic back pain111 compared to pain-free controls. Individuals with chronic pain also tend to have clinical comorbidities that are commonly associated with abnormal reward processing, such as depression112,113, substance abuse114–116, obesity117, and sleep disturbance118,119.
Because pain is an aversive stimulus, individuals are motivated to seek removal of this stimulus. In fact, analgesia is described as a type of negative reinforcement. If pain relief is not substantively rewarding, however, motivation to engage in pain self-management strategies might be attenuated in individuals with chronic pain. Supporting this notion are studies demonstrating increased NAc, ACC, and vmPFC activity at the offset of a noxious thermal stimulus in healthy individuals99,120–122. Humans and animals with chronic pain, however, show a reduction in NAc activity during pain offset123,124, suggesting attenuated reward from relief. As a result of diminished negative reinforcement from relief, it is possible that individuals with chronic pain experience reduced motivation to engage in pain relief behaviors, negatively influencing adherence.
Testable Hypotheses for the Neurobiologically-Informed Motivational Model for Pain Self-Management
Stemming from the reviewed extant literature, we propose testable hypotheses for an updated version of the MMPSM that incorporates putative neurobiological mechanisms contributing to motivation for pain self-management (Figure 2). Given that the problem of nonadherence continues to exist among patients with chronic pain, the goal of our updated model is to determine whether potential neurobiological deficiencies contributing to poor motivation feed into observed nonadherence. If supported, this line of research will ideally inform development of novel, adjunctive therapies correcting mesocorticolimbic deficiencies, with the hope that administration during critical treatment timepoints will promote long-term practice of effective pain self-management strategies.
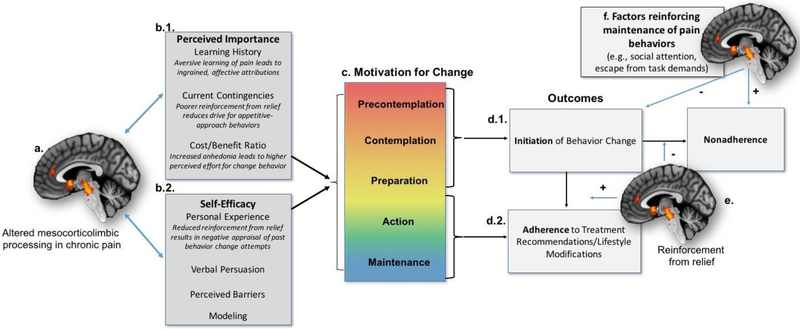
Figure 2.
A Neurobehavioral Model of Pain, Mesocorticolimbic Circuitry, and Treatment Adherence: The present model demonstrates how neurobiological factors might contribute to the Motivational Model for Pain Self-Management. First, previous evidence has demonstrated altered functioning among brain circuitry related to hedonic processing and motivation (i.e., mesocorticolimbic system), reflecting changes in reward and motivational processes in a chronic pain state (a.). As a result, pain self-management strategies might seem less important (b.1.) or the individual might have poorer self-efficacy to implement such strategies (b.2.). Motivation for behavior change/treatment adherence occurs across a gradient based on these factors (c.), and movement through these stages ultimately leads to initiation of self-management strategies (d.1.). If enough reinforcement from pain relief is processed via mesocorticolimbic circuitry (e.), initiation and subsequent adherence are increased. However, if reinforcement from relief is not sufficient, or if competing reinforcers maintain pain behaviors (f), then nonadherence is the primary outcome.
The MMPSM broadly states that in order for individuals with chronic pain to adhere to pain self-management techniques, adequate motivation for change is necessary26. Motivation is influenced by the perceived importance of engaging in a target behavior, as well as self-efficacy for the ability to effectively engage in the therapy. The literature reviewed above strongly supports this model, and demonstrates that the mesocorticolimbic system tracks effectual and aberrant motivational states15–17, including chronic pain101,107,111,123,125,126.
Mesocorticolimbic function contributes to two important constructs of motivation for treatment adherence: perceived importance of self-management strategies and perceived self-efficacy (Figure 2.a.). Previous findings also show aberrant learning history with pain and processing of contingencies (i.e., reinforcers and punishers) in chronic pain patients105,127, contributing to low perceived importance of engaging in pain self-management strategies (Figure 2.b.1). Second, individuals’ personality characteristics or mindset significantly influences their perceived self-efficacy for self-management of pain71,128–132 (Figure 2.b.2.). We hypothesize that:
H1: Mesocorticolimbic function subserves treatment-related learning history, contingency processing, and cost/benefit analysis. Individuals with mesocorticolimbic dysfunction will have lower perceived importance of symptom self-management.
