Abstract
Rationale
Whereas co-use of alcohol and marijuana is prevalent in adolescents, the effects of such drug co-exposure on ingestive and cognitive behaviors remain largely unexplored. We hypothesized that co-exposure to alcohol and Δ9-tetrahydrocannabinol (THC), the main psychoactive constitute of marijuana, alters feeding behavior and cognition differently from either drug alone.
Methods
Male rats received daily THC (3–20 mg/kg/day) or oil vehicle through subcutaneous injection or consumption of a cookie with access to saccharin or saccharin-sweetened alcohol during adolescence (P30–45). Barnes maze and sucrose preference tests were applied to assess spatial memory and behavioral flexibility and abstinence-related anhedonia, respectively.
Results
Subcutaneous THC did not affect alcohol intake, but dose-dependently increased acute (3-h) chow intake and reduced weight gain. Moderate alcohol consumption reduced the acute hyperphagic effect of subcutaneous THC. By contrast, oral THC at a dose > 5 mg/kg robustly reduced alcohol intake without affecting 3-h chow intake. At this dose, some rats stopped consuming the THC-laced cookies. Furthermore, oral THC reduced weight gain, and co-exposure to alcohol alleviated this effect. Chronic subcutaneous, but not oral, THC reduced sucrose intake during abstinence. Neither treatment impaired cognitive behaviors in the Barnes maze.
Conclusion
Moderate alcohol and THC consumption can interact to elicit unique outcomes on ingestive behaviors and energy balance. Importantly, this study established a novel model of voluntary alcohol and THC consumption for studying mechanisms underlying the consequences of adolescent onset co-use of the two drugs.
Keywords: Alcohol, Δ9-tetrahydrocannabinol, Adolescence, Polydrug use, Ingestive behavior, Cognitive function, Barnes maze, Sucrose preference
Introduction
Alcohol and marijuana/hashish are among the most widely used drugs. The 2016 National Survey on Drug Use and Health estimates that 81% and 44% of individuals aged 12 or older in the United States have used alcohol or marijuana/hashish at least once in their lifetime, respectively (SAMHSA 2017). The ease of access to alcohol and the growing popularity and decriminalization of marijuana increase the likelihood for both drugs to be used in combination (Mechoulam and Parker 2003). Despite this increasing trend, how alcohol and marijuana co-use uniquely affects ingestive and cognitive behaviors in adolescents and young adults remain unclear.
In the human and animal literature, reports on the effects of alcohol on appetite and cognition are mixed (Dry et al. 2012; Spear 2018; Yeomans 2010). We previously showed that the route of alcohol administration (oral vs. parenteral) and the time course of blood ethanol concentration (BEC) dictate the effects of alcohol on food intake in rats (Nelson et al. 2016). Rats that drank moderate amounts of alcohol aptly reduced their food intake to maintain their daily caloric intake and weight gain at the level of controls. Furthermore, most rodent studies that reported memory impairments administered a dose of alcohol that rapidly elevates BEC above the defined intoxication threshold of 80 mg/dl (Coleman et al. 2014; Kuzmin et al. 2012; Pascual et al. 2007). By contrast, we recently showed that chronic moderate alcohol consumption from adolescence to young adulthood did not alter object recognition memory in rats (Nelson et al. 2017). How the effects of moderate alcohol consumption on food intake and cognitive behavior will be modified by simultaneous marijuana use is yet to be explored.
Δ9-tetahydrocannabinoid (THC) is one of over 70 known phytocannabinoids derived from the marijuana plant (Cannabis sativa). The psychoactive effects of marijuana are mediated mainly by the actions of THC within the brain endocannabinoid system (Kirkham et al. 2002; Manwell et al. 2014b). THC activates cannabinoid receptor 1 (CB1R) in the central nervous system and in select peripheral tissues (Di Marzo and Matias 2005; Merroun et al. 2009) to modulate appetite, cognition, and mood (Di Marzo and Matias 2005; Earleywine 2002). In healthy human volunteers, smoked marijuana increased consumption of palatable food (Foltin et al. 1988). In rats, exogenous cannabinoids (THC, WIN 55,212–2, and CP 55,940) dose-dependently increased not only food intake (Merroun et al. 2009; Williams and Kirkham 2002; Williams et al. 1998), but also operant responding for sucrose and alcoholic beverages (Gallate et al. 1999; Malinen and Hyytia 2008). Other rodent studies, however, concluded that CB1R agonists either decreases or have no effect on food intake (Drews et al. 2005; Graceffo and Robinson 1998; Rubino et al. 2008). These observations underscore the need for more research aimed at dissecting the factors that predict the effects of THC on ingestive behaviors. To our knowledge, the potential for the route of THC administration to dictate the outcome of its interaction with popular appetite-altering compounds (e.g., alcohol) has not been explored.
The effects of THC on cognitive functions are equivocal. The smoking of a 20 mg/kg THC cigarette impaired performance in tests of attention and reaction time (Leirer et al. 1991) whereas medicinal marijuana use is associated with modest improvement in some aspects of executive function (Gruber et al. 2016). Notwithstanding, establishing cause-effect relationship between marijuana use and cognitive deficits in humans can be difficult (Earleywine 2002). Experiments with rodents have also documented cognitive impairment (Han et al. 2012; Murphy et al. 2017), lack of impairment (Cha et al. 2007), and even improved spatial memory (Bilkei-Gorzo et al. 2017) following THC exposure. These reports reinforce the idea that, amongst others, the effects of THC may depend on subtle experimental paradigms like the THC dosage, duration and age of exposure, sensitivity of cognitive tasks employed, and timing of drug exposure relative to behavioral testing.
The purpose of this study is to establish a rodent model of alcohol and cannabinoid co-administration to explore how the route of THC administration (parenteral vs. oral) interacts with the effects of moderate alcohol to modify ingestive and cognitive behaviors. We hypothesized that moderate alcohol consumption would suppress acute chow intake with no effect on daily total caloric intake. Regardless of administration route, THC would dose-dependently increase acute and daily caloric intake, and alcohol would lessen the hyperphagic effect of THC. We further predicted that memory impairments and abstinence-related anhedonia will be severe in rats exposed to combinations of alcohol and THC compared with those only exposed to either drug alone.
Materials and methods
Animal husbandry
Preadolescent male Long-Evans rats weighing 35–50 g (Envigo, Indianapolis, IN, USA) were semi-pair housed in large polyethylene tubs with transparent Plexiglas cage dividers that contained 6 spaced-out holes (~ 4 mm radius) that permitted visual, tactile, and olfactory interactions between cage pairs. This arrangement enabled us to track individual rats’ food and fluid intake. All animals arrived at postnatal day 22 (P22) except those used for Experiment 2a which arrived at P25. A total of 148 rats were used – 40 each in Experiments 1a and 1b, and 32 and 36 in Experiments 2a and 2b, respectively. Colony was maintained in a temperature and humidity-controlled vivarium on a 12-h:12-h light/dark cycle (lights on at 11:30 AM). Rats had ad libitum access to tap water and a standard rodent chow (3.1 kcal/g; 75.2% carbohydrate, 18.6% protein, and 6.2% fat from soybean oil; 2018 Teklad global rodent diet, Indianapolis, IN, USA) and varying accesses to fluids as described in the Procedures below. Daily animal handling and care occurred at 8:00 AM when body weight, food, and water intakes were recorded. All experiments were approved by IACUC at the University of Illinois at Urbana-Champaign, and they conformed to the guidelines stipulated in the Guide for the care and use of laboratory animals by the National Research Council.
Drug
The contents of 10 mg dronabinol capsules, a commercially-available synthetic Δ9-tetrahydrocannabinol (Actavis Pharm, Inc.; Parsippany, NJ, USA), were suspended in sesame oil vehicle (Fisher Scientific) for the 3, 5, and 10 mg/kg subcutaneous (s.c.) injections. In Experiment 1, the 3 mg/kg THC doses were administered in a volume of 3 ml/kg body weight, while both the 5 and 10 mg/kg doses were administered at a volume of 1.5 ml/kg body weight. In Experiment 2, dronabinol to be overlain on cookies (Goldfish Grahams Fudge Brownie, Pepperidge Farm; Norwalk, CT, USA) was diluted with sesame oil for the 1.5, 3, 5, and 10 mg/kg doses. The 1.5 and 3 mg/kg THC doses were applied onto cookie at a volume of 0.383 ml/kg body weight; the 5 and 10 mg/kg doses were respectively applied at 0.639 and 0.444 ml/kg body weight. Oil vehicle was administered isovolumetrically to the different THC doses.
Test fluids: saccharin and alcohol
Saccharin and sweetened alcohol solutions were prepared and presented to the rats as described in the Supplemental Information.
