Abstract
Objective:
Despite no clear evidence from randomized trials, surgical intervention of spontaneous intracerebral hemorrhage (ICH) still occurs. We sought to describe the characteristics of patients undergoing surgical intervention in ICH.
Methods:
Data from the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study were analyzed, and ICH patients were categorized into surgical intervention or nonoperative management groups. Patients with primary intraventricular hemorrhage (IVH) and those without data regarding the use of surgical intervention data were excluded.
Results:
The study cohort comprised 2,947 patients, and surgical intervention was performed in 289 (10%). Younger age (OR=0.967, p<0.001), lower baseline modified Rankin Scale (mRS; OR=0.728, p<0.001), higher admission Glasgow Coma Scale (GCS; OR=1.059, p=0.007), larger ICH volume (OR=1.037, p<0.001), infratentorial ICH location (OR=5.966, p<0.001), lobar ICH location (OR=1.906, p=0.001), lack of IVH (OR=0.567, p=0.001), ICP monitoring (OR=5.022, p<0.001), and mannitol use (OR=2.389, p<0.001) were independent predictors of surgical intervention. Younger age (OR=0.953, p<0.001), lower baseline mRS score (OR=0.713, p=0.002), larger ICH volume (OR=1.033, p<0.001), lobar ICH location (OR=2.467, p<0.001), ICP monitoring (OR=3.477, p<0.001), and mannitol use (OR=2.139, p<0.001) were independent predictors of surgical interventions in supratentorial ICHs. Larger ICH volume (OR=1.078, p<0.001), ICP monitoring (OR=6.099, p<0.001), and mannitol use (OR=2.952, p=0.005) were independent predictors of surgical interventions in infratentorial ICHs.
Conclusions:
We identified multiple factors associated with surgical intervention for patients with ICH. Younger age, good neurological function at baseline, large ICH volume on presentation, and lobar or infratentorial hematomas were independently associated with surgical intervention in ICH patients.
Keywords: surgery, predictors, intracerebral hemorrhage, neurosurgery, evacuation
Introduction
Spontaneous intracerebral hemorrhage (ICH) is associated with the highest rates of mortality and morbidity among all stroke subtypes, and it occurs with an annual incidence of 15 to 25 per 100,000 persons.1–5 Despite efforts to target the mechanisms of primary brain injury caused by ICH, the benefit of surgical intervention in ICH patients remains unproven.6–8 With the exception of infratentorial ICHs, the American Heart Association (AHA)/American Stroke Association (ASA) recommendations for the role of surgery in ICH management remain weak (Class IIb).9 Nevertheless, ICH surgery continues to be performed at medical centers in the United States. However, the selection criteria for which surgical treatment is employed in the contemporary management of ICH patients are elusive, highly variable across different institutions and individual physicians, and incompletely defined.10 Therefore, the aim of this multicenter, retrospective cohort study is to identify predictors of surgical intervention in patients with spontaneous ICH.
Methods
Patient Cohort and Selection
The Ethnic/Racial variations of IntraCerebral Hemorrhage (ERICH) study protocol has been previously described in detail.11 In brief, the ERICH study was a multicenter, prospective, case-control study designed to recruit 1,000 non-Hispanic whites, 1,000 nonHispanic blacks and 1,000 Hispanics with spontaneous ICH. Participants were derived from 19 United States sites comprising 42 hospitals. Institutional review board (IRB) approval and written informed consents were obtained at each site and from all patients (or legal guardians for patients who were unable to provide consent) participating in the study, respectively. All patients or legal guardians were subjected to a standardized data collection protocol, including a personal interview and medical chart abstraction. Data from each respective site were de-identified and pooled for analysis.
The present study comprised patients who were derived from the ICH case cohort of the ERICH study, and their data were retrospectively analyzed. ICH patients with available data pertaining to surgical intervention (i.e., craniotomy or craniectomy for decompression or evacuation of ICH and minimally invasive surgery for ICH evacuation) were included in the study cohort. Patients with primary intraventricular hemorrhage (IVH) were excluded. Patients who underwent ICH surgery were categorized into the surgical intervention group, whereas those who did not undergo ICH surgery were categorized into the nonoperative management group.
Baseline Data and Variables
Baseline demographic and clinical data included age, sex, race/ethnicity, baseline modified Rankin Scale (mRS), and admission Glasgow Coma Scale (GCS). Serum laboratory data obtained on admission included international normalized ratio (INR), partial thromboplastin time (PTT), and platelet count. Antiplatelet and anticoagulation medication use prior to ICH were recorded. Neuroimaging and treatment data included admission ICH volume, ICH location (categorized as infratentorial vs. supratentorial and lobar vs. deep), presence of IVH, intracranial pressure (ICP) monitoring, mannitol use, and hypertonic saline use.
