Abstract
Skin fibrosis is a chronic debilitating feature of several skin diseases that lead to characteristic increases in dermal fibroblast proliferation and collagen deposition through upregulation in components of the transforming growth factor beta (TGF-B)/SMAD pathway. In contrast to ultraviolet phototherapy, high-fluence light-emitting diode-generated red light (HF-LED-RL, 633 nm ± 15 nm) is a safe, economic, and non-invasive therapy with in vitro evidence that supports modulation of the key cellular characteristics involved in the pathogenesis of skin fibrosis. Limited data exists pertaining to the effects of HF-LED-RL on human skin fibroblast microRNA (miRNA). Herein, we explored the effects of HF-LED-RL on fibroblast miRNA levels using RNA-seq and miRNA expression analysis. Using RNA-seq analysis we found that HF-LED-RL at 320 and 640 J/cm2 increased transcription of key miRNA that are involved in skin fibrosis including miRNA-29, miRNA-196a, and Let-7a, and decreased transcription of miRNA-21, miRNA-23b, miRNA-31. These microRNA findings provide insight into the molecular underpinnings of HF-LED-RL and highlight potential therapeutic targets of interest for the treatment of skin fibrosis. Additional research on the specific molecular mechanisms underlying HF-LED-RL effects on fibroblasts may provide further mechanistic insight into this therapy and may reveal additional future therapeutic targets for skin fibrosis.
Introduction
Skin fibrosis is a chronic debilitating feature of several skin conditions that leads to characteristic increases in dermal fibroblast proliferation and collagen deposition through significant upregulation in the transforming growth factor beta (TGF-B)/SMAD pathway (1–3). Skin fibrosis impacts approximately 100 million individuals worldwide and leads to a significant economic burden and a decrease in quality-of-life (4). The skin diseases characterized by skin fibrosis include scleroderma, morphea, keloids, hypertrophic scars, and chronic graft-versus-host disease, and these conditions have limited treatment options (5, 6).
Ultraviolet phototherapy is currently used to treat some forms of fibrotic skin diseases, however, the efficacy of this therapy varies widely and also leads to DNA damage associated with skin cancer and aging (5, 6). In contrast, high-fluence light-emitting diode-generated red light (HF-LED-RL, 633 nm ± 15 nm) is a safe, economic, and non-invasive therapy with significant in vitro evidence for its ability to modulate the key cellular characteristics involved in the pathogenesis of skin fibrosis (7–10). Our group has previously demonstrated that HF-LED-RL can decrease cellular proliferation, cellular migration speed, collagen 1A levels, and TGF-Beta pathway activation (10). MicroRNA (miRNA), such as miRNA-21 and miRNA-29, are known to have roles in the cellular cascade leading to skin fibrosis and their modulation may be potential therapeutic targets of interest in diseases such as scleroderma (11).
However, the effects of HF-LED-RL on modulation of miRNAs on skin fibroblasts has yet to be explored (11). Herein, we explored the effects of HF-LED-RL on human skin fibroblasts using miRNA-seq and miRNA expression analysis. We hypothesized that treatment of human skin fibroblasts with HF-LED-RL would lead to measurable alterations in miRNA levels associated with skin fibrosis and may reveal potential targets of interest. Herein, we identify Let-7A, miRNA-29, and miRNA-196a as three key anti-fibrotic miRNA of interest that are upregulated by HF-LED-RL and miRNA-21, miRNA-23b, and miRNA-31 as key pro-fibrotic miRNA that are downregulated by HF-LED-RL.
2. MATERIALS AND METHODS
Tissue culture dishes (35-mm) were obtained from Corning (Tewksbury, MA). The cellular culture media, Dulbecco’s Modified Eagle’s Medium, fetal bovine serum, phosphate buffered saline (PBS), and antibiotic-antimycotic mixture (penicillin, streptomycin, and amphotericin B) were purchased from Invitrogen Life Technology (Invitrogen, Carlsbad, CA).
Light Source Characteristics
The light source used was a commercially available Omnilux New-U hand-held LED array (PhotoTherapeutics, CA). This LED unit has a 4.7 cm × 6.1 cm rectangular aperture and emits visible red light (633 nm ± 30 nm) at a power density of 360.2 W/m2 at room temperature and a distance of 10 mm from the bottom of the tissue culture dish to the LED array as previously described (6, 10, 12).
