Abstract
Background:
The loss of tactile feedback in minimally invasive robotic surgery remains a major challenge to the expanding field. With visual cue compensation alone, tissue characterization via palpation proves to be immensely difficult. This work evaluates a bi-modal vibro-tactile system as a means of conveying applied forces to simulate haptic feedback in two sets of studies simulating an artificial palpation task using the da Vinci surgical robot.
Methods:
Subjects in the first study were tasked with localizing an embedded vessel in a soft tissue phantom using a single-sensor unit. In the second study, subjects localized tumor-like structures using a three-sensor array. In both sets of studies, subjects completed the task under three trial conditions: no feedback, normal force tactile feedback, and hybrid vibro-tactile feedback. Recordings of correct localization, incorrect localization, and time-to-completion, were used to evaluate performance outcomes.
Results:
With the addition of vibro-tactile and pneumatic feedback, significant improvements in the percentage of correct localization attempts were detected (p=0.0001 and 0.0459, respectively) during the first experiment with phantom vessels. Similarly, significant improvements in correct localization were found with the addition of vibrotactile (p=2.57E-5) and pneumatic significance (p=8.54E-5) were observed in the second experiment involving tumor phantoms.
Conclusions:
This work demonstrates not only the superior benefits of a multi-modal feedback over traditional single modality feedback, but also the effectiveness of vibration in providing haptic feedback to artificial palpation systems.
Keywords: haptic feedback, robotic surgery, robotic palpation, vibrotactile feedback, haptics, minimally invasive surgery
Introduction.
Despite advantages such as shorter recovery time and reduced postoperative pain, minimally-invasive surgical approaches like laparoscopic and robotic surgery have been associated with a compromise or loss of tactile feedback, defined as the ability to recreate the sense of touch by applying forces and/or vibrations to the surgeon. Tactile feedback has been found to be essential for delicate surgical tasks including tissue retraction, dissection, and manipulation1–3. A large part of a minimally invasive surgeon’s learning curve is spent on adopting to this loss with compensation via visual cues. By partially restoring tactile feedback to the surgeon, studies have found decreased number of errors during blunt dissection tasks3 and reduced frequency of intraoperative tissue damages4,5.
One important yet infrequently explored utility of tactile feedback lies in tissue characterization6,7.During open surgery, abnormal tissues, such as tumor or inflamed soft tissue, are detected by their different mechanical properties from neighboring structures8. Such differences in mechanical properties are detectable only by the surgeon’s fingers but not eyes. Furthermore, the ability to localize anatomical structures buried under soft tissue, such as a mesentery vessel, the ureter, or a hidden lymph node, is critical in order to avoid injuring key structures9. In order to effectively distinguish anatomical structures from background soft tissue, a force sensitive instrument capable of providing artificial force feedback is required.
Haptic feedback technologies to date are comprised of two primary components, sensors and actuators. Sensors detect forces applied to the tissue by the surgical instrument, while actuators relay this information to the surgeon’s fingertips. Most existent haptic feedback systems rely on sensors that require significant modification of robotic instruments, thereby limiting their application in clinical settings10–13. In our current report, we seek to utilize sensors installed directly on the robotic instruments as an add-on component, allowing a wider range of compatibility with different robotic systems. In addition, most existent haptic feedback systems rely on auditory or visual cues for conveying the sense of touch to the surgeon, i.e. sensory substitution10,11,14. However, sensory substitution requires additional processing time by the surgeon and may lead to sensory overload in noisy environments like the operating room15. Surgeons have been found to prefer haptic feedback to sensory substitution as it is perceived as being more intuitive16. Furthermore, most systems focused on providing kinesthetic feedback only, i.e. sense of proprioception provided via sensors in large muscle tendons and joints, ignoring the role of tactile feedback, i.e. fine touch and vibration sensations provided by sensors in the epidermis and dermis of the fingertips17,18.
In the current ex-vivo study, we seek to evaluate the efficacy of tactile feedback restoration in improving the ability of the surgeon to quickly localize structures hidden in soft tissue. Furthermore, we evaluated whether the addition of a vibrotactile feedback, i.e. an on-off vibratory component, to a graded normal force feedback, provided additional discriminatory power for the surgeon in discerning underlying anatomical structure.
