Summary
Tuberculosis claims more human lives than any other infectious disease. This alarming epidemic has fueled the development of novel antimicrobials and diagnostics however, public health interventions that interrupt transmission have been slow to emerge, particularly in HIV-endemic settings. Transmission of tuberculosis is complex, involving various environmental, bacteriologic, and host factors among which concomitant HIV infection is of critical importance. Preventing person-to-person spread is central to halting the epidemic and consequently, tuberculosis transmission is now being studied with renewed interest. Herein, we review recent advances in the understanding of tuberculosis transmission from the view of source case infectiousness, inherent susceptibility of exposed individuals, appending tools for predicting risk of disease progression, the biophysical nature of the contagion and the environments wherein transmission occurs and is sustained in populations. We focus specifically on how HIV infection affects these aspects with a view to describing novel transmission blocking strategies for HIV-endemic settings.
Background
Despite worldwide vaccination, potent therapeutic intervention and the development of faster diagnostics, tuberculosis defiantly stands out as the most lethal infectious disease humanity faces today, wreaking havoc on impoverished communities in the developing world. In Africa, HIV co-infection has remained a key driver of the tuberculosis epidemic for almost two decades as the two diseases synergize to present unique programmatic and treatment challenges.1,2 To achieve a rapid reduction in the burden of tuberculosis, new interventions to reduce transmission are needed; however, efforts to develop these have been thwarted by a relatively poor understanding of how transmission occurs and how this process is affected by HIV co-infection.3
Successful transmission is reliant on many factors, including specific features of the index case, the susceptibility of the exposed host, behavior of bio-aerosols, pathogen inherent factors, and the environment wherein transmission occurs.4–6 Sustained community-wide transmission also requires certain social mixing elements and the associated movement of infectious bacilli between individuals.7 In light of this, eliminating transmission depends on the ability to detect and treat infected individuals at highest risk of becoming infectious, using new prognostic and diagnostic biomarkers to target preventive and curative therapies. Herein, we review these key features of transmission in the context of HIV co-infection with an outlook to developing novel transmission blocking strategies. This review represents a synthesis of discussions that took place at an NIH/SAMRC/Bill and Melinda Gates Foundation-funded workshop on tuberculosis transmission in HIV-endemic settings in South Africa, May 1–2, 2017.
Why is a focus on tuberculosis transmission important in HIV-endemic settings?
In HIV-endemic settings, the increased risk of active and recurrent tuberculosis due to HIV infection is well established.8–10 However, many questions remain about the mechanisms through which HIV affects tuberculosis transmission, as well as the influence of antiretroviral therapy (ART) in mitigating this effect, at the individual and population level. Increasing attention on vulnerable populations in tuberculosis and HIV-endemic settings has provided opportunities for identifying concentrated tuberculosis epidemics and therefore transmission hotspots or reservoirs.11–13 These vulnerable populations include miners, children, migrants, prisoners, sex workers, people who use drugs or alcohol, and people living with HIV.14–19 There is also evidence suggesting substantial transmission between these populations and the general population.20–22 Thus, targeted interventions to limit transmission in these populations may provide substantive benefit in the fight to improve tuberculosis control in the broader population.20,23,24
Tuberculosis transmission
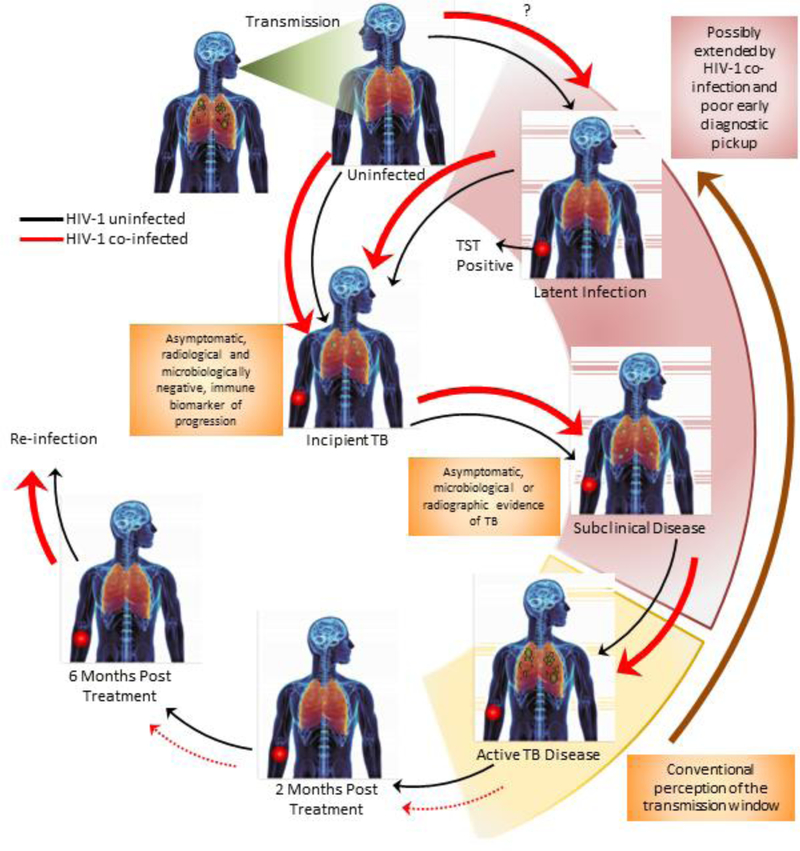
The increase in the incidence of tuberculosis concurrently with the surge of the HIV pandemic in sub-Saharan Africa implies noteworthy transmission among HIV-positive individuals.25 Transmission of tuberculosis requires the expulsion of viable tubercle bacilli from an active source case in the form of aerosolized droplet particles.26 These bio-aerosols are then taken up by an exposed, susceptible host through the normal process of breathing. Following uptake, two scenarios can occur. The first is elimination of bacteria with no lasting immunological signature of infection. Alternatively, a combination of adaptive and innate immunity can ultimately lead to containment of bacteria in the form of latent tuberculosis infection (LTBI, figure 1).27 In a small subset estimated at 5–10%, and much higher for HIV-infected individuals, containment is lost, resulting in active disease.27,28 This can be preceded by a phase of incipient tuberculosis, defined as early asymptomatic disease that is microbiologically and radiologically negative, yet may be identified by an immune biomarker for progression from infection to disease.29,30 This state is followed by subclinical disease, defined as asymptomatic, pulmonary disease that is radiologically/microbiologically detectable (figure 1).30 Progression to active disease results in destruction of infected lung tissue leading to the formation of cavities, which then leak bacteria into the airways to allow for transmission.31 Considering this, the progression and intensity of lung damage in an infected individual will directly affect their transmission potential. HIV coinfection severely alters disease presentation and pathology; how this affects the ability to transmit remains unresolved.1,32–34 HIV infection may enhance the proportion of successful transmission events, leading to infection and/or disease, and thus plays a significant role in remodeling transmission dynamics in the community.
Figure 1. Effects of HIV-1 infection on the progression of tuberculosis disease and the transmission window.
Progression to active, pulmonary tuberculosis disease involves a complex sequence of events that is dependent on initial exposure intensity and the interplay between innate and acquired immunity. Thicker arrows represent an enhanced risk and broken arrows indicate poor progression/reversion to the next/preceding phase. Red arrows indicate the sequence in the case of tuberculosis-HIV infection. Most cases of exposure lead to the development of asymptomatic latent tuberculosis infection (LTBI), which is characterized by an immunological response to mycobacterial antigens (using the tuberculin skin test [TST]). This can progress to incipient tuberculosis, which is defined as asymptomatic disease with no radiological or microbiological signs of disease but can be distinguished from LTBI by an immune biomarker progression from infection to disease. Subclinical disease develops thereafter and is classified as asymptomatic disease associated with either radiological or microbiological confirmation of tuberculosis. In some cases, individuals can spontaneously convert their TST to become uninfected again. HIV co-infection alters the dynamics of immune control and can favour progression to infection, in exposed individuals, and/or progression to incipient tuberculosis. It remains unclear if HIV infection increases the risk of becoming infected with LTBI. The risk of progression to active pulmonary tuberculosis can also be substantively enhanced in the presence of HIV co-infection. Conventionally, the transmission window (shown in yellow) has been assumed to circumscribe the development of granulomatous tuberculosis disease, with a high bacterial load and cavities. HIV co-infection can potentially extend this window (shown in pink) by (I) creating a larger proportion of individuals with incipient/subclinical tuberculosis, within whom the propensity to transmit is as yet unclear (II) creating a larger pool of actively diseased individuals who can transmit and (III) presenting poor treatment outcomes due to extra-pulmonary tuberculosis and drug-drug interactions.
