Abstract
Pediatric cataract represents an important cause of pediatric visual impairment. While both genetic and environmental causes for pediatric cataract are known, a large proportion remains idiopathic. The purpose of this review is to discuss genes involved in isolated pediatric cataract, with a focus on variable inheritance patterns within genes. Mutations in over 52 genes are known to cause isolated pediatric cataract, with a major contribution from genes encoding for crystallins, transcription factors, membrane proteins, and cytoskeletal proteins. Interestingly, both dominant and recessive inheritance patterns have been reported for mutations in 13 different cataract genes. For some genes, dominant and recessive alleles represent distinct types of mutations, but for many, especially missense variants, there are no clear patterns to distinguish between dominant and recessive alleles. Further research into the functional effects of these mutations, as well as additional data on the frequency of the identified variants, is needed to clarify variant pathogenicity. Whole exome sequencing continues to be successful in identifying novel genes associated with congenital cataract but is hindered by the extreme genetic heterogeneity of this condition. The large number of idiopathic cases suggests that more genes and potentially novel mechanisms of gene disruption remain to be identified.
Keywords: pediatric cataract, congenital cataract, isolated cataract, genetic, transcription factors, inheritance
Introduction
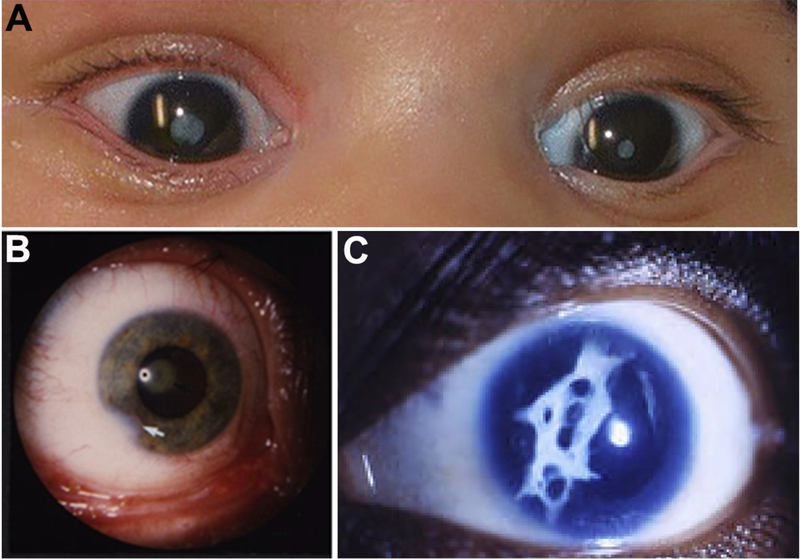
Cataracts are light scattering opacities in the lens of the eye that often result in vision impairment and affect individuals of all age groups. Pediatric cataracts, particularly congenital or infantile (onset within the first year of life), exemplify the most severe end of the spectrum (Figure 1A); congenital/ infantile cataracts currently explain ~10% of childhood blindness worldwide and represent the primary cause of blindness in some countries with a prevalence of 2.2–13.6 per 10,000 births (Khandekar 2008; Wu et al. 2016). Timely diagnosis and prompt surgical intervention in affected children are critical to avoid irreversible amblyopia and allow for normal visual development. Cataracts may be unilateral or bilateral and globally cases are split evenly between the two groups (Wu et al. 2016). Other ocular features that frequently coincide with cataracts in pediatric cases (microcornea, anterior segment defects (Figure 1B), microphthalmia, persistent fetal vasculature) as well as surgical complications (inflammatory response, secondary glaucoma, posterior capsule opacification (Figure 1C)) present additional challenges and affect visual prognosis (Lawrence et al. 2005; Whitman and Vanderveen 2014).
Figure 1:
Ocular photographs of previously reported patients with cataracts. A. Photograph showing bilateral dense central congenital cataract in an individual with a homozygous deletion of GCNT2 (reprinted with permission from Figure 1A of (Happ et al. 2016)); B. Photograph of the eye of an individual affected with a heterozygous mutation in PITX3 showing cataracts and corneal defects (white arrow) (reprinted with permission from Figure 1 of (Semina et al. 1998)); C. Photograph showing development of a fibrous posterior capsular reaction after lens removal for cataract in an individual with aniridia and cataract due to a nonsense mutation in PAX6 (reprinted with permission from Figure 2 of (Bremond-Gignac et al. 2010)).
Based on their position and morphology, pediatric cataracts can be further classified into total/complete, nuclear, anterior polar, posterior (cortical/subcapsular or polar), and other subtypes (punctate, sutural, lamellar); of these groups, total, nuclear, and posterior subcapsular are the most commonly reported (Haargaard et al. 2004; Wu et al. 2016). While visually significant cataracts need to be removed to preserve visual development, some subtypes of cataract, such as anterior polar, do not interfere with vision in most cases (Dixit et al. 2017). A cataract type may be indicative of gene’s expression and function during lens development; however, significant genetic and phenotypic heterogeneity is observed with family members sharing the same mutation displaying differing types of cataract and identical types of cataracts being caused by mutations in different genes (Shiels and Hejtmancik 2017).
Approximately 10–29% of pediatric cataract cases are currently attributed to genetic causes (Haargaard et al. 2004; Trumler 2011). Known environmental causes of cataracts include intrauterine infections, especially by so-called TORCH agents (Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes simplex), trauma, inflammation, metabolic disease (i.e. diabetes), and steroid or radiation treatments (Lloyd et al. 1992; Lu and Yang 2016). A large portion of pediatric cataract cases (up to 2/3 in some studies) remain idiopathic; while unilateral and bilateral cases are evenly distributed overall, unilateral cases are much more likely to be idiopathic compared to bilateral cases (Haargaard et al. 2004; Wu et al. 2016). With the recent successes in next generation sequencing-based genetic testing and an increasing number of newly identified factors with a significant role in lens development/function, it is likely that the number of cataract cases attributed to genetic causes will grow and explain some of the currently idiopathic disease.
General overview of genes associated with pediatrics cataracts
Over 200 syndromes have been associated with pediatric cataract in at least some cases, including numerous metabolic disorders such as lysosomal storage diseases, mitochondrial conditions, and congenital disorders of glycosylation. For some syndromes, such as Oculofaciocardiodental (BCOR), Marinesco-Sjogren (SIL1), and Lowe (OCRL) syndromes, congenital cataract is the major ocular finding (Bokenkamp and Ludwig 2016; Krieger et al. 2013; Zhou et al. 2017), while for others, such as Branchiooculofacial syndrome (TFAP2A), Norrie disease (NDP) and Warburg Micro syndrome (RAB3GAP1, RAB3GAP2, RAB18, or TBC1D20) cataracts are seen as part of generalized ocular dysgenesis along with other developmental ocular anomalies such as microphthalmia, anterior segment dysgenesis, and coloboma (Al-Dosari et al. 2010; Handley and Sheridan 1993; Sims 1993). Pediatric cataracts can also be seen in many chromosomal disorders, including Down syndrome (Trisomy 21) (Haargaard and Fledelius 2006), Cri-du-chat syndrome (5p deletion) (Farrell et al. 1988), Trisomy 18 (Correia et al. 2017), and 22q11.2 deletion syndrome (McDonald-McGinn et al. 1993), and in retinal disorders such as retinitis pigmentosa (Fahim et al. 1993). For nonsyndromic cases, cataracts may accompany a more complex ocular condition such as, for example, microcornea, myopic chorioretinal atrophy, and telecanthus (MMCAT) associated with mutations in ADAMTS18 (Aldahmesh et al. 2013), or appear as a more specific finding. Since others have reviewed syndromic causes of pediatric cataract (Trumler 2011), for the purposes of this review, we will focus on genes reported to cause isolated pediatric cataract in at least one family or for which isolated pediatric cataract may be the initially presenting feature.
Mutations in over 52 genes are known to cause isolated pediatric cataract (Table 1). Of these, 35 are significantly associated with isolated pediatric cataract (Table 1A), either only reported in isolated cataract or in multiple families with isolated cataracts while being syndromic in other cases (CRYAB (myopathy), GCNT2 (blood group i), MAF (Ayme-Gripp syndrome), TDRD7 (cataract)). Inheritance is variable with dominant inheritance only reported for 15 of these genes, recessive inheritance only for 7 factors, and both dominant and recessive inheritance patterns described for 13 genes. Mutations in crystallin genes are the most frequently identified genetic cause of isolated cataracts. Crystallin proteins are an important component of the human lens and provide both transparency as well as refractive power (Bassnett et al. 2011). Crystallins are categorized as α-, β-, and γ-crystallins, and each crystallin is further subdivided into individual protein components, eg, αA and αB crystallin proteins are encoded by separate genes, CRYAA and CRYAB. Mutations within crystallin genes that result in congenital cataract are thought to cause rapid protein aggregation (Shiels and Hejtmancik 2017). Other major gene families involved in isolated pediatric cataract include transcription factors (HSF4, PITX3, MAF, PAX6, FOXE3), membrane proteins (GJA8, GJA3, MIP, LIM2, CHMP4B, EPHA2, SLC16A12), cytoskeletal proteins (VIM, BFSP2, BFSP2), and numerous additional genes with other/unknown functions (Anand et al. 2018; Evers et al. 2015; Shiels et al. 2010).