Cell Culture
Commercially available, normal human skin dermal fibroblast cultures (AG13145, Coriell Cell Repositories) were maintained at 37°C in 5% CO2 with Dulbecco’s Modified Eagle Medium, 10% FBS, 1% antibiotic as previously described (12). For experiments, fibroblasts were plated at 2 × 104 cells per 35-mm dish. All experiments were performed on cell cultures passage 8 or less.
Fibroblast Irradiation
Fibroblasts were seeded and allowed to adhere for 24 hours. Fibroblasts were then irradiated with the LED array at doses that were selected based upon previous pilot irradiation studies. Each plate was matched with a temperature regulated “bench control plate” (BCP), to ensure that the measured effect was a result of LED-RL treatment and not due to environmental factors. These BCPs were protected from the LED light source and placed on a heating plate set to maintain the temperature of 34°C while on the bench top. Total RNA was collected by pooling four dishes from identical conditions at 4 hours post-irradiation based on prior studies demonstrating optimal HF-LED-RL effects at this time point (12).
Small RNA-Sequencing (RNA-Seq) and Analysis Methods
Total RNA samples were submitted to the UC Davis Comprehensive Cancer Center’s Genomics Shared Resource (GSR) for miRNA expression profiling with small RNA-Sequencing (RNA-Seq). Briefly, indexed, small RNA-Seq libraries were prepared with NEBNext Multiplex Small RNA Library (New England BioLabs) kitted reagents for 3′ adaptor ligation, 5′ adaptor ligation, first-strand cDNA synthesis, and high-fidelity PCR enrichment of the libraries with indexed primers according to the manufacturers’ and GSR’s standard protocols (13). Libraries were then pooled and submitted for multiplex sequencing on an Illumina HiSeq 3000 sequencing system (50-bp, single read, 15–20 libraries/lane). Analysis of the small RNA-Seq data (FASTQ format) was performed using the CAP-miRSeq pipeline (14), which accomplished read pre-processing, alignment with Bowtie (15), detection and quantification of mature/precursor/novel miRNAs with miRDeep2 (16), and identification of differentially-expressed miRNAs with edgeR (17).
3. RESULTS
HF-LED-RL Modulates Key miRNA Associated with Skin Fibrosis
HF-LED-RL increases transcription of key anti-fibrotic miRNA including Let-7a, miRNA-29, miRNA-196a in human skin fibroblasts as measured by miRNA expression analysis. 320 J/cm2 LED-RL increased levels Let-7a, miRNA-29, and miRNA-196a in human skin fibroblasts by 1.76 to 2.21- fold compared to matched controls (Figure 1A). 640 J/cm2 LED-RL increased levels of Let-7a, miRNA-29, miRNA-196a by 1.10 to 2.04-fold compared to matched controls (Figure 1B).
Figure 1: HF-LED-RL increases transcription of key miRNA that are involved in skin fibrosis including Let-7a, miRNA-29, miRNA-196a.