H2: Mesocorticolimbic function subserves appraisal of behavior change adeptness. Individuals with mesocorticolimbic dysfunction will have poorer self-efficacy for symptom self-management.
As a result of altered perceptions for treatment importance and self-efficacy, motivation for behavior change is affected (Figure 2.c.). The MMPSM describes motivation as graded along five stages originally described by Prochaska and DiClemente133: 1) “precontemplation” occurs when individuals have scant interest in changing behavior, 2) “contemplation” occurs when individuals have considered behavior change for the distant future, 3) “preparation” occurs when individuals actively consider attempting behavior change, 4) “action” occurs when individuals engage in behavior change, and finally, 5) “maintenance” occurs when individuals sustain already changed behaviors over a long period. We hypothesize that:
H3: Magnitude of mesocorticolimbic dysfunction will correlate with reported treatment motivation, so that greater dysfunction is associated with poorer readiness for change.
H4: Self-reported treatment motivation moderates the relationship between pre-treatment mesocorticolimbic function and adherence. Individuals with higher treatment motivation demonstrate a stronger relationship between mesocorticolimbic function and adherence.
As the individual moves through these stages of motivation, behavioral and mesocorticolimbic processes can influence the slope and plateau of changes in motivation. If the patient enters the preparation and action phase, then initiation in treatment is achieved (Figure 2.d.1.). If the individual’s treatment-induced analgesia is adequately reinforcing, then motivation for adherence increases, leading to maintenance over time (Figure 2.d.2.). In individuals with chronic pain, attenuated reinforcement from analgesia, which is associated with altered mesocorticolimbic functioning, might result in poorer self-efficacy and perceived importance of the therapy, increasing the likelihood of treatment nonadherence (Figure 2.e.).
H5: Practice of a pain management strategy will be associated with mesocorticolimbic activity via reinforcement. Individuals with high reinforcement from this practice (optimal mesocorticolimbic function) will have greater motivation for future strategy practice, leading to better adherence. Individuals with poor reinforcement from strategy practice (attenuated mesocorticolimbic function) will have lower motivation for future strategy practice, leading to poorer adherence.
An alternative pathway to treatment nonadherence may also emerge if competing reinforcers (e.g., social attention or escape from task demands)134 are in place and elicit greater unconscious reinforcement than treatment-related analgesia (Figure 2.f.). We hypothesize that:
H6: Factors that maintain pain behaviors (e.g., social attention or escape from task demands) will moderate the relationship between therapy-related reinforcement and adherence, so that individuals with high reinforcement from pain-maintaining factors will have a weaker relationship between therapy-related reinforcement and adherence.
Implications for Future Research and Intervention
The goal of psychological pain interventions is to teach patients symptom self-management strategies that they will practice over the course of treatment and continue to regularly implement as adopted lifestyle habits. However, nonadherence to these skills during the initiation/course of intervention and following treatment is prevalent. Current interventions to improve adherence fall short. A meta-analysis of adherence interventions demonstrated that only 18 of the 42 trials examined yielded significantly improved adherence across patients135. Additional predictors and potentially modifiable factors should be explored to set patients up for optimal benefit from psychological pain interventions.
If supported by substantial evidence, this neurobiologically-informed MMPSM presents translational avenues to refine existing or develop novel adherence interventions. Specifically, we envision these adjunctive interventions to be very brief and targeted to patients at-risk for poor adherence. Further, the adjunctive intervention would only be administered at key points during treatment. At-risk patients can be identified through well-validated questionnaires probing mesocorticolimbic function. Rather than spend several pain intervention sessions troubleshooting adherence from a solely behavioral standpoint, individuals at-risk for poor mesocorticolimbic function would be referred for adjunctive interventions at the initiation of treatment to promote optimal reward processing during engagement of intervention skills. Improved mesocorticolimbic function could potentially then promote an individual’s perceived importance of treatment and self-efficacy of applying skills in a feedforward loop.