The transmission window
The spectrum of disease presentation and pathology suggests that tuberculosis symptoms are likely to develop over a protracted time frame, rather than during a small window.35–37 Individuals may expectorate organisms prior to the onset of symptoms36 and may further transmit for months before to seeking medical care. This delay in treatment initiation can prolong the transmission window.38,39 This is particularly problematic in high prevalence areas when associated with social tendencies that promote transmission to large numbers of people such as the use of public transport.40 Additionally, after individuals present to the healthcare system, there are often delays in diagnosis and initiation of treatment, due to weak health systems,41 which can further extend the transmission window. It is unclear whether HIV infection shortens or prolongs the transmission window (figure 1).42–44
The source case and transmission: Impact of HIV co-infection
Prevailing evidence suggests that HIV is associated with reduced infectiousness for various reasons, including decreased sputum bacillary load, a reduction in cavitary disease, and shorter duration of exposing others due to more rapid progression to death or diagnosis45,46 (table 1). A meta-analysis on the infectiousness of HIV-infected individuals demonstrated that overall rates of tuberculin skin test conversion of household contacts were similar regardless of HIV sero-status of index cases (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.23–1.84). Furthermore, the likelihood of infected contacts to develop active disease was also similar regardless of HIV sero-status of index cases (OR, 1.17; 95% CI, 0.78–1.56), suggesting that HIV-infected tuberculosis patients are not more infectious compared to their HIV uninfected counterparts.47 A meta-analysis incorporating 37 studies reported that smear positivity and cavitary disease resulted in a higher likelihood of infectivity (adjusted OR, 2.15 [95% CI; 1.47–3.17], I2=38% and 1.9 [95% CI 1.26–2.84], I2=63% respectively). Both of these attributes are known to be reduced in HIV-infected individuals, hence in this meta-analysis, the infectiousness of HIV-infected individuals was reduced (AOR, 0.45 [CI 0.26– 0.80], I2=52%).48 A study in Uganda reported decreased infectiousness of HIV infected tuberculosis index cases with smear-negative tuberculosis or non-cavitary disease, compared to HIV-uninfected tuberculosis cases. However, there was no difference in infectiousness among HIV infected tuberculosis index cases who had positive sputum smears or cavitary disease.49 This suggests that disease severity of the index case may modify the infectiousness of tuberculosis cases coinfected with HIV. Furthermore, a study on intravenous drug users revealed that smear positive pulmonary tuberculosis patients who were HIV-seropositive were more likely to transmit tuberculosis, suggesting that bacterial load in the sputum is an important indicator of transmissibility, even in the context of HIV.50 However, this study assessed smear positive cases, with no comparison to smear negative tuberculosis.
Table 1.
Recent Studies Investigating the Differential Infectiousness of Tuberculosis Patients, based on their HIV-status and determined by a positive TST in household contacts of tuberculosis cases
| Study | HIV index strata* | Relative Risk**(95% CI) | Adjusted Relative Risk**(95% CI) |
|---|---|---|---|
| Previous Meta- analysis | |||
| Cruciani, 200147 | HIV-negative | 1 (Referent) | – |
| HIV-positive | 0.66 (0.60 – 0.72) | – | |
| Recent studies¶ | |||
| Huang, 201433 | HIV-negative | 1 (Referent) | 1 (Referent) |
| HIV-positive with a CD4 count ≥250 | 0.9 (0.6 – 1.3) | 0.9 (0.5 – 1.5)† | |
| HIV-positive with a CD4 count <250 | 0.6 (0.4 – 1.1) | 0.5 (0.3 – 0.9)† | |
| Martinez, 201649 | HIV-negative with smear positivity | 1 (Referent) | 1 (Referent) |
| HIV-positive with smear positivity | 0.94 (0.86 – 1.04) | 0.93 (0.85 – 1.01)† | |
| HIV-negative with smear negative results | 1 (Referent) | 1 (Referent) | |
| HIV-positive with smear negative results | 0.75 (0.63 – 0.90) | 0.76 (0.64 – 0.90)† | |
| HIV-negative with cavitary disease | 1 (Referent) | 1 (Referent) | |
| HIV-positive with cavitary disease | 1.07 (0.97 – 1.17) | 1.03 (0.96 – 1.12)† | |
| HIV-negative with cavitary disease | 1 (Referent) | 1 (Referent) | |
| HIV-positive with noncavitary disease | 0.75 (0.65 – 0.87) | 0.74 (0.65 – 0.85)† | |
| Khan, 201754 | HIV-negative | 1 (Referent) | 1 (Referent) |
| HIV-positive with ART for ≥1 year | 0.3 (0.1–0.9) | 0.4 (0.1–1.3)†† | |
| HIV-positive with no ART or ART<1 years | 0.2 (0.1–0.7) | 0.5 (0.2–1.3)†† |
All index cases have tuberculosis disease. This column stratifies tuberculosis index cases by their HIV status and other secondary modifying variables related to the severity of HIV or tuberculosis disease.
Adjusted for age, sex, smoking status, alcohol intake, nutritional status, number of BCG scars, household smoke exposure, relation to the tuberculosis case from household contacts; age, sex, cavitary lung disease, smear status, and treatment delay from tuberculosis cases.
Adjusted for age, education level, and alcohol status of the household contact; sputum smear and cavitary status of the tuberculosis case; and the number of individuals in the household.
Adjusted for age, sex of the household contact; degree of exposure of the household contact to the index case; sputum smear, age, and sex of the tuberculosis index case; whether index case was the mother of the contact, number of adults in household, household clustering; household socioeconomic status and duration of symptoms.
A positive tuberculin skin test was defined as an induration reaction ≥10-millimeters for all three recent studies. For Huang, 2014, a positive tuberculin skin test for household contacts that were HIV-positive was differentially defined as an induration reaction ≥5-millimeters. Khan, 2017 included only child household contacts 2–10 years of age while Huang, 2014 and Martinez, 2016 included contacts of all ages.
Measures of association here represent the relationship between positive tuberculin skin test results in household contacts exposed to HIV-negative index cases and household contacts exposed to varying types of HIV-positive index cases (high versus low CD4 count; cavitary versus noncavitary tuberculosis disease; antiretroviral therapy versus no or recent antiretroviral therapy). All crude models here are adjusted for household clustering while Huang, 2014 is also adjusted for age of the household contact. Martinez, 2016 and Huang, 2014 use modified Poisson regression models to derive relative risks while Khan, 2017 calculated crude and adjusted odds ratios. The Khan, 2017 manuscript reports odds ratios with “HIV, no ART and ART<1 year” as the reference category, not HIV-negative index cases as is shown here. Odds ratios shown here were kindly supplied by the authors.
In two studies in Peru and Botswana, household contacts of HIV-positive tuberculosis cases with low CD4 counts had substantially less LTBI compared to household contacts of HIV-negative index cases.33,51 This suggests that, infectiousness may be reduced in the setting of advanced HIV disease. It has been hypothesized that a depleted CD4 T-cell count in advanced HIV is associated with reduced lung inflammation leading to a reduction in cavities and sputum bacillary load, both of which impact infectiousness. In support of this, in South African mineworkers living with HIV, the proportion of patients with lung cavitation increased as CD4 counts increased; however, sputum smear bacillary load was not linearly associated with CD4 count52. Taken together, these studies suggest that HIV may alter infectiousness in some settings by reducing the probability that individuals develop smear-positive or cavitary disease, and that this effect may be particularly important in the context of advanced HIV disease.
ART may have implications for the infectiousness of HIV-positive tuberculosis patients through the restoration of immune function, which may increase the risk of smear-positive and cavitary disease. However, in a study in South Africa, ART was not associated with increased sputum smear positivity or lung cavitation, indicating that the ability to generate large amounts of bacteria in sputum is driven by a complex array of factors.53 A recent household contact study in Malawi found no difference in infectiousness between HIV-positive patients on and off ART54 (Table 1). This study however did not measure CD4 count.55 As ART has rendered HIV infection a chronic disease, the sheer number of these survivors with tuberculosis, and the length of time that they remain in the community, may be sufficient to contribute substantially to the overall tuberculosis burden.56 Further research is needed to illuminate the effect of ART of tuberculosis transmission dynamics in settings with a high burden of both HIV and tuberculosis.