Table 1:
Current summary of genes reported to cause isolated pediatric cataracts
| Gene | Full name | OMIM | Locus | Inheritance | References (AD and/or AR) |
|---|---|---|---|---|---|
| A: Genes significantly associated with isolated pediatric cataracts | |||||
| BFSP1 | Beaded filament structural protein 1 | 603307 | 20p12.1 | AR, AD | (Ramachandran et al. 2007; Wang et al. 2013) |
| BFSP2 | Beaded filament structural protein 2 | 603212 | 3q22.1 | AR, AD | (Aldahmesh et al. 2011; Conley et al. 2000) |
| CHMP4B | CHMP family, chromatin-modifying protein 4b | 610897 | 20q11.22 | AD | (Shiels et al. 2007) |
| CRYAA | Crystallin, alpha-a | 123580 | 21q22.3 | AD, AR | (Litt et al. 1998; Pras et al. 2000) |
| CRYAB* | Crystallin, alpha-b | 123590 | 11q23.1 | AD, AR | (Berry et al. 2001; Safieh et al. 2009) |
| CRYBA1 | Crystallin, beta-a1 | 123610 | 17q11.2 | AD, AR | (Gillespie et al. 2014; Kannabiran et al. 1998) |
| CRYBA2 | Crystallin, beta-a2 | 600836 | 2q35 | AD | (Reis et al. 2013) |
| CRYBA4 | Crystallin, beta-a4 | 123631 | 22q12.1 | AD | (Billingsley et al. 2006) |
| CRYBB1 | Crystallin, beta-b1 | 600929 | 22q12.1 | AD, AR | (Cohen et al. 2007; D. S. Mackay et al. 2002) |
| CRYBB2 | Crystallin, beta-b2 | 123620 | 22q11.23 | AD | (Litt et al. 1997) |
| CRYBB3 | Crystallin, beta-b3 | 123630 | 22q11.23 | AR, AD | (Reis et al. 2013; Riazuddin et al. 2005) |
| CRYGB | Crystallin, gamma-b | 123670 | 2q33.3 | AD | (AlFadhli et al. 2012) |
| CRYGC | Crystallin, gamma-c | 123680 | 2q33.3 | AD | (Heon et al. 1999) |
| CRYGD | Crystallin, gamma-d | 123690 | 2q33.3 | AD | (Stephan et al. 1999) |
| CRYGS | Crystallin, gamma-s | 123730 | 3q27.3 | AD | (H. Sun et al. 2005) |
| EPHA2 | Ephrin receptor epha2 | 176946 | 1p36.13 | AD, AR | (Aldahmesh, Khan, Mohamed, Hijazi et al. 2012; Shiels et al. 2008) |
| FOXE3 | Forkhead box e3 | 601094 | 1p33 | AD, AR | (Semina et al. 2001; Valleix et al. 2006) |
| FYCO1 | Fyve and coiled-coil domain containing 1 | 607182 | 3p21.31 | AR | (J. Chen et al. 2011) |
| GCNT2* | Glucosaminyl (n-acetyl) transferase 2, i-branching enzyme | 600429 | 6p24.3p24.2 | AR | (L. C. Yu et al. 2001) |
| GJA3 | Gap junction protein, alpha-3 | 121015 | 13q12.11 | AD | (D. Mackay et al. 1999) |
| GJA8 | Gap junction protein, alpha-8 | 600897 | 1q21.2 | AD, AR | (Ponnam et al. 2007; Shiels et al. 1998) |
| HSF4 | Heat-shock transcription factor 4 | 602438 | 16q22.1 | AD, AR | (Bu et al. 2002; Smaoui et al. 2004) |
| LEMD2 | Lem domain-containing protein 2 | 616312 | 6p21.31 | AR | (Boone et al. 2015) |
| LIM2 | Lens intrinsic membrane protein 2, 19-kd | 154045 | 19q13.41 | AR | (Pras et al. 2002) |
| LSS | Lanosterol synthase | 600909 | 21q22.3 | AR | (Zhao et al. 2015) |
| MAF* | V-MAF avian musculoaponeurotic fibrosarcoma oncogene homolog | 177075 | 16q23.2 | AD | (Jamieson et al. 2002) |
| MIP | Major intrinsic protein of lens fiber | 154050 | 12q13.3 | AD, AR | (Berry et al. 2000; J. Chen et al. 2017) |
| PITX3 | Paired-like homeodomain transcription factor 3 | 602669 | 10q24.32 | AD, AR | (Aldahmesh et al. 2011; Semina et al. 1998) |
| RRAGA | Ras-related gtp-binding protein a; | 612194 | 9p22.1 | AD | (J. H. Chen et al. 2016) |
| SIPA1L3 | Sipa1-like protein 3 | 616655 | 19q13.1 | AR, AD? | (Evers et al. 2015; Greenlees et al. 2015) |
| SLC16A12 | Solute carrier family 16 (monocarboxylic acid transporter), member 12 | 611910 | 10q23.31 | AD | (Kloeckener-Gruissem et al. 2008) |
| TDRD7* | Tudor domain-containing protein 7 | 611258 | 9q22.33 | AR | (Lachke et al. 2011) |
| TRPM3 | Transient receptor potential cation channel, subfamily m, member 3 | 608961 | 9q21.12q21.13 | AD | (Bennett et al. 2014) |
| UNC45B | Unc45, c. elegans, homolog of, b | 611220 | 17q12 | AD | (Hansen et al. 2014) |
| VIM | Vimentin | 193060 | 10p13 | AD | (Muller et al. 2009) |
| B. Genes associated with syndromic/panocular conditions with occasional reports of isolated pediatric cataract | |||||
| AGK | Acylglycerol kinase | 610345 | 7q34 | AR | (Aldahmesh, Khan, Mohamed, Alghamdi et al. 2012) |
| COL4A1 | Collagen, type IV, alpha-1 | 120130 | 13q34 | AD | (Coupry et al. 2010) |
| COL4A2 | Collagen, type IV, alpha-2 | 120090 | 13q34 | AD | (Ha et al. 2016) |
| CTDP1 | C-terminal domain of rna polymerase ii subunit a, phosphatase of, subunit 1 | 604927 | 18q23 | AR | (Tzifi et al. 2011) |
| CYP27A1 | Cytochrome p450, subfamily XXVIIa, polypeptide 1 | 606530 | 2q35 | AR | (A. O. Khan et al. 2015) |
| EYA1 | Eyes absent 1 | 601653 | 8q13.3 | AD | (Azuma et al. 2000) |
| FTL | Ferritin light chain | 134790 | 19q13.33 | AD | (Beaumont et al. 1995) |
| GALK1 | Galactokinase 1 | 604313 | 17q25.1 | AR | (Stambolian et al. 1995) |
| LONP1 | lon peptidase 1, mitochondrial | 605490 | 19p13.3 | AR | (A. O. Khan et al. 2015) |
| NF2 | Neurofibromin 2 | 607379 | 22q12.2 | AD | (Ragge et al. 1997) |
| NHS | NHS gene | 300457 | Xp22.2 | XL | (W. Sun et al. 2014) |
| PAX6 | Paired box gene 6 | 607108 | 11p13 | AD | (Ma et al. 2016) |
| PEX7 | Rhizomelic chondrodysplasia punctate, type 1/Adult Refsum Disease | 215100 | 6q23.3 | AR | (Braverman et al. 2002) |
| PEX11B | Peroxisome biogenesis factor 11b | 603867 | 1q21.1 | AR | (Taylor et al. 2017) |
| SIL1 | sil1, S. cerevisiae, homolog of | 608005 | 5q31.2 | AR | (Krieger et al. 2013) |
| SLC40A1 | Solute carrier family 40 (iron-regulated transporter), member 1 | 604653 | 2q32.2 | AD | (Yamakawa et al. 2016) |
| WFS1 | WFS1 gene | 606201 | 4p16.1 | AD | (Berry et al. 2013) |
Non-ocular anomalies present in some cases
An additional 17 genes that typically result in syndromic cataract or broader developmental ocular anomalies have been occasionally reported to cause isolated pediatric cataracts or may have isolated pediatric cataract as the first clinical condition diagnosed with additional features identifiable only through specific testing or developing with time (Table 1B). It is important for ophthalmologists to be aware of syndromic conditions which may initially present as isolated pediatric cataract since correct diagnosis will allow for prompt management of possible additional features. Two conditions deserve special attention as there are specific and effective treatments available. Cerebrotendinous xanthomatosis, caused by mutation in CYP27A1, is a disorder which affects the metabolism of cholesterol and bile acid and leads to progressive clinical features including cataract, diarrhea, cognitive/neurological impairment, and xanthomas. Treatment with oral chenodeoxycholic acid (CDCA) therapy improves the syndromic features of the condition, but is most effective if begun early, before significant neurological disease is present. Because early-onset cataract is one of the most specific and consistent early signs of this condition, it should be considered in the differential for pediatric cataract, especially in a patient with diarrhea (Duell et al. 2018). Galactokinase deficiency, caused by recessive mutations in the GALK1 gene, results in cataracts which are generally reversable (or preventable) with initiation of a lactose-free, galactose-restricted diet (Hennermann et al. 2011). While some regions include galactokinase deficiency in newborn screening panels, this is not universal and thus cannot be assumed to be ruled out in all children with pediatric cataract.
The proportion of cataracts that can be explained by currently known genes has varied between studies. Whole exome sequencing (WES) of hereditary pediatric cataracts identified causative mutations in 43–50% of cases in two studies (Reis et al. 2013; W. Sun et al. 2014) while next generation sequencing panels of cataract genes have indicated success rates of 63–75% (Gillespie et al. 2014; Ma et al. 2016; Zhai et al. 2017); it is not clear whether this discrepancy reflects a true increase in detection rates for panels over WES or variable criteria for pathogenicity and/or population differences. Additional possible loci for isolated pediatric cataracts have been identified through linkage studies or chromosomal rearrangements and are still awaiting gene discovery (Shiels et al. 2010).
Variable inheritance patterns within genes causing pediatric cataracts
The presence of both dominant and recessive alleles in the same gene is observed in a high proportion of genes associated with isolated pediatric cataracts- 13/35 (37%). Genes from each of the four major gene families (Transcription factors, Crystallins, Cytoskeletal proteins, and Membrane proteins) have been reported to be associated with both dominant and recessive inheritance and specific inheritance/allele types are compared below for each family with a focus on transcription factors as this group demonstrates more distinguishable patterns. Interestingly, C-terminal extension alleles in genes from different families (FOXE3, PITX3, CRYBB1, CRYBB3, and EPHA2), are universally reported to cause dominant cataracts.
• Transcription factors
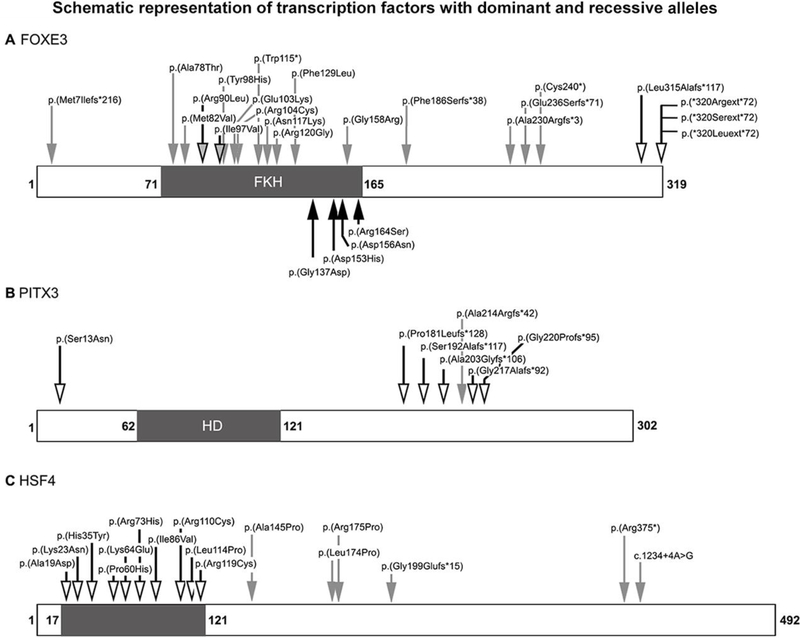
FOXE3 was one of the first ocular genes to show both dominant and recessive mutations (Table 2; Figure 2A). Heterozygous dominant mutations resulting in an erroneous protein extension have been identified in families affected with congenital cataract with or without anterior segment dysgenesis (ASD) (Bremond-Gignac et al. 2010; Doucette et al. 2011; Iseri et al. 2009; Semina et al. 2001). To date, four different extension mutations have been identified; three affect the stop codon directly and the fourth occurs four amino acids before the stop codon but results in a slightly longer erroneous extension. These alleles are clearly deleterious and not present in control populations. The contribution of heterozygous FOXE3 missense mutations to congenital cataract/ASD is less clear. Four heterozygous missense mutations have been reported as possibly associated with ocular disease. Two rare alleles, c.269G>T, p.(Arg90Leu) and c.289A>G, p.(Ile97Val), were initially reported as disease-causing in patients with Peters anomaly or congenital cataract (Gillespie et al. 2014; Ormestad et al. 2002) but were subsequently identified in heterozygote state in unaffected individuals (Islam et al. 2015; Plaisancie et al. 2017; Ullah et al. 2016). The other two heterozygous alleles noted in some cases with ocular disease appear to be population- specific variants of uncertain significance. The first, c.146G>C, p.(Gly49Ala), has a 4.3% allele frequency in African populations (including 5 homozygotes) in gnomAD (Lek et al. 2016) and is predicted benign by 4/5 in silico programs. The second, c.601G>A, p.(Val201Met), is seen in 4% of Hispanic alleles in gnomAD (including 6 homozygotes) and is similarly predicted benign by 4/5 programs. Both alleles have been reported to show possible increased frequency in cases affected with ocular disorders, but their significance is unclear (Reis et al 2010, Garcia-Montalvo et al 2014; Iseri et al. 2009). Recessive mutations in FOXE3 typically result in microphthalmia, aphakia, and/or sclerocornea with glaucoma in many cases (Valleix et al. 2006). Seventeen different recessive mutations have been reported to date, including six truncating and eleven missense mutations (The Human Gene Mutation Database (HGMD) at http://www.hgmd.cf.ac.uk, Stenson et al. 2014; Plaisancie et al. 2017); all pathogenic recessive variants are very rare (allele frequency <0.02%) and predicted damaging by in silico programs (Table 2). Functional analysis of several recessive alleles (truncating and missense) and two dominant extension alleles showed reduced transactivation function for all mutants, loss of DNA-binding for three of the four recessive mutations (with the exception of p.(Arg90Leu) also reported as dominant, see above), and normal DNA binding but abnormal migration pattern for dominant extension alleles (Islam et al. 2015); this data suggests that while loss-of-function is the most likely disease mechanism for recessive alleles, dominant extension mutations may result in a yet undiscovered gain of function.
