Abstract
Purpose:
Local ablative treatment to oligometastatic patients can result in long-term disease-free survival in some cancer patients. The importance of this treatment paradigm in prostate cancer is a rapidly evolving field. Herein we report on the safety and preliminary clinical outcomes of a modern cohort of oligometastatic prostate cancer (OPC) patients treated with consolidative stereotactic ablative radiation (SABR).
Methods:
Records of men with OPC who underwent consolidative SABR at our institution were reviewed. SABR was delivered in 1–5 fractions of 5–18 Gray. Kaplan-Meier estimates of local progression-free survival (LPFS), biochemical progression-free survival (bPFS; PSA nadir+2), distant progression free survival (DPFS) and time-tonext intervention (TTNI) were calculated.
Results:
In total, 66 OPC patients were identified with consolidative SABR delivered to 134 metastases: 89 bone, 40 nodal, and 5 viscera. The majority of men (49/66) had hormone sensitive prostate cancer (HSPC). Crude Grade 1 and 2 acute toxicities were 36% and 11% respectively with no ≥ Grade 3 toxicity. At 1-year LPFS was 92% and bPFS and DPFS were 69%. Of the 18 men with HSPC who had hormone therapy deferred, 11 (56%) remain disease free following SABR (1-year ADT-FS was 78%). In 17 castration resistant men, 11 had > 50% PSA declines with 1year TTNI of 30%.
Conclusions:
Consolidative SABR in OPC is feasible and well tolerated. The heterogeneity and small size of our series limits extrapolation of clinically meaningful outcomes following consolidative SABR in OPC, but our preliminary data suggests this approach warrants continued prospective study.
Keywords: Oligometastatic prostate cancer, stereotactic ablative radiation therapy, androgen deprivation therapy
Introduction
Originally proposed by Hellman and Weichselbaum [1], the oligometastatic or low volume metastatic state is currently defined as having fewer than 3 to 5 metastatic lesions [2]. As the collective understanding of this state evolves, biological parameters may someday inform or supplant this descriptive clinical definition [3–5]. Improvements in imaging and metastasis-directed therapies (MDT) have placed new emphasis on the oligometastatic state [6]. Local ablative treatment for limited metastatic disease has been correlated retrospectively with improvements in long term disease-free survival in lung, breast, colorectal, and sarcoma patients [6] and mounting prospective evidence suggests improvements in progression-free survival for oligometastatic lung cancers and hormone sensitive oligorecurrent prostate cancer [7–10].
Traditionally, metastatic prostate cancer has been, regardless of tumor burden, treated systemically with androgen deprivation therapy (ADT) and/or chemotherapy [11]. The importance of consolidating all macroscopic tumor deposits in men with oligometastatic prostate cancer is an area of active investigation [9,12,13], but the observation that men with lower numbers of metastases have better outcomes [14–16] supports a fundamental difference between oligometastatic prostate cancer (OPC) and higher volume disease. By this logic, recent studies have explored MDT of OPC by ablative and surgical treatments [17–20], as well as stereotactic ablative radiation (SABR) [21], also known as stereotactic body radiation therapy (SBRT) [22]. This highly targeted, high-dose approach provides millimeter-scale precision while minimizing collateral tissue damage and has shown efficacy in the management of OPC [17–19,21,23,24].
Studies to date have been conducted predominately in patients with a single metastasis [18,19,17,23,24,9]. Herein we review our early institutional outcomes with SABR for OPC in 66 consecutively treated patients with the majority having ≥ 2 metastases. We demonstrate that SABR is well tolerated and can significantly delay time to subsequent intervention.