Methods and Procedures
Experimental Design
We employed a repeated measures study design, whereby study subjects were asked to palpate soft tissue phantoms with the artificial palpation system mounted on a da Vinci robot (Intuitive Surgical, Sunnyvale, CA), in an attempt to localize the underlying hidden structure. Each subject was asked to perform the same task three times: once with no tactile feedback (i.e. visual feedback alone), once with graded normal force feedback, and once with both graded normal force feedback and vibrotactile feedback, i.e. bi-modal feedback. The order of the three trial conditions was randomized to eliminate any bias arising from the subject gaining experience with the artificial palpation system.
Prior to commencement of the trials, subjects were allowed to palpate a separate soft tissue phantom sample with native fingers to familiarize themselves with the shape and size of the target structure. Subsequently, they were instructed to scan the entire surface of the phantom, ensuring that the sensor array remained parallel to the surface of the table where the phantom was mounted. Subjects would then either point out the location of the target structure or simply state that no target organ was present (in case none could be found). Subjects were not told that all phantoms did in fact contain the target structure. Furthermore, even though subjects were told that a different phantom may have been used in each trial, the same phantom was simply repositioned in between the trials to change relative location of the target structure in relation to the camera. This approach eliminated any bias that could be introduced from variation in the depth and size of the target structure between phantom samples.
Using similar experimental designs, two separate trials were conducted. The first trial involved the use of a single force sensor in detection of a long tubular structure while the second trial sought to localize a small discrete tumor using a three-sensor array.
Artificial Palpation System Designs
In order to evaluate the impact of tactile feedback in tissue characterization, we have independently developed an artificial palpation system composed of a sensor component installed at the tip of a robotic instrument and a bi-modal haptic actuator, capable of providing both graded normal force feedback and vibrotactile feedback. The graded normal force feedback was provided via the use of pneumatic balloons in touch with the surgeon’s fingertips. The balloons inflate in correspondence with the force value detected by the sensor on the instrument tips. The vibrotactile feedback was provided with the use of a vibratory motor, which is activated in a binary manner based on thresholds that could be programmed depending on its utility (Figure 1a). The system is modified from previously reported haptic feedback systems from our research group, which was originally developed for minimizing robotic grasp force19–21 (Figure 1b). It is fully compatible with the da Vinci robotic surgical system and could potentially be modified to be used with most commercially available robotic surgical systems.
Figure 1:
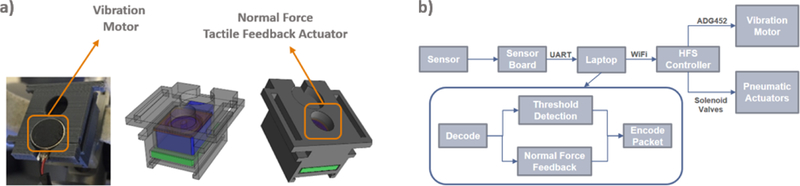
(a) 3D Printed Pneumatic Actuators with Vibration Motors (b) HFS Control System Overview
The primary modifications made to the previously reported system that allowed the execution of the below-described experiments were a newly-designed sensor array as described below.
Trial 1: Single Sensor for Detection of Tubular Structure
Sensor Design:
The initial artificial palpation prototype utilized a single FlexiForce A201 force sensor to detect forces applied to the tissue. The sensor was mounted at the end of da Vinci Fenestrated Bipolar forceps using a custom 3-D printed support (Figure 2a). The control system (Figure 1b) for the bi-modal vibrotactile HFS was implemented by configuring the logic engine of the HFS control software with two sets of rules. The first ruleset controlled the behavior of the pneumatic normal force feedback actuators, whereby incremental inflation of the pneumatic balloon in contact with the surgeon’s fingertip was induced corresponding to the force value detected by the single sensor on the bipolar forcep. The second ruleset controlled a vibratory motor that provided vibration to the surgeon’s fingertip when the detected force value exceeded the 75th quartile of the force range recorded.