Table 2.
Summary of disease-associated alleles in FOXE3.
| Mode | DNA effecta | Protein effect | Phenotype | Functional prediction | gnomAD frequency | Reference |
|---|---|---|---|---|---|---|
| REC | c.21_24delGG AT | p.(Met7Ilefs*216) | Microphthalmia, aphakia, sclerocornea, ASD | Premature truncation | 1/65338 (0.002%) | (Iseri et al. 2009) |
| VUS | c.146G>C | p.(Gly49Ala) | Microphthalmia, coloboma | Damaging by 1/5 (F) | 361/26504 (1.4%); 355/8250 African (4.3%); 5 homozygotes |
(Iseri et al. 2009) |
| REC | c.232G>A | p.(Ala78Thr) | Microphthalmia, sclerocornea | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Plaisancie et al. 2017) |
| REC | c.244A>G | p.(Met82Val) | Microphthalmia, sclerocornea, aphakia, ASD | Damaging by 5/5 (S, PP, F, MA, MT) | 24/259912 (0.009%) | (Iseri et al. 2009) |
| DOMb REC | c.269G>T | p.(Arg90Leu) | Peters anomaly Aphakia, corneal opacity | Damaging by 5/5 (S, PP, F, MA, MT) | 24/268362 (0.009%) | (Ormestad et al. 2002) (Islam et al. 2015) |
| DOMb REC | c.289A>G | p.(Ile97Val) | Cataract, syndromic Microphthalmia, corneal opacity | Damaging by 5/5 (S, PP, F, MA, MT) | 5/243212 (0.002%) | (Gillespie et al. 2014; Ullah et al. 2016) (Ullah et al. 2016) |
| REC | c.292T>C | p.(Tyr98His) | Sclerocornea, non-syndromic, bilateral, total | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Ali et al. 2010) |
| REC | c.307G>A | p.(Glu103Lys) | Congenital cataract | Damaging by 4/5 (S, PP, F, MT) | 1/29030 (0.003%) | (S. Y. Khan et al. 2016) |
| REC | c.310C>T | p.(Arg104Cys) | Microphthalmia, sclerocornea | Damaging by 5/5 (S, PP, F, MA, MT) | 1/244576 (0.0004%) | (Plaisancie et al. 2017) |
| REC | c.345G>A | p.(Trp115*) | Microphthalmia, sclerocornea | Premature truncation | NP | (Plaisancie et al. 2017) |
| REC | c.351C>G | p.(Asn117Lys) | Congenital cataract | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (S. Y. Khan et al. 2016) |
| REC | c.358C>G | p.(Arg120Gly) | Aphakia, corneal opacity | Damaging by 5/5 (S, PP, F, MA, MT) | 1/245968 (0.0004%) | (Islam et al. 2015) |
| REC | c.387C>G | p.(Phe129Leu) | Abnormality of the eye | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Retterer et al. 2016) |
| DOM | c.410G>A | p.(Gly137Asp) | Thoracic aortic aneurysms and dissections | Damaging by 4/5 (S, PP, F, MT) | 6/244140 (0.002%) | (Kuang et al. 2016) |
| DOM | c.457G>C | p.(Asp153His) | Thoracic aortic aneurysms and dissections | Damaging by 4/5 (S, PP, F, MT) | 1/29224 (0.003%) | (Kuang et al. 2016) |
| DOM | c.466G>A | p.(Asp156Asn) | Thoracic aortic aneurysms and dissections | Damaging by 5/5 (S, PP, F, MA, MT) | 76/262358 (0.03%) | (Kuang et al. 2016) |
| REC | c.472G>C | p.(Gly158Arg) | Microphthalmia, aphakia, staphyloma malformation | Damaging by 5/5 (S, PP, F, MA, MT) | 1/29234 (0.003%) | (Saboo et al. 2017) |
| DOM | c.490C>A | p.(Arg164Ser) | Thoracic aortic aneurysms and dissections | Damaging by 4/5 (PP, F, MA, MT) | NP | (Kuang et al. 2016) |
| REC | c.557delT | p.(Phe186Serfs*38) | Microphthalmia, aphakia, sclerocornea | Premature truncation | NP | (Reis et al. 2010) |
| VUS | c.601G>A | p.(Val201Met) | Possible risk factor for eye malformations | Damaging by 1/5 (F) | 272/86326 (0.3%); 236/5842 Hispanic (4.0%); 7 homozygotes | (Garcia-Montalvo et al. 2014) |
| REC | c.679_686dup | p.(Ala230Argfs*3) | Microphthalmia, sclerocornea | Premature truncation | NP | (Chassaing et al. 2014) |
| REC | c.705delC | p.(Glu236Serfs*71) | Microphthalmia, aphakia, sclerocornea | Premature truncation | NP | (Reis et al. 2010) |
| REC | c.720C>A | p.(Cys240*) | Microphthalmia, aphakia, sclerocornea | Premature truncation | 7/42902 (0.02%) | (Valleix et al. 2006) |
| DOM | c.942dupG | p.(Leu315Alafs*117) | Anterior segment ocular dysgenesis and cataracts | Erroneous extension | NP | (Semina et al. 2001) |
| DOM | c.958T>C | p.(*320Argext*72) | Cataract, ASD, unilateral micro | Erroneous extension | NP | (Iseri et al. 2009) |
| DOM | c.959G>C | p.(*320Serext*72) | Cataract, congenital | Erroneous extension | NP | (Bremond-Gignac et al. 2010) |
| DOM | c.959G>T | p.(*320Leuext*72) | Anterior segment dysgenesis | Erroneous extension | NP | (Doucette et al. 2011) |
Reference sequence: NM_012186.2; Non-ocular anomalies indicated in italics
Heterozygous alleles also reported in unaffected individuals
DOM, Dominant; REC, Recessive; VUS, heterozygous variant of uncertain significance reported as causative/associated in at least one paper; PP, Polyphen2; S, SIFT; F, FATHMM; MA, Mutation Assessor; MT, Mutation Taster; NP, Not Present
Figure 2: Schematic representation of transcription factors with dominant and recessive alleles.
Schematic representation of the FOXE3 (A), PITX3 (B), and HSF4 (C) proteins showing the pathogenic alleles reported in the literature and recorded in Tables 2–4. Dominant alleles are indicated with arrows with a bold outline and an empty-head, recessive alleles are marked with grey arrows without an outline, and alleles reported as both dominant and recessive are shown with grey-head arrows with a bold outline. Alleles associated with ocular phenotypes are indicated on the top, while Thoracic Aortic Aneurysms and Dissections (TAAD) alleles (for FOXE3) are marked on the bottom with black arrows.
PITX3 shows a similar pattern of dominant mutations associated with cataract/ASD and homozygous mutations associated with microphthalmia with corneal opacity (Table 3; Figure 2B). The majority of reported cases are dominant mutations resulting in congenital cataract with or without ASD, including one early missense and five frameshift mutations which all result in truncation of the normal protein sequence and addition of a long erroneous protein tail that leads to an extension of the total protein length by 5–11 amino acids (to 307–313 amino acids). The most commonly seen pathogenic allele is a recurrent 17-bp duplication reported in 12 families (Reis and Semina 2011; Verdin et al. 2014). Two families with homozygous mutations have been reported. The first, c.650delG, caused congenital cataract in heterozygous individuals and microphthalmia, corneal opacity, and neurological impairment in homozygous family members (Bidinost et al. 2006). The second, c.640_656del17, which deletes the same 17-bp involved in the recurrent duplication, was reported to cause microphthalmia with sclerocornea in homozygous individuals while heterozygotes were unaffected (Aldahmesh et al. 2011). It is interesting to note that while all of the frameshift mutations truncate the normal protein sequence within the C-terminal region, the 17-bp deletion is the only allele with recessive inheritance and also the only one which results in a shorter final protein product and not its extension (Figure 2A). Functional analysis of three dominant alleles, the recurrent 17-bp deletion, a 1-bp deletion (c.573delC), and the missense allele, showed significantly decreased DNA-binding and transactivation activity for the two proteins with altered protein length and mildly decreased binding/transactivation for the missense mutant protein (Sakazume et al. 2007; Verdin et al. 2014).
Table 3.
Summary of disease-associated alleles in PITX3.
| Mode | DNA effecta | Protein effect | Phenotype | Functional prediction | gnomAD frequency | Reference |
|---|---|---|---|---|---|---|
| DOM | c.38G>A | p.(Ser13Asn) | Cataract | Damaging by 3/5 (PP, MT, F) | 1/22,5438 (0.0004%) | (Semina et al. 1998) |
| DOM | c.542delC | p.(Pro181Leufs*128) | Cataract | Truncation of normal protein and erroneous protein tail resulting in extension (5 aa) | NP | (Berry et al. 2011) |
| DOM | c.573delC | p.(Ser192Alafs*117) | Cataract, Anterior segment dysgenesis | Truncation of normal protein and erroneous protein tail resulting in extension (5 aa) | NP | (Verdin et al. 2014) |
| DOM | c.608delC | p.(Ala203Glyfs*106) | Cataract | Truncation of normal protein and erroneous protein tail resulting in extension (5 aa) | NP | (H. Liu et al. 2017) |
| REC | c.640_656del17 | p.(Ala214Argfs*42) | Microphthalmia, sclerocornea | Protein truncation | 8/104,350 (0.008%) | (Aldahmesh et al. 2011) |
| DOMb | c.650delG | p.(Gly217Alafs*92) | Cataract bMicrophthalmia, corneal opacity, and neurological impairment | Truncation of normal protein and erroneous protein tail resulting in extension (5 aa) | NP | (Berry et al. 2004; Bidinost et al. 2006) |
| DOMc | c.640_656dup17 | p.(Gly220Profs*95) | Cataract, Anterior segment dysgenesis | Truncation of normal protein and erroneous protein tail resulting in extension (11 aa) | NP | (Semina et al. 1998) |
Reference sequence: NM_005029.3 (please note that some variants were renamed from the original publication to match their location on this transcript)
Homozygous mutations resulted in more severe phenotype in children of affected parents
Recurrent mutation seen in 12 families to date
DOM, Dominant; REC, Recessive; PP, Polyphen2; S, SIFT; F, FATHMM; MA, Mutation Assessor; MT, Mutation Taster; NP, Not Present
Both FOXE3 and PITX3 have also been reported to play a role in non-ocular disease. Several rare damaging heterozygous missense variants in the forkhead domain of FOXE3 were recently linked with thoracic aortic aneurysms and dissections (TAAD) in several families (Kuang et al. 2016); no ocular defects were reported in the TAAD families and, likewise, the cardiac phenotype has not been reported in patients with FOXE3 ocular phenotypes. The TAAD damaging missense mutations all occur within the second half of the forkhead domain between amino acids 137–164 (Kuang et al. 2016) whereas the ocular disease-associated missense alleles typically occur in the earlier in the forkhead domain (Figure 2A). The one exception to this pattern is a recessive missense mutation, c.472G>C, p.(Gly158Arg), reported to cause ocular disease; no cardiac phenotype is reported (Saboo et al. 2017). With regards to PITX3, a possible association with features of Parkinson’s disease has been noted by several studies. A heterozygous ~310 kb deletion of PITX3 (and 7 other genes) was identified in a patient with behavioral and neurological anomalies resembling Smith-Magenis syndrome and decreased L-DOPA in cerebrospinal fluid (but no ocular anomalies) (Derwinska et al. 2012). Several studies published association of polymorphisms in PITX3 with increased risk of Parkinson’s disease but a meta-analysis did not support this association other than a possible link with early-onset Parkinson’s disease in Caucasians (Jimenez-Jimenez et al. 2014). Additionally, a recent population study suggested a higher rate of dementia in patients with PITX3 polymorphism and Parkinson’s disease (Backstrom et al. 2017).
