Abstract
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis of unknown etiology, and currently the cellular and molecular interactions that dictate its pathogenesis remain elusive. A role of the interleukin-23 (IL-23)/IL-23R (IL-23 receptor) interaction in the development of psoriasis and PsA is well established. As IL-23 regulates the differentiation and activation of innate and adaptive immunity, it pertains to a very complex pathophysiology involving a plethora of effectors and transducers. In this review, we will discuss recent advances on the cellular and molecular pathophysiological mechanisms that regulate the initiation and progression of PsA as well as new therapeutic approaches for IL-23/IL-23R targeted therapeutics.
Keywords: Psoriatic arthritis, skin and joint inflammation, cytokines, IL-23/IL-23R pathways, human monoclonal IL-23 antibodies, therapeutics
1. Introduction
In the United States, an estimated 294,000 children under the age of 18 are affected by arthritis, including psoriatic arthritis [1] and up to 15% of patients who have psoriasis also develop inflammatory arthritis [2]. Psoriatic arthritis (PsA) is a systemic inflammatory disease, which is characterized by inflammation of the skin and joints [3]. Epidermal hyperplasia, synovial inflammation, and bone loss are hallmarks of PsA [3]. Although there is no specific genetic marker for diagnosis of PsA, the disease could be diagnosed through clinical and imaging features, which include psoriasis, peripheral joint disease, axial disease, enthesitis, and dactylitis [2, 4]. Neutralization of pro-inflammatory cytokines of the IL-23/IL-17 axis has shown great promise in combating the disease [5–7]. Ustekinumab (IL-23p40) and secukinumab (IL-17A) are human monoclonal antibodies that specifically bind to and neutralize IL-23p40 and IL-17A, respectively [6, 7]. These antibodies have been in clinical trials for the treatment of PsA and improved signs and symptoms of PsA, suggesting that IL-23/IL-17 has an important role in the pathogenesis of PsA. We will describe the current understanding of pathogenesis of PsA and ongoing therapeutic developments for treatment of PsA.
2. Clinical and pathophysiological features of PsA
2.1. Clinical features
In human, skin is composed of three layers including the epidermis, dermis, and hypodermis. The layer of the epidermis includes the stratum corneum (the outermost layer), stratum granulosum, stratum spinosum, and stratum basale. The dermis layer contains connective tissue, hair follicles, and sweat glands whereas hypodermis is made of fat and connective tissue. During pathogenesis of psoriasis and PsA, each of these layers becomes altered which is one of major features for the diseases include thinned suprapapillary plates, collections of neutrophils in the stratum corneum (Munro’s microabscesses), psoriasiform epithelial hyperplasia; elongation and edema of dermal papillae; parakeratosis, retained nuclei in the stratum corneum [2, 4]. In addition, bone and cartilage destruction with pathologic new bone formation is one of the most distinctive features of PsA [2]. Several clinical features of PsA has been characterized by Moll and Wright such as distal interphalangeal joints, arthritis mutilans, symmetric polyarthritis, ankylosing spondylitis, asymmetric distal interphalangeal joints [4].
2.2. Cellular Pathophysiology
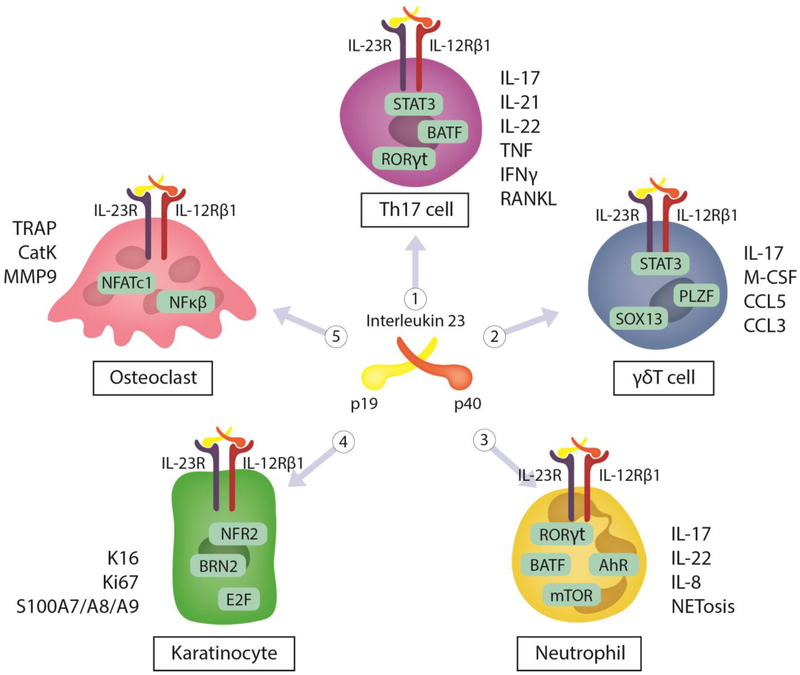
Although the precise cellular mechanisms of PsA are not completely understood, it is likely that genetic, immunologic, and environmental factors are involved in the activation of multiple cell types including T cells, neutrophils, keratinocytes, and osteoclasts (Figure 1). In this part, we will focus on the immunopathogenesis of PsA.
Figure 1. Cellular pathophysiology of PsA.
In PsA, Th17, γδT cells, neutrophils, keratinocytes, and osteoclast are involved in the initiation of the disease. 1) Th17 cells activated by STAT3 lead to the release of IL-17, IL-21, IL-22, TNF, IFNγ and RANKL. 2) γδT cells are also activated by STAT3 to release of IL-17, M-CSF, CCL5, and CCL3. 3) In neutrophils, IL-23 induces the expression of IL-23R, and a number of transcriptional activators including STAT3-dependent RORγt, NF-κB, aryl-hydrocarbon receptor (AhR), mammalian target of rapamycin (mTOR) and Basic Leucine Zipper ATF-Like Transcription Factor (BATF) pathways that regulate IL-17A, IL-17F and IL-22. NETs, IL-1β and IL-8 also contribute to neutrophil activity in psoriasis. 4) In keratinocytes, the transcription factor Nrf2 promotes proliferation of keratinocyte through controlling expression of K6, K16, and K17. IL-23 induces directly and/or indirectly (IL-17/IL-22) the expression of K16/Ki67 or S100A7–9 leads to induce proliferation of keratinocytes, hyperplasia, and skin inflammation. 5) In osteoclasts, IL-23 induces the expansion of osteoclast precursors and activates NFATc1 leading to the expression of TRAP, CatK, MMP9 that facilitate bone resorption.
