Abstract
Human pluripotent stem cells (hPSCs) have been suggested as a potential source for the de novo production of blood cells for transfusion, immunotherapies and transplantation. However, even with advanced hematopoietic differentiation methods, the primitive and myeloid-restricted waves of hematopoiesis dominate in hPSC differentiation cultures while cell-surface markers to distinguish these waves of hematopoiesis from lympho-myeloid hematopoiesis remain unknown. In the embryo, hematopoietic stem cells (HSCs) arise from hemogenic endothelium (HE) lining arteries, but not veins. This observation led to a longstanding hypothesis that arterial specification is an essential prerequisite to initiate HSC program. It has also been established that lymphoid potential in yolk sac and extraembryonic vasculature is mostly confined to arteries, while myeloid-restricted hematopoiesis is not specific to arterial vessels. Here we review how the link between arterialization and subsequent definitive multilineage hematopoietic program can be exploited to identify hemogenic endothelium enriched in lymphoid progenitors and aid in in vitro approaches to enhance the production of lymphoid cells and potentially HSCs from hPSCs. We also discuss alternative models of hematopoietic specification at arterial sites and the recent advances in understanding hematopoietic development and producing engraftable hematopoietic cells from hPSCs.
Category for the Table of Contents:
Stem Cells (hematopoietic, mesenchymal, embryonic and induced pluripotent stem cells); Normal Hematopoiesis (myelopoiesis, erythropoiesis, lymphopoiesis, megakaryocytopoiesis)
Introduction
Derivation of human embryonic stem cells (hESCs) 20 years ago [1] followed by advances in cellular reprogramming to generate human induced pluripotent stem cells (hiPSCs) [2–5] have created alternative platforms for producing blood cells for transfusion, immunotherapies and transplantation. Although the feasibility of generating myeloid, T lymphoid, and engraftable blood cells from human pluripotent stem cells (hPSCs) has been demonstrated [6–14], scalable production of definitive hematopoietic cells, including adult-type red blood cells, megakaryocytes, T cells, and hematopoietic stem cells (HSCs) with robust multilineage engraftment potential remains a significant challenge. Even with advanced hematopoietic differentiation methods, the primitive and myeloid-restricted waves of hematopoiesis dominate in hPSC differentiation cultures while lympho-myeloid progenitors with multilineage potential are produced in low frequency [15–18]. Moreover, key specification requirements for the development of lympho-myeloid progenitors and HSCs, as well as specific markers that distinguish these cells from myeloid-restricted progenitors and primitive wave of hematopoiesis remain largely obscure. Embryonic developmental studies in avian, mammalian, and zebrafish models have identified hemogenic endothelium (HE) as the immediate precursor of blood cells in the vasculature at many extraembryonic and embryonic sites (reviewed in [16, 19–21]). It has become evident that HE at different sites possess distinct hematopoietic lineage potential and that development of definitive multilineage hematopoietic progenitors are restricted to arterial vessels [22–25]. This review will outline current knowledge and controversies about the link between arterial specification and the definitive hematopoietic program. Exploring this link will aid in identifying and enhancing lympho-myeloid hematopoietic progenitors and eventually lead to generating engraftable HSCs from hPSC cultures.
Hematopoietic development in the arterial and non-arterial embryonic vasculature
It has been established that hematopoietic development in the vertebrate embryo occurs in multiple waves. The first transient wave of hematopoiesis takes place in the yolk sac blood islands that give rise only to primitive erythroid, megakaryocytic and macrophage cells that are different from their corresponding adult counterparts. In contrast, subsequent waves of definitive hematopoiesis produce adult-type erythro-myeloid progenitors (EMPs), lymphomyeloid cells, and HSCs (reviewed in [15, 26, 27]). While HSCs possess multilineage engraftment potential, other types of emerging definitive hematopoietic progenitors are lineage-restricted and do not reconstitute the entire hematopoietic system following transplantation. Thus, for clarity, we specify the type of definitive hematopoietic development to distinguish definitive erythro-myelopoiesis, lympho-myeloid hematopoiesis, and the development of HSC with multilineage engraftment potential.
Most of the HSCs in the mammalian embryo arise in the intraembryonic dorsal aorta within the intra-aortic hematopoietic clusters (IAHCs) [23, 25, 28, 29]. Lineage tracing experiments and real-time in vivo observations documented that IAHCs are formed from a distinct population of endothelium lining the ventral wall of the dorsal aorta through a unique morphogenic process called endothelial-to-hematopoietic transition (EHT) [22, 30–33]. During EHT, flat endothelial cells gradually acquire round hematopoietic morphology and phenotype and HSC potential.
Although the concept of HE was initially developed based on studies of hematopoiesis in the developing aorta, it became clear that endothelium in other embryonic sites such as endocardium [24, 34, 35], head vasculature [24, 36], and possibly somitic vessels [24] also possess hemogenic potential. In addition, multiple studies demonstrated that blood formation from the earliest primitive hematopoietic progenitor, the hemangioblast, also proceed through hemogenic endothelial intermediates [37–39]. When definitive erythro-myeloid and lymphomyeloid hematopoiesis establishes in the yolk sac, HE becomes a major source of adult-type blood cells formed within the extraembryonic vasculature, including vitelline, umbilical [25, 40], placental [41] and yolk sac [42–47] vasculature. Although blood cells arise almost exclusively from arterial HE within the embryo proper, EHT in extraembryonic sites is observed from HE lining arterial, venous, and capillary vessels [25, 42–45]. Interestingly, distinguishing extraembryonic umbilical and vitelline vasculature into venous and arterial compartments reveals HSC potential localized exclusively to arterial vessels [25]. When Yzaguirre and Speck [24] performed careful morphological and functional analysis of hematopoietic clusters arising within E9.5 and E10.5 mouse yolk sac vasculature, they found that cells with lymphoid potential are mostly restricted to arterial vessels. In contrast, the first wave of yolk sac erythro-myelopoiesis, which lack lymphoid potential, is not specific to the arterial vessels [24, 43]. In addition, murine embryonic studies of the role of the core binding factor beta (CBFβ) gene demonstrated that EMPs and HSCs emerge from distinct HE populations. Rescued expression of CBFβ in Cbfb−/− mice under control of the pan-endothelial gene, Tie2, rescued yolk sac HE and EMPs, but not HSC development from the AGM. In contrast, rescued expression of CBFβ expression under the control of the HSC-specific gene, Ly6a, restored HSC formation in the AGM, but not EMPs in yolk sac [48]. These results support the notion that not all HE are equal and that hematopoietic lineage potential is already predetermined at the HE stage. Overall, the above observations suggest that blood formation through endothelial intermediates is a central process during the development of the entire hematopoietic system, and that arterial versus non-arterial specification of HE may have a significant impact on hemogenic progeny and hematopoietic competence.
