Abstract
Background and aims:
High concentrations of low density lipoprotein (LDL) triglycerides have been associated with prevalent angiographic coronary artery disease. The present analysis was designed to investigate the association of LDL triglycerides with cardiovascular mortality and to explore possible mechanisms that may link LDL triglycerides to cardiovascular risk.
Methods:
LDL triglycerides were measured in 3,140 participants of the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. They were prospectively followed for cardiovascular mortality (median duration 9.9 years). Genome wide association data for LDL triglycerides were available for 2,900 LURIC participants. Genetic data and measurements of hepatic lipase activity were available for 478 participants of the HERITAGE Family study. Genome wide association data for cardiovascular disease were available for 184,305 participants of the CARDIoGRAMplusC4D consortium.
Results:
There was a continuous positive association between LDL triglycerides and cardiovascular mortality (hazard ratio for 5th vs. 1st quintile = 2.53, p < 0.001) and this association was similar in males and females. Genome wide association analysis in LURIC revealed that LDL triglycerides were strongly associated with variation in the hepatic lipase region (p < 10−15 for rs1800588 and rs10468017). The LDL triglyceride raising alleles in rs1800588 and rs10468017 were associated with low hepatic lipase activity in HERITAGE and increased cardiovascular risk in CARDIoGRAMplusC4D. Two-sample Mendelian randomization analysis (HERITAGE and CARDIoGRAMplusC4D) using rs1800588 and rs10468017 as instrumental variable suggested that low hepatic lipase activity may cause increased cardiovascular risk (p = 0.013).
Conclusions:
Low hepatic lipase activity may link high LDL triglycerides to increased cardiovascular risk.
Keywords: Cardiovascular mortality, HDL, hepatic lipase, LDL, triglycerides
Introduction
The role of triglycerides as a cardiovascular risk has been debated for decades. Elevated concentrations of circulating triglycerides have repeatedly been shown to associate with increased cardiovascular risk in epidemiologic studies, even in statin treated patients (1, 2). However, the association between triglycerides and cardiovascular risk has lost its statistical significance after multivariate adjustment in the largest pertinent meta-analysis including 302,430 participants from 68 long-term prospective studies (3). On the other hand, Mendelian randomization studies have suggested a causal role for triglycerides in pathophysiology of coronary heart disease (4).
Interventional studies on lipids have primarily focused on low density lipoprotein (LDL) cholesterol (5, 6), which per the classical definition of LDL by the Friedewald equation and β quantification is inclusive of intermediate-density lipoprotein (IDL) (7). Consequently, LDL cholesterol is also used in the guidelines for the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk of the American Heart Association and the American College of Cardiology and in the guidelines for the management of dyslipidaemias of the European Atherosclerosis Society and the European Society of Cardiology (8, 9). In contrast to cholesterol, triglycerides have usually been measured and reported as total triglycerides instead of accounting for their distribution amongst distinct lipoprotein classes (10, 11). However, we have previously shown that especially LDL triglycerides, which also include the IDL fraction, were associated with systemic low-grade inflammation and coronary artery disease in a cross-sectional approach (12).
The present analyses were performed 1) to investigate the associations of LDL triglycerides with cardiovascular mortality and 2) to explore possible mechanisms that may link LDL triglycerides to cardiovascular risk. Analyses were performed using data from the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study (12–14), the HEalth, RIsk factors, exercise Training, And GEnetics (HERITAGE) Family study (15–18), and the CARDIoGRAMplusC4D consortium (19).
Patients and methods
Study design, participants and clinical characterization
The LURIC study is a cohort of 3,316 patients, who were referred for coronary angiography to the Ludwigshafen Heart Center in South-West Germany from 1997 to 2000 (12–14). Inclusion criteria were: German ancestry, clinical stability except for acute coronary syndromes, and the availability of a coronary angiogram. Indications for angiography in individuals with clinically sTabledisease were chest pain and/or noninvasive test results suggestive of myocardial ischemia. Individuals suffering from any acute illness other than acute coronary syndromes, chronic non-cardiac diseases, or malignancy within the five past years, and those unable to understand the purpose of the study were excluded. A total of 3,140 participants with available measurements of total, LDL, high density lipoprotein (HDL), and very low density lipoprotein (VLDL) triglycerides were included in the present analyses. Diabetes mellitus was diagnosed according to the current criteria of the American Diabetes Association (20). Moreover, patients with a history of diabetes and those using oral anti-diabetics or insulin were also considered diabetic. Hypertension was diagnosed if the systolic and/or diastolic blood pressure exceeded 140 and/or 90 mmHg or if there was a history of hypertension, evident by treatment with antihypertensive drugs (12, 13). There was a follow-up for cardiovascular death. Information on the vital status was obtained from local population registries. Cardiovascular mortality was defined as death due to fatal myocardial infarction, sudden cardiac death, death after cardiovascular intervention, stroke and other causes of death caused by cardiovascular diseases (14). Assessment of death certificates was done by two experienced clinicians. In a few cases of disagreement or uncertainty concerning the coding of a specific cause of death, classification was made by a principal investigator of the LURIC study (W. M.) (14). The median (inter-quartile range) duration of the follow-up was 9.9 (8.7-10.7) years (mean [standard deviation] = 8.8 [3.0] years).
The HERITAGE Family Study is a cohort of healthy and sedentary white and black families who were recruited by a consortium of five universities in the United States and Canada from 1992 to 1997. The HERITAGE Family Study comprises 100 families with all members of European descent (both parents and at least 2 adult offspring) plus 100 families of African descent (at least one parent and two siblings) (15). We excluded individuals without complete genotype or phenotype data resulting in a cohort of 478 white participants for the present analyses.
Ethics approval and written informed consent
The LURIC and the HERITAGE Family studies were approved by the ethics committee of the Physicians Chamber of Rheinland-Pfalz and each institutional review board of the HERITAGE Family Study research consortium, respectively. Both studies were performed in accordance with the declaration of Helsinki. All participants gave written informed consent (12–14, 15–18).
Laboratory analyses
All analyses in the LURIC and HERITAGE studies were performed in fasting blood samples (12–14, 15–18).