If effective, the initial costs to apply these adjunctive therapies would likely outweigh the long-term costs and patient/provider frustration associated with nonadherence. Although research is still needed to address this topic, potential avenues for such therapies include neurofeedback, pharmacological interventions, behavioral re-training, and neurostimulation [e.g., repetitive transcranial magnetic stimulation (rTMS), transcranial direct-current stimulation (tDCS), and deep brain stimulation (DBS)]. Studies have shown modest efficacy of such methods in improving motivation via mesocorticolimbic targeting in healthy participants136–138, as well as individuals with intractable depression and anxiety spectrum disorders139–147.
In considering this model, certain limitations should also be weighed. To the best of our knowledge, no study has experimentally tested the specificity of the mesocorticolimbic system in treatment motivation and adherence in individuals with chronic pain. However, the testable hypotheses listed above provide avenues for research on this topic. Second, there are limitations in the measurement of motivation and adherence, such as bias in questionnaire wording and inconsistency in adherence endpoints51,148. We encourage future research to better characterize these aspects of treatment motivation and adherence.
Third, fMRI reliability has been documented as fair to good in the general neuroimaging literature149–151 and pain neuroimaging152–155. Future efforts should examine the reliability of neuroimaging findings over time in understanding this potential mechanistic link. Fourth, our model does not comprehensively include non-patient factors that can influence treatment motivation and adherence, such as provider characteristics or sociodemographic barriers. If the testable hypotheses in our model are supported, we encourage future work to incorporate this information into a larger framework that describes the influence of all these variables to explain as much variance as possible in treatment adherence. Finally, there is no clear evidence to dictate which set of self-management strategies most effectively improves symptoms. Future adaptive trials might help provide a clearer set of guidelines for which self-management strategies might be most effective with optimal adherence for a given population.
Conclusions
Motivation and associated adherence are critical for successful symptom management in individuals with chronic pain. Given that mesocorticolimbic circuitry is linked with motivation and demonstrates aberrant function among chronic pain patients, perceived importance of treatment and self-efficacy might be skewed in these individuals. Mesocorticolimbic dysfunction might lead to poorer reinforcement from analgesia, further decreasing patients’ treatment motivation in a feedforward loop. As such, mesocorticolimbic circuitry presents a possible target to improve patient clinical outcomes via motivation and adherence to symptom self-management strategies.
Highlights.
Adherence to chronic pain interventions requiring lifestyle changes is challenging
Mesocorticolimbic function is related to motivation, a key predictor of adherence
Chronic pain patients demonstrate altered mesocorticolimbic function and anhedonia
This system’s dysfunction might result in difficulty adhering to pain interventions
We offer a neurobehavioral model of pain, mesocorticolimbic function, and adherence
Acknowledgments
Funding:
This work was supported by the following grants from the National Institutes of Health: NHLBI F32HL143941 (JEL), NCCIH R01AT007176 (DAS), NIMHD R01MD009063 (CMC), and NIDA K23DA035915 (PHF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest:
none
References
- 1.Cordier L & Diers M Learning and Unlearning of Pain. Biomedicines 6, 67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flor H New developments in the understanding and management of persistent pain. Curr. Opin. Psychiatry 25, 109–113 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Larsson A et al. Pain and fear avoidance partially mediate change in muscle strength during resistance exercise in women with fibromyalgia. J. Rehabil. Med 49, 744–750 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Lorig KR & Holman H Self-management education: history, definition, outcomes, and mechanisms. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine 26, 1–7 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Grady PA & Gough LL Self-management: a comprehensive approach to management of chronic conditions. Am. J. Public Health 104, e25–e31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King DE, Mainous AG, Carnemolla M & Everett CJ Adherence to healthy lifestyle habits in US adults, 1988–2006. Am. J. Med 122, 528–534 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Reis BF & Brown LG Reducing psychotherapy dropouts: Maximizing perspective convergence in the psychotherapy dyad. Psychother. Theory, Res. Pract. Train 36, 123 (1999). [Google Scholar]
- 8.Dowell D, Haegerich TM & Chou R CDC guideline for prescribing opioids for chronic pain—United States, 2016. Jama 315, 1624–1645 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leider HL, Dhaliwal J, Davis EJ, Kulakodlu M & Buikema AR Healthcare costs and nonadherence among chronic opioid users. Am. J. Manag. Care 17, 32–40 (2011). [PubMed] [Google Scholar]