An alternate explanation of why tuberculosis HIV-infected cases seem to infect fewer close contacts is that persons living with HIV on ART may be diagnosed with tuberculosis sooner due to increased contact with the healthcare system. As ART is lifelong, regular follow-up visits offer intensified tuberculosis case finding and prompt treatment, thus reducing the risk of transmission at a population level.57 As HIV epidemics continue to mature, and due to the uncertainties concerning the influence of further uptake of ART, additional studies are needed to improve understanding of tuberculosis transmission in HIV-endemic settings.58
Impact of HIV on susceptibility to tuberculosis
The impact of HIV infection on the risk of developing active tuberculosis is unmistakable, often demonstrated by increased estimates of HIV prevalence among those with tuberculosis compared to those without it.59,60 A study among South African gold miners showed a marked increase in HIV prevalence accompanied by a quadrupling of annual tuberculosis case-notification rates during the same time period.61 The relationship between HIV and susceptibility to tuberculosis infection rather than disease progression is more difficult to demonstrate, though suggested when recent tuberculosis transmission can be proven. A study in a Catalonian prison with a high proportion of HIV-positive individuals indicated recent transmission rather than reactivation of LTBI as a driver of tuberculosis incidence.62 A meta-analysis of tuberculosis incidence in HIV-positive individuals showed that incidence rates were higher in individuals with lower CD4 counts, which was dependent on the tuberculosis burden in the immediate community, suggesting that reduced immunity enhances LTBI acquisition.63
Another phenomenon of interest is the high rates of tuberculosis recurrence among patients in tuberculosis and HIV high-burden settings, reported among 5 to 20% of patients who have completed tuberculosis treatment.64–67 A number of molecular epidemiological studies in these settings found that the prevailing underlying mechanism for this depends on the time since the end of treatment. Relapse is more common in the first year, which is superseded by reinfection thereafter.68–72 HIV infection was strongly associated with increased risk of reinfection in three of four studies.64,66,69,71 Tuberculosis incidence is significantly reduced in HIV-positive individuals starting ART with CD4 counts above 500 cells/µl.73,74 Coupled with early detection, early ART initiation is strongly protective, reducing the risk of active tuberculosis in HIV-infected children.75
Host genetic factors that modulate susceptibility to infection
Susceptibility to tuberculosis infection is primarily determined by assessing immunological response to the Mycobacterium tuberculosis complex. In this case, there is a disproportionate reliance on CD4+ T cell acquired immunity against mycobacterial antigens either by the TST or interferon-γ release assays (IGRAs). In HIV-positive patients these assays are less sensitive which reduces their negative predictive value in high transmission settings.76 The presence of apparently infection-resistant persons, termed “resistors”, is historically well documented in HIV-negative individuals.77,78 In contrast, the impact of HIV-positive infection resistors on TB transmission is understudied. As HIV-positive individuals display significantly increased susceptibility to tuberculosis, it is clear that the virus abrogates critical host resistance mechanisms.
In HIV negative subjects, a significant contribution of host genetic factors to TST and IGRA reactivity have been demonstrated,79–81 leading to studies aimed at identifying mediators of infection resistance (table 2). A genome-wide linkage analysis of TST-negativity in Uganda identified protective determinants on chromosomes 2 and 5.82 Another candidate gene study in West Africa implicated an IL10 haplotype associated with low circulating levels of the IL10 cytokine with M. tuberculosis infection resistance.83 A genome-wide linkage study in Cape Town, South Africa, linked TST negativity to a major locus termed TST1 on loci 11p14. TST response (induration in mm) was linked to another locus termed TST2 on loci 5p15.84 The TST1 locus was confirmed in a second independent sample from the Paris region85 and was genetically indistinguishable from an independently mapped locus termed TNF186 suggesting that infection resistance may be linked to the secretion of TNF, a well-established anti-tuberculosis effector molecule. Implication of TNF in infection resistance is supported by the observation of genetic variants in the ULK1 and TOLLIP genes as risk factors for TST-positivity.87,88 ULK1 is involved in the regulation of TNF secretion while TOLLIP is part of the TLR signaling cascade leading to TNF production.
Table 2.
Summary of host genetic factors that affect outcome of TB transmission
| Chromosome | Gene | TST/IGRA | Resistance/Susceptibility | Possible Mechanism |
|---|---|---|---|---|
| 2 and 5 | IL10 haplotype | TST | Resistance | Unresolved82 |
| Resistance | Low circulating IL1083 | |||
| 11p14 | TST1 | TST | Resistance | Controls lack of TST response84,85 |
| 5p15 | TST2 | TST | Resistance | Controls extent of TST response84 |
| 6p25 | DRB1/DQA1 | TST | Susceptibility | TST-positivity144 |
| 11p14 | TNF1 | TST | Resistance? | Controls TNF production upon exposure to BCG86 |
| ULK1 | TST | Susceptibility | Role in TNF production88 | |
| TOLLIP | TST | Susceptibility | Role in TNF production87 | |
| IL9 | TST | Resistance | Mast cells89 | |
| 8q12-q22 | IGRA | Resistance | IFNᵧ production on BCG exposure90 | |
| 3q 13-q22 | IGRA | Resistance? | IFNᵧ production on BCG and ESAT-6 exposure90 | |
| ZXDC | IGRA | Resistance? | IFNᵧ release in response to ESAT-691 |
A recent study, focused on a small sample of HIV-positive subjects who remain uninfected in the presence of documented exposure, reported strong genetic association with degree of TST positivity in the vicinity of the IL9 gene. This implicated mast cells in infection resistance, which is an attractive proposal as IL9 variants have previously been associated with pulmonary hyper-responsiveness.89 While the main locus appears specific for HIV-infected patients, the study also detected the loci on chromosomes 2 and 5 previously identified in HIV-negative patients.
Genome-wide linkage analyses in HIV-negative subjects identified two major loci, one on chromosome region 8q12-q22, which impacted on interferon-γ production following stimulation of whole blood with live BCG, and a second on 3q13-q22 was implicated in interferon-γ release following stimulation with ESAT-6 (table 2).90 The association of chromosome 3 with interferon-γ production via the ZDXC gene has been confirmed in a separate study.91 These preliminary findings underscore the need for more comprehensive analysis of protective genomic biomarkers. Moreover, in people who do not resist infection, but are able to contain it in the form of LTBI, biomarkers for identifying those individuals who are likely to lose bacterial containment and progress to disease would be of tremendous utility in targeting resources for preventive therapy.
Transcriptomic biomarkers for early diagnosis and screening for prevention of transmission
There is now a growing realization that individuals with asymptomatic and undiagnosed subclinical disease may also contribute to transmission.36,37,92 This raises the question as to whether all individuals with LTBI should be treated preventatively in order to interrupt the cycle of transmission. However, in high incidence HIV-tuberculosis-endemic settings where force of infection may exceed 10% per annum, with between 60–80% of adults infected, such an approach would not be feasible.93 Rather, attention has shifted to identification of individuals who are at high risk of imminent progression from LTBI to disease, which may provide an opportunity for targeted intervention.29,30
Recent studies have described prognostic transcriptomic signatures of risk for tuberculosis progression that may identify a state of incipient or subclinical tuberculosis, clinically indistinguishable from LTBI, but differentiated on the basis of an evolving host RNA signature.30,94 A 16-gene tuberculosis risk classification model, or Correlate of Risk (COR), was discovered within a study of more than 5000 South African adolescents and validated using a qRT-PCR platform to allow high volume testing in a research setting.94 The COR was able to discriminate between HIV-uninfected adults who would or would not progress to active tuberculosis with 66% sensitivity and 80% specificity for disease occurring within 12 months of testing.94 A number of diagnostic transcriptomic signatures have also been described in both HIV-infected and HIV-uninfected people.95–98 When applied to published microarray data from tuberculosis diagnostic studies, the COR demonstrates an area under the receiver operating characteristic curve ranging from 0·86–0·99 for discrimination between active tuberculosis and LTBI or uninfected individuals.94 These performance characteristics suggest that a biomarker-targeted screen & treat strategy might be feasible, using the COR to triage persons for definitive sputum investigation, followed by curative therapy for those sputum positive or preventive therapy for those sputum negative; and a negative COR result to identify those who need no intervention.99 Inclusion of HIV-infected individuals in such a strategy is crucial for impact in endemic countries.