Methods
Criteria:
With approval from of our institutional review board, we reviewed our retrospectively collected Johns Hopkins prostate cancer (PCa) radiation oncology database for consecutively SABR treated patients from 02/07/2011 to 1/15/2017. Inclusion criteria were: biopsy-proven PCa, diagnostic imaging consistent with ≤ 5 metastases, and follow-up with post-SABR prostate specific antigen (PSA) testing. Three patients with ≤ 5 initially observed metastases who were found during treatment planning to have 6 metastases were included. Of the 84 total men during this period with OPC treated with SABR we only examined the first 66 men to allow for a minimum of 4.5months of follow-up.
SABR technique:
During CT-simulation patients were immobilized using an Alpha Cradle (Smithers Medical Products, Inc, North Canton, Ohio) or equivalent device. Magnetic resonance imaging (MRI), bone scan, NaF or DCFPyL positron emission tomography [25]/CT images were used to delineate the gross tumor volume (GTV) and when possible fused with the planning CT. As per our institutional policy, if patients had ≥ 3-mm breathing motion on 4dimensional CT, active breathing control (ABC) was used for motion management. If breathing was < 3 mm, patients were treated free breathing (FB) with an internal target volume based on the 0% and 60% phases of the breathing cycle. GTV was defined as the sum of the abnormalities noted on PET, MRI T1 post-gadolinium sequences, bone scan and/or CT. In general, the clinical tumor volume (CTV) was equal to the GTV. Most commonly, the planning target volumes (PTV) were the GTV with the addition of 3–5 mm used as the volume to which the prescription was assigned [17]. If men were treated with CyberKnife, then image-guidance was per that device. During non-CyberKnife SABR delivery, a cone beam CT scan was co-registered (spine) with the FB or ABC simulation scan. To verify tumor positioning before SABR, patients were shifted to align with the GTV for each beam based on fluoroscopy, cone beam CT, or kV images.
Follow-up:
Patients were scheduled for serial follow-up exams every 3–6 months with history and physical, PSA, testosterone and imaging every 6–12 months unless a shorter interval was clinically indicated. Repeat imaging with NaF or DCFPyL PET/CT, MRI, or bone scans was conducted at 3–6 month intervals after SABR. The majority of patients were treated by a limited number of Johns Hopkins medical oncologists will similar practice patterns. So broadly, the indication for next intervention was determined by disease objective evidence of disease progression by PSA and/or radiographic testing or symptomatic progression.
Statistical analysis:
Clinical endpoints reported include biochemical progression-free survival (bPFS), local progression-free survival (LPFS), distant progression-free survival (DPFS), overall survival (OS) [25], ADT-free survival (ADT-FS), and time-to-next intervention (TTNI); all were calculated from the date of SABR. Biochemical failure was defined as: (1) PSA rise ≥ 2 above nadir following SABR or (2) an increase of PSA following SABR with failure to decline initially. LPFS was defined as the lack of tumor progression within the treated planning tumor volume (PTV) margin on follow-up imaging. DPFS was defined as the lack of new metastases on follow-up imaging. For both local and distant progression-free survival, clinically significant events (e.g. bone fractures) in a previously stable lesion were considered as clinical progression. TTNI was defined as the interval to next local or systemic intervention due to progression. The Kaplan-Meier method was used to estimate the rates of bPFS, LPFS, DPFS, OS, ADT-FS and TTNI. Analysis of LPFS was performed with each treated lesion considered individually (n = 134), while all other endpoints were analyzed with each patient considered individually.
Results
Patient and disease characteristics:
Sixty-six men with 134 metastatic PCa lesions were identified and analyzed. Prior treatment of metastatic tumors (including radiation and surgery) did not exclude patients from this review. Patients were generally asymptomatic or had minimal pain associated with their metastatic burden. Thirty-eight percent of men presented with synchronous or de novo oligometastases while 62% developed metachronous oligometastases between the conclusion of definitive treatment for their prostate cancer and consultation for SABR treatment. Prior chemotherapy was allowed with 55% (n = 36) of patients receiving chemotherapy prior to or concurrently with treatment. Forty-nine (or 74%) of men had hormone sensitive prostate cancer (HSPC) at the time of SABR and 17 (or 26%) had castration resistant prostate cancer (CRPC). Initial Gleason scores (GS) were as follows: GS 6 (n = 7), GS 7 (n = 16), GS 8 (n = 18), GS 9 (n = 22), and GS 10 (n = 3). Additional patient and disease characteristics are shown in Table 1.
