Abstract
Alcohol use disorders, affective disorders and their comorbidity are sexually dimorphic in humans. However, it is difficult to disentangle the interactions between subject factors influencing alcohol sensitivity in studies of humans. Herein, we combined murine models of unpredictable, chronic, mild stress (UCMS) and voluntary binge-drinking to examine for sex differences in the interactions between prior histories of excessive ethanol-drinking and stress upon ethanol-induced changes in motor behavior and subsequent drinking. In Experiment 1, female mice were insensitive to the UCMS-induced increase in ethanol-induced locomotion and ethanol intake under continuous alcohol-access. Experiment 2 revealed interactions between ethanol dose and sex (females > males), binge-drinking history (water > ethanol), and UCMS history (UCMS > controls), with no additive effect of a sequential prior history of both binge drinking and UCMS observed. We also observed an interaction between UCMS history and sex for righting recovery. UCMS history potentiated subsequent binge-drinking in water controls of both sexes and in male binge-drinking mice. Conversely, a prior binge-drinking history increased subsequent ethanol intake in females only, irrespective of prior UCMS history. In Experiment 3, a concurrent history of binge-drinking and UCMS did not alter ethanol intake, nor did it influence the ethanol dose-locomotor response function, but it did augment alcohol-induced sedation and reduced subsequent alcohol intake over that produced by binge-drinking alone. Thus, the subject factors of biological sex, prior stressor history and prior binge-drinking history interact in complex ways in mice to impact sensitivity to alcohol’s motor-stimulating, -incoordinating and intoxicating effects, as well as to influence subsequent heavy drinking.
Keywords: Binge-drinking, Unpredictable chronic mild stress, Sex differences, Locomotion, Righting reflex, Rotarod
1. Introduction
Alcohol Use Disorders (AUDs) exhibit a very high degree of comorbidity with all affective disorders, with the life-time co-prevalence rates ranging from 20 to 47% (e.g., [45,46,56,62,77,98]). However, the median age of onset for comorbid AUD-affective disorder is estimated to be 10 years younger than that for a substance abuse disorder alone; alcoholics are 2–3 times more likely than non-alcoholics to be diagnosed with an affective disorder and have a 4-fold increase in the risk for a depressive episode, even during alcoholism recovery (e.g., [33,40,41,44,62,77]). Conversely, individuals suffering from affective disorders are more likely to develop AUDs than the general population (e.g., [8,41,44,54,62]) and report affective symptom relief as a motivating factor for their problematic drinking (e.g., [2,15,30,47,80,95,104]). Thus, it is unclear from the epidemiological data which disorder precedes the other in comorbid individuals.
Complicating matters, AUDs, affective disorders & their comorbidity are sexually dimorphic in humans (e.g., [45,46,56,57,62–64,77]) and the closing of the gender gap in heavy ethanol-drinking (e.g., [36,49,64,96,106]) raises major concern as the development of an AUD and related psychiatric disturbances tends to follow an accelerated course in women (e.g., [18,43,48,55,61,64,74,81,92,93]). While the increased risk for AUD and affective disorder comorbidity in women vs. men is posited to be a function of sex differences in the neurocircuitry governing emotionality, as well as gender differences in environmental insults (e.g., [3,12,13,36]), it is unethical to conduct cause-effect studies on how environmental insults impact the human brain to result in behavioral pathology. Furthermore, a large number of confounding variables (including other drug abuse or therapeutic drug treatment, trauma & /or frequent aversive life events, frequency/amount of ethanol consumption, duration of drug/alcohol abstinence) render it impossible to systematically disentangle cause-effect relations between biological sex, brain function and emotionality, as well as ethanol history & cellular/molecular perturbations in the brains of humans in an experimentally-controlled fashion.
In this regard, rodent models of repeated stress (e.g., post-weanling isolation, repeated social defeat and unpredictable chronic mild stress or UCMS) demonstrate very consistently that a prior stress history augments voluntary ethanol consumption in laboratory rodents (e.g., [1,17,28,31,42,52,72,84,86,89,90]) of relevance to understanding the etiology of alcoholism and the high prevalence of comorbidity between AUDs and affective disorders. Conversely, rodent models of excessive ethanol exposure support greater stressor reactivity in alcohol-experienced animals (c.f., [4,5,47,66]). Although a large proportion of this aforementioned literature is derived from studies of forced ethanol exposure (e.g., vapor chambers, gavage administration), voluntary ethanol consumption can produce a long-lasting increase in negative affect in both laboratory rats and mice [38,50,65,68,69,83,97]. However, we know relatively little regarding how biological sex interacts with prior histories of stress and ethanol consumption to impact subsequent behavioral sensitivity to ethanol and ethanol intake. While one might presume from the limited behavioral sensitization and stress-drug cross-sensitization literature (c.f., [5,19,39,58,88]; see also [84]) that a sequential or concurrent history of stress and excessive ethanol-drinking would produce additive or synergistic effects upon ethanol sensitivity, particularly in females (c.f., [19,88]). However, to the best of our knowledge, no study has tested this hypothesis directly.
Thus, the goal of the present study was several-fold. In both humans and laboratory animals, sensitivity to ethanol’s sedative-hypnotic effects is typically inversely related to the propensity to drink excessively (e.g., [5,11,25,79,91]). Thus, the primary goal of this study was to extend our earlier results from male mice [84] to female subjects and examine for sex differences in the effects of a history of unpredictable, chronic, mild stress (UCMS) upon ethanol-induced motor behavior and intake. We recently demonstrated that a 2-week period of voluntary binge-drinking is sufficient to induce a negative affective state in male C57BL/6J mice [68,69]. Thus, the second goal of the study was to extend these results to another mouse strain to examine for the generalization of the effect and to determine whether or not a prior binge-drinking history modified the effects of UCMS upon ethanol behavioral sensitivity and intake in a sex-dependent manner. To accomplish this second goal, we examined the effects of both sequential and concurrent histories of binge-drinking and stress upon our variables of interest in male and female mice on a hybrid C57BL/6J-129X1/SvJ genetic background. The results of this work argue a complex interaction between the subject factors of stress history, binge-drinking history and biological sex that may inform the etiology and treatment of AUDs and affective disorder comorbidity.
2. Materials and methods
2.1. Subjects
The subjects were male and female mice on a mixed C56BL/6J X 129Xi/SvJ (B6.129) background that were bred in-house at the University of California Santa Barbara. B6.129 were selected for this study primarily because we have demonstrated both alcohol-induced locomotor sensitization [100] and UCMS-alcohol locomotor cross-sensitization in mice on this genetic background [84]. Furthermore, B6.129 mice exhibit a more moderate level of alcohol intake under both continuous- and limited-access drinking procedures than inbred C57BL/6J mice, with BACs approaching, but rarely exceeding, the 80 mg/dL criterion for an animal model of binge-drinking (e.g., [21–23,73,100]). Thus, we rationalized that their more moderate propensity to consume alcohol would reduce the likelihood of a ceiling effect upon alcohol intake following histories of stress and/or binge-drinking in B6.129 mice and the employ of subjects bred in-house eliminated concerns regarding any impact of transportation/relocation-related stress upon our variables of interest. To avoid litter confounds, mice from at least 3 different litters were used for each study. In addition, mice were always tested in adulthood between 7 and 15 weeks of age. Unless mandated by UCMS procedures (see below), mice were housed in groups of 2–5 on ventilated racks. Mice tested for alcohol consumption were housed under a reversed 12-h light:dark cycle (lights off at 10:00 h), whereas all other mice were housed in a distinct colony room, under a regular 12-h light:dark cycle (lights off at 19:00 h). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of California Santa Barbara and were consistent with the guidelines set by the NIH.
2.2. Unpredictable chronic mild stress (UCMS) procedures
As conducted recently [84], experimental mice in Experiments 1 and 2 were subjected to our 11-day UCMS procedures (see Table 1), while control animals received daily saline injections (vol = 10 ml/kg) for 11 days to habituate them to handling and injection procedures. The UCMS procedures were modified slightly for the experimental mice in Experiment 3 to accommodate simultaneous drinking procedures (see Table 2).
Table 1.
Summary of the UCMS procedures employed in Experiments 1 and 2.
| Day | Time | Procedure |
|---|---|---|
| 1 | O/N | Exposure to damp bedding |
| 2 | AM | 1-h immobilization in restraint tube |
| 3 | PM | 5-min exposure to 2% isoflurane anesthesia |
| O/N | Cage tilt | |
| 4 | AM | 1-h exposure to soiled rat bedding |
| PM | 5-min forced swim | |
| 5 | All day | Exposure to damp bedding |
| O/N | Food and water deprivation | |
| 6 | AM | 5-min forced swim |
| PM | 12-h housing under shifted light cycle | |
| 7 | PM | 1-h immobilization in restraint tube |
| O/N | Individual/isolation housing | |
| 8 | AM | 5-min exposure to 2% isoflurane anesthesia |
| PM | 1-h exposure to brightly lit inescapable arena | |
| O/N | Housing with bedding from strange males | |
| 9 | AM | 1-h immobilization in restraint tube |
| PM | 1-h exposure to soiled rat bedding | |
| O/N | Food and water deprivation | |
| 10 | PM | 15-min forced swim |
| O/N | Cage tilt | |
| 11 | AM | 1-h exposure to bobcat urine |
| PM | 3-h housing under shifted light cycle | |
| O/N | Housing with bedding from strange males |
Table 2.
Summary of the modified UCMS procedures employed in Experiment 3. Changes from the original protocol are designated with an asterisk (*).
| Day | Time | Procedure |
|---|---|---|
| 1 | O/N | Exposure to damp bedding |
| 2 | AM | 1-h immobilization in restraint tube |
| 3 | AM* | 5-min exposure to 2% isoflurane anesthesia |
| O/N | Cage tilt | |
| 4 | AM | 1-h exposure to soiled rat bedding |
| AM* | 5-min forced swim | |
| 5 | All day | Exposure to damp bedding |
| O/N | Food and water deprivation | |
| 6 | AM | 5-min forced swim |
| PM | 12-h housing under shifted light cycle | |
| 7 | PM | 1-h immobilization in restraint tube |
| O/N | Individual/isolation housing | |
| 8 | AM | 5-min exposure to 2% isoflurane anesthesia |
| PM | 1-h exposure to brightly lit inescapable arena | |
| O/N | Housing with bedding from strange males | |
| 9 | AM | 1-h immobilization in restraint tube |
| PM | 1-h exposure to soiled rat bedding | |
| O/N | Food and water deprivation | |
| 10 | AM* | 15-min forced swim |
| O/N | Cage tilt | |
| 11 | AM* | 3-h housing under shifted light cycle |
| PM* | 1-h exposure to bobcat urine | |
| O/N | Housing with bedding from strange males |
2.3. Monitoring of health outcomes
All animals were weighed prior to the beginning of each experiment and again after UCMS/saline procedures. As conducted in our prior study [84], all UCMS animals were weighed daily throughout UCMS procedures and coat condition was assessed daily by visual inspection of the face, top of head, abdomen, shoulders, back, and left and right flanks for signs of lack of self-care. Scores were assigned to each body part as follows: 0 = well-groomed, 0.5 = moderate degradation, 1 = unkept. Low scores indicated the animals were grooming properly, whereas higher scores were indicative of low self-care. Consistent with our previous work [84] and supporting the mildness of our UCMS procedures, no animal lost significant weight and no animal received a coat condition score higher than 0.5.