Studying the process of transmission: Aerobiology and the effect of HIV-infection
The current state of the art on airborne transmission of tuberculosis has been built on the pioneering studies of Richard Riley and colleagues nearly seventy years ago.100,101 Vented air from patients with pulmonary tuberculosis was shown to produce tuberculosis infection in guinea pigs and the concept of infectious ‘quanta’ was developed by defining the infectious dose required to produce a guinea pig infection. The numerical value of quanta of infection was defined within a specific animal study incorporating many parameters such as particle size, environmental conditions, sampling volume, ventilation rate, lung deposition fraction, and time dependent bacterial survival.102 More recent experimental measurements of bio-aerosol infectivity have shown a wide variation in infectivity between tuberculosis diagnosed individuals. Riley and colleagues reported that bio-aerosols from 8 of 61 tuberculosis patients (13%) were able to infect guinea pigs with an estimated mean infectious dose production of 1·25 per hour.101 Recently, similar studies have been extended to encompass HIV-infected individuals. In Peru, a study of tuberculosis ward admissions, all of whom were HIV-infected, revealed a substantive variation in infectiousness.103 In a subsequent study of ward admissions (82% HIV-positive), only a small proportion of HIV-infected MDR cases were responsible for 90% of infection transmitted to guinea pigs.104 These observations point to drug resistance and poor treatment as substantive drivers of the transmission potential of HIV-infected tuberculosis patients. However, pathogen related factors may be important in transmission as various animal models of tuberculosis disease have demonstrated that some strains are more transmissible than others.105,106
Studies of surfactant concentrations in exhaled bio-aerosols have shown that different respiratory activities generate bio-aerosols from different airway regions. Cough and deep sighs (exhalation after deep inspiration) generate particles from large airways and peripheral airways respectively.107 A strong cough will lead to a wider trajectory, particularly of large particles derived from the trachea and large airways. Exhalation after deep inspiration will produce larger numbers of small particles derived from the peripheral lung spaces, which will remain airborne for longer. How HIV infection affects the strength and frequency of respiratory maneuvers remains an unresolved question. HIV co-infection and reducing CD4 T-cell counts may also affect the architecture of the tuberculosis infected lung and impact on bio-aerosol particle production. Monitoring cough strength and frequency, together with bio-aerosol quantity and particle composition, in both HIV-infected and non-infected individuals could help identify infectious persons and implement measures for prevention of transmission.108–110
Bio-aerosol characterization of 34 newly diagnosed untreated tuberculosis cases (50% of whom were HIV-co-infected) in a Respiratory Aerosol Sampling Chamber (RASC) revealed that total bio-aerosol production was found to vary between 50 μm3 and 500 μm3 per liter of exhaled air.109 Electron microscopy of impacted bio-aerosols demonstrated multiple structures <5 microns consisting of organic material and the infrequent observation of bacilli-like structures.110 Fennelly and colleagues isolated M. tuberculosis colony forming units (CFUs) during 5 minutes of forced coughing from 27·7% of patients with a mean of 16 CFUs and a wide distribution that varied from 1–701 CFUs.111 A follow-up study of patients demonstrated that isolation of M. tuberculosis positive cough aerosols was associated with ongoing household transmission of tuberculosis, suggesting that the presence of CFUs was necessary for transmission.112 Similarly, Pattersen and colleagues isolated M. tuberculosis CFUs from 42·8%, and a combination of DNA and CFUs from 77%, of newly diagnosed tuberculosis patients using a RASC.110 As tuberculosis-HIV co-infected patients carry lower lung bacterial loads, these observations suggest that bio-aerosols from these patients are likely to harbour fewer culturable bacilli.
Differences in the proportion of patients identified with culturable bacilli in their bio-aerosols may in part be due to low sensitivity and the volume of bio-aerosol sampled. Considering this, a combination of high sensitivity detection and large air sampling volumes will be required in order to detect patients with lower levels of infectivity, such as sputum smear negative individuals, with HIV co-infection and low CD4 T-cell counts. An alternate explanation for the varying levels of CFUs detected could be that the tubercle bacilli in bio-aerosols adopt a differentially culturable state. Several studies have highlighted the presence of a population of differentially culturable tubercle bacilli in the sputum of treatment naïve individuals.113,114 These organisms are unable to grow on plates but can be recovered in liquid media supplemented with culture filtrate as a source of growth stimulatory molecules.113,114 Whether such organisms are present in bio-aerosols and whether they are important for the transmission process remains an open and exciting area for future study. Further resolution on this is important as individuals with low CFUs may contribute to endemic tuberculosis transmission in crowded, poorly ventilated environments.115
Transmission of tuberculosis in the community and institutional amplifiers
Achieving a clear understanding of where tuberculosis is transmitted and between whom, remains a vexing problem due to key attributes of tuberculosis described above. In this regard, molecular epidemiological tools such as genotyping with IS6110 elements or more recently, whole genome sequencing has allowed for greater understanding of transmission dynamics in the community.116,117 A particular problem with tuberculosis is the prolonged and highly variable duration of infectiousness, providing numerous opportunities for transmission, combined with airborne infection, which may occur without close contact. As a result, in endemic settings, only a small minority of transmission events can be linked between individuals using social network and/or molecular epidemiologic methods.118–120 Recently, several studies have identified high-risk environments for transmission potential in high HIV burden settings by mapping social interactions, including age-specific mixing patterns, in various private and public environments, as well integrating data on ventilation in these settings.118,121,122 These studies have implicated bars, schools, workplaces and public transit as sites for transmission potential. In this context, targeting interventions to populations within institutions that may drive tuberculosis has emerged as a potentially promising avenue for tuberculosis control.11,20,24,122
“Institutional amplifiers” or “reservoirs” of tuberculosis transmission represent settings where disease and transmission are concentrated within communities123. These institutions or locations are typically characterized by environments that are highly conducive to tuberculosis transmission, including high rates of indoor contact, poor ventilation, comorbidities influencing risk of infection and/or disease, and limited access to timely diagnosis and treatment. Several types of institutional drivers have been described in the literature including mines, hospitals, prisons, slums and homeless shelters. Many of these institutions are also highly HIV endemic.124–126
Prisons have been consistently identified as high tuberculosis burden institutions throughout the world, from low- to high-income countries.23,127–129 A recent systematic review found that tuberculosis incidence rates in prisons were a median of 23 times greater than that of their reference populations.127 Crowding, poor ventilation, malnutrition, poor access to tuberculosis care, HIV co-infection, smoking and drug use have all been cited as common factors driving the extraordinarily high incidence of tuberculosis infection and disease reported in prisons globally. While prisons may account for less than 10% of tuberculosis cases in the general population from low- and middle-income countries,23,130 their impact on community tuberculosis rates may be underestimated due to disease that occurs after release from prison, driving spillover of tuberculosis from prisons into communities.131 This was demonstrated in the context of a large tuberculosis outbreak in the United States in the mid-1990s, in which community tuberculosis cases genotypically matched those seen in the prisons.132,133 A recent study from Brazil demonstrated that one quarter of community tuberculosis cases occurred among ex-prisoners, and that over half of the remaining cases were genetically linked to strains observed among prisoners.134 A study from Moldova found that multidrug-resistant tuberculosis in the community was geographically congregated in areas with high numbers of former prisoners.135 This suggests that prisons in Eastern Europe, known to be seedbeds for transmission of drug resistant tuberculosis,129,136 may be contributing to the spread of multidrug resistant tuberculosis upon prisoner release. Despite these recent suggestive results, the amount of tuberculosis that is attributable to prisons may be, at least partially, dependent on setting-specific features such as the overall prisoner population, contact between prisoner or ex-prisoner populations and the general community, and average duration of incarceration. Further study is needed to determine whether, and to what extent, tuberculosis in prisons leads to meaningful spillover into the general population.
Mines in sub-Saharan Africa have extraordinarily high tuberculosis transmission rates due to crowding in poorly ventilated environments, exposure to silica dust, high HIV prevalence and low socioeconomic status.137,138 Seasonal migratory patterns by miners put the general population at substantial risk for tuberculosis transmission spillover from mines. In one study, the number of mines in a population was directly correlated to population tuberculosis incidence, prevalence, and mortality.139 Similarly, healthcare facilities have been recognized as institutional drivers of tuberculosis for over a century. Nosocomial transmission was identified as a key driver in MDR-tuberculosis outbreaks in New York in the 1990s; more recently, XDR-tuberculosis was associated with large hospital outbreaks in South Africa. In both settings, HIV co-infection played a major role.140–142 Recent reports have found that “programmatically incurable” XDR-tuberculosis patients, the vast majority of whom are HIV-positive, from hospital settings are released back into the community, possibly contributing to further transmission.141
Understanding institutional amplifiers and how HIV infection interplays with this phenomenon could lead to more efficient deployment of interventions. Using a mathematical model and drawing upon tuberculosis incidence data across Rio de Janeiro, Dowdy and colleagues demonstrated that interventions targeted at few high-burden slums, containing just six percent of the city’s population, could have the same overall impact as achieving tuberculosis control in the remaining population.143 To understand the potential for institutions or settings to serve as important amplifiers of tuberculosis incidence at the population level, several key attributes must be understood. These include (1) the relative size of the high-risk population compared with the overall population; (2) relative incidence of LTBI and disease (for which HIV is an important factor); (3) the level of in- and outflow migration from the institution or setting and the general population; and (4) degree of social mixing between members of the institution and the general population after re-joining the general population. Among these, perhaps the most challenging to estimate are the contact rates between the high-risk population and the general population. Molecular epidemiologic studies may provide one avenue for understanding transmission between these groups.