2.2.1. T cells
IL-23 has been implicated in the regulation of conventional (αβ) and non-conventional (γδ) T cells and the production of IL-17. A number of reports suggest that IL-23 is critical for differentiation, survival, and expansion of Th17 and γδT cells [8, 9]. γδT cells are significantly increased, in the dermis of psoriatic skin lesions, and in peripheral blood and synovium of PsA patients, compared with healthy control [10–12]. A number of different Th17 cell “flavors” have been described by the literature and shown mainly by intracellular staining to produce a number of cytokines as previously reviewed [13]. Th17 cells secreted their signature cytokines such as IL-17, TNF, IL-22 (Figure 1) [14, 15] and also produced RANKL [16] which have been shown to contribute to the pathology observed in PsA [17]. In rheumatoid arthritis, IL-22 regulates osteoclastogenesis by up-regulation of RANKL in human synovial fibroblasts [18] and recombinant IL-22 induced a significant increase in expression of antimicrobial peptides (S100A8, S100A9, defensin β1) and inflammatory cytokines (IL-1α, IL-6, and TNF-α) and keratinocyte hyperplasia in a mouse model of psoriasis-like skin inflammation [19]. RANKL secreted by Th17 cells also directly promotes osteoclastogenesis, while the effect of RANKL on the skin is been linked with regulatory functions of peripheral CD4+CD25+ regulatory T cells [16, 20]. Undoubtedly the most investigated cytokine of the Th17 cells is IL-17 itself, which is produced by both CD4+ T cells and γδ T cells. IL-17 producing CD4+ T cells are elevated in the synovial fluid of both psoriatic and PsA patients [17] and in a related animal model of IL-17 gene transfer in vivo it was shown that IL-17 induces bone loss and epidermal hyperplasia associated with PsA [21]. In keeping with these observations it was also demonstrated that γδ T cells were increased in human psoriatic skin lesions [10]. A recent study highlighted that TRAV15N-1 (Vδ6.3) γδT cells were clonally expanded in mice and expressed specific cytokines, M-CSF, CCL5, CCL3 (Figure 1), which are known to act on myeloid cells, indicating that this γδ T cell subset might have distinct functions in myelopoiesis [22]. We also observed that IL-17 gene transfer induced epidermal hyperplasia was persistent in the absence of conventional (αβ Th17 cells) and non-conventional (γδ) T cells but dependent on the activation of neutrophils (CD11b+Gr1highLy6G+), suggesting that IL-17 pathology may occur independently of T cells [23] as discussed next.
2.2.2. Neutrophils
Although IL-23 promotes the secretion of IL-17 by CD4+ T cells [24], neutrophils, not T cells, are the predominant cells producing IL-17 in human skin [25]. Interestingly, other groups have identified that neutrophils are the main source of IL-23 in the colon of patients with inflammatory bowel disease [26]. Additionally, IL-23 induces the expression of IL-23R, IL-17 and IL-22 on neutrophils (Figure 1) [27]. Furthermore, neutrophil direct role in epidermal hyperplasia is supported by the distinct presence of neutrophil exudates (Munro’s microabscesses) in the stratum corneum of the epidermis in psoriatic patients [28, 29], suggesting that myeloid cells (neutrophil and monocytes) contribute directly to development of pathogenesis of psoriasis and PsA. We and others have shown that neutrophil depletion reduces thickness of epidermis and Munro’s microabscess formation in mice [23, 30]. Neutrophil extracellular traps (NETs) was increased in human psoriatic skin, correlated with psoriasis disease severity, and induced the expression of β-defensin-2 [31]. β-defensin-2, a serum biomarker for psoriasis, is strongly expressed in psoriatic epidermis [32]. Another study also showed that neutrophil-derived proteases such as cathepsin G, elastase, and proteinase-3 processed and activated IL-36 [33]. IL-36 and its receptor (IL-36R or IL-1RL2) are able to stimulate the expression of inflammatory cytokines including IL-17, IL-23, TNF, IL-8, and IL-6 in human psoriatic lesional skin [34, 35]. However, many questions remain regarding the signaling pathway(s) involved and the interplay of the IL-23/IL-17 axis with neutrophils.
2.2.3. Keratinocytes
In the skin, uncontrolled hyperproliferation and differentiation of keratinocytes results in epidermal hyperplasia associated with parakeratosis, which is one of the most significant features in human PsA [2, 4]. Therefore, markers of keratinocyte differentiation and hyperproliferation such as keratin 16 (K16) and Ki67 have effectively been used as biomarkers in psoriasis [36]. In psoriasis, the transcription factor Nrf2 promotes proliferation of keratinocyte through controlling expression of K6, K16, and K17 (Figure 1) [37]. Inflammation of keratinocyte induces synthesis of chemokines (CXCL9, CXCL10, and CXCL11) that can recruit Th1 cells into the skin whereas CCL20, CXCL1, CXCL2, and CXCL8/IL-8 recruit Th17 and neutrophils [12]. Moreover, S100A7-S100A9, calcium-binding proteins, are specifically expressed in psoriasis and loss of S100A7-S100A9 improves symptoms of psoriasis-like disease [38]. We and others have previously shown that IL-23 can induce epidermal hyperplasia and stimulate the synthesis of leukotriene B4 (LTB4), a biologically active lipid inflammatory mediator, important in skin inflammation [39]. LTB4R1 is a high-affinity receptor for LTB4 predominantly expressed on neutrophils and macrophages [40] and it is also involved in joint pathology [41]. The interplay between IL-23 and the release of pro-inflammatory mediators in innate immunity is relatively understudied, and remains an area of increased interest.
2.2.4. Osteoclasts
In addition to T cells and neutrophils, osteoclasts also play a crucial role in the development of bone destruction [42]. Osteoclasts are multinucleated cells that perform the unique function of bone resorption [42]. During physiological bone remodeling, macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-B ligand (RANKL) regulate the differentiation of osteoclasts from their precursors [43]. RANKL selectively induces expression of nuclear factor of activated T cells cytoplasmic 1 (NFATc1) in the presence of M-CSF; importantly, NFATc1-deficient embryonic stem cells fail to differentiate into osteoclasts, and ectopic expression of NFATc1 causes precursor cells to undergo efficient differentiation without RANKL signaling indicating that NFATc1 is required for osteoclast differentiation [44]. Osteoclasts also highly express cathepsin K, matrix metalloproteinases (MMPs) which contribute differently to activities of osteoclast [45]. Bone resorption results in the release of tartrate-resistant acid phosphatase (TRAP) from osteoclasts, thus TRAP is considered as a marker of osteoclast function [46]. These factors (cathepsin K, TRAP, MMPs) mediate the activity of osteoclast and their increased expression is associated with several bone diseases serve as effective biomarkers of bone destruction. In psoriatic arthritis, expression of CD16 (FcRγIII) was considered as a potential marker of osteoclast precursors [47]. In addition, CD16high monocytes showed a higher activity of bone erosion than CD16int and CD16low monocytes, supporting that the level of CD16 expression on monocytes/osteoclast correlated with the bone resorption activity of osteoclast in psoriatic arthritis [47]. Interestingly, we recently showed that IL-23 induces NFATc1 in the absence of exogenous RANKL and stimulates the differentiation of MDL-1+/DAP12+ CD16+ cells corroborating the previous findings discussed herein [48, 49].