Experimental designs
Experiment 1: Chronic moderate alcohol drinking and subcutaneous THC administration, alone or in combination
Experiment 1a: Spatial learning after chronic alcohol and THC exposure
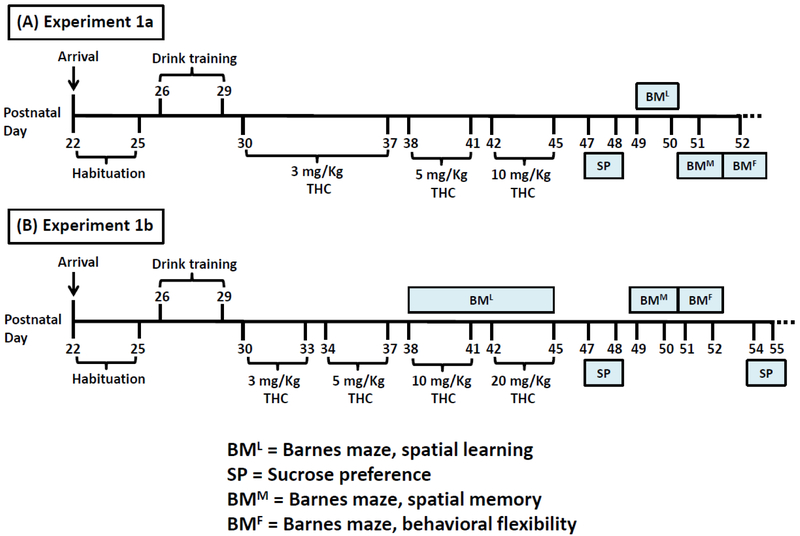
Forty pair-housed rats were habituated to the vivarium for 4 days. At 8:00 AM on the fourth habituation day, a cage divider was inserted in the center of each cage to enable separate tracking of individual animals’ intake. Over the next four days (P26–29), the animals were entrained to consume 0.1% saccharin along with chow during the last 3-h of the dark cycle (8:30–11:30 AM). After 11:30 AM measurements, saccharin bottles were replaced with water bottles. Rats were assigned to one of four groups (n = 10/group) by matching average body weight and saccharin intake during entrainment: control (CTL) given saccharin and oil injection, ethanol (EtOH) given 10% ethanol and oil injection, THC given saccharin and THC injection, and combination (COM) given 10% ethanol and THC injection. At 8:30 AM from P30–45, animals received daily subcutaneous THC or oil injections immediately before test fluid access in their home cages. Chow and test fluid intakes were measured at 11:30 AM (3-h intakes) when test fluids were supplanted by tap water. Drug and test fluid exposures spanned 16 days (P30–45) that overlapped with the onset of adolescence. The 3 mg/kg dose lasted for 8 days, while 5 and 10 mg/kg doses each lasted for 4 days (Rubino et al. 2009; Rubino et al. 2008). From P32, preputial separation – the complete manual retraction of the prepuce from the glans penis of male rats – was assessed during 11:30 AM measurements to determine how drug exposure would affect puberty onset (Korenbrot et al. 1977). One rat in the EtOH group was discovered dead at 8:00 AM on P42. Another EtOH rat was accidentally injected with 10 mg/kg THC on P43. The subsequent data from this rat was excluded from analysis. Except during the behavioral tests, chow and water were freely available for one week after the last drug exposure day. The experimental timeline is summarized in Fig. 1A.
Fig. 1.
Timeline of chronic subcutaneous THC injection and voluntary alcohol drinking. (A) In Experiment 1a (EtOH, n = 8; CTL, THC, & COM, n = 10/group), 3 mg/kg/day THC lasted for 8 days, while the 5 and 10 mg/kg/day THC each lasted for 4 days. Sucrose preference (SP) and Barnes maze (BM) tests were performed as indicated. (B) In Experiment 1b (n = 10/group), the four THC doses (3, 5, 10, & 20 mg/kg/day) each spanned 4 days. BM & SP tests were performed as indicated. Spatial learning (BML) occurred during the last 8 days of drug treatment.
Blood ethanol concentration (BEC)
To determine how low-to-moderate THC doses would affect BEC in rats exposed to both drugs concurrently, we measured BEC at different timepoints. The procedures are detailed in the Supplemental Information.
Behavioral tests
Sucrose preference
During abstinence from THC and alcohol, sucrose preference test was used to assess intake of a mildly rewarding sucrose solution (Carlin et al. 2016; Nelson et al. 2017). On the test days (Fig. 1A), rats were presented with two bottles, 1% sucrose vs. tap water, immediately after 8:00 AM measurements. The positions of the bottles were alternated on each day to prevent the development of location preference for drinking. After the sucrose preference tests, all rats were returned to ad libitum water access. Sucrose preference (%) was calculated as .
Barnes maze
Rats were submitted to the Barnes maze (BM) test to examine the effects of periadolescent alcohol and THC exposure on spatial learning, memory, and cognitive flexibility. The BM has been used by other researchers to assess such behaviors following different experimental manipulations (Coleman et al. 2014; Kuzmin et al. 2012). Specifications of the apparatus and procedural details can be found in the Supplemental Information.
Experiment 1b: Spatial learning during alcohol and THC exposure
During the BM procedure in Experiment 1a, we noticed that drug exposure during adolescence had no effect on performance. We reasoned that the drug might interfere with BM performance if: (1) we increased the cumulative THC dose administered to the rats, (2) drug exposure overlapped with training in the maze, and (3) spatial memory and cognitive flexibility were assessed a few days after BM training. The 40 rats (n = 10/group) were housed under identical conditions as in Experiment 1a. Habituation and drug administration procedure were also consistent with that of Experiment 1a, except that the THC and COM groups in Experiment 1b received 3, 5, 10, and 20 mg/kg THC injections (s.c.), each dose lasting for 4 days for a total of 16 drug exposure days (Fig. 1B). Importantly, to minimize THC-induced locomotor depression (Manwell et al. 2014b; Sanudo-Pena et al. 2000), the 20 mg/kg dose was administered as two separate 10 mg/kg doses. The first was administered at 8:30 AM as per the usual protocol, while the second was given at 9:30 PM (i.e., 2-h before dark onset). Two 48-h sucrose preference tests were performed (Fig. 1B). Details of Barnes maze procedures in this experiment can be found in the Supplemental Information
Experiment 2: Chronic moderate alcohol drinking and oral THC consumption, alone or in combination
Experiment 2a: Spatial learning during voluntary alcohol and THC consumption
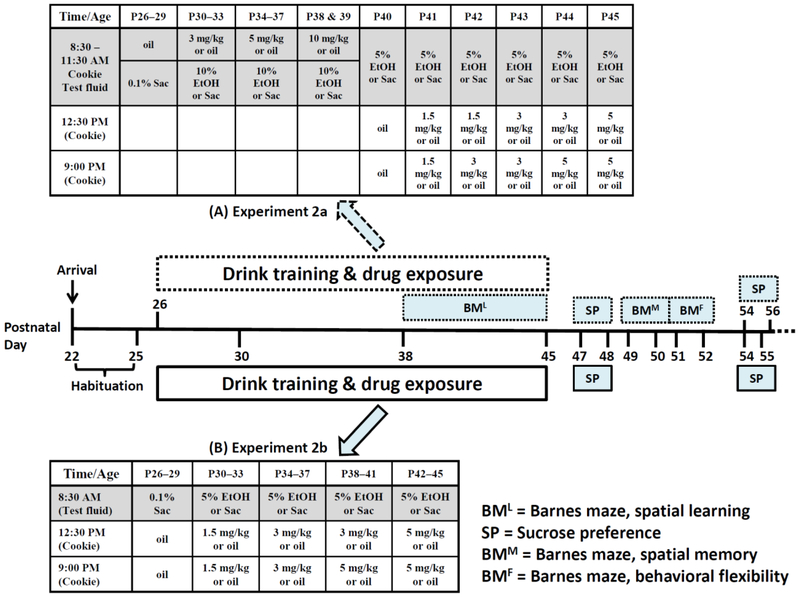
Although smoking or vaping is the preferred route of human THC administration, consumption of edibles laced with cannabis extracts is becoming popular (Grella et al. 2014; National Academies of Sciences 2017). THC-containing edible products like beverages (tea, coffee), confectionaries (cookie, brownies, candy, chocolate), and cooking condiments are available in states where recreational and medicinal marijuana are legal (Grella et al. 2014; Vandrey et al. 2015). To enhance the relevance of our model by mimicking this growing pattern of THC ingestion, we sought to administer it to the rats via cookies. The 32 rats (n = 8/group) were aged P25 at arrival. The drug treatment timeline employed here was consistent with that of Experiment 1b, except that drink training began on P26. The initial plan was to present rats with cookie overlaid with 3, 5, 10, and 20 mg/kg/day THC in their home cages. Each dose was to last for 4 days, and the 20 mg/kg/day THC was to be given as two 10 mg/kg doses. After the first 5 mg/kg/day THC consumption, THC and COM rats consumed less test fluids which became pronounced after the first two 10 mg/kg/day doses when food intake also reduced (see Results). Consequently, we modified our protocol to where lower THC doses were provided to the same rats twice a day: 1-h after the end of test fluid consumption, i.e., 1-h after light onset (12:30 PM), and 3-h before dark onset (9:00 PM). We also reduced the concentration of sweetened alcohol solution from 10% to 5% to mitigate any potential aversive effects caused by the interaction of THC (and its metabolites) and the higher alcohol concentration (Fig. 2A). The 5% alcohol solution was first introduced to the EtOH and COM rats on P40 when oil cookie was provided to all groups. Under this modified protocol (P41–45), rats were first presented with 3-h access to saccharin or 5% alcohol solution (8:30–11:30 AM). After chow and test fluid intakes were recorded, the first and second THC-laden cookies of the day were respectively presented at 12:30 and 9:00 PM. Barnes maze set-up and timeline were consistent with that of Experiment 1b. Except for days of sucrose preference tests (Fig. 2A), chow and water were freely available during a 2-week abstinent period.