In the LURIC study triglycerides and cholesterol were quantified with enzymatic reagents from WAKO (Neuss, Germany) on a WAKO 30R analyzer. For triglycerides, this assay has a lower limit of detection of 1 mg/dl. Lipoproteins were separated using a combined ultracentrifugation-precipitation method (β-quantification) in all 3140 LURIC participants: Plasma was ultracentrifuged at a density of d = 1.0063 kg/l (30,000 rpm for 18 h). VLDL in the supernatant were removed. In the remainder, LDL (and IDL) were precipitated with phosphotungstic acid/MgCl2. Centrifugation (10,000 rpm for 5 min) was the performed to separate precipitated LDL (and IDL) from HDL in the remainder. LDL triglycerides were calculated as the difference between triglycerides in the remainder before and after separation of LDL (11). The intra-day and inter-day coefficients of variation were <3% for LDL triglycerides (Supplementary table 1). Apolipoprotein B was measured by turbidimetry (Rolf-Greiner Biochemica, Flacht, Germany). Interleukin-6 was measured using a high sensitivity enzyme immunoassay (R&D Systems, Wiesbaden, Germany). C-reactive protein was measured protein was measured by immunonephelometry on a Behring Nephelometer II (N High Sensitivity CRP, Dade Behring, Marburg, Germany). Glucose was measured with an enzymatic assay on a Hitachi 717 analyzer.
In the HERITAGE Family study cholesterol and triglycerides were measured by enzymatic methods using the Technicon RA-1000 analyzer. In accordance with the LURIC study, LDL, HDL, and VLDL triglycerides were quantified with an ultracentrifugation-precipitation method in the 478 participants of the HERITAGE Family study. Post-heparin (10 minutes after 60 IU/kg body weight) hepatic lipase activity was measured by the modified Nilsson-Ehle and Ekman method. Hepatic lipase activity is expressed as nmoles of oleic acid released per ml of plasma per minute (18). Plasma glucose was enzymatically determined using a reagent kit distributed by Diagnostic Chemicals Ltd. (Oxford, United States). C-reactive protein was measured with a high-sensitivity solidphase chemiluminescent immunometric assay (IMMULITE 2000 High Sensitivity CRP, Diagnostic Products Corporation, Los Angeles, CA) implemented on an automated immunoassay instrument (Diagnostic Products Corporation, Los Angeles, United States).
Genotyping and quality control
In the LURIC study genotyping was performed us the Affymetrix Human SNP Array 6.0. Genotype imputation was performed using MACH 1.0 and HapMap II CEU (release 22, NCBI build 36, dbSNP 126) samples as a reference. After imputation, 2,543,887 SNPs were available. SNPs with a squared correlation of ≥ 0.3 between imputed and true genotypes were considered well imputed.
In the HERITAGE study genotyping was performed using the TaqMan allelic discrimination method (18) and with the Illumina Human CNV370-Quad v3.0 BeadChip on an Illumina BeadStation 500GX platform.
Genetic association with coronary artery disease and myocardial infarction
Risk estimates for genetic association with coronary artery disease and myocardial infarction were downloaded from the CARDIoGRAMplusC4D consortium (www.cardiogramplusc4d.org). This database consolidates genetic information from 60,801 coronary artery disease and myocardial infarction case subjects and 123,504 control subjects of mixed ancestry across 48 studies (19).
Statistical analysis
Baseline characteristics are presented as numbers and percentages of subjects in cases of categorical data and as means and standard deviations in cases of continuous data according to quintiles of total, LDL, HDL, and very VLDL triglycerides and genetic variants. The χ2-test and ANalysis Of VAriance were used to compare distributions of categorical and continuous parameters across quintiles of triglycerides and genetic variants. Moreover, Spearman correlations for the associations of total triglycerides and triglycerides in lipoprotein classes with clinical and metabolic parameters were calculated. In addition, the LDL triglyceride concentration was compared between men and women. Total triglycerides, triglycerides in lipoprotein classes, interleukin-6, and C-reactive protein were transformed logarithmically due to their skewed distribution. The Cox proportional hazards model was used to examine the association of total triglycerides and triglycerides in lipoprotein classes with cardiovascular death. Model 1 included adjustment for sex and age. Model 2 included adjustment for sex, age, and LDL cholesterol. Model 3 included adjustments for sex, age, body mass index, hypertension, diabetes mellitus, LDL cholesterol, HDL cholesterol, smoking, and use of statin and non-statin lipid lowering drugs. The net reclassification index for adding LDL triglycerides to the covariates of model 3 was calculated with the R-package ‘Hmisc’ (v 3.17-0). In addition, a hazard ratio plot for the associations of LDL triglycerides with cardiovascular mortality (model 2) was calculated using the R-package ‘rms’ (v 4.4-0). We also tested for interaction between sex and triglyceride quintiles with regard to cardiovascular death and we performed for sex-stratified analyses. SNPs were evaluated for associations with LDL triglycerides using linear regression analyses implemented in ProbABEL in the LURIC study (discovery study) (21). A Manhattan plot was drawn for the results. The P value for genome-wide significance was set at p < 5 × 10−8, which corresponds to an α of 0.05 with a Bonferroni correction for one million tests. Hardy-Weinberg equilibrium was tested with the χ2-test. Association between SNPs was tested with the χ2-test test. Beta coefficients for the associations of SNPs with hepatic lipase activity were calculated with linear regression. We used the beta coefficients for the associations between SNPs and hepatic lipase activity in the HERITAGE Family study, the beta coefficients for the associations between SNPs and LDL triglycerides in the LURIC study, and the respective beta coefficients for the associations between SNPs and coronary artery disease in the CARDIoGRAM consortium (www.cardiogramplusc4d.org) as input for two-sample Mendelian randomization. We used the R-package ‘TwoSampleMR’ (0.4.14) (22). The SPSS 23 (SPSS Inc., Chicago, USA) and the R v3.3.2 (www.r-project.org) statistical packages were used.
Results
Baseline characteristics of the LURIC study
Total triglycerides were positively associated with the body mass index, diabetes mellitus, fasting glucose, total and VLDL cholesterol, VLDL, LDL, and HDL triglycerides (Supplementary tables 2 and 3). Total triglycerides were inversely related to HDL cholesterol (Supplementary tables 2 and 3).
LDL triglycerides were positively associated with diabetes mellitus, total and LDL cholesterol, LDL apolipoprotein B, total triglycerides, and C-reactive protein (Supplementary tables 4 and 5). LDL triglycerides were inversely related to the proportion of males (Supplementary table 4). The mean (standard deviation) concentration of LDL triglycerides was higher in females (34 [13] mg/dl) than in males (30 [11] mg/dl) (p < 0.001).
HDL triglycerides were positively associated with diabetes mellitus, and total, LDL, and VLDL triglycerides (Supplementary tables 6 and 7). HDL triglycerides were inversely related to the proportions of males (Supplementary tables 6 and 7).
VLDL triglycerides were positively associated with the body mass index, diabetes mellitus, total and VLDL cholesterol, and total and HDL triglycerides (Supplementary tables 8 and 9). VLDL triglycerides were inversely related to HDL cholesterol (Supplementary tables 8 and 9) (Table 1).