2.4. Locomotor activity testing
On day 12 of each experiment (i.e., the day following the last UCMS manipulation or saline injection), mice were tested for locomotor hyperactivity. On the first day of locomotor testing, animals were administered an intraperitoneal (IP) injection of saline (vol = 20 ml/kg), prior to being placed into Plexiglas activity chambers (20 W cm × 37 cm L × 25 cm H) for 15 min. The total distance traveled was recorded using digital video-tracking and ANYMaze software (Stoelting Company, Wood Dale, IL). On day 13, the same animals were administered a 2 g/kg ethanol injection – a dose demonstrated previously to reveal stress-ethanol cross-sensitization in Swiss Webster and B6.129 mice [84]. To generate a more complete dose-response function, locomotor activity testing continued over the next 3 days, during which time animals were injected with 1, 3 and 4 g/kg ethanol, respectively.
2.5. Rotarod testing
In addition to testing for UCMS effects upon locomotor activity, we assayed also for effects upon alcohol intoxication using a fixed-speed (10 rpm) rotarod test (IIT Life Science, Woodland Hills, CA), using procedures similar to those described previously by our group [84], with the exception that training procedures were conducted shortly following testing for saline-induced locomotor activity on day 12. Mice were initially habituated to walking on the apparatus in a 2-min session, during which mice were placed immediately back onto the rotarod if they fell. Following habituation, mice underwent a series of 3-min training trials, during which mice remained on the floor of the apparatus if they fell until the next trial began. When an animal could remain on the rotarod for three, 3-min, trials, it was considered “trained”. The number of falls during the 2-min habituation session, as well as the number of trials taken for each mouse to reach training criterion, served to index group differences in basal motor coordination. Testing for ethanol-induced intoxication/motor incoordination was conducted, once daily, on days 13–15, at ~15 min post-ethanol injection (i.e., immediately following locomotor activity testing for 1–3 g/kg ethanol). During testing, each mouse was subjected to three, 3-min, rotarod testing trials (30 s apart), during which a mouse was left on the floor of the apparatus if it fell until the next trial began and the latency of each fall was recorded and used as an index of alcohol intoxication. As mice injected with 4 g/kg ethanol were too incapacitated to remain on the rotarod for any measurable amount of time, this dose was not assayed in this paradigm.
2.6. Regain of righting reflex
In lieu of rotarod testing, mice injected with 4 g/kg ethanol were tested for their ability to regain their righting reflex at ~15 min post-testing for locomotor activity. Each mouse was placed in a supine position in an empty standard mouse cage and allowed three, 10-s, trials to right themselves (as defined by all 4 paws on the ground). For each trial, the mice were assigned a “righting reflex score” according to the following rating scale and the average of the three tests was used for data analysis. 0 = unable to place in a supine position; 1 = immediate righting after being placed in a supine position (i.e., < 5 s); 2 = slow righting after being placed in a supine position (i.e., 5 = 10 s)
3 = transitions to recovery position (lying on side) but does not right; 4 = remains supine but moves limbs; 5 = remains supine with no limb movement.
2.7. Ethanol-drinking procedures
In Experiment 1, male and female UCMS and control mice were individually housed following locomotor activity testing. Mice were then continuously presented with 4 sipper tubes containing 0, 5, 10 and 20% ethanol solutions (v/v) [102], 5 days/week over the course of two weeks (10 drinking days total). At the same time each day, the sipper tubes were weighed to calculate ethanol intake over each 24 h-period and the amount of ethanol consumed was expressed as a function of the animals’ body weight. Experiments 2 and 3 extended the results for ethanol intake under continuous-access procedures to an animal model of binge-drinking. For this, we employed our modified Drinking-in-the-Dark (DID) procedures [22], in which mice were presented with 5, 10, 20 and 40% (v/v) alcohol for 2 h/day, beginning at 3 h into the dark phase of the circadian cycle. Bottles were weighed before and after each 2-h drinking session and the amount of ethanol consumed was expressed as a function of the animals’ body weight. The duration of the binge-drinking sessions in Experiments 2 and 3 varied as described in Section 2.8 below.
2.8. Experimental designs
2.8.1. Experiment 1
Experiment 1 was an exploratory study designed to extend our earlier demonstration of stress-alcohol cross-sensitization of locomotor activity and stress-induced potentiation of alcohol intake in males [84] to female subjects. For this study, groups of male and female B6.129 mice underwent UCMS or control procedures and then were tested for locomotion induced by a 2 g/kg ethanol injection. As in our earlier study [84], sex differences in the effects of a prior history of UCMS upon alcohol intake were determined under a continuous alcohol-drinking procedure in which mice were presented with 0, 5%, 10% and 20% (v/v) bottles for 5 days/week for 2 weeks.
2.8.2. Experiment 2
The goal of Experiment 2 was two-fold. Given that females are reported to be more sensitive to alcohol’s locomotor effects (c.f., [19]), the first goal was to determine whether or not the failure of the female mice in Experiment 1 to exhibit a UCMS-induced potentiation of alcohol-related behavior reflected a ceiling effect upon behavior. We addressed this possibility by examining for sex differences in the effect of UCMS upon the dose-response function for ethanol-induced locomotion (0–4 g/kg, IP), as well as ethanol-induced motor incoordination on a rotarod and ethanol-induced sedation under a loss of righting re-flex procedure. As a history of binge-drinking is well-characterized to produce hyper-anxiety in adult mice [68–70], the second goal was to determine whether or not a prior history of binge-drinking might potentiate UCMS’s effects upon ethanol-related behavior. Thus, male and female mice underwent 2 weeks (M-F) of our modified DID procedures (5%, 10%, 20% and 40% v/v ethanol), followed by 11 days of UCMS or saline injections. One subset of these animals then underwent dose-response testing for ethanol-induced changes in locomotor activity, rotarod performance (1–3 g/kg ethanol) and loss of righting reflex (4 g/kg ethanol). A second subset of these mice underwent 8 consecutive days of binge-drinking under our modified DID procedures (5%, 10%, 20% and 40% ethanol) to examine for UCMS effects upon subsequent binge-drinking.
2.8.3. Experiment 3
The design of Experiment 2 enabled assessment of the effects of sequential histories of binge-drinking and stress upon our behavioral measures. In Experiment 3, we determined the effect of a simultaneous history of binge-drinking and stress upon behavioral sensitivity to ethanol. For this, male and female mice underwent our modified DID procedures with 5%, 10%, 20% and 40% (v/v) ethanol during the 11 days UCMS or saline injections. To accommodate the 2-h drinking period, we rearranged the timing or the nature of some of the stressor procedures, as indicated in Table 2.
2.9. Statistical analyses
The data were analyzed using Sex × Stress ANOVAs, with repeated measures on the Day or Dose factors, when appropriate. Significant interactions were deconstructed by ANOVAs or t-tests, as appropriate, followed by LSD post-hoc tests. α = 0.05 for all analyses.
3. Results
3.1. Experiment 1: Exploratory study of sex differences in stress-ethanol cross-sensitization
3.1.1. Health outcomes
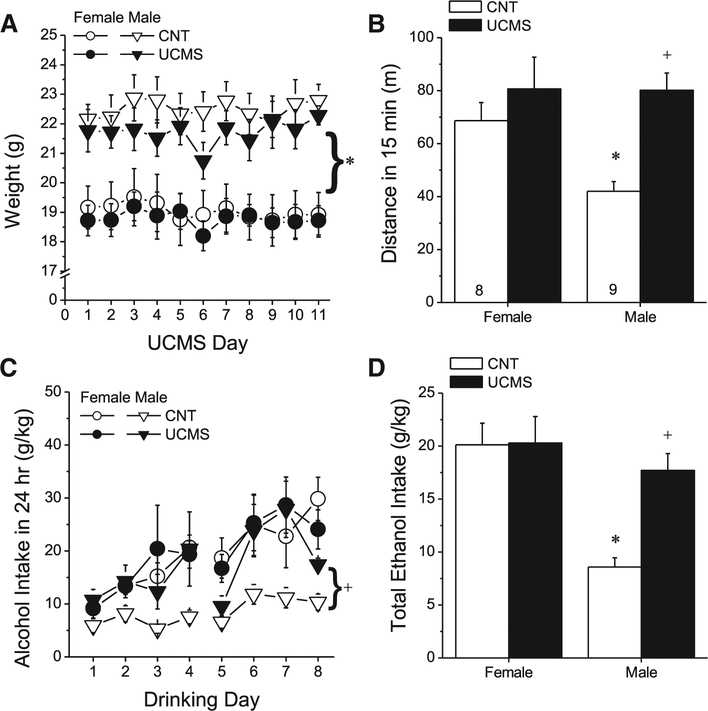
As expected, female B6.129 mice consistently weighed less than their male counterparts throughout the 11-day UCMS procedure (Fig. 1A) [Sex effect: F(1,28) = 23.62, p < 0.0001; Sex × Day: p = 0.66]. However, as reported previously [84], UCMSs history did not influence the average body weight of either sex at any point during stressor exposure [Day effect: F(10,280) = 2.13, p = 0.02; no Stress effect or interactions, p > 0.16]. Consistent with this, our UCMS procedures also did not influence coat condition scores in either sex (Sex × UCMS × Day ANOVA, all p’s > 0.75). These data do not support sex differences in the effects of UCMS history upon these gross measures of general health and well-being.
Fig. 1.
Females do not exhibit an UCMS-induced potentiation of alcohol-induced locomotor activity. A) Overall, female B6.129 mice weighed less than males, but the body weight of neither sex was significantly influenced by our 11-day UCMS procedures (UCMS), relative to that exhibited by saline-injected controls (CNT). *p < 0.05, main Sex effect. (B) Whereas no effect of UCMS was observed upon the locomotor response to 2 g/kg alcohol in female mice (left), UCMS doubled alcohol-induced locomotion in males (right). *p < 0.05 vs. respective female; + p < 0.05 vs. same-sex CNT. (C) Alcohol intake escalated in all groups over the course of the 8 days of continuous alcohol-access, with CNT males exhibiting consistently lower intake than their UCMS counterparts. +p < 0.05, main UCMS effect (males only). (D) The sex difference in UCMS effects upon alcohol intake was made more apparent when the average total daily alcohol intake was considered. *p < 0.05 vs. females; +p < 0.05 vs. CNT. Data represent the means ± SEMs of the number of mice indicated in Panel B.
3.1.2. Locomotor response to 2 g/kg ethanol
Control (CNT) females exhibited a greater locomotor response to 2 g/kg ethanol, compared to their male counterparts (Fig. 1B) [t(15) = 3.55, p = 0.003]. However, no sex difference was apparent in UCMS animals (t-test: p = 0.98). The lack of a sex difference in the responsiveness of UCMS animals to 2 g/kg reflected a significant stress-induced potentiation of ethanol-induced locomotor activity in male subjects [t(13) = 5.51, p < 0.0001], that was not apparent in females (Fig. 1B; t-test, p = 0.42). These data provided our first indication that prior stressor history and ethanol interact differently in male versus female subjects.