Fig. 2.
Timeline of chronic oral THC consumption and voluntary alcohol drinking. Treatments indicated in the shaded part of the tables occurred during the last 3-h of the dark cycle. (A) Manipulations in Experiment 2a (CTL & EtOH, n = 8/group; THC_C, n = 5; THC_A, n = 3; COM_C & COM_A, n = 4) are indicated in the dotted boxes and top table. The “A” or “C” following the underscores denote “Avoiders” and “Consumers” of THC-laden cookie, respectively. The transition from paradigm 1 to 2 occurred on P40.. (B) Manipulations in Experiment 2b (CTL, n = 10; EtOH & THC, n = 8/group; COM, n = 7) are indicated in the solid boxes and bottom table. Barnes maze testing was not performed.
Experiment 2b: Testing the modified voluntary alcohol and THC consumption paradigm
Experiment 2b tested the modified drug administration paradigm developed in Experiment 2a in a new cohort of rats. After habituation to the animal facility, the 36 rats (CTL and COM, n = 10/group; EtOH and THC, n = 8/group) were entrained to the new drug exposure schedule. Immediately after daily care during the 4-day training period, rats had 3-h access to 0.1% saccharin solution and chow (last 3-h of the dark cycle, 8:30–11:30 AM). Following measurement of chow and saccharin consumption, the first and second oil-laden cookies were respectively provided at 12:30 PM and 9:00 PM. During the 16-day treatment period, rats first had 3-h access to saccharin or sweetened 5% alcohol solution followed by a cookie laced with oil or THC (1.5, 3, or 5 mg/kg) presented twice per day at 12:30 PM and 9:00 PM (Fig. 2B). Each cumulative daily dose of 3, 6, 8, and 10 mg/kg THC lasted for 4 days. To ascertain how oral THC consumption would affect BEC kinetics, we measured BEC at different timepoints (see Supplemental Information). Barnes maze test was not performed in Experiment 2b. Except for days of sucrose preference tests (Fig. 2B), rats had ab libitum access to chow and water for 3 weeks following the last drug exposure day.
Plasma levels of THC and its metabolites
In Experiments 1b and 2b, tail blood was collected shortly after light onset on the day after the last THC exposure (i.e., 14-h after). This timing for blood collection was chosen mainly because it coincided with a period when daily measurements were performed. The blood samples were centrifuged, and plasma was immediately stored at −80°C. Lipid components of the plasma samples were extracted, and levels of THC, 11-OH-THC, and THC-COOH were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as we have previously done (Arnold et al. 2018; Tiscione et al. 2016).
Statistical analyses
In Experiment 1, daily caloric intake in CTL and THC rats consisted of calories from chow (3.1 kcal/g), while EtOH and COM rats consumed calories from chow and alcohol (7 kcal/g). The loss of one rat and the mishap during THC injection in Experiment 1a reduced the number of EtOH rats to 8. In Experiment 2, daily caloric intake in CTL and THC rats included calories from chow and cookie (4.7 kcal/g), while EtOH and COM rats consumed calories from chow, cookie, and alcohol. The inconsistency with which rats consumed THC cookies prompted us to apply separation or exclusion criteria (see Experiment 2 results below). In Experiment 2a, the respective final sample sizes of the THC and COM cookie-consuming groups (THC_C & COM_C) were 5 and 4, and that of the cookie-avoiding groups (THC_A & COM_A) were 3 and 4. In Experiment 2b, the final sample size of the COM group was 7. The dose-response effects of THC on alcohol, saccharin, and caloric intakes were analyzed using the average value from the respective doses. These intake analyses were repeated with body weight-adjusted intakes, which were computed as intake per 100 g body weight of individual rat.
Alcohol, saccharin, and caloric intake during treatments were analyzed by one-way ANOVA (paradigm 2 of Experiment 2a) or by two-way repeated measures ANOVA with treatment as a between-subject factor and THC dose or day as the repeated measure. Likewise, Barnes maze data were analyzed by two-way repeated measures ANOVA with treatment as a between-subject factor and trial session as the repeated measure. Significant main effects and interactions (p < 0.05) were accompanied with Tukey’s HSD post hoc tests. The effects of specific THC dose on caloric intake, alcohol intake, and BEC were determined by one-way ANOVA, as was analysis of weight gain during treatment, and caloric intake and weight gain during abstinence. Sucrose preference and fluid intakes during the preference tests were also analyzed one-way ANOVA. Plasma levels of THC and its major metabolites were analyzed via Student’s t-test. Pearson correlation was used to assess the linear relationship between ethanol dose and the ensuing BEC. Data are presented as mean ± standard error of the mean (SEM). Analyses were conducted in Statistica 13.1 (TIBCO Software Inc., Palo Alto, CA, USA).
Results
Experiment 1: Chronic moderate alcohol drinking and subcutaneous THC administration, alone or in combination
Subcutaneous THC reduced saccharin intake, but had no effect on alcohol intake and BEC
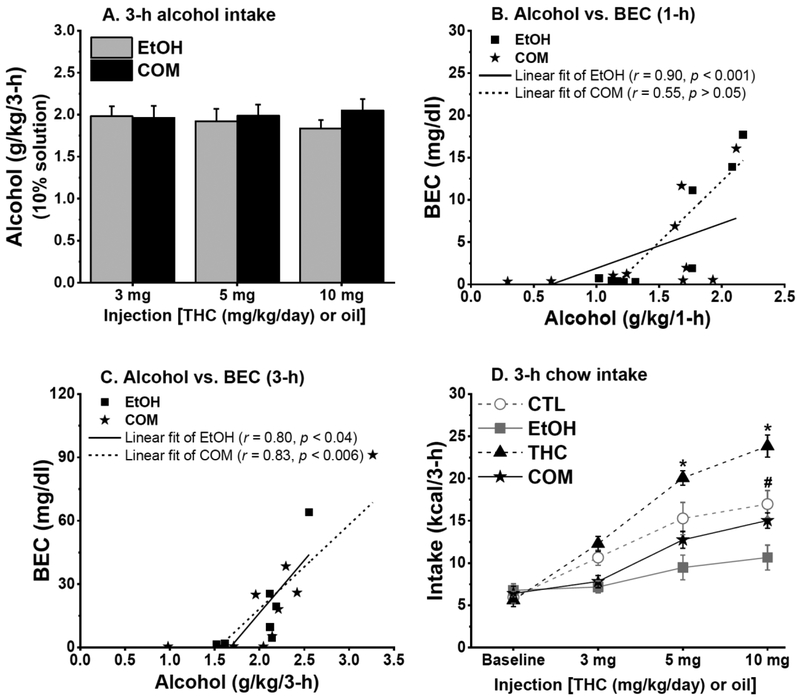
In Experiments 1a and 1b, THC and CTL groups increased their saccharin intake across treatment days [F(2,36) = 40.33 and F(3,54) = 21.24, respectively; both p < 0.0001]. Compared with the CTLs, subcutaneous THC reduced saccharin intake in Experiment 1b [F(1,18) = 7.83, p < 0.02], but not in 1a. The above result held true for body weight-adjusted saccharin intakes. Ethanol consumed by EtOH and COM rats did not differ at any of the administered THC dose (Fig. 3A & 5A). Subcutaneous THC had no effect on BEC, which mostly correlated with the ingested alcohol dose (Fig. 3B & 3C). More details are included in the Supplemental Information.