Table 1.
Patient and disease charateritics at diagnosis (n=66)
| Characteristic | Value |
|---|---|
| Age at diagnosis (y), median (range) | 65 (47–84) |
| Gleason score, n (%) | |
| 6 | 7 (10.6) |
| 7 | 16 (24.2) |
| 8 | 18 (27.3) |
| 9 | 22 (33.3) |
| 10 | 3 (4.5) |
| PSA | |
| Initial value (ng/mL), median (range) | 8.7 (1.9–2155) |
| PSA value (ng/mL), n (%) | |
| 0–5 | 9 (13.6) |
| >5–10 | 24 (36.4) |
| >10–20 | 9 (13.6) |
| >20 | 14 (21.2) |
| Unknown | 10 (15.2) |
| pT stage, n (%) | |
| pT1 | 0 (0.0) |
| pT2 | 15 (22.7) |
| pT3 | 31 (47.0) |
| pT4 | 0 (0.0) |
| Unknown | 3 (4.5) |
| cT stage, n (%) | |
| cT1 | 5 (7.6) |
| cT2 | 6 (9.1) |
| cT3 | 5 (7.6) |
| cT4 | 1 (1.5) |
| N stage, n (%) | |
| N0 | 50 (75.8) |
| Synchronous N1 | 16 (24.2) |
| M stage, n (%) | |
| M0, Metachronous | 41 (62.1) |
| Synchronous M1 | 25 (37.9) |
| Primary treatment, n (%) | |
| RP | 48 (72.7) |
| RT | 17 (25.8) |
| Post-RP XRT | 30 (45.5) |
| RT and salvage RP | 0 (0.0) |
| Chemotherapy, n (%) | |
| Prior or concurrent | 36 (54.5) |
| Adjuvant androgen-deprivation therapy, n (%) | |
| Neoadjuvant | 53 (80.3) |
| Concurrent | 53 (80.3) |
| Adjuvant | 48 (72.7) |
| Sensitivity to hormone therapy, n (%) | |
| HSPC | 49 (74.2) |
| CRPC | 17 (25.8) |
Abbreviations: PSA = prostate-specific antigen; RP = radical prostatectomy: RT = radiation therapy; XRT = external beam radiation therapy; HSPC = hormone sensitive prostate cancer; CRPC = castration resistant prostate cancer
The median and mean PSA reported prior to SABR were 1.2 ng/mL and 9.0 ng/mL, respectively. The majority of patients, 68% (n = 45), underwent SABR with a PSA ≤ 5. Fifty-three percent of men (n= 35) had ≥ 2 metastases. Consolidative SABR was delivered to a total of 134 metastases, with 89 osseous (66%), 40 nodal (30%) and 5 visceral (4%) lesions. The mean GTV was 9.1 cm3 (range 0.1–152.3 cm3). Additional patient characteristics at time of SABR are shown in Table 2. SABR was delivered to all lesions with a total dose in the range of 15 Gy to 55 Gy. The majority of lesions (72%) were treated with total doses > 15 Gy with varied fractionation; while 28% of lesions were treated with 15 Gy as a single fraction (Supplemental Table 1).
Table 2.
