Abstract
Objectives
Cancer cachexia, characterized by weight loss and sarcopenia, leads to a decline in physical function and is associated with poorer survival. Cancer cachexia remains poorly described in older adults with cancer. This study aims to characterize cancer cachexia in older adults by assessing its prevalence utilizing standard definitions and evaluating associations with components of the geriatric assessment (GA) and survival.
Materials and Methods:
Patients with cancer older than 65 years of age who underwent a GA and had baseline CT imaging were eligible in this cross-sectional study. Cancer cachexia was defined by the international consensus definition reported in 2011. Sarcopenia was measured using cross-sectional imaging and utilizing sex-specific cut-offs. Associations between cachexia, sarcopenia, and weight loss with survival and GA domains were explored.
Results:
Mean age of 100 subjects was 79.9 years (66–95) and 65% met criteria for cancer cachexia. Cachexia was associated with impairment in instrumental activities of daily living (IADL) (p=.017); no significant association was found between sarcopenia or weight loss and IADL impairment. Cachexia was significantly associated with poorer survival (median 1.0 vs 2.1 years, p=0.011).
Conclusions:
Cancer cachexia as defined by the international consensus definition is prevalent in older adults with cancer and is associated with functional impairment and decreased survival. Larger prospective studies are needed to further describe cancer cachexia in this population.
Keywords: cachexia, sarcopenia, geriatric oncology, geriatric assessment, weight loss, functional impairment
Introduction
Cancer cachexia is a prevalent syndrome across tumor types, occurring in 60% of patients with lung cancer and 50–85% of patients with gastrointestinal tract (GI) cancers(1). Characterized by loss of weight, muscle mass, and fat mass, cancer cachexia leads to reduced physical performance, functional impairment, and decreased quality of life, and it is associated with a poorer prognosis(1, 2). Despite its frequency in oncology practice, cancer cachexia is understudied and has no effective treatments(3). Over the past several years, however, heightened awareness of cancer cachexia has followed the publication of an international consensus panel definition and the reporting of two large phase III cachexia intervention trials (ROMANA trials)(2, 4). Considered the current gold standard, the international consensus panel defines cachexia as >5% weight loss in the preceding six months or >2% weight loss with one of the following: Body Mass index (BMI) of <20 kg/m2 or sarcopenia based on imaging analysis. Though the definition of sarcopenia has evolved over the past ten years to include, by some, the requirement of reduced muscle strength or physical performance along with muscle loss(5), the international consensus definition of cancer cachexia uses the term sarcopenia to indicate muscle loss alone. Overlap between the definitions of cachexia and sarcopenia exists; however, the definition described by the international consensus panel distinguishes cancer cachexia from sarcopenia by combining changes in weight, BMI and muscle in its diagnostic criteria, but omitting performance or functional measures. Though limitations may exist, establishment of an accepted, standardized, and validated definition of cancer cachexia has been crucial in the enrollment of more homogeneous populations in cachexia biomarker, observational, and intervention studies.
The prevalence of sarcopenia has been reported in geriatric oncology populations(6); however, the syndrome of cachexia is poorly described in older adults with cancer. No randomized cancer cachexia intervention trials in the geriatric oncology population have been published; the mean age in the ROMANA trial, a negative intervention trial investigating a ghrelin-agonist, was 63 years (range 30–88 years)(4). This lack of data in older adults with cancer is troubling, as cancers with the highest incidence of cachexia are diagnosed predominantly in those with advanced age. Currently, it is unclear if the international consensus definition of cancer cachexia is associated with objective physical performance, physical function, quality of life and clinical outcomes in older adults. The validation study of the consensus definition was developed from a comprehensive evaluation of survival, nutritional measures, inflammatory biomarkers and patient-reported outcomes in 861 cachectic and noncachectic patients with cancer(7). Unfortunately, this study consisted of a cohort with less than a quarter of its subjects over the age of 70. A significant knowledge gap exists in the understanding of cachexia in the geriatric oncology population.
The geriatric assessment (GA) is a “comprehensive evaluation of a patient’s physical, functional, social and psychological well-being” that can help guide the care and treatment of older adults(8). Given the breadth of data collected during this evaluation, the GA may be a very useful tool to evaluate the relationship between cachexia and outcomes in older patients with cancer. Combining data obtained in the GA with novel methods to study body composition could aid in healthcare decision making and heighten the ability to study cachexia in this population. The recent development of software that can accurately assess muscle and fat mass by analyzing a single cross-sectional image slice, typically obtained from the lumbar spine region, provides a quick, cost-effective measure to study body morphometrics(9, 10). Obtaining body composition measurements from computed tomography (CT) or magnetic resonance imaging (MRI) is now considered the preferred method for assessing muscle and fat mass, rather than dual energy x-ray absorptiometry (DEXA) and bioelectrical impedance (BIA)(2).