Statistical Analysis
All statistical analyses were performed using Stata (version 14.2, College Station, TX). Baseline, clinical, radiologic, and treatment characteristics were compared between the surgical intervention and nonoperative management groups. Student’s t or Wilcoxon rank-sum tests were used to compare continuous variables, and Pearson’s χ2 or Fisher’s exact tests were used to compare categorical variables, where appropriate. To assess for independent predictors of surgical intervention, a multivariable logistic regression model was developed using surgical intervention as the dependent variable and covariates with p<0.10 in univariate comparisons as the independent variables. The fit of the model was assessed using the Hosmer-Lemeshow goodness-of-fit test. To avoid listwise deletions due to missing data, a second multivariable logistic regression model was built using multiply imputed data. The multiple imputation was performed using chained equations with m=50. Imputed values for baseline mRS (0.4%), admission GCS (2.4%), presence of IVH (3.2%), ICH volume (3.2%), mannitol use (0.1%), and hypertonic saline use (0.2%) were generated using conditional regression models based on these auxiliary variables: age, sex, surgical intervention, lobar ICH location, infratentorial ICH location, and ICP monitoring. Parameter estimates from analyzing the imputed datasets were pooled according to Rubin’s rules.12 Subgroup analyses for supratentorial and infratentorial ICHs were performed. Statistical significance was defined as p<0.05, and all tests were two-tailed.
Results
Comparison of Baseline Characteristics
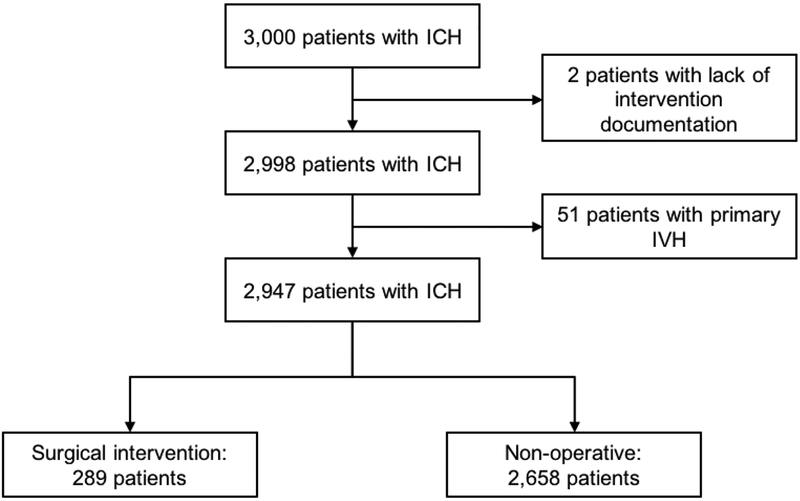
Of the 3,000 patients with spontaneous ICH who were enrolled in the ERICH study, 53 were excluded from the present analysis (two patients excluded for lack of documentation regarding surgical intervention and 51 patients excluded for primary IVH). The study cohort comprised the remaining 2,947 ICH patients, who were categorized into the surgical intervention (n=289) versus nonoperative management (n=2,658) groups (Figure 1).
Figure 1.
Flow diagram showing the patient selection process.
Table 1 compares the baseline characteristics between ICH patients who underwent surgical intervention versus nonoperative management. Patients in the surgical intervention cohort were younger (mean 57 vs. 62 years old, p<0.001), and they had lower admission GCS (median 13 vs. 15, p<0.001) scores. Distributions of race/ethnicity (p<0.001) and baseline mRS scores (p<0.001) were different between the surgical intervention and nonoperative management groups. Patients who underwent surgical evacuation had larger ICH volumes (mean 46 vs. 18 cm3, p<0.001), and they were more likely to have an infratentorial ICH (23% vs. 12%, p<0.001), lobar ICH (45% vs. 30%, p<0.001), and IVH (48% vs. 41%, p=0.021). ICP monitoring (52% vs. 15%, p<0.001), mannitol (49% vs. 13%, p<0.001), and hypertonic saline (10% vs. 3%, p<0.001) were more frequently used in the surgical intervention group.
Table 1.
Comparison of baseline demographic, clinical, radiologic, and treatment characteristics between ICH patients who underwent nonoperative management versus surgical intervention.