Is arterial specification of HE required for HSC formation and specification of definitive multilineage hematopoiesis?
The observation that HSCs originate particularly from arterial vessels led to the longstanding hypothesis that arterial specification is an essential prerequisite for initiating the definitive hematopoietic program [49] (Figure 1A). This hypothesis is supported by the direct observation of HSC formation from aortic endothelium through EHT [22, 30–33], and the demonstration of shared requirements of Notch, VEGF, and Hedgehog signaling for both arterial fate acquisition and HSC development [50–56]. Additional evidence supporting the arterial specification-dependent model of HSC development came from studies in mice with knock-out of the artery-specific gene, Ephrin B2 (EfnB2), which is essential for the vascular remodeling and repulsive sorting of arterial- and venous-fated endothelial cells [57]. EfnB2−/− mice, in addition to defects in the vascular network, revealed selective impairment of hematopoiesis in the dorsal aorta, while primitive and erythro-myeloid hematopoiesis in yolk sac was not affected [58].
Figure 1. Models of hematopoietic specification in AGM region.
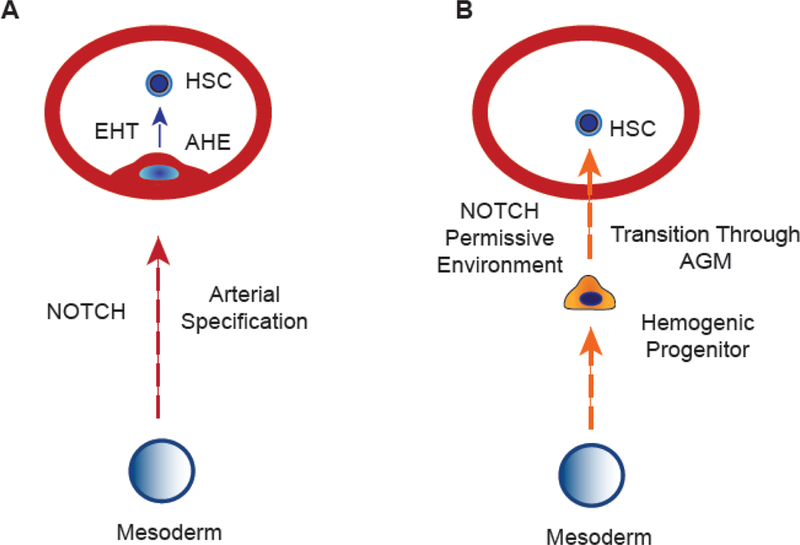
(A) Classical arterial fate acquisition-dependent model of HSC development. HE progenitor arising from mesoderm undergoes an arterial specification that then allows for the HSC program to initiate and lead to EHT. AHE is arterial-type HE. (B) Arterial-independent model of HSC specification. According to this model, HE are hematopoietic-restricted progenitors expressing endothelial markers rather than true endothelial cells. These progenitors undergo HSC specification and maturation following transition through the permissive arterial environment. This model assumes that HSC specification is uncoupled from the arterial program.
However, this hypothesis has been challenged by the identification of hematopoietic specification mechanisms that are uncoupled from arteriovenous specification. Notch1−/− mice display altered arteriovenous specification with dorsal aorta malformations [59]. Although paraaortic splanchnopleural cells in these mutants did not produce blood cells [55, 60], hematopoiesis was restored from these cells in vitro by overexpressing RUNX1 [60]. It has been demonstrated that the NOTCH ligand Jag1 is not involved in arterial specification, but is required for blood formation in mouse AGM [54]. Zebrafish studies revealed that Jag1 expression is regulated by TGFβ which affects hematopoiesis in dorsal aorta independently of arterial development [61]. Zebrafish tbx16 mutant lacking axial vascular organization also have impaired HSC function. However, overexpression of Vegf and Notch is sufficient to induce hematopoiesis in these mutants despite a lack of axial vascular organization [53]. These findings led to the hypothesis that HSC development could be uncoupled from arterial specification of the HE (Figure 1B). According to this hypothesis, HE cells are distinct, hematopoietic-restricted progenitors expressing endothelial markers rather than bona-fide endothelial cells. These progenitors acquire HSC potential following transition through AGM which provides a permissive environment for the acquisition of the self-renewal program [15]. Observation of migrating subaortic mesenchymal/mesodermal cells through aortic endothelial cells to become hematopoietic cells [31, 62] is consistent with arterial specification-independent model for HSC development. In addition, the demonstration that HE isolated from murine AGM produce almost exclusively blood cells in cultures with hematopoietic cytokines, but never both blood and endothelium [63], has been used to define HE as a hematopoietic-restricted progenitor.