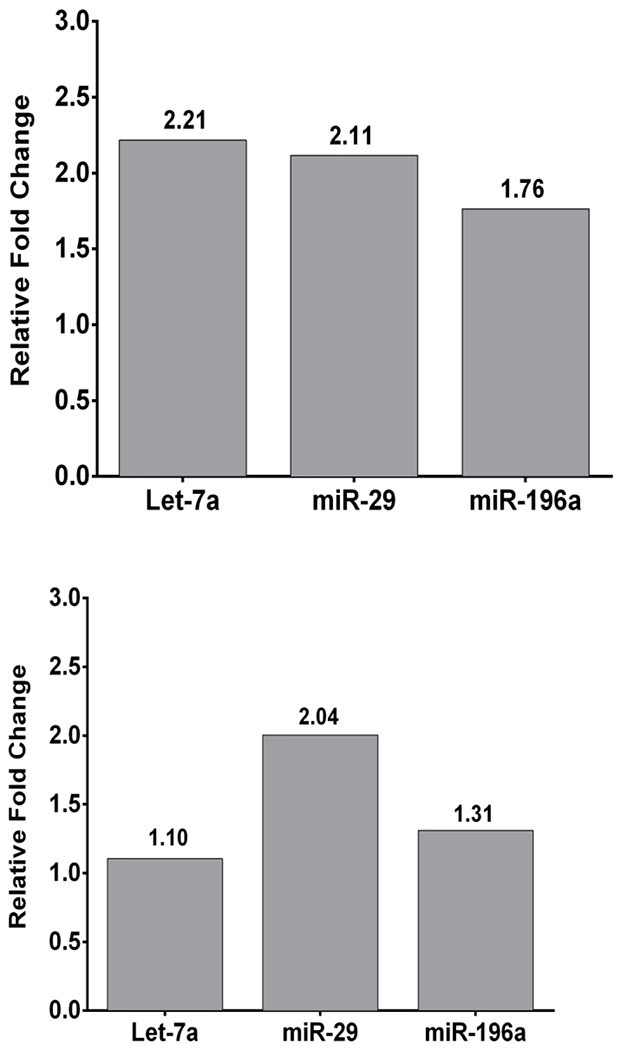
(A) 320 J/cm2 LED-RL increased transcription of Let-7a, miRNA-29, miRNA-196a in human skin fibroblasts as measured by microRNA expression analysis. Fold changes were: Let-7a = 2.21, miRNA-29 = 2.11, miRNA-196a = 1.76. Bars represent fold change compared to matched control. (B) 640 J/cm2 LED-RL increased transcription of Let-7a, miRNA-29, miRNA-196a in human skin fibroblasts as measured by microRNA expression analysis. Fold changes were: Let-7a = 1.10, miRNA-29 = 2.04, miRNA-196a = 1.31. Bars represent fold change compared to matched control.
Furthermore, HF-LED-RL decreased transcription of key profibrotic miRNA involved in skin fibrosis including miRNA-21, miRNA-23b, and miRNA-31. 320 J/cm2 LED-RL decreased levels of miRNA-21, miRNA-23b, and miRNA-31 to 0.21 to 0.89 compared to matched control (Figure 2A). 640 J/cm2 LED-RL decreased levels of miRNA-21, miRNA-23b, miRNA-31 to 0.64 to 0.91 compared to matched control (Figure 2B).
Figure 2: HF-LED-RL decreased transcription of key miRNA involved in skin fibrosis including miRNA-21, miRNA-23b, miRNA-31.
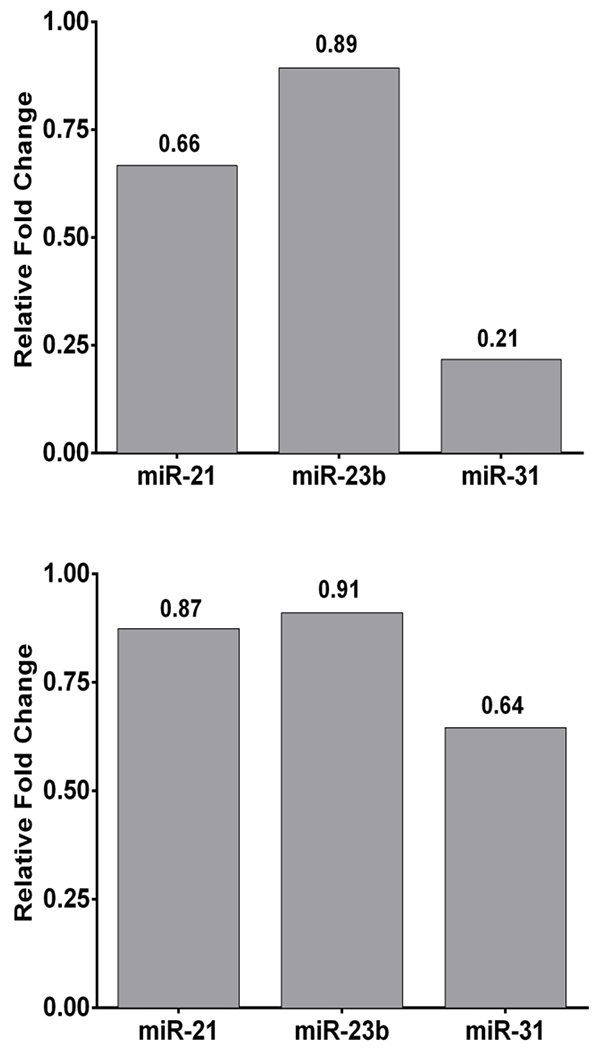
(A) 320 J/cm2 LED-RL decreased transcription of miRNA-21, miRNA-23b, miRNA-31 in human skin fibroblasts as measured by microRNA expression analysis. Fold changes were: miRNA-21 = 0.66, miRNA-23b = 0.89, miRNA-31 = 0.21. Bars represent fold change compared to matched control. (B) 640 J/cm2 LED-RL decreased transcription of miRNA-21, miRNA-23b, miRNA-31 in human skin fibroblasts as measured by microRNA expression analysis. Fold changes were: miRNA-21 = 0.87, miRNA-23b = 0.91, miRNA-31 = 0.64. Bars represent fold change compared to matched control.