2.3. Molecular Pathophysiology
2.3.1. IL-23/IL-23R
In 2000, Oppmann and colleagues discovered that IL-23 (p19/p40) is a novel heterodimeric cytokine of the IL-12 family and is predominantly produced by dendritic cells and macrophages [50]. IL-23 and IL-12 have a mutual p40 subunit whereas p19 is a unique for IL-23 and p35 for IL-12. IL-23 binds specifically to and signals through its heterodimeric receptor assembly which is composed of IL-12Rβ1 and IL-23R subunits [51].
IL-23 signals via its cognate receptor IL-23R and the shared receptor IL-12Rβ1 and is thought to mediate a stoichiometric ternary complex with the ectodomains of the two receptors. While IL-12Rβ1 orthologues consistently display five extracellular domains, mammalian IL-23R consist of just three extracellular domains compared to evolutionarily more distant vertebrates, which have two to three additional membrane-proximal fibronectin type III (FnIII) domains. Current models for the assembly of cell-surface complexes mediated by IL-12 family cytokines have been largely based on the structural principles derived from the interleukin-6 (IL-6) complex with interleukin-6 receptor subunit alpha (IL-6Rα) and glycoprotein 130 (gp130) and other gp130 complexes [52, 53].
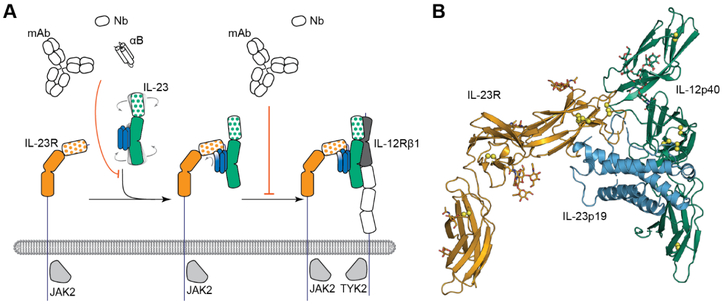
The structure of IL-23 in complex with its cognate receptor IL23R has been recently determined [54] providing unprecedented insights into the assembly principles underlying cytokine-receptor complexes of IL-12 family. IL-23 deploys only its IL-23p19 subunit to contact IL-23R at its N-terminal Ig-like domain, which in turn reaches out via its terminal peptide to grab onto the IL-23 scaffold (Figure 2A). The structural and functional hotspot of this interaction partially restructures the helical IL-23p19 subunit of IL-23 and leads to restraining of its IL-12p40 subunit to enable cooperative binding of the shared receptor IL-12Rβ1 with high affinity. The IL-23p19 is likely not involved in binding IL-12Rβ1 at all, so the low-affinity interaction between IL-23 and IL-12Rβ1 is exclusively mediated by the p40 subunit (Figure 2B). Moreover, the experimental evidence supports a sequential model of assembly mechanism, in which a stable binary IL-23:IL-23R complex is the mechanistic prerequisite for the recruitment of the shared receptor IL-12Rβ1 to complete the ternary cytokine-receptor complex [54]. Furthermore, the dimeric disulfide-linked form of the IL-12p40 subunit, IL-12p80, which was shown to have both agonistic signaling properties as well as antagonistic behavior over a range of activities such as macrophage chemotaxis, inflammatory responses, and protective activity in a mycobacterial model, binds IL-12Rβ1 while lacking an α-helical subunit [55].
Figure 2. Schematic representation of the assembly mechanism of the IL-23-mediated receptor complex.
(A) The signaling complex mediated by IL-23 proceeds via the sequential recruitment of the two cognate receptors and involves conformational selection and restructuring of IL-23 (blue and green) by IL-23R (orange) to recruit IL-12Rβ1 (black and white) with high affinity (Bloch et. al., 2018, modified). (B) Cartoon representation of the crystal structure of the IL-23:IL-23R complex (PDB 5mzv).
Using IL-23RGFP+/+ reporter mice, other studies showed that IL-23R is expressed in Th17, natural killer T (NKT) cells, γδT cells, group 3 innate lymphoid cells (ILC), dendritic cells, macrophages, and recently neutrophils [56, 57].
2.3.2. IL-23 signaling
Although the cytoplasmic domain of IL-23R does not have kinase activity, it has seven tyrosine residues [51]. Three of these tyrosines are potential candidates for phosphorylation and binding-sites for Src homology 2 domain [51]. Upon engagement with IL-23, the IL-23 receptor assembly will recruit and associate with Jak2/Tyk2 leading to activation of Jak2/Tyk2 and STAT3 [58]. Moreover, NF-κB and PI3K/Akt pathways are activated by IL-23 in T cells, leading to phosphorylation of STAT3 by PI3K/Akt which have an important role in regulation of IL-17 gene expression [58]. In Th17 cells, retinoid-related orphan receptor (ROR) γT, RORα, and signal transducer and activator of transcription (STAT3) are crucial transcription factors which bind the IL-17 promoter and activate gene expression cascades [59]. In addition, interaction of IL-23 with IL-23R also induces activation of nuclear factor of kappa light chain enhancer of activated B cells (NF-κB) and translocation of NF-κB into nucleus through regulation of inhibitory subunit of nuclear factor kappa B alpha (IκBα) [60]. Activation of NF-κB will induce expression of several inflammatory genes such as TNF, IL-1β, IL-6, and CXCLs [61].
2.3.3. IL-23 in the skin
In murine model of IL-23-mediated psoriasis-like epidermal hyperplasia, IL-23 treatment induced a dermal infiltration of T cells, dendritic cells (CD11c+), macrophages (F4/80+), and neutrophils (Gr1+LY6G+) [62]. Moreover, IL-23 regulates and stabilizes the Th17 phenotype and secretion of IL-17, IL-21 and IL-22 by Th17 cells (Figure 1), which mediate the epidermal hyperplasia, keratinocyte differentiation in psoriasis [63]. Injection of IL-23 induced the expression of CCR6 (a CCL20 receptor, expressed by Th17 cells and activated neutrophils), in the epidermis layer and Ccr6–/– mice did not develop IL-23-induced psoriasis-like inflammation, indicating that IL-23 induced psoriasis-like inflammation is dependent on CCR6 [64]. Moreover, CCL20-derived antagonists block Th17 cell homing and prevents the signs of psoriasis in a mouse model [65].
As previously mentioned IL-23 expansion of γδT cells in the dermis layer is a key component in the pathogenesis of psoriasis [10]. CD69 is an activation marker of skin γδT cells, and controls the aryl hydrocarbon receptor (AhR)-dependent secretion of interleukin 22 (IL-22) by γδ T cells, which contributes to the development of psoriasis induced by IL-23 [66]. Indeed, CD69-deficient mice had lower expression of epidermal IL-22 and STAT3 and attenuated skin inflammation induced by IL-23, compared with wild-type mice, suggesting that CD69 is a key mediator of psoriasis pathogenesis [66].