In this study, we aimed to describe the prevalence of cancer cachexia in older adults by assessing weight loss and using cross-sectional imaging to evaluate muscle. Furthermore, by examining associations between cancer cachexia, GA impairments, and survival, we hoped to further characterize the clinical implications of cachexia in older adults with cancer. Lastly, we explore outcomes of subgroups of patients by stratifying for muscle loss alone or weight loss alone to examine if these measures are better predictors of outcomes in older adults with cancer than cachexia.
Materials and Methods
Eligibility Criteria
Subjects were recruited from the Specialized Oncology Care and Research in the Elderly (SOCARE) clinic at the University of Rochester between February 2013 and February 2015 on an institutional review board (IRB)-approved protocol. The SOCARE clinic provides primary oncologic care and consultative geriatric oncology services for the University of Rochester and community oncology practices in Western New York. Subjects had a confirmed cancer diagnosis and completed both a GA and CT imaging of the abdomen and pelvis within six weeks of each other. Patients with prostate cancer were excluded, because the most common treatment (androgen deprivation therapy) significantly reduces muscle mass, particularly in men ≥70 years(11).
Definition of Cachexia, Sarcopenia and Variables of Interest
In this study, the following diagnostic criteria based on the international consensus definition were employed for cancer cachexia: a) weight loss greater than 5% in the previous six months, b) weight loss greater than 2% in the previous six months and a BMI less than 20 kg/m2, or c) weight loss greater than 2% in the previous six months and sarcopenia as determined by CT imaging analysis(2). Self-reported weight loss was collected as part of the GA. For consistency in measuring cachexia according to standard criteria, sarcopenia was defined as muscle loss alone in this study. Imaging analysis to detect sarcopenia was performed by utilizing a computer software program, Slice-O-Matic 5.1 (Tomovision, Canada), to calculate muscle area in the region of the third lumbar vertebra; this method has been shown to predict whole body muscle mass(10). Muscle was identified and quantified by using the Hounsfield unit scale ranging from −30 to −150 Hounsfield units. Sarcopenia was determined by the skeletal muscle index, which was calculated by muscle area (cm2)/ height (m2) and stratified using validated sex-specific cut-offs (Female 38.5 cm2/m2, Male 52.4 cm2/m2)(9); BMI cut-offs were not used for skeletal muscle index as a large majority of subjects had a BMI <30 kg/m2. Other measures of interest included weight loss in the previous six months, stratified at levels of >5% and >10%, regardless of change in muscle.
The following demographic and clinical measures were collected: age, gender, cancer type, and cancer stage. GA measures collected included falls, instrumental activities of daily living (IADL), Short Physical Performance Battery (SPPB), the Geriatric Depression Scale (GDS), self-reported fatigue, and engagement in exercise. Exercise, defined in the questionnaire as “any planned physical activity performed to increase physical fitness,” is self-reported as yes or no. GA variables were dichotomized into impaired and non-impaired groups based on cut-off scores established in the literature(12); cut-offs used in previous SOCARE studies were specifically utilized for consistency.
Statistical Analysis
Descriptive statistics were used to characterize the distribution of cachexia, sarcopenia, weight loss, and demographic and clinical covariates. The chi-square test was used to assess bivariate relations of demographic, clinical characteristics, and GA impairments with 1) cachexia (defined per the international consensus definition), 2) sarcopenia (defined by low skeletal muscle index described above), and 3) weight loss of >5% and >10%. Overall survival (OS) was estimated using the Kaplan-Meier method with time measured from the date of confirmed tissue diagnosis to date of death or date of last follow-up. The association of cachexia, as established by the international consensus definition, sarcopenia or weight loss as binary variables with OS was appraised by log-rank test in univariate analyses and further tested in multivariate analyses using the proportional hazards model. Proportionality assumption was checked by the log(-log(survival)) versus log (time) plots. Statistical analysis was conducted via SAS Version 9.4 (SAS Institute Inc., Cary, North Carolina). Statistical significance was set at P ≤ 0.05.