Conclusions and forecast
Transmission of tuberculosis remains a multifaceted, poorly understood process that is further complicated by HIV infection, which impacts on the ability of individuals to transmit while at the same time creating a large pool of high susceptible individuals to drive further disease in the community setting. Understanding the effect of antiretroviral therapy on the infectiousness of HIV-positive tuberculosis cases, together with determining how HIV infection modulates the risk of reinfection or relapse in individuals with a history of tuberculosis, should feature as an important and immediate priority. Intensifying integration of HIV-tuberculosis control programs is also likely to have a great impact on limiting diagnostic delays, early case detection, prompt treatment onset and ultimately reduction of transmission. Effort should also be focused on investigating the proportion of tuberculosis cases in the general population that are attributable to institutional amplifiers and what interventions can be directed towards these amplifiers to lower the intra-institution high force of infection and subsequently prevent future community-based cases. Collectively, a greater focus on limiting transmission promises to deliver significant gains in controlling tuberculosis.
Methods for literature searches
We searched PubMed for English-language articles with the search terms “Tuberculosis”[Mesh] AND “HIV”[Mesh] AND transmission, which returned 99 results. After reviewing the results for relevance, we generalized the PubMed search with the terms “Tuberculosis”[Mesh] AND “HIV”[Mesh] only, which returned 2163 results. We reviewed results from the previous five years, with additional review of references from relevant manuscripts.
Box: Critical Knowledge Gaps.
Does antiretroviral therapy increase the infectiousness of HIV-positive tuberculosis cases?
How does HIV infection modulate the risk of reinfection or relapse in individuals with a history of tuberculosis treatment?
What are optimal ways to measure tuberculosis transmission and tuberculosis reinfection, at the individual- and population-level?
Are genetic determinants of infection resistance altered in HIV-infected tuberculosis cases?
Will early detection of subclinical disease in HIV-infected and uninfected cases improve linkage to care and reduce transmission?
How does HIV-infection alter the manifestation of pulmonary disease and the capacity of diseased individuals to transmit?
What proportion of tuberculosis cases in the general population are attributed to different institutional amplifiers (mines, hospitals, prisons, etc.)?
What interventions can be directed towards institutional amplifiers to lower the intra-institution high force of infection and subsequently prevent future community cases?
Box 2: Urgent unmet needs.
Development of technologies to distinguish a) patients at high- and low-risk for transmitting tuberculosis, and b) patients at high risk for tuberculosis infection and subsequently at high risk for progressing to active tuberculosis disease, based on both host and bacillary characteristics.
Determine optimal methods to evaluate interventions intended to halt tuberculosis transmission in HIV-endemic settings, which requires improvements in tuberculosis program monitoring capabilities and application of appropriate modeling strategies.
Further integration of HIV and tuberculosis programming in sub-Saharan Africa and other areas with substantial burden of both HIV and tuberculosis. Programmatic practices that are useful for integration include HIV-testing for individuals at high-risk of tuberculosis, infection control in areas with high levels of tuberculosis, initiation of ART therapy, providing isoniazid preventive therapy to all HIV-positive patients exposed to tuberculosis, and intensified tuberculosis case finding
Acknowledgements
The authors wish to thank Thomas Scriba for critical evaluation of the manuscript.
Role of funding sources
This publication and workshop have been funded in whole or in part with Federal Funds from the Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), US National Institutes of Health (NIH), Department of Health and Human Services under contract number HHSN272201600001G Research Support Services for the Division of AIDS. Funding support was also provided in part by NIH grants: [to JRA (K01 AI104411, R01 AI30058 and DP2 AI131082), ES (R01 AI124349), LM (T32 AI 052073), and YvdH (K08 AI106420)]. BDK was supported by funding from an International Early Career Scientist Award from the Howard Hughes Medical Institute and the South African National Research Foundation. BDK, JP, MH, and RW were supported by the South African Medical Research Council with funds received from the National Department of Health. The authors acknowledge support from the Bill and Melinda Gates Foundation [to BDK (OPP1100182), MH (OPP1137034 and OPP1151915), SH (STBP/TBREACH/GSA/W5–29), JP, and RW]. SH receives salary support from the Stop TB Partnership’s TB REACH initiative co-funded by the Government of Canada. LM was supported by the Ruth L. Kirschstein National Research Service Award. ES was supported by the Canadian Institutes of Health Research (CIHR; FDN-143332). YvdH was supported by the U.S. Civilian Research and Development Foundation (CRDF) OISE-16–62061-1. The South African Medical Research Council co-hosted the workshop. The Bill and Melinda Gates foundation and National Institutes of Health provided funding support for some participants travel to the workshop. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
All authors declare no conflicts of interest.
References
- 1.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 2011; 24(2): 351–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankar EM, Vignesh R, Ellegard R, et al. HIV-Mycobacterium tuberculosis co-infection: a ‘danger-couple model’ of disease pathogenesis. Pathog Dis 2014; 70(2): 110–8. [DOI] [PubMed] [Google Scholar]
- 3.Auld SC, Kasmar AG, Dowdy DW, et al. Research Roadmap for Tuberculosis Transmission Science: Where Do We Go From Here and How Will We Know When We’re There? J Infect Dis 2017; 216(suppl_6): S662–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathema B, Andrews JR, Cohen T, et al. Drivers of Tuberculosis Transmission. J Infect Dis 2017; 216(suppl_6): S644–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Churchyard G, Kim P, Shah NS, et al. What We Know About Tuberculosis Transmission: An Overview. J Infect Dis 2017; 216(suppl_6): S629–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong BC, Hill PC, Aiken A, et al. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J Infect Dis 2008; 198(7): 1037–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews JR, Morrow C, Walensky RP, Wood R. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. J Infect Dis 2014; 210(4): 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zumla A, Malon P, Henderson J, Grange JM. Impact of HIV infection on tuberculosis. Postgrad Med J 2000; 76(895): 259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallory KF, Churchyard GJ, Kleinschmidt I, De Cock KM, Corbett EL. The impact of HIV infection on recurrence of tuberculosis in South African gold miners. Int J Tuberc Lung Dis 2000; 4(5): 455–62. [PubMed] [Google Scholar]
- 10.Pawlowski A, Jansson M, Skold M, Rottenberg ME, Kallenius G. Tuberculosis and HIV co-infection. PLoS Pathog 2012; 8(2): e1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrestha S, Chihota V, White RG, Grant AD, Churchyard GJ, Dowdy DW. Impact of Targeted Tuberculosis Vaccination Among a Mining Population in South Africa: A Model-Based Study. Am J Epidemiol 2017; 186(12): 1362–9. [DOI] [PubMed] [Google Scholar]
- 12.Martinez L, le Roux DM, Barnett W, Stadler A, Nicol MP, Zar HJ. Tuberculin skin test conversion and primary progressive tuberculosis disease in the first 5 years of life: a birth cohort study from Cape Town, South Africa. Lancet Child Adolesc Health 2018; 2(1): 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, Scano F. Tuberculosis incidence in prisons: a systematic review. PLoS Med 2010; 7(12): e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stop TB Partnership. Data for Action for Tuberculosis Key, Vulnerable and Underserved Populations; 2017. [Google Scholar]
- 15.Shakoor S, Hasan R. Tuberculosis in vulnerable populations in Eastern Mediterranean Region-Implications for control. Int J Mycobacteriol 2016; 5 Suppl 1: S15. [DOI] [PubMed] [Google Scholar]
- 16.Dara M, Acosta CD. Tuberculosis prevention and control in prisons: do we know enough? Int J Tuberc Lung Dis 2014; 18(7): 758–9. [DOI] [PubMed] [Google Scholar]
- 17.MacIntyre CR, Kendig N, Kummer L, Birago S, Graham NM. Impact of tuberculosis control measures and crowding on the incidence of tuberculous infection in Maryland prisons. Clin Infect Dis 1997; 24(6): 1060–7. [DOI] [PubMed] [Google Scholar]