IL-23 regulates not only T cells but also NK cell populations in murine psoriasis-like disease [63]. Depletion of macrophages significantly reduced the skin inflammation and inflammatory cytokines in IL-23 mediated psoriasiform skin inflammation [67]. Moreover, IL-23 regulates granulopoiesis and homeostasis of neutrophil in mice [68]. In mouse model, injection of IL-23 is able to stimulate keratinocytes proliferation and induce epidermal hyperplasia [69].
It has been showed that IL-23 induced accumulation and differentiation of Langerhans cells which may be an important mechanism in regulation of cutaneous immune responses [70]. LIM-domain only protein 4 (LMO4), a transcription factor, regulates differentiation and proliferation of keratinocyte during embryogenesis. IL-23 increased the expression of LMO4 through Jak2/Akt/ STAT3 pathway and knockdown of LMO4 using shRNA had negative effects on differentiation and proliferation of keratinocytes in the ears of IL-23-injected mice [71]. Biomarkers of keratinocytes proliferation and differentiation such as Ki67 and K16, respectively as well as skin inflammatory markers (S100A7-S100A9, TNF-α, IL-19) was induced by IL-23 in mouse skin [72].
2.3.4. IL-23 in the joint
In joint autoimmune inflammation, IL-23 induced and prolonged expression of inflammatory cytokines including IL-1β, IL-6, IL-17, and TNF and IL-23 deficient mice (p19−/−) did not exhibit any clinical symptoms of disease and were resistant to the development of joint inflammation [24]. Our group has shown that overexpression of IL-23 using in vivo gene delivery induced chronic arthritis, severe bone loss, and myelopoiesis in the bone marrow and spleen, which resulted in increased osteoclast differentiation and systemic bone loss [73]. IL-23 activates the synthesis and production of leukotriene B4 (LTB4) that exacerbates synovial inflammation in vivo and bone resorption in vitro, suggesting IL-23 might induce synovial inflammation through leukotriene B4 [39, 41]. In human PBMCs, IL-23 induces osteoclast differentiation through activation of DNAX activating protein of 12 kDa and its ITAMs [48] and up-regulates activation of osteoclast-associated genes (TRAP, CalCR, MMP9) through osteoclast transcription factor NFATc1 [48] (Figure 1). Although our group could not replicate the results others have shown that IL-23 increased expression of receptor activator of NF-kappa B (RANK) in primary murine bone marrow macrophages and promoted RANKL mediated osteoclast differentiation [74].
3. IL-23/IL-23R blockade in PsA
Currently, several antibodies against IL-23 are being developed for the treatment of psoriasis and PsA such as ustekinumab, briakinumab, tildrakizumab, guselkumab, and risankizumab (Table 1). Although clinical trials of IL-23p40 antibodies has been done and approved earlier than IL-23p19, IL-23p40 antibodies are not only inhibiting IL-23 signaling but also the IL-12 pathway, which is not required to achieve efficacy in these patients. In fact, inhibition of IL-12 is not necessary in the treatment of psoriasis and may even have negative effects and potential risks in tumor immune surveillance and in host defense against infectious diseases [75]. On the other hand, inhibition of IL-23p19 does not increase the risks of cancer development as well as bacterial/parasite infection [76]. Therefore, targeting IL-23p19 alone may be a promising treatment approach in PsA patients, by achieving a selective down-regulation of Th17 and Th22 cell responses. In this part, we will discuss about human monoclonal antibodies, which have been approved and/or are under clinical trial for PsA treatment. In contrast to antibody-based therapeutics, non-IgG based scaffolds [77] are an attractive alternative to IgG-based antibodies and at least five non-IgG based scaffolds have been developed to target IL-23: Adnectins [78], Albumin-binding domains [79, 80], Alphabodies [81], Atrimers [82] and Nanobodies [83](Figure 3).
Table 1.
IL-23/IL-23R targeted therapeutics for psoriasis and PsA
| Drug name (Reference) | Sponsor | Target | Type | Dose (mg) | Side effects | Clinical Development Status |
|---|---|---|---|---|---|---|
| Ustekinumab [84] | Janssen Biotech | IL-23p40 | Fully human IgG1κ monoclonal antibody | 45 90 | High risks infection and cancer | FDA approval (2009) |
| Briakinumab [75] | Abbott Laboratories | IL-23p40 | Fully human IgG1 monoclonal antibody | 100 200 | Serious infection and cancer | FDA withdraw (2017) |
| Tildrakizumab [87] | Sun Pharma and Merck & Co. Inc | IL-23p19 | Humanised, IgG1κ mono antibody | 100 200 | Non reported | FDA approval (2017) |
| Guselkumab [91] | Janssen | IL-23p19 | Fully human IgG1λ monoclonal antibody | 100 | Non reported | FDA approval (2017) |
| Risankizumab [92] | Abb Vie Inc | IL-23p19 | Fully human IgG1 monoclonal antibody | 90 180 | Non reported | Phase III Clinical trial |
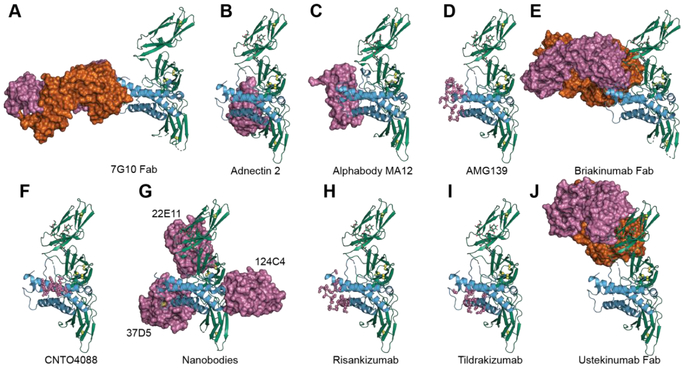
Figure 3. Various antagonists developed to neutralize IL-23.
A) Crystal structure of the IL-23 in complex with neutralizing 7G10 Fab (PDB 3D85), B) Structure of Adnectin 2, an Adnectin recognizing IL-23 (PDB 3qwr), C) Structure of MA12, an Alphabody recognizing IL-23p19 (PDB 5mj4), D) binding epitope of human antibody AMG139 mapped onto the IL-23 structure, E) Structure of briakinumab Fab, an IgG1 antibody recognizing IL-12p40 (PDB 5n2k), F) binding epitope of a recombinant mouse anti-IL23 antibody CNTO 4088 mapped onto the IL-23 structure, G) human IL-23 in complex with three nanobodies (PDB: 4grw) H) binding epitope of a humanized murine mAb Risankizumab targeting IL-23p19, mapped onto the IL-23 structure I) binding epitope of a humanized anti-IL23p19 mAb Tildrakizumab, mapped onto the IL-23 structure J) Structure of Ustekinumab-Fab, human monoclonal antibody recognizing IL-12p40 (PDB: 3hmx). In green: IL-12p40, in blue: IL-23p19, IL-23 antagonists are depicted in pink and orange surface representations.
