Abstract
A broad spectrum of pathology affects the rectum, anus and perineum, and understanding of relevant anatomy is important in accurate reporting, particularly in rectal cancer. In this pictorial review, correlative imaging, endoscopic, pathologic and operative images are presented to illustrate normal anorectal anatomy and neoplastic conditions that affect the anus and rectum. A particular case-based focus is given to rectal adenocarcinoma with pelvic MR and surgical histopathology. Additionally, carcinoid tumor, gastrointestinal stromal tumor, condylomata acuminata, squamous cell carcinoma, melanoma, and metastatic disease about the rectum or anus are reviewed.
Keywords: Rectum, anorectal, rectal cancer, colorectal adenocarcinoma, Computed tomography, magnetic resonance imaging
Introduction
Anorectal Anatomy
Knowledge of the anorectal region anatomy is essential for radiologists to identify disease processes and evaluate their local extension. This concept is of value for accurate tumor staging and treatment strategy. The anus and rectum comprises the distal end of the gastrointestinal tract and acts as a fecal reservoir and facilitates defecation. The rectum measures approximately 13–15 cm in length and tapers to a 3–4 cm in the anal canal after traversing the levator ani. The levator ani is muscle group comprised of the puborectalis, pubococcygeus, and iliococcygeus muscles which support the pelvic viscera, contribute to defecation and maintain fecal continence (Figure 1).1,2
Figure 1.
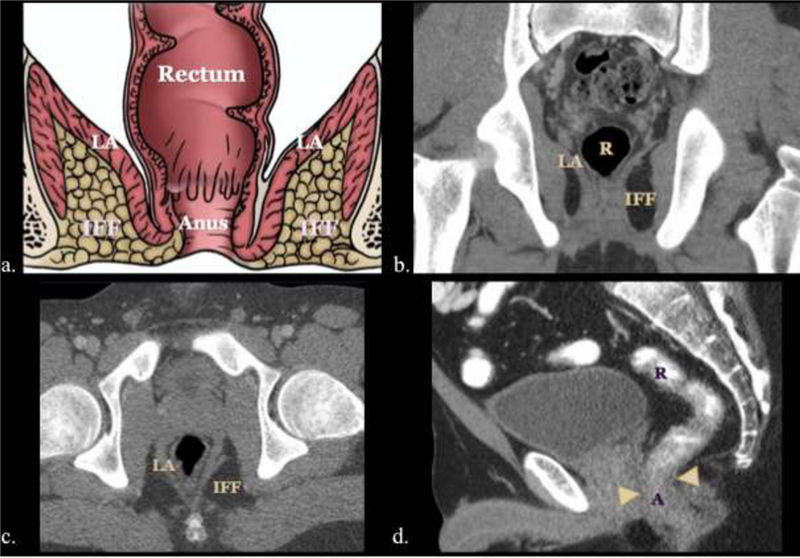
Illustrative diagram (a) and multiplanar CT images (b, c, d) of the rectum, anus, levator ani (LA) muscle group, and ischiorectal fossa (IF).
The rectoanal region extends caudal from the rectosigmoid junction to the anal verge. The rectosigmoid junction projects at the level of the sacral promontory. The transition from the rectum to the anus also has different opinions: the dentate line by some, whereas a surgical transition may be defined as the proximal border of the anal sphincter complex. In the latter definition, the rectum ends anterior-inferior to the tip of coccyx tapering into a sharp posterior angulation (anorectal flexure) as it penetrates the pelvic diaphragm about the levator ani muscle.1,2
The anal canal is approximately 4 cm in length and is bound bilaterally by the ischiorectal fossa, anteriorly by the perineal muscles and vagina in women or urethra in men, and posteriorly by the coccyx. In the anal transition zone, the dentate line denotes the junction of embryologic endodermal and ectodermal origins, and transitions from autonomic to somatic innervation, mesenteric to systemic vascular supply and superior rectal to inguinal lymphatic drainage. In this area, vertical mucosal folds form the columns of Morgagni. Between these columns, anal glands open into sinuses or crypts. The rectal columnar mucosa changes through a short transitional zone near the dentate line to anal squamous epithelium. The distal anal epithelium becomes thicker and continuous with hair and sweat gland bearing perianal skin (Figure 2–3).1,2 The rectoanal region has distinct anatomic spaces. These include the perianal, ischiorectal, intersphincteric, and supralevator spaces. The ischiorectal space is contiguous with the surrounding fascial planes, bordered laterally by the levator ani muscles, and contains the pudendal nerve branches and arterial branches/venous tributaries of the internal pudendal vessels. The presence of these pudendal nerves is important to consider when mass-effect or infiltrative process involve the ischiorectal fat, as this may manifest as pain.1,2
Figure 2.
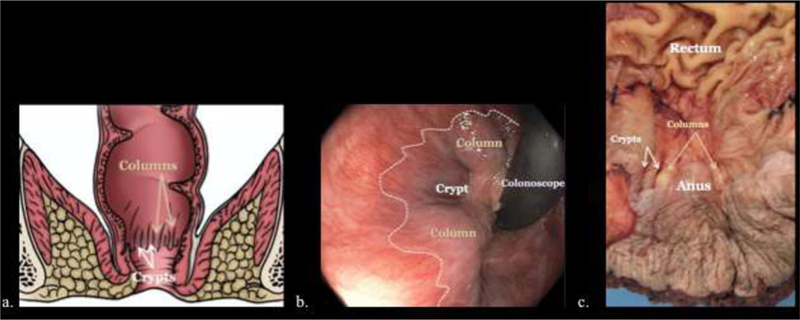
Illustrative diagram (a), endoscopic image (b), and gross pathologic specimen (c) demonstrating the columns of Morgagni (gold arrows, a, c), anal crypts (white arrows, a, c) between the columns and dentate line (dashed line, b).
Figure 3.
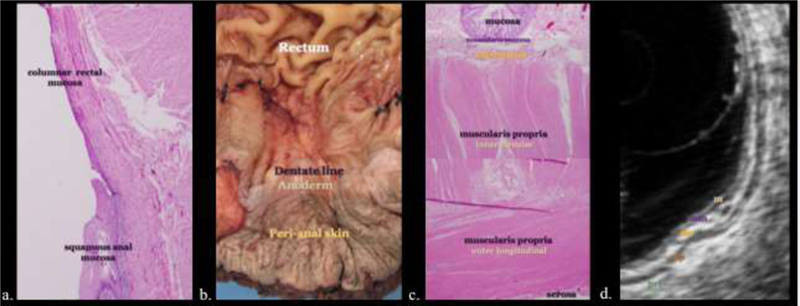
Histology of the anorectal area with pathologic and sonographic correlation. a, b) Rectal columnar mucosa changes through a short transitional zone near the dentate line to anal squamous epithelium. The distal anal epithelium becomes thicker and continuous with hair and sweat gland bearing perianal skin. c, d) The rectal and anal walls are composed of concentric layers of mucosa (m), muscularis mucosa (mm), submucosa (sm), muscularis propria (mp) and serosa or perirectal fat (prf). These histologically distinct layers appear alternately hyper- and hypoechoic at sonography, the so called “gut signature.”
The internal and external anal sphincters work as two entities to control fecal continence. The internal anal sphincter is a continuation of the involuntary circular smooth muscle layer while the external anal sphincter is voluntary/skeletal muscle continuation of the puborectalis muscle (Figure 4).1,2
Figure 4.
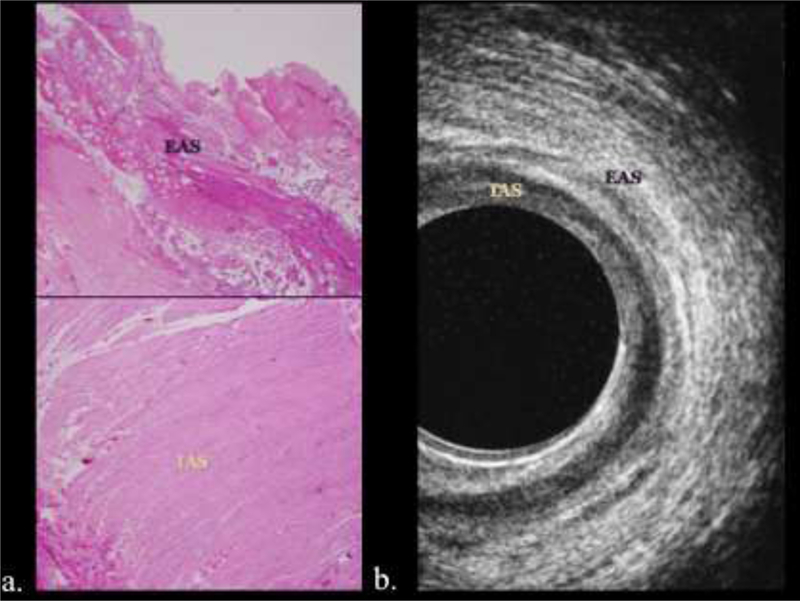
Histology and sonographic correlation of the internal and external anal sphincter. a, b) The internal anal sphincter (IAS) is a continuation of the involuntary circular smooth muscle layer and appears hypoechoic at endoluminal ultrasound. The external anal sphincter (EAS) is voluntary/skeletal muscle continuation of the puborectalis muscle and of mixed echogenicity.