- 18.Reid SE, Topp SM, Turnbull ER, et al. Tuberculosis and HIV control in sub-Saharan African prisons: “thinking outside the prison cell”. J Infect Dis 2012; 205 Suppl 2: S265–73. [DOI] [PubMed] [Google Scholar]
- 19.van Hest NA, Aldridge RW, de Vries G, et al. Tuberculosis control in big cities and urban risk groups in the European Union: a consensus statement. Euro Surveill 2014; 19(9). [DOI] [PubMed] [Google Scholar]
- 20.Sacchi FP, Praca RM, Tatara MB, et al. Prisons as reservoir for community transmission of tuberculosis, Brazil. Emerg Infect Dis 2015; 21(3): 452–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourdillon PM, Goncalves CC, Pelissari DM, et al. Increase in Tuberculosis Cases among Prisoners, Brazil, 2009–2014(1). Emerg Infect Dis 2017; 23(3): 496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stead WW. Undetected tuberculosis in prison. Source of infection for community at large. JAMA 1978; 240(23): 2544–7. [DOI] [PubMed] [Google Scholar]
- 23.Bourdillon PM, Goncalves CC, Pelissari DM, et al. Increase in Tuberculosis Cases among Prisoners, Brazil, 2009–20141. Emerg Infect Dis 2017; 23(3): 496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews JR, Basu S, Dowdy DW, Murray MB. The epidemiological advantage of preferential targeting of tuberculosis control at the poor. Int J Tuberc Lung Dis 2015; 19(4): 375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdool Karim SS, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet 2009; 374(9693): 921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner RD, Bothamley GH. Cough and the transmission of tuberculosis. J Infect Dis 2015; 211(9): 1367–72. [DOI] [PubMed] [Google Scholar]
- 27.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol 2013; 31: 475–527. [DOI] [PubMed] [Google Scholar]
- 28.Cadena AM, Flynn JL, Fortune SM. The Importance of First Impressions: Early Events in Mycobacterium tuberculosis Infection Influence Outcome. MBio 2016; 7(2): e00342–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petruccioli E, Scriba TJ, Petrone L, et al. Correlates of tuberculosis risk: predictive biomarkers for progression to active tuberculosis. Eur Respir J 2016; 48(6): 1751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scriba TJ, Penn-Nicholson A, Shankar S, et al. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog 2017; 13(11): e1006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menzies NA, Wolf E, Connors D, et al. Progression from latent infection to active disease in dynamic tuberculosis transmission models: a systematic review of the validity of modelling assumptions. Lancet Infect Dis 2018. [DOI] [PMC free article] [PubMed]
- 32.de Noronha AL, Bafica A, Nogueira L, Barral A, Barral-Netto M. Lung granulomas from Mycobacterium tuberculosis/HIV-1 co-infected patients display decreased in situ TNF production. Pathol Res Pract 2008; 204(3): 155–61. [DOI] [PubMed] [Google Scholar]
- 33.Huang CC, Tchetgen ET, Becerra MC, et al. The effect of HIV-related immunosuppression on the risk of tuberculosis transmission to household contacts. Clin Infect Dis 2014; 58(6): 765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diedrich CR, Flynn JL. HIV-1/Mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun 2011; 79(4): 1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallgren A The time-table of tuberculosis. Tubercle 1948; 29(11): 245–51. [DOI] [PubMed] [Google Scholar]
- 36.Esmail H, Dodd PJ, Houben R. Tuberculosis transmission during the subclinical period: could unrelated cough play a part? Lancet Respir Med 2018; 6(4): 244–6. [DOI] [PubMed] [Google Scholar]
- 37.Meintjes G, Wilkinson RJ. Undiagnosed active tuberculosis in HIV-infected patients commencing antiretroviral therapy. Clin Infect Dis 2010; 51(7): 830–2. [DOI] [PubMed] [Google Scholar]
- 38.Dowdy DW, Basu S, Andrews JR. Is passive diagnosis enough? The impact of subclinical disease on diagnostic strategies for tuberculosis. Am J Respir Crit Care Med 2013; 187(5): 543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng S, Chen W, Yang Y, et al. Effect of Diagnostic and Treatment Delay on the Risk of Tuberculosis Transmission in Shenzhen, China: An Observational Cohort Study, 1993–2010. PLoS One 2013; 8(6): e67516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horna-Campos OJ, Sanchez-Perez HJ, Sanchez I, Bedoya A, Martin M. Public transportation and pulmonary tuberculosis, Lima, Peru. Emerg Infect Dis 2007; 13(10): 1491–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Getnet F, Demissie M, Assefa N, Mengistie B, Worku A. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: systematic review and meta-analysis. BMC Pulm Med 2017; 17(1): 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008; 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med 2007; 175(1): 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekandi JN, Zalwango S, Martinez L, et al. Four Degrees of Separation: Social Contacts and Health Providers Influence the Steps to Final Diagnosis of Active Tuberculosis Patients in Urban Uganda. BMC Infect Dis 2015; 15: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaisson RE, Schecter GF, Theuer CP, Rutherford GW, Echenberg DF, Hopewell PC. Tuberculosis in patients with the acquired immunodeficiency syndrome. Clinical features, response to therapy, and survival. Am Rev Respir Dis 1987; 136(3): 570–4. [DOI] [PubMed] [Google Scholar]
- 46.Elliott AM, Hayes RJ, Halwiindi B, et al. The impact of HIV on infectiousness of pulmonary tuberculosis: a community study in Zambia. AIDS 1993; 7(7): 981–7. [DOI] [PubMed] [Google Scholar]
- 47.Cruciani M, Malena M, Bosco O, Gatti G, Serpelloni G. The impact of human immunodeficiency virus type 1 on infectiousness of tuberculosis: a meta-analysis. Clin Infect Dis 2001; 33(11): 1922–30. [DOI] [PubMed] [Google Scholar]
- 48.Melsew YA, Doan TN, Gambhir M, Cheng AC, McBryde E, Trauer JM. Risk factors for infectiousness of patients with tuberculosis: a systematic review and meta-analysis. Epidemiol Infect 2018; 146(3): 345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez L, Sekandi JN, Castellanos ME, Zalwango S, Whalen CC. Infectiousness of HIV-Seropositive Patients with Tuberculosis in a High-Burden African Setting. Am J Respir Crit Care Med 2016; 194(9): 1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cayla JA, Garcia de Olalla P, Galdos-Tanguis H, et al. The influence of intravenous drug use and HIV infection in the transmission of tuberculosis. AIDS 1996; 10(12): 1446–7. [DOI] [PubMed] [Google Scholar]
- 51.Kenyon TA, Creek T, Laserson K, et al. Risk factors for transmission of Mycobacterium tuberculosis from HIV-infected tuberculosis patients, Botswana. Int J Tuberc Lung Dis 2002; 6(10): 843–50. [PubMed] [Google Scholar]
- 52.van Halsema CL, Fielding KL, Chihota VN, et al. Brief Report: The Effect of Antiretroviral Therapy and CD4 Count on Markers of Infectiousness in HIV-Associated Tuberculosis. J Acquir Immune Defic Syndr 2015; 70(1): 104–8. [DOI] [PubMed] [Google Scholar]
- 53.Bassett IV, Wang B, Chetty S, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis 2010; 51(7): 823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan PY, Crampin AC, Mzembe T, et al. Does antiretroviral treatment increase the infectiousness of smear-positive pulmonary tuberculosis? Int J Tuberc Lung Dis 2017; 21(11): 1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borgdorff MW, De Cock KM. Provision of ART to individuals infected with HIV: impact on the epidemiology and control of tuberculosis. Int J Tuberc Lung Dis 2017; 21(11): 1091–2. [DOI] [PubMed] [Google Scholar]
- 56.Yates TA, Khan PY, Knight GM, et al. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect Dis 2016; 16(2): 227–38. [DOI] [PubMed] [Google Scholar]
- 57.Wood R, Lawn SD. Antiretroviral treatment as prevention: impact of the ‘test and treat’ strategy on the tuberculosis epidemic. Curr HIV Res 2011; 9(6): 383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dodd PJ, Knight GM, Lawn SD, Corbett EL, White RG. Predicting the long-term impact of antiretroviral therapy scale-up on population incidence of tuberculosis. PloS one 2013; 8(9): e75466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PloS one 2012; 7(3): e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2006; 42(7): 1040–7. [DOI] [PubMed] [Google Scholar]
- 61.Corbett EL, Charalambous S, Fielding K, et al. Stable incidence rates of tuberculosis (TB) among human immunodeficiency virus (HIV)-negative South African gold miners during a decade of epidemic HIV-associated TB. J Infect Dis 2003; 188(8): 1156–63. [DOI] [PubMed] [Google Scholar]
- 62.March F, Coll P, Guerrero RA, Busquets E, Cayla JA, Prats G. Predictors of tuberculosis transmission in prisons: an analysis using conventional and molecular methods. AIDS 2000; 14(5): 525–35. [DOI] [PubMed] [Google Scholar]