Patient and disease charateritics at time of stereotactic ablative radiotherapy
| Characteristic | Patient (n=66) | Lesion (n=134) |
|---|---|---|
| Pre-SABR PSA (ng/mL), | 1.2 (0.01–95.8) | |
| median (range) and mean | 9.0 | |
| PSA (ng/mL), n (%) | ||
| 0–5 | 45 (68.2) | |
| >5–10 | 5 (7.6) | |
| >10–20 | 7 (10.6) | |
| >20 | 9 (13.6) | |
| Total no. of metastases, no. of patients (%) | ||
| 1 | 31 (47.0) | |
| 2 | 19 (28.8) | |
| 3 | 7 (10.6) | |
| 4 | 4 (6.1) | |
| 5 | 2 (3.0) | |
| 6 | 3 (4.5) | |
| Regions treated, n (%) | ||
| Bone | 41 (62.1) | 89 (66.4) |
| Node | 24 (36.4) | 40 (29.9) |
| Other | 3 (4.5) | 5 (3.7) |
| Lesion gross tumor volume, cm3 (n=134) | ||
| Mean (Range) | 9.09 (0.1–152.3) | |
| Median | 2.45 |
Abbreviations: SABR = stereotactic ablative radiotherapy; PSA = prostate specific antigen
Clinical outcomes:
Median and mean follow-up were 61 and 66 weeks, respectively. Acute toxicities were noted in 46% (n = 30) of patients; whereas late toxicities were noted in 27% (n = 18) of patients with the most common toxicities being fatigue and hot flashes (Supplemental Table 2). In total, grade 1 toxicities were noted in 24 patients and grade 2 toxicities were noted in 7. There were no grade 3 or higher toxicities observed.
The average time to any failure was 35 weeks. Overall, LPFS at 1-year was 92% (Figure 1A) with LPFS of 100% for SABR biologically equivalent doses (BED with α/β =10) of > 50. The bPFS and DPFS for the entire cohort at 1-year were each 69% (Figure 1B–C). For all types of failure, men with oligometastatic HSPC had better outcomes than those with CRPC (Figure 1A–C). Twenty-two men had a new intervention secondary to progression of their disease post-SABR, with median TTNI not reached overall with our current follow-up of 15-months (Figure 2A). Of the 18 men with HSPC who were not treated with adjuvant hormone therapy, 11 (61%) remain free of disease following SABR corresponding to a 1-year ADT-FS of 78% (Figure 2B) and a median ADT-FS that was not reached. Of the 17 men with oligometastatic CRPC treated with SABR, 11 had > 50% PSA declines with a 1year TTNI of 38% (Figure 2A) and a median TTNI of 45 weeks. This was congruent with a 1-year bPFS and DPFS of 43% and 33%, respectively (Figure 1B–C) in men with CRPC. During the study period, two patients died: one of complications related to their metastatic CRPC and the other from unconfirmed causes not likely related to prostate cancer.
Figure 1.
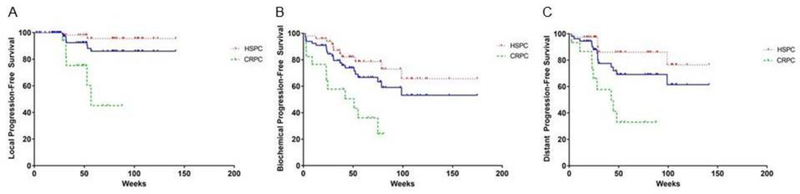
The effect of stereotactic ablative radiotherapy on clinical progression in men with oligometastatic prostate cancer. (A) Local progression-free survival of the entire cohort (blue), HSPC [34] and CRPC (green). (B) Biochemical progression-free survival of the entire cohort (blue), HSPC [34] and CRPC (green). (C) Distant progression-free survival of the entire cohort (blue), HSPC [34] and CRPC (green).
Figure 2.