3.1.3. Ethanol intake under continuous-access procedures
Analysis of the change in the dose-response function for ethanol intake over the course of 8-day drinking period indicated a significant Sex × Stress × Dose × Day interaction [F(14,392) = 2.59, p = 0.001]. Thus, this interaction was deconstructed along the Dose factor to examine for changes in subject factor interactions over days. Although we observed no sex differences in water intake, female B6.129 mice exhibited greater alcohol intake than males over the course of our continuous-access drinking procedures (Fig. 1C) [Sex effect: F(1,24) = 10.24, p = 0.004; Day effect: F(7168) = 10.06, p < 0.0001; Sex × Day: p = 0.15]. UCMS potentiated alcohol intake, overall [Stress effect: F(1,24) = 4.50, p = 0.04]. Although the Stress × Sex interaction was shy of statistical significance [Stress × Sex: F(1,24) = 3.92, p = 0.06]. Indeed, follow-up analyses, conducted separately for males and females, detected a UCMS effect in males only [for males: Day effect: F(7,77) = 6.52, p < 0.0001; Stress effect: F(1,11) = 24.87, p < 0.0001; Stress × Day: p = 0.09; for females: Day effect: F(7,91) = 6.20, p < 0.0001; no Stress effect or Stress × Day interaction, p’s > 0.75]. Thus, the stress-induced potentiation of both alcohol-induced psychomotor-activity and alcohol intake is sex-specific, with males seemingly more vulnerable to the effects of stress than females.
Finally, an analysis of BACs attained at 3 h into the dark phase of the circadian cycle during continuous-access drinking revealed relatively low BACs, with females exhibiting lower BACs overall than males [Sex effect: F(1,29) = 6.88, p = 0.01]. Although inspection of Fig. 1D suggested that UCMS potentiated BACs selectively in female mice, the UCMS effect was not statistically reliable [UCMS effect and interaction: p’s > 0.07]. Thus, the sex-dependent effect of UCMS upon alcohol sensitivity does not appear to be related in any obvious manner to changes in BACs.
3.2. Experiment 2: Sex differences in the effects of sequential histories of binge-drinking and stress upon behavioral sensitivity to ethanol
3.2.1. Ethanol-induced locomotor-stimulation
Consistent with their alcohol intake under continuous-access conditions (see Fig. 1D), female B6.129 mice consumed a greater amount of alcohol under our modified 4-bottle DID procedures than did males [Sex effect: F(1,46) = 4.42, p = 0.04]. In both cases, mice of both sexes consumed quantities of alcohol that are predicted to result in BACs ≥ 0.08 mg% [85], with males consuming 8.00 ± 0.61 g/kg and females consuming 9.61 ± 0.60 g/kg, on average, during the 2-h sessions. Importantly, for neither sex did we detect a difference in alcohol intake between mice slated to undergo UCMS versus control procedures and alcohol intake was stable over the course of the 2-week drinking period (Stress effect and interactions, p’s > 0.07; Day effect and interactions, p’s > 0.35).
As expected, the dose-response function for ethanol-induced loco-motor activity was shifted as a function of Sex [Sex × Dose: F(4,360) = 6.10, p < 0.0001], UCMS history [Stress × Dose: F(4,360) = 5.53, p < 0.0001], as well as a function of binge-drinking history [Binge × Dose: F(4,360) = 4.23, p = 0.002]. Although a sex difference was not apparent in the effects of prior binge history or UCMS upon the ethanol dose-locomotor response function (Sex × Dose × Binge: p = 0.31; Sex × Dose × Stress: p = 0.96), prior histories of binge-drinking and stress interacted to alter locomotor sensitivity to alcohol [Binge × Stress × Dose: F(4,360) = 3.47, p = 0.008], in sex-dependent manner [Sex × Binge × Stress × Dose: F (4,360) = 2.87, p = 0.02]. This significant 4-way interaction was then constructed along both the Binge and Stress factors, in order to examine for sex differences in the effects of UCMS and prior binge-drinking history upon the ethanol dose-locomotor response function (Fig. 2).
Fig. 2.
Effects of a prior history of sequential binge-drinking and stress upon the alcohol dose-locomotor response function. Irrespective of prior UCMS history, alcohol-naïve (Water) females (A) locomoted a greater distance in response to injections of 0, 2 and 3 g/kg alcohol, relative to their male counterparts (B), and UCMS potentiated the locomotor responses to 1 and 2 g/kg alcohol, irrespective of sex. Irrespective of prior UCMS history, binge-drinking (Binge) females (C) locomoted more in response to injections of 1, 2 and 3 g/kg alcohol, than their male counterparts (D), and alcohol-induced locomotion was lower overall in Binge-CNT mice, relative to Water-CNT, irrespective of sex. In contrast, Binge-UCMS mice exhibited a lower loco-motor response to 2 g/kg alcohol than Water-UCMS animals. The data represent the means ± SEMs of the number of mice indicated in parentheses. *Denotes main Sex effect; +denotes main Stress effect; #denotes main Binge effect.
In ethanol-naïve (water-drinking) mice (Fig. 2A, B), the ethanol dose-locomotor response function was shifted in females relative to males [Sex × Dose: F(4,152) = 4,85, p = 0.001], with females exhibiting greater locomotor responsiveness to 0, 2 and 3 g/kg ethanol [for 0 g/kg: t(40) = 2.60, p = 0.01; for 2 g/kg: t(40) = 2.71, p = 0.01; for 3 g/kg: t(40) = 3.61, p = 0.001; other doses: p’s > 0.10]. In ethanol-naïve mice, UCMS shifted further the dose-response function [Stress × Dose: F(4152) = 6.85, p < 0.0001] and while inspection of Fig. 2A versus 2B suggested that the UCMS effect was greater in male versus female water-drinkers, the Sex × Stress × Dose interaction was not statistically significant (p = 0.16). Collapsing across sex, ethanolnaïve UCMS mice exhibited a greater locomotor response to the 1 and 2 g/kg doses, relative to unstressed controls [for 1 g/kg: t(40) = 2.49, p = 0.02; for 2 g/kg: t(40) = 2.48, p = 0.02; other doses: p’s > 0.12]. Thus, a prior history of repeated stress augments sensitivity to ethanol-induced locomotor hyper-activity in both male and female mice.
A comparable analysis of ethanol-binging mice also supported greater ethanol-induced locomotion in female versus male mice (Fig. 2C, D) [Sex × Dose: F(4,208) = 2.56, p = 0.04], with females exhibiting greater locomotor hyperactivity in response to 1, 2 and 3 g/kg ethanol [1 g/kg: t(55) = 2.77, p = 0.008; 2 g/kg: t(55) = 2.83, p = 0.006; 3 g/kg: t(55) = 4.64, p < 0.0001; other doses, p’s > 0.35]. Interestingly, we detected no UCMS effect in binge-drinking animals of either sex [Stress effect: p = 0.08; Stress × Dose: p = 0.06; Sex × Stress × Dose: p = 0.24]. These data do not support an additive or synergistic effect of a sequential history of binge-drinking and UCMS upon sensitivity to ethanol’s locomotor-stimulatory effects.
An inspection of the data for ethanol-naïve versus-binging mice (Fig. 2 top vs. bottom) suggested that a history of ethanol-binging blunted ethanol-induced motor activity. Thus, we next deconstructed the significant 4-way interaction along the Stress factor to examine for sex differences in the effects of prior binge-drinking history upon ethanol locomotor sensitivity. In stress-naïve CNT mice, prior binge-drinking history shifted the dose-response function downward [Binge effect: F(1,37) = 4.87, p = 0.03; Binge × Dose, p = 0.31], irrespective of sex [Sex × Dose: F(4,148) = 2.72, p = 0.03; Sex × Binge × Dose: p = 0.13]. In UCMS animals, prior ethanol-binging also altered the ethanol dose-locomotor response function in a sex-independent manner; however, in this case of UCMS mice, the binge effect varied with ethanol dose [Binge × Dose: F(4,212) = 7.71, p < 0.0001; Sex × Dose: F(4,212) = 3.50, p = 0.009; Sex × Binge × Dose: p = 0.07]. Collapsing the data for UCMS animals across sex, we detected a significant binge-induced reduction in the locomotor response to the 2 g/kg ethanol dose only [t(55) = 3.41, p = 0.001; other doses: p’s > 0.08]. The outcomes from the Sex X Binge X Dose analysis corroborate those from the Sex X UCMS X Dose analysis above, providing evidence that a prior history of sequential binge-drinking and stress interact in a complex fashion to influence ethanol-induced locomotion that is clearly not additive nor synergistic.
3.2.2. Ethanol-induced motor incoordination
As the dose-response function for ethanol-induced locomotion was inverted U-shaped (Fig. 2), we then tested mice for behavioral signs of incoordination immediately following the 15-min test of locomotor activity. When tested on a fixed speed rotarod, female mice exhibited less sensitivity than males to the motor incoordinating effects of ethanol (Fig. 3A) [Sex effect: F(1,91) = 15.34, p < 0.0001; Sex × Dose: F (2,91) = 4.71, p = 0.01]. Post-hoc comparisons indicated the sex difference in ethanol sensitivity was apparent at all doses tested, although the sex difference was most obvious at 3 g/kg ethanol (Fig. 3A) [for 1 g/kg: t(97) = 1.96, p = 0.05; for 2 g/kg: t(97) = 2.87, p = 0.005; for 3 g/kg: t(97) = 3.17, p = 0.002]. Irrespective of sex, a prior history of stress reduced sensitivity to ethanol-induced motor incoordination (Fig. 3A) [UCMS effect: F(1,91) = 8.91, p = 0.004; UCMS × Dose: F (2182) = 5.80, p = 0.004; Sex × UCMS × Dose: p = 0.91] and post-hoc comparisons indicated a significant UCMS effect at the 3 g/kg dose [t(97) = 0.004], but not at the other doses tested (t-tests, p’s > 0.25). A prior history of binge-drinking did not influence the dose-response function for ethanol-induced motor incoordination, nor did it interact with the other independent variables to influence the latency to fall from the rotarod (Fig. 3A; DID effect and interactions, all p’s > 0.60).
Fig. 3.
Effects of a prior history of sequential binge-drinking and stress upon behavioral measures of alcohol intoxication and subsequent binge-drinking. (A) Irrespective of prior binge-drinking or stressor history, females were less sensitive than males to the motor-in-coordinating effects of alcohol when assayed by rotarod. UCMS improved rotarod performance at the 3 g/kg alcohol dose similarly in males and females. (B) Prior histories of both binge-drinking and stressor exposure reduced and increased alcohol-induced sedation, respectively, in female and male subjects. For neither sex were the effects of these manipulations additive. (C) A prior history of stressor exposure augmented subsequent binge-drinking, irrespective of sex or prior binge-drinking history. However, only female bingers exhibited greater subsequent alcohol intake. The data represent the means ± SEMs of the number of mice indicated in parentheses in Fig. 2. +p < 0.05 vs. respective CNT; #p < 0.05 vs. respective Water.