| Variable | Nonoperative Management (n=2,658) | Surgical Intervention (n=289) | p-value |
|---|---|---|---|
| Age, mean yr (SD) | 62.1 (14) | 57.1 (13.4) | <0.001 |
| Male sex, n (%) | 1,550/2,658 (58.3) | 183/289 (63.3) | 0.100 |
| Race/Ethnicity, n (%) | <0.001 | ||
| White | 890/2,658 (33.5) | 86/289 (29.8) | |
| Black | 920/2,658 (34.6) | 69/289 (23.9) | |
| Hispanic | 848/2,658 (31.9) | 134/289 (46.4) | |
| Baseline mRS, n (%) | <0.001 | ||
| 0 | 1,837/2,648 (69.4) | 238/288 (82.6) | |
| 1 | 317/2,648 (12) | 22/288 (7.6) | |
| 2 | 267/2,648 (10.1) | 16/288 (5.6) | |
| 3 | 134/2,648 (5.1) | 6/288 (2.1) | |
| 4 | 77/2,648 (2.9) | 4/288 (1.4) | |
| 5 | 16/2,648 (0.6) | 2/288 (0.7) | |
| Admission GCS, median (IQR) | 15 (11–15) | 13 (8–15) | <0.001 |
| Antiplatelet use, n (%) | 1,166/2,631 (44.3) | 116/285 (40.7) | 0.243 |
| Anticoagulant use, n (%) | 261/2,621 (10) | 34/283 (12) | 0.277 |
| INR, mean (SD) | 1.2 (0.8) | 1.3 (0.8) | 0.147 |
| PTT, mean sec (SD) | 29.5 (8.4) | 29.5 (6.6) | 0.983 |
| Platelet count, mean k/uL (SD) | 225.4 (75.9) | 224.9 (82.5) | 0.921 |
| ICH volume, mean cm3 (SD) | 18.2 (23.4) | 46.2 (29.3) | <0.001 |
| Infratentorial ICH location, n (%) | 326/2,658 (12.3) | 67/289 (23.2) | <0.001 |
| Lobar ICH location, n (%) | 802/2,658 (30.2) | 130/289 (45) | <0.001 |
| Presence of IVH, n (%) | 1,057/2,594 (40.8) | 126/262 (48.1) | 0.021 |
| ICP monitoring, n (%) | 398/2,658 (15) | 149/289 (51.6) | <0.001 |
| Mannitol use, n (%) | 335/2,654 (12.6) | 142/289 (49.1) | <0.001 |
| Hypertonic saline use, n (%) | 78/2,658 (2.9) | 29/289 (10) | <0.001 |
n = number; yr = year; uL = microliter; k = ×1,000; ICH = intracerebral hemorrhage; IVH = intraventricular hemorrhage; GCS = Glasgow Coma Scale; IQR = interquartile range; mRS = modified Rankin Scale; ICP = intracranial pressure; INR = international normalized ratio; PTT = partial thromboplastin time.
Predictors of Surgical Intervention
Table 2 details the multivariable analyses for independent predictors of surgical intervention in patients with ICH. In the non-imputed multivariable model, younger age (OR=0.969 [0.957–0.980], p<0.001), lower baseline mRS (OR=0.722 [0.598–0.872], p=0.001), higher admission GCS (OR=1.059 [1.015–1.105], p=0.009), larger ICH volume (OR=1.036 [1.030–1.042], p<0.001), infratentorial ICH location (OR=5.316 [3.447–8.199], p<0.001), lobar ICH location (OR=1.922 [1.292–2.859], p=0.001), lack of IVH (OR=0.541 [0.378–0.776], p=0.001), ICP monitoring (OR=5.809 [4.035–8.361], p<0.001), and mannitol use (OR=2.135 [1.503–3.035], p<0.001) were associated with surgical intervention. These predictors remained significant in the multiply imputed model.
Table 2.
Multivariable analyses for independent predictors of surgical intervention in patients with ICH.
| Predictors | Odds ratio† | 95% CI | p-value | Odds ratio* | 95% CI* | p-value* |
|---|---|---|---|---|---|---|
| Age | 0.969 | 0.957–0.980 | <0.001 | 0.967 | 0.957–0.979 | <0.001 |
| Baseline mRS | 0.722 | 0.598–0.872 | 0.001 | 0.728 | 0.610–0.868 | <0.001 |
| Race/Ethnicity‡ | ||||||
| White | — | — | — | — | — | — |
| Black | 0.767 | 0.502–1.173 | 0.221 | 0.704 | 0.472–1.049 | 0.084 |
| Hispanic | 1.440 | 0.982–2.112 | 0.062 | 1.316 | 0.919–1.886 | 0.134 |
| Admission GCS | 1.059 | 1.015–1.105 | 0.009 | 1.059 | 1.016–1.103 | 0.007 |
| ICH volume | 1.036 | 1.030–1.042 | <0.001 | 1.037 | 1.031–1.043 | <0.001 |
| Infratentorial ICH location | 5.316 | 3.447–8.199 | <0.001 | 5.966 | 3.981–8.942 | <0.001 |
| Lobar ICH location | 1.922 | 1.292–2.859 | 0.001 | 1.906 | 1.308–2.780 | 0.001 |
| Presence of IVH | 0.541 | 0.378–0.776 | 0.001 | 0.567 | 0.401–0.803 | 0.001 |
| ICP monitoring | 5.809 | 4.035–8.361 | <0.001 | 5.022 | 3.556–7.092 | <0.001 |
| Mannitol use | 2.135 | 1.503–3.035 | <0.001 | 2.389 | 1.719–3.321 | <0.001 |
| Hypertonic saline use | 0.867 | 0.479–1.570 | 0.637 | 0.793 | 0.448–1.405 | 0.427 |
ICH = intracerebral hemorrhage; GCS = Glasgow Coma Scale; CI = confidence interval; mRS = modified Rankin Scale; IVH = intraventricular hemorrhage; ICP = intracranial pressure
Values based on pooled parameter estimates from multiply imputed data using chained equations with m=50.
Hosmer-Lemeshow goodness-of-fit test χ2 (8)=9.27, p=0.320.
White as reference category.