- 10.Ng JYY et al. Self-determination theory applied to health contexts: A meta-analysis. Perspect. Psychol. Sci 7, 325–340 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Kähkönen O et al. Motivation is a crucial factor for adherence to a healthy lifestyle among people with coronary heart disease after percutaneous coronary intervention. J. Adv. Nurs 71, 2364–2373 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Kerns RD, Bayer LA & Findley JC Motivation and adherence in the management of chronic pain. Handb. pain Syndr. Biopsychosoc. Perspect 99–121 (1999). [Google Scholar]
- 13.Kim S-H, McDonald S, Kim S, Foster C & Fidler S Importance of self-motivation and social support in medication adherence in HIV-infected adolescents in the United Kingdom and Ireland: a multicentre HYPNet Study. AIDS Patient Care STDS 29, 354–364 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Heider J, Kock K, Sehlbrede M & Schroder A Readiness to change as a moderator of therapy outcome in patients with somatoform disorders. Psychother. Res 1–12 (2017). doi: 10.1080/10503307.2016.1265686 [DOI] [PubMed] [Google Scholar]
- 15.Salamone JD et al. in Behavioral Neuroscience of Motivation 231–257 (Springer, 2015). [Google Scholar]
- 16.Salamone JD & Correa M The mysterious motivational functions of mesolimbic dopamine. Neuron 76, 470–485 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz N et al. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science (80-. ). 345, 535–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bromberg-Martin ES, Matsumoto M & Hikosaka O Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey MR, Simpson EH & Balsam PD Neural substrates underlying effort, time, and risk-based decision making in motivated behavior. Neurobiol. Learn. Mem 133, 233–256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apkarian AV, Baliki MN & Farmer MA Predicting transition to chronic pain. Curr. Opin. Neurol 26, 360–367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porreca F & Navratilova E Reward, motivation, and emotion of pain and its relief. Pain 158, S43–S49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navratilova E & Porreca F Reward and motivation in pain and pain relief. Nat. Neurosci 17, 1304–1312 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navratilova E et al. Positive emotions and brain reward circuits in chronic pain. J. Comp. Neurol 524, 1646–1652 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moulton EA et al. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J. Neurosci 31, 3795–3804 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor AMW, Becker S, Schweinhardt P & Cahill C Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain 157, 1194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen MP, Nielson WR & Kerns RD Toward the development of a motivational model of pain self-management. J. Pain 4, 477–492 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Rosenstock IM, Strecher VJ & Becker MH Social learning theory and the health belief model. Health Educ. Q 15, 175–183 (1988). [DOI] [PubMed] [Google Scholar]
- 28.Hochbaum G, Rosenstock I & Kegels S Health belief model. United States Public Heal. Serv (1952). [Google Scholar]
- 29.Fishbein M A theory of reasoned action: some applications and implications. (1979). [PubMed]
- 30.Ajzen I in Action control 11–39 (Springer, 1985). [Google Scholar]
- 31.Jensen MP A neuropsychological model of pain: research and clinical implications. J. Pain 11, 2–12 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Angelides NH, Gupta J & Vickery TJ Associating resting-state connectivity with trait impulsivity. Soc. Cogn. Affect. Neurosci 12, 1001–1008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortensen JA, Lehn H, Evensmoen HR & Håberg AK Evidence for an antagonistic interaction between reward and punishment sensitivity on striatal activity: A verification of the Joint Subsystems Hypothesis. Pers. Individ. Dif 74, 214–219 (2015). [Google Scholar]
- 34.Wacker J, Dillon DG & Pizzagalli DA The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 46, 327–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller J et al. Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. J. Psychiatr. Res 47, 1319–1328 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Simon JJ et al. Neural reward processing is modulated by approach- and avoidancerelated personality traits. Neuroimage 49, 1868–1874 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Von Korff M et al. United States national pain strategy for population research: Concepts, definitions, and pilot data. J. Pain 17, 1068–1080 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Johnston M, Foster M, Shennan J, Starkey NJ & Johnson A The effectiveness of an Acceptance and Commitment Therapy self-help intervention for chronic pain. Clin. J. Pain 26, 393–402 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Thomas VN, Wilson-Barnett J & Goodhart F The role of cognitive-behavioural therapy in the management of pain in patients with sickle cell disease. J. Adv. Nurs 27, 1002–1009 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Mann EG, Lefort S & Vandenkerkhof EG Self-management interventions for chronic pain. Pain Manag. 