Figure 2:
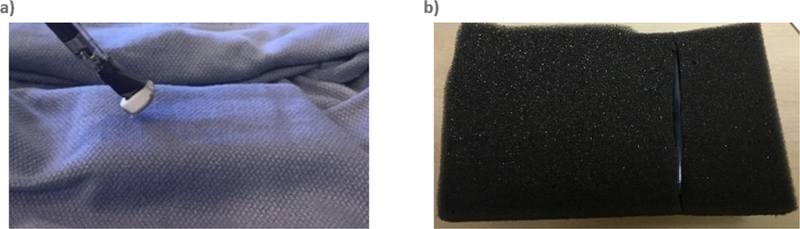
(a) Normal Force Sensor Installed on da Vinci Fenestrated Bipolar Forceps (b) Phantom Made of a Polyurethane Tube Hidden Inside a Soft EVA Foam
Phantom Design:
To simulate the presence of a tubular structure inside soft tissue, akin to ureter in the retroperitoneal fat or mesentery vessels in the mesentery, a phantom was created by placing a semi-compressible, silicone-based tube, inside a soft Eva foam material and then covering it up with a soft, but thick, patterned cloth (Figure 2b). The cloth made visual localization of the tubular structure challenging.
Trial 2: Sensor Array for Detection of Discrete Tumor
Sensor Design:
The use of a single sensor in Trial 1 showed that sequential palpation of background tissue, target organ, and background tissue and accurate retrospective comparison of the gathered force information was required in order to localize the target organ. This is markedly different from native human fingers, which could simultaneously detect thousands of data points in neighboring regions covered by the surface area of the finger and localize the underlying structure based on its distinct force value from its neighboring background tissue. Based on this observation, we developed the second iteration of the artificial palpation prototype, which sought to further enhance its discriminatory power with the use of a three-sensor array (Figure 3a) in place of a single sensor. This configuration utilized the two outer sensors in the array as reference sensors and the middle sensor as a data sensor. This approach ensured that detection of semi- and non-compressible structures surrounded by soft tissue could be more easily achieved. The algorithm that controlled the incremental inflation of the pneumatic balloon now provided balloon inflation correspondent with the maximal force value detected by the three sensors. The threshold for activating the vibratory motor has been programmed in such a way that, instead of using an arbitrary force value threshold, the vibratory motor was now activated when the data sensor recorded higher forces than the two reference sensors (figure 3b).
Figure 3:
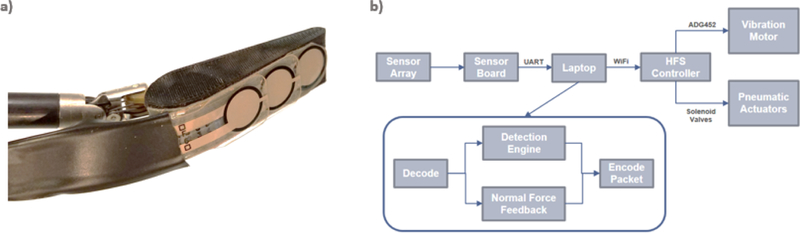
(a) Sensor Array with Two Outer Reference Sensors and a Center Data Sensor (b) Control System of Vibro-Tactile Hybrid HFS using Normal Force Sensor Array
Phantom Design:
A solid tumor phantom was created by injecting epoxy inside a low-density soft sponge material measuring approximately 15×10 cm, simulating a tumor approximately 1cm in diameter that could be easily palpated but not visualized from the sponge surface (Figure 4a & 4b).
Figure 4:
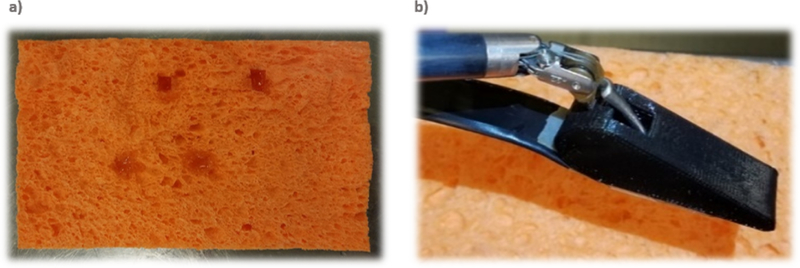
(a) View of Phantom from Underneath. Phantom is Made of Soft Sponge Material with Injected Epoxy Used to Simulate Tumors. (b) Soft Sponge Phantom with Spherical Tumor-Like Structures Hidden Inside, Palpated Using Normal Force Sensor Array
Data Analysis
For both trials, three outcomes of interest were recorded: correct localization, false localization (failure to localize the structure or localizing it to the wrong site) and the time-to-completion. For the first two outcomes, statistical analysis of the ordinal data (i.e. detections, faults) would ideally be performed using One-way Repeated Ordinal Regression22. However, this test would fail due to the binary nature of the data in our current study. For this reason, Friedman’s test, which is a non-parametric alternative to Repeated Measures ANOVA, was used instead. For time-to-completion, Log2 transformation was used to achieve normality, followed by statistical analysis using Repeated Measures ANOVA. Follow up post-hoc analysis was performed using Tukey correction method when the p-value was < 0.05.