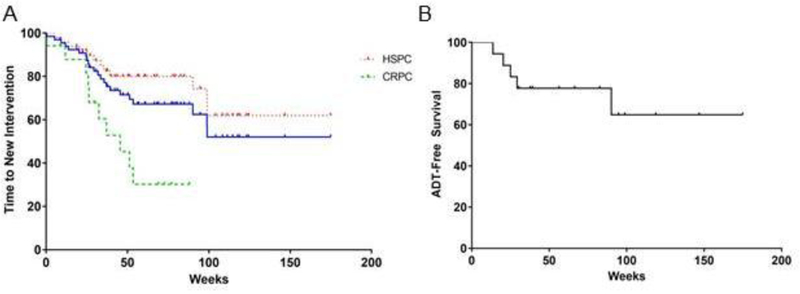
The effect of stereotactic ablative radiotherapy on delaying subsequent therapy in men with oligometastatic prostate cancer. (A) Time to next therapy of the entire cohort (blue), HSPC [34] and CRPC (green). (B) Time to ADT initiation in men with oligometastatic HSPC.
Discussion
This study demonstrates consolidative SABR in men with OPC is well tolerated and appears to have a clinical signal adding to a growing literature of evidence validating its use. We report 1-year LPFS of 92% and bPFS and DPFS of 69% and in those with HSPC not treated with hormone therapy, 61% remain free of disease following SABR. Furthermore, treatment was very well tolerated with 24 patients experiencing grade 1 toxicity, 7 with grade 2 toxicities and no grade 3 or higher toxicities observed.
Perhaps the most notable contributor to the subjective wellbeing of a man with metastatic HSPC is the delaying or deferring of ADT, the side effects of which can significantly hamper a patient’s lifestyle and systemic health [11]. With ADT, in addition to decreases in subjective feelings of wellness associated with androgen loss, there has been reported increased risk of osteoporosis, cardiovascular-related mortality and diabetes [26]. Consistent with prior retrospective reports [12,27] and the recently reported randomized phase II STOMP trial [9], our study also suggests that SABR can substantially delay initiation of ADT in men with oligometastatic HSPC for up to a year. Additionally, unlike ADT, SABR is associated with mild side effects without an associated decrement in quality of life measured in both acute and long term follow up [9].
STOMP represents the first published prospective randomized trial of MDT in OPC and validated the efficacy of SABR for MDT by demonstrating a superior ADT-FS (21 months vs 13 months) compared to observation [9,13]. Justification for its advent was supported by an accumulation of retrospective and observational studies reporting durable LPFS, DPFS, and ADT-FS with the use of MDT [17–19,23,24]. The results of our institutional experience are concordant with these reports and in addition add novel information to the already established body of literature. Specifically, the majority of men in this report (53%) had ≥2 metastases and thus our study examines a population of OPC with a heavier disease burden then has been explored in the current literature [9,17,19,28]. Rates of two year distant and progression free survival reported in the literature following SABR range from 30–40% and in a subset of those with oligometastatic progression retreated with SABR, the proportion of individuals remaining progression free is as high as 50% at last follow up [28]. Herein we report a one-year DPFS rate of 69%, which compares favorably to the aforementioned studies and therefore, may justify the more aggressive use of MDT in men with several oligometastatic foci.
Thus far the outcomes of interest and treatment intent of SABR in OPC have, for the most part, been aimed at delaying the start of palliative ADT. With growing support for its efficacy in this realm, the aims and goals MDT may shift from a palliative nature to a definitive one by offering these men a second (and in the context of salvage radiation a third) chance for cure. In alignment with the Hellman-Weichselbaum spectrum theory of metastasis, the validation and subsequent adoption of more sensitive imaging modalities such as NaF, Choline C-11, and DCFPyL PET/CT scan can allow for earlier detection of low volume metastatic disease that subsequently that can be treated with local therapy, possibly with definitive intent. Justification for such a paradigm is also supported by retrospective evidence that MDT improves prostate cancer cause specific survival in a propensity matched cohort [29]. Therefore, treatment intensification aimed at simultaneously eradicating other sites of microscopic metastatic disease through the use of concurrent ADT, which is retrospectively associated with improved DPFS when combined with SABR [17], non-castrating antiandrogens such as enzalutamide, chemotherapies such as docetaxel and radiopharmaceuticals may be justified.