Fig. 3.
Chronic subcutaneous THC and voluntary alcohol consumption, alone or when combined, differently affected caloric intake and weight gain (Experiment 1a: EtOH, n = 8; CTL, THC, & COM, n = 10/group). (A) EtOH & COM rats consumed similar doses of sweetened 10% alcohol. (B) Blood ethanol concentration (BEC) correlated with 1-h alcohol intake in the EtOH group. (C) BEC correlated with 3-h alcohol intake in the EtOH group, and with 3-h alcohol intake in the COM group after subcutaneous 10 mg/kg THC. (D) THC injection dose-dependently stimulated 3-h chow intake. Alcohol consumption reduced 3-h chow intake and blunted the hyperphagic effect of THC. THC vs. EtOH & COM: *p < 0.004; CTL vs. EtOH & THC: #p < 0.03.
Subcutaneous THC stimulated short-term chow intake, but reduced weight gain
Subcutaneous THC dose-dependently increased while moderate alcohol consumption reduced 3-h chow intake in Experiments 1a [group and group × time, F(3,35) = 14.16 and F(6,70) = 5.47, respectively; both p < 0.0002; Fig. 3D] and 1b [group and group × time, F(3,36) = 11.46 and F(9,108) = 3.55, respectively; both p < 0.0007; Fig. 5B]. In both experiments, the reduced caloric intake by the EtOH group relative to the CTL group (post hoc, both p < 0.02) did not persist when alcohol calorie was included. The respective hyperphagic and hypophagic effect of THC and alcohol appeared to oppose each other as chow intake of the COM group did not differ from that of the CTLs in both experiments. The significant main effects in 3-h chow intake outlined above persisted when intake was adjusted to individual body weight.
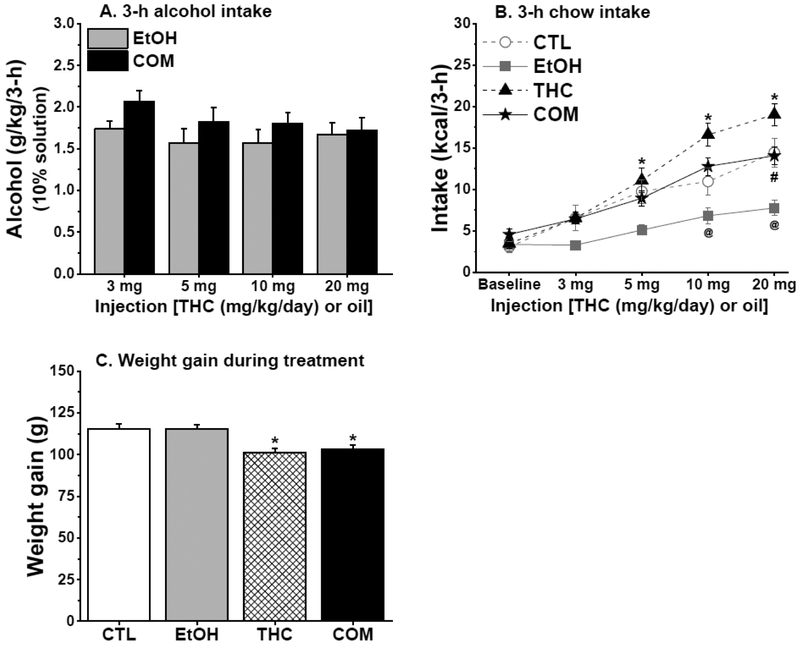
Fig. 5.
Chronic subcutaneous THC and voluntary alcohol consumption, alone or when combined, differently affected caloric intake and weight gain (Experiment 1b: n = 10/group). (A) EtOH & COM rats consumed similar doses of sweetened 10% alcohol solution. (B) THC injection dose-dependently increased 3-h chow intake only in the COM relative to the EtOH group. Alcohol consumption reduced 3-h chow intake, but THC averted the hypophagic effect of alcohol. THC vs. EtOH: *p < 0.05; EtOH vs. COM: @p < 0.05; CTL vs. EtOH: #p < 0.02. (C) Subcutaneous THC suppressed weight gain in the THC & COM groups. THC or COM vs. CTL or EtOH: *p < 0.02.
Regardless of body weight adjustment, daily caloric intake did not differ between groups. In parallel with the total daily caloric intake results of Experiment 1a, there were no group differences in weight gain during drug exposure. In Experiment 1b, however, THC and COM rats gained less weight than did CTL and EtOH rats [F(3,36) = 7.84, p < 0.0004; post hoc, all p < 0.02; Fig. 5C].
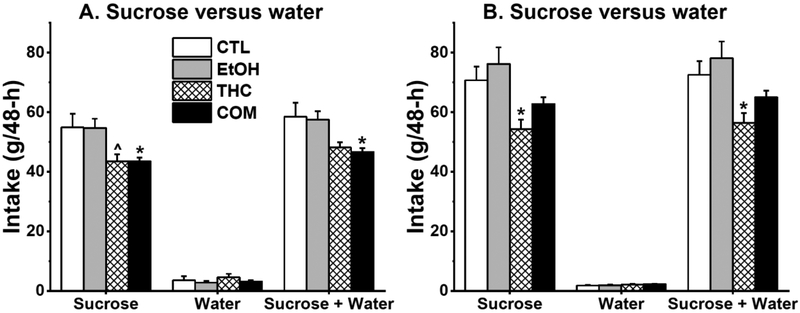
Subcutaneous THC reduced sucrose intake during abstinence
Abstinence from chronic subcutaneous THC and voluntary alcohol drinking had no effect on sucrose preference as all groups preferred 1% sucrose solution to plain water during the 48-h sucrose preference tests. In Experiment 1a, subcutaneous THC, but not moderate alcohol drinking, reduced sucrose consumption in the THC and COM groups compared with the CTL group [F(3,33) = 4.46, p < 0.01; post hoc, p = 0.058 and < 0.05, respectively; Fig. 4A]. THC and COM groups consumed less total fluid compared with the CTL group [F(3,33) = 4.25, p < 0.02; post hoc, p = 0.086 and < 0.04, respectively]. These effects persisted with body weight-adjusted analyses.
Fig. 4.
Chronic subcutaneous THC alone or when combined with voluntary alcohol consumption reduced sucrose and total fluid intake during 48-h sucrose preference tests. (A) Results from Experiment 1a (EtOH, n = 8; CTL, THC, & COM, n = 10/group). THC vs. CTL: ^p = 0.058; COM vs. CTL: *p < 0.05. (B) Results from the second test in Experiment 1b (n = 10/group). THC vs. CTL & EtOH: *p < 0.05.
In the first sucrose preference test of Experiment 1b (P47–48), THC rats consumed less sucrose and total fluid than did the CTL and EtOH rats [sucrose: F(3,36) = 8.37, p < 0.0003; post hoc, p < 0.02 and 0.0004, respectively; total fluid: F(3,36) = 10.21, p < 0.0001; post hoc, p < 0.02 and 0.0002, respectively; Supplemental Fig. S4]. In the second sucrose preference test (P54–55), THC rats also consumed less sucrose and total fluid compared with the CTL and EtOH groups [sucrose: F(3,36) = 5.37, p < 0.004; post hoc, p < 0.04 and 0.004, respectively; total fluid: F(3,36) = 5.18, p < 0.005; post hoc, p < 0.05 and 0.004, respectively; Fig. 4B]. All the significant main findings outlined above held true for body weight-adjusted sucrose and total fluid intakes.
Subcutaneous THC, moderate alcohol, and their combination had no effect on Barnes maze performance
A synopsis of the results can be found in the Supplemental Information (Fig. S2 & S3).
Experiment 2: Chronic moderate alcohol drinking and oral THC consumption, alone or in combination
Individual differences in voluntary THC consumption
Simultaneous cookie and test fluid exposure (paradigm 1): during the 16-day treatment period, all CTL and EtOH rats that received oil cookie consumed it within 1-h. Six of the eight THC rats and five of the eight COM rats that received cookie overlaid with 3 mg/kg, 5 mg/kg, and the first 10 mg/kg THC consumed it within 3-h. One THC and three COM rats utterly avoided the 10 mg/kg THC cookie.
Cookie exposure following test fluid access (paradigm 2 and Experiment 2b): when this schedule of drug exposure was implemented during the last five days of Experiment 2a (Fig. 2A), three THC and four COM rats sporadically had bits of leftover THC-laden cookies. Thus, we separated both groups into cookie consumers (THC_C, n = 5; COM_C, n = 4) and cookie avoiders (THC_A, n = 3; COM_A, n = 4). The “cookie avoiders” were rats with left-over cookies on two or more days. Throughout Experiment 2b (Fig. 2B), except for three COM rats that intermittently had bits of leftover cookies, all rats that received THC cookie consumed it before the next cookie was provided.