3, 211–222 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Brawley LR & Culos-Reed SN Studying adherence to therapeutic regimens: overview, theories, recommendations. Control. Clin. Trials 21, S156–S163 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Butow P & Sharpe L The impact of communication on adherence in pain management. Pain 154 Suppl 1, S101–7 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Nicholas MK et al. Is adherence to pain self-management strategies associated with improved pain, depression and disability in those with disabling chronic pain? Eur. J. Pain 16, 93–104 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Busch AJ, Schachter CL, Overend TJ, Peloso PM & Barber KAR Exercise for fibromyalgia: a systematic review. J. Rheumatol 35, 1130–1144 (2008). [PubMed] [Google Scholar]
- 45.Kerns RD & Haythornthwaite JA Depression among chronic pain patients: cognitivebehavioral analysis and effect on rehabilitation outcome. J. Consult. Clin. Psychol 56, 870 (1988). [DOI] [PubMed] [Google Scholar]
- 46.Cinciripini PM & Floreen A An evaluation of a behavioral program for chronic pain. J. Behav. Med 5, 375–389 (1982). [DOI] [PubMed] [Google Scholar]
- 47.Medina-Mirapeix F et al. Predictive factors of adherence to frequency and duration components in home exercise programs for neck and low back pain: an observational study. BMC Musculoskelet. Disord 10, 155 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolt GS & McEvoy JF Adherence to rehabilitation in patients with low back pain. Man. Ther 8, 110–116 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Timmerman L, Stronks DL, Groeneweg JG & Huygen FJ Prevalence and determinants of medication non- adherence in chronic pain patients: a systematic review. Acta Anaesthesiol. Scand 60, 416–431 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Turk DC & Rudy TE Neglected topics in the treatment of chronic pain patients—relapse, noncompliance, and adherence enhancement. Pain 44, 5–28 (1991). [DOI] [PubMed] [Google Scholar]
- 51.Jordan JL, Holden MA, Mason EE & Foster NE Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev 20, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin LR, Williams SL, Haskard KB & DiMatteo MR The challenge of patient adherence. Ther. Clin. Risk Manag 1, 189 (2005). [PMC free article] [PubMed] [Google Scholar]
- 53.Iuga AO & McGuire MJ Adherence and health care costs. Risk Manag. Healthc. Policy 7, 35–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiMatteo MR, Giordani PJ, Lepper HS & Croghan TW Patient adherence and medical treatment outcomes a meta-analysis. Med. Care 794–811 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Nicholas MK et al. Cognitive exposure versus avoidance in patients with chronic pain: Adherence matters. Eur. J. Pain 18, 424–437 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Cecchi F et al. Predictors of response to exercise therapy for chronic low back pain: result of a prospective study with one year follow-up. Eur. J. Phys. Rehabil. Med 50, 143–151 (2014). [PubMed] [Google Scholar]
- 57.Turk DC & Okifuji A Psychological factors in chronic pain: evolution and revolution. J. Consult. Clin. Psychol 70, 678 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Turk DC et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 106, 337–345 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Kerns RD et al. Can we improve cognitive-behavioral therapy for chronic back pain treatment engagement and adherence? A controlled trial of tailored versus standard therapy. Health Psychol. 33, 938–947 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Sabaté E Adherence to long-term therapies: evidence for action. (World Health Organization, 2003). [PubMed] [Google Scholar]
- 61.Anderson RJ, Hurley RW, Staud R & Robinson ME Cognitive-motivational influences on health behavior change in adults with chronic pain. Pain Med. 17, 1079–1093 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Green EC & Murphy E Health belief model. Wiley Blackwell Encycl. Heal. illness, Behav. Soc (2014). [Google Scholar]
- 63.Mathes T, Jaschinski T & Pieper D Adherence influencing factors–a systematic review of systematic reviews. Arch. Public Heal 72, 37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bennett G Miller WR and Rollnick S (1991) Motivational interviewing: Preparing people to change addictive behavior New York: Guilford Press, 1991. Pp. xvii + 348. £24.95 hardback, £11.50 paper. ISBN 0–89862–566–1. J. Community Appl. Soc. Psychol 2, 299–300 (1992). [Google Scholar]
- 65.Biller N, Arnstein P, Caudill MA, Federman CW & Guberman C Predicting completion of a cognitive-behavioral pain management program by initial measures of a chronic pain patient’s readiness for change. Clin. J. Pain 16, 352–359 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Dorflinger L, Kerns RD & Auerbach SM Providers’ roles in enhancing patients’ adherence to pain self management. Transl. Behav. Med 3, 39–46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mata J et al. Motivational “spill-over” during weight control: Increased self-determination and exercise intrinsic motivation predict eating self-regulation. (2011). [DOI] [PubMed]