Neoplasms of the anus and rectum – Rectal cancer
Colorectal cancer is the third most frequent new cancer diagnosis and the third leading cause of cancer death in the United States.3 Rectal cancer treatment strategies, epidemiology, and risk factors differ from the tumors affecting the proximal colon. However, since most rectal cancer is misclassified in cancer registries as colon cancers, they are reported in large registries together as colorectal cancer. One accepted definition of rectal cancer is cancer arising within 16 cm from the anal verge.4 Screening examinations with optical colonoscopy and virtual colonography improves the detection of early disease. In terms of colorectal malignancies, adenocarcinoma is by far the most common histology (98%). Other rare subtypes are discussed in later sections.4 Surgical resection with negative margins was historically the only locally curative therapy for rectal cancer, although non-operative management chemoradiation therapy can have long-term success in selected patients.5–7 The initial local staging is performed to determine which patients require preoperative chemoradiation therapy (CRT) or to plan surgery. For tumors in the upper two thirds of the rectum, the standard procedure is low anterior resection with total mesorectal excision wherein the rectum (except the distal portion) and the surrounding mesorectum are removed. When the tumor locates in the distal one third of the rectum, depending on local extension, sphincter sparing surgeries or abdominoperineal resection surgeries are attempted.4
Treatment strategies aim at complete resection, often after attempts at downstaging with neoadjuvant chemoradiation. Total mesorectal excision is the most accepted surgical approach, in which the entire mesorectal compartment is removed, including its fascia. Local tumor recurrence is directly related to inadequate resection.4 The mainstay of rectal cancer greater than stage 1 is neoadjuvant chemoradiation and many patients may have a downstaging response to this treatment, with few achieving a complete response.8 In this situation, MRI plays a central role in pre and post treatment staging. The benefits of downstaging and downsizing locally advanced rectal cancer include: improvement in resectability, better local control, decreased rates of local recurrence, and improved overall survival.
Neoplasms are staged relative to tumor size (T), status of regional lymph nodes (N) and distant metastases (M) utilizing the American Joint Committee on Cancer (AJCC system) (Table 1).9 MRI may demonstrate asymmetric T2 high signal intense mural thickening about the rectum. Local staging of rectal adenocarcinoma can be accomplished with high resolution MR in pretreatment, post-chemoradiation, and postoperative stages. Conventional and high-resolution oblique T2WI are the most important sequence for tumor staging. Ensuring the proper plane is important, as non-oblique T2 images can falsely upstage tumor. The use of diffusion weighted MR imaging (DWI) may help in the assessment of response to CRT and may improve the accuracy of MRI for detection of rectal cancers. DWI can occasionally the primary lesion if it is not well visualized in the other sequences10,11; some studies have suggested DWI can predict or assess the response of rectal cancer to neoadjuvant chemoradiation.12 Metastatic lymph nodes may restrict diffusion, however, MR has a poor to moderate sensitivity in characterizing lymph node involvement. The use of MR to assess response after chemoradiation therapy has been the focus of several prospective and retrospective investigations.
Table 1.
Rectal cancer primary T staging by MR criteria.
| T Stage | Process of T Staging with MRI High-Resolution |
|---|---|
| Tx: | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor invades submucosa. Replacement of submucosal layer by abnormal signal not extending into circular muscle layer. |
| T2 | Tumor invades but does not penetrate muscularis propria. Intermediate T2 signal intensity in muscularis that does not extend beyond outer longitudinal rectal muscle wall into mesorectal fat. (Figure 5) |
| T3 | Tumors invades subserosa throught muscularis propria. Nodular or broad-based bulge projection or Intermediate T2WI projecting into the mesorectal fat. a) Tumors extends < 1mm beyond muscularis propria. (Figure 6) b)Tumors extends 1–5 mm beyond muscularis propria. (Figure 7) c) Tumors extends 5–15 mm beyond muscularis propria. d) Tumors extends >15 mm beyond muscularis propria. |
| T4 | a) Tumor penetrates the visceral peritoneum. Abutment or penetration of abnormal signal intensity beyond the peritoneum b) Tumor directly invades or is adherent to other organs or structures (Figure 8). |
Interpretation of rectal cancer MR after chemoradiation therapy
T Response, assessing for residual tumor:
Intermediate to high signal intensity on T2WI MRI similar to that of baseline tumor indicates residual tumor. Careful review of high-resolution images will enable delineation of small foci of intermediate signal intensity tumor within areas of low signal intensity fibrosis.
Fibrotic changes.
On T2WI, areas of fibrosis have low signal intensity similar to that of muscularis propria. The fibrous tissue present after treatment causes thickening of the rectal wall in most cases.
Desmoplastic Reaction.
Desmoplastic reaction, also called “reactive fibrosis”, is a reactive process evident on imaging that does not contain tumor. On post-CRT T2WI, this findings manifests as low-intensity spicules or strands in the perirectal fat radiating from residual tumor. It can be difficult to discern between these tendrils of the desmoplastic reaction and residual tumor.13
Mucinous Change in Tumor (Figure 6 and 7).
Figure 6.
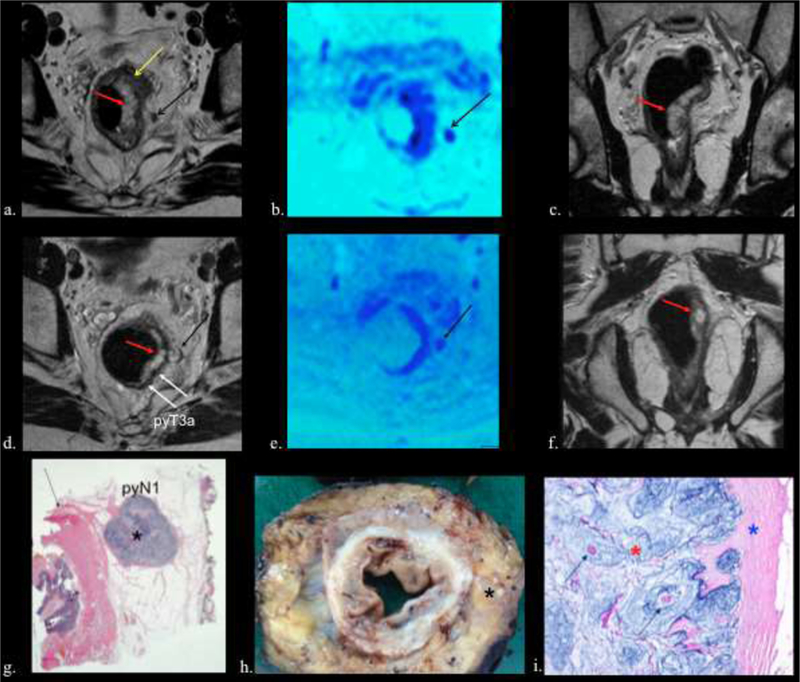
70-year-old man with mucinous rectal adenocarcinoma. pyT3a N1.
a) Pretreatment axial T2w image , b) fusion T2w-DW images and c) coronal T2w images show areas of mucin (red arrow) and areas of intermediate signal intensity solid cellular tumor (yellow arrow). Also note the positive lymph node in mesorectum (black arrows). d) Postreatment axial T2w image , e) fusion T2w-DW images and f) coronal T2w images shows tumor downsizing with residual areas of acellular mucin (red arrows). Observe that after CRT the node (black arrow) has mucin formation. Note the nodular configuration of the rectal wall advancing into mesorectal fat (T3) (white arrows). g, h, and i). Histologic (g and i) and gross (h) resection specimens showing ganglion with abundant mucin (black asterisk) and the rectal wall (black arrow). Higher magnification (i) shows mucin (red asterisk), fibrosis (blue asterisk), and isolated islands of tumor cells (green arrows).
Figure 7.
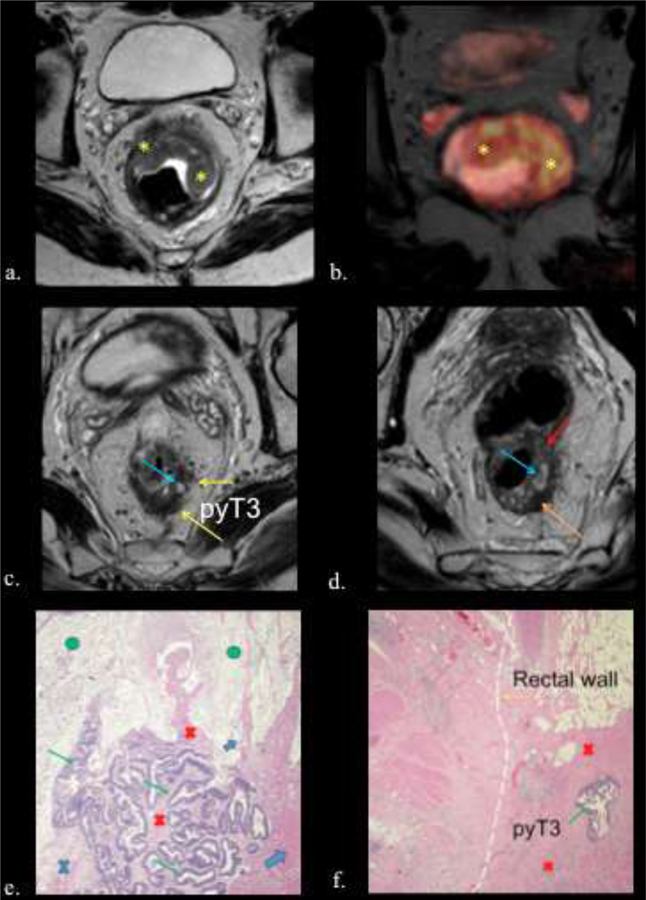
56-year-old male with rectal adenocarcinoma. yp T3b N0. a) Baseline axial high- spatial resolution T2 weighted image shows a semiannular tumor (yellow asterisk). b) Fusion T2w-DW images (yellow asterisk) c and d) Postreatment axial high-spatial resolution T2-w images. Areas of extracellular mucin (light blue arrows) and fibrosis (orange arrow) are observed. Note areas of residual tumor with intermediate signal intensity greater than signal of the gluteal muscles and similar to the signal of the original tumor (red arrow). Observe tumor bulging in to the mesorectal fat, suggesting residual tumor invading the mesorectal fat (T3) (yellow arrows). e) Photograph of histopathologic sections (H and E, x100) shows areas of acellular mucin (green circle) and residual tumor cells (green arrow) surrounded by fibrosis (red cross), inflammatory infiltrate (blue cross) and muscularis propria (light blue arrows). f) Photograph of histopathologic sections (H and E, x40) shows residual tumor cells (green arrow.) surrounded by fibrosis (red cross). Dotted lines shows the original limit of normal rectal wall.