However, this model implies that HSC development does not proceed through an arterial endothelial stage, which is challenged by the fact that HE cells in the aorta are highly similar to non-HE and express typical arterial-specific genes including EfnB2, Dll4, Notch4, and Sox17 [54, 56, 64, 65]. HE are also capable of forming vascular tubes in the presence of VEGF and the absence of hematopoietic cytokines [63], suggesting that aortic HE are bona fide endothelial cells and their capacity to make blood or endothelial cells is determined by appropriate milieu of signaling factors. Moreover, conclusions about restoring HSC function in the setting of perturbed arteriovenous specification were made almost exclusively on the morphological observation of blood formation at anatomical sites of HSC emergence or in vitro functional analysis, without assessing functionality of the rescued blood cells in vivo. It is quite possible that HE mis-specified in the setting of disrupted arterial program can still undergo EHT under certain conditions, but ultimately fail to form functional HSCs. In fact, Guiu et al showed that increasing levels of Notch signaling increased EHT and the number of hematopoietic cells in the mouse AGM lumen, but decreased the number of functional HSCs due to persistent GATA2 expression [66]. Their findings emphasize the importance of functional assays of HSCs and that simply observing EHT occurring in AGM does not necessarily mean that the resulting blood cells possess self-renewal potential.
While existence of unique mechanisms regulating HSC development independent of arterial specification is commonly used to justify an arterial-independent model of HSC specification, this argument does not consider the complexity of HE and HSC development. HE are likely specified independently of non-HE during early mesodermal partitioning [67, 68] and blood formation from HE includes the EHT step. Thus, inhibition of blood formation in dorsal aorta in the setting of preserved arterial specification could be explained by the selective effect of a particular signal on HE specification or EHT, and does not exclude the need for arterial specification by HE to become HSCs.
Similar to the embryo proper, arterial specification of extraembryonic vessels is regulated by NOTCH signaling [69–72]. Although it has been well documented that EMPs in the yolk sac do not require NOTCH signaling [55, 73], little is known about the effect of NOTCH signaling on arterial specification of HE and lympho-myeloid hematopoiesis in the yolk sac and extraembryonic vasculature. Studies by Hirschi’s lab demonstrated that NOTCH signaling plays an important role in the specification of HE with retinoic acid signaling-dependent definitive hematopoiesis [74], suggesting that HE in extraembryonic sites may also undergo an arterial-intermediate.
Major advances in understanding HE development in hPSC differentiation cultures
In the last two decades, significant progress has been made in understanding HE and blood development from hPSCs. One of the important milestones was the identification of CD43 as an early marker of all hematopoietic cells generated from hPSCs [75]. This allowed for the precise separation of CD43+ hematopoietic cells from preceding VE-cadherin(VEC)+CD43− HE progenitors. It also became clear that endothelium generated from hPSCs are heterogenous and at least three different clonogenic progenitors with varying endothelial potential are formed from hPSCs committed to lateral plate mesoderm: 1) mesenchymoangioblast (endothelial and mesenchymal potentials) [76, 77], 2) hemangioblast (endothelial and hematopoietic potentials) [77, 78], and 3) cardiovascular progenitors (endothelial and cardiomyocyte potentials) [79], suggesting that VEC+CD43− may include a heterogenous population of endothelial cells with varying levels of hemogenic and non-hemogenic potential.
Indeed, functional analysis of the VEC+ populations in hPSC cultures revealed that HE are a distinct endothelial lineage which can be distinguished from non-HE cells by the lack of CD73 expression [17, 18]. Extensive phenotypic and molecular profiling analyses demonstrated that HE and non-HE progenitors are very similar phenotypically and express typical endothelial surface markers, including CD31, CD34, KDR, TEK, ESAM, CD49d, CD54, CD141, CD146, CD151, CD166, CD201, and APLNR. However HE in contrast to non-HE express plentiful CD226 and high levels of RUNX1, GFI1, RHAG, NTS and BMPER genes [17]. Based on these findings, HE was characterized as VEC+CD43−CD73− cells with primary endothelial characteristics lacking hematopoietic CFC potential, but capable of producing blood cells following secondary culture on stromal cells or extracellular matrix in the presence of hematopoietic cytokines [17].
Functional analysis of single hPSC-derived HE cells revealed that not all HE cells are functionally the same. Only less than 10% of HE cells that produced blood in presence of OP9DLL4 stroma were able to generate lympho-myeloid progenitors, while the remaining HE cells produced lineage-restricted blood cells [18], thus suggesting that the hematopoietic potential of HE is already predetermined at the HE stage. Similar conclusions can be made from our direct HE programming studies, which revealed that HE with erythro-megakaryocytic or panmyeloid potentials can be induced directly from hPSCs using different sets of transcription factors TAL1/GATA2 and ETV2/GATA2 respectively [80].
Studies by the Keller group revealed that specification of HE with primitive and definitive hematopoietic potential from mesoderm in hPSC cultures is regulated by distinct signaling. Primitive hematopoiesis from hPSCs requires Nodal/activin signaling and inhibition of the WNT/β-catenin pathway. Specification of definitive HE occurs in the absence of Nodal/activin signaling and requires activation of the WNT/β-catenin signaling [81].
Specification of arterial-type HE from hPSCs
As discussed above, development of definitive hematopoiesis with lympho-myeloid and HSC potentials is restricted to arterial vessels. It has been established that NOTCH signaling is the most critical determinant of arterial specification in embryonic [51] and extraembryonic yolk sac vasculature [69–72]. However, it remains unclear whether NOTCH signaling plays a similar role in the specification of vasculogenic and hemogenic endothelia. Moreover, the arterial specification-independent model of HSC development outlined in the prior section questions the necessity for HE to acquire an arterial fate in order to enable HSC development.