- 68.Stewart JA et al. Motivation for Psychological Treatment Predicts Favorable Outcomes in Multimodal Interdisciplinary Treatment for Chronic Somatoform Pain. Psychother. Psychosom 86, 60–61 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Kratz AL, Molton IR, Jensen MP, Ehde DM & Nielson WR Further evaluation of the motivational model of pain self-management: coping with chronic pain in multiple sclerosis. Ann. Behav. Med 41, 391–400 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Molton IR, Jensen MP, Nielson W, Cardenas D & Ehde DM A preliminary evaluation of the motivational model of pain self-management in persons with spinal cord injury–related pain. J. Pain 9, 606–612 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guite JW et al. Pain beliefs and readiness to change among adolescents with chronic musculoskeletal pain and their parents before an initial pain clinic evaluation. Clin. J. Pain 30, 27–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Habib S, Morrissey S & Helmes E Preparing for pain management: a pilot study to enhance engagement. J. Pain 6, 48–54 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Salamone JD The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav. Brain Res 61, 117–133 (1994). [DOI] [PubMed] [Google Scholar]
- 74.Cardinal RN, Parkinson JA, Hall J & Everitt BJ Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev 26, 321–352 (2002). [DOI] [PubMed] [Google Scholar]
- 75.Naqvi NH & Bechara A The hidden island of addiction: the insula. Trends Neurosci. 32, 56–67 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jarcho JM, Mayer EA, Jiang ZK, Feier NA & London ED Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. PAIN® 153, 744–754 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC & Wager TD Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.du Hoffmann J & Nicola SM Activation of Dopamine Receptors in the Nucleus Accumbens Promotes Sucrose-Reinforced Cued Approach Behavior. Front. Behav. Neurosci 10, 144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friston KJ Functional and effective connectivity in neuroimaging: a synthesis. Hum. Brain Mapp 2, 56–78 (1994). [Google Scholar]
- 80.Fox MD & Greicius M Clinical applications of resting state functional connectivity. Front. Syst. Neurosci 4, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greicius M Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol 21, 424–430 (2008). [DOI] [PubMed] [Google Scholar]
- 82.Hillary FG et al. Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology 29, 59–75 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Schultz AP et al. Phases of Hyperconnectivity and Hypoconnectivity in the Default Mode and Salience Networks Track with Amyloid and Tau in Clinically Normal Individuals. J. Neurosci 37, 4323–4331 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camara E, Rodriguez-Fornells A, Ye Z & Münte TF Reward networks in the brain as captured by connectivity measures. Front. Neurosci 3, 350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikemoto S Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci. Biobehav. Rev 35, 129–150 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kringelbach ML The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci 6, 691–702 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Ballard IC et al. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J. Neurosci 31, 10340–10346 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zweynert S et al. Motivational salience modulates hippocampal repetition suppression and functional connectivity in humans. Front. Hum. Neurosci 5, 144 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Satterthwaite TD et al. Common and Dissociable Dysfunction of the Reward System in Bipolar and Unipolar Depression. Neuropsychopharmacology 40, 2258–2268 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furman DJ, Hamilton JP & Gotlib IH Frontostriatal functional connectivity in major depressive disorder. Biol. Mood Anxiety Disord 1, 11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trost S et al. Disturbed Anterior Prefrontal Control of the Mesolimbic Reward System and Increased Impulsivity in Bipolar Disorder. Neuropsychopharmacology 39, 1914–1923 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S & Brady KT Brain activation to cocaine cues and motivation/treatment status. Addict. Biol 19, 240–249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moreno-Lopez L, Albein-Urios N, Martinez-Gonzalez JM, Soriano-Mas C & Verdejo-Garcia A Prefrontal Gray Matter and Motivation for Treatment in CocaineDependent Individuals with and without Personality Disorders. Front. psychiatry 5, 52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Le Berre A-P et al. Readiness to change and brain damage in patients with chronic alcoholism. Psychiatry Res. Neuroimaging 213, 202–209 (2013). [DOI] [PubMed] [Google Scholar]