Mucin formation occurs in three circumstances: i-post therapeutic mucinous response in non-mucinous tumors; ii-pathologically mucinous rectal tumor comprises lakes of extracellular mucin lined by columns of malignant cells, cords, and vessels; and iii-in mucinous tumors nonresponse is reflected pathologically as persistent columns of malignant cells and cords. In any case, mucin is hyperintense on T2WI and is difficult to differentiate among them.14 Comparison of the tumor’s appearance prior to treatment is key in interpreting post-CRT rectal MR examinations. Homogenous T2WI hyperintensity is characteristic of acellular mucin. Intermediate signal intensity within the T2WI hyperintense mucin indicates cellular mucin whereas areas of low signal intensity indicate mucin surrounding fibrotic change. Nonmucinous tumors that develop post-CRT mucin on MR can be interpreted as treatment response with confidently identified pure acellular mucin as an area of complete treatment response. Mucinous rectal cancer, defined as abundant extra-cellular mucin comprising the majority (>50%) of the tumor at pathologic analysis or tumors with a substantial proportion of pre-treatment mucin are more difficult to interpret on post-CRT MR examinations.10, 11 Park et al.15 reported an inter-reader agreement study that proposed a tumor regression grading system based on presence/absence of residual soft tissue components of pathologically confirmed rectal mucinous adenocarcinomas. In their retrospective cohort of 59 patients, their post-CRT grading system was the only significant MR imaging finding predictive of treatment response.
Pseudotumor.
After CRT the original tumor can show fibrotic changes, low in signal intensity, and less bulky. These changes may result in near normal thickness of treated rectal wall but the unaffected submucosa and mucosa can became edematous, thickened, and of intermediate intensity, which may lead to a false positive interpretation.11
Roles of Diffusion-weighted and Perfusion MRI:
Diffusion-weighted sequences have been proposed to be indicative of treatment response. As a tumor responds to treatment, the apparent diffusion coefficient (ADC) is likely to rise at first, but re equilibrium may occur after a time leading to decrease in ADC at the end of treatment.16 At the end of treatment tumor response as fibro inflammatory tissues can manifest as increased ADC values (Figure 8).
Figure 8.
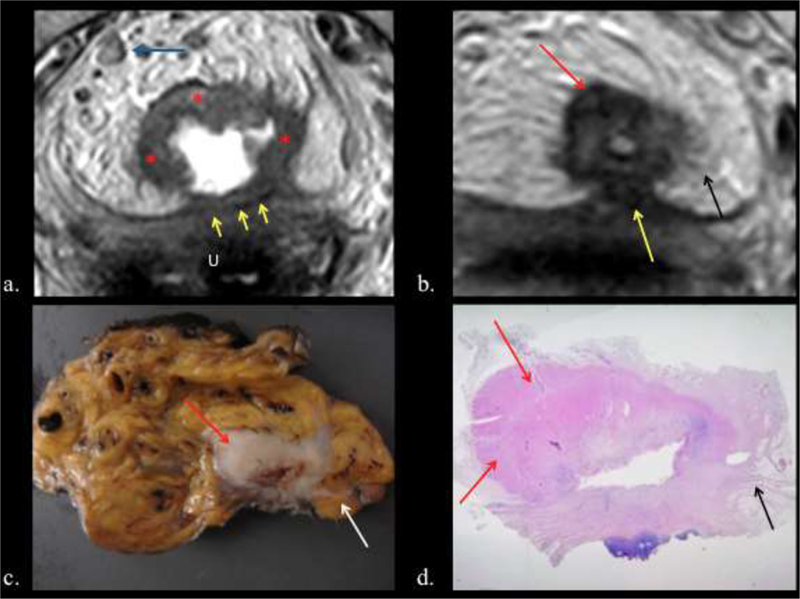
a.) Baseline oblique-axial high-spatial resolution T2 weighted image shows stage T4b invasive tumor (Red asterisks) infiltrating the uterus (U) (Yellow arrows). Blue arrow= positive node abutting the MRC, note its irregular border. b.) Post-CRT oblique-axial high- spatial resolution T2 weighted image shows tumor regression within rectal wall. Fibrotic low- signal-intensity scar (red arrows). Low signal intensity spicules in perirectal fat are consistent with desmoplastic reaction (Black arrow). Note the peritoneum retracted by fibrotic reaction (yellow arrow). c). The corresponding tissue slice confirms fibrosis in the rectal wall (Red arrow) and desmoplastic reaction (white arrow). d) Photograph of histopathologic section (H and E, x 0,4) shows fibrosis (red asterisks) and desmoplastic reaction (Black arrow).
Volumetric analysis with DWI has been shown and subsequently prospectively validated to be a means to assess response of tumor size after chemoradiation therapy. Lambregts et al.17 used these methods in 112 patients with rectal cancer who pelvic MRs before and after volumetric analyses with both DWI and T2WI, using predefined parameters for complete response that are beyond the scope of this work. DWI offered better sensitivity for accurate tumor measurements compared to T2WI. Other studies, including a meta-analysis, have demonstrated that DWI significantly improves MR accuracy of restaging rectal cancer following neoadjuvant therapy.18,19 MR has poor to moderate sensitivity for detecting regional metastatic lymph nodes. In one prospective study that matched 205 lymph nodes in 40 patients with rectal cancer, MR had a sensitivity of 58% and positive predictive value of 62% for detecting node-by-node metastases.20 Other studies in which surgeons have planned their operative lymph node dissection based on imaging findings have shown similar poor to moderate sensitivities of MR’s detection of nodal disease in rectal cancer.21
Neoplasms of the anus and rectum – Polyps
Epithelial polyps can be non-neoplastic (hyperplastic and inflammatory) or neoplastic. Most colon cancers develop from adenomatous polyps. Adenomatous polyps are classified as tubular, tubulovillous or villous, and may be sessile or pedunculated (Figure 5). Malignant risk is related to histology (highest with villous lesion) and increases with polyp size over 1 cm. Overall malignant transformation risk is 6% in the general population but 100% in familial adenomatous polyposis syndrome. Small polyps can be removed via colonoscopy while larger lesions require surgery.22,23
Figure 5.

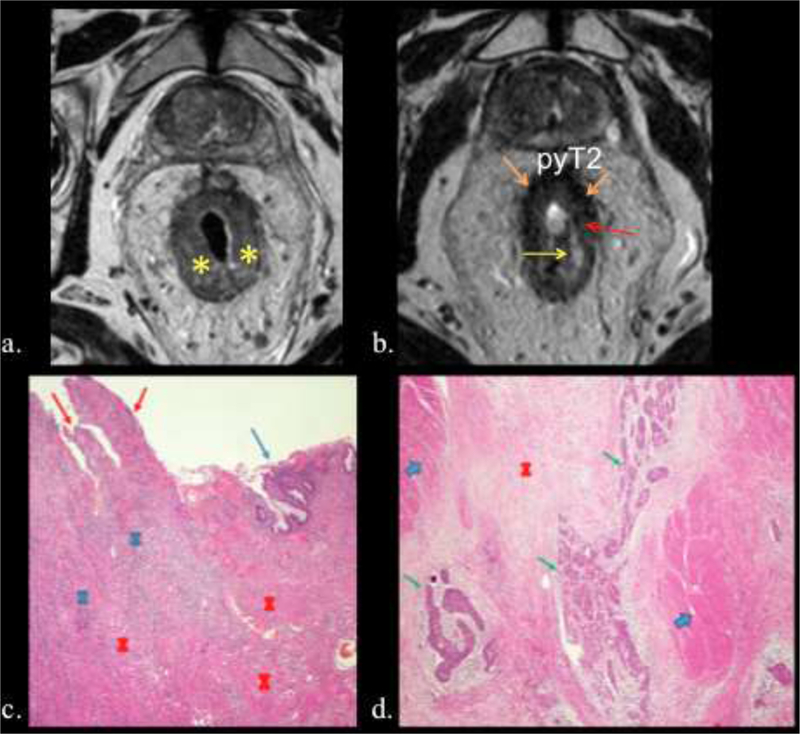
68-year-old man with rectal adenocarcinoma pyT2 N0. TRG 3. a.) Post-CRT axial high-spatial resolution T2 weighted image of stage T2 tumor, shows semiannular tumor at 12 to 7 o´clock position (outlined in red). b and c). The tissue slices corresponding to A show tumor in the rectal wall (Outlined in black) f and g). Photographs of histopathologic section (H and E, x 0,4) show tumor infiltration into the muscularis propria with mild fibrosis (outlined in black).