DISCUSSION
MicroRNAs (miRNA) are key regulators in the pathogenesis of skin fibrosis (11). Modulation of miRNAs also has therapeutic potential for the treatment of skin fibrosis (11). Fibrosis-related miRNA modulate TGF-beta signaling, extracellular matrix (ECM) deposition, and fibroblast proliferation or differentiation (11). Specific miRNAs may serve either a pro-fibrotic or anti-fibrotic role depending on their target and the direction of their fold change (11). Our analyses identified several miRNAs modulated by HF-LED-RL that have known important functions in skin fibrosis. We found that HF-LED-RL reduced the transcription of miRNA-21, miRNA-23b, and miRNA-31 that are involved with promoting skin fibrosis and the TGF-beta pathway. Meanwhile, we found that HF-LED-RL also led to increases in levels of Let-7a, miRNA-29 and miRNA-196a that have antifibrotic effects. These miRNA findings support HF-LED-RL’s anti-fibrotic potential for prevention and treatment of fibrotic skin disease.
There is a growing body of scientific data on the effects of HF-LED-RL that suggest that visible light may represent an important modality for the treatment of skin fibrosis (7, 8, 10). The TGF-Beta pathway and subsequent SMAD pathway activation is a primary driver of skin fibrosis (1, 8, 18). We have previously reported that fibroblasts treated with HF-LED-RL demonstrate inhibition of the TGF-beta/SMAD pathway (19). Specifically, HF-LED-RL treated fibroblasts demonstrate decreased TGF-beta 1 ligand levels, decreased pSMAD2 levels, and subsequent decreased fibroblast proliferation and collagen 1A production (10, 19). However, the mechanisms underlying these changes are not fully explored. Our observed microRNA alterations resulting from HF-LED-RL highlights the potential role that HF-LED-RL induced miRNA changes mediating skin fibrosis.
Upregulated microRNAs included Let-7a, miRNA-29, and miRNA-196a. MiRNA-29 is known to be a key negative regulator of ECM protein synthesis (20–22). MiRNA-29 has been studied in scleroderma patients and was found to be consistently downregulated in patient-derived skin fibroblasts and is suggested to play a role in the pathogenesis of scleroderma (22). Specifically, miRNA-29 has been shown to be a key post-transcriptional regulator of collagen production, suggesting that increases may be beneficial in skin fibrosis (23). Furthermore, the synthesis of collagen is regulated by miRNA-150 and miRNA-196a at the level of collagen synthesis (24, 25). Our results suggest that the mitigation of collagen production by HF-LED-RL may be due to be in part to modulation of these key miRNA (23).