- 95.Apkarian AV et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci 24, 10410–10415 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuchinad A et al. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J. Neurosci 27, 4004–4007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Puiu T et al. Alterations in Gray Matter Volume are Associated with Reduced EvokedPain Connectivity following Acute Pregabalin Administration. Arthritis Rheumatol. (Hoboken, N.J.) 68, 1511–1521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bauch EM, Rausch VH & Bunzeck N Pain anticipation recruits the mesolimbic system and differentially modulates subsequent recognition memory. Hum. Brain Mapp 35, 4594–4606 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leknes S, Lee M, Berna C, Andersson J & Tracey I Relief as a reward: hedonic and neural responses to safety from pain. PLoS One 6, e17870 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jensen J et al. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 40, 1251–1257 (2003). [DOI] [PubMed] [Google Scholar]
- 101.Loggia ML et al. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. (Hoboken, N.J.) 66, 203–212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baliki MN et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci 15, 1117–1119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Petre B et al. Smoking increases risk of pain chronification through shared corticostriatal circuitry. Hum. Brain Mapp 36, 683–694 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mutso AA et al. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol 111, 1065–1076 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Flor H, Knost B & Birbaumer N The role of operant conditioning in chronic pain: an experimental investigation. Pain 95, 111–118 (2002). [DOI] [PubMed] [Google Scholar]
- 106.Elvemo NA, Landrø NI, Borchgrevink PC & Håberg AK Reward responsiveness in patients with chronic pain. Eur. J. Pain 19, 1537–1543 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wood PB et al. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J. Pain 8, 51–58 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Ledermann K et al. Relation of dopamine receptor 2 binding to pain perception in female fibromyalgia patients with and without depression--A [(1)(1)C] raclopride PET-study. Eur. Neuropsychopharmacol 26, 320–330 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Albrecht DS et al. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 10, 829–839 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hagelberg N et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain 101, 149–154 (2003). [DOI] [PubMed] [Google Scholar]
- 111.Martikainen IK et al. Chronic back pain is associated with alterations in dopamine neurotransmission in the ventral striatum. J. Neurosci 35, 9957–9965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tremblay LK et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch. Gen. Psychiatry 62, 1228–1236 (2005). [DOI] [PubMed] [Google Scholar]
- 113.Redlich R et al. Reward processing in unipolar and bipolar depression: a functional MRI study. Neuropsychopharmacology 40, 2623–2631 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Volkow ND, Fowler JS, Wang GJ, Baler R & Telang F Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56, 3–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beck A et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry 66, 734–742 (2009). [DOI] [PubMed] [Google Scholar]
- 116.Garland EL, Bryan CJ, Nakamura Y, Froeliger B & Howard MO Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology (Berl). 234, 621–629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nummenmaa L et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One 7, e31089 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gujar N, Yoo S-S, Hu P & Walker MP Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J. Neurosci 31, 4466–4474 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hasler BP et al. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. J. Sleep Res 21, 515–526 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Becerra L & Borsook D Signal valence in the nucleus accumbens to pain onset and offset. Eur. J. pain 12, 866–869 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Becerra L, Navratilova E, Porreca F & Borsook D Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans. J. Neurophysiol 110, 1221–1226 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wanigasekera V et al. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc. Natl. Acad. Sci. U. S. A 109, 17705–17710 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baliki MN, Geha PY, Fields HL & Apkarian AV Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 66, 149–160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kato T, Ide S & Minami M Pain relief induces dopamine release in the rat nucleus accumbens during the early but not late phase of neuropathic pain. Neurosci. Lett 629, 73–78 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Jääskeläinen SK et al. Role of the dopaminergic system in chronic pain–a fluorodopaPET study. Pain 90, 257–260 (2001). [DOI] [PubMed] [Google Scholar]