HSF4, another transcription factor, is also associated with both dominant and recessive inheritance (Table 4; Figure 2C), but in this case both types of alleles result in isolated congenital cataract. Ten missense mutations within the first 120 amino acids have been reported in families with dominant cataracts; functional analysis of five of these variants demonstrated inhibition of DNA binding for all (Enoki et al. 2010). A heterozygous splicing variant upstream of the coding region was also reported in a small Chinese family with congenital cataract (Zhai et al. 2017), but seems likely to be a polymorphism based on the 0.4% East Asian carrier frequency in gnomAD. Three missense mutations later in the gene, within the hydrophobic repeat domain (aa 129–203) (The UniProt Consortium 2017), and three truncating mutations (one frameshift, one nonsense, and one splicing in the final intron) were reported to cause recessive cataracts; functional assessment of the truncating alleles demonstrated a loss of function for all three (Merath et al. 2013).
Table 4:
Summary of disease-associated alleles in HSF4
| Mode | DNA effecta | Protein effect | Phenotype | Functional prediction | gnomAD frequency | Reference |
|---|---|---|---|---|---|---|
| DOM | c.56C>A | p.(Ala19Asp) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Bu et al. 2002) |
| DOM | c.69G>T | p.(Lys23Asn) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Lv et al. 2014) |
| DOM | c.103C>T | p.(His35Tyr) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | 2/222498 (0.0009%) | (Gillespie et al. 2014) |
| DOMb | c.179C>A | p.(Pro60His) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | 1/ 245860 (0.0004%) | (Li et al. 2016) |
| DOM | c.190A>G | p.(Lys64Glu) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Berry et al. 2018) |
| DOM | c.218G>A | p.(Arg73His) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Ke et al. 2006) |
| DOM | c.256A>G | p.(Ile86Val) | Cataract | Damaging by 2/5 (F, MT) | NP | (Bu et al. 2002) |
| DOM | c.328C>T | p.(Arg110Cys) | Cataract | Damaging by 2/5 (F, MT) | NP | (L. Liu et al. 2015) |
| DOM | c.341T>C | p.(Leu114Pro) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Bu et al. 2002) |
| DOM | c.355C>T | p.(Arg119Cys) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Bu et al. 2002) |
| REC | c.433G>C | p.(Ala145Pro) | Cataract | Damaging by 3/5 (PP, F, MT) | NP | (J. Chen et al. 2017) |
| REC | c.521T>C | p.(Leu174Pro) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Behnam et al. 2016) |
| REC | c.524G>C | p.(Arg175Pro) | Cataract | Damaging by 5/5 (S, PP, F, MA, MT) | NP | (Forshew et al. 2005) |
| REC | c.596_600del GGCCG | p.(Gly199Glufs*15) | Cataract | Protein truncation | NP | (Forshew et al. 2005) |
| REC | c.1123C>T | p.(Arg375*) | Cataract | Protein truncation | 1/245696 (0.004%) | (Sajjad et al. 2008) |
| REC | c.1234+4A>G | Splicing disruption | Cataract | Exon skipping and protein truncation | 3/273010 (0.001%) | (Smaoui et al. 2004) |
Reference sequence: NM_001538.3 (please note that some variants were renamed from the original publication to match their location on this transcript)
Patient also has a heterozygous CYRGC nonsense allele, so the significance of this allele is uncertain
DOM, Dominant; REC, Recessive; PP, Polyphen2; S, SIFT; F, FATHMM; MA, Mutation Assessor; MT, Mutation Taster; NP, Not Present
• Crystallin genes
Dominant inheritance is reported for all 12 crystallin genes and recessive alleles have also been identified in five crystallins to date: CRYAA, CRYAB, CRYBA1, CRYBB1, and CRYBB3 (Table 1). As noted above, C-terminal extension mutations are always associated with dominant disease (CRYBB1 and CRYBB3).
As previously shown for other genes, nonsense mediated decay (NMD) seems likely to play a role in determining whether mutant alleles result in recessive or dominant effects (Khajavi et al. 2006). For several crystallins, truncating alleles occurring early in the gene and predicted to be subject to NMD are associated with recessive disease while mutations in the final exon (or late in the penultimate exon), which are predicted to escape NMD, are linked with dominant disease. For example, early truncating mutations in CRYAA and CRYBB1 are associated with recessive disease while truncating mutations in the final exon of CRYBB1 cause dominant cataracts (no truncating mutations have been reported in the final exon CRYAA). Similarly, nonsense mutations in the final exon of CRYGD and CRYBB2 are associated with dominant disease while an earlier truncating allele in CRYGD, c.51T>G, p.(Tyr17*), was shown not to co-segregate with a dominant cataract phenotype in one family (Reis et al. 2014). This pattern is not consistent among all crystallins, however; truncating mutations in both the final exon as well as exon 2 (of 3 exons) of CRYGC are associated with dominant inheritance and CRYBA1 shows the opposite pattern with truncating and splicing mutations throughout the gene associated with dominant disease while a missense and possible in-frame deletion are associated with recessive cataracts (HGMD; (Stenson et al. 2014)).
The effect of missense mutations in crystallin genes is more difficult to ascertain. For some genes, such as CRYBB1, CRYBA2, CRYBA4, and CRYGS missense mutations have only been associated with dominant disease. For others, like CRYAA, CRYAB, and CRYBB3, recessive and dominant missense mutations have been reported in adjacent amino acids with no clear genotype-phenotype correlations (HGMD;(Stenson et al. 2014)).
Out of all crystallins, CRYAB is probably the most unusual as it can cause isolated congenital cataract, isolated skeletal and/or cardiac myopathy, or both. Mutations associated with myofibrillar myopathy and/or dilated cardiomyopathy consist of both truncating and missense alleles, seem to cluster in the central conserved α-crystallin domain and C-terminal region, and can be either dominant or recessive, with the recessive condition being lethal in infancy (Dimauro et al. 2017). Mutations associated with pediatric cataract are primarily missense alleles; while the majority of cataract mutations are within the N-terminal region, several, including one frameshift allele, occur in the α-crystallin domain and C-terminal region (HGMD;(Stenson et al. 2014)). Both recessive and dominant mutations are noted, including missense mutations in identical or adjacent amino acids which show differing inheritance patterns; a c.32G>A, p.(Arg11His) mutation co-segregated with dominant cataracts in 6 members of a family affected with congenital nuclear cataract while c.31C>T, p.(Arg11Cys) and c.34C>T, p.(Arg12Cys) mutations segregated with recessive congenital cataract in two consanguineous families (Q. Chen et al. 2009; Jiao et al. 2015).
• Cytoskeletal proteins
BFSP1 and BFSP2 are components of the beaded filament, an important component of the lens cytoskeleton (Alizadeh et al. 2003). Dominant and recessive mutations resulting in congenital cataract have been reported for both genes. For BFSP1, there is no clear distinction between mutations types; while most truncating mutations, including in the final exon, are recessive, a splicing mutation in the final intron is reported to be dominant and both dominant and recessive missense mutations are reported. For BFSP2, a clearer pattern is emerging with missense/in-frame mutations associated with dominant disease and truncating alleles with recessive (HGMD;(Stenson et al. 2014)).
• Membrane proteins
Three membrane proteins associated with congenital cataract also show both dominant and recessive mutations. For EPHA2, extending mutations are always dominant and truncating mutations have been recessive; missense mutations can be either dominant or recessive with no discernable pattern. For GJA8, the majority of mutations are dominant missense mutations but several recessive truncating mutations as well as one recessive missense mutation were also reported. Similarly, the majority of mutations in MIP are dominant missense or truncating mutations; one recessive homozygous missense mutation was reported (HGMD;(Stenson et al. 2014)).
Novel cataract genes, new pathways
In the last 3 years, use of whole exome sequencing with or without prior linkage analysis identified several new cataract genes with possible roles in the PI3K/AKT/mTOR signaling, cholesterol biosynthesis or other pathways.
Dominant missense or splicing mutations in RagA GTPase (RRAGA) were identified in three unrelated families with dominant congenital or juvenile cataract (J. H. Chen et al. 2016). Rag (Ras-related GTPase) GTPases are believed to facilitate the re-localization of mTORC1 to the peri-nuclear region and/or large vesicular structures of the cell. The cellular localization of mTORC1 appears to be critical to its activation in the presence of amino acids. Consistent with this, RRAGA mutations were shown to disrupt mTORC1 signaling (J. H. Chen et al. 2016).
A homozygous missense mutation in LEMD2 co-segregated in a large family affected with juvenile Hutterite cataract (initially identified in the Hutterites of North America nearly 30 years ago) and, in some individuals, sudden cardiac death. LEMD2 is a member of the LEM domain-containing proteins which localize to the inner nuclear membrane and function in nuclear structure organization, cell signaling and differentiation. LEMD2 has been shown to directly interact with A type lamins and help to anchor the lamina polymer to the inner nuclear membrane. Also, in mouse and cell culture models, loss of Lemd2 led to the activation of Akt as well as Erk1/2 and other MAP kinases, with embryonic lethality in homozygous animals. Human LEMD2 and mouse Lemd2 genes have been shown to be expressed in the developing and postnatal lenses (Boone et al. 2015).
Linkage analysis followed by whole exome sequencing in a family with siblings affected with isolated congenital dense white cataracts identified nonsense mutations in Signal-Induced Proliferation-Associated 1 Like 3, SIPA1L3 (Evers et al. 2015). The SIPA1L3 protein contains several domains, including RapGAP, PDZ, two actin-binding domains, and a coiled-coil leucine zipper; the protein was shown to mediate cell adhesion/polarity and organization of the cytoskeleton. SIPA1L3 had been identified as a candidate for cataracts through comparison of genes enriched in the lens from the iSyTE project with previously mapped cataract loci (Lachke et al. 2012), however the previously linked family showed dominant inheritance. Concurrently, heterozygous disruption of SIPA1L3 was identified in two families with congenital cataract including a patient with a de novo translocation disrupting SIPA1L3 and additional ocular anomalies and a second patient with a missense variant in SIPA1L3 (Greenlees et al. 2015). Further work in mice and zebrafish confirmed the importance of SIPA1L3 in ocular development and lens formation (Greenlees et al. 2015; Walker et al. 2017).
Recessive missense mutations in the LSS gene encoding lanosterol synthase, an important enzyme in the cholesterol biosynthesis pathway, which is expressed in the lens, were identified in two families with isolated congenital cataract and in one family with additional syndromic features of small penis, baldness and absence of eyebrows (X. Chen and Liu 2017; Zhao et al. 2015). Interestingly, treatment of naturally occurring cataracts in rabbit lenses (in vitro) and dogs (in vivo) with lanosterol showed reduction of cataract severity and increase in lens clarity in both (Zhao et al. 2015), suggesting that while identifying new genes responsible for congenital cataract is likely to diagnose only a small number of new cases due to the extreme genetic heterogeneity, this identification may lead to new treatment options for a broader range of cataract patients. Consistent with this, a recent study used this work with lanosterol to guide their identification of pharmacological chaperones and found a novel sterol which was effective in reducing cataract in lenses with both specific crystallin mutations as well as age related cataract (Makley et al. 2015).