3.2.3. Ethanol intoxication
As animals injected with 4 g/kg ethanol were too physically in capacitated to be tested on the rotarod following their test for loco-motor activity (i.e., the latency to fall was 0 s), we examined for sex differences in the effects of a sequential history of binge-drinking and UCMS upon their ability to regain their righting reflex, once placed in a supine position. In contrast to the negative results for locomotor hyper-activity (Fig. 2), analyses of the average righting score indicated a significant Sex × DID × UCMS interaction [F(1,83) = 7.49, p = 0.008]. We first deconstructed this interaction along the Sex factor and in females, we failed to detect any significant effects of either a prior history of binge-drinking or stress upon intoxication (Fig. 3B; DID × UCMS ANOVA, all p’s > 0.10). However, in males, a significant interaction was observed [F(1,38) = 4.74, p = 0.04] that reflected a stress-induced potentiation of alcohol intoxication in water-drinking controls [t(18) = 2.31, p = 0.04] that was not apparent in mice with a prior binge-drinking history (Fig. 3B; t-test, p = 0.25). Deconstruction of the 3-way interaction along the DID factor revealed a significant Sex × UCMS interaction in water-drinking controls (Fig. 3B) [F(1,41) = 4.81, p = 0.03] that reflected a lower righting score in stressed vs. unstressed females [t(20) = 2.59, p = 0.02] versus the higher righting score in stressed vs. unstressed males mentioned above.
3.2.4. Subsequent binge-ethanol intake
In a separate cohort, mice with prior histories of binge-drinking and/or repeated stress were assayed for sex differences in binge ethanol intake under a 2-bottle procedure (20 and 40%). As we have observed repeatedly, female mice continued to consume higher amounts of ethanol than males [Sex effect: F(1,97) = 12.02, p = 0.001] and as we reported previously in males under continuous ethanol-access procedures [84], a prior history of repeated stress potentiated subsequent ethanol intake (Fig. 3C) [UCMS effect: F(1,79) = 11.14, p = 0.001]. Interestingly, we observed a sex-dependent effect of a prior history of binge drinking that varied with prior stress history (Fig. 3C) [Sex × DID × UCMS: F(1,97) = 5.86, p = 0.02]. Deconstruction of this 3-way interaction along the Sex factor revealed a significant DID × UCMS interaction in females only (Fig. 3C) [F(1,44) = 4.77, p = 0.04], that reflected a stress-induced potentiation of binge-drinking in water controls [t(20) = 2.36, p = 0.03] but not in the female mice with a prior history of binge-drinking (t-test, p = 0.60). In males, prior stress augmented subsequent binge drinking, irrespective of prior drinking history (Fig. 3C) [UCMS effect: F(1,52) = 11.33, p = 0.001; no DID effect or interaction, p’s > 0.25] and t-tests con-firmed significant CNT-UCMS differences in mice with prior histories of both water [t(27) = 2.3, p = 0.03] and ethanol-drinking [t(22) = 2.41, p = 0.03]. Deconstruction of the 3-way interaction along the DID factor did not support a sex difference in either ethanol intake or the ability of prior stress to potentiate ethanol intake in ethanol history-negative controls [UCMS effect: F(1,50) = 11.42, p = 0.001; Sex effect and interaction, p’s > 0.20]. However, in ethanol history-positive animals, prior stress history potentiated alcohol intake in males only [Sex effect: F(1,46) = 12.10, p = 0.001; Sex × UCMS: F(1,46) = 4.75, p = 0.04].
3.3. Experiment 3: Sex differences in the effects of a simultaneous history of binge-drinking and stress upon behavioral sensitivity to ethanol
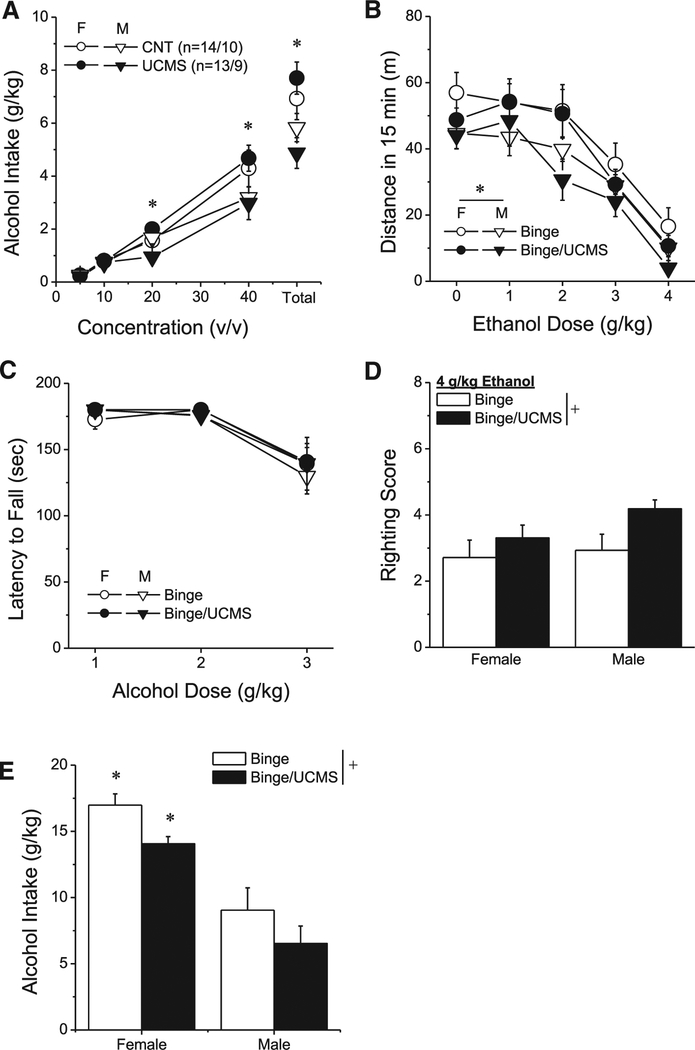
3.3.1. Binge-drinking during UCMS
When provided daily opportunity to consume ethanol from sipper tubes containing 5, 10, 20, 40% alcohol (v/v), B6.129 mice consumed, on average, > 4 g/kg ethanol within a 2-h (Fig. 4A). As this level of ethanol intake is predicted to result in BACs > 80 mg% [85], by definition, both the male and female mice in this study were engaged in binge-drinking behavior. As expected (Boehm studies), female mice binge-drank more ethanol than males [Sex effect: F(1,42) = 8.08, p = 0.007; Sex × Day: F(9378) = 1.96, p = 0.04] and this sex difference reflected increased intake of 40% ethanol (Fig. 4A) [Sex × Dose: F (3126) = 4.37, p = 0.006; Sex × Dose × Day: p = 0.20]. Interestingly, however, the binge-drinking exhibited by neither sex was unaffected by concurrent stressor exposure, as indicated by no main effect of, or interactions with, the Stress factor (Fig. 4A; all p’s > 0.15). Thus, while a prior stress history augments ethanol intake under both continuous-access ([84]; Fig. 1D) and limited-access conditions (Fig. 3C), concurrent stressor exposure does not significantly augment binge-drinking under our procedures in either male or female mice.
Fig. 4.
Effects of a prior history of concurrent binge-drinking and stress upon alcohol-induced changes in behavior. (A) Females binge-drank more high-dose alcohol than males, but concurrent stressor exposure did not systemically alter alcohol intake in either sex. *p < 0.05 for females vs. males. (B) Binging females locomoted more than binging males, but concurrent stressor exposure did not alter the alcohol dose-locomotor response function. *Denotes a main Sex effect. (C) No sex or UCMS effects were observed for rotarod performance in binging mice. (D) Irrespective of sex, binging mice with a history of concurrent stressor exposure exhibited greater alcohol-induced intoxication, relative to unstressed binging animals. *Denotes a main History effect.
3.3.2. Ethanol-induced locomotor activity
To our surprise, we observed absolutely no effect of UCMS upon the dose-response function for ethanol-induced locomotion in mice with a binge-drinking history (Fig. 4B) [Dose effect: F(2,84) = 25.82, p < 0.0001; all other p’s > 0.50]. These data indicate that a concurrent history of binge-drinking and UCMS does not influence ethanol-induced locomotion above that produced by a history of binge-drinking alone.
3.3.3. Ethanol-induced motor incoordination
As expected, alcohol dose-dependently reduced rotarod performance in mice with a prior history of binge-drinking [Dose effect: F (2,84) = 25.82, p < 0.00001]. Notably, the binge-drinking mice in this study performed much better on the task than did the animals in Experiment 2, most notably at the 3 g/kg dose (see Fig. 3A vs. 4C), which likely reflects the development of tolerance to ethanol-induced motor-impairment produced by their 10-day binge-drinking history. Also in contrast to the results of Experiment 2, rotarod performance in binge-experienced mice was completely unaffected by concurrent stressor exposure (Fig. 4C; Sex × Dose × Stress ANOVA, all other p’s > 0.50), which may reflect a floor effect upon behavior related to their apparent tolerance.
3.3.4. Ethanol-induced intoxication
Consistent with the notion that a 10-day history of binge-drinking induces tolerance to ethanol’s intoxicating properties, the average righting reflex score exhibited by both male and female mice in Experiment 3 was lower than that observed in the ethanol-naïve controls in Experiment 2 (Fig. 3B vs. Fig. 4D). However, whereas a prior history of binge-drinking did not significantly alter the effects of UCMS upon the righting reflex (see Binge mice in Fig. 4D), UCMS impaired the capacity of mice with a concurrent history of binge-drinking to right themselves following an injection of 4 g/kg ethanol (Fig. 4D) [Stress effect: F(1,45) = 3.99, p = 0.05]. Although an inspection of Fig. 4D suggests that this UCMS effect was more robust in male versus female mice, we detected no main Sex effect or Sex × Stress interaction (p’s > 0.20). These rotarod and righting reflex data provide novel evidence that coincident versus sequential binge-drinking/stressor exposure produce distinct, sex-related, effects upon alcohol intoxication that highlight the importance of the timing/temporal patterning of these subject-history factors for sex differences in behavioral sensitivity to this drug.
3.3.5. Subsequent binge-drinking
Finally, we determined whether or not binge-drinking of high-dose ethanol solutions (20 and 40% v/v) would be augmented in mice with a prior history of concurrent binge-drinking and stress. Consistent with the results from Experiments 1 and 2 (Figs. 1C and 3C), female mice consumed more alcohol than males (Fig. 4E) [Sex effect: F(1,45) = 50.75, p < 0.0001]. However, irrespective of sex, mice with a prior history of concurrent binge-drinking and stress exhibited less alcohol intake than mice with a prior history of binge-drinking alone (Fig. 4E) [Stress effect: F(1,45) = 6.25, p = 0.02; interaction: p = 0.85]. These data provide additional evidence that sequential and simultaneous histories of binge-drinking and stress produce distinct effects upon the propensity to consume ethanol.