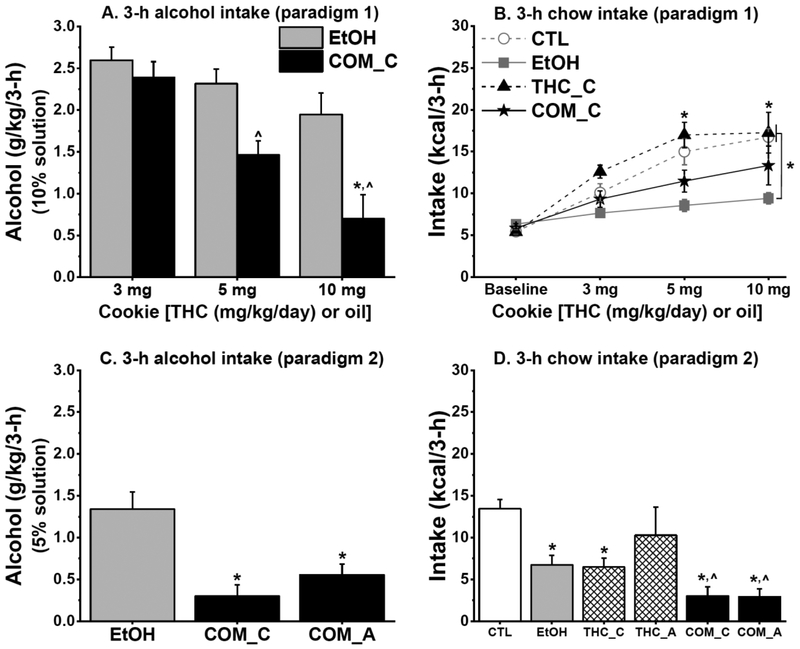
Oral THC dose-dependently reduced alcohol intake when alcohol was simultaneously consumed with THC-laden cookie
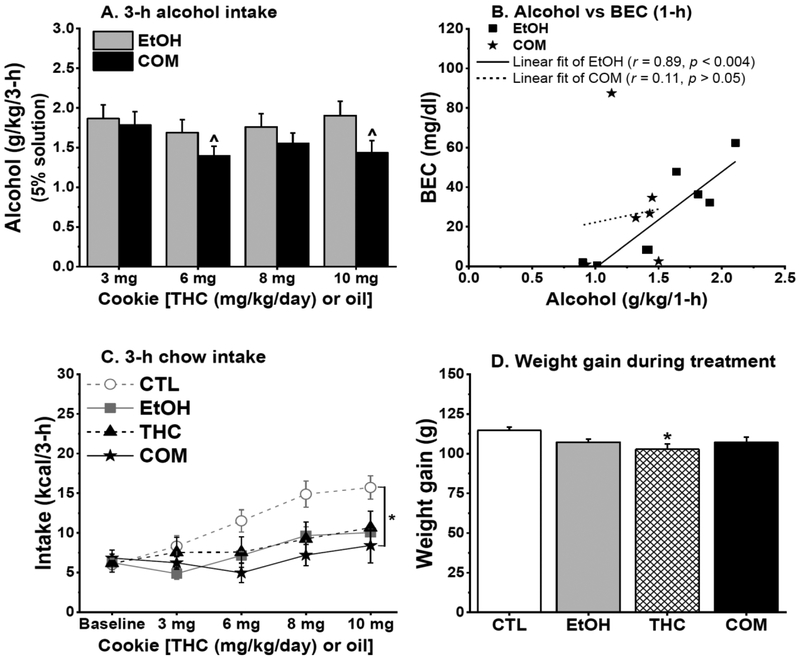
In Experiments 2a and 2b, THC and CTL rats increased their saccharin intake across treatment days [F(2,24) = 9.52 and F(3,48) = 42.51, respectively; both p < 0.001]. Weight-adjusted analyses of saccharin intake revealed no significant group differences, but THC rats tended to consume less saccharin in Experiment 2a [F(1,12) = 3.60, p = 0.08]. The effect of oral THC on alcohol consumption depended on the temporal order of their consumption. Under paradigm 1 of Experiment 2a when sweetened 10% alcohol solution and THC-laden cookies of increasing doses were simultaneously available, COM_C rats progressively reduced their alcohol consumption compared with the EtOH rats [group and group × time, F(1,11) = 8.33 and F(2,22) = 5.37, p < 0.02, respectively; both p < 0.02; Fig. 6A]. Throughout paradigm 2 of Experiment 2a when rats consumed sweetened 5% alcohol solution before THC-laden cookies, both COM_C and COM_A rats continued to consume less alcohol compared with the EtOH rats [F(2,103 = 8.00, p < 0.006; Fig 6C]. Although EtOH rats appeared to reduce their alcohol intake when the concentration of alcohol solution was reduced from 10% (cookie simultaneously available) to 5% (cookie not simultaneously available), the COM rats still consumed significantly less alcohol. When alcohol was presented prior to THC-laden cookies of increasing doses in Experiment 2b, the EtOH and COM rats consumed similar alcohol doses i.e., intake was not THC dose-dependent (Fig. 7A). Oral THC had no effect on BEC (Fig. 7B) and see the Supplemental Information for more details.
Fig. 6.
Chronic oral THC consumption reduced alcohol intake, while alcohol alone or when combined with oral THC reduced 3-h chow intake (Experiment 2a: CTL & EtOH, n = 8/group; THC_C, n = 5; THC_A, n = 3; COM_C & COM_A, n = 4). (A) In paradigm 1 (P30–39), COM_C rats consumed lower doses of sweetened 10% alcohol solution than did EtOH rats: *p < 0.009. COM_C rats also consumed reduced alcohol doses during 5 & 10 mg/kg/day THC consumption than they did during 3 mg/kg/day THC consumption: ^p < 0.03. (B) Moderate 10% alcohol consumption suppressed 3-h chow intake in EtOH rats. EtOH vs. CTL & THC_C: *p < 0.03. (C) When THC or oil cookie was consumed after sweetened 5% alcohol access in paradigm 2 (P40–45), COM_C & COM_A rats continued to consume less alcohol than the EtOH rats did. EtOH vs. COM_C & COM_A: *p < 0.05. (D) The EtOH, THC_C, COM_C, & COM_A groups consumed significantly lower 3-h chow compared with the CTLs. CTL vs. EtOH, THC_C, COM_C, & COM_A: *p < 0.008; THC_A vs. COM_C & COM_A: ^p = 0.055 & 0.050, respectively.
Fig. 7.
Chronic oral THC consumption mildly reduced alcohol intake and suppressed caloric intake and weight gain (Experiment 2b: CTL, n = 10; EtOH & THC, n = 8/group; COM, n = 7). (A) EtOH and COM rats consumed similar doses of 5% alcohol solution. COM rats consumed lower alcohol doses during 6 & 10 mg/kg/day THC consumption than during 3 mg/kg/day THC consumption: ^p < 0.008. (B) BEC correlated with 1-h alcohol intake in the EtOH group. (C) Drinking 5% alcohol reduced 3-h chow intake and augmented the somewhat hypophagic effect of oral THC. CTL vs. EtOH: p = 0.086; CTL vs. COM: *p < 0.03. (D) THC consumption suppressed weight gain in the THC group compared with the CTLs: *p < 0.02.
Oral THC, moderate alcohol, and their interaction reduced short-term chow intake
The temporal order of THC and alcohol consumption differently affected 3-h chow intake. In paradigm 1 of Experiment 2a, the EtOH group consumed less 3-h chow compared with the CTL and THC groups [F(3,23) = 8.93, p < 0.0005; post hoc, both p < 0.004; Fig 6B]. But in paradigm 2, EtOH, THC, and COM groups consumed less 3-h chow relative to the CTLs [F(5,26) = 9.72, p < 0.0001; post hoc, all p < 0.008; Fig. 6D]. In Experiment 2b, although THC-laden cookies were not available during the last 3-h of the dark cycle, COM and EtOH rats consumed less 3-h chow compared with the CTLs [F(3,29) = 3.64, p < 0.03; post hoc, p < 0.03 and = 0.086, respectively; Fig. 7C]. However, this treatment effect was only marginal (p = 0.089) with body weight-adjusted analyses.