In addition to preventing the site-specific complications associated with local progression of metastases, SABR is a complimentary local treatment to maximize durability of systemic approaches. SABR has already been shown to improve disease control in lung cancer patients treated with systemic oncogene-directed therapies through ablation of resistant clones [7,30,31]. The ability to maintain men with CRPC on 2nd-line hormonal agents that are oligoprogressing and delay chemotherapy adds significantly to the clinical options for these patients. Our data showing that SABR can delay distant progression and time to initiation of the next line of therapy (median 42–45 weeks) compares very favorably to any of the approved systemic agents in the CRPC space [32,35]. Finally, the highly conformal nature of SABR limits radiation-associated immunosuppression [33] and may be well suited for concurrent use with immune therapies.
Our study has all the intrinsic limitations of a small retrospective series, heterogeneity, and inclusion of shorter follow-up timeframes in our series limits definitive conclusions following consolidative SABR in OPC. In addition, a compelling weakness is that attempts to define the oligometastatic state by radiographic enumeration of lesions will always be limited by the sensitivity and specificity of the imaging modality and other patient-disease factors that are not well understood. As molecular imaging modalities such as prostate specific membrane antigen (PSMA)-targeted imaging mature, the value of MDT in the oligometastatic state may increase further as patients previously defined as oligometastatic based on conventional imaging may have additional detectable tumor deposits and require reclassification as polymetastatic [36]. Regardless, our preliminary data suggests SABR in OPC men warrants continued prospective study. In addition to the recent reporting of the Belgian STOMP trial, more definitive conclusions await the completion and reporting of other prospective randomized studies such as our Baltimore ORIOLE trial (NCT02680587).
Supplementary Material
Acknowledgements
PT Tran was supported by the Nesbitt-McMaster Foundation, Ronald Rose & Joan Lazar; Movember Foundation, Prostate Cancer Foundation; Commonwealth Foundation; NIH/NCI (R01CA166348 and U01CA2120007).
Footnotes
Disclosure of Potential Conflicts of Interest
Conflict of Interest: The authors declare that they have no conflict of interest
Research involving Human Participants and/or Animals
For this type of study formal consent is not required
Informed Consent
For this type of study formal consent is not required
References
- 1.Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13 (1):8–10. [DOI] [PubMed] [Google Scholar]
- 2.Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM (2017) Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol 14 (1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uppal A, Wightman SC, Mallon S, Oshima G, Pitroda SP, Zhang Q, Huang X, Darga TE, Huang L, Andrade J, Liu H, Ferguson MK, Greene GL, Posner MC, Hellman S, Khodarev NN, Weichselbaum RR (2015) 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget 6 (6):3540–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong AC, Watson SP, Pitroda SP, Son CH, Das LC, Stack ME, Uppal A, Oshima G, Khodarev NN, Salama JK, Weichselbaum RR, Chmura SJ (2016) Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer 122 (14):2242–2250. [DOI] [PubMed] [Google Scholar]
- 5.Reyes DK, Pienta KJ (2015) The biology and treatment of oligometastatic cancer. Oncotarget 6 (11):8491–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma DA, Salama JK, Lo SS, Senan S, Treasure T, Govindan R, Weichselbaum R (2014) The oligometastatic state - separating truth from wishful thinking. Nat Rev Clin Oncol 11 (9):549–557. [DOI] [PubMed] [Google Scholar]
