Abstract
Rheumatic musculoskeletal manifestations are increasingly recognized as a major cause of morbidity and impaired quality of life in patients with inflammatory bowel diseases (IBDs). IBDs are associated with a variety of musculoskeletal pathologies, from peripheral arthritis to axial involvement, and from localized or regional pathologies to diffuse metabolic disorders. Recent advances, especially in imaging techniques, allow a better understanding of these pathologies, and assist their recognition even in the preclinical phase. This review aims to describe the musculoskeletal clinical and imaging manifestations in IBD with special emphasis on the current concepts and the updated radiological work-up.
Keywords: Spondyloarthritis, Enthesitis, Sacroiliitis, Magnetic resonance
1. Introduction
Musculoskeletal manifestations represent a major cause of morbidity and impaired quality of life in patients with inflammatory bowel diseases (IBDs), including both ulcerative colitis and Crohn disease. IBDs have been associated with a variety of musculoskeletal pathologies, ranging from peripheral arthritis to axial involvement and even to diffuse bone metabolic diseases. Many advances have been made over the last decades, especially in understanding, classifying, and diagnosing these pathologies, resulting partly from the great progress in imaging techniques. Furthermore, radiological studies have shown occult rheumatic manifestations, such as enthesitis and sacroiliitis, even in clinically asymptomatic IBD patients, although their clinical repercussions remain unclear [1,2]. The main purpose of the present review is to describe current concepts in musculoskeletal clinical manifestations in IBD and their updated radiological work-up.
2. Epidemiology
The prevalence of IBDs in Western Europe ranges from 50 to 100 per 100,000 [3]; their incidence is estimated to be 6‑15/100,000 [4]. The association between IBD and arthritis has long been observed, but only more recently has the concept of spondyloarthritis (SpA) appeared [5]: SpA comprises idiopathic ankylosing spondylitis (AS), psoriatic arthritis, IBD-related SpA, and reactive arthritis [6]. The most frequently described rheumatic manifestations in IBD are sacroiliitis, in 10%‑30% of cases [7,8]; AS in 3%‑10%; enthesitis, ranging from 1% to 54% among different studies; and dactylitis, described in 0% to 6% of IBD patients [8]. The wide range of reported frequencies of rheumatic manifestations in IBDs could depend on the population studied and inclusion criteria, but also on the lack of standardization of diagnostic approach, of validated diagnostic criteria, or of case definitions and terminology [9]. Musculoskeletal manifestations can precede the diagnosis of IBD; they could appear simultaneously with or after the diagnosis [9]. Risk factors for developing SpA in IBD patients are active bowel disease, a family history of IBD, a history of appendectomy, smoking, or extra-intestinal manifestations such as erythema nodosum and pyoderma gangrenosum [10,11]. A large study following 470 patients with IBD for 20 years found a high prevalence of inflammatory axial disorders occurring late in the course of the disease. Among these disorders, AS was diagnosed in 4.5% of the patients, non-radiographical SpA in 7.7%, whereas inflammatory back pain was present in 46.8% of the patients. Axial SpA was more frequent in patients having a chronic course of the intestinal disease; positivity for Human Leukocyte Antigen B27 (HLA-B27) was a predisposing factor in these patients [12].