Subgroup analysis of Supratentorial ICH
Table 3 compares the baseline characteristics between patients with supratentorial ICH who underwent surgical intervention versus nonoperative management. Patients in the surgical intervention group were younger (mean 56 vs. 62 years old, p<0.001), and they had lower admission GCS (median 12 vs. 15, p<0.001) scores. Distributions of race/ethnicity (p<0.001) and baseline mRS scores (p<0.001) were different between the two groups. Patients who underwent surgical intervention had larger ICH volumes (mean 54 vs. 20 cm3, p<0.001), and they were more likely to have a lobar ICH (59% vs. 34%, p<0.001). ICP monitoring (49% vs. 15%, p<0.001), mannitol (49% vs. 13%, p<0.001), and hypertonic saline (10% vs. 3%, p<0.001) were more frequently used in the surgical intervention group.
Table 3.
Comparison of baseline demographic, clinical, radiologic, and treatment characteristics between patients with supratentorial ICH who underwent nonoperative management versus surgical intervention.
| Variable | Nonoperative Management (n=2,332) | Surgical Intervention (n=222) | p-value |
|---|---|---|---|
| Age, mean yr (SD) | 62.3 (14) | 55.7 (13.5) | <0.001 |
| Male sex, n (%) | 1,367/2,332 (58.6) | 140/222 (63.1) | 0.198 |
| Race/Ethnicity, n (%) | <0.001 | ||
| White | 797/2,332 (34.2) | 68/222 (30.6) | |
| Black | 804/2,332 (34.5) | 49/222 (22.1) | |
| Hispanic | 731/2,332 (31.4) | 105/222 (47.3) | |
| Baseline mRS, n (%) | <0.001 | ||
| 0 | 1,611/2,322 (69.4) | 185/222 (83.3) | |
| 1 | 285/2,322 (12.3) | 19/222 (8.6) | |
| 2 | 231/2,322 (10) | 10/222 (4.5) | |
| 3 | 118/2,322 (5.1) | 3/222 (1.4) | |
| 4 | 64/2,322 (2.8) | 3/222 (1.4) | |
| 5 | 13/2,322 (0.6) | 2/222 (0.9) | |
| Admission GCS, median (IQR) | 15 (12–15) | 12 (8–15) | <0.001 |
| Antiplatelet use, n (%) | 1,030/2,307 (44.7) | 91/219 (41.6) | 0.378 |
| Anticoagulant use, n (%) | 216/2,298 (9.4) | 22/218 (10.1) | 0.739 |
| INR, mean (SD) | 1.2 (0.8) | 1.2 (0.6) | 0.874 |
| PTT, mean sec (SD) | 29.5 (8.6) | 29.1 (5.9) | 0.535 |
| Platelet count, mean k/uL (SD) | 225.3 (76.3) | 226.6 (87.5) | 0.811 |
| ICH volume, mean cm3 (SD) | 19.7 (24.4) | 53.5 (29) | <0.001 |
| Lobar ICH location, n (%) | 802/2,332 (34.4) | 130/222 (58.6) | <0.001 |
| Presence of IVH, n (%) | 961/2,278 (42.2) | 96/203 (47.3) | 0.159 |
| ICP monitoring, n (%) | 353/2,332 (15.1) | 108/222 (48.7) | <0.001 |
| Mannitol use, n (%) | 299/2,328 (12.8) | 108/222 (48.7) | <0.001 |
| Hypertonic saline use, n (%) | 75/2,327 (3.2) | 23/222 (10.4) | <0.001 |
n = number; yr = year; uL = microliter; k = ×1,000; ICH = intracerebral hemorrhage; IVH = intraventricular hemorrhage; GCS = Glasgow Coma Scale; IQR = interquartile range; mRS = modified Rankin Scale; ICP = intracranial pressure; INR = international normalized ratio; PTT = partial thromboplastin time.
Table 4 details the multivariable analyses for independent predictors of surgical intervention in patients with supratentorial ICH. Younger age (OR=0.955 [0.942–0.969], p<0.001), lower baseline mRS (OR=0.676 [0.537–0.851], p=0.001), larger ICH volume (OR=1.032 [1.026–1.039], p<0.001), lobar ICH location (OR=2.441 [1.650–3.609], p<0.001), ICP monitoring (OR=3.806 [2.586–5.600], p<0.001), and mannitol use (OR=2.007 [1.354–2.974], p=0.001) were significantly associated with surgical intervention. These predictors remained significant in the multiply imputed model.
Table 4.
Multivariable analyses for independent predictors of surgical intervention in patients with supratentorial ICH.