- 7.Gomez DR, Blumenschein GR Jr., Lee JJ, Hernandez M, Ye R, Camidge DR, Doebele RC, Skoulidis F, Gaspar LE, Gibbons DL, Karam JA, Kavanagh BD, Tang C, Komaki R, Louie AV, Palma DA, Tsao AS, Sepesi B, William WN, Zhang J, Shi Q, Wang XS, Swisher SG, Heymach JV (2016) Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 17 (12):1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, Pulipparacharuvil S, Choy H, Timmerman RD (2018) Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 4 (1):e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, Lambert B, Delrue L, Bultijnck R, Claeys T, Goetghebeur E, Villeirs G, De Man K, Ameye F, Billiet I, Joniau S, Vanhaverbeke F, De Meerleer G (2018) Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 36 (5):446–453. [DOI] [PubMed] [Google Scholar]
- 10.Collen C, Christian N, Schallier D, Meysman M, Duchateau M, Storme G, De Ridder M (2014) Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol 25 (10):1954–1959. [DOI] [PubMed] [Google Scholar]
- 11.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, van der Poel HG, van der Kwast TH, Rouviere O, Wiegel T, Mottet N (2016) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol [DOI] [PubMed]
- 12.Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, Roach M 3rd, Briganti A (2015) Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol 67 (5):852–863. [DOI] [PubMed] [Google Scholar]
- 13.Phillips RM, Hayman J, Tran PT (2018) STOMPing Out Hormone-Sensitive Metastases With Local Therapies in Prostate Cancer. J Clin Oncol 36 (5):435–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweizer MT, Zhou XC, Wang H, Yang T, Shaukat F, Partin AW, Eisenberger MA, Antonarakis ES (2013) Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann Oncol 24 (11):2881–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ost P, Decaestecker K, Lambert B, Fonteyne V, Delrue L, Lumen N, Ameye F, De Meerleer G (2014) Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate 74 (3):297–305. [DOI] [PubMed] [Google Scholar]
- 16.Sridharan S, Steigler A, Spry NA, Joseph D, Lamb DS, Matthews JH, Atkinson C, Tai KH, Duchesne G, Christie D, Attia J, Holliday EG, Denham JW (2016) Oligometastatic bone disease in prostate cancer patients treated on the TROG 03.04 RADAR trial. Radiother Oncol 121 (1):98–102. [DOI] [PubMed] [Google Scholar]
- 17.Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, Henderson D, Casamassima F, Orecchia R, Surgo A, Brown L, Tree A, Miralbell R, De Meerleer G (2016) Progression-free Survival Following Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Treatment-naive Recurrence: A Multi-institutional Analysis. Eur Urol 69 (1):9–12. [DOI] [PubMed] [Google Scholar]
- 18.Muacevic A, Kufeld M, Rist C, Wowra B, Stief C, Staehler M (2013) Safety and feasibility of imageguided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol 31 (4):455–460. [DOI] [PubMed] [Google Scholar]
- 19.Muldermans JL, Romak LB, Kwon ED, Park SS, Olivier KR (2016) Stereotactic Body Radiation Therapy for Oligometastatic Prostate Cancer. Int J Radiat Oncol Biol Phys 95 (2):696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnes RJ, Murphy CR, Bergstralh EJ, DiMonte G, Cheville JC, Lowe VJ, Mynderse LA, Kwon ED (2015) Salvage lymph node dissection for prostate cancer nodal recurrence detected by 11C-choline positron emission tomography/computerized tomography. J Urol 193 (1):111–116. [DOI] [PubMed] [Google Scholar]
- 21.De Bleser E, Tran PT, Ost P (2017) Radiotherapy as metastasis-directed therapy for oligometastatic prostate cancer. Curr Opin Urol 27 (6):587–595. [DOI] [PubMed] [Google Scholar]
- 22.Loo BW Jr., Chang JY, Dawson LA, Kavanagh BD, Koong AC, Senan S, Timmerman RD (2011) Stereotactic ablative radiotherapy: what’s in a name? Pract Radiat Oncol 1 (1):38–39. [DOI] [PubMed] [Google Scholar]