Table 1. Baseline characteristics of the entire LURIC cohort.
| Number | 3140 |
|---|---|
| Male sex | 2190 (69.7) |
| Age, years | 62.6 (10.6) |
| Body mass index, kg/m2 | 27.5 (4.1) |
| Hypertension | 2297 (73.2) |
| Systolic blood pressure, mmHg | 141 (23) |
| Diastolic blood pressure, mmHg | 81 (11) |
| Diabetes mellitus | 1241 (39.5) |
| Fasting glucose, mg/dl | 113 (35) |
| Smoking | |
| Never | 1136 (36.2) |
| Former smoker | 1405 (44.7) |
| Current smoker | 599 (19.1) |
| Lipids and apolipoproteins | |
| Total cholesterol, mg/dl | 193 (39) |
| LDL cholesterol, mg/dl | 117 (34) |
| HDL cholesterol, mg/dl | 39 (11) |
| VLDL cholesterol, mg/dl | 37 (26) |
| Total triglycerides, mg/dl | 173 (119) |
| LDL triglycerides, mg/dl | 32 (12) |
| HDL triglycerides, mg/dl | 16 (7) |
| VLDL triglycerides, mg/dl | 126 (114) |
| LDL apolipoprotein B, mg/dl | 85 (22) |
| Inflammatory parameters | |
| Interleukin-6, pg/ml | 5.2 (6.1) |
| C-reactive protein, mg/l | 9.0 (18.4) |
| Lipid lowering drug | |
| Statin | 1459 (46.5) |
| Non-statin lipid lowering drug | 78 (2.5) |
Values are numbers (percentages) for categorical variables and means (standard deviations) for continuous variables; to convert values for cholesterol to millimoles per liter, multiply by 0.02586; to convert values for triglycerides to millimoles per liter, multiply by 0.01129.
Triglycerides in lipoprotein classes and cardiovascular mortality in the LURIC study
A total of 589 participants died from cardiovascular diseases.
In the entire cohort, high total triglycerides were associated with modestly increased cardiovascular mortality adjusted for sex and age, and after additional adjustment for LDL cholesterol. This association was attenuated after multivariate adjustment (Table 2). There was significant interaction of between sex and total triglyceride quintiles in predicting cardiovascular mortality (p = 0.031). Performing stratified analysis, total triglycerides were significantly associated with cardiovascular mortality in females but not in males (Table 3). There was a continuous positive association of LDL triglyceride quintiles with cardiovascular mortality adjusted for sex and age (Table 2 and Fig. 1). This relationship remained significant after additional adjustment for LDL cholesterol and after multivariate adjustment (Table 2). Inclusion of LDL triglycerides into a model including the variables of model 3 improved the net reclassification (95 % confidence interval) by 18 (9 – 27) (p < 0.001). There was no interaction between LDL triglyceride quintiles and sex with regard to cardiovascular mortality (p = 0.874). Performing stratified analyses, LDL triglyceride quintiles were similarly associated with cardiovascular mortality in males and females (Table 3).
Table 2. Cardiovascular mortality according to triglyceride quintiles in the entire LURIC cohort.
| Model 1a |
Model 2b |
Model 3c |
||||||
|---|---|---|---|---|---|---|---|---|
| N | CD (%) | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Total triglycerides | ||||||||
| 1st quintile | 634 | 125 (19.7) | 1.14 (0.88-1.48) | 0.309 | 1.14 (0.88-1.47) | 0.326 | 1.23 (0.95-1.60) | 0.112 |
| 2nd quintile | 648 | 111 (17.1) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 3rd quintile | 616 | 118 (19.2) | 1.22 (0.94-1.58) | 0.138 | 1.22 (0.94-1.58) | 0.139 | 1.07 (0.82-1.39) | 0.616 |
| 4th quintile | 617 | 118 (19.1) | 1.24 (0.96-1.61) | 0.103 | 1.24 (0.96-1.61) | 0.105 | 1.07 (0.82-1.39) | 0.614 |
| 5th quintile | 625 | 117 (18.7) | 1.41 (1.09-1.84) | 0.010 | 1.40 (1.07-1.81) | 0.013 | 1.07 (0.82-1.40) | 0.629 |
| LDL triglycerides | ||||||||
| 1st quintile | 614 | 90 (14.7) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 2nd quintile | 674 | 118 (17.5) | 1.24 (0.94-1.63) | 0.122 | 1.32 (1.00-1.74) | 0.049 | 1.24 (0.94-1.63) | 0.136 |
| 3rd quintile | 623 | 108 (17.3) | 1.25 (0.94-1.65) | 0.121 | 1.39 (1.04-1.85) | 0.026 | 1.29 (0.97-1.73) | 0.082 |
| 4th quintile | 614 | 120 (19.5) | 1.44 (1.10-1.90) | 0.009 | 1.65 (1.24-2.19) | 0.001 | 1.42 (1.06-1.89) | 0.019 |
| 5th quintile | 615 | 153 (24.9) | 2.08 (1.60-2.71) | <0.001 | 2.53 (1.90-3.35) | <0.001 | 1.99 (1.47-2.69) | <0.001 |
| HDL triglycerides | ||||||||
| 1st quintile | 627 | 107 (17.1) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 2nd quintile | 724 | 140 (19.3) | 1.08 (0.84-1.39) | 0.546 | 1.08 (0.84-1.38) | 0.571 | 1.16 (0.90-1.50) | 0.240 |
| 3rd quintile | 627 | 108 (17.2) | 0.97 (0.74-1.27) | 0.800 | 0.96 (0.73-1.25) | 0.741 | 0.99 (0.75-1.30) | 0.939 |
| 4th quintile | 546 | 94 (17.2) | 1.04 (0.78-1.37) | 0.799 | 1.02 (0.73-1.25) | 0.876 | 1.06 (0.80-1.40) | 0.709 |
| 5th quintile | 616 | 140 (22.7) | 1.33 (1.03-1.73) | 0.029 | 1.31 (1.01-1.70) | 0.041 | 1.34 (1.03-1.74) | 0.031 |
| VLDL triglycerides | ||||||||
| 1st quintile | 631 | 134 (21.2) | 1.30 (1.01-1.68) | 0.045 | 1.29 (1.00-1.67) | 0.049 | 1.41 (1.09-1.83) | 0.009 |
| 2nd quintile | 634 | 105 (16.6) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 3rd quintile | 623 | 126 (20.2) | 1.30 (1.01-1.69) | 0.045 | 1.30 (1.00-1.68) | 0.048 | 1.15 (0.89-1.50) | 0.280 |
| 4th quintile | 624 | 118 (18.9) | 1.23 (0.95-1.60) | 0.123 | 1.22 (0.94-1.59) | 0.134 | 1.08 (0.82-1.40) | 0.595 |
| 5th quintile | 628 | 106 (16.9) | 1.30 (0.99-1.71) | 0.055 | 1.28 (0.97-1.68) | 0.081 | 0.97 (0.74-1.29) | 0.851 |
N number, CD cardiovascular death; HR hazard ratio (calculated with Cox regression).