Downregulated microRNAs included miRNA-21, miRNA-23b, and miRNA-31. MiRNA-21 is a key miRNA associated with skin fibrosis and likely important target for therapeutic consideration as TGF-beta signaling upregulates miRNA-21 transcription. miRNA-21 upregulation reduces the expression of anti-fibrotic SMAD7 releasing the inhibition on the TGF-beta pathway to create a feed-forward loop (26). Interrupting this feed-forward loop is one proposed therapeutic approach to treating skin fibrosis. Furthermore, miRNA-23b increases TGF-beta receptor expression and increased levels of miRNA-23b is associated with scleroderma (27). The down-regulation of these key pro-fibrotic miRNA by HF-LED-RL may contribute to our previously described HF-LED-RL effects on fibroblast proliferation and collagen production (10). One limitation of this study is that all fibroblast cells were isolated from normal human skin. Further research is needed to investigate and confirm the role of these miRNAs in HF-LED-RL modulation of skin fibroblasts derived from fibrotic skin or other lesions and to confirm these findings in vivo. Limitations to this study include that it was in vitro and that samples were pooled to obtain sufficient miRNA levels. Although the in vitro design and sample pooling were both necessary given the experimental design, further studies are needed to validate that these miRNA changes are also observed in vivo.
Skin fibrosis is a significant medical problem with limited available treatment modalities. HF-LED-RL represents a potentially new therapeutic modality with a supporting body of in vitro evidence demonstrating its ability to modulate the key cellular characteristics associated with skin fibrosis. These findings are promising and early human clinical trials are underway to investigate HF-LED-RL (28, 29). Herein, we explored the mechanism behind HF-LED-RL photobiomodulation of fibroblasts using miRNA-Seq and miRNA expression analysis and found significant alterations in transcription of miRNA involved in skin fibrosis. The miRNA we found modulated were mechanistically consistent with our previous findings that HF-LED-RL decreases fibroblast proliferation and collagen production through modulation of the TGF-beta pathway. To the best of our knowledge, this is the first study investigating the HF-LED-RL effects on skin fibroblasts miRNA levels. Additional research on the specific mechanisms and roles of miRNAs in mediating HF-LED-RL’s effects on skin fibroblasts may provide further mechanistic insight into this therapy and may reveal additional therapeutic targets for prevention and treatment of skin fibrosis.
Acknowledgments
Funding Source:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked awards TL1 TR000133 and KL2 TR000134. The UC Davis Comprehensive Cancer Center Genomics Shared Resource is supported by Cancer Center Support Grant P30 CA093373 (Lara) from the National Cancer Institute. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number K23GM117309.
Jared Jagdeo serves on the scientific advisory board for GlobalMed Technologies.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature medicine. 2012;18(7):1028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu CS, Wu PH, Fang AH, Lan CC. FK506 inhibits the enhancing effects of transforming growth factor (TGF)-beta1 on collagen expression and TGF-beta/Smad signalling in keloid fibroblasts: implication for new therapeutic approach. The British journal of dermatology. 2012;167(3):532–41. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft KJ, Syed F, Bayat A. Site-Specific Keloid Fibroblasts Alter the Behaviour of Normal Skin and Normal Scar Fibroblasts through Paracrine Signalling. PloS one. 2013;8(12):e75600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ. 2003;326(7380):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitiello M, Abuchar A, Santana N, Dehesa L, Kerdel FA. An Update on the Treatment of the Cutaneous Manifestations of Systemic Sclerosis: The Dermatologist’s Point of View. The Journal of clinical and aesthetic dermatology. 2012;5(7):33–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Mamalis AD, Lev-Tov H, Nguyen DH, Jagdeo JR. Laser and light-based treatment of Keloids--a review. Journal of the European Academy of Dermatology and Venereology : JEADV. 2014;28(6):689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamalis A, Jagdeo J. The Combination of Resveratrol and High-Fluence Light Emitting Diode-Red Light Produces Synergistic Photobotanical Inhibition of Fibroblast Proliferation and Collagen Synthesis: A Novel Treatment for Skin Fibrosis. Dermatol Surg. 2017;43(1):81–6. [DOI] [PubMed] [Google Scholar]