Ustekinumab
(Stelara; Janssen Biotech, Inc.) is a human IgG1κ monoclonal antibody that binds to the common p40 subunit of IL-12 and IL-23. Ustekinumab was approved in 2009 by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of moderate-to-severe psoriasis in adult patients. In fact, the initial studies with ustekinumab were designed for the treatment of psoriasis and later studies evaluated efficacy and safety of ustekinumab for PsA treatment. Ustekinumab is subcutaneously injected into PsA patients (45 or 90 mg to ≤100 kg or >100 kg, respectively) at week 0 and week 4, and thereafter every 12 weeks [84]. Phase II clinical trial studies showed that ustekinumab significantly reduced signs and symptoms of PsA and diminished skin lesions compared with placebo [7]. In a phase III PSUMMIT 2 trial, ustekinumab significantly improved active PsA in a diverse population of patients with active PsA, including anti-TNF-experienced PsA patients [84, 85]. In 2013, FDA approved ustekinumab in the treatment of active PsA. Thus, anti-p40 antibody ustekinumab is considered effective for the treatment of psoriasis and PsA.
Briakinumab
(ABT-874; Abbott Laboratories) is another human IgG1λ monoclonal antibody that targets the shared p40 subunit of IL-12 and IL-23. Briakinumab is designed for the treatment of autoimmune diseases including psoriasis [75]. However, no study has been done to evaluate efficacy and safety of briakinumab for PsA treatment. Thus, we will summary the results on efficacy and safety of briakinumab for psoriasis because psoriasis and PsA have several mutual clinical and pathological features. The clinical trial study compared efficacy and safety of briakinumab with methotrexate, a traditional systemic agent, in patients with psoriasis [75]. The results showed that briakinumab has a higher efficacy than methotrexate in reducing the signs and symptoms of moderate-to-severe psoriasis. However, patients in the briakinumab group have developed serious infections and cancers [75]. Thus, this could be one of reasons leading to withdraw from application with the FDA due to safety concerns [86].
Tildrakizumab
(MK-3222, Sun Pharma and Merck & Co. Inc.) is a high-affinity, humanised, IgG1κ monoclonal antibody targeting IL-23p19 and designed to selectively block the IL-23 signaling pathway and it has no affinity for IL-12 [87, 88] (Figure 3). Among IL-23p19 inhibitors, tildrakizumab is the first one to show positive results in phase-3 clinical trials for the treatment of moderate-to-severe plaque psoriasis. Phase 3 trials showed that tildrakizumab (100mg and 200mg) has a higher efficiency in treatment of moderate-to-severe plaque psoriasis, compared with placebo and etanercept (a TNF inhibitor) [87]. Tildrakizumab was generally safe and well tolerated, suggesting that IL-23p19 is a crucial target for inhibition of psoriasis [88]. In 2017, tildrakizumab was approved to treat patients with moderate-to-severe plaque psoriasis.
Guselkumab
(CNTO 1959, Janssen) is a fully human IgG1λ monoclonal antibody that targets the unique IL-23p19 subunit, thus inhibiting IL-23–specific intracellular and downstream signaling [89]. In 2017, Guselkumab was approved by FDA for treatment of moderate to severe plaque psoriasis in adults [90]. Guselkumab (100 mg) is administrated through a subcutaneous injection into the patients every eight weeks (except for the second dose, which is given four weeks after the first dose) [91]. However, efficacy of guselkumab for the treatment of PsA is currently being determined in clinical trials.
Risankizumab
(BI-655066 Boehringer Ingelheim, currently ABBV-066 AbbVie) is a fully human IgG1 mAb specific for the IL-23 p19 subunit [76]. Single doses of risankizumab reduced hyperkeratosis with parakeratosis, epidermal acanthosis, and inflammation in the dermis and epidermis [76]. Moreover, treatment with risankizumab decreased expression of biomarkers associated with skin inflammation and IL-23/IL-17 signaling pathways such as K16, Ki67, β-defensin 2, and S100A7 as well as reduction of dermal infiltration by neutrophils, T cells, and dendritic cells [76]. Phase II trials showed that specific blockade of IL-23 with risankizumab had superior clinical responses in patients with moderate-to-severe plaque psoriasis, compared with ustekinumab (an IL-12 and IL-23p40 antibody) [92]. Moreover, there were no serious side effects in the 180-mg risankizumab group whereas two basal-cell carcinomas and one major cardiovascular adverse event were observed in ustekinumab group [92]. The results of phase III trials on risankizumab have not yet been reported.
4. Insights and Conclusions
Psoriatic arthritis is a type of chronic inflammatory arthritis that occurs in patients with psoriasis. Neutrophils and T cells play necessary roles in the development as well as the resolution of inflammation and hence can evoke and/or subside the pathological features that are observed in PsA patients. IL-23 activates neutrophils and T cells which in turn interplay with other immune cells to evoke PsA pathologies in skin and bone. IL-23 is also involved in regulation of IL-17 producing cells, which have effects directly/indirectly on keratinocyte proliferation, skin inflammation, and bone loss during development of PsA. We are very hopeful that future studies will shed more light on the pathogenic mechanisms relevant to human inflammatory PsA disease and further understanding of T cells and neutrophils participation in PsA will open new avenues of treatment for PsA patients.
Highlights:
IL-23 is critical in the pathogenesis of PsA.
IL-23R signaling influences adaptive and innate immunity.
Understanding the pathogenesis of PsA will lead to novel therapeutic strategies
Acknowledgements
The authors thank Thanh Nguyen for assistance with graphic design.
Financial support
This work was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01AR062173, and a National Psoriasis Foundation Translational Research grant to I.E.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
IEA has received grants, salary, consulting fees from Schering Plough Biopharma/Merck, Novartis, Pfizer and Tanabe Research Labs USA. The authors have no other conflicts of interest to declare.