Double contrast barium enema exams have lower yield for the detection of polyps as compared to an adequate optical colonoscopy. However, barium enema does have its utility after failed or incomplete endoscopic exams, as does CT colonography. Sessile polyps appear either as filling defects on the dependent portion of bowel or ring shadows on the nondependent portion of the bowel. Whereas sessile polyps will have a degree of uniformity, pedunculated polyps can be seen on barium enema by the presence of a stalk.24 Kung et al.25 reported a retrospective review of 276 patients who received screening double contrast barium enema and, when available, compared it with endoscopic and pathologic findings for polypoid lesions. The overall diagnostic yield in their study was 6.2% for advanced neoplastic lesions of any size.
In 2016, CT colonography (CTC) received recognition by the U.S. Preventive Services Task Force as a recommended colorectal screening modality for patient 50–75 years. However, at the time of writing, one challenge to more widespread implementation of CTC is the lack of Medicare reimbursement.26 CTC has similar cancer detections rates to optical colonoscopy and offers the advantage of not requiring sedation and the ability to demonstrate extracolonic findings. In one study that compared over 3,000 patients who either received CTC or optical colonoscopy as a screening showed no significant difference in cancer detection rates (3.2% vs 3.4%, respectively; p=0.69). In the CTC group, approximately 8% went to optical colonoscopy and the overall number of polytpectomies were significantly lower in the CTC group (561 polyps in 31210 patients for CTC vs 2434 polyps in the optical colonoscopy group, p<0.01).27 There are many other studies that demonstrate the value and detail the nuances of CTC, which is beyond the scope of this work. Excellent CTC dedicated reviews are available.28–31
Neoplasms of the anus and rectum – Carcinoid tumors
Carcinoid tumors of the gastrointestinal tract are masses that arise from neuroendocrine cells within gastrointestinal mucosa and submucosa.32 Since the implementation of screening colonoscopy, rectal carcinoids are diagnosed more frequently compared to small intestinal carcinoid tumors.33 In contrast to colonic carcinoid tumors, which tend to be 2 cm or greater, rectal carcinoids smaller than 1.0 cm can often be resected endoscopically (Figure 8).34 One systematic review that included over 4,500 patients with rectal carcinoid reported that approximately 80% of these tumors are found at <1 cm size.35 In the absence of local or distant invasion, tumors larger than 2.0 cm require surgical resection, and tumors between 1.0 cm and 2.0 cm remain an area of controversy. Larger tumor size correlates with a high risk for metastasis.35 CT and MR may show a submucosal or intraluminal small plaque-like mural or polypoid mass, depending on size. MR manifests as homogenously enhancing mass isointense on T1WI and iso to hyperintense on T2WI. In the authors’ experience, no particular imaging feature helps in differentiating carcinoid to adenocarcinoma or other diagnoses; visualization and sampling by endoscopy is need in the absence of a suggestive history.36 Local invasion is uncommon in rectal carcinoids but can be seen with larger masses and cause regional fibrosis leading to bowel or urinary tract obstruction.34,36
Neoplasms of the anus and rectum – Gastrointestinal stromal tumor
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the gastrointestinal tract. These tumors are thought to arise from interstitial cell of Cajal and express CD117 (c-kit) on immunohistochemistry.37 The majority (up to 90%) of GIST occurs in the stomach and small intestine and less than 5% occur in the anorectal area.38 Rectal GISTs often present as mural masses that thicken the rectal wall (Figure 9). MRI shows intermediate intensity of T1-weighted image and heterogeneous high signal intensity on T2-weighted images. In one study of 14 patients with pathologically confirmed rectal GISTs, imaging characteristics on CT and MRI were most often rounded or oval exophytic masses with heterogeneous and moderate enhancement.39 Treatment principles include surgical excision when possible. Imatinib can be used to downstage GIST with hopes of future resection or as monotherapy in those who are not surgical candidates.40
Figure 9.
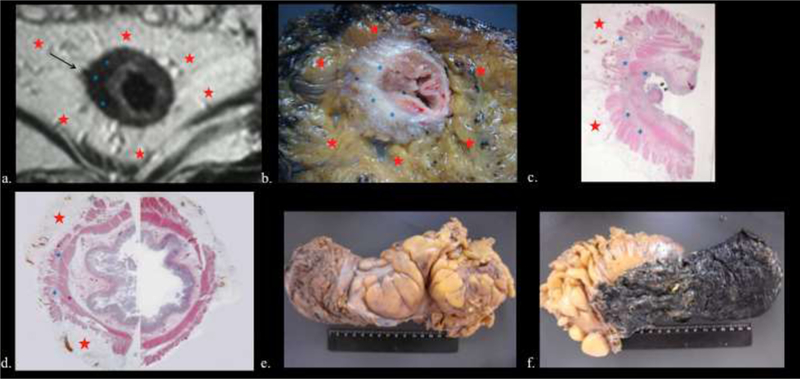
59-year-old man with rectal adenocarcinoma pyT0 N0. TRG1. A: Posttreatment axial T2weighted image shows a wide fibrotic scar with low signal intensity (Blue asterisk) between 7 and 11 o´clock positions and desmoplastic reaction, which is seen as a fine low- signal-intensity strand (Black arrow). Red stars = mesorectal fat. B: The tissue slice shows fibrosis (Blue asterisk), surrounded by normal mesorectal fat (Red asterisk). C and D: Photographs of histopathologic section (H and E x 0,4) corresponding to A and B confirms the presence of fibrosis (Blue asterisk). Red asterisk= mesorectal fat. E and F: Rectal tissues after TME.
Neoplasms of the anus and rectum – Condylomata acuminata
Condyloma acuminata is a collection of anogential warts caused by sexual transmission of human papilloma virus (HPV). The strand of HPV that cause condylomata acuminata are not typically those that transform into squamous cell carcinoma.41,42 These lesions can be treated medically with topical agents or with surgical excision in selected cases. As these anogenital warts are clinically apparent, imaging is rarely indicated or performed. However, imaging can be helpful to determine level of extent for large condylomata acuminata to assist with surgical planning (Figure 10).43 On CT and MR, condylomata acuminata manifest as exophytic mucosal lesion arising from the anorectal area or perineum.44 Rarely, a large collection may manifest as giant condyloma acuminatum (Buschke-Loewenstein tumor), which is locally invasive.45
Figure 10.
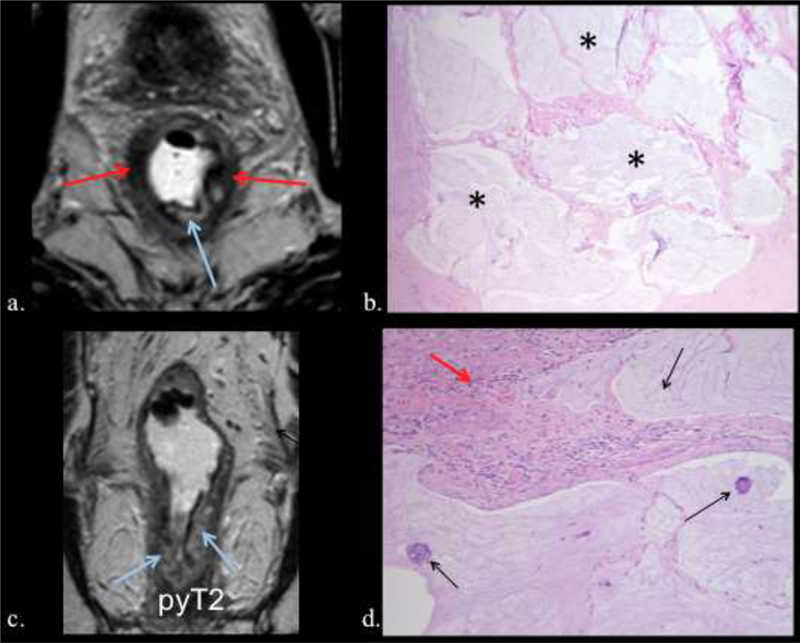
85-year-old man with rectal adenocarcinoma. pyT2N0. TRG1. a) Axial and b) coronal postreatment T2W MRI posttreatment axial T2 shows low signal intensity fibrosis (red arrow) and acellular mucin indicated by areas of high signal intensity (ligh blue arrow). c) Photograph of histopathologic sections (H and E, x100) shows acellular mucin (black asterisk). d) Photograph of histopathologic sections (H and E, x400) shows residual tumor cells (black arrow) and fibrosis (red arrow).