To determine whether NOTCH signaling affects HE specification, we employed a 2D chemically-defined, feeder and xeno-free hPSC differentiation system in which all stages of hematopoietic development are temporally, phenotypically and functionally defined [82]. In this system, the first VEC+CD43−CD73− arose on day 4 (D4) of differentiation. Since D4 HE cells retain expression of HAND1 mesodermal gene, we defined them as immature or primordial HE. These cells expressed NOTCH1, but not DLL4 or DLL1 NOTCH ligands [83]. When primordial HE were isolated and cultured in the presence DLL1-Fc or DAPT to activate or inhibit NOTCH signaling respectively [83], NOTCH activation led to the formation of CD144+CD43−CD73− HE expressing the earliest arterial marker DLL4 [84]. Molecular profiling studies demonstrated that DLL4+ HE in contrast to DLL4− HE had an arterial molecular signature as signified by high expression of NOTCH1, NOTCH4, JAG2, HEY1, HEY2, SOX17 and EFNB2.
To determine whether NOTCH activation and formation of DLL4+ HE is associated with establishing the definitive hematopoietic program, we engineered a H1 hESC reporter line in which the Runx+23 enhancer [85, 86] linked to the minimal β-globin promoter and fluorescent protein is inserted into AAVS1 locus [83]. Previously, the Runx1+23 enhancer was found to be active in HE found in regions where definitive hematopoiesis emerges, including the para-aortic splanchnopleura, AGM region, vitelline, and umbilical arteries [85–89]. Using this reporter cell line, we found that NOTCH activation leads to increased Runx1+23 reporter signal, and is mostly restricted to DLL4+ HE. Hemogenic capacity of DLL4+ HE was strictly dependent on NOTCH signaling and required stroma.
Functional analysis of hematopoietic progenitors obtained from DLL4+ HE revealed an enrichment of lymphoid progenitors and the production of erythrocytes expressing increased levels of adult β and decreased levels of embryonic ζ and ε hemoglobins compared to blood cells generated from DLL4− HE [83]. In addition, molecular profiling of lin-CD34+CD45+ hematopoietic progenitors generated from DLL4+ and DLL4− HE cultured on OP9-DLL4 revealed upregulation of genes essential for AGM and fetal liver hematopoiesis and lymphoid development, including MECOM, GFI1B, ERG, ARID5B, and BCOR [83]. Studies using GATA2 knockout hESC lines with conditional GATA2 expression revealed that GATA2 deficiency does not affect specification of arterial and non-arterial HE indicating that arterial vs non-arterial HE fate is likely predetermined by mechanisms upstream of GATA2 [90].
Overall these findings suggest that following exposure to NOTCH signaling, primordial HE generated from hPSCs specify into VEC+CD43−CD73−DLL4+ arterial-type HE which requires NOTCH signaling to undergo EHT and produce definitive lympho-myeloid and erythroid cells (Figure 2). The demonstration that NOTCH-mediated arterialization of HE is an essential prerequisite for establishing the definitive hematopoietic program provides supports for an arterial specification-dependent model of definitive hematopoietic development. In addition, these findings demonstrate the utility of DLL4 as marker for identifying definitive hematopoietic wave from hPSC cultures at the HE stage of development.
Figure 2. Specification of arterial-type HE and definitive multilineage hematopoiesis in hPSC cultures.
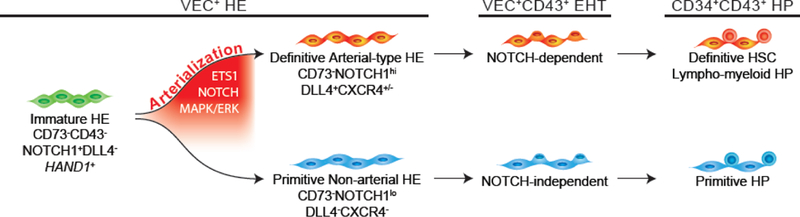
HE that undergo EHT to give rise to lympho-myeloid HPs mature through an arterialization process, which can be identified by the upregulation of arterial markers, NOTCH1 (N1), DLL4, and CXCR4. In contrast, HE that give rise to primitive, erythro-myeloid restricted HPs do not mature through an arterialization process and undergo EHT through a NOTCH-independent procedure. Arterial specification of HE can be promoted by modulation of pathways involved in arteriovenous specification during embryonic development, including ETS1 and MAPK/ERK signaling.
Enhancing definitive hematopoiesis from hPSCs through arterial specification
Since arterial specification of HE has been found to be critical for establishing lymphomyeloid hematopoiesis, it is logical to assume that definitive multilineage hematopoiesis in hPSC cultures can be enhanced through promoting the arterial program. During vascular development, arterial fate is controlled by a number of key signaling pathways including Hedgehog, VEGF, NOTCH, MAPK/ERK, Wnt/B-catenin signaling pathways and ETS, SOXF and FOXC1/C2 transcription factors (reviewed in [91]). In vertebrate embryo, arterial specification is initiated by the induction of DLL4 expression [84] through an arterial-specific enhancer located within the third intron of DLL4 that is controlled by ETS, SOXF and RBPJk factors [92, 93]. In hPSC cultures, overexpression of ETS1 during the mesodermal stage of development dramatically enhances the formation of arterial-type HE expressing DLL4 and CXCR4. Blood cells generated from arterial HE were more than 100-fold enriched in the frequency of T cell precursors and possessed the capacity to produce B lymphocytes and red blood cells expressing high levels of BCL11a and β-globin [94]. The effect of ETS1 was mostly mediated through activating NOTCH signaling. Following ETS1 overexpression, regulon activity for NOTCH1, SOXF (SOX17, SOX18), KLF5 and BCL6B genes in DLL4+ arterial-type HE was increased while ETS1 regulon signal was poor, which is consistent with findings that the effect of ETS1 is primarily mediated through the activation of an arterial-specific enhancer leading to upregulation of NOTCH1 signaling and SOXF transcription factors, rather than from any immediate downstream signaling of ETS1.