4. Discussion
Human biomedical research critically depends upon basic animal science for insight into the etiology and of disease. This is particularly true with respect to disentangling how interactions between subject factors, such as prior histories of stress and drug/ethanol use, impact mental disease. Through the study of murine models of unpredictable stress and binge-drinking, we demonstrate clearly that a prior history of either binge-drinking or unpredictable stress increases behavioral sensitivity to ethanol. However, in neither males nor females, does a combined alcohol-stress history exert any greater effect upon behavioral sensitivity to ethanol than that of either history alone. Further, we demonstrate that a combined alcohol-stress history does not alter the subsequent propensity to consume ethanol, above and beyond that produced by either history alone. These results are discussed below within the context of the extant literature pertaining to sex differences in ethanol- and stressor-reactivity, as well as ethanol-stress interactions.
4.1. Subject factor interactions in the locomotor stimulatory effects of alcohol
The motor response to ethanol is sexually dimorphic in humans, with females exhibiting greater signs of intoxication in response to comparable alcohol doses than males [78,108]. Although not as well characterized as for other drugs of abuse (e.g., cocaine, amphetamines and opioids), the acute motor response to ethanol and its capacity to sensitize with repeated alcohol treatment is also sexually dimorphic in laboratory rodents. However, in contrast to stimulants and opioids, to which adult female rodents are hyper-sensitive (e.g., [6,58,88]), the data pertaining to sex differences in alcohol-induced changes in loco-motor hyper-activity are mixed and vary as a function of species and strain (e.g., [32,53,60,75,76,94]; c.f., [19]). This being said, females tend not to differ from males with respect to behavioral measures of ethanol motor-incoordination and/or intoxication and while the severity of ethanol-induced intoxication varies considerably with strain in mice, the absence of male-female differences in intoxication is consistent across different mouse strains (e.g., [9,10,24,25,59,75,76,103]).
Our prior ethanol-related studies of male and female mice on a B6.129 hybrid genetic background were too underpowered to reliably detect sex-related differences in sensitivity to ethanol’s locomotor-hyper-activating, in-coordinating and intoxicating effects [100]. Examining mice on a heterogeneous B6.129 genetic background for sex-related differences in behavior is important, considering that (1) the genetic background of humans is heterogeneous and (2) this particular hybrid strain is commonly employed in transgenic studies of neuropsychiatric disease [26,27], but their alcohol-related phenotype has not been characterized extensively. Herein, low to moderate alcohol doses (1–3 g/kg) were more effective at stimulating locomotor hyper-activity in females, than in males, and this sex difference was observed in all three experiments. Furthermore, this sex difference was apparent, irrespective of the prior binge-drinking and/or UCMS history of the mice. These data are consistent, in part, with the results of an earlier report by Melón et al. [76], in which a prior history of binge-drinking under a procedurally-distinct Drinking-in-the-Dark Multiple-Access-Schedule paradigm was found not to influence the greater locomotor responsiveness of female C57BL/6J mice to an injection of 1.75 g/kg ethanol.
4.2. Subject factor interactions in the intoxicating properties of alcohol
In contrast to our prior results for Swiss-Webster male mice [84], a prior UCMS history blunted, rather than enhanced, sensitivity to the intoxicating properties of alcohol as measured by the latency to fall from a rotarod (Fig. 3A). This UCMS effect was apparent in both male and female B6.129 mice and was unaffected by a prior history of binge-drinking. Thus, while prior UCMS history sensitizes Swiss-Webster mice to both the locomotor-activating and -intoxication/sedating effects of ethanol [84], a UCMS-ethanol cross-sensitization is apparent in B6.129 hybrid for locomotor-activity only. The precise explanation for the polar-opposite effects of our UCMS procedures upon rotarod performance of Swiss-Webster vs. B6.129 mice is not clear as there were several procedural differences between this and the previous study, which could have contributed to the disparate outcomes. Strain differences in sensitivity to ethanol-induced intoxication are well-described (e.g., [24,25,105]), as are differences in the behavioral reactivity to stressors (e.g., [20,27,51,105,107]). However, to the best of our knowledge, no study has examined explicitly for strain differences in the ability of repeated stress to cross-sensitize with ethanol’s loco-motor, intoxicating or sedating effects. Alternatively, although both studies trained the mice to walk on the rotarod prior to ethanol-testing, the Swiss-Webster mice in Quadir et al. [84] were tested only once for ethanol-induced changes in performance (3 g/kg) and locomotor and rotarod testing was conducted in separate cohorts of mice. Given its larger and more complex design, Experiment 2 employed within-subjects testing procedures, both across paradigms (locomotion then rotarod/righting reflect) and across ethanol doses (1, 2 and 3 g/kg, once a day across 3 days) to generate a dose-response function while conserving animal numbers and increasing the statistical power of our analyses. Thus, the possibility exists that the effects of UCMS upon ethanol-induced impairments in rotarod performance vary as a function of motor-learning under the influence of the drug (i.e., behavioral tolerance), with UCMS being less detrimental for animals trained to perform under the influence of ethanol, than for sober animals. Unfortunately, as all mice in Experiment 2 underwent our dose-response testing procedures, the interaction between prior UCMS and/or drinking history upon the development of behavioral tolerance on the rotarod remains to be determined.
Consistent with the fact that females were more sensitive to the locomotor-activating effects of ethanol in all three experiments, the female mice in Experiment 2 were less sensitive than their male counterparts to the locomotor in-coordinating effects of low to moderate ethanol doses, as indicated by a longer latency to fall from the rotarod across all doses tested (Fig. 3A). Again, this sex difference was apparent irrespective of prior binge-drinking and/or UCMS history. This being said, absolutely no sex difference in rotarod performance was observed in Experiment 3, in which all mice had a prior history of binge-drinking. This finding was unexpected as the statistical analyses of the results of Experiment 2 indicated that the interactions between sex, ethanol dose and prior UCMS history were independent of prior binge-drinking history (see Section 3.2.2). It is clear from an inspection of the data between the two experiments (Fig. 3A vs. Fig. 4D) that the rotarod responses of the mice were comparable at 1 and 2 g/kg ethanol, but that the mice in Experiment 2 were considerably more sensitive to the intoxicating properties of the 3 g/kg dose than the mice in Experiment 3. Further, ethanol induced less sedation in Experiment 3 versus Experiment 2 (Fig. 4D vs. Fig. 3B). Together, these data argue that the binge-drinking exhibited by the mice in Experiment 3 produced tolerance to drug-induced intoxication. Binge-drinking under limited-access procedures has been reported to induce a tolerance to alcohol’s intoxicating effects across several strains of mice [29,71] and in the present study, the alcohol dose-locomotor response functions for binge-experienced mice in Experiment 2 were shifted downwards of those of water controls (Fig. 2 top row vs. bottom row). Indeed, the locomotor activity expressed by the ethanol-drinking mice in Experiment 3 was comparable to that observed in the binge-experienced animals in Experiment 2 (Fig. 4B vs. Fig. 2 bottom row), supporting the notion that our binge-drinking procedures were sufficient to reduce the efficacy of alcohol to stimulate locomotor activity.
This being said, the inter-experiment differences in sensitivity to the intoxicating/sedative properties of alcohol cannot be readily explained by the development of tolerance, particularly given that Experiment 3 mice consumed considerably less alcohol prior to behavioral testing than did the mice in Experiment 2 (Section 3.2.1 vs. Fig. 4A). Although the precise reason for the difference in alcohol intake across the two experiments is not entirely clear, it is not unprecedented to observe a waxing and waning of alcohol intake across different cohorts of animals under limited-access Drinking-in-the-Dark binge-drinking procedures, particularly in mice on a mixed B6.129 background (e.g., [23] vs. [73]). Nevertheless, given the inter-experiment differences in ethanol intake, the possibility exists that the higher ethanol consumption sensitized Experiment 2 mice to the intoxicating effects of alcohol, while the in-take exhibited by Experiment 3 mice was insufficient to induce the neuroplasticity necessary for the manifestation of a sensitized response. Following this logic, we would predict the greatest alcohol-induced intoxication in females given that they consistently consumed more alcohol than males prior to behavioral testing; this simply was not the case (Figs. 3A, 4B). While a complete parametric analysis of the relationship between biological sex, the amount of binge-alcohol intake and the dose-response function for alcohol-induced intoxication is beyond the scope of this report, it is abundantly clear from the rotarod results of both Experiments 2 and 3 that the effects of a combined history of stress and alcohol (either simultaneous or sequential) upon alcohol-induced intoxication/sedation are no different from those produced by a prior history of either stress or alcohol-drinking alone. Further, it seems that biological sex may interact with the amount of alcohol consumption to influence sensitivity to the behavioral effects of higher alcohol doses.
4.3. Subject factor interactions in the sedative properties of high-dose alcohol
Previously, we reported that UCMS history increases the sleep-time of Swiss-Webster males injected acutely with a sedative 5 g/kg dose of ethanol [84]. Herein, when B6.129 mice were tested for their ability to right themselves ~20 min post-injection with 4 g/kg dose, binge/UCMS-naïve females exhibited greater intoxication than males as indicated by their higher righting score (Fig. 3B). This sex difference in the ability to right for binge/UCMS-naïve animals contrasts with a failure to detect a sex difference in the duration of the loss of righting reflex reported recently for inbred C57BL/6J mice following injection with 3.6 g/kg ethanol [10]. This discrepancy in findings might relate to the contribution of the 129X1/SvJ strain to genetic background of our subjects, the fact that our animals were disturbed at 15 min post-injection prior to testing for righting and/or a difference in the dependent variable measured. Interestingly and unlike ethanol-induced locomotor hyper-activity and changes in rotarod performance, a prior history of binge-drinking and/or UCMS reversed the direction of the sex difference in righting in Experiment 2, such that females now exhibited lower righting scores than males (Fig. 3B). These data indicate that a prior history of either binge-drinking and/or UCMS reduced sensitivity to ethanol-induced sedation selective in female subjects. A similar, but non-significant, sex-related trend in righting scores was observed in the ethanol-experienced mice in Experiment 3 (Fig. 4D), which bolsters the argument that the magnitude of a sex differences in ethanol-induced hypnosis varies as a function of prior ethanol intake, with more robust sex differences observed between male and female mice engaged in heavy binge-drinking. While our rotarod results for male B6.129 mice did not replicate our prior findings for male Swiss-Webster mice [84], UCMS history increased righting score in the male mice in Experiment 2 – a finding in-line with the potentiation of ethanol-induced sleep-time observed in our prior work [84]. Together, these results argue a more important role for biological sex than genetic background in the effect of a prior stress history upon the sedative-hypnotic properties of alcohol, although this hypothesis remained to be vetted.