3. Pathogenesis
Joint inflammation and bowel disease have long been associated. Histological studies have shown a resemblance between the intestinal biopsies in patients with AS and patients with Crohn disease, even if clinical manifestations of Crohn disease were not present in the AS group. One of the main hypotheses proposed to explain the relationship between the gut and the joints is that intestinal bacteria are directly involved in the pathogenesis of joint inflammation, and that gut lymphocytes and activated macrophages are also recruited to the joints [13]. The predisposing mechanisms are, however, more complex, and genetic and environmental factors act in addition to the susceptibility to develop both IBD and rheumatic manifestations as AS. A class I molecule of the major histocompatibility complex, HLA-B27 is located in the short arm of chromosome 6 [14]. HLA-B27 acts as a presenting antigen molecule that initiates immune responses [15]. There are more than 160 subtypes of HLA-B27, with the most common disease associated subtypes being B27-02, B27-05 and B27-04 depending on the patient’s race and ethnicity [16]. The importance of HLA-B27 as a predisposing factor for AS is well recognized, but the role of HLA-B27 in IBD-related SpA remains to be determined [17] because the rate of positivity ranges between 25% and 75% in Crohn patients [18]. Patients with IBD-related SpA tend to be HLA-B27 negative as compared with those whose SpA is not related to IBD, but patients who do present the gene have a higher risk of developing sacroiliitis and AS, and also a more severe course of the axial disease. Conversely, peripheral arthritis seems to be independent of HLA-B27 status [12]. Idiopathic AS patients who are HLA-B27 positive show a younger age at the onset of the disease, a better clinical response to anti-tumor necrosis factor inhibitor (anti-TNF), a familial segregation, a higher risk for acute anterior uveitis, but a lower risk for psoriasis and IBD. There is, however, an association between intestinal inflammation and SpA independent of HLA-B27 [16]. Other genes are currently being studied to explain SpA manifestations in IBD patients that are HLA-B27 negative [17]. Endoplasmic reticulum aminopeptidase 1 (ERAP1) polymorphism that results in an altered enzymatic activity is associated with SpA [19]. Danoy et al discovered variants at chr1q32 and Stat3 that appear to link AS and Crohn disease; these authors proposed that shared pathogenesis might be related to the presence of those genes [20].
4. Clinical musculoskeletal manifestations in IBD and their imaging work-up (Table 1)
Table 1.
Clinical musculoskeletal manifestations of inflammatory bowel diseases.
| Spondyloarthritis spectrum [3,17] |
| Peripheral spondyloarthritis manifestations |
| - Type 1 peripheral arthritis |
| - Type 2 peripheral arthritis |
| - Enthesitis |
| - Dactylitis |
| Axial spondyloarthritis manifestations |
| - Sacroiliitis |
| - Ankylosing spondylitis |
| - Inflammatory back pain (IBP) without radiological manifestations |
| Metabolic and treatment related pathologies [25] |
| - Osteoporosis and osteoporotic fractures |
| - Osteonecrosis |
| - Paradoxical arthritis occurring during anti-TNFi treatment for IBD |
| Muscular manifestations [25, 53] |
| - Sarcopenia |
| - Myopathy |
| - Gastrocnemius myalgia syndrome |
| Miscellaneous [56,57] |
| - Fibromyalgia |
| - Arthralgia without arthritis |
| “Forgotten” manifestations [3,58] |
| - Granulomatous synovitis |
| - Hypertrophic osteoarthropathy |
Idiopathic inflammatory bowel diseases may be associated with extra-intestinal manifestations in up to 50% of cases; the musculoskeletal system is one of the most prevalent sites of involvement [21,22].
4.1. Spondyloarthritis spectrum in inflammatory bowel disease
IBD-related SpA is part of the spondyloarthritis category, representing a group of diseases with clinical features and a genetic background in common [23]. The diagnosis of IBD-related SpA is based on the Assessment in Spondyloarthritis International Society (ASAS) criteria [24], which were developed as classification criteria but are regularly used in clinical practice for diagnostic purposes. Based on these criteria, a patient with IBD is classified as having axial SpA if the patient has had inflammatory back pain for at least 3 months, presents with either sacroiliitis (radiographical or on magnetic resonance), or fulfils the clinical arm that requires both the presence of the HLA-B27 molecule and another SpA feature such as arthritis, heel enthesitis, uveitis, dactylitis, psoriasis, good response to nonsteroidal anti-inflammatory drugs (NSAIDs), family history for SpA, or elevated C-reactive protein. For IBD-related peripheral SpA, the diagnosis is validated if a patient with known IBD presents with arthritis, enthesitis or dactylitis without needing any other criterion. The sensitivity and specificity of the two sets of criteria are good (79.5% and 83.3 %, respectively) [24].