Results
Trial 1: Single Sensor for Detection of Tubular Structure
A total of 19 subjects participated in trial 1, each repeating the same trial under three separate conditions: 1) when no tactile feedback was provided, i.e. using visual cues alone, there were 4 (21%) instances of mis-localizations, 10 (53%) instances of subject not finding the tube, and 5 (26%) correct detections of the tube when no tactile feedback. 2) When graded normal force feedback was provided, there were 3 (16%) mis-localizations, 4 (21%) instances of subject not finding the tube, and 12 (63%) correct detections. 3) When the vibrotactile feedback was added to the graded normal force feedback,i.e. bi-modal feedback, there were no instances of mis-localizations, 2 (11%) instances of the subject not being able to locate the tube, and 17 (89%) correct detections.
For the purposes of statistical analysis, a fault/false-positive was identified as either a subject not being able to locate the tube or incorrectly identifying the location of the tube. A correct detection was defined as correct localization of the vessel-like structure (Figure 5). The bi-modal feedback performed significantly better than no feedback with regards to mis-localizations (p = 0.0001, Friedman) and correct detections (p = 0.0001, Friedman). When comparing the unimodal graded normal force feedback with the no feedback conditions, a significant reduction in faults (p = 0.0457, t-test, Tukey) and correct detections (p = 0.0459, t-test, Tukey) could also be observed. No significant difference was observed between the bi-modal and unimodal graded normal force feedback with regards to fault (p = 0.206, t-test, Tukey) nor correct localization of the tube (p = 0.206, t-test, Tukey).
Figure 5:
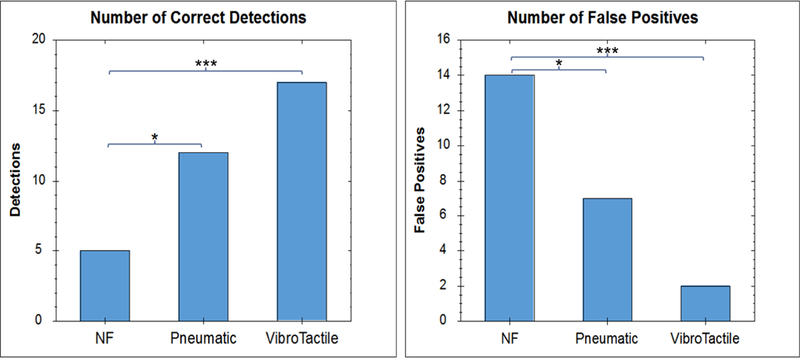
Comparison of Correctness Score (Number of Correct Detections) and Number of Faults (False Positives and Not Detected) in Detecting Non-Compressible Structure in Soft Tissue, Under Different Feedback Conditions
With regards to the time-to-completion of the tasks (Figure 6), the bi-modal feedback performed significantly better (shorter completion times) than both the no feedback (p = 0.0033, t-test, Tukey) and the unimodal graded normal force feedback (p = 0.029, t-test, Tukey) conditions. No significant difference was present between the time-to-completion time between the no feedback and unimodal graded normal force feedback conditions (p = 0.762, t-test, Tukey).