Arterial specification is also controlled by several signaling cascades operating downstream of the VEGF signaling pathway. Among them are the MAPK/ERK signaling cascade. It has been shown that indirect ERK activation through inhibition of Phosphoinositide 3-kinase (PI3K) downstream of VEGF signaling, enhances arterial specification in zebrafish, while inhibition of the ERK signaling pathway blocks arterial specification [95, 96]. Modulation of this signaling in hPSC cultures revealed that indirect MAPK/ERK activation promotes DLL4+CXCR4+/− arterial-type HE and lympho-myeloid progeny, while its inhibition causes the opposite effect [94].
In hPSC cultures, arterial specification of vascular endothelium has been also achieved using TGFβ inhibitors immediately after initiating mesoderm formation [97]. The use of TGFβ inhibitors along with the WNT activator, CHIR99021, was essential to induce SOX17+CD34+ HE with arterial identity that express HOXA genes and possess hemogenic activity closely resembling human AGM hematopoiesis [98].
Overall, these findings strongly indicate that exploiting mechanisms critical for arterial specification could be an important strategy to enhance definitive multilineage hematopoiesis, from hPSC cultures. Although it was hoped that arterial specification of the primordial HE would be sufficient to generate engraftable hematopoietic cells, these expectations were not realized [83, 94, 98]. This reflects the complexity of the mechanisms involved in embryonic HSC formation which still has not been reproduced in vitro. Several other signaling pathways, including those uncoupled from aortic specification, such as TGFβ [61], retinoic acid [99, 100], inflammation [101, 102], hormone [103, 104], and blood flow induced shear stress [105, 106], have all been shown to serve critical roles during HSC development, and may be aberrantly modulated during differentiation from hPSC culture in vitro. It remains to be determined how these pathways identified in vivo can be integrated into in vitro hPSC differentiation cultures to successfully generate engraftable HSCs de novo.
Advances in understanding specification of hematopoietic lineages and producing engraftable hematopoietic cells from hPSCs
Differentiation and generation of hematopoietic cells from hPSC cultures reflects the multiple waves of embryonic hematopoiesis; phenotypic markers for which still remain obscure [15]. The hPSC-derived CD34+CD43+ hematopoietic progenitors are heterogeneous and include populations of CD235a+CD41a+ cells enriched in erythro-megakaryocytic progenitors and linCD43+ CD90+CD45RA−CD45+/− multipotent progenitors with broad lympho-myeloid potentials [75, 107–109]. Although specific phenotypic markers to distinguish primitive versus definitive hematopoietic progenitors remain largely unknown, different waves of and hematopoiesis in hPSC cultures can be identified based on functional assays.
The first wave of FGF2- and VEGF-dependent mesodermal progenitor with hematopoietic and endothelial potential can be identified in hPSC cultures using a blast colony forming assay [77, 78, 110]. Hematopoietic cells within blast colonies develops through an endothelial intermediate (cores), and their differentiation potential is restricted to erythrocytes, megakaryocytes and macrophages [17, 39, 77], thereby suggesting that blast colonies reflects the first primitive wave of hematopoiesis. The definitive wave of hematopoiesis with lympho-myeloid potential can be identified based on their lymphoid potential, i.e. capacity to produce all types of myeloid cells and T lymphocytes [111].
Lympho-myeloid specification from hPSC can be promoted by inhibiting activin signaling or activating WNT signaling, or by combining both immediately after primitive streak formation [81, 98, 111]. This treatment simultaneously suppresses primitive hematopoiesis while promoting lympho-myeloid hematopoiesis. Similarly, promotion of lympho-myeloid and suppression of primitive hematopoiesis was achieved through forced expression of CDX4 during mesodermal specification [112].
Definitive myeloid-restricted progenitors, EMPs, have not been formally characterized in humans. However, distinguishing this wave of hematopoiesis from primitive hematopoiesis could be made based on their capacity to produce granulocytes, while the lack of lymphoid potential could separate EMPs from definitive lympho-myeloid cells. In defined 2D differentiation systems, cells with EMP properties can be identified by CD34+CD41a/CD235a+CD45+ phenotype [82]. It was also suggested that EMP in human embryo and hPSC cultures can be distinguished from lympho-myeloid progenitors based on the expression of HOXA genes [98].
Despite these advances in understanding hematopoiesis from hPSCs, generating cells with robust engraftment and multilineage-reconstitution potential in vivo remains a significant challenge. It has been shown that low/lack of HOXA cluster gene expression in hPSC-derived hematopoietic progenitors distinguishes them from their fetal liver and AGM counterparts [98, 100, 113]. Although knockdown of HOXA genes in fetal liver HSCs make them more similar to hESC-derived hematopoietic progenitors, which are incapable of engraftment in NSG mice [100], overexpression of HOXA5 and HOXA7 genes in hPSC-derived CD34+ cells [100] or induction of HOXA genes in hPSC differentiation cultures by treatment with TGFβ-inhibitor and WNT activator [98], or retinoic acid agonists [100], fail to endow hPSC-derived hematopoietic progenitors with engraftment potential. However, overexpression of ERG, RUNX1, LCOR and SPI1 transcription factors in addition to HOXA5, HOXA7 and HOXA9 in hPSC-derived HE produced cells capable of multilineage hematopoietic engraftment in primary and secondary mouse recipients [6]. In addition, hematopoietic engraftment in newborn NSG mice was achieved following overexpression of MLL-AF4 lymphoblastic leukemia fusion protein in hiPSC-derived hematopoietic progenitors [114]. Both types of engineered cells still demonstrated molecular and functional differences to bona fide HSCs in their robustness of engraftment and spectrum of terminal differentiation and their potential for leukemic transformation remains unknown.