In Experiment 2a, daily caloric intake and weight gain were unaffected during drug exposure and the 2-week abstinent period. In Experiment 2b, THC rats consumed fewer daily calories compared with the CTLs [F(3,29) = 3.71, p < 0.03; post hoc, p < 0.03], but this effect became insignificant when the intake data was adjusted by body weight. However, the THC rats gained less weight [F(3,29) = 3.77, p < 0.03; post hoc, p < 0.02; Fig. 7D] compared with the CTLs. Such group difference in weight gain did not persist into the third week of abstinence.
Oral THC, moderate alcohol, and their combination had no effect on sucrose intake during abstinence
A synopsis of the results can be found in the Supplemental Information (Fig. S5).
Oral THC, moderate alcohol, and their combination had no effect on Barnes maze performance
A synopsis of the results (Experiment 2a) can be found in the Supplemental Information.
THC, alcohol, or their combination did not alter age of puberty onset
Pre-pubertal drug treatment had no effect on the age of puberty onset in Experiments 1 and 2. The average age of puberty onset was 37 days (P34–41 among rats in all four groups) in Experiment 1, and 38 days (P33–42) in Experiment 2.
Subcutaneous and oral THC resulted in significant plasma cannabinoid levels
Significant plasma levels of THC and THC-COOH were detected 14-h after injection of 10 mg/kg (Experiment 1b) or access to a 5 mg/kg THC-laced cookie (Experiment 2b) in both THC and COM rats (Table 1). However, significant levels of 11-OH-THC were detected only in rats that received THC injection. Finally, 11-OH-THC levels were significantly lower in the COM group compared with the THC group [t (9) = 3.96, p < 0.01] when THC was injected subcutaneously.
Table 1.
Plasma levels of THC and its major metabolites in Experiments 1b and 2b
| Group╲Route | Subcutaneous THC injection | Oral THC consumption | ||||
|---|---|---|---|---|---|---|
| THC | 11-OH-THC | THC-COOH | THC | 11-OH-THC | THC-COOH | |
| THC | 16.9 (2.89) | 5.0 (0.66)* | 7.1 (1.02) | 1.3 (0.28) | nd | 1.8 (0.44) |
| COM | 15.1 (1.29) | 2.1 (0.36)* | 5.5 (1.79) | 8.6 (5.11) | nd | 3.1 (0.81) |
Data are mean (± SEM) concentrations (ng/ml) in each treatment group (n = 5–6).
significantly different from each other according to Student’s t-test (p < 0.01). nd: non-detectable.
Discussion
The present study is the first to examine the effects of adolescent alcohol and THC exposure, alone or when combined, on ingestive and cognitive behaviors in adolescent male rats. It revealed that the effects of co-exposure to the two drugs on ingestive behaviors depend on the route of THC administration. Consistent with our hypothesis, moderate alcohol suppressed acute chow intake and subcutaneous THC dose-dependently induced acute hyperphagia without affecting alcohol intake. By contrast, oral THC can alleviate the hypophagic effect of moderate alcohol, but doses higher than 5 mg/kg blunted alcohol intake. These drug administrations did not significantly change total daily caloric intake. However, higher cumulative doses of subcutaneous and oral THC suppressed weight gain. Inconsistent with our hypothesis, co-exposure to alcohol and subcutaneous or oral THC did not impair spatial learning and memory in a Barnes maze. Finally, chronic subcutaneous but not oral THC reduced intake of 1% sucrose solution during abstinence, with no significant interaction with alcohol.
Caloric intake
The route and schedule of THC administration dictated its dose-dependent effects on caloric intake in rats. Results of Experiment 1 indicate that rats compensated for the immediate hyperphagic effect of THC by reducing chow intake during the subsequent period of the day. The acute hyperphagic effects of subcutaneous THC injection concur with early studies with low dose (< 1 mg/kg) intraperitoneal THC injection in rats (Jarbe and DiPatrizio 2005). Other studies with THC doses > 1 mg/kg, however, reported no change or reduced food intake immediately following intraperitoneal THC injection (Drewnowski and Grinker 1978; Graceffo and Robinson 1998). Thus, compared with intraperitoneal injection, higher doses (> 5 mg/kg) of subcutaneous THC are required to produce immediate increase in appetite.
Previous studies have shown that oral gavage of THC increases short-term (within 2-h) food intake at a dose as low as 0.50 mg/kg in adult rats (Farrimond et al. 2010; Williams et al. 1998). By contrast, in Experiment 2a, voluntary oral THC consumption did not produce immediate (3-h) hyperphagia. The progressive increase in food intake during adolescence may mask the moderate hyperphagic effects of oral THC that may appear given a larger sample size. Furthermore, the use of calorie-rich cookie (~ 4 kcal/cookie) to deliver THC might have precluded a hyperphagic response by making the rats consume less chow. But it is unlikely that the calorie from cookie masked the hyperphagic effect of THC because the rats could consume greater than 20 kcal as shown in Experiment 1 or greater than half of their daily energy intake within 15 min when they are hyperphagic (Liang et al. 2013).
The divergent effects of subcutaneous versus oral THC on caloric intake may relate to the bioavailability and pharmacokinetic profiles of THC. Compared with injected or inhaled THC, orally-consumed THC peaks slowly in plasma (Grotenhermen 2003; Manwell et al. 2014a). The lipophilic nature of THC permits its easy permeation across the blood brain barrier. Thus, the timeline of plasma THC and its major active metabolite (11-OH-THC) reflects changes to brain levels (Tseng et al. 2004; Wiley and Burston 2014) where they act on select brain regions that regulate appetite and motivation (Di Marzo and Matias 2005; Kirkham et al. 2002; Wenzel and Cheer 2018). THC can either stimulate or suppress appetite depending on the relative proportion of presynaptic glutamatergic or GABAergic terminals on which it acts, and the ensuing effects on hormone signaling (Bellocchio et al. 2010; Gatta-Cherifi and Cota 2015). Moreover, cannabinoid activity in mitochondria and glia, hepatocytes, adipocytes, myocytes, and other tissue types are involved in energy balance regulation [reviewed in (Gatta-Cherifi and Cota 2015)]. The effects of THC on food intake in rodents is complex and can be dictated by an array of biological and methodological factors. Our novel voluntary alcohol and THC co-use model will be useful for studies aimed to explore the underlying biological mechanisms that mediate the effects of oral drug consumption. As an added advantage, our model can be adapted to explore dose-dependent effects of alcohol and THC co-use on behavior and energy balance.
Saccharin and alcohol intake
The results that THC administered subcutaneously or orally could reduce saccharin intake contradict previous reports that systemic or central administration of endocannabinoid or THC increases intake and palatability of sweet solutions (Gallate et al. 1999; O’Brien et al. 2013; Shinohara et al. 2009). Likewise, that oral THC can robustly reduce alcohol intake contrasts with previous findings where systemic injection of CB1R agonists increases voluntary ethanol drinking (Colombo et al. 2002; Linsenbardt and Boehm 2009). These intake suppression effects, however, are in line with the notion that THC can be aversive for rodents. Intraperitoneal injection of THC can support conditioned taste as well as place aversion in both rats and mice (Han et al. 2017; Manwell et al. 2014b; Wakeford and Riley 2014). Although the procedures in our experiments were not specifically designed to examine the aversive property of THC, the results of alcohol intake support that rats are more tolerant to the aversive effects of subcutaneous than of oral THC. Conversely, alcohol may enhance the aversive effects of THC because more COM than THC rats stopped eating THC-laced cookies in Experiments 2a (4 vs. 3 rats) and 2b (3 vs. 0 rats). This phenomenon, colloquially termed being “crossfaded”, can manifest in individuals who simultaneously use alcohol and cannabis to achieve an enhanced high (Patrick et al. 2018). It may result from high-potency or -dose THC interacting with alcohol to reverse the rewarding effects or to potentiate the aversive effects of their combined use. The dose-dependent effect of oral THC in reducing alcohol intake suggests that the phenomenon is not likely due to cookie availability. Additionally, the unchanged alcohol intake following subcutaneous 20 mg/kg/day THC administration that resulted in higher plasma levels of THC and its active metabolite than oral 10 mg/kg/day THC did suggests that the sedative effect of THC played a minor role in the robust alcohol intake reduction following oral THC administration.
Sucrose preference
As we previously observed (Nelson et al. 2017), abstinence from chronic moderate alcohol consumption did not elicit behavioral anhedonia measured by sucrose preference (Kang et al. 2016). Here we did not observe an effect on sucrose preference following abstinence from chronic THC, alone or when combined with moderate alcohol intake. Our findings are consistent with the report that chronic combined moderate alcohol consumption and marijuana smoking had no influence on mood in humans (Chait and Perry 1994). We also found that rats previously exposed to subcutaneous, but not oral, THC consumed less sucrose during abstinence compared with controls. Such behavioral manifestation may resemble an endophenotype of psychophathology and a sequela of drug abstinence (e.g., avolition) (Renard et al. 2014; Renard et al. 2017). However, other factors such as changes in taste detection and perception can contribute to the reduced sucrose intake. This is the first study to demonstrate changes in sucrose intake during abstinence from THC and its combined use with alcohol. The differential effects of subcutaneous and oral THC and their interaction with moderate alcohol to affect sucrose intake during abstinence warrant further investigation.