Adjusted for sex and age;
model 1 with additional adjustment for LDL cholesterol;
model 1 with additional adjustment for body mass index, hypertension, diabetes, LDL cholesterol, HDL cholesterol, smoking, and use of statin and non-statin lipid lowering drugs.
Table 3. Cardiovascular mortality according to triglyceride quintiles stratified for gender.
| Model 1a |
Model 2b |
Model 3c |
||||||
|---|---|---|---|---|---|---|---|---|
| N | CD (%) | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Total triglycerides | ||||||||
| Males | ||||||||
| 1st quintile | 420 | 96 (22.9) | 1.11 (0.83-1.49) | 0.484 | 1.11 (0.83-1.49) | 0.481 | 1.21 (0.90-1.62) | 0.213 |
| 2nd quintile | 438 | 86 (19.6) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 3rd quintile | 443 | 95 (21.4) | 1.23 (0.92-1.65) | 0.166 | 1.23 (0.92-1.65) | 0.166 | 1.09 (0.81-1.46) | 0.578 |
| 4th quintile | 445 | 81 (18.2) | 1.05 (0.77-1.42) | 0.774 | 1.05 (0.77-1.42) | 0.772 | 0.92 (0.67-1.25) | 0.573 |
| 5th quintile | 444 | 71 (16.0) | 1.08 (0.79-1.49) | 0.619 | 1.09 (0.79-1.50) | 0.606 | 0.85 (0.62-1.19) | 0.345 |
| Females | ||||||||
| 1st quintile | 214 | 29 (13.6) | 1.26 (0.74-2.15) | 0.395 | 1.22 (0.72-2.09) | 0.462 | 1.33 (0.77-2.28) | 0.310 |
| 2nd quintile | 210 | 25 (11.9) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 3rd quintile | 173 | 23 (13.3) | 1.09 (0.62-1.92) | 0.770 | 1.08 (0.61-1.90) | 0.795 | 0.97 (0.55-1.71) | 0.909 |
| 4th quintile | 172 | 37 (21.5) | 1.89 (1.14-3.14) | 0.014 | 1.88 (1.13-3.12) | 0.015 | 1.59 (0.94-2.68) | 0.082 |
| 5th quintile | 181 | 46 (25.4) | 2.38 (1.46-3.88) | <0.001 | 2.26 (1.39-3.69) | 0.001 | 1.67 (1.00-2.79) | 0.050 |
| LDL triglycerides | ||||||||
| Males | ||||||||
| 1st quintile | 486 | 76 (15.6) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 2nd quintile | 497 | 94 (18.9) | 1.27 (0.94-1.72) | 0.119 | 1.32 (0.97-1.79) | 0.078 | 1.26 (0.92-1.71) | 0.145 |
| 3rd quintile | 427 | 75 (17.6) | 1.19 (0.87-1.64) | 0.284 | 1.26 (0.91-1.75) | 0.160 | 1.22 (0.87-1.70) | 0.246 |
| 4th quintile | 410 | 86 (21.0) | 1.43 (1.05-1.95) | 0.023 | 1.54 (1.12-2.13) | 0.008 | 1.40 (1.01-1.94) | 0.046 |
| 5th quintile | 370 | 98 (26.5) | 2.14 (1.58-2.89) | <0.001 | 2.36 (1.71-3.26) | <0.001 | 1.98 (1.41-2.78) | <0.001 |
| Females | ||||||||
| 1st quintile | 128 | 14 (10.9) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 2nd quintile | 177 | 24 (13.6) | 1.10 (0.57-2.12) | 0.786 | 1.25 (0.65-2.43) | 0.506 | 1.09 (0.56-2.13) | 0.809 |
| 3rd quintile | 196 | 33 (16.8) | 1.36 (0.73-2.54) | 0.336 | 1.66 (0.88-3.12) | 0.118 | 1.43 (0.75-2.73) | 0.281 |
| 4th quintile | 204 | 34 (16.7) | 1.42 (0.76-2.64) | 0.275 | 1.81 (0.96-3.43) | 0.066 | 1.36 (0.70-2.65) | 0.362 |
| 5th quintile | 245 | 55 (22.4) | 1.88 (1.04-3.38) | 0.036 | 2.95 (1.58-5.51) | 0.001 | 1.96 (1.00-3.85) | 0.051 |
| HDL triglycerides | ||||||||
| Males | ||||||||
| 1st quintile | 526 | 94 (17.9) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 2nd quintile | 546 | 105 (19.2) | 1.02 (0.77-1.34) | 0.914 | 1.02 (0.77-1.35) | 0.900 | 1.05 (0.80-1.40) | 0.711 |
| 3rd quintile | 433 | 83 (19.2) | 1.05 (0.78-1.41) | 0.766 | 1.05 (0.78-1.42) | 0.737 | 1.05 (0.78-1.42) | 0.742 |
| 4th quintile | 352 | 66 (18.8) | 1.05 (0.77-1.44) | 0.768 | 1.06 (0.77-1.45) | 0.732 | 1.04 (0.76-1.43) | 0.807 |
| 5th quintile | 333 | 81 (24.3) | 1.30 (0.96-1.75) | 0.085 | 1.31 (0.97-1.77) | 0.077 | 1.28 (0.94-1.74) | 0.116 |
| Females | ||||||||
| 1st quintile | 101 | 13 (12.9) | 1.0 reference | 1.0 reference | 1.0 reference | |||
| 2nd quintile | 178 | 35 (19.7) | 1.38 (0.73-2.61) | 0.320 | 1.34 (0.71-2.53) | 0.372 | 1.69 (0.89-3.23) | 0.110 |
| 3rd quintile | 194 | 25 (12.9) | 0.78 (0.40-1.52) | 0.456 | 0.73 (0.37-1.43) | 0.361 | 0.84 (0.43-1.65) | 0.604 |
| 4th quintile | 194 | 28 (14.4) | 1.05 (0.54-2.03) | 0.887 | 0.98 (0.51-1.90) | 0.949 | 1.21 (0.62-2.37) | 0.575 |
| 5th quintile | 283 | 59 (20.8) | 1.43 (0.78-2.64) | 0.244 | 1.33 (0.73-2.44) | 0.353 | 1.66 (0.89-3.09) | 0.112 |
| VLDL triglycerides | ||||||||
| Males | ||||||||
| 1st quintile | 401 | 97 (24.2) | 1.16 (0.87-1.56) | 0.307 | 1.16 (0.87-1.56) | 0.306 | 1.26 (0.94-1.69) | 0.128 |
| 2nd quintile | 412 | 86 (20.9) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 3rd quintile | 453 | 97 (21.4) | 1.14 (0.85-1.52) | 0.390 | 1.14 (0.85-1.52) | 0.390 | 1.02 (0.76-1.36) | 0.916 |
| 4th quintile | 462 | 82 (17.7) | 0.95 (0.70-1.28) | 0.713 | 0.95 (0.70-1.28) | 0.716 | 0.84 (0.62-1.14) | 0.254 |
| 5th quintile | 462 | 67 (14.5) | 0.93 (0.68-1.29) | 0.673 | 0.93 (0.67-1.30) | 0.682 | 0.73 (0.52-1.01) | 0.059 |
| Females | ||||||||
| 1st quintile | 230 | 37 (16.1) | 1.96 (1.13-3.41) | 0.017 | 1.92 (1.10-3.33) | 0.021 | 2.11 (1.21-3.70) | 0.009 |
| 2nd quintile | 222 | 19 (8.6) | 1.0 reference | - | 1.0 reference | - | 1.0 reference | - |
| 3rd quintile | 170 | 29 (17.1) | 2.00 (1.12-3.57) | 0.019 | 1.93 (1.08-3.44) | 0.027 | 1.73 (0.96-3.10) | 0.066 |
| 4th quintile | 162 | 36 (22.2) | 2.63 (1.51-4.58) | 0.001 | 2.59 (1.49-4.52) | 0.001 | 2.25 (1.27-3.98) | 0.005 |
| 5th quintile | 166 | 39 (23.5) | 2.92 (1.69-5.05) | <0.001 | 2.70 (1.55-4.70) | <0.001 | 1.98 (1.12-3.50) | 0.020 |
N number, CD cardiovascular death; HR hazard ratio (calculated with Cox regression).