4.2. Manifestations of peripheral spondyloarthritis in inflammatory bowel disease
4.2.1. Arthritis
Peripheral arthropathies occurring in IBD are part of the SpA group of conditions [24,25]. The prevalence is about 15% of the patients [26]. In 1998, the Oxford group [27] distinguished two types of peripheral arthritis in patients with IBD. Type 1 peripheral arthritis evolves in a parallel fashion with the active intestinal disease and is characterized by an asymmetrical, acute and often self-remitting non-destructive oligo-arthritis, involving mostly weight bearing joints. Type 1 arthritis has a prevalence of about 4%, is more frequent in females, is associated with the HLA-B27 carriage, and with colonic involvement [25,28]. IBD complications as abscesses, fistulae, and hemorrhages are considered risk factors [3]. The most important differential diagnosis for Type 1 arthritis, as opposed to Type 2, is infection, given the higher likelihood of septic arthritis in patients suffering from an inflammatory systemic disease and undergoing immunosuppressive treatment. Type 2 arthritis runs an course independent of the intestinal disease, and has no proven association with HLA-B27 status [29]. It resembles rheumatoid arthritis: it has a similar clinical and even radiological potentially erosive pattern with symmetrical, progressive polyarthritis and a predilection for the small joints of the upper extremities [28,29]. Type 2 arthritis can precede or coexist with the gut manifestations. Peripheral arthritis should be differentiated from arthralgia, which is more prevalent but has a more favorable outcome [25].
4.2.2. Enthesitis
Enthesitis, the hallmark of all SpAs, represents inflammation and pain at the site of the insertion of a tendon, ligament, or capsule to the bone. It accounts for a high burden of disease and for a decrease in quality of life. Enthesitis is clinically diagnosed with a frequency of around 20% [30,31], which makes it an under-recognized manifestation. Enthesitis related to IBD has a similar pattern of involvement as idiopathic SpA. There is, however, a predilection for the insertion of weight-bearing tendons of the lower limb such as the Achilles tendon, the plantar fascia, and the patellar tendon. The assessment of enthesitis indices represents a useful outcome measure that reflects disease activity in SpA. Standardization is important to insure a homogeneous approach [28]. There are, however, no formal studies assessing the enthesitis in IBD. Most of the papers on enthesitis are written based on studies performed in patients with psoriatic arthritis (PsA).
4.2.3. Dactylitis
Dactylitis is the term used to describe the swelling and pain of an entire finger or toe (Figure 1). The clinical examination is usually revealing of the condition and in most cases will be sufficient for the diagnosis [28].
Figure 1.
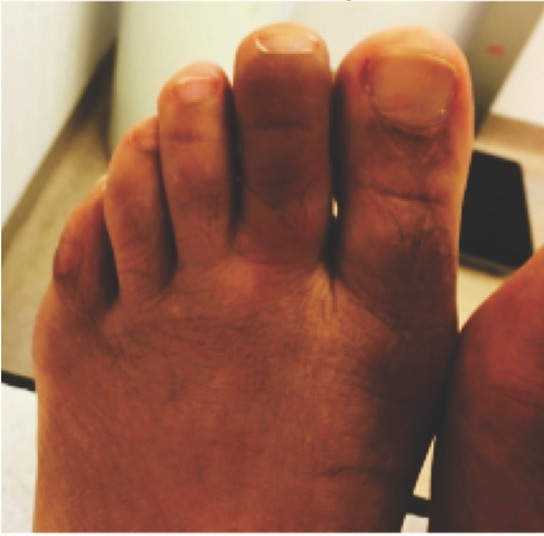
Diffuse swelling of the second right toe (“sausage” like) consistent with dactylitis.
4.3. Axial spondyloarthritis manifestations in inflammatory bowel disease
Axial involvement is equal in prevalence between sexes and forms of IBD [25]. The axial involvement seems to precede intestinal manifestations in Crohn patients [3]. Inflammatory back pain is defined by the ASAS experts as at least four out of the following five parameters: age at onset, insidious onset, improvement with exercise, no improvement with rest, pain at night with improvement upon getting up [24].
Axial involvement in IBD patients could be symptomatic or asymptomatic, affecting the spine or the sacroiliac joints. Occult sacroiliitis refers to the presence of radiographical or magnetic resonance imaging (MRI) signs of sacroiliitis in asymptomatic patients. Detection of occult sacroiliitis is considered important to prevent future disability and to ensure a long-term good quality of life in IBD patients [2].