Figure 6:
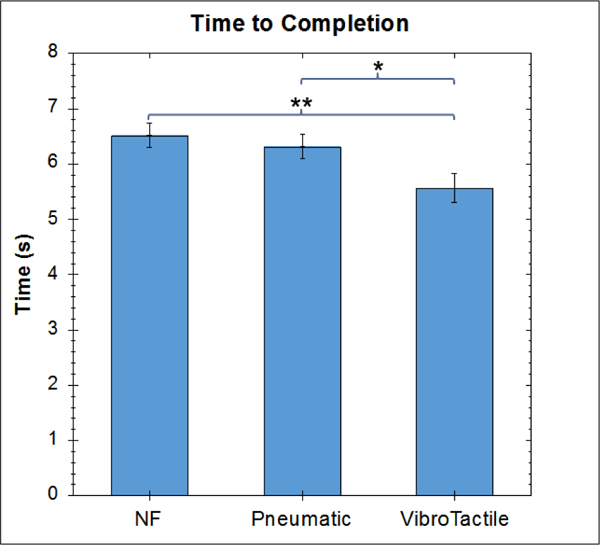
Comparison of Time-to-Completion for Localization of Non-Compressible Tubular Structure Under Different Feedback Conditions
Trial 2: Sensor Array for Detection of Discrete Tumor
A total of 14 subjects participated in trial 2, with each subject repeating the same task three times, each time with three different tactile feedback conditions, i.e. no tactile feedback, unimodal graded normal force feedback alone, or bimodal vibrotactile feedback.
Similar to trial 1, the results of trial 2 showed (Figure 7) that the ability of the subject to detect the tumors was significantly improved compared to the no feedback conditions, both when using unimodal normal force feedback (p = 8.54E-5, t-test, Holm) and bi-modal vibro-tactile feedback (p = 2.57E-5, t-test, Holm). The bimodal vibrotactile feedback also performed better than the unimodal pneumatictactile feedback with regard to the number of correctly detected tumors (p = 0.019, t-test, Holm). Looking at the total number of correctly detected tumors, the no feedback condition resulted in 3 correctly identified tumors out of a total of 56. Providing unimodal normal force feedback increased this number to 33/56 and providing bimodal vibro-tactile feedback resulted in 44/56 correctly identified tumors.
Figure 7:
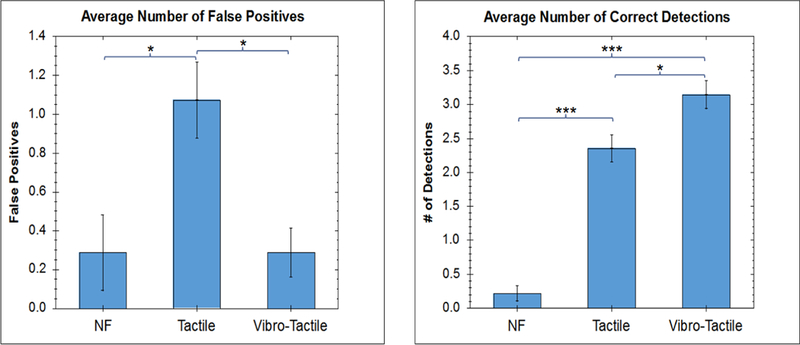
Comparison of the Number of Faults and Correctness Score (Number of Correct Detections) for Artificial Palpation in Robotic Surgery, Under Different Feedback Conditions
With regard to the number of mis-localizations, the bimodal vibrotactile feedback system performed significantly better than the unimodal normal force feedback (p = 0.025 t-test, Holm). This corresponds to total false positives of 4/56, 15/56, and 4/56 for no feedback, unimodal feedback and bimodal feedback conditions, respectively.
It is worth mentioning that while there is also a significant difference between the unimodal normal force feedback and the no feedback conditions with regard to the number of mis-localizations, in reality this value does not correctly represent the observations of the experiment. In nearly all no feedback cases, the subject simply gave up on finding a tumor in the phantom and reported that the phantom contained no tumors at all, therefore leading to a low mis-localization value.
Discussions.
While expert surgeons are able to compensate for the loss of haptic feedback during minimally-invasive procedures with visual cues in many respects23, tissue characterization remains an irreplaceable utility of tactile sensation24. The increased incidence of biliary duct injury associated with laparoscopic cholecystectomy is frequently attributable to the surgeons’ compromised ability to localize firm structures behind adhesions and fat25. In addition, surveys of robotic surgeons have demonstrated consensus of need for enhanced detection of tissue consistency differences and localization of hidden vessel, tumor or lymph nodes26. In order to address this need, researchers have developed artificial palpation devices specifically for the characterization of prostate tumors, arterial stenotic lesions, and kidney stones8,27,28. Furthermore, recent efforts have focused on the development of a general-purpose artificial palpation system compatible with commonly used laparoscopic and/or robotic instruments that allows simultaneous tissue characterization during the performance of clinically relevant tasks29–31. Nearly all such systems to date rely on customized and modified versions of existing laparoscopic and robotic instruments, making them incompatible with existing clinically used solutions. Here we describe a general-purpose solution, implemented as an add-on, and compatible with existing robotic surgical systems and instruments. This system can be used to provide feedback without requiring any modification to existing robotic systems. Additionally, rather than relying on a single modality of feedback, this study has investigated the benefits that utilizing additional feedback modalities can provide to artificial palpation systems.