Adjusted for sex and age;
model 1 with additional adjustment for LDL cholesterol;
model 1 with additional adjustment for body mass index, hypertension, diabetes, LDL cholesterol, HDL cholesterol, smoking, and use of statin and non-statin lipid lowering drugs.
Figure 1. Hazard ratio plot for cardiovascular mortality according to LDL triglycerides.
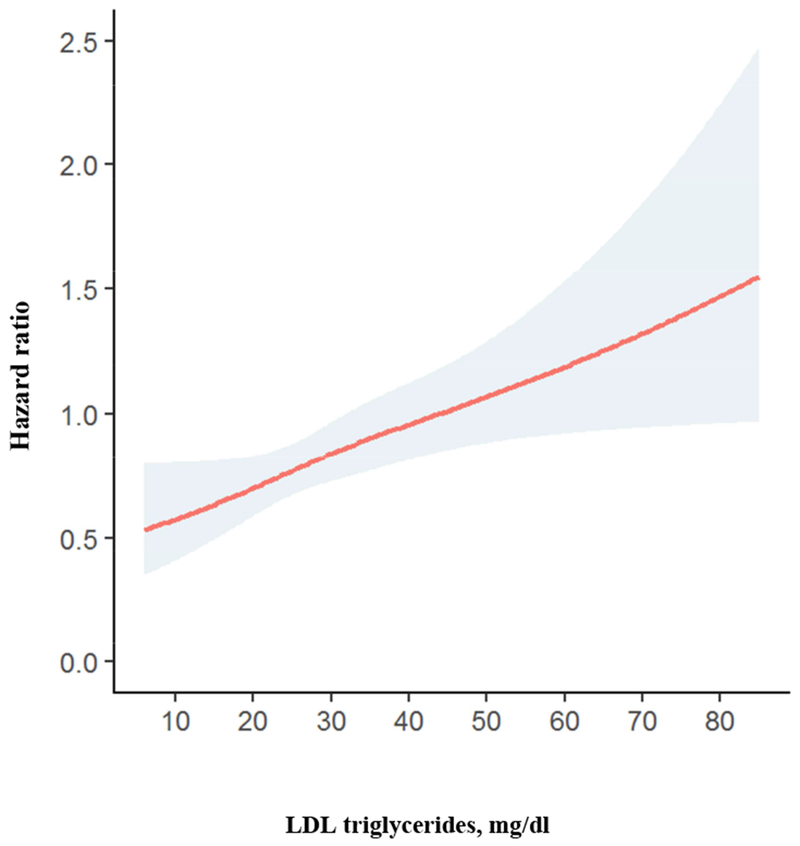
High HDL triglycerides were associated with modestly increased cardiovascular mortality (Table 2). This relationship did not remain significant after multivariate adjustment and was similar in males and females with no interaction (p = 0.284) (Table 3).
In the entire cohort, there was a modest trend towards a U-shaped association between VLDL triglycerides and cardiovascular mortality in models adjusted for sex and age (Table 2). In the multivariate adjusted model, the association between low VLDL triglycerides and increased cardiovascular mortality remained statistically significant (Table 2). The relationship between high VLDL triglycerides and increased cardiovascular mortality lost statistical significance after multivariate adjustment (Table 2). There was significant interaction of between sex and VLDL triglyceride quintiles in predicting cardiovascular mortality (p = 0.011). Performing stratified analysis, VLDL triglycerides were associated with cardiovascular mortality in females but not in males (Table 3).
Genome wide association study for LDL triglycerides in the LURIC study
Genome wide association analysis revealed a strong association of LDL triglycerides with variation in the gene locus encoding hepatic triglyceride lipase (p < 10−15) (Fig. 2A). There were 2 loci in the promoter region of the hepatic lipase gene (Fig. 2B) and in a transcript named LOC101928635 nearby the hepatic lipase gene (Fig. 2C) on chromosome 15 with the lead SNPs rs2070895 and rs10468017, respectively. Rs2070895 was in strong linkage disequilibrium with rs1800588 (R = 0.979; p < 0.001), another SNP located in the promoter region of the hepatic lipase gene (Supplementary table 10). Due to availability of rs1800588 in the HERITAGE Family study, subsequent analyses were performed for this SNP (18). All SNPs were in Hardy-Weinberg equilibrium (p > 0.4). In the entire LURIC study, the T alleles in rs1800588 (Supplementary table 11) and rs10468017 (Supplementary table 12) were associated with increased LDL triglycerides. Similar associations were observed when we separately analyzed males and females (Supplementary table 11 and 12). Together, the 2 SNPs explained 4.5 % of the variance of LDL triglycerides.
Figure 2. Genetic associations of LDL triglycerides.