The clinical manifestations of AS are the identical, regardless of the association with IBD. Importantly, there is no correlation between the activity and progression of the two diseases. There seems to be a slight difference regarding the age of onset: idiopathic AS tends to manifest itself before 40 years of age, whereas IBD-associated AS can become apparent even at older ages—probably because of the high number of occult sacroiliitis cases that ultimately evolve into AS; there is no sex distribution difference for IBD associated AS [29].
4.4. Imaging techniques in spondyloarthritis spectrum
4.4.1. Conventional radiography
Conventional radiography of the spine and pelvis show the typical findings of AS and sacroiliitis, but these features appear only after several years of progression of the disease (Figure 2). It is nevertheless an important exam to start with and should be performed systematically when dealing with low back pain in IBD patients. The typical AS lesions of the sacroiliac joint include periarticular sclerosis, particularly on the iliac side, irregularities and erosions of the sacroiliac joint, pseudo-widening of the sacroiliac joint space, joint space narrowing, bridging and ankylosis. The differential diagnosis of radiological sacroiliitis includes mechanical changes, osteitis condensans ilii, pseudoankylosis of idiopathic skeletal hyperostosis, hyperparathyroidism, and subacute septic sacroiliitis [25,32].
Figure 2.
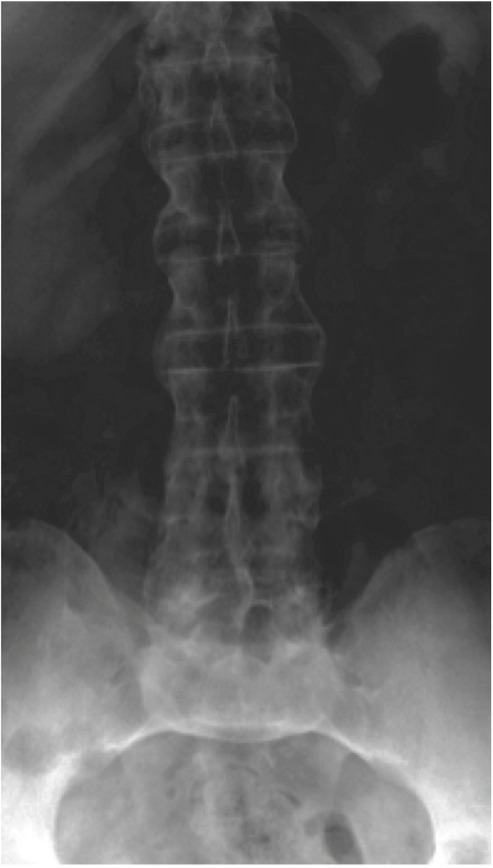
Postero-anterior radiography showing the ankylosis of sacroiliac joints (grade IV) and multiples syndesmophytes of the vertebral bodies (“bamboo” spine) in an asymptomatic patient. The radiography was performed when discovering a Schober test of 0 cm.
Radiological sacroiliitis is present in up to 50% of patients with IBD even if syndesmophytes and progressive ankylosing spondylitis have been reported in only 1%–10% of patients [25]. In a series of 81 patients with IBD without coexistent musculoskeletal manifestations, the radiographic exam showed sacroiliac abnormalities in 27% of the patients, and at 3 years of follow-up 4 out of the 22 patients had developed inflammatory back pain and active sacroiliitis on MRI [2].
Radiographies of the peripheral articulations are performed routinely and can show a wide range of anomalies. Soft tissue swelling and erosions can appear in type 2 peripheral arthritis and are indistinguishable from the erosions characteristic of rheumatoid arthritis, although it seems that osteoporosis is less frequent. New bone formation can sometimes be observed. There are no pathognomonic features to indicate the presence of arthritis due to IBD on the radiographic exam. Enthesitis can be detected in early stages, as osteopenia and erosions, soft tissue calcifications, tendon insertion calcifications, and bone cortex irregularities that can progress to enthesophytes in later stages. The hallmark of enthesitis is the combination of erosions and bone proliferation but visible only in latestage disease [3,28].