- 23.Schick U, Jorcano S, Nouet P, Rouzaud M, Vees H, Zilli T, Ratib O, Weber DC, Miralbell R (2013) Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol 52 (8):1622–1628. [DOI] [PubMed] [Google Scholar]
- 24.Ingrosso G, Trippa F, Maranzano E, Carosi A, Ponti E, Arcidiacono F, Draghini L, Di Murro L, Lancia A, Santoni R (2017) Stereotactic body radiotherapy in oligometastatic prostate cancer patients with isolated lymph nodes involvement: a two-institution experience. World J Urol 35 (1):45–49. [DOI] [PubMed] [Google Scholar]
- 25.Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, Blazeby JM, Peters TJ, Holding P, Bonnington S, Lennon T, Bradshaw L, Cooper D, Herbert P, Howson J, Jones A, Lyons N, Salter E, Thompson P, Tidball S, Blaikie J, Gray C, Bollina P, Catto J, Doble A, Doherty A, Gillatt D, Kockelbergh R, Kynaston H, Paul A, Powell P, Prescott S, Rosario DJ, Rowe E, Davis M, Turner EL, Martin RM, Neal DE, Protec TSG (2016) Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 375 (15):1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford ED, Moul JW (2015) ADT risks and side effects in advanced prostate cancer: cardiovascular and acute renal injury. Oncology (Williston Park) 29 (1):55–58, 65–56. [PubMed] [Google Scholar]
- 27.Berkovic P, De Meerleer G, Delrue L, Lambert B, Fonteyne V, Lumen N, Decaestecker K, Villeirs G, Vuye P, Ost P (2013) Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer 11 (1):27–32. [DOI] [PubMed] [Google Scholar]
- 28.Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, De Vos F, Huysse W, Hautekiet A, Maes G, Ost P (2014) Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steuber T, Jilg C, Tennstedt P, De Bruycker A, Tilki D, Decaestecker K, Zilli T, Jereczek-Fossa BA, Wetterauer U, Grosu AL, Schultze-Seemann W, Heinzer H, Graefen M, Morlacco A, Karnes RJ, Ost P (2018) Standard of Care Versus Metastases-directed Therapy for PET-detected Nodal Oligorecurrent Prostate Cancer Following Multimodality Treatment: A Multi-institutional Case-control Study. Eur Urol Focus [DOI] [PubMed]
- 30.Gan GN, Weickhardt AJ, Scheier B, Doebele RC, Gaspar LE, Kavanagh BD, Camidge DR (2014) Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys 88 (4):892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA Jr., Aisner DL, Gaspar LE, Kavanagh BD, Doebele RC, Camidge DR (2012) Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 7 (12):1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handy CE, Antonarakis ES (2016) Sequencing Treatment for Castration-Resistant Prostate Cancer. Curr Treat Options Oncol 17 (12):64. [DOI] [PubMed] [Google Scholar]
- 33.Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, Hacker-Prietz A, Assadi RK, Saeed AM, Pawlik TM, Jaffee EM, Laheru DA, Tran PT, Weiss MJ, Wolfgang CL, Ford E, Grossman SA, Ye X, Ellsworth SG (2016) Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 94 (3):571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotecha R, Djemil T, Tendulkar RD, Reddy CA, Thousand RA, Vassil A, Stovsky M, Berglund RK, Klein EA, Stephans KL (2016) Dose-Escalated Stereotactic Body Radiation Therapy for Patients With Intermediate- and High-Risk Prostate Cancer: Initial Dosimetry Analysis and Patient Outcomes. Int J Radiat Oncol Biol Phys 95 (3):960–964. [DOI] [PubMed] [Google Scholar]
- 35.Beauval JB, Loriot Y, Hennequin C, Rozet F, Barthelemy P, Borchiellini D, Schlurmann Constans F, Gross E, Maillet D, Pasticier G, et al. Loco-regional treatment for castration-resistant prostate cancer: Is there any rationale? A critical review from the AFU-GETUG. Crit Rev Oncol Hematol 2018. February;122:144–9. [DOI] [PubMed] [Google Scholar]
- 36.Bouchelouche K, Choyke PL. PSMA PET in prostate cancer – a step towards personalized medicine. Current opinion in oncology 2016;28(3):216–221. 10.1097/CCO.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