Interestingly, the sex-dependent effect of UCMS history upon righting score was nearly identical to that produced by a history of binge-drinking (Fig. 2B), indicating that female binge-drinkers are less sensitive than males to the sedative-hypnotic properties of high-dose alcohol. However, as was observed for our other dependent variables, in neither sex, did a sequential history of binge-drinking and UCMS exert additive or synergistic effects upon this measure (Fig. 2B). In contrast, a simultaneous history of ethanol intake and UCMS augmented the sedative effects of ethanol, above that produced by an ethanol-drinking experience alone and this effect was sex-independent (Fig. 4D). These particular results argue that the specific interactions between biological sex and prior ethanol/stress history upon the se-dative-hypnotic effects of high-dose ethanol vary not only as a function of having a prior ethanol/stress, but also as a function of the temporal patterning or coincidence of the ethanol/stress history. Obviously, the design of Experiment 3 does permit conclusions regarding whether or not the effects of simultaneous ethanol/stress upon ethanol-induced hypnosis are additive or synergistic and the present results need to be replicated using more conventional measures of ethanol-induced sedation (e.g., sleep-time or latency to right). Nevertheless, they provide novel behavioral evidence of very complex interactions between the subject factors of sex, prior ethanol-drinking history, and prior stress history in regulating behavioral sensitivity to high-dose ethanol and argue that the neuroadaptations produced by a sequential versus simultaneous history of ethanol-drinking and repeated stress are distinct.
4.4. Subject factor interactions in withdrawal-induced binge-drinking
In both humans and laboratory animals, sensitivity to the intoxicating, sedative-hypnotic properties of ethanol are inversely corrected with vulnerability to engage in problem-drinking and develop an AUD (e.g., [37,91]). However, in our prior study, UCMS potentiated not only motor sensitivity to ethanol, but also augmented ethanol intake under continuous-access conditions in male Swiss-Webster mice [84]. Consistent with our prior work in Swiss-Webster mice, UCMS history potentiated ethanol intake by male B6.129 mice; this potentiation was observed under both continuous-access (Fig. 1C, D) and limited-access conditions (Fig. 3C, left) and the magnitude of the increase was unaffected by a prior binge-drinking history (Fig. 3C, left). Interestingly, however, a prior binge-drinking history alone was insufficient to augment subsequent ethanol intake by B6.129 males (Fig. 3C). This finding is consistent with our prior observations [21–23,68,69,101] and those of others (e.g., [29,34,37,71,75,76,85]) that adult male mice on a B6 background exhibit a very stable pattern of ethanol consumption under limited-access, binge-drinking procedures and extend these results by demonstrating that stable alcohol intake is maintained in ethanol-experienced mice even following a 14-day hiatus from drinking (11 days of UCMS control procedures + 3 days of ethanol dose-response testing). The failure to observe an alcohol-deprivation effect (i.e., greater ethanol intake following a period of withdrawal) in our binge-drinking males was somewhat surprising, but may reflect the fact that mice were administered ethanol injections for the 3 days prior to the resumption of drinking. Nevertheless, it is clear from the present results of Experiment 2 that, unlike a prior UCMS history, a prior 2-week history of binge-drinking does not sensitize ethanol intake in male mice, as there was no difference in the average alcohol consumption exhibited by binge-experienced males and those consuming ethanol for the first time (Fig. 3C, left). Thus, as observed in Swiss-Webster mice [84], males with a prior history of repeated stress exhibit greater ethanol intake, despite an increased sensitivity to the sedative-hypnotic properties of this drug.
When assessed under continuous-access conditions, we observed no effect of UCMS upon the ethanol intake of female mice (Fig. 1C, D). This negative result is attributed to a ceiling effect upon ethanol intake, rather than a female-specific resiliency to the intake-enhancing effects of UCMS, as UCMS clearly potentiated to the binge-alcohol intake exhibited by both ethanol-naïve and binge-experienced females when they were tested under limited-access procedures (Fig. 3C, right). Further, as observed in males, a prior history of binge-drinking did not influence the magnitude of the UCMS-potentiated binge-drinking in females (Fig. 3C). However, in contrast to males, prior binge-drinking history produced a quantitatively similar increase in subsequent ethanol intake following withdrawal as that produced by UCMS either alone or in combination with drinking (Fig. 3C, right). These results further the argument that a sequential history of binge-drinking and repeated stress does not produce any additive or synergistic effect upon ethanol intake in either sex. Furthermore, as ethanol intake under limited-access conditions did not vary systemically as function of intake in either sex (Section 3.2.1), these data provide novel evidence that a sex difference exists with respect to a sensitization of ethanol intake during protracted withdrawal from a history of voluntary binge-drinking history. Although the design of Experiment 3 did not include an ethanol-naïve comparison group, the withdrawal-induced drinking exhibited by the binge-only females in this experiment was more than double that observed during their initial drinking phase (6.93 ± 0.79 vs. 16.99 ± 0.85 g/kg), while that of the Experiment 3 males was elevated, but to a much lesser extent (5.83 ± 0.53 vs. 9.05 ± 1.67 g/kg) (Fig. 4A total vs. Fig. 4E). Thus, the results of Experiment 3 replicate those of Experiment 2 by indicating greater withdrawal-induced binge-drinking in females with a prior binge-drinking history than their male counterparts. These results are consistent with clinical evidence that binge-drinking females are more susceptible than males to alcohol’s neuropsychiatric effects (e.g., [18,43,48,55,61,64,74,81,92,93]). Given that the gender gap in binge-drinking is closing in humans (e.g., [36,49,64,96,106]), it is imperative that we start to understand the biopsychological mechanisms underpinning this sex difference in withdrawal-induced binge-drinking.
While sex differences in initial ethanol intake is a likely mediator of the severity of withdrawal symptoms driving subsequent alcohol intake, it is notable that the binge-drinking female controls in Experiment 3 consumed on average ~ 3 g/kg less alcohol per day than the males in Experiment 2 (Fig. 4E vs. Section 3.2.1), yet these females exhibited a robust ethanol deprivation effect (Fig. 4E), while the males did not (Fig. 3C). Thus, the sex difference in withdrawal-induced drinking is not a mere reflection of greater alcohol intake in female versus male mice. Alternatively, the potentiation of withdrawal-induced binge-drinking observed in females with prior histories of UCMS and/or binge-drinking (Fig. 3C) might reflect their lower sensitivity to the se-dative-hypnotic effects of ethanol (Fig. 3B). Indeed, a lower sensitivity to these more aversive, motor-incapacitatings effects of ethanol could readily explain the increased capacity of females with prior histories of binge-drinking and/or repeated stress to consume more ethanol during withdrawal. In females, both alcohol intake and sensitivity to ethanol’s motor effects are sensitive to reproductive cycle (e.g., [14,35,67,87]). However, the variability in withdrawal-induced drinking exhibited by binge-experienced male and female mice in Experiment 2 was comparable (Fig. 3C), while the variability in the data for the females in Experiment 3 was actually lower than that observed in their male counterparts (Fig. 4E). Likewise, the variability in alcohol-induced locomotor activity, intoxication and sedation was also comparable between males and females throughout study. Thus, it is not likely that the higher withdrawal-induced drinking observed in our female subjects reflects simply their reproductive status at the time of testing, although it is likely that ovarian hormones contribute to the increased binge-intake exhibited by female subjects both during initial drinking and following a period of withdrawal and a hormonal bases for our observed sex differences in withdrawal-induced drinking will be pursued in future studies.
Fascinatingly, while the effects of a sequential history of binge-drinking + repeated stress upon withdrawal-induced drinking were either greater than (males), or similar to (females), those produced by a history of binge-drinking alone (Fig. 3C), withdrawal-induced drinking was lower in both male and female mice with a simultaneous binge/stress history than in their counterparts with a history binge-drinking alone (Fig. 4E). This finding is intriguing as concurrent UCMS experience did not alter initial ethanol intake by either sex (Fig. 4A), despite our repeated demonstration that a prior history of UCMS potentiates subsequent ethanol intake under both continuous- and limited-access procedures (Figs. 1C, D, 3C, 4D; [84]). At the present time, the precise mechanism underlying this “protective” effect of simultaneous stress history upon withdrawal-induced drinking is completely unknown, but it would appear that certain neuroadaptations induced by our UCMS procedures may counteract or reverse those produced by binge-alcohol consumption when these procedures are temporally coincident. We know very little regarding the neurobiological consequences of our UCMS procedures and our studies to date have focused on glutamate-related signaling molecules within the nucleus accumbens (NAC) as glutamate hyperactivity within this region is well-characterized to drives the positive reinforcing properties of ethanol (c.f., [7]) and more recent work by our laboratory implicates a hyper-glutamate state within the NAC shell in mediating also the negative reinforcing properties of ethanol withdrawal in binge-drinking mice [70]. However, our limited study to date indicate that UCMS alone produces surprisingly few changes in the total protein expression of Group1 mGlu receptors, NMDA receptor subunits or their major Homer scaffolding proteins [84]. That being said, UCMS was found to reduce PLCβ expression within the NAC shell and to reverse an ethanol-induced increase in ERK activity within NAC subregions [84]. This latter result is particularly interesting given that considerable evidence indicates a necessary role for ERK induction in regulating addiction-related behavior (c.f., [16,99,109]). Thus, ERK activation within NAC subregions will be a central focus of future work designed to understand how the temporal patterning of prior histories of stress and binge-drinking influence behavioral sensitivity to ethanol and binge-ethanol intake.
4.5. Conclusions
The issue of subject factor interactions with respect to the etiology and severity of AUDs, affective disorders and their comorbidity cannot be readily disentangled in studies of humans. The results of the present series of experiments indicate that even with the experimental control afforded by studying murine models of ethanol intake and repeated, unpredictable stress, the subject factors of biological sex, prior stressor history and prior binge-drinking history interact in complex, sometimes unpredictable, ways to impact behavioral sensitivity to ethanol and heavy drinking. This being said, the present results provide clear evidence that while prior histories of repeated stress or binge-drinking influence alcoholism-related behaviors, sometimes in a sex-dependent manner, their combined effects are not additive nor are they synergistic. Further, the present data highlight that the direction of certain behavioral consequences of prior histories of repeated stress and binge-drinking, notably withdrawal-induced drinking, varies not only with biological sex, but also with their temporal patterning. These results have important clinical ramification for understanding how important subject factors interact to influence alcoholism-related behaviors of relevance to our understanding of the etiology and prognosis of AUDS, affective disorders and their co-morbidities.
Acknowledgements
The authors would like to thank Daniel Colin, Praveena Motupali and Michal Coelho for their technical assistance. Funding for this work was provided by NIAAA grant AA024044 to KKS, the UCSB Faculty Research Assistance Program to SGQ, MAP, DLM and EG, as well as the UCSB McNair Scholars Program to EG.