The benefits of the vibro-tactile HFS over single-modality feedback solution highlight the value of vibration as a feedback modality. Vibration feedback, when applied in a continuous fashion, can be distracting and lead to increased mental load. When used in an intermittent fashion however, vibration feedback is extremely effective in directing the brain’s attention to a specific event or area of the body32. This unique benefit is what allows the robotic operator to rapidly scan a large area of tissue and quickly identify potential structures. Ultimately, the graded feedback provided by the pneumatic normal force feedback actuators is a must for palpating the target area and identifying the margins and/or tissue characteristics. However, as seen in the results of these studies, such graded feedback modalities alone, cannot improve the time-to-completion of such surgical tasks.
In addition to the single-sensor unit, a three-sensor array was designed to allow kinetic scanning of a soft tissue surface as opposed to point-by-point indentation of the surface. Outcomes were further improved with the observed reduction of the instances of false positives. However, future efforts are required to estimate the optimal sophistication of sensor array required to achieve adequate tissue characterization fidelity and limit device footprint and cost.
It is worth mentioning that the experimental design of the second set of experiments involving detection of tumors led to a much more challenging task compared to the more straightforward detection of vessels in the initial vibro-tactile HFS study. This is clear from the mere 5% correct detection rate for the no feedback condition, demonstrating the inadequacy of visual inspection (i.e. without feedback) for localizing structures hidden in soft tissue. On the other hand, providing tactile feedback increases this number to 59%, while the multi-modal feedback raises this number further to 79%. Considering that this system relies on a low-resolution array consisting of only three normal force sensors, this value demonstrates how effective multi-modal haptic feedback systems can be in resolving one of the most critical limitations facing robotic surgical systems. Furthermore, these results also highlight the benefits of multi-modal feedback systems over traditional single modality feedback methods.
The bi-modal vibro-tactile system investigated in this work was representative of only a proof of concept implementation of HFS for artificial palpation. Despite this, it clearly shows the benefits of a multi-modal haptic feedback system over single-modality solutions. The synergistic relationship between the two feedback modalities presented in this vibro-tactile system resembles how fast adapting mechanoreceptors in the skin work together with slow adapting pressure sensors to allow rapid scanning of an area and identification of various structures not visible to the naked eye.
The outcomes of this work demonstrate that even a single-sensor, naïve implementation of a tactile feedback system can significantly improve the surgeons’ tissue characterization capabilities. This implementation, once further enhanced with better sensing and software detection algorithms, clearly outlines the benefits of tactile feedback systems for artificial palpation applications in robotic surgery. Most importantly, the results show that simulating the symbiotic relationship of multiple sensory modalities involved in human touch can significantly impact the effectiveness of artificial palpation systems. Ultimately, the results of this work highlight the importance of further research into haptic feedback solutions that can easily integrate into existing surgical robotics solutions and utilize multiple modalities of feedback to provide a more natural artificial palpation solution.