Neoplasms of the anus and rectum – Anal squamous cell carcinoma
Squamous-cell carcinoma (SCC) of the anus is an uncommon malignancy that is more prevalent in patients with sexually transmitted diseases and promiscuous sexual practices, namely individuals with human immunodeficiency virus. The majority of patients present with anal bleeding. Physical exam may reveal a mass and endoscopic or proctoscopic examination allows for direct visualization and biopsy.46 Imaging of anal SCC includes MRI, endoanal ultrasound, CT, and PET/CT. Akin to the imaging strategy of rectal cancer, MRI is a preferred modality to accurately assess the characteristics of the primary tumor and local regional lymph node involvement (Figure 11).47,48 Endoanal ultrasound is useful in assessing the depth of tumor before and after chemoradiation treatment.49,50 PET/CT has been shown to be more sensitive than CT in imaging the primary tumor of anal SCC and detecting lymph node involvement. Cotter et el.51 reported a clinical study of 41 patient with biopsy proven anal carcinoma who underwent both PET/CT and CT. PET/CT visualized 91% of the primary tumors compared to only 59% with CT alone. Unlike many solid tissue malignancies, SCC of the anus can often be adequately treated nonoperatively. The mainstay of treatment in patients with anal canal squamous cell carcinoma is the Nigro protocol, which is chemotherapy and radiation treatments followed by surgical excision, if necessary.52 Patients who fail to respond to neoadjuvant therapy have high morbidity, even with appropriately timed surgery.53
Figure 11.
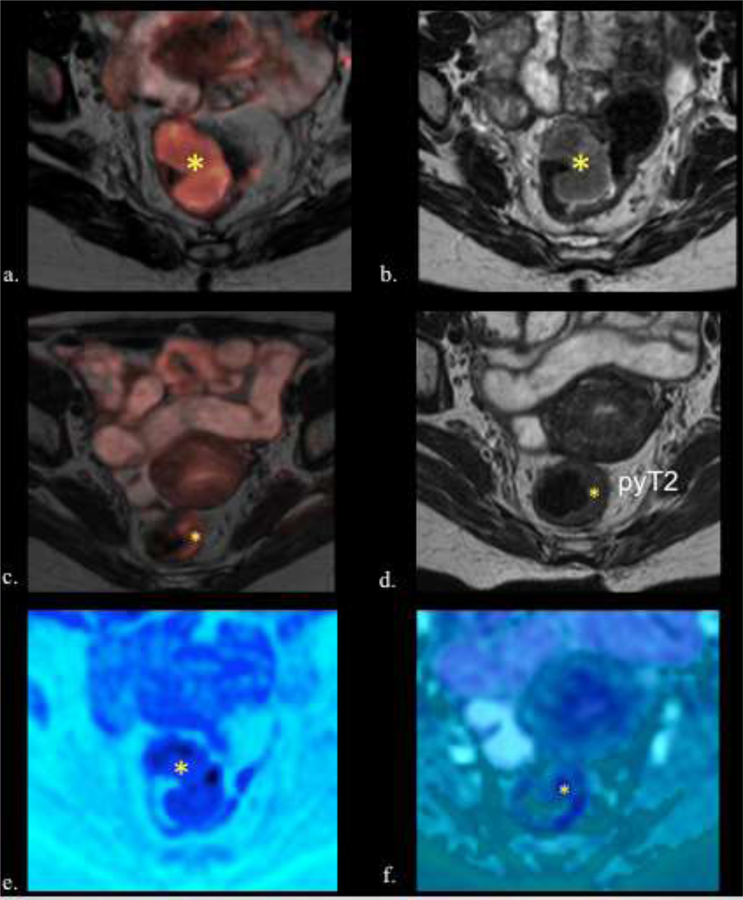
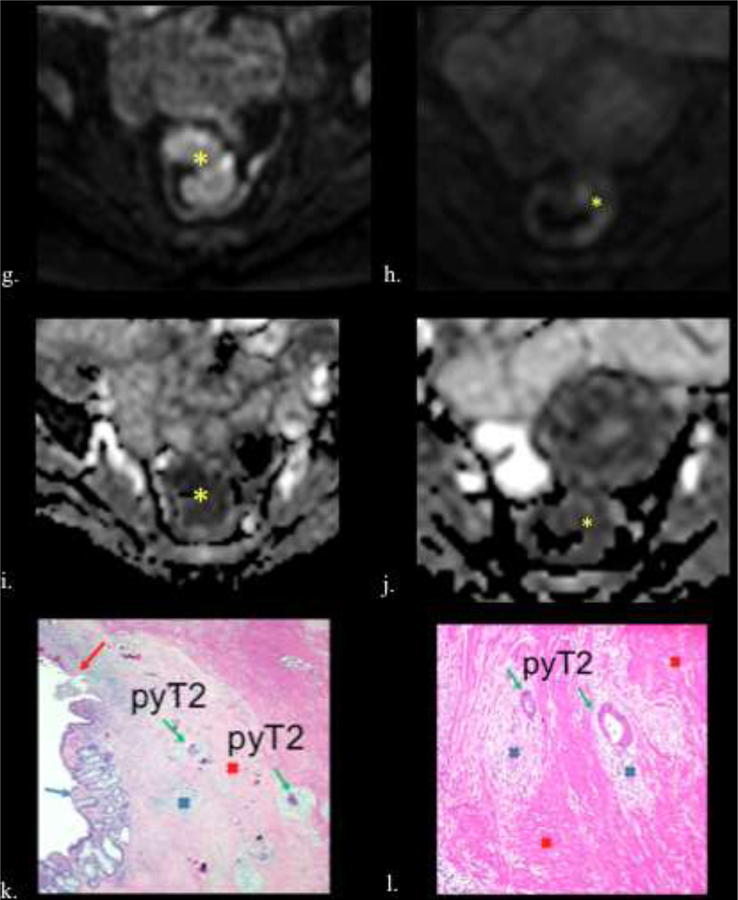
58-year-old female with rectal adenocarcinoma. ypT2N0. TRG 1. a) Fusion T2w- DW images and b) Axial T2w image shows the tumor before treatment with intermediate signal intensity tumor (asterisk). c) Fusion T2w-DW images and d) Axial T2WI after CRT shows significant downsizing. However residual intermediate signal intensity wall thickening is seen (asterisk) (T2). No low signal intensity of fibrosis is seen, but the border of the wall is smooth. e) Pre and f) postreatment axial fusion T2w-DW images show downsizing (asterisk). On DWI obtained g) before and h) after CRT the tumor shows restriction (asterisk) with a decreased signal intensity after treatment. Observe that the tumor appears dark on ADC maps (asterisk) i) before CRT, with an increased in the ADC signal intensity j) after CRT (asterisk). k) Photograph of histopathologic sections (H and E, x40) shows residual tumor cells (green arrow) surrounded by inflammatory infiltrate (blue cross) and fibrosis (red cross), which causes a complex and heterogeneous signal in the MR. Calcifications (Black arrow), normal mucosa (blue arrow), ulceration (red arrow). l) Photograph of histopathologic sections (H and E, x400) shows residual tumor cells (green arrow), inflammatory infiltrate (blue cross) and muscularis propria (light blue arrows).
Neoplasms of the anus and rectum – Rare primary tumors and metastases
The vast majority of rectal cancer is adenocarcinoma and most anal cancer is squamous cell carcinoma. Adenocarcinomas, sarcomas and small cell carcinomas have rarely been reported in the anal canal, with poor prognosis. Lesions arising in adjacent organs or anatomic spaces may produce mass effect upon or invade the anorectal canal. Anorectal melanoma is rare with a poorly understood pathogenesis. It constitutes the third most common site of melanoma, after skin and eye. Because of the mucosal spread and often lack of a clinically evident lesion, and certainly one not visible to the patient, anorectal melanoma is often advanced at diagnosis. Unlike rectal adenocarcinoma, neoadjuvant therapy has no role as surgery is the mainstay of treatment.54 There are few imaging series of anorectal melanoma, namely an MR series of 3 patients55 and a CT series of 8 patients.56 On MR, as with melanoma located elsewhere anatomically, melanin may showed scattered foci of high signal on T1WI.57 Metastatic lymph nodes may be hemorrhagic, producing increased signal on most sequences. T2WI can help assess the depth of the tumor. On CT, the primary tumor may appear as a fungating or polypoid lesion and concurrent regional lymphadenopathy is often preset.56 Regardless, sampling is required for diagnosis. The lesion may be amelanotic or appear similar to a thrombosed hemorrhoid at endoscopy (Figure 18).
Figure 18.
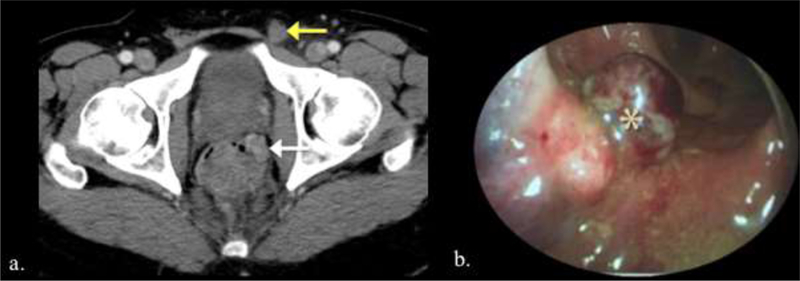
Two different patients with anorectal melanoma. a. Patient with biopsy-proven rectal melanoma. CT demonstrate an enhancing mass in the left lateral wall of the rectum (white arrow) and an enlarged left inguinal lymph node (yellow arrow). b. Endoscopic image of lobulated red-brown anal melanoma.