Additional candidate genes identified through whole exome sequencing of non-syndromic pediatric cataract cases include recessive genes TAPT1, WDR87, MFSD6L, AKR1E2, RNLS, and CYP51A1 and dominant EZR (Aldahmesh, Khan, Mohamed, Hijazi et al. 2012; Patel et al. 2016; Zhai et al. 2017).
Concluding remarks and future directions
The genetics of isolated pediatric cataract is marked by extreme heterogeneity and frequently variable inheritance patterns for alleles within the same gene. This relatively rare genetic phenomenon complicates interpretation of sequencing results in dominant disease as it is not always clear whether a heterozygous variant in a proband represents a disease-causing mutation or carrier status for recessive disease. While location or functional data can distinguish between recessive and dominant alleles in some genes, for many, especially missense mutations, inheritance cannot yet be predicted. Further research will be needed to determine whether some of the reported mutations may be rare benign variants that co-segregated by chance in small families, whether the variability of inheritance patterns is related to differing conformational/functional effects of the specific missense mutations (ie. dominant negative vs loss of function), or whether other genetic and/or environmental factors are contributing to cataract formation and influencing whether the mutation appears dominant or recessive. Despite the significant number of new genes identified in the past 10 years with the application of whole exome sequencing, a large number of hereditary cataract cases/loci remain genetically unexplained (Reis et al. 2013; Shiels and Hejtmancik 2017). Further study of candidate genes and pathways identified through animal models of cataracts (Zeiss 2013), databases such as iSyte which analyze the expression of various genes at different stages of lens development (Kakrana et al. 2018), and genetic analysis of human patients, including whole exome sequencing, whole genomic sequencing, and microRNA analysis (X. Yu et al. 2017), is likely to continue to identify new factors involved in hereditary pediatric cataract. These studies will explain additional pediatric cataract cases and extend/connect pathways involved in lens development.
Footnotes
Conflict of interest: Authors report no conflict of interest.
References
- Aldahmesh MA, Khan AO, Mohamed J, Alkuraya FS (2011) Novel recessive BFSP2 and PITX3 mutations: Insights into mutational mechanisms from consanguineous populations. Genet Med 13:978–981. doi: 10.1097/GIM.0b013e31822623d5 [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, Khan AO, Mohamed JY, Alghamdi MH, Alkuraya FS (2012) Identification of a truncation mutation of acylglycerol kinase (AGK) gene in a novel autosomal recessive cataract locus. Hum Mutat 33:960–962. doi: 10.1002/humu.22071 [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, Khan AO, Mohamed JY, Hijazi H, Al-Owain M, Alswaid A, Alkuraya FS (2012) Genomic analysis of pediatric cataract in Saudi Arabia reveals novel candidate disease genes. Genet Med doi: 10.1038/gim.2012.86 [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, Alshammari MJ, Khan AO, Mohamed JY, Alhabib FA, Alkuraya FS (2013) The syndrome of microcornea, myopic chorioretinal atrophy, and telecanthus (MMCAT) is caused by mutations in ADAMTS18. Hum Mutat 34:1195–1199. 10.1002/humu.22374 [DOI] [PubMed] [Google Scholar]
- Al-Dosari MS, Almazyad M, Al-Ebdi L, Mohamed JY, Al-Dahmash S, Al-Dhibi H, Al-Kahtani E, Al-Turkmani S, Alkuraya H, Hall BD, Alkuraya FS (2010) Ocular manifestations of branchio-oculo-facial syndrome: report of a novel mutation and review of the literature. Mol Vis 16:813–818. [PMC free article] [PubMed] [Google Scholar]
- AlFadhli S, Abdelmoaty S, Al-Hajeri A, Behbehani A, Alkuraya F (2012) Novel crystallin gamma B mutations in a Kuwaiti family with autosomal dominant congenital cataracts reveal genetic and clinical heterogeneity. Mol Vis 18:2931–2936. [PMC free article] [PubMed] [Google Scholar]
- Ali M, Buentello-Volante B, McKibbin M, Rocha-Medina JA, Fernandez-Fuentes N, Koga-Nakamura W, Ashiq A, Khan K, Booth AP, Williams G, Raashid Y, Jafri H, Rice A, Inglehearn CF, Zenteno JC (2010) Homozygous FOXE3 mutations cause non-syndromic, bilateral, total sclerocornea, aphakia, microphthalmia and optic disc coloboma. Mol Vis 16:1162–1168. [PMC free article] [PubMed] [Google Scholar]
- Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG (2003) Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Invest Ophthalmol Vis Sci 44:5252–5258. [DOI] [PubMed] [Google Scholar]
- Anand D, Agrawal SA, Slavotinek A, Lachke SA (2018) Mutation update of transcription factor genes FOXE3, HSF4, MAF, and PITX3 causing cataracts and other developmental ocular defects. Hum Mutat doi: 10.1002/humu.23395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M (2000) Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet 9:363–366. [DOI] [PubMed] [Google Scholar]
- Backstrom D, Domellof ME, Granasen G, Linder J, Mayans S, Elgh E, Mo SJ, Forsgren L (2017) PITX3 genotype and risk of dementia in Parkinson’s disease: A population-based study. J Neurol Sci 381:278–284. doi: 10.1016/j.jns.2017.08.3259 [DOI] [PubMed] [Google Scholar]
- Bassnett S, Shi Y, Vrensen GF (2011) Biological glass: structural determinants of eye lens transparency. Philos Trans R Soc Lond B Biol Sci 366:1250–1264. doi: 10.1098/rstb.2010.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont C, Leneuve P, Devaux I, Scoazec JY, Berthier M, Loiseau MN, Grandchamp B, Bonneau D (1995) Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat Genet 11:444–446. doi: 10.1038/ng1295-444 [DOI] [PubMed] [Google Scholar]
- Behnam M, Imagawa E, Chaleshtori AR, Ronasian F, Salehi M, Miyake N, Matsumoto N (2016) A novel homozygous mutation in HSF4 causing autosomal recessive congenital cataract. J Hum Genet 61:177–179. doi: 10.1038/jhg.2015.127 [DOI] [PubMed] [Google Scholar]
- Bennett TM, Mackay DS, Siegfried CJ, Shiels A (2014) Mutation of the melastatin-related cation channel, TRPM3, underlies inherited cataract and glaucoma. PLoS One 9:e104000. doi: 10.1371/journal.pone.0104000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S (2000) Missense mutations in MIP underlie autosomal dominant ‘polymorphic’ and lamellar cataracts linked to 12q. Nat Genet 25:15–17. doi: 10.1038/75538 [DOI] [PubMed] [Google Scholar]
- Berry V, Francis P, Reddy MA, Collyer D, Vithana E, MacKay I, Dawson G, Carey AH, Moore A, Bhattacharya SS, Quinlan RA (2001) Alpha-B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am J Hum Genet 69:1141–1145. doi: 10.1086/324158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry V, Francis PJ, Prescott Q, Waseem NH, Moore AT, Bhattacharya SS (2011) A novel 1-bp deletion in PITX3 causing congenital posterior polar cataract. Mol Vis 17:1249–1253. [PMC free article] [PubMed] [Google Scholar]
- Berry V, Gregory-Evans C, Emmett W, Waseem N, Raby J, Prescott D, Moore AT, Bhattacharya SS (2013) Wolfram gene (WFS1) mutation causes autosomal dominant congenital nuclear cataract in humans. Eur J Hum Genet 21:1356–1360. doi: 10.1038/ejhg.2013.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry V, Pontikos N, Moore A, Ionides ACW, Plagnol V, Cheetham ME, Michaelides M (2018) A novel missense mutation in HSF4 causes autosomal-dominant congenital lamellar cataract in a British family. Eye (Lond) 32:806–812. doi: 10.1038/eye.2017.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry V, Yang Z, Addison PK, Francis PJ, Ionides A, Karan G, Jiang L, Lin W, Hu J, Yang R, Moore A, Zhang K, Bhattacharya SS (2004) Recurrent 17 bp duplication in PITX3 is primarily associated with posterior polar cataract (CPP4). J Med Genet 41:e109. doi: 10.1136/jmg.2004.020289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidinost C, Matsumoto M, Chung D, Salem N, Zhang K, Stockton DW, Khoury A, Megarbane A, Bejjani BA, Traboulsi EI (2006) Heterozygous and homozygous mutations in PITX3 in a large Lebanese family with posterior polar cataracts and neurodevelopmental abnormalities. Invest Ophthalmol Vis Sci 47:1274–1280. doi: 10.1167/iovs.05-1095 [DOI] [PubMed] [Google Scholar]
- Billingsley G, Santhiya ST, Paterson AD, Ogata K, Wodak S, Hosseini SM, Manisastry SM, Vijayalakshmi P, Gopinath PM, Graw J, Heon E (2006) CRYBA4, a novel human cataract gene, is also involved in microphthalmia. Am J Hum Genet 79:702–709. doi: 10.1086/507712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokenkamp A, Ludwig M (2016) The oculocerebrorenal syndrome of Lowe: an update. Pediatr Nephrol 31:2201–2212. doi: 10.1007/s00467-016-3343-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone PM, Yuan B, Gu S, Ma Z, Gambin T, Gonzaga-Jauregui C, Jain M, Murdock TJ, White JJ, Jhangiani SN, Walker K, Wang Q, Muzny DM, Gibbs RA, Hejtmancik JF, Lupski JR, Posey JE, Lewis RA (2015) Hutterite-type cataract maps to chromosome 6p21.32-p21.31, cosegregates with a homozygous mutation in LEMD2, and is associated with sudden cardiac death. Mol Genet Genomic Med 4:77–94. doi: 10.1002/mgg3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman N, Chen L, Lin P, Obie C, Steel G, Douglas P, Chakraborty PK, Clarke JT, Boneh A, Moser A, Moser H, Valle D (2002) Mutation analysis of PEX7 in 60 probands with rhizomelic chondrodysplasia punctata and functional correlations of genotype with phenotype. Hum Mutat 20:284–297. doi: 10.1002/humu.10124 [DOI] [PubMed] [Google Scholar]
- Bremond-Gignac D, Bitoun P, Reis LM, Copin H, Murray JC, Semina EV (2010) Identification of dominant FOXE3 and PAX6 mutations in patients with congenital cataract and aniridia. Mol Vis 16:1705–1711. [PMC free article] [PubMed] [Google Scholar]
- Bu L, Jin Y, Shi Y, Chu R, Ban A, Eiberg H, Andres L, Jiang H, Zheng G, Qian M, Cui B, Xia Y, Liu J, Hu L, Zhao G, Hayden MR, Kong X (2002) Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat Genet 31:276–278. doi: 10.1038/ng921 [DOI] [PubMed] [Google Scholar]
- Chassaing N, Causse A, Vigouroux A, Delahaye A, Alessandri JL, Boespflug-Tanguy O, Boute-Benejean O, Dollfus H, Duban-Bedu B, Gilbert-Dussardier B, Giuliano F, Gonzales M, Holder-Espinasse M, Isidor B, Jacquemont ML, Lacombe D, Martin-Coignard D, Mathieu-Dramard M, Odent S, Picone O, Pinson L, Quelin C, Sigaudy S, Toutain A, Thauvin-Robinet C, Kaplan J, Calvas P (2014) Molecular findings and clinical data in a cohort of 150 patients with anophthalmia/microphthalmia. Clin Genet 86:326–334. doi: 10.1111/cge.12275; 10.1111/cge.12275 [DOI] [PubMed] [Google Scholar]
- Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, Kantorow M, Pras E, Frydman M, Pras E, Riazuddin S, Riazuddin SA, Hejtmancik JF (2011) Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am J Hum Genet 88:827–838. doi: 10.1016/j.ajhg.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang Q, Cabrera PE, Zhong Z, Sun W, Jiao X, Chen Y, Govindarajan G, Naeem MA, Khan SN, Ali MH, Assir MZ, Rahman FU, Qazi ZA, Riazuddin S, Akram J, Riazuddin SA, Hejtmancik JF (2017) Molecular Genetic Analysis of Pakistani Families With Autosomal Recessive Congenital Cataracts by Homozygosity Screening. Invest Ophthalmol Vis Sci 58:2207–2217. doi: 10.1167/iovs.17-21469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Huang C, Zhang B, Yin S, Liang J, Xu C, Huang Y, Cen LP, Ng TK, Zheng C, Zhang S, Chen H, Pang CP, Zhang M (2016) Mutations of RagA GTPase in mTORC1 Pathway Are Associated with Autosomal Dominant Cataracts. PLoS Genet 12:e1006090. doi: 10.1371/journal.pgen.1006090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ma J, Yan M, Mothobi ME, Liu Y, Zheng F (2009) A novel mutation in CRYAB associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis 15:1359–1365. doi: 143 [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu L (2017) Congenital cataract with LSS gene mutations: a new case report. J Pediatr Endocrinol Metab 30:1231–1235. doi: 10.1515/jpem-2017-0101 [DOI] [PubMed] [Google Scholar]
- Cohen D, Bar-Yosef U, Levy J, Gradstein L, Belfair N, Ofir R, Joshua S, Lifshitz T, Carmi R, Birk OS (2007) Homozygous CRYBB1 deletion mutation underlies autosomal recessive congenital cataract. Invest Ophthalmol Vis Sci 48:2208–2213. doi: 10.1167/iovs.06-1019 [DOI] [PubMed] [Google Scholar]
- Conley YP, Erturk D, Keverline A, Mah TS, Keravala A, Barnes LR, Bruchis A, Hess JF, FitzGerald PG, Weeks DE, Ferrell RE, Gorin MB (2000) A juvenile-onset, progressive cataract locus on chromosome 3q21-q22 is associated with a missense mutation in the beaded filament structural protein-2. Am J Hum Genet 66:1426–1431. doi: 10.1086/302871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia JD, da Rosa EB, Silveira DB, Correia EP, Lorenzen MB, Travi GM, Rosa RC, Zen PR, Zen TD, Rosa RF (2017) Trisomy 18 and eye anomalies. Am J Med Genet A 173:553–555. doi: 10.1002/ajmg.a.38036 [DOI] [PubMed] [Google Scholar]
- Coupry I, Sibon I, Mortemousque B, Rouanet F, Mine M, Goizet C (2010) Ophthalmological features associated with COL4A1 mutations. Arch Ophthalmol 128:483–489. doi: 10.1001/archophthalmol.2010.42 [DOI] [PubMed] [Google Scholar]
- Derwinska K, Mierzewska H, Goszczanska A, Szczepanik E, Xia Z, Kusmierska K, Tryfon J, Kutkowska-Kazmierczak A, Bocian E, Mazurczak T, Obersztyn E, Stankiewicz P (2012) Clinical improvement of the aggressive neurobehavioral phenotype in a patient with a deletion of PITX3 and the absence of L-DOPA in the cerebrospinal fluid. Am J Med Genet B Neuropsychiatr Genet 159B:236–242. doi: 10.1002/ajmg.b.32020 [DOI] [PubMed] [Google Scholar]
- Dimauro I, Antonioni A, Mercatelli N, Caporossi D (2017) The role of alphaB-crystallin in skeletal and cardiac muscle tissues. Cell Stress Chaperones doi: 10.1007/s12192-017-0866-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit L, Puente M, Yen KG (2017) Characteristics of Anterior Lens Opacities in Children. Open Ophthalmol J 11:84–88. doi: 10.2174/1874364101711010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette L, Green J, Fernandez B, Johnson GJ, Parfrey P, Young TL (2011) A novel, non-stop mutation in FOXE3 causes an autosomal dominant form of variable anterior segment dysgenesis including Peters anomaly. Eur J Hum Genet 19:293–299. doi: 10.1038/ejhg.2010.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duell PB, Salen G, Eichler FS, DeBarber AE, Connor SL, Casaday L, Jayadev S, Kisanuki Y, Lekprasert P, Malloy MJ, Ramdhani RA, Ziajka PE, Quinn JF, Su KG, Geller AS, Diffenderfer MR, Schaefer EJ (2018) Diagnosis, treatment, and clinical outcomes in 43 cases with cerebrotendinous xanthomatosis. J Clin Lipidol doi: S1933-2874(18)30266-6 doi: 10.1016/j.jacl.2018.06.008 [DOI] [PubMed] [Google Scholar]
- Enoki Y, Mukoda Y, Furutani C, Sakurai H (2010) DNA-binding and transcriptional activities of human HSF4 containing mutations that associate with congenital and age-related cataracts. Biochim Biophys Acta 1802:749–753. doi: 10.1016/j.bbadis.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Evers C, Paramasivam N, Hinderhofer K, Fischer C, Granzow M, Schmidt-Bacher A, Eils R, Steinbeisser H, Schlesner M, Moog U (2015) SIPA1L3 identified by linkage analysis and whole-exome sequencing as a novel gene for autosomal recessive congenital cataract. Eur J Hum Genet 23:1627–1633. doi: 10.1038/ejhg.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim AT, Daiger SP, Weleber RG (1993) Nonsyndromic Retinitis Pigmentosa Overview In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews((R)) University of Washington, Seattle: GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved, Seattle (WA) [Google Scholar]
- Farrell JW, Morgan KS, Black S (1988) Lensectomy in an infant with cri du chat syndrome and cataracts. J Pediatr Ophthalmol Strabismus 25:131–134. [DOI] [PubMed] [Google Scholar]
- Forshew T, Johnson CA, Khaliq S, Pasha S, Willis C, Abbasi R, Tee L, Smith U, Trembath RC, Mehdi SQ, Moore AT, Maher ER (2005) Locus heterogeneity in autosomal recessive congenital cataracts: linkage to 9q and germline HSF4 mutations. Hum Genet 117:452–459. doi: 10.1007/s00439-005-1309-9 [DOI] [PubMed] [Google Scholar]
- Garcia-Montalvo IA, Pelcastre-Luna E, Nelson-Mora J, Buentello-Volante B, Miranda-Duarte A, Zenteno JC (2014) Mutational Screening of FOXE3, GDF3, ATOH7, and ALDH1A3 in Congenital Ocular Malformations. Possible Contribution of the FOXE3 p.VAL201MET Variant to the Risk of Severe Eye Malformations. Ophthalmic Genet doi: 10.3109/13816810.2014.903983 [DOI] [PubMed] [Google Scholar]
- Gillespie RL, O’Sullivan J, Ashworth J, Bhaskar S, Williams S, Biswas S, Kehdi E, Ramsden SC, Clayton-Smith J, Black GC, Lloyd IC (2014) Personalized Diagnosis and Management of Congenital Cataract by Next-Generation Sequencing. Ophthalmology doi: 10.1016/j.ophtha.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Greenlees R, Mihelec M, Yousoof S, Speidel D, Wu SK, Rinkwitz S, Prokudin I, Perveen R, Cheng A, Ma A, Nash B, Gillespie R, Loebel DA, Clayton-Smith J, Lloyd IC, Grigg JR, Tam PP, Yap AS, Becker TS, Black GC, Semina E, Jamieson RV (2015) Mutations in SIPA1L3 cause eye defects through disruption of cell polarity and cytoskeleton organization. Hum Mol Genet 24:5789–5804. doi: 10.1093/hmg/ddv298 [DOI] [PubMed] [Google Scholar]
- Ha TT, Sadleir LG, Mandelstam SA, Paterson SJ, Scheffer IE, Gecz J, Corbett MA (2016) A mutation in COL4A2 causes autosomal dominant porencephaly with cataracts. Am J Med Genet A 170A:1059–1063. doi: 10.1002/ajmg.a.37527 [DOI] [PubMed] [Google Scholar]
- Haargaard B, Fledelius HC (2006) Down’s syndrome and early cataract. Br J Ophthalmol 90:1024–1027. doi: 10.1136/bjo.2006.090639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haargaard B, Wohlfahrt J, Fledelius HC, Rosenberg T, Melbye M (2004) A nationwide Danish study of 1027 cases of congenital/infantile cataracts: etiological and clinical classifications. Ophthalmology 111:2292–2298. doi: 10.1016/j.ophtha.2004.06.024 [DOI] [PubMed] [Google Scholar]
- Handley M, Sheridan E (1993) RAB18 Deficiency In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews((R)) University of Washington, Seattle: GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved, Seattle (WA) [PubMed] [Google Scholar]
- Hansen L, Comyn S, Mang Y, Lind-Thomsen A, Myhre L, Jean F, Eiberg H, Tommerup N, Rosenberg T, Pilgrim D (2014) The myosin chaperone UNC45B is involved in lens development and autosomal dominant juvenile cataract. Eur J Hum Genet 22:1290–1297. doi: 10.1038/ejhg.2014.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happ H, Weh E, Costakos D, Reis LM, Semina EV (2016) Case report of homozygous deletion involving the first coding exons of GCNT2 isoforms A and B and part of the upstream region of TFAP2A in congenital cataract. BMC medical genetics 17:64. doi: 10.1186/s12881-016-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennermann JB, Schadewaldt P, Vetter B, Shin YS, Monch E, Klein J (2011) Features and outcome of galactokinase deficiency in children diagnosed by newborn screening. J Inherit Metab Dis 34:399–407. doi: 10.1007/s10545-010-9270-8 [DOI] [PubMed] [Google Scholar]
- Heon E, Priston M, Schorderet DF, Billingsley GD, Girard PO, Lubsen N, Munier FL (1999) The gamma-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet 65:1261–1267. doi: 10.1086/302619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseri SU, Osborne RJ, Farrall M, Wyatt AW, Mirza G, Nurnberg G, Kluck C, Herbert H, Martin A, Hussain MS, Collin JR, Lathrop M, Nurnberg P, Ragoussis J, Ragge NK (2009) Seeing clearly: the dominant and recessive nature of FOXE3 in eye developmental anomalies. Hum Mutat 30:1378–1386. doi: 10.1002/humu.21079 [DOI] [PubMed] [Google Scholar]
- Islam L, Kelberman D, Williamson L, Lewis N, Glindzicz MB, Nischal KK, Sowden JC (2015) Functional analysis of FOXE3 mutations causing dominant and recessive ocular anterior segment disease. Hum Mutat 36:296–300. doi: 10.1002/humu.22741 [DOI] [PubMed] [Google Scholar]
- Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, Heon E, Wirth MG, van Heyningen V, Donnai D, Munier F, Black GC (2002) Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet 11:33–42. [DOI] [PubMed] [Google Scholar]
- Jiao X, Khan SY, Irum B, Khan AO, Wang Q, Kabir F, Khan AA, Husnain T, Akram J, Riazuddin S, Hejtmancik JF, Riazuddin SA (2015) Missense Mutations in CRYAB Are Liable for Recessive Congenital Cataracts. PLoS One 10:e0137973. doi: 10.1371/journal.pone.0137973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Jimenez FJ, Garcia-Martin E, Alonso-Navarro H, Agundez JA (2014) PITX3 and risk for Parkinson’s disease: a systematic review and meta-analysis. Eur Neurol 71:49–56. doi: 10.1159/000353981 [DOI] [PubMed] [Google Scholar]