4.4.2. Ultrasonography
Ultrasonography (US) is a non-invasive, reproducible, and inexpensive method for detecting inflammatory lesions of the peripheral joints and periarticular structures such as enthesitis, synovitis, bursitis, and tenosynovitis. US is increasingly used by rheumatologists and radiologists for diagnostic purposes in IBD-related conditions, and also for treatment-guided injections. The main disadvantage of this technique is that it is operator dependent and has a slow learning curve. However, US is highly informant when performed by trained and experienced users. US can show a loss of normal fibrillar echogenicity of the tendon, hypoechoic thickening, bone erosions, or new bone formations. Notably, US can assess the hypervascularization in the form of abnormal Doppler signals (Figure 3), offering an insight into the disease’s inflammation activity [33]. For the time being, there is no standardization of the definition of enthesitis or which entheses should be examined, but US is an accessible and reliable tool for detection of early or occult disease despite not the present lack of understanding the significance and the evolution of the occult enthesitis. Future studies, should, however determine whether occult enthesitis is the precursor of the clinical disease or whether there is a correlation with disease activity. The sensitivity of this technique is rapidly evolving, being proportional to the increasing frequency and performance of the US transducers [33,34].
Figure 3.
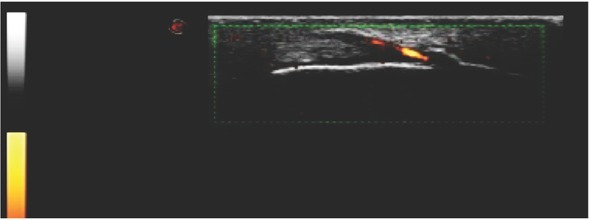
Ultrasound examination showing decreased echogenicity of the tendon, new bone formation at the insertion of the tendon and hypervascularization visualized on Power Doppler exam, indicating enthesitis.
4.4.3. Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is considered the gold standard imaging modality for assessing inflammatory diseases of joints and spine [35,36]. MRI has been used for more than two decades in axial and sacroiliac joint imaging, and its place in the study of SpA has grown ever since, demonstrating inflammatory lesions in many patients with normal radiography. MRI is used in SpA to assess the presence of active sacroiliac inflammatory lesions, of axial inflammatory manifestations, to evaluate the existence of structural lesions early in the course of the disease, and to monitor disease activity [36,37].
ASAS classification criteria for MRI use in SpA were first published in 2009 [37], and an update by the ASAS MRI working group was published in 2016 [32]. According to ASAS definitions, MRI anomalies include structural changes and inflammatory lesions. Structural lesions include bone erosion, sclerosis, fatty conversion of bone marrow, bony bridges, and ankyloses [38,39]. Interestingly, MRI structural changes in AS patients occur early in the disease course and are observed even in patients with disease duration of less than 2 years, whereas radiographical abnormalities occur several years later [40]. Inflammatory lesions include bone marrow edema, capsulitis, and enthesitis [24]. Bone marrow edema is defined as an area of high signal on T2-weighted imaging (WI) with fat saturation or short tau inversion recovery (STIR) sequences, localized in the subchondral bone, particularly [35] (Figure 4). Capsulitis is defined as thickening of the joint capsule and ligaments together with a hyperintense signal on T2WI or STIR images. The European Society of Skeletal Radiology (ESSR) Arthritis Subcommittee published in 2015 their “Recommendations for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases” wherein they suggest that what is interpreted as capsulitis and synovitis of the sacroiliac joints could actually be enthesitis because the histological analysis of the sacroiliac joints shows that there are no capsule or synovium in the proximal two-thirds of the joint [38,39]. Synovitis is defined by the ASAS group by a hyperintense signal on contrast-enhanced T1WI with fat saturation, in the synovial part of the sacroiliac joint [24,37,40]. The current definition of typical inflammatory sacroiliitis must include bone marrow edema on STIR images and located in the subchondral bone marrow in particular; any other structural or inflammatory lesion helps in the process of diagnosis but it is not mandatory according to ASAS [32,37]. The intensity of the signal is dependent on the activity of the inflammation. Conversely, other authorities consider bone marrow edema and fat infiltration as being nonspecific for the diagnosis of sacroiliitis, even if extended into several consecutive slices, and they put the erosions on the first place as specificity for SpA [32,40] (Figure 5). Therefore, controversy exists regarding the definition of active sacroiliitis on MRI between different groups of experts.