References
- [1].Advani T, Hensler JG, Koek W, Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice, Int. J. Neuropsychopharmacol 10 (2007) 595–607. [DOI] [PubMed] [Google Scholar]
- [2].Annis HM, Sklar SM, Moser AE, Gender in relation to relapse crisis situations, coping, and outcome among treated alcoholics, Addict. Behav 23 (1998) 127–131. [DOI] [PubMed] [Google Scholar]
- [3].Anthenelli RM, Maxwell RA, Geracioti TD Jr., Hauger R, Stress hormone dysregulation at rest and after serotonergic stimulation among alcohol-dependent men with extended abstinence and controls, Alcohol. Clin. Exp. Res 25 (2001) 692–703. [PubMed] [Google Scholar]
- [4].Becker HC, Influence of stress associated with chronic alcohol exposure on drinking, Neuropharmacology (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Becker HC, Lopez MF, Doremus-Fitzwater TL, Effects of stress on alcohol drinking: a review of animal studies, Psychopharmacology 218 (2011) 131–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Becker JB, McClellan ML, Reed BG, Sex differences, gender and addiction, J. Neurosci. Res 95 (1–2) (2017) 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bell RL, Hauser SR, McClintick J, Rahman S, Edenberg HJ, Szumlinski KK, McBride WJ, Ethanol-associated changes in glutamate reward neurocircuitry: a minireview of clinical and preclinical genetic findings, Prog. Mol. Biol. Transl. Sci 137 (2016) 41–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, Wang S, Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions, Drug Alcohol Depend. 132 (2013) 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Blednov YA, Benavidez JM, Black M, Leiter CR, Osterndorff-Kahanek E, Johnson D, Johnson D, Borghese CM, Hanrahan JR, Johnston GAR, Chebib M, Harris RA, GABAA receptors containing ρ1 subunits contribute to in vivo effects of ethanol in mice, PLoS One 9 (1) (2014) e85525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Blednov YA, Black M, Benavidez JM, Stamatakis EE, Harris RA, PPAR agonists: II. Fenofibrate and tesaglitazar alter behaviors related to voluntary alcohol consumption, Alcohol. Clin. Exp. Res 40 (3) (2016) 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brabant C, Guarnieri DJ, Quertemont E, Stimulant and motivational effects of alcohol: lessons from rodent and primate models, Pharmacol. Biochem. Behav 122C (2014) 37–52. [DOI] [PubMed] [Google Scholar]
- [12].Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall RK, Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder, Alcohol. Clin. Exp. Res 30 (2006) 938–946. [DOI] [PubMed] [Google Scholar]
- [13].Brady KT, Waldrop AE, McRae AL, Back SE, Saladin ME, Upadhyaya HP, Anton RF, Randall RK, The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response, J. Stud. Alcohol 67 (2006) 700–706. [DOI] [PubMed] [Google Scholar]
- [14].Brot MD, Koob GF, Britton KT, Anxiolytic effects of steroid hormones during the estrous cycle. Interactions with ethanol, Recent Dev. Alcohol 12 (1995) 243–259. [DOI] [PubMed] [Google Scholar]
- [15].Brown JM, Williams J, Bray RM, Hourani L, Postdeployment alcohol use, aggression, and post-traumatic stress disorder, Mil. Med 177 (2012) 1184–1190. [DOI] [PubMed] [Google Scholar]
- [16].Cahill E, Salery M, Vanhoutte P, Caboche J, Convergence of dopamine and glutamate signaling onto striatal ERK activation in response to drugs of abuse, Front. Pharmacol 4 (2014) 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Caldwell EE, Riccio DC, Alcohol self-administration in rats: modulation by temporal parameters related to repeated mild social defeat stress, Alcohol 44 (2010) 265–274. [DOI] [PubMed] [Google Scholar]
- [18].Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF, Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory, Alcohol Alcohol. 40 (2005) 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Camarini R, Pautassi RM, Behavioral sensitization to ethanol: neural basis and factors that influence its acquisition and expression, Brain Res. Bull 125 (2016) 53–78. [DOI] [PubMed] [Google Scholar]
- [20].Clément Y, Calatayud F, Belzung C, Genetic basis of anxiety-like behaviour: a critical review, Brain Res. Bull 57 (1) (2002) 57–71 (Jan 1, 2002). [DOI] [PubMed] [Google Scholar]
- [21].Cozzoli DK, Courson J, Caruana AL, Miller BW, Thompson AB, Wroten M, Zhang PW, Xiao B, Hu J-H, Klugmann M, Metten P, Worley PW, Crabbe JC, Szumlinski KK, Accumbens shell metabotropic glutamate receptor 5-associated signaling regulates binge alcohol drinking: evidence from Drinking-in-the-Dark studies, Alcohol. Clin. Exp. Res 36 (2012) 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK, Binge alcohol drinking by mice requires intact Group1 metabo-tropic glutamate receptor signaling within the central nucleus of the amygdala, Neuropsychopharmacology 39 (2014) 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cozzoli DK, Courson J, Rostock C, Wroten MG, Caruana AL, Zhang PW, Xiao B, Hu J-H, Worley PF, Crabbe JC, Finn DA, Szumlinski KK, Extended amygdala protein kinase C epsilon signaling mediates binge alcohol consumption, Biol. Psychiatry 79 (2016) 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Crabbe JC, Metten P, Yu CH, Schlumbohm JP, Cameron AJ, Wahlsten D, Genotypic differences in ethanol sensitivity in two tests of motor incoordination, J. Appl. Physiol 95 (4) (1985) 1338–1351. [DOI] [PubMed] [Google Scholar]
- [25].Crabbe JC, Metten P, Cameron AJ, Wahlsten D, An analysis of the genetics of alcohol intoxication in inbred mice, Neurosci. Biobehav. Rev 28 (2005) 785–802. [DOI] [PubMed] [Google Scholar]
- [26].Crawley JN, Behavioral phenotyping strategies for mutant mice, Neuron 57 (6) (2008) 809–818. [DOI] [PubMed] [Google Scholar]
- [27].Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R, Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies, Psychopharmacology 132 (1997) 107–124. [DOI] [PubMed] [Google Scholar]
- [28].Croft AP, Brooks SP, Cole J, Little HJ, Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist, Psychopharmacology 183 (2005) 163–170. [DOI] [PubMed] [Google Scholar]
- [29].Cronise K, Finn DA, Metten P, Crabbe JC, Scheduled access to ethanol results in motor impairment and tolerance in female C57BL/6J mice, Pharmacol. Biochem. Behav 81 (4) (2005) 943–953. [DOI] [PubMed] [Google Scholar]
- [30].Dawson DA, Grant BF, Ruan WJ, The association between stress and drinking: modifying effects of gender and vulnerability, Alcohol Alcohol. 40 (2005) 453–460. [DOI] [PubMed] [Google Scholar]
- [31].Delis F, Thanos PK, Rombola C, Rosko L, Grandy D, Wang G-J, Volkow ND, Chronic mild stress increases alcohol intake in mice with low dopamine D2 receptor levels, Behav. Neurosci 127 (2013) 95–105. [DOI] [PubMed] [Google Scholar]
- [32].DuBose CS, Chesler EJ, Goldowitz D, Hamre KM, Use of the expanded panel of BXD mice narrow QTL regions in ethanol-induced locomotor activation and motor incoordination, Alcohol. Clin. Exp. Res 37 (2013) 170–183. [DOI] [PubMed] [Google Scholar]
- [33].Fava M, Abraham M, Alpert J, Nierenberg AA, Pava JA, Rosenbaum JF, Gender differences in Axis I comorbidity among depressed outpatients, J. Affect. Disord 38 (1996) 129–133. [DOI] [PubMed] [Google Scholar]
- [34].Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC, A procedure to produce high alcohol intake in mice, Psychopharmacology 178 (4) (2005) 471–480 (2005 Apr). [DOI] [PubMed] [Google Scholar]
- [35].Ford MM, Eldridge JC, Samson HH, Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns, Alcohol. Clin. Exp. Res 26 (5) (2002) 635–643. [PubMed] [Google Scholar]
- [36].Fox HC, Sinha R, Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women, Harv. Rev. Psychiatry 17 (2009) 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fritz BM, Cordero KA, Barkley-Levenson AM, Metten P, Crabbe JC, Boehm SL II, Genetic relationship between predisposition for binge alcohol consumption and blunted sensitivity to adverse effects of alcohol in mice, Alcohol. Clin. Exp. Res 38 (5) (2014) 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gilpin NW, Karanikas CA, Richardson NH, Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats, PLoS One 7 (2012) e31466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goeders NE, The impact of stress on addiction, Eur. Neuropsychopharmacol 13 (6) (2003) 435–441 (Dec 2003). [DOI] [PubMed] [Google Scholar]
- [40].Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K, Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions, Arch. Gen. Psychiatry 61 (2004) 807–816. [DOI] [PubMed] [Google Scholar]
- [41].Grant BF, Hasin DS, Stinson FS, Dawson DA, Patricia Chou S, June Ruan W, Huang B, Co-occurrence of 12-month mood and anxiety disorders and personality disorders in the US: results from the national epidemiologic survey on alcohol and related conditions, J. Psychiatr. Res 39 (2005) 1–9. [DOI] [PubMed] [Google Scholar]
- [42].Hall FS, Huang S, Fong GW, Pert A, Linnoila M, Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats, Psychopharmacology 139 (1998) 210–216. [DOI] [PubMed] [Google Scholar]
- [43].Harford TC, Yi HY, Faden VB, Chen CM, The dimensionality of DSM-IV alcohol use disorders among adolescent and adult drinkers and symptom patterns by age, gender, and race/ethnicity, Alcohol. Clin. Exp. Res 33 (2009) 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hasin DS, Grant BF, Major depression in 6050 former drinkers: association with past alcohol dependence, Arch. Gen. Psychiatry 59 (2004) 794–800. [DOI] [PubMed] [Google Scholar]
- [45].Hasin DS, Goodwin RD, Stinson FS, Grant BF, Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions, Arch. Gen. Psychiatry 62 (2005) 1097–1106. [DOI] [PubMed] [Google Scholar]
- [46].Hasin DS, Stinson FS, Ogburn E, Grant BF, Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions, Arch. Gen. Psychiatry 64 (2007) 830–842. [DOI] [PubMed] [Google Scholar]
- [47].Heilig M, Egli M, Crabbe JC, Becker HC, Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict. Biol 15 (2010) 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hesselbrock MN, Meyer RE, Keener JJ, Psychopathology in hospitalized alcoholics, Arch. Gen. Psychiatry 42 (1985) 1050–1055. [DOI] [PubMed] [Google Scholar]
- [49].Hesselbrock VM, Segal B, Hesselbrock MN, Alcohol dependence among Alaska Natives entering alcoholism treatment: a gender comparison, J. Stud. Alcohol 61 (2000) 150–156. [DOI] [PubMed] [Google Scholar]
- [50].Holleran KM, Wilson HH, Fetterly TL, Bluett RJ, Centanni SW, Gilfarb RA, Rocco LE, Patel S, Winder DG, Ketamine and MAG lipase inhibitor-dependent reversal of evolving depressive-like behavior during forced abstinence from alcohol drinking, Neuropsychopharmacology 41 (8) (2016) 2062–2071 (2016 Jul). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN, Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice, Genes Brain Behav. 1 (1) (2002) 55–69 (2002 Jan). [DOI] [PubMed] [Google Scholar]
- [52].Hwa LS, Holly EN, DeBold JF, Miczek KA, Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice, Psychopharmacology 233 (4) (2016) 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Itzhak Y, Anderson KL, Ethanol-induced behavioral sensitization in adolescent and adult mice: role of the nNOS gene, Alcohol. Clin. Exp. Res 32 (10) (2008) 1839–1848. [DOI] [PubMed] [Google Scholar]