- 8.Mamalis A, Siegel D, Jagdeo J. Visible Red Light Emitting Diode Photobiomodulation for Skin Fibrosis: Key Molecular Pathways. Curr Dermatol Rep. 2016;5:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamalis A, Koo E, Jagdeo J. Resveratrol Prevents Reactive Oxygen Species-Induced Effects of Light-Emitting Diode-Generated Blue Light in Human Skin Fibroblasts. Dermatol Surg. 2016;42(6):727–32. [DOI] [PubMed] [Google Scholar]
- 10.Mamalis A, Koo E, Garcha M, Murphy WJ, Isseroff RR, Jagdeo J. High fluence light emitting diode-generated red light modulates characteristics associated with skin fibrosis. J Biophotonics. 2016;9(11–12):1167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babalola O, Mamalis A, Lev-Tov H, Jagdeo J. The role of microRNAs in skin fibrosis. Arch Dermatol Res. 2013;305(9):763–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lev-Tov H, Mamalis A, Brody N, Siegel D, Jagdeo J. Inhibition of fibroblast proliferation in vitro using red light-emitting diodes. Dermatol Surg. 2013;39(8):1167–70. [DOI] [PubMed] [Google Scholar]
- 13.Pan CX, Zhang H, Tepper CG, Lin TY, Davis RR, Keck J, et al. Development and Characterization of Bladder Cancer Patient-Derived Xenografts for Molecularly Guided Targeted Therapy. PloS one. 2015;10(8):e0134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Z, Evans J, Bhagwate A, Middha S, Bockol M, Yan H, et al. CAP-miRSeq: a comprehensive analysis pipeline for microRNA sequencing data. BMC Genomics. 2014;15:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature methods. 2012;9(4):357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedlander MR, Mackowiak SD, Li N, Chen W, Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40(1):37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shroff A, Mamalis A, Jagdeo J. Oxidative Stress and Skin Fibrosis. Curr Pathobiol Rep. 2014;2(4):257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamalis AD, Jagdeo JR. High-Fluence Light-Emitting Diode–Generated Red Light Modulates the Transforming Growth Factor-Beta Pathway in Human Skin Fibroblasts. Dermatol Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 20.Cheng J, Wang Y, Wang D, Wu Y. Identification of collagen 1 as a post-transcriptional target of miR-29b in skin fibroblasts: therapeutic implication for scar reduction. Am J Med Sci. 2013;346(2):98–103. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Li Z, Wang Y, Li L, Wang D, Zhang W, et al. Overexpression of miR-29b reduces collagen biosynthesis by inhibiting heat shock protein 47 during skin wound healing. Transl Res. 2016;178:38–53 e6. [DOI] [PubMed] [Google Scholar]
- 22.Peng WJ, Tao JH, Mei B, Chen B, Li BZ, Yang GJ, et al. MicroRNA-29: a potential therapeutic target for systemic sclerosis. Expert Opin Ther Targets. 2012;16(9):875–9. [DOI] [PubMed] [Google Scholar]
- 23.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62(6):1733–43. [DOI] [PubMed] [Google Scholar]
- 24.Honda N, Jinnin M, Kajihara I, Makino T, Makino K, Masuguchi S, et al. TGF-beta-mediated downregulation of microRNA-196a contributes to the constitutive upregulated type I collagen expression in scleroderma dermal fibroblasts. J Immunol. 2012;188(7):3323–31. [DOI] [PubMed] [Google Scholar]
- 25.Honda N, Jinnin M, Kira-Etoh T, Makino K, Kajihara I, Makino T, et al. miR-150 down-regulation contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin beta3. Am J Pathol. 2013;182(1):206–16. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H, Luo H, Li Y, Zhou Y, Jiang Y, Chai J, et al. MicroRNA-21 in scleroderma fibrosis and its function in TGF-beta-regulated fibrosis-related genes expression. J Clin Immunol. 2013;33(6):1100–9. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Yang R, Fan X, Gu T, Zhao Z, Chang D, et al. MicroRNA array analysis of microRNAs related to systemic scleroderma. Rheumatol Int. 2012;32(2):307–13. [DOI] [PubMed] [Google Scholar]
- 28.Jagdeo J, Austin E, Mamalis A, Wong C, Ho D, Siegel DM. Light-emitting diodes in dermatology: A systematic review of randomized controlled trials. Lasers Surg Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho D, Koo E, Mamalis A, Jagdeo J. A Systematic Review of Light Emitting Diode (LED) Phototherapy for Treatment of Psoriasis: An Emerging Therapeutic Modality. J Drugs Dermatol. 2017;16(5):482–8. [PubMed] [Google Scholar]