- Kakrana A, Yang A, Anand D, Djordjevic D, Ramachandruni D, Singh A, Huang H, Ho JWK, Lachke SA (2018) iSyTE 2.0: a database for expression-based gene discovery in the eye. Nucleic Acids Res 46:D875–D885. doi: 10.1093/nar/gkx837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannabiran C, Rogan PK, Olmos L, Basti S, Rao GN, Kaiser-Kupfer M, Hejtmancik JF (1998) Autosomal dominant zonular cataract with sutural opacities is associated with a splice mutation in the betaA3/A1-crystallin gene. Mol Vis 4:21. [PubMed] [Google Scholar]
- Ke T, Wang QK, Ji B, Wang X, Liu P, Zhang X, Tang Z, Ren X, Liu M (2006) Novel HSF4 mutation causes congenital total white cataract in a Chinese family. Am J Ophthalmol 142:298–303. doi: 10.1016/j.ajo.2006.03.056 [DOI] [PubMed] [Google Scholar]
- Khajavi M, Inoue K, Lupski JR (2006) Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease. Eur J Hum Genet 14:1074–1081. doi: 10.1038/sj.ejhg.5201649 [DOI] [PubMed] [Google Scholar]
- Khan AO, Aldahmesh MA, Alkuraya FS (2015) Phenotypes of Recessive Pediatric Cataract in a Cohort of Children with Identified Homozygous Gene Mutations (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 113:T71–T715. [PMC free article] [PubMed] [Google Scholar]
- Khan SY, Vasanth S, Kabir F, Gottsch JD, Khan AO, Chaerkady R, Lee MC, Leitch CC, Ma Z, Laux J, Villasmil R, Khan SN, Riazuddin S, Akram J, Cole RN, Talbot CC, Pourmand N, Zaghloul NA, Hejtmancik JF, Riazuddin SA (2016) FOXE3 contributes to Peters anomaly through transcriptional regulation of an autophagy-associated protein termed DNAJB1. Nat Commun 7:10953. doi: 10.1038/ncomms10953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandekar R (2008) Visual disabilities in children including childhood blindness. Middle East Afr J Ophthalmol 15:129–134. doi: 10.4103/0974-9233.51988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloeckener-Gruissem B, Vandekerckhove K, Nurnberg G, Neidhardt J, Zeitz C, Nurnberg P, Schipper I, Berger W (2008) Mutation of solute carrier SLC16A12 associates with a syndrome combining juvenile cataract with microcornea and renal glucosuria. Am J Hum Genet 82:772–779. doi: 10.1016/j.ajhg.2007.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M, Roos A, Stendel C, Claeys KG, Sonmez FM, Baudis M, Bauer P, Bornemann A, de Goede C, Dufke A, Finkel RS, Goebel HH, Haussler M, Kingston H, Kirschner J, Medne L, Muschke P, Rivier F, Rudnik-Schoneborn S, Spengler S, Inzana F, Stanzial F, Benedicenti F, Synofzik M, Lia Taratuto A, Pirra L, Tay SK, Topaloglu H, Uyanik G, Wand D, Williams D, Zerres K, Weis J, Senderek J (2013) SIL1 mutations and clinical spectrum in patients with Marinesco-Sjogren syndrome. Brain 136:3634–3644. doi: 10.1093/brain/awt283 [DOI] [PubMed] [Google Scholar]
- Kuang SQ, Medina-Martinez O, Guo DC, Gong L, Regalado ES, Reynolds CL, Boileau C, Jondeau G, Prakash SK, Kwartler CS, Zhu LY, Peters AM, Duan XY, Bamshad MJ, Shendure J, Nickerson DA, Santos-Cortez RL, Dong X, Leal SM, Majesky MW, Swindell EC, Jamrich M, Milewicz DM (2016) FOXE3 mutations predispose to thoracic aortic aneurysms and dissections. J Clin Invest 126:948–961. doi: 10.1172/JCI83778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, Howell GR, Saadi I, Cavallesco R, Yue Y, Tsai AC, Nair KS, Cosma MI, Smith RS, Hodges E, Alfadhli SM, Al-Hajeri A, Shamseldin HE, Behbehani A, Hannon GJ, Bulyk ML, Drack AV, Anderson PJ, John SW, Maas RL (2011) Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 331:1571–1576. doi: 10.1126/science.1195970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Ho JW, Kryukov GV, O’Connell DJ, Aboukhalil A, Bulyk ML, Park PJ, Maas RL (2012) iSyTE: integrated Systems Tool for Eye gene discovery. Invest Ophthalmol Vis Sci 53:1617–1627. doi: 10.1167/iovs.11-8839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MG, Kramarevsky NY, Christiansen SP, Wright MM, Young TL, Summers CG (2005) Glaucoma following cataract surgery in children: surgically modifiable risk factors. Trans Am Ophthalmol Soc 103:46–55. [PMC free article] [PubMed] [Google Scholar]
- Lek M et al. (2016) Analysis of protein-coding genetic variation in 60,706 humans Nature 536:285–291. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Wang S, Ye H, Tang Y, Qiu X, Fan Q, Rong X, Liu X, Chen Y, Yang J, Lu Y (2016) Distribution of gene mutations in sporadic congenital cataract in a Han Chinese population. Mol Vis 22:589–598. [PMC free article] [PubMed] [Google Scholar]
- Litt M, Carrero-Valenzuela R, LaMorticella DM, Schultz DW, Mitchell TN, Kramer P, Maumenee IH (1997) Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum Mol Genet 6:665–668. [DOI] [PubMed] [Google Scholar]
- Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG (1998) Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet 7:471–474. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu H, Tang J, Lin Q, Sun Y, Wang C, Yang H, Khan MR, Peerbux MW, Ahmad S, Bukhari I, Zhu J (2017) Whole Exome Sequencing Identifies a Novel Mutation in the PITX3 Gene, Causing Autosomal Dominant Congenital Cataracts in a Chinese Family. Ann Clin Lab Sci 47:92–95. [PubMed] [Google Scholar]
- Liu L, Zhang Q, Zhou LX, Tang ZH (2015) A novel HSF4 mutation in a Chinese family with autosomal dominant congenital cataract. J Huazhong Univ Sci Technolog Med Sci 35:316–318. doi: 10.1007/s11596-015-1430-5 [DOI] [PubMed] [Google Scholar]
- Lloyd IC, Goss-Sampson M, Jeffrey BG, Kriss A, Russell-Eggitt I, Taylor D (1992) Neonatal cataract: aetiology, pathogenesis and management. Eye (Lond) 6 ( Pt 2):184–196. doi: 10.1038/eye.1992.37 [DOI] [PubMed] [Google Scholar]
- Lu B, Yang Y (2016) Detection of TORCH pathogens in children with congenital cataracts. Exp Ther Med 12:1159–1164. doi: 10.3892/etm.2016.3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Huang C, Zhang J, Liu Z, Zhang Z, Xu H, You Y, Hu J, Li X, Wang W (2014) A novel HSF4 gene mutation causes autosomal-dominant cataracts in a Chinese family. G3 (Bethesda) 4:823–828. doi: 10.1534/g3.113.009860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma AS, Grigg JR, Ho G, Prokudin I, Farnsworth E, Holman K, Cheng A, Billson FA, Martin F, Fraser C, Mowat D, Smith J, Christodoulou J, Flaherty M, Bennetts B, Jamieson RV (2016) Sporadic and Familial Congenital Cataracts: Mutational Spectrum and New Diagnoses Using Next-Generation Sequencing. Hum Mutat 37:371–384. doi: 10.1002/humu.22948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S (1999) Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet 64:1357–1364. doi: 10.1086/302383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DS, Boskovska OB, Knopf HL, Lampi KJ, Shiels A (2002) A nonsense mutation in CRYBB1 associated with autosomal dominant cataract linked to human chromosome 22q. Am J Hum Genet 71:1216–1221. doi: 10.1086/344212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makley LN, McMenimen KA, DeVree BT, Goldman JW, McGlasson BN, Rajagopal P, Dunyak BM, McQuade TJ, Thompson AD, Sunahara R, Klevit RE, Andley UP, Gestwicki JE (2015) Pharmacological chaperone for alpha-crystallin partially restores transparency in cataract models. Science 350:674–677. doi: 10.1126/science.aac9145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Emanuel BS, Zackai EH (1993) 22q11.2 Deletion Syndrome In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews((R)) University of Washington, Seattle: GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved, Seattle (WA) [Google Scholar]
- Merath K, Ronchetti A, Sidjanin DJ (2013) Functional analysis of HSF4 mutations found in patients with autosomal recessive congenital cataracts. Invest Ophthalmol Vis Sci 54:6646–6654. doi: 10.1167/iovs.13-12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Bhattacharya SS, Moore T, Prescott Q, Wedig T, Herrmann H, Magin TM (2009) Dominant cataract formation in association with a vimentin assembly disrupting mutation. Hum Mol Genet 18:1052–1057. doi: 10.1093/hmg/ddn440 [DOI] [PubMed] [Google Scholar]
- Ormestad M, Blixt A, Churchill A, Martinsson T, Enerback S, Carlsson P (2002) Foxe3 haploinsufficiency in mice: a model for Peters’ anomaly. Invest Ophthalmol Vis Sci 43:1350–1357. [PubMed] [Google Scholar]
- Patel N, Anand D, Monies D, Maddirevula S, Khan AO, Algoufi T, Alowain M, Faqeih E, Alshammari M, Qudair A, Alsharif H, Aljubran F, Alsaif HS, Ibrahim N, Abdulwahab FM, Hashem M, Alsedairy H, Aldahmesh MA, Lachke SA, Alkuraya FS (2016) Novel phenotypes and loci identified through clinical genomics approaches to pediatric cataract. Hum Genet doi: 10.1007/s00439-016-1747-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisancie J, Ragge NK, Dollfus H, Kaplan J, Lehalle D, Francannet C, Morin G, Colineaux H, Calvas P, Chassaing N (2017) FOXE3 mutations: genotype-phenotype correlations. Clin Genet doi: 10.1111/cge.13177 [DOI] [PubMed] [Google Scholar]
- Ponnam SP, Ramesha K, Tejwani S, Ramamurthy B, Kannabiran C (2007) Mutation of the gap junction protein alpha 8 (GJA8) gene causes autosomal recessive cataract. J Med Genet 44:e85. doi: 10.1136/jmg.2007.050138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras E, Frydman M, Levy-Nissenbaum E, Bakhan T, Raz J, Assia EI, Goldman B, Pras E (2000) A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Jewish Persian family. Invest Ophthalmol Vis Sci 41:3511–3515. [PubMed] [Google Scholar]
- Pras E, Levy-Nissenbaum E, Bakhan T, Lahat H, Assia E, Geffen-Carmi N, Frydman M, Goldman B, Pras E (2002) A missense mutation in the LIM2 gene is associated with autosomal recessive presenile cataract in an inbred Iraqi Jewish family. Am J Hum Genet 70:1363–1367. doi: 10.1086/340318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragge NK, Baser ME, Riccardi VM, Falk RE (1997) The ocular presentation of neurofibromatosis 2. Eye (Lond) 11 ( Pt 1):12–18. doi: 10.1038/eye.1997.3 [DOI] [PubMed] [Google Scholar]
- Ramachandran RD, Perumalsamy V, Hejtmancik JF (2007) Autosomal recessive juvenile onset cataract associated with mutation in BFSP1. Hum Genet 121:475–482. doi: 10.1007/s00439-006-0319-6 [DOI] [PubMed] [Google Scholar]