- [54].Jacobsen LK, Southwick SM, Kosten TR, Substance use disorders in patients with posttraumatic stress disorder: a review of the literature, Am. J. Psychiatry 158 (2001) 1184–1190. [DOI] [PubMed] [Google Scholar]
- [55].Jacobson R, The contributions of sex and drinking history to the CT brain scan changes in alcoholics, Psychol. Med 16 (1986) 547–559. [DOI] [PubMed] [Google Scholar]
- [56].Johnston LD, O’Malley PM, Bachman JG, National Survey Results on Drug Use From the Monitoring the Future Study, 1975–1997 Volume I: Secondary School Students (NIH Publication No. 98–4345), National Institute on Drug Abuse, Rockville, MD, 1999. [Google Scholar]
- [57].Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, National Survey Results on Drug Use From the Monitoring the Future Study, 1975–2007 Volume I: Secondary School Students (NIH Publication No. 08–6418A), National Institute on Drug Abuse, Bethesda, MD, 2008. [Google Scholar]
- [58].Kalivas PW, Stewart J, Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity, Brain Res. Rev 16 (3) (1991) 223–244 (1991 Sep–Dec). [DOI] [PubMed] [Google Scholar]
- [59].Kamens HM, Silva C, McCarthy R, Cox RJ, Ehringer MA, No evidence of a role of the β4 subunit of the nicotinic acetylcholine receptor in alcohol-related behaviors, BMC Res. Notes 10 (1) (2017) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kawakami SE, Quadros IM, Takahashi S, Suchecki D, Long maternal separation accelerates behavioural sensitization to ethanol in female, but not in male mice, Behav. Brain Res 184 (2) (2007) 109–116. [DOI] [PubMed] [Google Scholar]
- [61].Kessler RC, Crum RM, Warner LA, Nelson CG, Schulenberg J, Anthony JC, Lifetime co-occurrence of DSM–III–R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey, Arch. Gen. Psychiatry 54 (1997) 313–321. [DOI] [PubMed] [Google Scholar]
- [62].Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM, Prevalence and treatment of mental disorders. 1990 to 2003, N. Engl. J. Med 352 (2005) 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Keyes KM, Grant BF, Hasin DS, Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population, Drug Alcohol Depend. 93 (2008) 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Keyes KM, Martins SS, Blanco C, Hasin DS, Telescoping and gender differences in alcohol dependence: new evidence from two National surveys, Am. J. Psychiatry 167 (2010) 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Knapp DJ, Saiers JA, Pohorecky LA, Observations of novel behaviors as indices of ethanol withdrawal-induced anxiety, Alcohol Alcohol. Suppl 2 (1992) 489–493. [PubMed] [Google Scholar]
- [66].Koob GF, Addiction is a reward deficit and stress surfeit disorder, Front. Psychiatry 4 (2013) 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lancaster FE, Gender differences in animal studies. Implications for the study of human alcoholism, Recent Dev. Alcohol 12 (1995) 209–215. [PubMed] [Google Scholar]
- [68].Lee KM, Coelho M, McGregor HA, Waltermire RS, Szumlinski KK, Binge alcohol drinking elicits persistent negative affect in mice, Behav. Brain Res 291 (2015) 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK, Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens, Front. Cell. Neurosci 10 (2016) 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lee KM, Coelho MA, Sern KR, Class MA, Bocz MD, Szumlinski KK (2017) Anxiolytic effects of buspirone and MTEP in the Porsolt forced swim test. Chronic Stress (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL II, Tolerance to ethanol’s ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model, Alcohol. Clin. Exp. Res 35 (7) (2011) 1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lodge DJ, Lawrence AJ, The effect of isolation rearing on volitional ethanol consumption and central CCK/dopamine systems in Fawn-Hooded rats, Behav. Brain Res 141 (2003) 113–122. [DOI] [PubMed] [Google Scholar]
- [73].Lum EN, Campbell RR, Rostock C, Szumlinski KK, mGluR1 receptors within the nucleus accumbens regulate alcohol intake in mice under limited-access conditions, Neuropharmacology 79 (2014) 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF, Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects, Alcohol. Clin. Exp. Res 32 (2008) 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Melón LC, Boehm SL 2nd, Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/2J and C57BL/6J inbred mouse strains, Alcohol. Clin. Exp. Res 35 (7) (2011) 1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Melón LC, Wray KN, Moore EM, Boehm SL 2nd, Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence, Pharmacol. Biochem. Behav 104 (2013) 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Merikangas KR, Ames M, Cui L, Stang PE, Ustun TB, Von Korff M, Kessler RC, The impact of comorbidity of mental and physical conditions on role disability in the US adult household population, Arch. Gen. Psychiatry 6 (2007) 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Miller MA, Weafer J, Fillmore MT, Gender differences in alcohol impairment of simulated driving performance and driving-related skills, Alcohol Alcohol. 44 (2009) 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Newlin DB, Thomson JB, Alcohol challenge with sons of alcoholics: a critical review and analysis, Psychol. Bull 108 (1990) 383–402. [DOI] [PubMed] [Google Scholar]
- [80].Noone M, Dua J, Markham R, Stress, cognitive factors, and coping resources as predictors of relapse in alcoholics, Addict. Behav 24 (1999) 687–693. [DOI] [PubMed] [Google Scholar]
- [81].van Noorden MS, Giltay EJ, den Hollander-Gijsman ME, van der Wee NJ, van Veen T, Zitman FG, Gender differences in clinical characteristics in a naturalistic sample of depressive outpatients: the Leiden Routine Outcome Monitoring Study, J. Affect. Disord 125 (2010) 116–123. [DOI] [PubMed] [Google Scholar]
- [83].Pang TY, Renoir T, Du X, Lawrence AJ, Hannan AJ, Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running, Eur. J. Neurosci 37 (2013) 1803–1810. [DOI] [PubMed] [Google Scholar]
- [84].Quadir SG, Santos JR, Campbell RR, Wroten MG, Singh N, Holloway JJ, Bal SK, Camarini R, Szumlinski KK, Homer2 regulates alcohol and stress cross-sensitization, Addict. Biol 21 (3) (2016) 613–633 (2016 May). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC, Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice, Physiol. Behav 84 (1) (2005) 53–63. [DOI] [PubMed] [Google Scholar]
- [86].Riga D, Schmitz LJ, van der Harst JE, van Mourik Y, Hoogendijk WJ, Smit AB, De Vries TJ, Spijker S, A sustained depressive state promotes a guanfacine reversible susceptibility to alcohol seeking in rats, Neuropsychopharmacology 39 (2014) 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF, Estrous cycle effects on operant responding for ethanol in female rats, Alcohol. Clin. Exp. Res 22 (7) (1998) 1564–1569. [PubMed] [Google Scholar]
- [88].Robinson TE, Becker JB, Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis, Brain Res. 396 (2) (1986) 157–198. [DOI] [PubMed] [Google Scholar]
- [89].Rodriguez-Arias M, Navarrete F, Blanco-Gandia MC, Arenas MC, Bartoll-Andrés A, Aguilar Ma, Rubio G, Miñarro J, Manzanares J, Social defeat in adolescent mice increases vulnerability to alcohol consumption, Addict. Biol 21 (2014) 87–97. [DOI] [PubMed] [Google Scholar]
- [90].Schenk S, Gorman K, Amit Z, Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats, Alcohol 7 (1990) 321–326. [DOI] [PubMed] [Google Scholar]
- [91].Schuckit MA, Studies of men at High Risk for Future Alcoholism, in: von Wartburg JP, Magnenat P, Muller R, Wyss S (Eds.), Currents in Alcohol Research and the Prevention of Alcohol Problems, Toronto’ Hans Huber Publishers, Berne Stuttgart, 1985, pp. 45–51. [Google Scholar]
- [92].Schuckit MA, Daeppen JB, Tipp JE, Hesselbrock M, Bucholz KK, The clinical course of alcohol-related problems in alcohol dependent and nonalcohol dependent drinking women and men, J. Stud. Alcohol 59 (1998) 581–590. [DOI] [PubMed] [Google Scholar]
- [93].Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I, Effects of alcoholism and gender on brain metabolism, Am. J. Psychiatry 160 (2003) 1180–1183. [DOI] [PubMed] [Google Scholar]
- [94].Sershen H, Hashim A, Vadasz C, Strain and sex differences in repeated ethanol treatment-induced motor activity in quasi-congenic mice, Genes Brain Behav. 1 (3) (2002) 156–165. [DOI] [PubMed] [Google Scholar]
- [95].Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM, Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals, Neuropsychopharmacology 34 (2009) 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sonne SC, Back SE, Diaz Zuniga C, Randall CL, Brady KT, Gender differences in individuals with comorbid alcohol dependence and post-traumatic stress disorder, Am. J. Addict 12 (2003) 412–423. [PubMed] [Google Scholar]
- [97].Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW, Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice, Neuropsychopharmacology 34 (2009) 1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Substance Abuse and Mental Health Services Administration, Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10–4586), (2010).
- [99].Sun WL, Quizon PM, Zhu J, Molecular mechanism: ERK signaling, drug addiction, and behavioral effects, Prog. Mol. Biol. Transl. Sci 137 (2016) 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During MT, Worley PF, Middaugh LD, Kalivas PW, Homer2 is necessary for ethanol-induced neuroplasticity, J. Neurosci 25 (2005) 7054–7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS, Accumbens neurochemical adaptations produced by binge-like alcohol consumption, Psychopharmacology 190 (2007) 415–431. [DOI] [PubMed] [Google Scholar]
- [102].Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE, Accumbens Homer2 over-expression facilitates alcohol-induced neuroplasticity in C57BL/6J mice, Neuropsychopharmacology 33 (2008) 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, Finn DA, Sex differences in ethanol’s anxiolytic effect and chronic ethanol withdrawal severity in mice with a null mutation of the 5α-reductase type 1 gene, Behav. Genet 45 (3) (2015) 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Uhart M, Wand GS, Stress, alcohol and drug interaction: an update of human research, Addict. Biol 14 (2009) 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wahlsten D, Metten P, Phillips TJ, Boehm SL II, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC, Different data from different labs: lessons from studies of gene-environment interaction, J. Neurobiol 54 (1) (2003) 283–311. [DOI] [PubMed] [Google Scholar]
- [106].Witt ED, Puberty, hormones, and sex differences in alcohol abuse and dependence, Neurotoxicol. Teratol 29 (2007) 81–95. [DOI] [PubMed] [Google Scholar]
- [107].Yilmazer-Hanke DM, Morphological correlates of emotional and cognitive behaviour: insights from studies on inbred and outbred rodent strains and their crosses, Behav. Pharmacol 19 (5–6) (2008) 403–434. [DOI] [PubMed] [Google Scholar]
- [108].York JL, Biederman I, Hand movement speed and accuracy in detoxified alcoholics, Alcohol. Clin. Exp. Res 15 (1991) 982–990. [DOI] [PubMed] [Google Scholar]
- [109].Zamora-Martinez ER, Edwards S, Neuronal extracellular signal-regulated kinase (ERK) activity as marker and mediator of alcohol and opioid dependence, Front. Integr. Neurosci 8 (2014) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]