REFERENCES
- [1].Sacks JJ, Helmick CG, Luo Y.h., Ilowite NT, Bowyer S, Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001–2004, Arthritis Rheum, 57 (2007) 1439–1445. [DOI] [PubMed] [Google Scholar]
- [2].Ritchlin CT, Colbert RA, Gladman DD, Psoriatic Arthritis N Engl J Med, 376 (2017) 957–970. [DOI] [PubMed] [Google Scholar]
- [3].Anandarajah AP, Ritchlin CT, The diagnosis and treatment of early psoriatic arthritis, Nat Rev Rheum, 5 (2009) 634–641. [DOI] [PubMed] [Google Scholar]
- [4].Moll JMH, Wright V , Psoriatic arthritis, Semin Arthritis Rheum 3(1973) 55–78. [DOI] [PubMed] [Google Scholar]
- [5].McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewé R, Conaghan PG, Gottlieb AB, Richards H, Pricop L, Ligozio G, Patekar M, Mpofu S, Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial, The Lancet, 386 (2015) 1137–1146. [DOI] [PubMed] [Google Scholar]
- [6].Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, Landewé R, Nash P, Pricop L, Yuan J, Richards HB, Mpofu S, Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis, N Engl J Med, 373 (2015) 1329–1339. [DOI] [PubMed] [Google Scholar]
- [7].Gottlieb A, Menter A, Mendelsohn A, Shen Y-K, Li S, Guzzo C, Fretzin S, Kunynetz R, Kavanaugh A, Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial, The Lancet, 373 (2009) 633–640. [DOI] [PubMed] [Google Scholar]
- [8].Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG, Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity, Immunity, 31 (2009) 331–341. [DOI] [PubMed] [Google Scholar]
- [9].McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ, The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17–producing effector T helper cells in vivo, Nat Immunol, 10 (2009) 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala Venkatakrishna R., Zhang H.-g., Wang T, Zheng J, Yan J, Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation, Immunity, 35 (2011) 596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guggino G, Ciccia F, Di Liberto D, Lo Pizzo M, Ruscitti P, Cipriani P, Ferrante A, Sireci G, Dieli F, Fourniè JJ, Giacomelli R, Triolo G, Interleukin (IL)‐9/IL‐9R axis drives γδ T cells activation in psoriatic arthritis patients, Clin Exp Immunol, 186 (2016) 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lowes MA, Suárez-Fariñas M, Krueger JG, Immunology of psoriasis, Annu Rev Immunol, 32 (2014) 227–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Adamopoulos IE, Bowman EP, Immune regulation of bone loss by Th17 cells, Arthritis Res Ther, 10 (2008) 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liang SC, Tan X-Y, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA, Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides, J Exp Med, 203 (2006) 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Korn T, Bettelli E, Oukka M, Kuchroo VK, IL-17 and Th17 Cells, Annu Rev Immunol, 27 (2009) 485–517. [DOI] [PubMed] [Google Scholar]
- [16].Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H, Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction, J Exp Med, 203 (2006) 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Benham H, Norris P, Goodall J, Wechalekar MD, FitzGerald O, Szentpetery A, Smith M, Thomas R, Gaston H, Th17 and Th22 cells in psoriatic arthritis and psoriasis, Arthritis Res Ther, 15 (2013) R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim K-W, Kim H-R, Park J-Y, Park J-S, Oh H-J, Woo Y-J, Park M-K, Cho M-L, Lee S-H, Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts, Arthritis Rheum, 64 (2012) 1015–1023. [DOI] [PubMed] [Google Scholar]
- [19].Ma H-L, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA, IL-22 is required for Th17 cell–mediated pathology in a mouse model of psoriasis-like skin inflammation, J Clin Invest, 118 (2008) 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S, Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells, Nat Med, 12 (2006) 1372. [DOI] [PubMed] [Google Scholar]
- [21].Adamopoulos IE, Suzuki E, Chao C-C, Gorman DAN, Adda S, Maverakis E, Zarbalis K, Geissler R, Asio A, Blumenschein WM, McClanahan T, De Waal Malefyt R, Gershwin ME, Bowman EP, IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis, Ann Rheum Dis, 74 (2015) 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mamedov MR, Scholzen A, Nair RV, Cumnock K, Kenkel JA, Oliveira JHM, Trujillo DL, Saligrama N, Zhang Y, Rubelt F, Schneider DS, Chien Y.-h., Sauerwein RW, Davis MM, A macrophage colony-stimulating factor-producing γδT cell subset prevents malarial parasitemic recurrence, Immunity, 8 (2018) 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Suzuki E, Maverakis E, Sarin R, Bouchareychas L, Kuchroo VK, Nestle FO, Adamopoulos IE, T-cell independent mechanisms associated with NETosis and selective autophagy in IL-17A-mediated epidermal hyperplasia, J Immunol, 197 (2016) 4403–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ, Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation, J Exp Med, 198 (2003) 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ, Bruce AT, Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis, J Immunol, 187 (2011) 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kvedaraite E, Lourda M, Ideström M, Chen P, Olsson-Åkefeldt S, Forkel M, Gavhed D, Lindforss U, Mjösberg J, Henter J-I, Svensson M, Tissue-infiltrating neutrophils represent the main source of IL-23 in the colon of patients with IBD, Gut, 65 (2016) 1632–1641. [DOI] [PubMed] [Google Scholar]
- [27].Chen F, Cao A, Yao S, Evans-Marin HL, Liu H, Wu W, Carlsen ED, Dann SM, Soong L, Sun J, Zhao Q, Cong Y, mTOR mediates IL-23 induction of neutrophil IL-17 and IL-22 production, J Immunol, 196 (2016) 4390–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nestle FO, Kaplan DH, Barker J, Psoriasis N Engl J Med, 361 (2009) 496–509.] [DOI] [PubMed] [Google Scholar]
- [29].Perera GK, Di Meglio P, Nestle FO, Psoriasis, Annu Rev Pathol, 7 (2012) 385–422. [DOI] [PubMed] [Google Scholar]
- [30].Schön M, Kubitza RC, Ruzicka T, Schön MP, Denzer D, Critical role of neutrophils for the generation of psoriasiform skin lesions in flaky skin mice, J Invest Dermatol, 114 (2000) 976–983. [DOI] [PubMed] [Google Scholar]
- [31].Hu SC-S, Yu H-S, Yen F-L, Lin C-L, Chen G-S, Lan C-CE, Neutrophil extracellular trap formation is increased in psoriasis and induces human β-defensin-2 production in epidermal keratinocytes, Sci Rep, 6 (2016) 31119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jansen PAM, Rodijk-Olthuis D, Hollox EJ, Kamsteeg M, Tjabringa GS, de Jongh GJ, van Vlijmen-Willems IMJJ, Bergboer JGM, van Rossum MM, de Jong EMGJ, den Heijer M, Evers AWM, Bergers M, Armour JAL, Zeeuwen PLJM, Schalkwijk J, Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin, PLoS ONE, 4 (2009) e4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Henry CM, Sullivan GP, Clancy DM, Afonina IS, Kulms D, Martin SJ, Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines, Cell Rep, 14 (2016) 708–722. [DOI] [PubMed] [Google Scholar]