Results
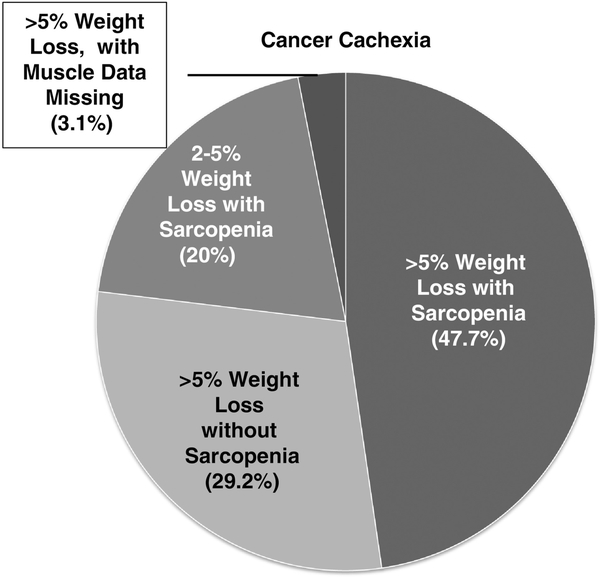
In total, 117 consecutive patients met eligibility from February 1, 2013, to February 28, 2015; 100 had detailed data on weight loss, and of those 100 subjects, 95 had cross-sectional imaging of adequate quality to evaluate muscle mass. Cachexia data are available for those five patients with poor quality imaging because they met diagnostic criteria based on weight loss alone. Mean age was 79.9 years (range: 66–95 years) and 55% were male. The cohort included mostly patients who had GI (49%) and lung (24%) cancers. Both early and advanced disease states were represented; 50% of the cohort had stage IV cancer. Most patients were treatment naïve at the time of the geriatric assessment. Only 7% and 8% had received chemotherapy or radiation in the past 6 months, respectively. In regards to surgical intervention, 21% underwent cancer surgery in the past year. Per international consensus definition, 65% of subjects had cancer cachexia; 80% of those with cachexia had greater than 5% weight loss and 20% had between 2% and 5% weight loss with evidence of sarcopenia (Figure 1). The average BMI and percent weight loss of those who met criteria for cachexia was 25.73 kg/m2 and 11.3%, respectively. There were no statistically significant differences found in prevalence of cachexia by gender, age, cancer type or stage (Table 1a). In regards to changes in muscle, sarcopenia was found in 57.9% of patients and was more prevalent in men and those with advanced stage cancers (Table 1b). Overall, 51%, and 29% were found to have >5% and >10% weight loss, respectively.
Figure 1.
Characterizing cancer cachexia: In those with cancer cachexia, the pie chart demonstrates what percentage of patients fit certain criteria for diagnosis relative to weight loss and sarcopenia.
Table 1.
Cancer cachexia and sarcopenia and their relationships to demographics, clinical characteristics and GA impairments.
| a) | ||||
|---|---|---|---|---|
| Cohort Description | Cachexia (per International consensus definition | |||
| Variable | Group | n | Cachexia (%) | P-value (chi-sq) |
| All (n=100) | All | 100 | 65.0 | |
| Gender (n=99) | Female | 44 | 59.1 | 0.219 |
| Male | 55 | 70.9 | ||
| Cancer type | Upper GI | 31 | 77.4 | 0.196 |
| Lower GI | 18 | 61.1 | ||
| Lung | 24 | 50.0 | ||
| GU | 11 | 81.8 | ||
| Breast | 5 | 40.0 | ||
| Other | 11 | 63.6 | ||
| Stage (n=91) | 1 | 11 | 54.5 | 0.415 |
| 2 | 10 | 70.0 | ||
| 3 | 25 | 60.0 | ||
| 4 | 45 | 75.6 | ||
| Stage (n=91) | 1–3 | 46 | 60.9 | 0.133 |
| 4 | 45 | 75.6 | ||
| Age (n=98) | <=75 | 25 | 68.0 | 0.872 |
| 76–80 | 27 | 66.7 | ||
| 81–85 | 30 | 66.7 | ||
| >=85 | 16 | 56.3 | ||
| Falls (n=97) | No | 61 | 62.3 | 0.476 |
| Yes | 36 | 69.4 | ||
| IADL (n=99) | 0 | 33 | 48.5 | 0.017 |
| >=1 | 66 | 72.7 | ||
| SPPB (n=89) | >=9 | 22 | 54.5 | 0.285 |
| <=8 | 67 | 67.2 | ||
| GDS (n=94) | <=4 | 65 | 69.2 | 0.187 |
| >4 | 29 | 55.2 | ||
| Fatigue (n=58) | No | 17 | 58.8 | 0.879 |
| Yes | 41 | 61.0 | ||
| b) | ||||
|---|---|---|---|---|
| Cohort Description | Sarcopenia (muscle loss alone) | |||
| Variable | Group | N | Sarcopenia (%) | P-value (chi-sq) |
| All (n=95) | All | 95 | 57.9 | |
| Gender | Female | 41 | 36.6 | <0.001 |
| Male | 54 | 74.1 | ||
| Cancer type | Upper GI | 31 | 61.3 | 0.425 |
| Lower GI | 16 | 62.5 | ||
| Lung | 23 | 43.5 | ||
| GU | 10 | 80.0 | ||
| Breast | 5 | 40.0 | ||
| Other | 10 | 60.0 | ||
| Stage (n=88) | 1 | 10 | 40.0 | 0.132 |
| 2 | 10 | 60.0 | ||
| 3 | 24 | 45.8 | ||
| 4 | 44 | 70.5 | ||
| Stage (n=88) | 1–3 | 44 | 47.7 | 0.030 |
| 4 | 44 | 70.5 | ||
| Age (n=95) | <=75 | 25 | 56.0 | 0.817 |
| 76–80 | 25 | 56.0 | ||
| 81–85 | 29 | 55.2 | ||
| >=85 | 16 | 68.8 | ||
| Falls (n=92) | No | 58 | 60.3 | 0.488 |
| Yes | 34 | 52.9 | ||
| IADL (n=94) | 0 | 30 | 46.7 | 0.148 |
| >=1 | 64 | 62.5 | ||
| SPPB (n=86) | >=9 | 21 | 52.4 | 0.716 |