References
- 1.Puangmali P, Liu H, Seneviratne LD, Dasgupta P, Althoefer K. Miniature 3-axis distal force sensor for minimally invasive surgical palpation. IEEE/ASME Trans Mechatronics. 2012;17(4):646–656. doi: 10.1109/TMECH.2011.2116033. [DOI] [Google Scholar]
- 2.Ahmadi R, Packirisamy M, Dargahi J, Cecere R. Discretely loaded beam-type optical fiber tactile sensor for tissue manipulation and palpation in minimally invasive robotic surgery. IEEE Sens J. 2012;12(1):22–32. doi: 10.1109/JSEN.2011.2113394. [DOI] [Google Scholar]
- 3.Wagner CR, Stylopoulos N, Howe RD. The role of force feedback in surgery: Analysis of blunt dissection. Proc - 10th Symp Haptic Interfaces Virtual Environ Teleoperator Syst HAPTICS 2002. 2002:68–74. doi: 10.1109/HAPTIC.2002.998943. [DOI] [Google Scholar]
- 4.Bethea BT, Okamura AM, Kitagawa M, et al. Application of Haptic Feedback to Robotic Surgery. J Laparoendosc AdvSurg Tech. 2004;14(3):191–195. doi: 10.1089/1092642041255441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diks J, Nio D, Linsen MA, Rauwerda JA, Wisselink W. Suture damage during robot-assisted vascular surgery: is it an issue? Surg Laparosc Endosc Percutan Tech. 2007;17(6):524–527. doi: 10.1097/SLE.0b013e318150e590. [DOI] [PubMed] [Google Scholar]
- 6.Van Der Meijden OAJ, Schijven MP. The value of haptic feedback in conventional and robot- assisted minimal invasive surgery and virtual reality training: A current review. Surg Endosc Other Interv Tech. 2009;23(6):1180–1190. doi: 10.1007/s00464-008-0298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitsch V, Farber B. A meta-analysis of the effects of haptic interfaces on task performance with teleoperation systems. IEEE Trans Haptics. 2013;6(4):387–398. doi: 10.1109/TOH.2012.62. [DOI] [PubMed] [Google Scholar]
- 8.Ahn B, Lorenzo EIS, Rha KH, Kim HJ, Kim J. Robotic palpation-based mechanical property mapping for diagnosis of prostate cancer. J Endourol. 2011;25(5):851–857. doi: 10.1089/end.2010.0468. [DOI] [PubMed] [Google Scholar]
- 9.Okamura AM, Simone C, O’Leary MD. Force modeling for needle insertion into soft tissue. IEEE Trans Biomed Eng. 2004;51(10):1707–1716. doi: 10.1109/TBME.2004.831542. [DOI] [PubMed] [Google Scholar]
- 10.Schostek S, Ho C-N, Kalanovic D, Schurr MO. Artificial tactile sensing in minimally invasive surgery - a new technical approach. Minim invasive Ther allied Technol MITAT. 2006;15(5):296–304. doi: 10.1080/13645700600836299. [DOI] [PubMed] [Google Scholar]
- 11.Ottermo MV, Stavdahl Ø, Johansen T a. A remote palpation instrument for laparoscopic surgery: design and performance. Minim Invasive Ther Allied Technol. 2009;18(1):259–272. doi: 10.1080/13645700903202314. [DOI] [PubMed] [Google Scholar]
- 12.Menciassi A, Eisinberg A, Carrozza MC, Dario P. Force sensing microinstrument for measuring tissue properties and pulse in microsurgery. IEEE/ASME Trans Mechatronics. 2003;8(1):10–17. doi: 10.1109/TMECH.2003.809153. [DOI] [Google Scholar]
- 13.Ottermo MV, Stavdahl O, Johansen T a. Palpation instrument for augmented minimally invasive surgery. 2004 IEEE/RSJ Int Conf Intell Robot Syst (IEEE Cat No04CH37566). 2004;4:3–7. doi: 10.1109/IROS.2004.1390033. [DOI] [Google Scholar]
- 14.Kitagawa M, Dokko D, Okamura AM, Yuh DD. Effect of sensory substitution on suture- manipulation forces for robotic surgical systems. J Thorac Cardiovasc Surg. 2005;129(1):151–158. doi: 10.1016/j.jtcvs.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Meli L, Pacchierotti C, Prattichizzo D. Sensory subtraction in robot-assisted surgery: Fingertip skin deformation feedback to ensure safety and improve transparency in bimanual haptic interaction. IEEE Trans Biomed Eng. 2014;61(4):1318–1327. doi: 10.1109/TBME.2014.2303052. [DOI] [PubMed] [Google Scholar]
- 16.Koehn JK, Kuchenbecker KJ. Surgeons and non-surgeons prefer haptic feedback of instrument vibrations during robotic surgery. Surg Endosc Other Interv Tech. 2015;29(10):2970–2983. doi: 10.1007/s00464-014-4030-8. [DOI] [PubMed] [Google Scholar]
- 17.Samur E, Sedef M, Basdogan C, Avtan L, Duzgun O. A robotic indenter for minimally invasive measurement and characterization of soft tissue response. Med Image Anal. 2007;11(4):361–373. doi: 10.1016/j.media.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. Vol 3; 2000. doi: 10.1036/0838577016. [DOI] [Google Scholar]
- 19.King C-H, Higa AT, Culjat MO, et al. A pneumatic haptic feedback actuator array for robotic surgery or simulation. Stud Health Technol Inform. 2007;125(February):217–222. [PubMed] [Google Scholar]
- 20.Wottawa CR, Genovese B, Nowroozi BN, et al. Evaluating tactile feedback in robotic surgery for potential clinical application using an animal model. Surg Endosc. 2016;30(8):3198–3209. doi: 10.1007/s00464-015-4602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King CH, Culjat MO, Franco ML, et al. Tactile feedback induces reduced grasping force in robot- assisted surgery. IEEE Trans Haptics. 2009;2:103–110. doi: 10.1109/TOH.2009.4. [DOI] [PubMed] [Google Scholar]
- 22.<Salvatore S. Mangiafico R Handbook: One-way Repeated Ordinal ANOVA with CLMM. http://rcompanion.org/handbook/G_08.html. Accessed May 6, 2017.