Conclusion and perspectives
Since the first derivation of hESC in 1998 and hiPSCs in 2007, remarkable progress has been made toward developing systems for efficient hematopoietic differentiation and understanding hematopoietic development from hPSCs. Almost all types of blood cells have been produced from hPSCs and the feasibility of using iPSC-derived T and NK cells for immunotherapy have been demonstrated [115, 116]. Recognizing HE as the immediate precursor of hematopoietic cells and the identification of HE in hPSC cultures has advanced our understanding of hematopoietic development and defined the future directions critical to improving definitive hematopoiesis from hPSC.
It has become clear that HE represents a unique endothelial lineage distinct from nonHE. Accumulating evidence suggest that hematopoietic specification is predetermined at the HE stage of differentiation. Thus, it is critical to define the conditions essential for differentiating HE specifically into cells with long-term engraftment and multilineage reconstitution potential. In vivo observations and recent in vitro hPSC studies demonstrated a close link between arterial specification and HSC development, and this understanding was utilized to define phenotypic markers specific to arterial-type HE with enhanced potential for lympho-myeloid progeny. Although hPSC-derived arterial HE display many features of HE found in the AGM, it remains to be determined whether the bottleneck in HSC specification is due to the lack of appropriate environmental signals for subsequent specification of arterial HE into pre-HSC and more mature HSCs, or whether arterial HE generated in vitro still retains properties of yolk sac arterial-type HE and therefore, inherently lacks engraftment potential. In vivo studies in animal models will be essential to fully understand the differences in hematoendothelial development and hematopoietic potential at different arterial and non-arterial sites of hematopoiesis and translate these findings toward advancing hPSC technologies for HSC production.
Highlightes.
Hemogenic endothelium (HE) is a distinct endothelial lineage
Arterial programming of HE is an essential prerequisite for lymphopoiesis
Arterial-specific markers can be used to identify HE with lympho-myeloid potential
Arterial programming of HE could aid to instruct lymphopoiesis from hPSCs
Alternative models of HSC specification at arterial sites are discussed
Acknowledgments
Funding Sources
IS and GU are supported by funds from the National Institute of Health (R01HL142665, R01HL116221, and P51 RR000167).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- [2].Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- [3].Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009;324:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–1920. [DOI] [PubMed] [Google Scholar]
- [5].Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008;451:141–146. [DOI] [PubMed] [Google Scholar]
- [6].Sugimura R, Jha DK, Han A, et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 2017;545:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rahman N, Brauer PM, Ho L, et al. Engineering the haemogenic niche mitigates endogenous inhibitory signals and controls pluripotent stem cell-derived blood emergence. Nat Commun 2017;8:15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell 2008;3:85–98. [DOI] [PubMed] [Google Scholar]
- [9].Wang L, Menendez P, Shojaei F, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med 2005;201:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vizcardo R, Masuda K, Yamada D, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell 2013;12:31–36. [DOI] [PubMed] [Google Scholar]
- [11].Nayak RC, Trump LR, Aronow BJ, et al. Pathogenesis of ELANE-mutant severe neutropenia revealed by induced pluripotent stem cells. J Clin Invest 2015;125:3103–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest 2009;119:2818–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dias J, Gumenyuk M, Kang H, et al. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev 2011;20:1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sim X, Jarocha D, Hayes V, et al. Identifying and enriching platelet-producing human stem cell-derived megakaryocytes using factor V uptake. Blood 2017;130:192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ditadi A, Sturgeon CM, Keller G. A view of human haematopoietic development from the Petri dish. Nat Rev Mol Cell Biol 2017;18:56–67. [DOI] [PubMed] [Google Scholar]
- [16].Slukvin II. Generating human hematopoietic stem cells in vitro -exploring endothelial to hematopoietic transition as a portal for stemness acquisition. FEBS Lett 2016;590:4126–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Choi KD, Vodyanik MA, Togarrati PP, et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep 2012;2:553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ditadi A, Sturgeon CM, Tober J, et al. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat Cell Biol 2015;17:580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gritz E, Hirschi KK. Specification and function of hemogenic endothelium during embryogenesis. Cell Mol Life Sci 2016;73:1547–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Swiers G, Rode C, Azzoni E, de Bruijn MF. A short history of hemogenic endothelium. Blood cells, molecules & diseases 2013;51:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dzierzak E, Bigas A. Blood Development: Hematopoietic Stem Cell Dependence and Independence. Cell Stem Cell 2018;22:639–651. [DOI] [PubMed] [Google Scholar]
- [22].Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 2010;464:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J 2000;19:2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yzaguirre AD, Speck NA. Insights into blood cell formation from hemogenic endothelium in lesser-known anatomic sites. Dev Dyn 2016;245:1011–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gordon-Keylock S, Sobiesiak M, Rybtsov S, Moore K, Medvinsky A. Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood 2013;122:2338–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ivanovs A, Rybtsov S, Ng ES, Stanley EG, Elefanty AG, Medvinsky A. Human haematopoietic stem cell development: from the embryo to the dish. Development 2017;144:2323–2337. [DOI] [PubMed] [Google Scholar]
- [27].Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol 2008;9:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rybtsov S, Ivanovs A, Zhao S, Medvinsky A. Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development 2016;143:1284–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].North T, Gu TL, Stacy T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 1999;126:2563–2575. [DOI] [PubMed] [Google Scholar]
- [30].Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 2010;464:116–120. [DOI] [PubMed] [Google Scholar]
- [31].Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010;464:112–115. [DOI] [PubMed] [Google Scholar]
- [32].Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 1998;125:4575–4583. [DOI] [PubMed] [Google Scholar]