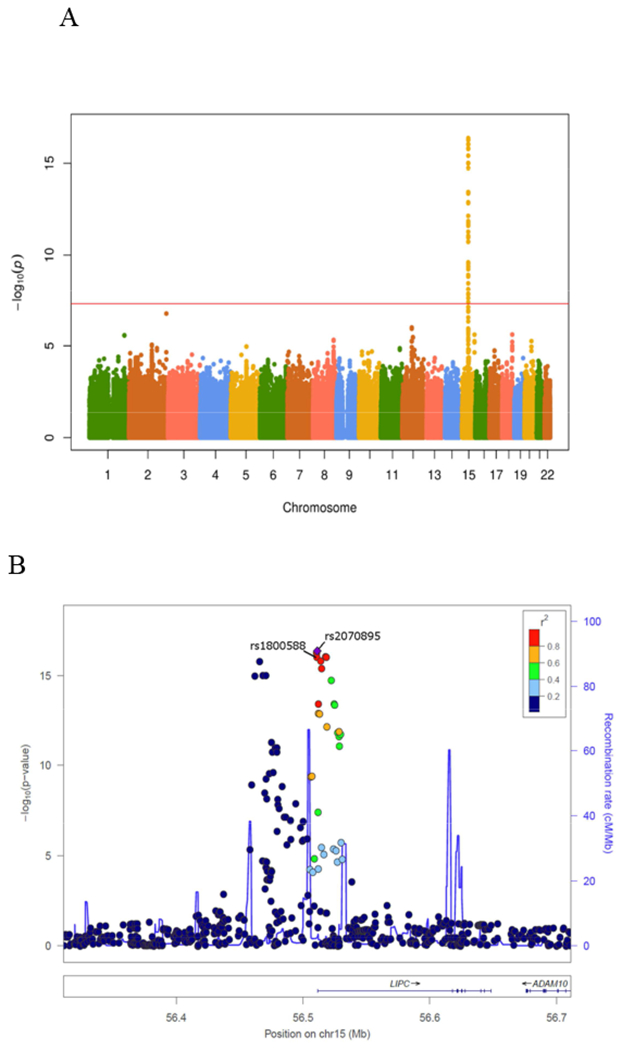
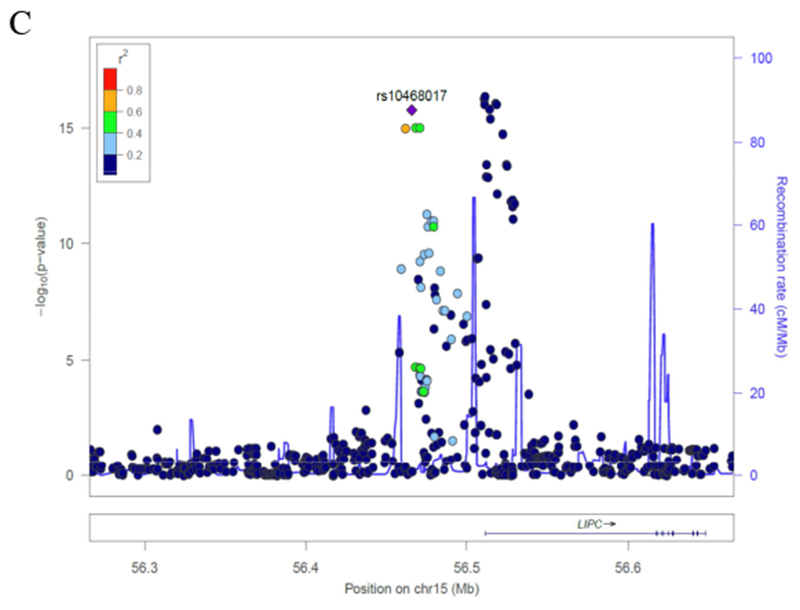
(A) Manhattan plot. (B) Genetic region of rs1800588 on chromosome 15. (C) Genetic region of rs10468017 on chromosome 15
Mendelian randomization analysis
In the entire LURIC study, the T alleles in rs1800588 (Supplementary table 11) and rs10468017 (Supplementary table 12) were associated with increased LDL triglycerides (beta = 2.997 and 2.733, standard error = 0.375 and 0.337, respectively). Similar associations were observed when we separately analyzed males and females (Supplementary table 11 and 12). Together, the 2 SNPs explained 4.5 % of the variance of LDL triglycerides.
In the entire HERITAGE Family study (Supplementary table 13), mean (standard deviation) hepatic lipase activity was higher in males than in females (243 [62] vs. 175 [62] nmol/min/ml, p < 0.001). The T alleles in rs1800588 and rs10468017 were associated with decreased hepatic lipase activity (beta = −29.325 and −27.902, standard error = 4.710 and 5.491, respectively). Mean (standard deviation) hepatic lipase activity was 221 (66) nmol/min/ml in the 307 rs1800588 CC homozygotes (253 [60] and 191 [58] in males and females, respectively), 190 (73) in the 150 heterozygotes (229 [64] and 149 [59] in males and females, respectively), and 169 (72) in the 21 TT homozygotes (217 [57] and 116 [45] in males and females, respectively) (p = 0.001) (18). Mean (standard deviation) hepatic lipase activity was 226 (71) nmol/min/ml in the 238 rs10468017 CC homozygotes (265 [59] and 192 [64] in males and females, respectively), 197 (67) in the 192 heterozygotes (230 [60] and 161 [55] in males and females, respectively), and 172 (56) in the 48 TT homozygotes (203 [47] and 128 [35] in males and females, respectively) (p = 0.001). Together, the 2 SNPs explained 13.8 % of the variance of hepatic lipase activity.
The T alleles in rs1800588 and rs10468017 were associated with increased prevalence of coronary artery disease and myocardial infarction in the CARDIoGRAMplusC4D consortium (beta = 0.038 and 0.015, standard error = 0.011 and 0.011, respectively).
The two-sample Mendelian randomization analysis using rs1800588 and rs10468017 as instrumental variable revealed that low hepatic lipase activity was causally related to higher risk of coronary artery disease and myocardial infarction (p = 0.013). The odds ratio for the risk of coronary artery disease and myocardial infarction per 1 nmol/min/ml decrease of hepatic lipase activity was 1.01. Two-sample Mendelian randomization using rs1800588 and rs10468017 as instrumental variable also indicated that high LDL triglycerides were associated with increased cardiovascular risk (p = 0.008).