- Reis LM, Semina EV (2011) Genetics of anterior segment dysgenesis disorders. Curr Opin Ophthalmol 22:314–324. doi: 10.1097/ICU.0b013e328349412b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Muheisen S, Raggio V, Salviati L, Han DP, Costakos D, Yonath H, Hall S, Power P, Semina EV (2013) Whole exome sequencing in dominant cataract identifies a new causative factor, CRYBA2, and a variety of novel alleles in known genes. Hum Genet 132:761–770. doi: 10.1007/s00439-013-1289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Schneider A, Bardakjian T, Stoler JM, Melancon SB, Semina EV (2010) FOXE3 plays a significant role in autosomal recessive microphthalmia. Am J Med Genet A 152A:582–590. doi: 10.1002/ajmg.a.33257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Semina EV (2014) Identification of a novel C-terminal extension mutation in EPHA2 in a family affected with congenital cataract. Mol Vis 20:836–842. [PMC free article] [PubMed] [Google Scholar]
- Retterer K, Juusola J, Cho MT, Vitazka P, Millan F, Gibellini F, Vertino-Bell A, Smaoui N, Neidich J, Monaghan KG, McKnight D, Bai R, Suchy S, Friedman B, Tahiliani J, Pineda-Alvarez D, Richard G, Brandt T, Haverfield E, Chung WK, Bale S (2016) Clinical application of whole-exome sequencing across clinical indications. Genet Med 18:696–704. doi: 10.1038/gim.2015.148 [DOI] [PubMed] [Google Scholar]
- Riazuddin SA, Yasmeen A, Yao W, Sergeev YV, Zhang Q, Zulfiqar F, Riaz A, Riazuddin S, Hejtmancik JF (2005) Mutations in betaB3-crystallin associated with autosomal recessive cataract in two Pakistani families. Invest Ophthalmol Vis Sci 46:2100–2106. doi: 10.1167/iovs.04-1481 [DOI] [PubMed] [Google Scholar]
- Saboo US, Penke D, Mahindrakar A, Uddaraju M, Sankurathri C, Gong X, Xing C, Mootha VV (2017) Exome sequencing reveals novel homozygous FOXE3 mutation in microphthalmos with staphylomatous malformation. Ophthalmic Genet 38:295–297. doi: 10.1080/13816810.2016.1217549 [DOI] [PubMed] [Google Scholar]
- Safieh LA, Khan AO, Alkuraya FS (2009) Identification of a novel CRYAB mutation associated with autosomal recessive juvenile cataract in a Saudi family. Mol Vis 15:980–984. [PMC free article] [PubMed] [Google Scholar]
- Sajjad N, Goebel I, Kakar N, Cheema AM, Kubisch C, Ahmad J (2008) A novel HSF4 gene mutation (p.R405X) causing autosomal recessive congenital cataracts in a large consanguineous family from Pakistan. BMC Med Genet 9:99–2350-9–99. doi: 10.1186/1471-2350-9-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakazume S, Sorokina E, Iwamoto Y, Semina EV (2007) Functional analysis of human mutations in homeodomain transcription factor PITX3. BMC Mol Biol 8:84. doi: 10.1186/1471-2199-8-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M (2001) Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet 10:231–236. [DOI] [PubMed] [Google Scholar]
- Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC (1998) A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet 19:167–170. doi: 10.1038/527 [DOI] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Hejtmancik JF (2010) Cat-Map: putting cataract on the map. Mol Vis 16:2007–2015. [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HL, Maraini G, Li A, Jiao X, Hejtmancik JF (2008) The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol Vis 14:2042–2055. [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HL, Yamada K, Yoshiura K, Niikawa N, Shim S, Hanson PI (2007) CHMP4B, a novel gene for autosomal dominant cataracts linked to chromosome 20q. Am J Hum Genet 81:596–606. doi: 10.1086/519980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Hejtmancik JF (2017) Mutations and mechanisms in congenital and age-related cataracts. Exp Eye Res 156:95–102. doi: 10.1016/j.exer.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S (1998) A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet 62:526–532. doi: 10.1086/301762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims KB (1999) NDP-related retinopathies. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews®. University of Washington, Seattle (WA) [Google Scholar]
- Smaoui N, Beltaief O, BenHamed S, M’Rad R, Maazoul F, Ouertani A, Chaabouni H, Hejtmancik JF (2004) A homozygous splice mutation in the HSF4 gene is associated with an autosomal recessive congenital cataract. Invest Ophthalmol Vis Sci 45:2716–2721. doi: 10.1167/iovs.03-1370 [DOI] [PubMed] [Google Scholar]
- Stambolian D, Ai Y, Sidjanin D, Nesburn K, Sathe G, Rosenberg M, Bergsma DJ (1995) Cloning of the galactokinase cDNA and identification of mutations in two families with cataracts. Nat Genet 10:307–312. doi: 10.1038/ng0795-307 [DOI] [PubMed] [Google Scholar]
- Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN (2014) The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet 133:1–9. doi: 10.1007/s00439-013-1358-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan DA, Gillanders E, Vanderveen D, Freas-Lutz D, Wistow G, Baxevanis AD, Robbins CM, VanAuken A, Quesenberry MI, Bailey-Wilson J, Juo SH, Trent JM, Smith L, Brownstein MJ (1999) Progressive juvenile-onset punctate cataracts caused by mutation of the gammaD-crystallin gene. Proc Natl Acad Sci U S A 96:1008–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Ma Z, Li Y, Liu B, Li Z, Ding X, Gao Y, Ma W, Tang X, Li X, Shen Y (2005) Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J Med Genet 42:706–710. doi: 10.1136/jmg.2004.028274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xiao X, Li S, Guo X, Zhang Q (2014) Exome sequencing of 18 Chinese families with congenital cataracts: a new sight of the NHS gene. PLoS One 9:e100455. doi: 10.1371/journal.pone.0100455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RL, Handley MT, Waller S, Campbell C, Urquhart J, Meynert AM, Ellingford JM, Donnelly D, Wilcox G, Lloyd IC, Mundy H, FitzPatrick DR, Deshpande C, Clayton-Smith J, Black GC (2017) Novel PEX11B Mutations Extend the Peroxisome Biogenesis Disorder 14B Phenotypic Spectrum and Underscore Congenital Cataract as an Early Feature. Invest Ophthalmol Vis Sci 58:594–603. doi: 10.1167/iovs.16-21026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The UniProt Consortium (2017) UniProt: the universal protein knowledgebase. Nucleic Acids Res 45:D158–D169. doi: 10.1093/nar/gkw1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumler AA (2011) Evaluation of pediatric cataracts and systemic disorders. Curr Opin Ophthalmol 22:365–379. doi: 10.1097/ICU.0b013e32834994dc [DOI] [PubMed] [Google Scholar]
- Tzifi F, Pons R, Athanassaki C, Poulou M, Kanavakis E (2011) Congenital cataracts, facial dysmorphism, and neuropathy syndrome. Pediatr Neurol 45:206–208. doi: 10.1016/j.pediatrneurol.2011.05.008 [DOI] [PubMed] [Google Scholar]
- Ullah E, Nadeem Saqib MA, Sajid S, Shah N, Zubair M, Khan MA, Ahmed I, Ali G, Dutta AK, Danda S, Lao R, Ling-Fung Tang P, Kwok PY, Ansar M, Slavotinek A (2016) Genetic analysis of consanguineous families presenting with congenital ocular defects. Exp Eye Res 146:163–171. doi: 10.1016/j.exer.2016.03.014 [DOI] [PubMed] [Google Scholar]
- Valleix S, Niel F, Nedelec B, Algros MP, Schwartz C, Delbosc B, Delpech M, Kantelip B (2006) Homozygous nonsense mutation in the FOXE3 gene as a cause of congenital primary aphakia in humans. Am J Hum Genet 79:358–364. doi: 10.1086/505654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin H, Sorokina EA, Meire F, Casteels I, de Ravel T, Semina EV, De Baere E (2014) Novel and recurrent PITX3 mutations in Belgian families with autosomal dominant congenital cataract and anterior segment dysgenesis have similar phenotypic and functional characteristics. Orphanet J Rare Dis 9:26–1172-9–26. doi: 10.1186/1750-1172-9-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LR, Tosky ER, Sutton KM, Griess R, Abebe MD, Barnes SY, Cunnigham T, Kachman SD, Nielsen MK, Ciobanu DC (2017) A 16.7 kb deletion in Sipa1l3 is associated with juvenile cataract in mice. Mamm Genome 28:515–519. doi: 10.1007/s00335-017-9720-9 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang T, Wu D, Zhang J (2013) A novel beaded filament structural protein 1 (BFSP1) gene mutation associated with autosomal dominant congenital cataract in a Chinese family. Mol Vis 19:2590–2595. [PMC free article] [PubMed] [Google Scholar]
- Whitman MC, Vanderveen DK (2014) Complications of pediatric cataract surgery. Semin Ophthalmol 29:414–420. doi: 10.3109/08820538.2014.959192 [DOI] [PubMed] [Google Scholar]
- Wu X, Long E, Lin H, Liu Y (2016) Prevalence and epidemiological characteristics of congenital cataract: a systematic review and meta-analysis. Sci Rep 6:28564. doi: 10.1038/srep28564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa N, Oe K, Yukawa N, Murakami K, Nakashima R, Imura Y, Yoshifuji H, Ohmura K, Miura Y, Tomosugi N, Kawabata H, Takaori-Kondo A, Mimori T (2016) A Novel Phenotype of a Hereditary Hemochromatosis Type 4 with Ferroportin-1 Mutation, Presenting with Juvenile Cataracts. Intern Med 55:2697–2701. doi: 10.2169/internalmedicine.55.6565 [DOI] [PubMed] [Google Scholar]
- Yu LC, Twu YC, Chang CY, Lin M (2001) Molecular basis of the adult i phenotype and the gene responsible for the expression of the human blood group I antigen. Blood 98:3840–3845. [DOI] [PubMed] [Google Scholar]
- Yu X, Zheng H, Chan MT, Wu WKK (2017) MicroRNAs: new players in cataract. Am J Transl Res 9:3896–3903. [PMC free article] [PubMed] [Google Scholar]
- Zeiss CJ (2013) Translational models of ocular disease. Vet Ophthalmol 16 Suppl 1:15–33. doi: 10.1111/vop.12065 [DOI] [PubMed] [Google Scholar]
- Zhai Y, Li J, Yu W, Zhu S, Yu Y, Wu M, Sun G, Gong X, Yao K (2017) Targeted Exome Sequencing of Congenital Cataracts Related Genes: Broadening the Mutation Spectrum and Genotype-Phenotype Correlations in 27 Chinese Han Families. Sci Rep 7:1219–017-01182–9. doi: 10.1038/s41598-017-01182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen XJ, Zhu J, Xi YB, Yang X, Hu LD, Ouyang H, Patel SH, Jin X, Lin D, Wu F, Flagg K, Cai H, Li G, Cao G, Lin Y, Chen D, Wen C, Chung C, Wang Y, Qiu A, Yeh E, Wang W, Hu X, Grob S, Abagyan R, Su Z, Tjondro HC, Zhao XJ, Luo H, Hou R, Perry JJ, Gao W, Kozak I, Granet D, Li Y, Sun X, Wang J, Zhang L, Liu Y, Yan YB, Zhang K (2015) Lanosterol reverses protein aggregation in cataracts. Nature 523:607–611. doi: 10.1038/nature14650 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wojcik A, Sanders VR, Rahmani B, Kurup SP (2017) Ocular findings in a patient with oculofaciocardiodental (OFCD) syndrome and a novel BCOR pathogenic variant. Int Ophthalmol doi: 10.1007/s10792-017-0754-5 [DOI] [PubMed] [Google Scholar]