- [33].Zovein AC, Hofmann JJ, Lynch M, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 2008;3:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nakano H, Liu X, Arshi A, et al. Haemogenic endocardium contributes to transient definitive haematopoiesis. Nat Commun 2013;4:1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jankowska-Steifer E, Madej M, Niderla-Bielinska J, et al. Vasculogenic and hematopoietic cellular progenitors are scattered within the prenatal mouse heart. Histochem Cell Biol 2015;143:153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li Z, Lan Y, He W, et al. Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell 2012;11:663–675. [DOI] [PubMed] [Google Scholar]
- [37].Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development 1998;125:725–732. [DOI] [PubMed] [Google Scholar]
- [38].Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 2004;432:625–630. [DOI] [PubMed] [Google Scholar]
- [39].Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 2009;457:892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development 2010;137:3651–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HKA. The placenta is a niche for hematopoietic stem cells. Developmental Cell 2005;8:365–375. [DOI] [PubMed] [Google Scholar]
- [42].Li W, Ferkowicz MJ, Johnson SA, Shelley WC, Yoder MC. Endothelial cells in the early murine yolk sac give rise to CD41-expressing hematopoietic cells. Stem Cells Dev 2005;14:4454. [DOI] [PubMed] [Google Scholar]
- [43].Frame JM, Fegan KH, Conway SJ, McGrath KE, Palis J. Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. Stem Cells 2016;34:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Goldie LC, Lucitti JL, Dickinson ME, Hirschi KK. Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood 2008;112:31943204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nadin BM, Goodell MA, Hirschi KK. Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood 2003;102:2436–2443. [DOI] [PubMed] [Google Scholar]
- [46].Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A 2011;108:1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yoshimoto M, Porayette P, Glosson NL, et al. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood 2012;119:5706–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen MJ, Li Y, De Obaldia ME, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 2011;9:541552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Clements WK, Traver D. Signalling pathways that control vertebrate haematopoietic stem cell specification. Nat Rev Immunol 2013;13:336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 2002;3:127–136. [DOI] [PubMed] [Google Scholar]
- [51].Lawson ND, Scheer N, Pham VN, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 2001;128:3675–3683. [DOI] [PubMed] [Google Scholar]
- [52].Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell 2005;8:389–400. [DOI] [PubMed] [Google Scholar]
- [53].Burns CE, Galloway JL, Smith AC, et al. A genetic screen in zebrafish defines a hierarchical network of pathways required for hematopoietic stem cell emergence. Blood 2009;113:5776–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Robert-Moreno A, Guiu J, Ruiz-Herguido C, et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J 2008;27:1886–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kumano K, Chiba S, Kunisato A, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 2003;18:699–711. [DOI] [PubMed] [Google Scholar]
- [56].Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development 2005;132:1117–1126. [DOI] [PubMed] [Google Scholar]
- [57].Adams RH, Wilkinson GA, Weiss C, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 1999;13:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chen II, Caprioli A, Ohnuki H, Kwak H, Porcher C, Tosato G EphrinB2 regulates the emergence of a hemogenic endothelium from the aorta. Sci Rep 2016;6:27195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Krebs LT, Xue Y, Norton CR, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- [60].Nakagawa M, Ichikawa M, Kumano K, et al. AML1/Runx1 rescues Notch1-null mutationinduced deficiency of para-aortic splanchnopleural hematopoiesis. Blood 2006;108:3329–3334. [DOI] [PubMed] [Google Scholar]
- [61].Monteiro R, Pinheiro P, Joseph N, et al. Transforming Growth Factor beta Drives Hemogenic Endothelium Programming and the Transition to Hematopoietic Stem Cells. Dev Cell 2016;38:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bertrand JY, Giroux S, Golub R, et al. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proceedings of the National Academy of Sciences of the United States of America 2005;102:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Swiers G, Baumann C, O’Rourke J, et al. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nat Commun 2013;4:2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Richard C, Drevon C, Canto PY, et al. Endothelio-mesenchymal interaction controls runx1 expression and modulates the notch pathway to initiate aortic hematopoiesis. Dev Cell 2013;24:600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lizama CO, Hawkins JS, Schmitt CE, et al. Repression of arterial genes in hemogenic endothelium is sufficient for haematopoietic fate acquisition. Nat Commun 2015;6:7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Guiu J, Shimizu R, D’Altri T, et al. Hes repressors are essential regulators of hematopoietic stem cell development downstream of Notch signaling. J Exp Med 2013;210:7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pardanaud L, Luton D, Prigent M, Bourcheix LM, Catala M, Dieterlen-Lievre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development 1996;122:1363–1371. [DOI] [PubMed] [Google Scholar]
- [68].Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell 2000;102:787–796. [DOI] [PubMed] [Google Scholar]
- [69].Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 2004;18:901911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gale NW, Dominguez MG, Noguera I, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A 2004;101:15949–15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Deng Y, Larrivee B, Zhuang ZW, et al. Endothelial RAF1/ERK activation regulates arterial morphogenesis. Blood 2013;121:3988–3996, S3981–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Duarte A, Hirashima M, Benedito R, et al. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 2004;18:2474–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bertrand JY, Cisson JL, Stachura DL, Traver D. Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood 2010;115:2777–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Marcelo KL, Sills TM, Coskun S, et al. Hemogenic endothelial cell specification requires cKit, Notch signaling, and p27-mediated cell-cycle control. Dev Cell 2013;27:504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 2006;108:2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kumar A, D’Souza SS, Moskvin OV, et al. Specification and Diversification of Pericytes and Smooth Muscle Cells from Mesenchymoangioblasts. Cell Rep 2017;19:1902–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Vodyanik MA, Yu J, Zhang X, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 2010;7:718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 2007;109:2679–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 2008;453:524–528. [DOI] [PubMed] [Google Scholar]