| Predictors | Odds ratio† | 95% CI | p-value | Odds ratio* | 95% CI* | p-value* |
|---|---|---|---|---|---|---|
| Age | 0.955 | 0.942–0.969 | <0.001 | 0.953 | 0.940–0.965 | <0.001 |
| Baseline mRS | 0.676 | 0.537–0.851 | 0.001 | 0.713 | 0.578–0.881 | 0.002 |
| Race/Ethnicity‡ | ||||||
| White | — | — | — | — | — | — |
| Black | 0.688 | 0.425–1.112 | 0.127 | 0.640 | 0.407–1.007 | 0.054 |
| Hispanic | 1.404 | 0.919–2.145 | 0.116 | 1.336 | 0.895–1.995 | 0.157 |
| Admission GCS | 1.034 | 0.986–1.084 | 0.171 | 1.036 | 0.990–1.085 | 0.128 |
| ICH volume | 1.032 | 1.026–1.039 | <0.001 | 1.033 | 1.027–1.039 | <0.001 |
| Lobar ICH location | 2.441 | 1.650–3.609 | <0.001 | 2.467 | 1.703–3.574 | <0.001 |
| ICP monitoring | 3.806 | 2.586–5.600 | <0.001 | 3.477 | 2.402–5.034 | <0.001 |
| Mannitol use | 2.007 | 1.354–2.974 | 0.001 | 2.139 | 1.473–3.105 | <0.001 |
| Hypertonic saline use | 0.787 | 0.413–1.502 | 0.468 | 0.698 | 0.373–1.307 | 0.262 |
ICH = intracerebral hemorrhage; GCS = Glasgow Coma Scale; CI = confidence interval; mRS = modified Rankin Scale; IVH = intraventricular hemorrhage; ICP = intracranial pressure.
Values based on pooled parameter estimates from multiply imputed data using chained equations with m=50.
Hosmer-Lemeshow goodness-of-fit test χ2 (8)=6.20, p=0.624.
White as reference category.
Subgroup analysis of Infratentorial ICH
Table 5 compares the baseline characteristics between patients with infratentorial ICH who underwent surgical intervention versus nonoperative management. Patients who underwent surgical intervention had higher INR values (mean 1.5 vs. 1.2, p=0.020) and larger ICH volumes (mean 21 vs. 8 cm3, p<0.001), and they were more likely to have IVH (51% vs. 30%, p=0.002). ICP monitoring (61% vs. 14%, p<0.001), mannitol (51% vs. 11%, p<0.001), and hypertonic saline (9% vs. 1%, p=0.001) were more frequently used in the surgical intervention group. Specifically, the majority of patients in this subgroup who underwent surgical intervention had cerebellar ICH (n=66/67; 98.5%).
Table 5.
Comparison of baseline demographic, clinical, radiologic, and treatment characteristics between patients with infratentorial ICH who underwent nonoperative management versus surgical intervention.
| Variable | Nonoperative Management (n=326) | Surgical Intervention (n=67) | p-value |
|---|---|---|---|
| Age, mean yr (SD) | 60.9 (14) | 61.6 (11.7) | 0.700 |
| Male sex, n (%) | 183/326 (56.1) | 43/67 (64.2) | 0.225 |
| Race/Ethnicity, n (%) | 0.497 | ||
| White | 93/326 (28.5) | 18/67 (26.9) | |
| Black | 116/326 (35.6) | 20/67 (29.9) | |
| Hispanic | 117/326 (35.9) | 29/67 (43.3) | |
| Baseline mRS, n (%) | 0.626 | ||
| 0 | 226/326 (69.3) | 53/66 (80.3) | |
| 1 | 32/326 (9.8) | 3/66 (4.6) | |
| 2 | 36/326 (11) | 6/66 (9.1) | |
| 3 | 16/326 (4.9) | 3/66 (4.6) | |
| 4 | 13/326 (4) | 1/66 (1.5) | |
| 5 | 3/326 (0.9) | 0/66 (0) | |
| Admission GCS, median (IQR) | 15 (10.5–15) | 14 (9–15) | 0.271 |
| Antiplatelet use, n (%) | 136/324 (42) | 25/66 (37.9) | 0.538 |
| Anticoagulant use, n (%) | 45/323 (13.9) | 12/65 (18.5) | 0.347 |
| INR, mean (SD) | 1.2 (0.7) | 1.5 (1.2) | 0.020 |
| PTT, mean sec (SD) | 29.1 (6) | 30.6 (8.4) | 0.098 |
| Platelet count, mean k/uL (SD) | 225.7 (72.9) | 219.2 (63.8) | 0.502 |
| ICH volume, mean cm3 (SD) | 8 (9.5) | 21.2 (10.3) | <0.001 |
| Presence of IVH, n (%) | 96/316 (30.4) | 30/59 (50.9) | 0.002 |
| ICP monitoring, n (%) | 45/326 (13.8) | 41/67 (61.2) | <0.001 |
| Mannitol use, n (%) | 36/326 (11) | 34/67 (50.8) | <0.001 |
| Hypertonic saline use, n (%) | 3/326 (0.9) | 6/67 (9) | 0.001 |
n = number; yr = year; uL = microliter; k = ×1,000; ICH = intracerebral hemorrhage; IVH = intraventricular hemorrhage; GCS = Glasgow Coma Scale; IQR = interquartile range; mRS = modified Rankin Scale; ICP = intracranial pressure; INR = international normalized ratio; PTT = partial thromboplastin time.
Table 6 details the multivariable analyses for independent predictors of surgical intervention in patients with infratentorial ICH. Larger ICH volume (OR=1.072 [1.038–1.107], p<0.001) and ICP monitoring (OR=7.567 [3.474–16.483], p<0.001) were associated with surgical intervention. These predictors remained significant in the multiply imputed model. Mannitol use (OR=2.952 [1.387–6.281], p=0.005) was also found to be an independent predictor of surgical intervention in the multiply imputed model.