- [34].Frey S, Derer A, Messbacher M-E, Baeten DLP, Bugatti S, Montecucco C, Schett G, Hueber AJ, The novel cytokine interleukin-36α is expressed in psoriatic and rheumatoid arthritis synovium, Ann Rheum Dis, 72 (2013) 1569–1574. [DOI] [PubMed] [Google Scholar]
- [35].Blumberg H, Dinh H, Dean C, Trueblood ES, Bailey K, Shows D, Bhagavathula N, Aslam MN, Varani J, Towne JE, Sims JE, IL-1RL2 and its ligands contribute to the cytokine network in psoriasis, J Immunol, 185 (2010) 4354–4362. [DOI] [PubMed] [Google Scholar]
- [36].Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN, Keratins (Kl6 and Kl7) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro, Br J Dermatol, 133 (1995) 501–511. [DOI] [PubMed] [Google Scholar]
- [37].Yang L, Fan X, Cui T, Dang E, Wang G, Nrf2 promotes keratinocyte proliferation in psoriasis through up-regulation of keratin 6, keratin 16, and keratin 17, J Invest Dermatol., 137 (2017) 2168–2176. [DOI] [PubMed] [Google Scholar]
- [38].Schonthaler Helia B., Guinea-Viniegra J, Wculek Stefanie K., Ruppen I, Ximénez-Embún P, Guío-Carrión A, Navarro R, Hogg N, Ashman K, Wagner Erwin F., S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3, Immunity, 39 (2013) 1171–1181. [DOI] [PubMed] [Google Scholar]
- [39].Dixit N, Wu DJ, Belgacem YH, Borodinsky LN, Gershwin ME, Adamopoulos IE, Leukotriene B4 activates intracellular calcium and augments human osteoclastogenesis, Arthritis Res Ther, 16 (2014) 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oyoshi Michiko K., He R, Li Y, Mondal S, Yoon J, Afshar R, Chen M, Lee David M., Luo Hongbo R., Luster Andrew D., Cho John S., Miller Lloyd S., Larson A, Murphy George F., Geha Raif S., Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation, Immunity, 37 (2012) 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bouchareychas L, Grössinger EM, Kang M, Qiu H, Adamopoulos IE, Critical role of LTB4/BLT1 in IL-23 induced synovial inflammation and osteoclastogenesis via NF-κB, J Immunol, 198 (2017) 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Teitelbaum SL, Bone Resorption by Osteoclasts, Science, 289 (2000) 1504–1508. [DOI] [PubMed] [Google Scholar]
- [43].Boyle WJ, Simonet WS, Lacey DL, Osteoclast differentiation and activation, Nature, 423 (2003) 337–342. [DOI] [PubMed] [Google Scholar]
- [44].Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J.-i., Wagner EF, Mak TW, Kodama T, Taniguchi T, Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts, Dev Cell, 3 (2002) 889–901. [DOI] [PubMed] [Google Scholar]
- [45].Jean‐Marie D, A. TL, E. MT, Kim H, Tine T, Laurence B, Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities, Microsc Res Tech, 61 (2003) 504–513. [DOI] [PubMed] [Google Scholar]
- [46].Minkin C, Bone acid phosphatase: Tartrate-resistant acid phosphatase as a marker of osteoclast function, Calcif Tissue Int, 34 (1982) 285–290. [DOI] [PubMed] [Google Scholar]
- [47].Chiu YG, Shao T, Feng C, Mensah KA, Thullen M, Schwarz EM, Ritchlin CT, CD16 (FcRγIII) as a potential marker of osteoclast precursors in psoriatic arthritis, Arthritis Res Ther, 12 (2010) R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shin H-S, Sarin R, Dixit N, Wu J, Gershwin ME, Bowman EP, Adamopoulos IE, Crosstalk among interleukin 23 and DNAX activating protein 12-dependent pathways promotes osteoclastogenesis, J Immunol, 194 (2015) 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Adamopoulos IE, Mellins ED, Alternative pathways of osteoclastogenesis in inflammatory arthritis, Nat Rev Rheumatol, 11 (2015) 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M.-r., Gorman D, Wagner J, Zurawski S, Liu Y-J, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA, Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12, Immunity, 13 (2000) 715–725. [DOI] [PubMed] [Google Scholar]
- [51].Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O’Farrell A-M, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW, A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R, J Immunol, 168 (2002) 5699–5708. [DOI] [PubMed] [Google Scholar]
- [52].Boulanger MJ, Chow D.-c., Brevnova EE, Garcia KC, Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex, Science, 300 (2003) 2101–2104. [DOI] [PubMed] [Google Scholar]
- [53].Skiniotis G, Boulanger MJ, Garcia KC, Walz T, Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor, Nat Struct Mol Biol, 12 (2005) 545–551. [DOI] [PubMed] [Google Scholar]
- [54].Bloch Y, Bouchareychas L, Merceron R, Składanowska K, Van den Bossche L, Detry S, Govindarajan S, Elewaut D, Haerynck F, Dullaers M, Adamopoulos IE, Savvides SN, Structural activation of pro-inflammatory human cytokine IL-23 by cognate IL-23 receptor enables recruitment of the shared receptor IL-12Rβ1, Immunity, (2018) 45–58.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cooper AM, Khader SA, IL-12p40: an inherently agonistic cytokine, Trends Immunol, 28 (2007) 33–38. [DOI] [PubMed] [Google Scholar]
- [56].Awasthi A, Riol-Blanco L, Jäger A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M, IL-23 receptor GFP reporter mice reveal distinct populations of IL-17-producing cells, J Immunol, 182 (2009) 5904–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zindl CL, Lai J-F, Lee YK, Maynard CL, Harbour SN, Ouyang W, Chaplin DD, Weaver CT, IL-22–producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis, Proc Natl Acad Sci U S A, 110 (2013) 12768–12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cho M-L, Kang J-W, Moon Y-M, Nam H-J, Jhun J-Y, Heo S-B, Jin H-T, Min S-Y, Ju J-H, Park K-S, Cho Y-G, Yoon C-H, Park S-H, Sung Y-C, Kim H-Y, STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice, J Immunol, 176 (2006) 5652–5661. [DOI] [PubMed] [Google Scholar]
- [59].Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou H-C, Beg AA, Chen YH, The Th17 immune response is controlled by the Rel–RORγ–RORγT transcriptional axis, J Exp Med, 208 (2011) 2321–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cheung PFY, Wong CK, Lam CWK, Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation, J Immunol, 180 (2008) 5625–5635. [DOI] [PubMed] [Google Scholar]
- [61].Pahl HL, Activators and target genes of Rel/NF-κB transcription factors, Oncogene, 18 (1999) 6853–6866. [DOI] [PubMed] [Google Scholar]
- [62].Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A, IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A, J Immunol, 186 (2011) 1495–1502. [DOI] [PubMed] [Google Scholar]
- [63].Nakajima K, Kanda T, Takaishi M, Shiga T, Miyoshi K, Nakajima H, Kamijima R, Tarutani M, Benson JM, Elloso MM, Gutshall LL, Naso MF, Iwakura Y, DiGiovanni J, Sano S, Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model, J Immunol, 186 (2011) 4481–4489. [DOI] [PubMed] [Google Scholar]