Although primary gastrointestinal lymphoma may represent up to 17% of small bowel tumors, primary anorectal lymphoma is exceedingly rare58 - 0.2% of rectal malignancies by one source.59 Associated with Non-Hodgkin’s lymphoma, primary anorectal lymphoma affects two distinct patient populations: those who are HIV carriers and the rest of the population.59 There are no described reproducible discerning findings for primary rectal lymphoma. The primary tumor will be indistinguishable from rectal carcinoma. If there is lymphadenopathy elsewhere as characteristic with lymphoma, that may be a potential clue.60 Management is controversial but surgical resection can be curative. At present, there is not good data to support or refute the role of chemotherapy for this disease.59
Metastasis about the rectum and anus is often by local invasion (Figure 19). Effect of this invasion on surgical candidacy and rates of obstruction and fistulization depend on the primary tumor.
Figure 19.
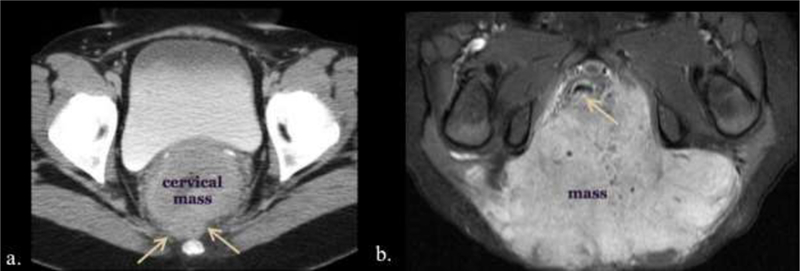
Two different patients with invasion and mass effect on the rectum by adjacent tumors. a. 43-year-old female presenting to the emergency care center with right abdominal pain. Large circumferential cervical mass obliterates the rectovaginal tissue plane (arrows) on CT. b. Axial T1 enhanced MR of a 2-year-old female with a large sacrococcygeal teratoma filling the ischiorectal fossae and obscuring the dorsal anorectal wall (arrow).
Conclusion
The radiologist plays a key role in staging malignancies about the rectum. Rectal cancer is the most common malignancy and has well described imaging features, some of which drive subsequent management. Less common malignancies are reviewed, and while their imaging appearances can be variable, a baseline understanding of anorectal anatomy and principles of rectal cancer staging can help in composing competent reports and diagnoses.
Figure 12.
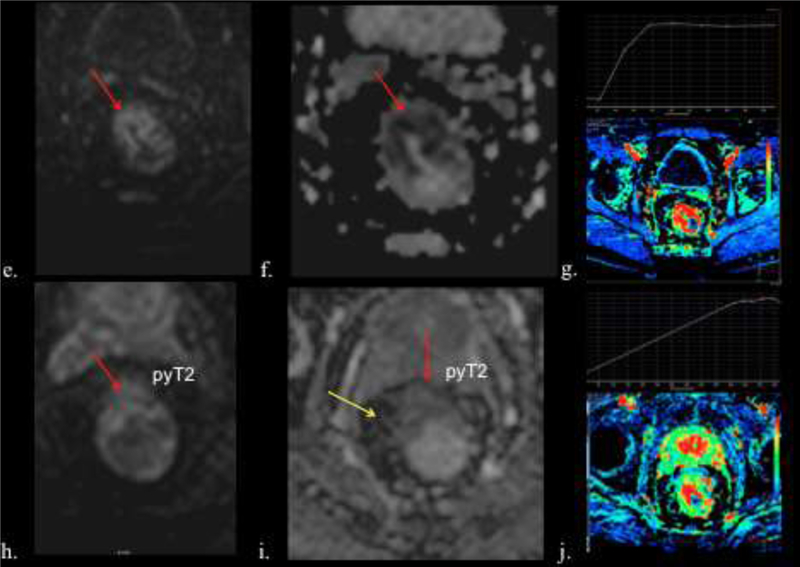
69-year-old male with rectal adenocarcinoma. T2 N0. a) Pretreatment axial high- spatial resolution T2-w image shows a semiannular tumor (asterisk). b) Posttreatment axial T2-w image shows ulceration with hyperintense signal, corresponding to inflammatory changes with edema and congestion (yellow arrow). Note hypointense signal intensity due to fibrosis (orange arrows) and intermediate signal intensity relative to residual tumor (red arrows). c) Photograph of histopathologic sections (H and E, x40) shows inflammatory infiltrate (blue cross) and fibrosis (red cross), ulceration (red arrows) and normal mucosa (blue arrow). d) Photograph of histopathologic sections (H and E, x100) shows residual tumor cells (green arrow), fibrosis (red cross) and normal muscles (light blue arrows). e) Pre- CRT axial DW MRI (b value of 800 sec/mm2) and f) corresponding ADC map show high signal intensity lesion in the rectum (arrow). g) Post-CRT axial DW MRI show faint hyperintensity signal in the rectal wall (arrow). h) Post-CRT map shows no hypointensity in most of the original tumor (red arrow). i) However, there was a residual hypointense area (yellow arrow). g and j.) Dynamic CE MRI shows Time Intensity Curve (TIC). Pre-CRT (g.) shows TIC type 3 (quick enhancement followed by a signal plateau) which has changed to TIC type 2 (j.) with slow enhancement followed by a signal plateau probably due to regression of tumor microcirculation with the presence of fibrosis and inflammatory changes.
Figure 13.
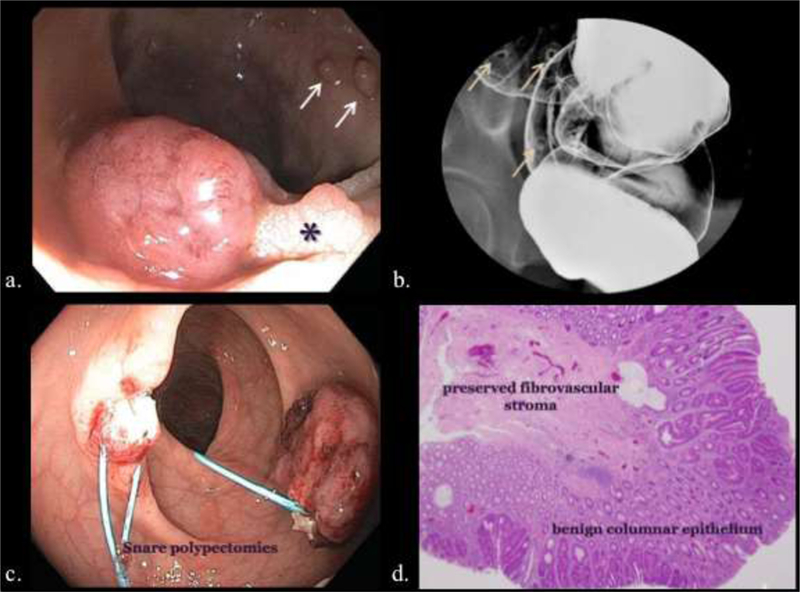
Endoscopic, enterographic, and histopathologic representations of rectal polyps. a). Screening colonoscopy revealing sessile polyps (arrows) and a pedunculated polyp (*). b). Barium enema showing features of sessile polyps. c). Snare polypectomy at colonoscopy. d). Histologic image of a benign adenomatous polyp demonstrating its columnar epithelium and fibrovascular stroma.
Figure 14.
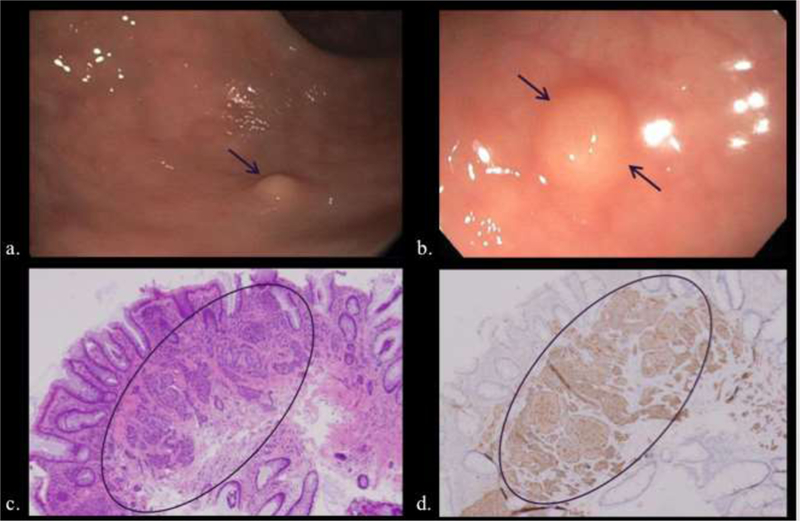
Two patients undergoing colonoscopy for history of colon polyps (a, b) and iron deficiency anemia (c. d), respectively. a, b) Small sub-mucosal sessile polypoid lesions (arrows) were removed with excisional biopsy. c, d). Hematoxylin and eosin (c) microscopy demonstrates cellular clusters of neuroendocrine cells in the submucosa and lamina propria (circled) that stain for synaptophysin (d).
Figure 15.