Learning, memory, and behavioral flexibility
Cognitive dysfunction is a predisposing factor for drug use disorder, and it contributes to poor addiction treatment outcomes (Spear 2018). Our study is the first to investigate the combined effects of chronic THC and moderate alcohol exposure on cognitive functions assessed in the Barnes maze during adolescence. Surprisingly, exposure to neither drugs impaired spatial learning, memory, and behavioral flexibility (see supplemental figures S1 & S2). Several factors may explain our inability to detect behavioral impairments in the rats, including the ease of the Barnes maze tasks we employed and the fact that THC is a partial CB1R agonist. In parallel with the second point, cognitive deficits occur when potent CB1R agonist such as WIN 55,212–2 and CP 55,940 are used (O’Shea et al. 2004; Tomas-Roig et al. 2017). Finally, the coincident peak blood THC and alcohol concentrations attained in this study might have been subthreshold to that required to uncover interactive effects between THC and moderate alcohol (Ramaekers et al. 2006).
Conclusion
Co-use of alcohol and cannabis is in vogue despite the lack of empirical data on how it affects brain and behavior. In establishing novel rodent models of THC and alcohol co-use, we found that injected or orally administered THC can dose-dependently interact with alcohol to differently influence feeding behaviors. Importantly, our established method of voluntary THC and alcohol ingestion is an improvement over two recent involuntary co-administration models (Khatri et al. 2018; Swartzwelder et al. 2012), and closely recapitulates the manner of human consumption. Our methods can be easily adapted for investigations of the behavioral, metabolic, and neurobiological mechanisms of joint alcohol and cannabinoid use in adolescent and adult subjects.
Supplementary Material
Acknowledgements
Startup funds from the Psychology Department of the University of Illinois at Urbana-Champaign (to NCL), and NIH grants R03DA043701 (to NCL), and R01GM115584–01A1 and R03DA042365–01A1 (to AD) supported this study. We thank Dr. Lucas Li of the Roy J. Carver Metabolomics Center (UIUC) for performing the LC-MS/MS analyses. We acknowledge the technical assistance of Firmino Pinto, Charles Shoemaker, Jacqueline Sanchez, and Sheel Vasavada, and are grateful to Dr. Justin Rhodes who provided access to the TopScan software. Portions of this work were presented at the 41st annual scientific meeting of the Research Society on Alcoholism in San Diego, CA, USA.
References
- Arnold WR, Weigle AT, Das A (2018) Cross-talk of cannabinoid and endocannabinoid metabolism is mediated via human cardiac CYP2J2 J Inorg Biochem 184:88–99 doi: 10.1016/j.jinorgbio.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L et al. (2010) Bimodal control of stimulated food intake by the endocannabinoid system Nat Neurosci 13:281–283 doi: 10.1038/nn.2494 [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A et al. (2017) A chronic low dose of Delta(9)-tetrahydrocannabinol (THC) restores cognitive function in old mice Nat Med 23:782–787 doi: 10.1038/nm.4311 [DOI] [PubMed] [Google Scholar]
- Carlin JL, McKee SE, Hill-Smith T, Grissom NM, George R, Lucki I, Reyes TM (2016) Removal of high-fat diet after chronic exposure drives binge behavior and dopaminergic dysregulation in female mice Neuroscience 326:170–179 doi: 10.1016/j.neuroscience.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS (2007) Sex differences in the effects of delta9-tetrahydrocannabinol on spatial learning in adolescent and adult rats Behav Pharmacol 18:563–569 doi: 10.1097/FBP.0b013e3282ee7b7e [DOI] [PubMed] [Google Scholar]
- Chait LD, Perry JL (1994) Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance Psychopharmacology (Berl) 115:340–349 [DOI] [PubMed] [Google Scholar]
- Coleman LG Jr., Liu W, Oguz I, Styner M, Crews FT (2014) Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility Pharmacol Biochem Behav 116:142–151 doi: 10.1016/j.pbb.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G et al. (2002) Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats Psychopharmacology (Berl) 159:181–187 doi: 10.1007/s002130100887 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I (2005) Endocannabinoid control of food intake and energy balance Nat Neurosci 8:585–589 doi: 10.1038/nn1457 [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Grinker JA (1978) Food and water intake, meal patterns and activity of obese and lean Zucker rats following chronic and acute treatment with delta9-tetrahydrocannabinol Pharmacol Biochem Behav 9:619–630 [DOI] [PubMed] [Google Scholar]
- Drews E, Schneider M, Koch M (2005) Effects of the cannabinoid receptor agonist WIN 55,212–2 on operant behavior and locomotor activity in rats Pharmacol Biochem Behav 80:145–150 doi: 10.1016/j.pbb.2004.10.023 [DOI] [PubMed] [Google Scholar]
- Dry MJ, Burns NR, Nettelbeck T, Farquharson AL, White JM (2012) Dose-related effects of alcohol on cognitive functioning PLoS One 7:e50977 doi: 10.1371/journal.pone.0050977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earleywine M (2002) Understanding Marijuana: A new look at the scientific evidence. Oxford University Press, New York [Google Scholar]
- Farrimond JA, Hill AJ, Whalley BJ, Williams CM (2010) Cannabis constituents modulate delta9-tetrahydrocannabinol-induced hyperphagia in rats Psychopharmacology (Berl) 210:97–106 doi: 10.1007/s00213-010-1821-z [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Byrne MF (1988) Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory Appetite 11:1–14 [DOI] [PubMed] [Google Scholar]
- Gallate JE, Saharov T, Mallet PE, McGregor IS (1999) Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist Eur J Pharmacol 370:233–240 [DOI] [PubMed] [Google Scholar]
- Gatta-Cherifi B, Cota D (2015) Endocannabinoids and Metabolic Disorders Handb Exp Pharmacol 231:367–391 doi: 10.1007/978-3-319-20825-1_13 [DOI] [PubMed] [Google Scholar]
- Graceffo TJ, Robinson JK (1998) Delta-9-tetrahydrocannabinol (THC) fails to stimulate consumption of a highly palatable food in the rat Life Sci 62:PL85–88 [DOI] [PubMed] [Google Scholar]
- Grella CE, Rodriguez L, Kim T (2014) Patterns of medical marijuana use among individuals sampled from medical marijuana dispensaries in los angeles J Psychoactive Drugs 46:267–275 doi: 10.1080/02791072.2014.944960 [DOI] [PubMed] [Google Scholar]
- Grotenhermen F (2003) Pharmacokinetics and pharmacodynamics of cannabinoids Clin Pharmacokinet 42:327–360 doi: 10.2165/00003088-200342040-00003 [DOI] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine MT, Smith RT, Lukas SE (2016) Splendor in the Grass? A Pilot Study Assessing the Impact of Medical Marijuana on Executive Function Front Pharmacol 7:355 doi: 10.3389/fphar.2016.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J et al. (2012) Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD Cell 148:1039–1050 doi: 10.1016/j.cell.2012.01.037 [DOI] [PubMed] [Google Scholar]
- Han X et al. (2017) CB1 Receptor Activation on VgluT2-Expressing Glutamatergic Neurons Underlies Delta(9)-Tetrahydrocannabinol (Delta(9)-THC)-Induced Aversive Effects in Mice Sci Rep 7:12315 doi: 10.1038/s41598-017-12399-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, DiPatrizio NV (2005) Delta9-THC induced hyperphagia and tolerance assessment: interactions between the CB1 receptor agonist delta9-THC and the CB1 receptor antagonist SR-141716 (rimonabant) in rats Behav Pharmacol 16:373–380 [DOI] [PubMed] [Google Scholar]
- Kang S, Wu MM, Galvez R, Gulley JM (2016) Timing of amphetamine exposure in relation to puberty onset determines its effects on anhedonia, exploratory behavior, and dopamine D1 receptor expression in young adulthood Neuroscience 339:72–84 doi: 10.1016/j.neuroscience.2016.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri D, Laroche G, Grant ML, Jones VM, Vetreno RP, Crews FT, Mukhopadhyay S (2018) Acute ethanol inhibition of adult hippocampal neurogenesis involves CB1 cannabinoid receptor signaling Alcohol Clin Exp Res doi: 10.1111/acer.13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V (2002) Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol Br J Pharmacol 136:550–557 doi: 10.1038/sj.bjp.0704767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI (1977) Preputial separation as an external sign of pubertal development in the male rat Biol Reprod 17:298–303 [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Liljequist S, Meis J, Chefer V, Shippenberg T, Bakalkin G (2012) Repeated moderate-dose ethanol bouts impair cognitive function in Wistar rats Addict Biol 17:132–140 doi: 10.1111/j.1369-1600.2010.00224.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leirer VO, Yesavage JA, Morrow DG (1991) Marijuana carry-over effects on aircraft pilot performance Aviat Space Environ Med 62:221–227 [PubMed] [Google Scholar]