- 126.Berger SE et al. Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC Res. Notes 7, 739 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Becker S, Kleinböhl D, Baus D & Hölzl R Operant learning of perceptual sensitization and habituation is impaired in fibromyalgia patients with and without irritable bowel syndrome. Pain 152, 1408–1417 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Gracely R et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 127, 835–843 (2004). [DOI] [PubMed] [Google Scholar]
- 129.Burgmer M et al. Cerebral activation and catastrophizing during pain anticipation in patients with fibromyalgia. Psychosom. Med 73, 751–759 (2011). [DOI] [PubMed] [Google Scholar]
- 130.Campbell CM & Edwards RR Mind–body interactions in pain: the neurophysiology of anxious and catastrophic pain-related thoughts. Transl. Res 153, 97–101 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Samwel HJA, Evers AWM, Crul BJP & Kraaimaat FW The role of helplessness, fear of pain, and passive pain-coping in chronic pain patients. Clin. J. Pain 22, 245–251 (2006). [DOI] [PubMed] [Google Scholar]
- 132.Jensen MP, Turner JA & Romano JM Self-efficacy and outcome expectancies: relationship to chronic pain coping strategies and adjustment. Pain 44, 263–269 (1991). [DOI] [PubMed] [Google Scholar]
- 133.Prochaska JO & DiClemente CC The transtheoretical approach. Handb. Psychother. Integr 300, 334 (1992). [Google Scholar]
- 134.Bastian B, Jetten J, Hornsey MJ & Leknes S The positive consequences of pain: A biopsychosocial approach. Personal. Soc. Psychol. Rev 18, 256–279 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Jordan JL, Holden MA, Mason EE & Foster NE Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev 2015, CD005956 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chib VS, Yun K, Takahashi H & Shimojo S Noninvasive remote activation of the ventral midbrain by transcranial direct current stimulation of prefrontal cortex. Transl. Psychiatry 3, e268 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Soutschek A, Kang P, Ruff CC, Hare TA & Tobler PN Brain stimulation over frontopolar cortex enhances motivation to exert effort for reward. Biol. Psychiatry (2017). [DOI] [PubMed] [Google Scholar]
- 138.Su L et al. Improving motivation through real-time fMRI based self-regulation of the nucleus accumbens. (2017). [DOI] [PubMed]
- 139.Holtzheimer PE 3rd, Russo J & Avery DH A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol. Bull 35, 149–169 (2001). [PubMed] [Google Scholar]
- 140.Gaynes BN et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J. Clin. Psychiatry 75, 477–89; quiz 489 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Leggett LE et al. Repetitive Transcranial Magnetic Stimulation for Treatment-Resistant Depression in Adult and Youth Populations: A Systematic Literature Review and MetaAnalysis. Prim. Care Companion CNS Disord 17, 10.4088/PCC.15r01807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xie C-L et al. Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in Parkinson disease: a meta-analysis of randomized controlled clinical trials. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol 36, 1751–1761 (2015). [DOI] [PubMed] [Google Scholar]
- 143.Malone DA et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol. Psychiatry 65, 267–275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bewernick BH, Kayser S, Sturm V & Schlaepfer TE Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology 37, 1975 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schlaepfer TE, Bewernick BH, Kayser S, Mädler B & Coenen VA Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry 73, 1204–1212 (2013). [DOI] [PubMed] [Google Scholar]
- 146.Bewernick BH et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 67, 110–116 (2010). [DOI] [PubMed] [Google Scholar]
- 147.Schlaepfer TE et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33, 368 (2008). [DOI] [PubMed] [Google Scholar]
- 148.Stirratt MJ et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl. Behav. Med 5, 470–482 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Braun U et al. Test–retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage 59, 1404–1412 (2012). [DOI] [PubMed] [Google Scholar]
- 150.Birn RM et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage 83, 550–558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bennett CM & Miller MB How reliable are the results from functional magnetic resonance imaging? Ann. N. Y. Acad. Sci 1191, 133–155 (2010). [DOI] [PubMed] [Google Scholar]
- 152.Letzen JE et al. Test-Retest Reliability of Pain-Related Brain Activity in Healthy Controls Undergoing Experimental Thermal Pain. J. Pain 15, 1008–1014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Letzen JE, Boissoneault J, Sevel LS & Robinson ME Test-retest reliability of pain-related functional brain connectivity compared to pain self-report. Pain (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Upadhyay J et al. Test–retest reliability of evoked heat stimulation BOLD fMRI. J. Neurosci. Methods 1–9 (2015). doi: 10.1016/j.jneumeth.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 155.Quiton RL, Keaser ML, Zhuo J, Gullapalli RP & Greenspan JD Intersession reliability of fMRI activation for heat pain and motor tasks. NeuroImage Clin. 5, 309–321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]