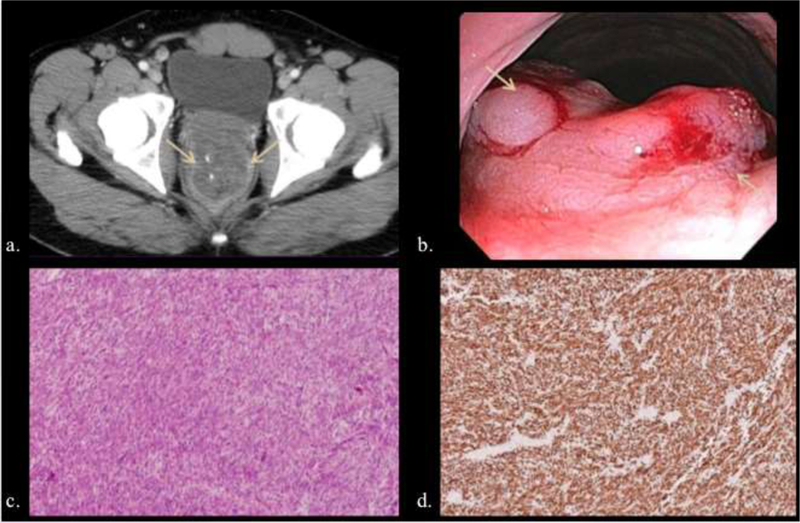
47-year-old male with complaints of constipation and rectal bleeding. a, b) A large lobulated submucosal rectal mass was evident at CT (arrows) (a) and colonoscopy (b) (arrows), and patient underwent surgical resection. c, d) Hematoxylin and eosin (c) and CD- 117 immunohistochemistry (d) illustrate spindle cell proliferation strongly positive on CD117 (c-kit) immunostain.
Figure 16.
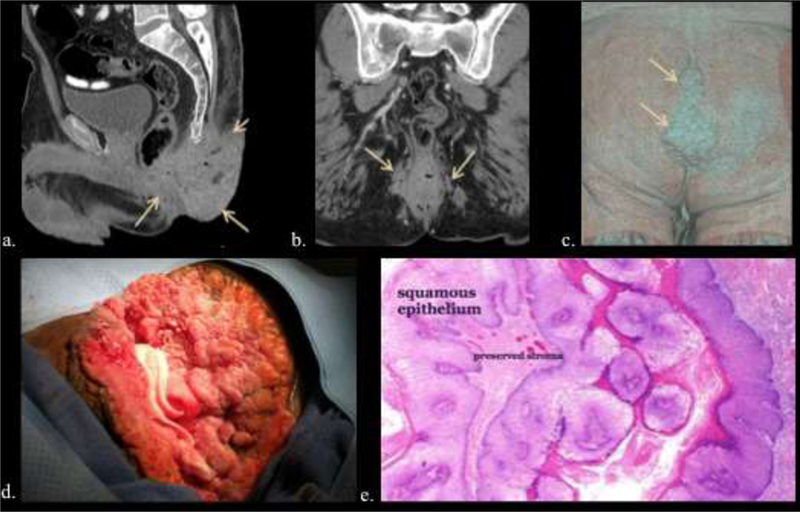
49-year-old male with 14-year history of increasing anal warts. a, b, c) Multi- planar CT (a, b) and volume rendered image (c) depict extensive lobulated anal and perianal disease extending from the gluteal cleft (arrows). d, e). Intraoperative image of giant condylomata (d) and low power microscopy of a typical non-invasive wart (e).
Figure 17.

64-year-old male with hematochezia for two months. a, b, c) Multi-plane T1 enhanced MR (a, b) and FDG PET (c) were positive for an avid lobulated left anal mass lesion (arrows). Biopsy returned high-grade squamous cell carcinoma and patient was treated with chemoradiation. d, e). Endoscopy (d) and microscopy (e) of different patients with anal squamous cell carcinoma illustrating an ulcerated anal canal mass (*), and squamous cell invasion into underlying stoma.
Table 2.
Rectal cancer MR imaging morphologic and signal intensity T staging criteria after chemoradiation therapy.
| Tumor | Morphologic and Signal Intensity Criterion after CRT |
|---|---|
| Active tumor | Intermediate signal intensity, higher than that of muscle. |
| Tumor response | Lower signal intensity relative to pre-CRT findings, higher signal intensity than that of fat relative to pre-CRT. |
| T0-T2 | Normal rectal wall with complete disappearance of tumor, hypointense thickened rectal wall due to fibrosis with or without hypointense espiculations (desmoplastic reaction) that extends to the mesorectum (Figure 9). |
| T3 | Broad-based pushing and/or nodular configuration of tumor margin with intermediate signal intensity extending into the perirectal fat (Figure 7) |
| T4 | Broad-based pushing and/or nodular configuration of tumor margin with intermediate signal intensity extending into an adjacent organ or peritoneum. |
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: All authors claim no conflicts of interest or disclosures.
Conference Presentation: Concepts and some cases from this review were presented as an educational exhibit at the 2014 RSNA meeting – Naeem S, Heldmann M, Dubose A, Thomas-Ogunniyi J, Sangster G, Cole P. Rectal, Perirectal and Perineal Diseases-a Multi-modality Pictorial Review with Histological, Endoscopic and Operative Correlation.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
References:
- 1.Fry R, Mahmoud N, Maron D, Bleier J. Chapter 52: Colon and rectum. In: Townsend C, Beauchamp R, Evers B, Mattox K, editors. Sabiston textbook of surgery 19th ed. Philadelphia, PA: Elsevier Saunders; 2012. [Google Scholar]
- 2.Nelson H Chapter 53: Anus. In: Townsend C, Beauchamp, Evers, Mattox K, editors. Sabiston textbook of surgery 19th ed. Philadelphia, PA: Elsevier Saunders; 2012. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 4.Quaia E, De Paoli L, Cova MA. Pretherapeutic Diagnosis and Staging. In Rectal Cancer 2013. (pp. 9–26). Springer Milan. [Google Scholar]
- 5.Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 2015;16:919–27 . 10.1016/S1470-2045(15)00120-5 [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014; 88:822–28 . 10.1016/j.ijrobp.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 7.Torok JA, Palta M, Willett CG, Czito BG. Nonoperative management of rectal cancer. Cancer 2016;122:34–41 . 10.1002/cncr.29735 [DOI] [PubMed] [Google Scholar]
- 8.Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg 2016;263:458–64 . 10.1097/SLA.0000000000000368 [DOI] [PubMed] [Google Scholar]
- 9.American Joint Committee on Cancer. AJCC cancer staging manual 7th ed. NewYork, NY: Springer; 2010. [Google Scholar]
- 10.Nougaret S, Reinhold C, Mikhael HW, et al. The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? Radiology 2013;268:330–44 . 10.1148/radiol.13121361 [DOI] [PubMed] [Google Scholar]
- 11.Kaur H, Choi H, You YN, et al. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. RadioGraphics 2012;32:389–409 . 10.1148/rg.322115122 [DOI] [PubMed] [Google Scholar]
- 12.Cai G, Xu Y, Zhu J, et al. Diffusion-weighted magnetic resonance imaging for predicting the response of rectal cancer to neoadjuvant concurrent chemoradiation. World J Gastroenterol 2013;19:5520–27 . 10.3748/wjg.v19.i33.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iafrate F, Laghi A, Paolantonio P, et al. Preoperative staging of rectal cancer with MR Imaging: correlation with surgical and histopathologic findings. RadioGraphics 2006;26:701–14 . 10.1148/rg.263055086 [DOI] [PubMed] [Google Scholar]
- 14.Matalon SA, Mamon HJ, Fuchs CS, et al. Anorectal Cancer: Critical Anatomic and Staging Distinctions That Affect Use of Radiation Therapy. RadioGraphics 2015;35:2090–107. 10.1148/rg.2015150037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SH, Lim JS, Lee J, et al. Rectal Mucinous Adenocarcinoma: MR Imaging Assessment of Response to Concurrent Chemotherapy and Radiation Therapy-A Hypothesis-generating Study. Radiology 2017;285:124–33. 10.1148/radiol.2017162657. [DOI] [PubMed] [Google Scholar]
- 16.Monguzzi L, Ippolito D, Bernasconi DP, et al. Locally advanced rectal cancer: value of ADC mapping in prediction of tumor response to radiochemotherapy. Eur J Radiol 2013;82:234–40 . 10.1016/j.ejrad.2012.09.027 [DOI] [PubMed] [Google Scholar]
- 17.Lambregts DMJ, Rao S-X, Sassen S, et al. MRI and Diffusion-weighted MRI Volumetry for Identification of Complete Tumor Responders After Preoperative Chemoradiotherapy in Patients With Rectal Cancer: A Bi-institutional Validation Study. Ann Surg 2015;262:1034–9 . 10.1097/SLA.0000000000000909 [DOI] [PubMed] [Google Scholar]
- 18.Song I, Kim SH, Lee SJ, et al. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br J Radiol 2012;85:577–86 . 10.1259/bjr/68424021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Paardt MP, Zagers MB, Beets-Tan RGH, et al. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology 2013;269:101–12 . 10.1148/radiol.13122833 [DOI] [PubMed] [Google Scholar]
- 20.Park JS, Jang Y-J, Choi G-S, et al. Accuracy of preoperative MRI in predicting pathology stage in rectal cancers: node-for-node matched histopathology validation of MRI features. Dis Colon Rectum 2014;57:32–38 . 10.1097/DCR.0000000000000004 [DOI] [PubMed] [Google Scholar]
- 21.Akiyoshi T, Ueno M, Matsueda K, et al. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol 2014;21:189–96 . 10.1245/s10434-013-3216-y [DOI] [PubMed] [Google Scholar]
- 22.Lee MK, Chen F, Esrailian E, et al. Combined endoscopic and laparoscopic surgery may be an alternative to bowel resection for the management of colon polyps not removable by standard colonoscopy. Surg Endosc 2013;27:2082–6 . 10.1007/s00464-012-2714-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein NS, Bhanot P, Odish E, Hunter S. Hyperplastic-like colon polyps that preceded microsatellite-unstable adenocarcinomas. Am J Clin Pathol 2003;119:778–96 . 10.1309/DRFQ-0WFU-F1G1-3CTK [DOI] [PubMed] [Google Scholar]