- [64].Hedrick MN, Lonsdorf AS, Shirakawa A-K, Lee C-CR, Liao F, Singh SP, Zhang HH, Grinberg A, Love PE, Hwang ST, Farber JM, CCR6 is required for IL-23–induced psoriasis-like inflammation in mice, J Clin Invest, 119 (2009) 2317–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Getschman AE, Imai Y, Larsen O, Peterson FC, Wu X, Rosenkilde MM, Hwang ST, Volkman BF, Protein engineering of the chemokine CCL20 prevents psoriasiform dermatitis in an IL-23–dependent murine model, Proc Natl Acad Sci U S A, 114 (2017) 12460–12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cibrian D, Saiz ML, de la Fuente H, Sánchez-Díaz R, Moreno-Gonzalo O, Jorge I, Ferrarini A, Vázquez J, Punzón C, Fresno M, Vicente-Manzanares M, Daudén E, Fernández-Salguero PM, Martín P, Sánchez-Madrid F, CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis, Nat Immunol, 17 (2016) 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang Y, Edelmayer R, Wetter J, Salte K, Gauvin D, Leys L, Lippert S, Su Z, Miller L, Huang S, Honore P, Kort M, McGaraughty S, Scott V, Gauld SB, Macrophages play a pathogenic role in IL-23 mediated psoriasiform skin inflammation, J Immunol, 198 (2017) 127.111. [Google Scholar]
- [68].Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K, IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice, J Immunol, 179 (2007) 8274–8279. [DOI] [PubMed] [Google Scholar]
- [69].Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, Gorman DM, Smith K, de Waal Malefyt R, Kastelein RA, McClanahan TK, Bowman EP, IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2–dependent mechanisms with implications for psoriasis pathogenesis, J Exp Med, 203 (2006) 2577–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kopp T, Lenz P, Bello-Fernandez C, Kastelein RA, Kupper TS, Stingl G, IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity, J Immunol, 170 (2003) 5438–5444. [DOI] [PubMed] [Google Scholar]
- [71].Tu Z, Zhang S, Zhou G, Zhou L, Xiang Q, Chen Q, Zhao P, Zhan H, Zhou H, Sun L, LMO4 is a disease-provocative transcription coregulator activated by IL-23 in psoriatic keratinocytes, J Invest Dermatol, (2017) 1078–1087. [DOI] [PubMed] [Google Scholar]
- [72].Lindroos J, Svensson L, Norsgaard H, Lovato P, Moller K, Hagedorn PH, Olsen GM, Labuda T, IL-23-mediated epidermal hyperplasia is dependent on IL-6, J Invest Dermatol, 131 (2011) 1110–1118. [DOI] [PubMed] [Google Scholar]
- [73].Adamopoulos IE, Tessmer M, Chao C-C, Adda S, Gorman D, Petro M, Chou C-C, Pierce RH, Yao W, Lane NE, Laface D, Bowman EP, IL-23 is critical for the induction of arthritis, osteoclast formation and maintenance of bone mass, J Immunol, 187 (2011) 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chen L, Wei X-Q, Evans B, Jiang W, Aeschlimann D, IL-23 promotes osteoclast formation by up-regulation of receptor activator of NF-κB (RANK) expression in myeloid precursor cells, Eur J Immunol, 38 (2008) 2845–2854. [DOI] [PubMed] [Google Scholar]
- [75].Reich K, Langley RG, Papp KA, Ortonne J-P, Unnebrink K, Kaul M, Valdes JM A 52-Week Trial Comparing Briakinumab with Methotrexate in Patients with Psoriasis, N Engl J Med 365 (2011) 1586–1596. [DOI] [PubMed] [Google Scholar]
- [76].Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, Lalovic B, Aslanyan S, Wang EEL, Hall D, Solinger A, Padula S, Scholl P, Anti–IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial, J Allergy Clin Immunol, 136 (2015) 116–124.e117. [DOI] [PubMed] [Google Scholar]
- [77].Škrlec K, Štrukelj B, Berlec A, Non-immunoglobulin scaffolds: a focus on their targets, Trends Biotechnol, 33 (2015) 408–418. [DOI] [PubMed] [Google Scholar]
- [78].Lipovšek D, Adnectins: engineered target-binding protein therapeutics, Protein Eng Des Sel, 24 (2011) 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Křížová L, Kuchař M, Petroková H, Osička R, Hlavničková M, Pelák O, Černý J, Kalina T, Malý P, p19-targeted ABD-derived protein variants inhibit IL-23 binding and exert suppressive control over IL-23-stimulated expansion of primary human IL-17+ T-cells, Autoimmunity, 50 (2017) 102–113. [DOI] [PubMed] [Google Scholar]
- [80].Kuchař M, Vaňková L, Petroková H, Černý J, Osička R, Pelák O, Šípová H, Schneider B, Homola J, Šebo P, Kalina T, Malý P, Human interleukin-23 receptor antagonists derived from an albumin-binding domain scaffold inhibit IL-23-dependent ex vivo expansion of IL-17-producing T-cells, Proteins, 82 (2014) 975–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Desmet J, Verstraete K, Bloch Y, Lorent E, Wen Y, Devreese B, Vandenbroucke K, Loverix S, Hettmann T, Deroo S, Somers K, Henderikx P, Lasters I, Savvides SN, Structural basis of IL-23 antagonism by an Alphabody protein scaffold, Nat Commun, 5 (2014) 5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Anke K-R, Martha W, Katherine B, Elise C, Daniela O, Maria G, Mili K, Polypeptides that bind to IL-23R Anaphore, Inc., USA Patent, 2010, pp. WO2011043835A2011043831.
- [83].Desmyter A, Spinelli S, Boutton C, Saunders M, Blachetot C, de Haard H, Denecker G, Van Roy M, Cambillau C, Rommelaere H, Neutralization of human Interleukin 23 by multivalent nanobodies explained by the structure of cytokine–nanobody complex, Front Immunol, 8 (2017) 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM, Doyle MK, Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial, Lancet, 382 (2013) 780–789. [DOI] [PubMed] [Google Scholar]
- [85].Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, Wang Y, Shen Y-K, Doyle MK, Mendelsohn AM, Gottlieb AB, Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial, Ann Rheum Dis, (2014) 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Traczewski P, Rudnicka L, Briakinumab for the treatment of plaque psoriasis, BioDrugs, 26 (2012) 9–20. [DOI] [PubMed] [Google Scholar]
- [87].Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, Nograles K, Mehta A, Cichanowitz N, Li Q, Liu K, La Rosa C, Green S, Kimball AB, Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials, The Lancet, 390 (2017) 276–288. [DOI] [PubMed] [Google Scholar]
- [88].Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, Gottlieb AB, Nakagawa H, Bowman EP, Mehta A, Li Q, Zhou Y, Shames R, Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial, Br J Dermatol, 173 (2015) 930–939. [DOI] [PubMed] [Google Scholar]
- [89].Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, Shen Y-K, Szapary P, Randazzo B, Reich K, A Phase 2 Trial of Guselkumab versus Adalimumab for Plaque Psoriasis, N Engl J Med, 373 (2015) 136–144. [DOI] [PubMed] [Google Scholar]
- [90].Markham A, Guselkumab: First Global Approval, Drugs, 77 (2017) 1487–1492. [DOI] [PubMed] [Google Scholar]
- [91].Blauvelt A, Papp KA, Griffiths CEM, Randazzo B, Wasfi Y, Shen Y-K, Li S, Kimball AB, Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator–controlled VOYAGE 1 trial, J Am Acad Dermatol, 76 (2017) 405–417. [DOI] [PubMed] [Google Scholar]
- [92].Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour J-P, Menter A, Philipp S, Sofen H, Tyring S, Berner BR, Visvanathan S, Pamulapati C, Bennett N, Flack M, Scholl P, Padula SJ, Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis, N Engl J Med, 376 (2017) 1551–1560. [DOI] [PubMed] [Google Scholar]