Table 6.
Multivariable analyses for independent predictors of surgical intervention in patients with infratentorial ICH.
| Predictors | Odds ratio† | 95% CI | p-value | Odds ratio* | 95% CI* | p-value* |
|---|---|---|---|---|---|---|
| INR | 1.311 | 0.801–2.147 | 0.281 | 1.360 | 0.855–2.162 | 0.194 |
| PTT | 0.996 | 0.939–1.056 | 0.888 | 0.997 | 0.943–1.054 | 0.926 |
| ICH volume | 1.072 | 1.038–1.107 | <0.001 | 1.078 | 1.046–1.110 | <0.001 |
| Presence of IVH | 1.086 | 0.488–2.419 | 0.840 | 1.058 | 0.504–2.220 | 0.882 |
| ICP monitoring | 7.567 | 3.474–16.483 | <0.001 | 6.099 | 2.988–12.447 | <0.001 |
| Mannitol use | 2.139 | 0.952–4.807 | 0.066 | 2.952 | 1.387–6.281 | 0.005 |
| Hypertonic saline use | 1.714 | 0.299–9.836 | 0.545 | 1.896 | 0.337–10.663 | 0.468 |
ICH = intracerebral hemorrhage; GCS = Glasgow Coma Scale; CI = confidence interval; mRS = modified Rankin Scale; IVH = intraventricular hemorrhage; ICP = intracranial pressure; INR = international normalized ratio; PTT = partial thromboplastin time.
Values based on pooled parameter estimates from multiply imputed data using chained equations with m=50.
Hosmer-Lemeshow goodness-of-fit test χ2 (8)=8.30, p=0.405.
Discussion
Given the unclear benefits of ICH surgery, it is important to understand the factors that influence the selection of ICH patients for surgical intervention in the modern era outside of clinical trial settings. The goals of ICH surgery include prevention or amelioration of cerebral herniation, relief of intracranial hypertension, reduction of locoregional mass effect and perihematomal edema, and clearance of cytotoxic and pro-inflammatory byproducts.13 However, randomized trials that have compared surgical hematoma evacuation to medical management for ICH have failed to demonstrate a clear benefit from surgical intervention.6–8,14–17 Other studies have suggested that decompressive craniectomy may improve outcomes in ICH patients with elevated ICP.18–21 Despite the lack of Level A evidence to support the surgical treatment of ICH, it continues to be performed in some ICH patients outside the context of clinical trials. As such, we sought to delineate the factors that currently influence the selection of ICH patients for surgery.
The Surgical Trial in Intracerebral Haemorrhage (STICH) comprised 1,033 patients from 83 centers in 27 countries, and it randomized patients with a supratentorial hematoma ≥2 cm in size and a GCS ≥5 to early surgery or initial conservative management.6 Subsequently, the Surgical Trial in Lobar Intracerebral Haematomas (STICH II) selected conscious patients (GCS motor component ≥5 and GCS eye component ≥2) with lobar ICHs within 1 cm from the cortical surface and 10–100 cm3 in volume.7 In the Minimally Invasive Surgery Plus Alteplase for Intracerebral Hemorrhage Evacuation (MISTIE) phase II trial, 96 patients aged 18–80 years with GCS ≤14 or NIHSS ≥6 and a premorbid mRS ≤1 who presented with a ICH ≥20 cm3 in volume were randomized to image-guided minimally invasive catheter-based hematoma evacuation plus alteplase thrombolysis or conservative management.8 The ongoing MISTIE phase III trial (ClinicalTrials.gov NCT01827046) has the same inclusion criteria, except the minimum ICH volume is 30 cm3 and the target enrollment is 500 patients. The ongoing Early MiNimally-Invasive Removal of IntraCerebral Hemorrhage (ENRICH) trial (ClinicalTrials.gov NCT02880878) seeks to recruit 300 patients aged 18–80 years who present with a supratentorial ICH 30–80cm3 in volume, GCS of 5–14, and premorbid mRS ≤1. The patients the ENRICH trial will be randomized to minimally invasive parafascicular surgery with the BrainPath (NICO, Indianapolis, IN) device or medical management. The Artemis in the Removal of Intracerebral Hemorrhage (MIND) trial seeks to randomized 500 patients aged 18–80 years with a supratentorial ICH 20–80 cm3 in volume, NIHSS ≥6, GCS 5–15, and baseline mRS ≤1 to minimally invasive surgery using the Artemis Neuro Evacuation Device (Penumbra Inc., Alameda, CA) or medical management.