- 24.Levine MS, Rubesin SE, Laufer I, Herlinger H. Diagnosis of colorectal neoplasms at double-contrast barium enema examination. Radiology 2000;216:11–18 . 10.1148/radiology.216.1.r00jl3311 [DOI] [PubMed] [Google Scholar]
- 25.Kung JW, Levine MS, Glick SN, et al. Colorectal cancer: screening double-contrast barium enema examination in average-risk adults older than 50 years. Radiology 2006;240:725–35 . 10.1148/radiol.2403051236 [DOI] [PubMed] [Google Scholar]
- 26.Yee J, McGlothlin A, Keysor KJ. Screening CT colonography reimbursement: triumphs and navigating a path forward. Abdom Radiol (NY) 2017;42:86–9 . 10.1007/s00261-016-0974-6 [DOI] [PubMed] [Google Scholar]
- 27.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007;357:1403–12 . 10.1056/NEJMoa070543 [DOI] [PubMed] [Google Scholar]
- 28.Mang T, Maier A, Plank C, et al. Pitfalls in multi-detector row CT colonography: a systematic approach. Radiographics 2007;27:431–54 . 10.1148/rg.272065081 [DOI] [PubMed] [Google Scholar]
- 29.Weiss JM, Kim DH, Smith MA, et al. Predictors of primary care provider adoption of CT colonography for colorectal cancer screening. Abdom Radiol (NY) 2017;42:1268–75 . 10.1007/s00261-016-0971-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol 2007;189:290–8 . 10.2214/AJR.07.2136 [DOI] [PubMed] [Google Scholar]
- 31.Cox K, Duszak R, Hemingway J, et al. Reassessing medicare trends in diagnostic CT colonography after achieving CPT code category I status. Abdom Radiol (NY) 2016;41:1357–62 . 10.1007/s00261-016-0636-8 [DOI] [PubMed] [Google Scholar]
- 32.Levy AD, Sobin LH. From the archives of the AFIP: Gastrointestinal carcinoids: imaging features with clinicopathologic comparison. Radiographics 2007; 27:237–57 . 10.1148/rg.271065169 [DOI] [PubMed] [Google Scholar]
- 33.Taghavi S, Jayarajan SN, Powers BD, et al. Examining rectal carcinoids in the era of screening colonoscopy: a surveillance, epidemiology, and end results analysis. Dis Colon Rectum 2013;56:952–9 . 10.1097/DCR.0b013e318291f512 [DOI] [PubMed] [Google Scholar]
- 34.Baxi AJ, Chintapalli K, Katkar A, et al. Multimodality Imaging Findings in Carcinoid Tumors: A Head-to-Toe Spectrum. Radiographics 2017;37:516–36 . 10.1148/rg.2017160113 [DOI] [PubMed] [Google Scholar]
- 35.McDermott FD, Heeney A, Courtney D, et al. Rectal carcinoids: a systematic review. Surg Endosc 2014;28:2020–6 . 10.1007/s00464-014-3430-0 [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Kim JH, Lim JS, et al. MRI findings of rectal submucosal tumors. Korean J Radiol 2011;12:487–98 . 10.3348/kjr.2011.12.4.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy AD, Remotti HE, Thompson WM, et al. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics 2003;23:283–304, 456; 10.1148/rg.232025146 [DOI] [PubMed] [Google Scholar]
- 38.Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer 2005;117:289–93 . 10.1002/ijc.21167 [DOI] [PubMed] [Google Scholar]
- 39.Jiang Z-X, Zhang S-J, Peng W-J, Yu B-H. Rectal gastrointestinal stromal tumors: imaging features with clinical and pathological correlation. World J Gastroenterol 2013;19:3108–16 . 10.3748/wjg.v19.i20.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol 2013;20:586–92 . 10.1245/s10434-012-2647-1 [DOI] [PubMed] [Google Scholar]
- 41.Jin F, Prestage GP, Kippax SC, et al. Risk factors for genital and anal warts in a prospective cohort of HIV-negative homosexual men: the HIM study. Sex Transm Dis 2007;34:488–93 . 10.1097/01.olq.0000245960.52668.e5 [DOI] [PubMed] [Google Scholar]
- 42.Sonnex C, Scholefield JH, Kocjan G, et al. Anal human papillomavirus infection in heterosexuals with genital warts: prevalence and relation with sexual behaviour. BMJ 1991;303:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman P, Halpert RD. Invasive squamous cell carcinoma of the anus arising in condyloma acuminatum: CT demonstration. Gastrointest Radiol 1991;16:267–70 [DOI] [PubMed] [Google Scholar]
- 44.Orlowski HLP, Mellnick VM, Dahiya N, et al. The imaging findings of typical and atypical genital and gynecologic infections. Abdom Radiol (NY) 2016;41:2294–309 . 10.1007/s00261-016-0749-0 [DOI] [PubMed] [Google Scholar]
- 45.Balthazar EJ, Streiter M, Megibow AJ. Anorectal giant condyloma acuminatum (Buschke-Loewenstein tumor): CT and radiographic manifestations. Radiology 1984;150:651–3 . 10.1148/radiology.150.3.6695063 [DOI] [PubMed] [Google Scholar]
- 46.Steele SR, Varma MG, Melton GB, et al. Practice parameters for anal squamous neoplasms. Dis Colon Rectum 2012;55:735–749 . 10.1097/DCR.0b013e318255815e [DOI] [PubMed] [Google Scholar]
- 47.Roach SC, Hulse PA, Moulding FJ, et al. Magnetic resonance imaging of anal cancer. Clin Radiol 2005;60:1111–9 . 10.1016/j.crad.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 48.Parikh J, Shaw A, Grant LA, et al. Anal carcinomas: the role of endoanal ultrasound and magnetic resonance imaging in staging, response evaluation and follow-up. Eur Radiol 2011;21:776–85 . 10.1007/s00330-010-1980-7 [DOI] [PubMed] [Google Scholar]
- 49.Tarantino D, Bernstein MA. Endoanal ultrasound in the staging and management of squamous-cell carcinoma of the anal canal: potential implications of a new ultrasound staging system. Dis Colon Rectum 2002;45:16–22 [DOI] [PubMed] [Google Scholar]
- 50.Martellucci J, Naldini G, Colosimo C, et al. Accuracy of endoanal ultrasound in the follow-up assessment for squamous cell carcinoma of the anal canal treated with radiochemotherapy. Surg Endosc 2009;23:1054–7 . 10.1007/s00464-008-0130-7 [DOI] [PubMed] [Google Scholar]
- 51.Cotter SE, Grigsby PW, Siegel BA, et al. FDG-PET/CT in the evaluation of anal carcinoma. Int J Radiat Oncol Biol Phys 2006;65:720–5 . 10.1016/j.ijrobp.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 52.Nigro ND. An evaluation of combined therapy for squamous cell cancer of the anal canal. Dis Colon Rectum 1984;27:763–6 [DOI] [PubMed] [Google Scholar]
- 53.Papaconstantinou HT, Bullard KM, Rothenberger DA, Madoff RD. Salvage abdominoperineal resection after failed Nigro protocol: modest success, major morbidity. Colorectal Dis 2006;8:124–9. 10.1111/j.1463-1318.2005.00911.x [DOI] [PubMed] [Google Scholar]
- 54.Stefanou A, Nalamati SPM. Anorectal melanoma. Clin Colon Rectal Surg 2011;24:171–6. 10.1055/s-0031-1286001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuoka H, Nakamura A, Iwamoto K, et al. Anorectal malignant melanoma: preoperative usefulness of magnetic resonance imaging. J Gastroenterol 2005;40:836–42 . 10.1007/s00535-005-1638-4 [DOI] [PubMed] [Google Scholar]
- 56.Kim KW, Ha HK, Kim AY, et al. Primary malignant melanoma of the rectum: CT findings in eight patients. Radiology 2004;232:181–6 . 10.1148/radiol.2321030909 [DOI] [PubMed] [Google Scholar]
- 57.Hoeffel CC, Azizi L, Mourra N, et al. MRI of rectal disorders. AJR Am J Roentgenol 2006;187:W275–84 . 10.2214/AJR.05.0508 [DOI] [PubMed] [Google Scholar]
- 58.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63–71 . 10.1097/SLA.0b013e31818e4641 [DOI] [PubMed] [Google Scholar]
- 59.Peralta EA. Rare anorectal neoplasms: gastrointestinal stromal tumor, carcinoid, and lymphoma. Clin Colon Rectal Surg 2009;22:107–14 . 10.1055/s-0029-1223842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghai S, Pattison J, Ghai S, et al. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics 2007;27:1371–88 . 10.1148/rg.275065151 [DOI] [PubMed] [Google Scholar]