- 63.Kufa T, Mabuto T, Muchiri E, et al. Incidence of HIV-associated tuberculosis among individuals taking combination antiretroviral therapy: a systematic review and meta-analysis. PLoS One 2014; 9(11): e111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 2001; 358(9294): 1687–93. [DOI] [PubMed] [Google Scholar]
- 65.Verver S, Warren RM, Beyers N, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med 2005; 171(12): 1430–5. [DOI] [PubMed] [Google Scholar]
- 66.Luzze H, Johnson DF, Dickman K, et al. Relapse more common than reinfection in recurrent tuberculosis 1–2 years post treatment in urban Uganda. Int J Tuberc Lung Dis 2013; 17(3): 361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood R, Lawn SD, Caldwell J, Kaplan R, Middelkoop K, Bekker LG. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PloS one 2011; 6(10): e25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charalambous S, Grant AD, Moloi V, et al. Contribution of reinfection to recurrent tuberculosis in South African gold miners. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2008; 12(8): 942–8. [PubMed] [Google Scholar]
- 69.Crampin AC, Mwaungulu JN, Mwaungulu FD, et al. Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS 2010; 24(3): 417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Middelkoop K, Bekker LG, Shashkina E, Kreiswirth B, Wood R. Retreatment tuberculosis in a South African community: the role of re-infection, HIV and antiretroviral treatment. Int J Tuberc Lung Dis 2012; 16(11): 1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guerra-Assuncao JA, Houben RM, Crampin AC, et al. Recurrence due to Relapse or Reinfection With Mycobacterium tuberculosis: A Whole-Genome Sequencing Approach in a Large, Population-Based Cohort With a High HIV Infection Prevalence and Active Follow-up. J Infect Dis 2015; 211(7): 1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marx FM, Dunbar R, Enarson DA, et al. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin Infect Dis 2014; 58(12): 1676–83. [DOI] [PubMed] [Google Scholar]
- 73.Bock P, Jennings K, Vermaak R, et al. Incidence of Tuberculosis Among HIV-Positive Individuals Initiating Antiretroviral Treatment at Higher CD4 Counts in the HPTN 071 (PopART) Trial in South Africa. J Acquir Immune Defic Syndr 2018; 77(1): 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015; 373(9): 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dodd PJ, Prendergast AJ, Beecroft C, Kampmann B, Seddon JA. The impact of HIV and antiretroviral therapy on TB risk in children: a systematic review and meta-analysis. Thorax 2017; 72(6): 559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cattamanchi A, Smith R, Steingart KR, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2011; 56(3): 230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verrall AJ, Netea MG, Alisjahbana B, Hill PC, van Crevel R. Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology 2014; 141(4): 506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma N, Zalwango S, Malone LL, et al. Clinical and epidemiological characteristics of individuals resistant to M. tuberculosis infection in a longitudinal TB household contact study in Kampala, Uganda. BMC Infect Dis 2014; 14: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cobat A, Gallant CJ, Simkin L, et al. High heritability of antimycobacterial immunity in an area of hyperendemicity for tuberculosis disease. J Infect Dis 2010; 201(1): 15–9. [DOI] [PubMed] [Google Scholar]
- 80.Cobat A, Barrera LF, Henao H, et al. Tuberculin skin test reactivity is dependent on host genetic background in Colombian tuberculosis household contacts. Clin Infect Dis 2012; 54(7): 968–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tao L, Zalwango S, Chervenak K, et al. Genetic and shared environmental influences on interferon-gamma production in response to Mycobacterium tuberculosis antigens in a Ugandan population. Am J Trop Med Hyg 2013; 89(1): 169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stein CM, Zalwango S, Malone LL, et al. Genome scan of M. tuberculosis infection and disease in Ugandans. PLoS One 2008; 3(12): e4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thye T, Browne EN, Chinbuah MA, et al. IL10 haplotype associated with tuberculin skin test response but not with pulmonary TB. PLoS One 2009; 4(5): e5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cobat A, Gallant CJ, Simkin L, et al. Two loci control tuberculin skin test reactivity in an area hyperendemic for tuberculosis. J Exp Med 2009; 206(12): 2583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cobat A, Poirier C, Hoal E, et al. Tuberculin skin test negativity is under tight genetic control of chromosomal region 11p14–15 in settings with different tuberculosis endemicities. J Infect Dis 2015; 211(2): 317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cobat A, Hoal EG, Gallant CJ, et al. Identification of a major locus, TNF1, that controls BCG-triggered tumor necrosis factor production by leukocytes in an area hyperendemic for tuberculosis. Clin Infect Dis 2013; 57(7): 963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah JA, Musvosvi M, Shey M, et al. A Functional Toll-Interacting Protein Variant Is Associated with Bacillus Calmette-Guerin-Specific Immune Responses and Tuberculosis. Am J Respir Crit Care Med 2017; 196(4): 502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Horne DJ, Graustein AD, Shah JA, et al. Human ULK1 Variation and Susceptibility to Mycobacterium tuberculosis Infection. J Infect Dis 2016; 214(8): 1260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sobota RS, Stein CM, Kodaman N, et al. A chromosome 5q31.1 locus associates with tuberculin skin test reactivity in HIV-positive individuals from tuberculosis hyper-endemic regions in east Africa. PLoS Genet 2017; 13(6): e1006710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jabot-Hanin F, Cobat A, Feinberg J, et al. Major Loci on Chromosomes 8q and 3q Control Interferon gamma Production Triggered by Bacillus Calmette-Guerin and 6-kDa Early Secretory Antigen Target, Respectively, in Various Populations. J Infect Dis 2016; 213(7): 1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jabot-Hanin F, Cobat A, Feinberg J, et al. An eQTL variant of ZXDC is associated with IFN-gamma production following Mycobacterium tuberculosis antigen-specific stimulation. Sci Rep 2017; 7(1): 12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Calligaro C, Esmail A, Mnguni T, Mottay L, Dheda K. The diagnostic gap: Characterising the profile of undiagnosed infectious tuberculosis patients in the community. S Afr Respir J 2016; 22(4): 93–8. [Google Scholar]
- 93.Andrews JR, Hatherill M, Mahomed H, et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. American journal of respiratory and critical care medicine 2015; 191(5): 584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zak DE, Penn-Nicholson A, Scriba TJ, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. The Lancet 2016. [DOI] [PMC free article] [PubMed]
- 95.Anderson ST, Kaforou M, Brent AJ, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. The New England journal of medicine 2014; 370(18): 1712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bloom CI, Graham CM, Berry MP, et al. Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PloS one 2013; 8(8): e70630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaforou M, Wright VJ, Oni T, et al. Detection of tuberculosis in HIV-infected and - uninfected African adults using whole blood RNA expression signatures: a case-control study. PLoS medicine 2013; 10(10): e1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sweeney TE, Braviak L, Tato CM, Khatri P. Genome-wide expression for diagnosis of pulmonary tuberculosis: a multicohort analysis. The Lancet Respiratory medicine 2016; 4(3): 213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Penn-Nicholson A, Scriba TJ, Hatherill M, White RG, Sumner T. A novel blood test for tuberculosis prevention and treatment. S Afr Med J 2016; 107(1): 4–5. [DOI] [PubMed] [Google Scholar]
- 100.Riley RL, Wells WF, Mills CC, Nyka W, McLean RL. Air hygiene in tuberculosis: quantitative studies of infectivity and control in a pilot ward. Am Rev Tuberc 1957; 75(3): 420–31. [DOI] [PubMed] [Google Scholar]
- 101.Riley RL, Mills CC, O’Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis 1962; 85: 511–25. [DOI] [PubMed] [Google Scholar]
- 102.Issarow CM, Wood R, Mulder N. Seminal Mycobacterium Tuberculosis in vivo Transmission Studies: Reanalysis Using Probabilistic Modelling. Mycobact Dis 2016; 6: 217. [Google Scholar]