| <=8 | 65 | 56.9 | ||
| GDS (n=88) | <=4 | 62 | 56.5 | 0.951 |
| >4 | 28 | 57.1 | ||
| Fatigue (n=57) | No | 17 | 41.2 | 0.096 |
| Yes | 40 | 65.0 | ||
The prevalence of cachexia was significantly different between subjects with no IADL impairments compared to those with ≥ 1 IADL impairment (48.5 vs. 72.5%, p=.017). No statistically significant associations between >5% weight loss alone and IADL dependence (56.1% vs. 42.2%, p=0.201) or sarcopenia alone and IADL dependence (62.5% vs 46.7%, p=0.147) were detected. Cachexia, sarcopenia, and >5% and >10% weight loss were not significantly associated with other metrics of the GA (falls, lower SPPB score, depression, fatigue or self-reported exercise (Table 1, weight loss data not shown).
Overall, 80 patients died during the study and follow-up; median time to death was 1.2 years and median follow-up among surviving patients was 3.4 years. Cachexia was associated with poorer overall survival when compared to non-cachectic older adults with cancer (median 1.0 vs 2.1 years, p=0.011, Figure 2). This association with poorer survival remained significant after adjusting for cancer type and stage (hazard ratio[HR]= 1.82; 95% CI: 1.06–3.11; p=0.029). In bivariate analysis, sarcopenia was also associated with worse overall survival (median: 0.9 vs. 1.6 years; p=0.028; Kaplan –Meier not shown). However, after adjusting for cancer type and stage, this association was no longer statistically significant (HR=1.50;95% CI: 0.91–2.48; p=0.116). We tested if >5% or >10% weight loss was associated with survival and found no statistically significant association (p= .0739 and p=.1541, respectively).
Figure 2.
Overall survival stratified by cachexia.
Discussion
This study is one of the first to evaluate the prevalence of cancer cachexia in older adults and its associated with GA domains and survival. Our results demonstrate that cancer cachexia, as defined by the international consensus definition published in 2011, is highly prevalent in the geriatric oncology population and is associated with functional impairment. Furthermore, cancer cachexia is negatively associated with survival in older adults with cancer across tumor types and different stages of disease. The diagnostic criteria that were developed and intended to describe a syndrome “of ongoing loss of skeletal muscle mass (with or without loss of fat mass) that … leads to progressive functional impairment”(2) have not been formally validated in the geriatric oncology population specifically. Our findings, though less comprehensive than a study designed to validate these criteria(7), indicate that this definition is useful in the study of cachexia in older adults given its association with functional impairment and worse survival.
We found that muscle loss alone may not be the best predictor of adverse outcomes in older adults with cancer, possibly due in part to the fact that sarcopenia, regardless of a pathologic diagnosis, is a geriatric syndrome and a physiologic process of normal aging. Evaluation specifically for the syndrome of cachexia, which combines weight loss, severely low BMI, and reduced muscle mass, is more comprehensive and appears to be a better predictor of adverse outcomes in older adults with cancer than the assessment of muscle loss or weight loss alone. A fair criticism of the international consensus definition of cancer cachexia is that it fails to incorporate physical performance or functional measures as does the European Consensus definition of sarcopenia(5). However, our demonstration of the association of cancer cachexia with functional impairment in older adults with cancer indicates that this may not be necessary. Nonetheless, the complexity in identifying cachexia and/or sarcopenia in this population underscores the importance of using the GA in the evaluation of nutrition and body composition in older adults with cancer.