- 23.Hagen ME, Meehan JJ, Inan I, Morel P. Visual clues act as a substitute for haptic feedback in robotic surgery. Surg Endosc. 2008;22(6):1505–1508. doi: 10.1007/s00464-007-9683-0. [DOI] [PubMed] [Google Scholar]
- 24.Lamata P, Gómez EJ, Sánchez-Margallo FM, Lamata F, Del Pozo F, Usón J. Tissue consistency perception in laparoscopy to define the level of fidelity in virtual reality simulation. Surg Endosc Other Interv Tech. 2006;20(9):1368–1375. doi: 10.1007/s00464-004-9269-z. [DOI] [PubMed] [Google Scholar]
- 25.Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko S-T, Airan MC. Complications of laparoscopic cholecystectomy: A national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 2017;165(1):9–14. doi: 10.1016/S0002-9610(05)80397-6. [DOI] [PubMed] [Google Scholar]
- 26.Alleblas CCJ, Vleugels MPH, Nieboer TE. Ergonomics of laparoscopic graspers and the importance of haptic feedback: the surgeons??? perspective. Gynecol Surg 2016;13(4):379–384. doi: 10.1007/s10397-016-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abouei Mehrizi A, Moini M, Afshari E, Kadkhodapour J, Sadjadian A, Najarian S. Application of artificial palpation in vascular surgeries for detection of peripheral arterial stenosis. J Med Eng Technol. 2014;38(4):169–178. doi: 10.3109/03091902.2014.891663. [DOI] [PubMed] [Google Scholar]
- 28.Afshari E, Najarian S, Simforoosh N. Application of artificial tactile sensing approach in kidney- stone-removal laparoscopy. In: Bio-Medical Materials and Engineering. Vol 20; 2010:261–267. doi: 10.3233/BME-2010-0640. [DOI] [PubMed] [Google Scholar]
- 29.Dalvand MM, Shirinzadeh B, Nahavandi S, Smith J. Effects of realistic force feedback in a robotic assisted minimally invasive surgery system. Minim Invasive Ther Allied Technol. 2014;23(3):127–135. doi: 10.3109/13645706.2013.867886. [DOI] [PubMed] [Google Scholar]
- 30.Moradi Dalvand M, Shirinzadeh B, Shamdani AH, Smith J, Zhong Y. An actuated force feedback- enabled laparoscopic instrument for robotic-assisted surgery. Int J Med Robot Comput Assist Surg. 2014;10(1):11–21. doi: 10.1002/rcs.1503. [DOI] [PubMed] [Google Scholar]
- 31.Bark K, McMahan W, Remington A, et al. In vivo validation of a system for haptic feedback of tool vibrations in robotic surgery. Surg Endosc Other Interv Tech. 2013;27(2):656–664. doi: 10.1007/s00464-012-2452-8. [DOI] [PubMed] [Google Scholar]
- 32.Giabbiconi C-M, Trujillo-Barreto NJ, Gruber T, Müller MM. Sustained spatial attention to vibration is mediated in primary somatosensory cortex. Neuroimage. 2007;35(1):255–262. doi: 10.1016/j.neuroimage.2006.11.022. [DOI] [PubMed] [Google Scholar]