The most recent AHA/ASA guidelines from 2015 noted that the efficacy of surgery for supratentorial ICH remained unproven (Class IIb recommendation; Level of Evidence A).9 In addition, the benefits from decompressive craniectomy with or without hematoma evacuation (Class IIb recommendation; Level of Evidence C) and minimally invasive ICH evacuation with or without the use of thrombolytics (Class IIb recommendation; Level of Evidence B) were deemed uncertain. In the present analysis of a large, multicenter, multiethnic cohort of ICH patients, we identified numerous independent predictors of surgical intervention. Younger age, better baseline functional status (i.e., lower baseline mRS), and those with a less debilitating neurological condition (i.e., higher admission GCS) were independently associated with surgical intervention, which suggests that clinicians may have deemed these patients to have a greater neurological reserve and capacity for eventual recovery and, thus, a lower likelihood of a poor outcome.22 Interestingly, despite a lower admission GCS score found in the surgical intervention group, higher admission GCS score was associated with surgical intervention after controlling for other covariates. Lobar and infratentorial hematomas, large volume clots, and those without associated IVH were more likely to be treated surgically. Taken together, these predictors and those found in the subgroup analysis of supratentorial ICHs concur with many of the inclusion and exclusion criteria used in both previously completed and actively enrolling clinical trials investigating ICH surgery.23 However, we acknowledge that it is not possible to determine the magnitude by which practice had been influenced or shaped by the reporting of these trials. While many of the RCTs, conducted in neurologically stable patients, have principally investigated the hypothesized benefit to surgery in mitigating the second phase of injury after ICH, the prevention of dangerous compartment shifts and cerebral herniation remains an important rationale. We also found ICP monitoring and administration of mannitol to be predictors, which may indicate that surgical intervention was more likely to be recommended for patients with clinical and/or radiologic signs of intracranial hypertension.
Due to the anatomic restrictions of the posterior fossa, cerebellar hematomas can cause rapid deterioration via obstructive hydrocephalus secondary to compression of the fourth ventricle or local mass effect on the brainstem. Several nonrandomized studies have suggested improved outcomes with surgery in patients with cerebellar hemorrhages >3 cm in diameter, brainstem compression, or hydrocephalus.24–26 In contrast, surgical treatment of brainstem hematomas is universally avoided, due to the unacceptably high risk of neurological morbidity. Given the lack of clinical equipoise, a randomized trial comparing surgery versus conservative management for infratentorial ICHs is unlikely to ever be conducted. As such, although surgery for cerebellar ICH is Level of Evidence B, the AHA/ASA guidelines recommendation for this procedure remains Class I.9 Furthermore, initial cerebrospinal fluid drainage, rather than surgery, in these patients is not recommended (Class III recommendation; Level of Evidence C).9 Our subgroup analysis of infratentorial ICH identified larger ICH volume, ICP monitoring, and mannitol as predictors of surgical intervention. Since ICP monitoring and hyperosmolar therapy are often considered less effective at guiding the treatment of infratentorial mass lesions, our findings suggests that some patients with cerebellar hematomas may be improperly or inefficiently managed within contemporary algorithms for ICH management.
We acknowledge that several limitations affect the validity and generalizability of our study. Our results are contingent upon the accuracy and reliability of the collected data, which were derived from patient self-report or legal guardians of incapacitated patients and from medical chart abstraction. Therefore, this study may be subject to reporting and recall biases. Because the ERICH study was not specifically designed to assess the surgical treatment of ICH, operative details (e.g., timing of surgery, reason for surgery, surgical technique or devices used, craniotomy vs. craniectomy, degree of hematoma evacuation, perioperative complications) were not captured. Although ICP monitoring and mannitol use may be surrogate indicators of elevated ICP, details regarding ICP values, waveform tracings, and presence of midline shift on neuroimaging were not available. Despite our best attempts to include variables that could govern the decision to perform ICH surgery, there may be other variables that were not captured or accounted for, including clinical deterioration days after admission that were not captured. Furthermore, the present analyses were not designed with the intent of comparing the outcomes of surgery versus conservative management for ICH. Given the observational design of the ERICH study, it is possible that some patients were enrolled in concurrent surgical ICH trials. We believe that any such juxtaposition using the available data may be difficult to rigorously perform and clearly interpret, due to the multitude of baseline differences in patient and ICH characteristics between the surgical intervention and nonoperative management cohorts that we have outlined in our findings. Nevertheless, we concede that defining the role of surgical treatment and either justifying or refuting its utilization remains one of the foremost priorities in the modern management of ICH. Lastly, it is important to note that the identified predictors represent selection bias of the treating neurosurgeon or center, and should not be taken as guidelines or selection criteria for ICH surgery.
Conclusions
Despite insufficient evidence to support the use of surgical intervention for ICH, this treatment continues to be employed in ICH patients. We clarified the selection bias for ICH surgery outside of the setting of clinical trials by identifying multiple predictors of surgical intervention. Younger ICH patients in good neurological condition at baseline and presentation with large volume, lobar or infratentorial hematomas were more likely to undergo surgery. Additional data from ongoing and future studies are necessary to ascertain the role of surgery in the management of ICH.
Acknowledgments
Sources of Funding: This study was supported by a grant from the National Institute of Neurological Disorders and Stroke (NINDS: U-01-NS069763).