Figure 4.
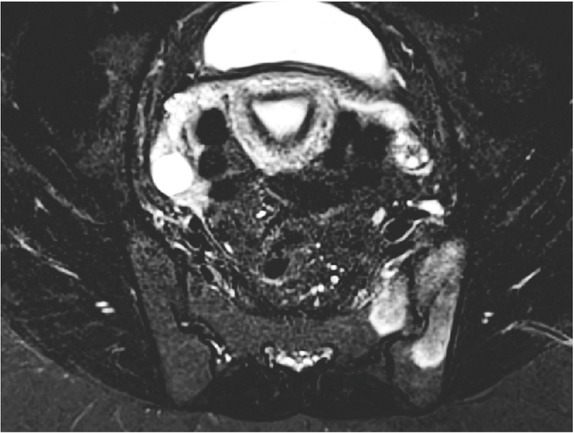
MRI demonstrating extensive subchondral bone marrow hyperintensities on axial STIR sequence involving both iliac and sacral bones on the left side, consistent with bone marrow edema.
Figure 5.
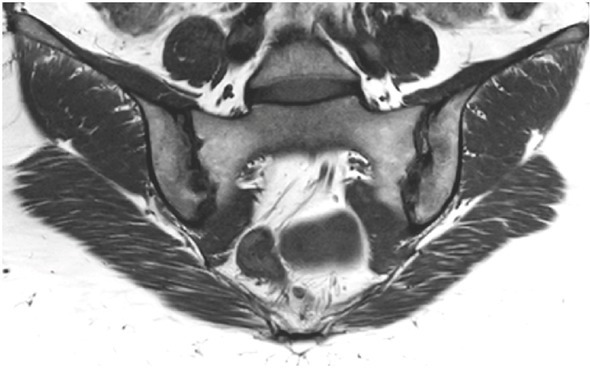
MRI showing multiples subchondral erosions on coronal T1-weighted imaging, predominantly on the right iliac bone.
Nonetheless, all the experts agree that the interpretation of MRI studies in patients with suspicion of SpA should be done by subspecialized musculoskeletal radiologists. They also agree that young patients with inflammatory back pain and negative radiography should undergo an MRI of the sacroiliac joint [25, 40]. Weber et al [40] showed that MRI of the sacroiliac joints has far greater diagnostic utility when using a standardized and systematic approach. The standard MRI protocol should include axial and coronal T1WI and STIR, oriented in the plane of the sacroiliac joint [35].
MRI in dactylitis shows flexor tenosynovitis or extensor tendonitis/paratendinitis but no bone edema has been reported so far. Another important feature is the subcutaneous edema has been described as a “honeycomb” appearance of the peritendinous soft tissue on T2WI [34,41]. Olivieri et all [34] showed increase in the volar bone-to-skin distance in dactylitic fingers caused mainly by the distension of the flexor synovial sheaths. Enlargement of the finger joint capsule is sometimes observed but does not represent a mandatory feature for the diagnosis [41].
Fat suppression sequences (STIR, T2WI with fat suppression) are the most sensitive method for detecting active enthesitis at any site [35]. The most prominent MRI features of enthesitis regardless of the site are changes in the structure of the tendon/swelling, bone marrow edema, erosions, and new bone formation. When synovitis and enthesitis occur in the same joint, the MRI features of the enthesitis may be at least partially obliterated by the synovitis. Inflammatory and mechanically induced enthesitis have similar MRI features at plantar fascia insertion showing bone marrow and soft tissue edema [42]. The MRI findings of Achilles tendon enthesitis in patients with SpA include a diffusely swollen tendon, loss of the round configuration, and increased signal intensity; retrocalcaneal bursitis; and subcutaneous and bone marrow edema. As in the case of the plantar fascia, the same features are also described for exercise-induced tendinopathy, making the differentiation between inflammatory and mechanically induced enthesopathy possible only when supported by clinical information [34,42].