Discussion
The present analysis in the LURIC study showed that LDL triglycerides associated positively with cardiovascular mortality, both in males and females. This finding is consistent with our previous cross-sectional analysis showing a higher prevalence of coronary artery disease in participants with elevated LDL triglycerides (12). The association between LDL triglycerides and cardiovascular mortality remained highly significant even after adjustment for LDL cholesterol, which could be anticipated as a relevant confounder. The association between LDL triglycerides and cardiovascular mortality also remained significant after adjustment for other potential confounders such as body mass index, hypertension, diabetes mellitus, HDL cholesterol, smoking, and use of lipid lowering drugs. Addition of LDL triglycerides to the multivariate model significantly improved net reclassification for the risk of cardiovascular death. There was also a modest association of high HDL triglycerides with increased cardiovascular mortality. The associations of total and VLDL triglycerides with cardiovascular mortality were weak with modest trends towards a U-shape. Notably, there was significant interaction between sex and total and VLDL triglyceride quintiles with regard to cardiovascular mortality. Total and VLDL triglycerides were associated with cardiovascular mortality in females only. This is in agreement with findings obtained from the Emerging Risk Factors Collaboration (3) and a Danish study (23). The sex-specific difference may be due to confounding by alcohol use in males but remains to be insufficiently explained (23).
To investigate the pathophysiology of the robust association between LDL triglycerides and cardiovascular mortality more in detail, we performed a genome wide association analyses for LDL triglycerides in the LURIC study. One locus in the promoter region of the hepatic lipase gene and another locus in close relation to the hepatic lipase gene were most strongly associated with LDL triglycerides. Rs2070895 and rs10468017 proved to be the respective lead SNPs. Rs2070895 was in strong linkage disequilibrium with rs1800588 which was used for subsequent analyses. Carriers of rs1800588 and rs10468017 alleles causing low hepatic lipase activity had high LDL triglycerides. This raises the possibility that low hepatic lipase activity might link high LDL triglycerides to increased cardiovascular risk. Indeed, low hepatic lipase activity has previously been related to a higher extent of coronary atherosclerosis in a cohort of 200 patients undergoing elective coronary angiography. In addition, lower hepatic lipase activity was found in patients with coronary artery disease compared to healthy controls. Low hepatic lipase activity was also associated with a higher burden of calcified lesions determined with spiral computed tomography in patients with heterozygous familial hypercholesterolemia (24). Lower hepatic lipase activity, potentially due to downregulation by estrogens and upregulation by testosterone (25), may account for higher LDL triglycerides in females than males. However, there was no interaction between LDL triglyceride quintiles and sex with regard to cardiovascular mortality. Moreover, stratified analyses showed concordant associations of LDL triglycerides with cardiovascular mortality in males and females.
The present two-sample Mendelian randomization analysis with exposure data obtained from the HERITAGE Family study and outcome data from CARDIoGRAMplusC4D consortium adds further support to the hypothesis that low hepatic lipase activity might represent a causal cardiovascular risk factor. This is in agreement with results from The Copenhagen City Heart Study (26).
However, a direct role of LDL triglycerides in the pathogenesis of atherosclerosis cannot be ruled out considering that triglycerides have been detected in the atherosclerotic plaque, be it to a much lesser extent than cholesterol (27). Considering their positive correlation with inflammatory parameters, that was not apparent for HDL and VLDL triglycerides, LDL triglycerides may also cause low grade systemic inflammation, which has been implicated in the pathophysiology of atherosclerosis (28). Vice versa, high LDL triglycerides may be a marker of systemic inflammation, which may mediate atherogenic effects via a reduction of hepatic lipase activity (29). Our Mendelian randomization data for LDL triglycerides add at least support that LDL triglycerides are on a pathway which may be causally related to cardiovascular disease.
This study provides information on the relationships between triglycerides in lipoprotein classes and cardiovascular mortality in a large cohort with a complete follow-up in all 3,140 participants. In addition, genome wide association data for LDL triglycerides are presented. Moreover, the study includes a two-sample Mendelian randomization analysis with data from the HERITAGE Family study, one of the very few studies with measurements of post-heparin hepatic lipase activity, and CARDIoGRAMplusC4D, the largest genetic consortium addressing the genetic basis of cardiovascular disease. This enabled us to address a possible pathophysiologic mechanism which might explain the association of LDL triglycerides with cardiovascular risk. Of relevance, rs1800588 and rsl0468017 explained 13.8 % of the variance in hepatic lipase activity. This is a higher degree of explained variance compared with previous Mendelian randomization studies on major lipids (30). The degree of variance explained by rs1800588 and rs10468017 is also higher for hepatic lipase activity than for LDL triglycerides.
It is a limitation of this study that the laboratory measurements in the LURIC cohort were performed at baseline only. Consequently, we were not able to evaluate possible changes of triglycerides in lipoprotein classes during the follow-up. In addition, the LDL fraction includes particles with densities of 1.006 through 1.063 kg/l and thus comprises IDL (1006 to 1.019 kg/l). Another limitation is that the LURIC cohort represents a selected patient group with clinical indications for coronary angiography. Therefore, studies investigating the relationships of triglycerides in lipoprotein classes with outcomes in other patient groups are encouraged. It is also a limitation of this work that the participants of the HERITAGE Family study are partly related. This may have impacted upon the data. However, the associations of rs1800588 and rs10468017 with hepatic lipase activity appeared robust when only unrelated participants of the HERITAGE Family study were analyzed. Finally, rs1800588 has previously been related to cardiovascular risk (26). However, we present findings from CARDIoGRAMplusC4D consortium, where this SNP initially did not show up because it did not reach the p-value threshold for overall genome-wide significance. Hence, our observations markedly increase the evidence that hepatic lipase activity may be related to cardiovascular disease.
In conclusion, there is a significant positive association of LDL triglycerides with cardiovascular risk. This association may be accounted for by atherogenic effects of low hepatic lipase activity. Increasing hepatic lipase activity may be instrumental to reduce cardiovascular risk.
Supplementary Material
Acknowledgments
Financial support
The LURIC study was supported by the 7th Framework Program (integrated projects AtheroRemo, Grant Agreement number 201668 and RiskyCAD, Project Number 305739) of the European Union, by the German Federal Ministry of Education and Research (project e:AtheroSysMed [Systems medicine of coronary heart disease and stroke], grant number 01ZX1313A-K). The work was also supported as part of the Competence Cluster of Nutrition and Cardiovascular Health (nutriCARD) which is funded by the German Federal Ministry of Education and Research (grant number 01EA1411A). The HERITAGE Family Study was funded by the National Institutes of Health (grant numbers HL-45670, HL-47323, HL-47317, HL-47327, HL-47321). C. Bouchard is partially supported by the John W. Barton Sr Chair in Genetics and Nutrition.