- [80].Elcheva I, Brok-Volchanskaya V, Kumar A, et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat Commun 2014;5:4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol 2014;32:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Uenishi G, Theisen D, Lee JH, et al. Tenascin C promotes hematoendothelial development and T lymphoid commitment from human pluripotent stem cells in chemically defined conditions. Stem cell reports 2014;3:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Uenishi GI, Jung HS, Kumar A, et al. NOTCH signaling specifies arterial-type definitive hemogenic endothelium from human pluripotent stem cells. Nat Commun 2018;9:1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chong DC, Koo Y, Xu K, Fu S, Cleaver O. Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev Dyn 2011;240:2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nottingham WT, Jarratt A, Burgess M, et al. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood 2007;110:4188–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tamplin OJ, Durand EM, Carr LA, et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 2015;160:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ng CE, Yokomizo T, Yamashita N, et al. A Runx1 intronic enhancer marks hemogenic endothelial cells and hematopoietic stem cells. Stem Cells 2010;28:1869–1881. [DOI] [PubMed] [Google Scholar]
- [88].Bee T, Ashley EL, Bickley SR, et al. The mouse Runx1 +23 hematopoietic stem cell enhancer confers hematopoietic specificity to both Runx1 promoters. Blood 2009;113:51215124. [DOI] [PubMed] [Google Scholar]
- [89].Swiers G, Baumann C, O’Rourke J, et al. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nat Commun 2013;4:2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kang H, Mesquitta WT, Jung HS, Moskvin OV, Thomson JA, Slukvin II. GATA2 Is Dispensable for Specification of Hemogenic Endothelium but Promotes Endothelial-to-Hematopoietic Transition. Stem cell reports 2018. [DOI] [PMC free article] [PubMed]
- [91].Fish JE, Wythe JD. The molecular regulation of arteriovenous specification and maintenance. Dev Dyn 2015;244:391–409. [DOI] [PubMed] [Google Scholar]
- [92].Sacilotto N, Monteiro R, Fritzsche M, et al. Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci U S A 2013;110:11893–11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wythe JD, Dang LT, Devine WP, et al. ETS factors regulate Vegf-dependent arterial specification. Dev Cell 2013;26:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Park MA, Kumar A, Jung HS, et al. Activation of the Arterial Program Drives Development of Definitive Hemogenic Endothelium with Lymphoid Potential. Cell Rep 2018;23:2467–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol 2006;16:13661372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Herbert SP, Huisken J, Kim TN, et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 2009;326:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhang J, Chu LF, Hou Z, et al. Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proc Natl Acad Sci U S A 2017;114:E6072–E6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ng ES, Azzola L, Bruveris FF, et al. Differentiation of human embryonic stem cells to HOXA(+) hemogenic vasculature that resembles the aorta-gonad-mesonephros. Nat Biotechnol 2016;34:1168–1179. [DOI] [PubMed] [Google Scholar]
- [99].Chanda B, Ditadi A, Iscove NN, Keller G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell 2013;155:215–227. [DOI] [PubMed] [Google Scholar]
- [100].Dou DR, Calvanese V, Sierra MI, et al. Medial HOXA genes demarcate haematopoietic stem cell fate during human development. Nat Cell Biol 2016;18:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].He Q, Zhang C, Wang L, et al. Inflammatory signaling regulates hematopoietic stem and progenitor cell emergence in vertebrates. Blood 2015;125:1098–1106. [DOI] [PubMed] [Google Scholar]
- [102].Li Y, Esain V, Teng L, et al. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev 2014;28:2597–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Heo HR, Chen L, An B, Kim KS, Ji J, Hong SH. Hormonal regulation of hematopoietic stem cells and their niche: a focus on estrogen. Int J Stem Cells 2015;8:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kim HR, Lee JH, Heo HR, et al. Improved hematopoietic differentiation of human pluripotent stem cells via estrogen receptor signaling pathway. Cell Biosci 2016;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kim PG, Nakano H, Das PP, et al. Flow-induced protein kinase A-CREB pathway acts via BMP signaling to promote HSC emergence. J Exp Med 2015;212:633–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].North TE, Goessling W, Peeters M, et al. Hematopoietic stem cell development is dependent on blood flow. Cell 2009;137:736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 2009;27:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Klimchenko O, Mori M, Distefano A, et al. A common bipotent progenitor generates theerythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood 2009;114:1506–1517. [DOI] [PubMed] [Google Scholar]
- [109].Paluru P, Hudock KM, Cheng X, et al. The negative impact of Wnt signaling on megakaryocyte and primitive erythroid progenitors derived from human embryonic stem cells. Stem Cell Res 2014;12:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Davis RP, Ng ES, Costa M, et al. Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood 2008;111:1876–1884. [DOI] [PubMed] [Google Scholar]
- [111].Kennedy M, Awong G, Sturgeon CM, et al. T lymphocyte potential marks the emergenceof definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep 2012;2:1722–1735. [DOI] [PubMed] [Google Scholar]
- [112].Creamer JP, Dege C, Ren Q, et al. Human definitive hematopoietic specification frompluripotent stem cells is regulated by mesodermal expression of CDX4. Blood 2017;129:29882992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Salvagiotto G, Zhao Y, Vodyanik M, et al. Molecular profiling reveals similarities and differences between primitive subsets of hematopoietic cells generated in vitro from human embryonic stem cells and in vivo during embryogenesis. Exp Hematol 2008;36:1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tan YT, Ye L, Xie F, et al. Respecifying human iPSC-derived blood cells into highly engraftable hematopoietic stem and progenitor cells with a single factor. Proc Natl Acad Sci U S A 2018;115:2180–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Themeli M, Kloss CC, Ciriello G, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol 2013;31:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018;23:181–192 e185. [DOI] [PMC free article] [PubMed] [Google Scholar]