4.5. Metabolic and treatment related pathologies
4.5.1. Osteoporosis and osteoporotic fractures
Osteoporosis and osteopenia appear in about 20% of IBD patients [25,26]. Osteoporosis is diagnosed in adults when there is at least a 2.5 standard deviation lower (T-score ≤ −2.5) bone mineral density than the general population by dual-energy X-ray absorptiometry (DEXA). The screening recommendations based on “The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease” are the same as in the general population. The patients suffering from IBD have a high number of risk factors, such as chronic inflammation, corticosteroid treatment, extensive small-bowel disease or resection, smoking, low physical activity, sarcopenia, and nutritional deficiencies [25].
Because abdominal computer tomography (CT) scans are usually performed in IBD patients to evaluate the intestinal disease and its complications, an opportunistic screening assessment for osteoporosis can be performed with very good results. For that purpose, sagittal L1 trabecular attenuation is measured and a definition of osteoporosis corresponds to a cut-off of less than 110 Hounsfield units (HU) (specificity >90% for osteoporosis on calibrated CT scanners at 120 kV(p). Simultaneously, the CT scan allows for the screening of the vertebral fractures applying the Genant scale [43].
Interestingly, most of the patients normalize their bone density if they achieve at least 3 years of stable remission; the treatment with anti-TNF improves the bone density as well. Nevertheless, a higher risk of vertebral fractures persists in IBD patients even in the presence of normalized bone mineral density [25]. Treatment with anti-TNF-α increases bone mass in IBD patients but without reducing the risk of new vertebral fractures. IBD patients have a considerably higher incidence of vertebral fractures than the general population, and the only predictive factor identified so far is the existence of previous fractures [44]. A recent meta-analysis found that the global risk of fracture was increased for patients with IBD versus controls (RR = 1.38, 95% CI 1.11-1.73; p = 0.005) with a particularly very high vertebral fracture risk (OR = 2.26, 95% CI 1.04-4.90; p < 0.001), which was not the case for non-vertebral fractures [45].
4.5.2. Osteonecrosis
Osteonecrosis represents the infarction of osseous tissue–usually in the subchondral regions of the bone, or, less commonly, in the diaphyseal regions (bone infarct). High dosage regimens of corticosteroid therapy are likely the most important etiological factor. Multifocal osteonecrosis with involvement of different locations seems particularly common in IBD patients [46]; most of the time, symptoms are limited to a single joint even though multiple joints are involved [47]. The exact threshold dose for the corticotherapy has not yet been determined, but it seems that the most important factor is the cumulative dose [47]. Kamata et al [48] found osteonecrosis of the femoral head in 4 out of 20 asymptomatic IBD patients when performing MRI. All patients had refractory inflammatory bowel disease requiring high dose and long-term corticosteroid treatment. It was also found that the administration of 50 mg prednisolone for more than 2 weeks was a risk factor for osteonecrosis in their group of IBD patients [46, 47, 48].
Technetium-99m methylene diphosphonate (99mTc-MDP) bone scintigraphy is a highly sensitive method for detecting osteonecrosis. 99mTc-MDP binds to the hydroxyapatite component of the osseous matrix; the blood flow influences the uptake of the radiotracer, making a change in bone turnover as low as 5% visible on bone scintigraphy (compared to radiography or bone CT where a loss of about 50% of the mineral density is needed to visualize the abnormalities). A patient with suspected osteonecrosis will undergo a three-phase study. In the acute phase (1 week), osteonecrosis generally appears as a photopenic area, and after 1 to 3 weeks there is an increase in the uptake of the radiotracer with a distribution in the subchondral bone. Osteonecrosis is visible on the bone scintigraphy a week after the initial event and has a very high sensitivity (up to 91% for the femoral neck), which makes it a very good tool for screening IBD patients with high risk for multifocal osteonecrosis [49].