- 103.Escombe AR, Oeser C, Gilman RH, et al. The detection of airborne transmission of tuberculosis from HIV-infected patients, using an in vivo air sampling model. Clin Infect Dis 2007; 44(10): 1349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Escombe AR, Moore DA, Gilman RH, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med 2008; 5(9): e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marquina-Castillo B, Garcia-Garcia L, Ponce-de-Leon A, et al. Virulence, immunopathology and transmissibility of selected strains of Mycobacterium tuberculosis in a murine model. Immunology 2009; 128(1): 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones-Lopez EC, Kim S, Fregona G, et al. Importance of cough and M. tuberculosis strain type as risks for increased transmission within households. PLoS One 2014; 9(7): e100984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Larsson P, Mirgorodskaya E, Samuelsson L, et al. Surfactant protein A and albumin in particles in exhaled air. Respir Med 2012; 106(2): 197–204. [DOI] [PubMed] [Google Scholar]
- 108.Barrat A, Cattuto C, Tozzi AE, Vanhems P, Voirin N. Measuring contact patterns with wearable sensors: methods, data characteristics and applications to data-driven simulations of infectious diseases. Clin Microbiol Infect 2014; 20(1): 10–6. [DOI] [PubMed] [Google Scholar]
- 109.Wood R, Morrow C, Barry CE 3rd, et al. Real-Time Investigation of Tuberculosis Transmission: Developing the Respiratory Aerosol Sampling Chamber (RASC). PLoS One 2016; 11(1): e0146658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patterson B, Morrow C, Singh V, et al. Detection of Mycobacterium tuberculosis bacilli in bio-aerosols from untreated TB Patients. Gates Open Research 2017; In press. [DOI] [PMC free article] [PubMed]
- 111.Fennelly KP, Jones-Lopez EC, Ayakaka I, et al. Variability of infectious aerosols produced during coughing by patients with pulmonary tuberculosis. Am J Respir Crit Care Med 2012; 186(5): 450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jones-Lopez EC, Namugga O, Mumbowa F, et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: a household contact study. Am J Respir Crit Care Med 2013; 187(9): 1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mukamolova GV, Turapov O, Malkin J, Woltmann G, Barer MR. Resuscitation-promoting factors reveal an occult population of tubercle Bacilli in Sputum. Am J Respir Crit Care Med 2010; 181(2): 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chengalroyen MD, Beukes GM, Gordhan BG, et al. Detection and Quantification of Differentially Culturable Tubercle Bacteria in Sputum from Patients with Tuberculosis. Am J Respir Crit Care Med 2016; 194(12): 1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Issarow CM, Mulder N, Wood R. Modelling the risk of airborne infectious disease using exhaled air. J Theor Biol 2015; 372: 100–6. [DOI] [PubMed] [Google Scholar]
- 116.Middelkoop K, Mathema B, Myer L, et al. Transmission of tuberculosis in a South African community with a high prevalence of HIV infection. J Infect Dis 2015; 211(1): 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dheda K, Limberis JD, Pietersen E, et al. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. Lancet Respir Med 2017; 5(4): 269–81. [DOI] [PubMed] [Google Scholar]
- 118.Andrews JR, Morrow C, Walensky RP, Wood R. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. The Journal of infectious diseases 2014; 210(4): 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Middelkoop K, Mathema B, Myer L, et al. Transmission of tuberculosis in a South African community with a high prevalence of HIV infection. The Journal of infectious diseases 2014; 211(1): 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Crampin AC, Glynn JR, Traore H, et al. Tuberculosis transmission attributable to close contacts and HIV status, Malawi. Emerging infectious diseases 2006; 12(5): 729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dodd PJ, Looker C, Plumb ID, et al. Age- and Sex-Specific Social Contact Patterns and Incidence of Mycobacterium tuberculosis Infection. Am J Epidemiol 2016; 183(2): 156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Andrews JR, Morrow C, Wood R. Modeling the role of public transportation in sustaining tuberculosis transmission in South Africa. Am J Epidemiol 2013; 177(6): 556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Basu S, Stuckler D, McKee M. Addressing institutional amplifiers in the dynamics and control of tuberculosis epidemics. The American journal of tropical medicine and hygiene 2011; 84(1): 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McElroy PD, Southwick KL, Fortenberry ER, et al. Outbreak of tuberculosis among homeless persons coinfected with human immunodeficiency virus. Clin Infect Dis 2003; 36(10): 1305–12. [DOI] [PubMed] [Google Scholar]
- 125.Beck-Sague C, Dooley SW, Hutton MD, et al. Hospital outbreak of multidrug-resistant Mycobacterium tuberculosis infections. Factors in transmission to staff and HIV-infected patients. JAMA 1992; 268(10): 1280–6. [DOI] [PubMed] [Google Scholar]
- 126.Dolan K, Wirtz AL, Moazen B, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet 2016; 388(10049): 1089–102. [DOI] [PubMed] [Google Scholar]
- 127.Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, Scano F. Tuberculosis incidence in prisons: a systematic review. PLoS medicine 2010; 7(12): e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nyangulu D, Harries A, Kang’Ombe C, et al. Tuberculosis in a prison population in Malawi. The Lancet 1997; 350(9087): 1284–7. [DOI] [PubMed] [Google Scholar]
- 129.Aerts A, Habouzit M, Mschiladze L, et al. Pulmonary tuberculosis in prisons of the ex-USSR state Georgia: results of a nation-wide prevalence survey among sentenced inmates. The International Journal of Tuberculosis and Lung Disease 2000; 4(12): 1104–10. [PubMed] [Google Scholar]
- 130.Stead WW. Undetected tuberculosis in prison: source of infection for community at large. Jama 1978; 240(23): 2544–7. [DOI] [PubMed] [Google Scholar]
- 131.Stuckler D, Basu S, McKee M, King L. Mass incarceration can explain population increases in TB and multidrug-resistant TB in European and central Asian countries. Proceedings of the National Academy of Sciences of the United States of America 2008; 105(36): 13280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jones TF, Woodley CL, Fountain FF, Schaffner W. Increased incidence of the outbreak strain of Mycobacterium tuberculosis in the surrounding community after an outbreak in a jail. Southern medical journal 2003; 96(2): 155–8. [DOI] [PubMed] [Google Scholar]
- 133.Jones TF, Craig AS, Valway SE, Woodley CL, Schaffner W. Transmission of tuberculosis in a jail. Annals of internal medicine 1999; 131(8): 557–63. [DOI] [PubMed] [Google Scholar]
- 134.Sacchi FP, Praça RM, Tatara MB, et al. Prisons as reservoir for community transmission of tuberculosis, Brazil. Emerging infectious diseases 2015; 21(3): 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jenkins HE, Plesca V, Ciobanu A, et al. Assessing spatial heterogeneity of multidrug-resistant tuberculosis in a high-burden country. European Respiratory Journal 2013; 42(5): 1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ruddy M, Balabanova Y, Graham C, et al. Rates of drug resistance and risk factor analysis in civilian and prison patients with tuberculosis in Samara Region, Russia. Thorax 2005; 60(2): 130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Godfrey-Faussett P, Sonnenberg P, Shearer S, et al. Tuberculosis control and molecular epidemiology in a South African gold-mining community. The Lancet 2000; 356(9235): 1066–71. [DOI] [PubMed] [Google Scholar]
- 138.Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. New England Journal of Medicine 2014; 370(4): 301–10. [DOI] [PubMed] [Google Scholar]
- 139.Stuckler D, Basu S, McKee M, Lurie M. Mining and risk of tuberculosis in sub-Saharan Africa. American journal of public health 2011; 101(3): 524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Andrews JR, Shah NS, Weissman D, Moll AP, Friedland G, Gandhi NR. Predictors of multidrug- and extensively drug-resistant tuberculosis in a high HIV prevalence community. PLoS One 2010; 5(12): e15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dheda K, Limberis JD, Pietersen E, et al. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. The lancet Respiratory medicine 2017; 5(4): 269–81. [DOI] [PubMed] [Google Scholar]
- 142.Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. The Lancet 2007; 370(9597): 1500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dowdy DW, Golub JE, Chaisson RE, Saraceni V. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proceedings of the National Academy of Sciences of the United States of America 2012; 109(24): 9557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sveinbjornsson G, Gudbjartsson DF, Halldorsson BV, et al. HLA class II sequence variants influence tuberculosis risk in populations of European ancestry. Nat Genet 2016; 48(3): 318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]