There are several limitations to consider when analyzing these data. This is a single institution study of a cohort of patients referred to a specialized geriatric oncology clinic by mostly medical oncologists with a referral base that may be more likely to be pre-frail or frail. Additionally, patients with hematologic malignancies were not represented. Although these patients were not intentionally excluded from this study, referrals of patients with hematologic malignancies to the SCOARE clinic was low. The cohort has a large representation of GI and lung cancers. While these cancers are among the most common, they are also the two types most commonly associated with cachexia(3). The small representation of breast cancer in our cohort, and the referral patterns described above, indicate that a cachexia prevalence of 65% could be an overestimate. Lastly, this study assessed endpoints (cachexia, sarcopenia, weight loss, and GA impairments) that were captured at one time-point and compared to each other and survival. Prospective longitudinal observation and intervention studies are needed to track changes in body composition, physical function, and patient-reported outcomes throughout the disease course to better understand the natural history of cancer cachexia in older adults.
In conclusion, cancer cachexia, as defined by the international consensus panel and reported in 2011, is prevalent and is associated with functional impairment and worse overall survival in older adults with cancer. Our findings indicate that cancer cachexia may be a better predictor of adverse outcomes than sarcopenia or weight loss alone. Though not routinely used in clinical practice, CT analysis for sarcopenia, in addition to the GA that includes detailed data on weight, may help guide clinical decision making or discussions regarding prognosis. A larger prospective study that incorporates cachexia-related symptoms, nutritional intake, and survival in addition to the above outcomes is needed to better understand cancer cachexia in this population and demonstrate how identifying and treating this syndrome may impact the care of older adults with cancer.
Acknowledgements:
We would like to thank Dr. Susan Rosenthal for her invaluable guidance in writing and language and for editing the manuscript.
Funding: The work was funded by the Wilmot Foundation for Cancer Research. The work was also funded through UG1 CA189961 from the National Cancer Institute, and R01 CA177592 from the National Cancer Institute. This work was made possible by the generous donors to the WCI geriatric oncology philanthropy fund. All statements in this report, including its findings and conclusions, are solely those of the authors.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart GD, Skipworth RJ, Fearon KC. Cancer cachexia and fatigue. Clin Med (Lond). 2006. Mar-Apr;6(2):140–3. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011. May;12(5):489–95. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 3.Dunne RF, Mustian KM, Garcia JM, Dale W, Hayward R, Roussel B, et al. Research priorities in cancer cachexia: The University of Rochester Cancer Center NCI Community Oncology Research Program Research Base Symposium on Cancer Cachexia and Sarcopenia. Curr Opin Support Palliat Care. 2017. December;11(4):278–86. PubMed PMID: Pubmed Central PMCID: PMC5658778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016. April;17(4):519–31. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010. July;39(4):412–23. PubMed PMID: Pubmed Central PMCID: 2886201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broughman JR, Williams GR, Deal AM, Yu H, Nyrop KA, Alston SM, et al. Prevalence of sarcopenia in older patients with colorectal cancer. J Geriatr Oncol. 2015. November;6(6):442–5. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, et al. Validation of the Consensus-Definition for Cancer Cachexia and evaluation of a classification model--a study based on data from an international multicentre project (EPCRC-CSA). Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014. August;25(8):1635–42. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 8.Mohile SG, Velarde C, Hurria A, Magnuson A, Lowenstein L, Pandya C, et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Canc Netw. 2015. September;13(9):1120–30. PubMed PMID: Pubmed Central PMCID: PMC4630807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. The Lancet Oncology. 2008. July;9(7):62935 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 10.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2008. October;33(5):997–1006. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TL, Ke C, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2012. September 10;30(26):3271–6. PubMed PMID: . Pubmed Central PMCID: PMC3434987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loh KP, Pandya C, Zittel J, Kadambi S, Flannery M, Reizine N, et al. Associations of sleep disturbance with physical function and cognition in older adults with cancer. Support Care Cancer. 2017. October;25(10):3161–9. PubMed PMID: . Pubmed Central PMCID: PMC5660663. [DOI] [PMC free article] [PubMed] [Google Scholar]