Abbreviations List
- ICH
intracerebral hemorrhage
- OR
odds ratio
- ERICH
Ethnic/Racial Variations of Intracerebral Hemorrhage
- IVH
intraventricular hemorrhage
- GCS
Glasgow Coma Scale
- ICP
intracranial pressure
- AHA
American Heart Association
- ASA
American Stroke Association
- IRB
Institutional review board
- mRS
modified Rankin Scale
- INR
international normalized ratio
- STICH
Surgical Trial in Intracerebral Haemorrhage
- STICH II
Surgical Trial in Lobar Intracerebral Haematomas
- MISTIE
Minimally Invasive Surgery Plus Alteplase for Intracerebral Hemorrhage Evacuation
- ENRICH
Early MiNimally-Invasive Removal of IntraCerebral Hemorrhage
- MIND
Artemis in the Removal of Intracerebral Hemorrhage
- RCT
randomized controlled trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. The New England journal of medicine. 1992;326(11):733–736. [DOI] [PubMed] [Google Scholar]
- 2.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41(7):1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24(6):796–800. [DOI] [PubMed] [Google Scholar]
- 4.Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40(2):394–399. [DOI] [PubMed] [Google Scholar]
- 5.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and metaanalysis. The Lancet Neurology. 2010;9(2):167–176. [DOI] [PubMed] [Google Scholar]
- 6.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet (London, England). 2005;365(9457):387–397. [DOI] [PubMed] [Google Scholar]
- 7.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet (London, England). 2013;382(9890):397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. The Lancet Neurology. 2016;15(12):1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. [DOI] [PubMed] [Google Scholar]
- 10.Gregson BA, Mendelow AD. International variations in surgical practice for spontaneous intracerebral hemorrhage. Stroke. 2003;34(11):2593–2597. [DOI] [PubMed] [Google Scholar]
- 11.Woo D, Rosand J, Kidwell C, McCauley JL, Osborne J, Brown MW, et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke. 2013;44(10):e120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin DB. Multiple Imputation for Nonresponse in Surveys. In Wiley Series in Probability and Statistics. New York: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 13.Fiorella D, Arthur A, Bain M, Mocco J. Minimally Invasive Surgery for Intracerebral and Intraventricular Hemorrhage: Rationale, Review of Existing Data and Emerging Technologies. Stroke. 2016;47(5):1399–1406. [DOI] [PubMed] [Google Scholar]
- 14.Wang WZ, Jiang B, Liu HM, Li D, Lu CZ, Zhao YD, et al. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. International journal of stroke : official journal of the International Stroke Society. 2009;4(1):11–16. [DOI] [PubMed] [Google Scholar]
- 15.Prasad K, Mendelow AD, Gregson B. Surgery for primary supratentorial intracerebral haemorrhage. The Cochrane database of systematic reviews. 2008(4):Cd000200. [DOI] [PubMed] [Google Scholar]
- 16.Vespa P, Hanley D, Betz J, Hoffer A, Engh J, Carter R, et al. ICES (Intraoperative Stereotactic Computed Tomography-Guided Endoscopic Surgery) for Brain Hemorrhage: A Multicenter Randomized Controlled Trial. Stroke. 2016;47(11):2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M, et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke. 2012;43(11):2923–2930. [DOI] [PubMed] [Google Scholar]
- 18.Fung C, Murek M, Z’Graggen WJ, Krahenbuhl AK, Gautschi OP, Schucht P, et al. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke. 2012;43(12):3207–3211. [DOI] [PubMed] [Google Scholar]
- 19.Heuts SG, Bruce SS, Zacharia BE, Hickman ZL, Kellner CP, Sussman ES, et al. Decompressive hemicraniectomy without clot evacuation in dominant-sided intracerebral hemorrhage with ICP crisis. Neurosurgical focus. 2013;34(5):E4. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi S, Wada K, Nagatani K, Otani N, Mori K. Decompressive hemicraniectomy for spontaneous intracerebral hemorrhage. Neurosurgical focus. 2013;34(5):E5. [DOI] [PubMed] [Google Scholar]
- 21.Hayes SB, Benveniste RJ, Morcos JJ, Aziz-Sultan MA, Elhammady MS. Retrospective comparison of craniotomy and decompressive craniectomy for surgical evacuation of nontraumatic, supratentorial intracerebral hemorrhage. Neurosurgical focus. 2013;34(5):E3. [DOI] [PubMed] [Google Scholar]
- 22.Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891–897. [DOI] [PubMed] [Google Scholar]
- 23.Gregson BA, Broderick JP, Auer LM, Batjer H, Chen XC, Juvela S, et al. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012;43(6):1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Da Pian R, Bazzan A, Pasqualin A. Surgical versus medical treatment of spontaneous posterior fossa haematomas: a cooperative study on 205 cases. Neurological research. 1984;6(3):145–151. [DOI] [PubMed] [Google Scholar]
- 25.Firsching R, Huber M, Frowein RA. Cerebellar haemorrhage: management and prognosis. Neurosurgical review. 1991;14(3):191–194. [DOI] [PubMed] [Google Scholar]
- 26.van Loon J, Van Calenbergh F, Goffin J, Plets C. Controversies in the management of spontaneous cerebellar haemorrhage. A consecutive series of 49 cases and review of the literature. Acta neurochirurgica. 1993;122(3–4):187–193. [DOI] [PubMed] [Google Scholar]