- Liang NC, Smith ME, Moran TH (2013) Palatable food avoidance and acceptance learning with different stressors in female rats Neuroscience 235:149–158 doi: 10.1016/j.neuroscience.2012.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL 2nd (2009) Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area Neuroscience 164:424–434 doi: 10.1016/j.neuroscience.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen H, Hyytia P (2008) Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats Alcohol Clin Exp Res 32:1976–1983 doi: 10.1111/j.1530-0277.2008.00786.x [DOI] [PubMed] [Google Scholar]
- Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE (2014a) A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part I: development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents J Pharmacol Toxicol Methods 70:120–127 doi: 10.1016/j.vascn.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Manwell LA, Ford B, Matthews BA, Heipel H, Mallet PE (2014b) A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part II: comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents J Pharmacol Toxicol Methods 70:112–119 doi: 10.1016/j.vascn.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker L (2003) Cannabis and alcohol--a close friendship Trends Pharmacol Sci 24:266–268 doi: 10.1016/S0165-6147(03)00107-X [DOI] [PubMed] [Google Scholar]
- Merroun I et al. (2009) Influence of intracerebroventricular or intraperitoneal administration of cannabinoid receptor agonist (WIN 55,212–2) and inverse agonist (AM 251) on the regulation of food intake and hypothalamic serotonin levels Br J Nutr 101:1569–1578 doi: 10.1017/S0007114508083530 [DOI] [PubMed] [Google Scholar]
- Murphy M, Mills S, Winstone J, Leishman E, Wager-Miller J, Bradshaw H, Mackie K (2017) Chronic Adolescent Delta9-Tetrahydrocannabinol Treatment of Male Mice Leads to Long-Term Cognitive and Behavioral Dysfunction, Which Are Prevented by Concurrent Cannabidiol Treatment Cannabis Cannabinoid Res 2:235–246 doi: 10.1089/can.2017.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences E, and Medicine (2017) The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research In: Sciences NAo (ed). The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press, Washington (DC). doi: 10.17226/24625 [DOI] [PubMed] [Google Scholar]
- Nelson NG, Suhaidi FA, DeAngelis RS, Liang NC (2016) Appetite and weight gain suppression effects of alcohol depend on the route and pattern of administration in Long Evans rats Pharmacol Biochem Behav 150–151:124–133 doi: 10.1016/j.pbb.2016.10.006 [DOI] [PubMed] [Google Scholar]
- Nelson NG, Suhaidi FA, Law WX, Liang NC (2017) Chronic moderate alcohol drinking alters insulin release without affecting cognitive and emotion-like behaviors in rats Alcohol 70:11–22 doi: 10.1016/j.alcohol.2017.12.001 [DOI] [PubMed] [Google Scholar]
- O’Brien LD, Wills KL, Segsworth B, Dashney B, Rock EM, Limebeer CL, Parker LA (2013) Effect of chronic exposure to rimonabant and phytocannabinoids on anxiety-like behavior and saccharin palatability Pharmacol Biochem Behav 103:597–602 doi: 10.1016/j.pbb.2012.10.008 [DOI] [PubMed] [Google Scholar]
- O’Shea M, Singh ME, McGregor IS, Mallet PE (2004) Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats J Psychopharmacol 18:502–508 doi: 10.1177/026988110401800407 [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C (2007) Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats Eur J Neurosci 25:541–550 doi: 10.1111/j.1460-9568.2006.05298.x [DOI] [PubMed] [Google Scholar]
- Patrick ME, Fairlie AM, Lee CM (2018) Motives for simultaneous alcohol and marijuana use among young adults Addict Behav 76:363–369 doi: 10.1016/j.addbeh.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G (2006) Cognition and motor control as a function of Delta9-THC concentration in serum and oral fluid: limits of impairment Drug Alcohol Depend 85:114–122 doi: 10.1016/j.drugalcdep.2006.03.015 [DOI] [PubMed] [Google Scholar]
- Renard J, Krebs MO, Le Pen G, Jay TM (2014) Long-term consequences of adolescent cannabinoid exposure in adult psychopathology Front Neurosci 8:361 doi: 10.3389/fnins.2014.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Szkudlarek HJ, Kramar CP, Jobson CEL, Moura K, Rushlow WJ, Laviolette SR (2017) Adolescent THC Exposure Causes Enduring Prefrontal Cortical Disruption of GABAergic Inhibition and Dysregulation of Sub-Cortical Dopamine Function Sci Rep 7:11420 doi: 10.1038/s41598-017-11645-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T et al. (2009) Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood Hippocampus 19:763–772 doi: 10.1002/hipo.20554 [DOI] [PubMed] [Google Scholar]
- Rubino T et al. (2008) Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates Neuropsychopharmacology 33:2760–2771 doi: 10.1038/sj.npp.1301664 [DOI] [PubMed] [Google Scholar]
- Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (2017) https://www.samhsa.gov/data/.
- Sanudo-Pena MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM (2000) Activational role of cannabinoids on movement Eur J Pharmacol 391:269–274 [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Inui T, Yamamoto T, Shimura T (2009) Cannabinoid in the nucleus accumbens enhances the intake of palatable solution Neuroreport 20:1382–1385 doi: 10.1097/WNR.0b013e3283318010 [DOI] [PubMed] [Google Scholar]
- Spear LP (2018) Effects of adolescent alcohol consumption on the brain and behaviour Nat Rev Neurosci 19:197–214 doi: 10.1038/nrn.2018.10 [DOI] [PubMed] [Google Scholar]
- Swartzwelder NA et al. (2012) Effects of ethanol, Delta(9)-tetrahydrocannabinol, or their combination on object recognition memory and object preference in adolescent and adult male rats Neurosci Lett 527:11–15 doi: 10.1016/j.neulet.2012.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscione NB, Miller R, Shan X, Sprague J, Yeatman DT (2016) An Efficient, Robust Method for the Determination of Cannabinoids in Whole Blood by LC-MS-MS J Anal Toxicol 40:639–648 doi: 10.1093/jat/bkw063 [DOI] [PubMed] [Google Scholar]
- Tomas-Roig J, Benito E, Agis-Balboa RC, Piscitelli F, Hoyer-Fender S, Di Marzo V, Havemann-Reinecke U (2017) Chronic exposure to cannabinoids during adolescence causes long-lasting behavioral deficits in adult mice Addict Biol 22:1778–1789 doi: 10.1111/adb.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Harding JW, Craft RM (2004) Pharmacokinetic factors in sex differences in Delta 9-tetrahydrocannabinol-induced behavioral effects in rats Behav Brain Res 154:77–83 doi: 10.1016/j.bbr.2004.01.029 [DOI] [PubMed] [Google Scholar]
- Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO (2015) Cannabinoid Dose and Label Accuracy in Edible Medical Cannabis Products JAMA 313:2491–2493 doi: 10.1001/jama.2015.6613 [DOI] [PubMed] [Google Scholar]
- Wakeford AG, Riley AL (2014) Conditioned taste avoidance induced by Delta(9)-tetrahydrocannabinol in the Fischer (F344) and Lewis (LEW) rat strains Pharmacol Biochem Behav 116:39–44 doi: 10.1016/j.pbb.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Cheer JF (2018) Endocannabinoid Regulation of Reward and Reinforcement through Interaction with Dopamine and Endogenous Opioid Signaling Neuropsychopharmacology 43:103–115 doi: 10.1038/npp.2017.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ (2014) Sex differences in Delta(9)-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats Neurosci Lett 576:51–55 doi: 10.1016/j.neulet.2014.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC (2002) Reversal of delta 9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine Pharmacol Biochem Behav 71:333–340 [DOI] [PubMed] [Google Scholar]
- Williams CM, Rogers PJ, Kirkham TC (1998) Hyperphagia in pre-fed rats following oral delta9-THC Physiol Behav 65:343–346 [DOI] [PubMed] [Google Scholar]
- Yeomans MR (2010) Alcohol, appetite and energy balance: is alcohol intake a risk factor for obesity? Physiol Behav 100:82–89 doi: 10.1016/j.physbeh.2010.01.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.