Abbreviations:
- HDL
high density lipoprotein
- HERITAGE
Health, Risk Factors, Exercise Training, and Genetics
- IDL
intermediate density lipoprotein
- LDL
low density lipoprotein
- LURIC
Ludwigshafen Risk and Cardiovascular Health
- SNP
single nucleotide polymorphism
- VLDL
very low density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Dr. Silbernagel reports grants and personal fees from Sanofi, grants and non-financial support from Amgen, grants from Numares, and non-financial support from Bayer, outside the submitted work. Dr. Landmesser reports personal fees from Sanofi, personal fees from Amgen, personal fees from Medicines Company, personal fees from Bayer, personal fees from Novartis, personal fees from Abbott, outside the submitted work. Dr. Laaksonen reports other from Zora Biosciences, outside the submitted work. Dr. März reports other from Synlab Services GmbH, other from Synlab Holding GmbH, grants and personal fees from Siemens Diagnostics, grants and personal fees from Aegerion Pharmaceuticals, grants and personal fees from Amgen, grants and personal fees from Astrazeneca, grants and personal fees from Danone Research, grants and personal fees from Sanofi, personal fees from Hoffmann LaRoche, personal fees from MSD, grants and personal fees from Pfizer, personal fees from Sanofi, personal fees from Synageva, grants and personal fees from BASF, grants from Abbott Diagnostics, grants and personal fees from Numares, outside the submitted work. Dr. Schunkert, Dr. Scharnagl, Dr. Kleber, Mrs. Delgado, Dr. Stojakovic, Dr. Erdmann, Dr. Rankinen, Dr. Bouchard, and Dr. Grammer have nothing to disclose.
References
- 1.Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H, Olsson AG. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65:2267–75. [DOI] [PubMed] [Google Scholar]
- 2.Colantonio LD, Bittner V, Reynolds K, Levitan EB, Rosenson RS, Banach M, Kent ST, Derose SF, Zhou H, Safford MM, Muntner P. Association of Serum Lipids and Coronary Heart Disease in Contemporary Observational Studies. Circulation. 2016;133:256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists’ (CTT) Collaboration., Fulcher J, O’Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–405. [DOI] [PubMed] [Google Scholar]
- 5.Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration, Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, Ouwehand W, Watkins H, Samani NJ, Saleheen D, Lawlor D, Reilly MP, Hingorani AD, Talmud PJ, Danesh J. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA. 2016;316:1289–97. [DOI] [PubMed] [Google Scholar]
- 7.Martin SS, Blaha MJ. Genetically low triglycerides and mortality: further support for “the earlier the better”? Clin Chem. 2014;60:705–7. [DOI] [PubMed] [Google Scholar]
- 8.Catapano AL, Graham I, De Backer G, et al. ; Authors/Task Force Members.; Additional Contributor. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 9.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934 [DOI] [PubMed] [Google Scholar]
- 10.Keech A, Simes RJ, Barter P, et al. ; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61. [DOI] [PubMed] [Google Scholar]
- 11.ACCORD Study Group., Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.März W, Scharnagl H, Winkler K, Tiran A, Nauck M, Boehm BO, Winkelmann BR. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health study. Circulation. 2004;110:3068–74. [DOI] [PubMed] [Google Scholar]
- 13.Winkelmann BR, März W, Boehm BO, Zotz R, Hager J, Hellstern P, Senges J; LURIC Study Group (LUdwigshafen Risk and Cardiovascular Health). Rationale and design of the LURIC study--a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics. 2001;2(1 Suppl 1):S1–73. [DOI] [PubMed] [Google Scholar]
- 14.Silbernagel G, Schottker B, Appelbaum S, et al. High-density lipoprotein cholesterol, coronary artery disease, and cardiovascular mortality. Eur Heart J. 2013;34:3563–71. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J. The HERITAGE family study. Aims, design, and measurement protocol. Med Sci Sports Exerc. 1995;27:721–9. [PubMed] [Google Scholar]
- 16.St-Amand J, Després JP, Lemieux S, Lamarche B, Moorjani S, Prud’homme D, Bouchard C, Lupien PJ. Does lipoprotein or hepatic lipase activity explain the protective lipoprotein profile of premenopausal women? Metabolism. 1995;44:491–8. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teran-Garcia M, Santoro N, Rankinen T, Bergeron J, Rice T, Leon AS, Rao DC, Skinner JS, Bergman RN, Després JP, Bouchard C; HERITAGE Family Study. Hepatic lipase gene variant −514C>T is associated with lipoprotein and insulin sensitivity response to regular exercise: the HERITAGE Family Study. Diabetes .2005;54:2251–5. [DOI] [PubMed] [Google Scholar]
- 19.Nikpay M, Goel A, Won HH, et al. ; CARDIoGRAMplusC4D Consortium. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39 Suppl 1:S13–22. [DOI] [PubMed] [Google Scholar]
- 21.Aulchenko YS, Struchalin MV, van Duijn CM. Probabel package for genome-wide association analysis of imputed data. BMC bioinformatics. 2010; 11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemani G, Zheng J, Elsworth B, Wade KH, Baird D, Haberland V, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Smith G Davey, Gaunt TR, Haycock PC, The MR-Base Collaboration.The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 24.Dugi KA, Brandauer K, Schmidt N, Nau B, Schneider JG, Mentz S, Keiper T, Schaefer JR, Meissner C, Kather H, Bahner ML, Fiehn W, Kreuzer J. Low hepatic lipase activity is a novel risk factor for coronary artery disease. Circulation. 2001;104:3057–62. [DOI] [PubMed] [Google Scholar]
- 25.Desmeules A, Couillard C, Tchernof A, Bergeron J, Rankinen T, Leon AS, Rao DC, Skinner JS, Wilmore JH, Despres JP, Bouchard C. Post-heparin lipolytic enzyme activities, sex hormones and sex hormone-binding globulin (SHBG) in men and women: The HERITAGE Family Study. Atherosclerosis. 2003;171:343–50. [DOI] [PubMed] [Google Scholar]
- 26.Andersen RV, Wittrup HH, Tybjaerg-Hansen A, Steffensen R, Schnohr P, Nordestgaard BG. Hepatic lipase mutations,elevated high-density lipoprotein cholesterol, and increased risk of ischemic heart disease: the Copenhagen City Heart Study. J Am Coll Cardiol. 2003;41:1972–82. [DOI] [PubMed] [Google Scholar]
- 27.Felton CV, Crook D, Davies MJ, Oliver MF. Relation of plaque lipid composition and morphology to the stability of human aortic plaques. Arterioscler Thromb Vasc Biol. 1997;17:1337–45. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43 [DOI] [PubMed] [Google Scholar]
- 29.Feingold KR, Memon RA, Moser AH, Shigenaga JK, Grunfeld C. Endotoxin and interleukin-1 decrease hepatic lipase mRNA levels. Atherosclerosis. 1999;142:379–87. [DOI] [PubMed] [Google Scholar]
- 30.White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, Sattar N, Humphries SE, Hingorani AD, Holmes MV. Association of Lipid Fractions With Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol. 2016. September 1;1(6):692–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