4.5.3. Paradoxical arthritis occurring during anti-TNF treatment for IBD
Paradoxical articular manifestations represent a condition that appears when using a treatment that is conventionally used to treat it. Since the introduction of anti-TNF, cases of arthritis or disabling arthralgia have been reported to occur in IBD-patients in intestinal remission under TNF. The prevalence is reported to be 3% for infliximab, and it poses a diagnostic challenge because its main differential diagnosis is SpA related to IBD. Recent articles have suggested measuring drug levels or adding methotrexate prior to eliminating the anti-TNF [50,51]. A recent and very interesting study documented and analyzed 10 cases of paradoxical arthritis occurring during anti-TNF treatment for IBD. The authors performed a synovial biopsy of all the patients and found similar histological findings in terms of synovial resident CD68+, CD21+, CD20+, CD3+ and CD117+ cells, comparable to the psoriatic arthritis. They suggest that changing the target of the treatment to an anti-IL12/IL23 may be an option for severe paradoxical articular manifestations [50].
4.6. Muscular manifestations
4.6.1. Sarcopenia
Sarcopenia or lean mass depletion is an emerging concept that refers to a generalized loss of skeletal muscle mass; it has been associated with a poor prognosis in IBD patients, notably higher risk of surgical intervention and hospitalization, as well as a worse response to treatment [52,53]. The diagnosis of sarcopenia requires an assessment of body mass and muscle strength. The whole body DEXA is the gold standard for the diagnosis; it is outstanding in terms of time, cost, and low radiation. The procedure is based on analyzing the attenuation of X-ray when passing over the tissues with the possibility of differentiating between bone, adipose tissue, and muscle [52,54].
4.7. Miscellaneous musculoskeletal manifestations
4.7.1. Fibromyalgia
Fibromyalgia has been initially thought to be associated with IBD [55]. A large retrospective cohort study was recently performed, analyzing 4,510 patients with IBD and 18,040 gender- and age-matched patients without history of IBD over a period of 12 years: it found no correlation between the 2 pathologies. These results, therefore, override the previous theory that IBD patients had higher risk of fibromyalgia [56].
4.8. “Forgotten” manifestations
4.8.1. Hypertrophic osteoarthropathy
The hypertrophic osteoarthropathy represents a “bulbous” deformity of the tips of the fingers and toes also known as clubbing of the fingers; it can have a primary idiopathic origin or a secondary cause. IBD is a cause of secondary hypertrophic osteoarthropathy, which has been associated with poor prognosis for the intestinal disease [3]. This clubbing of the fingers has historically been reported in as many as 38% of Crohn patients and in 15% of patients with ulcerative colitis [57]. However, there is a lack of recent updates on this observation, suggesting that the prevalence could have been diminished considerably with the emergence of newly effective therapies.
4.8.2. Granulomatous synovitis
Granulomatous synovitis is an entity that had been described in the 1970s but has not been reported in the past 30 years. Lindstrom et al. first described it in a young patient with Crohn disease in whom a surgical biopsy of the ankle had been performed. The histological exam showed a chronic, granulomatous inflammation with hyperplasic mesothelium, lymphocytic infiltration, and multiple non-necrotizing epithelioid cell granulomas with Langhans-type giant cells [58].
To summarize, rheumatic manifestations in inflammatory bowel diseases contribute substantially to the burden of the disease. Imaging advances and availability of the different techniques allow for the increasing recognition of occult rheumatic manifestations. The correct recognition of occult spondyloarthritis in IBD requires an integrated multidisciplinary approach, including rheumatologic, gastrointestinal, and radiological assessment to establish the appropriate diagnostic and therapeutic strategies. The use of imaging techniques should be included in the assessment of IBD patients to correctly evaluate occult spondyloarthritis, thus preventing future disability and worsening quality of life. Ultrasound is a reliable and accessible tool for detection of early superficial entheseal diseases, whereas MRI is preferred for the deep entheseal sites. Screening for osteoporosis in IBD patients could be performed using DEXA as in the general population or using an opportunistic abdominal CT scan previously performed for the assessment of the IBD. Additionally, the CT scan gives the possibility of vertebral fracture screening and assessment. Osteonecrosis tends to be multifocal and asymptomatic in IBD patients, so screening using scintigraphy could be useful prior to MRI assessment of multiple sites. Paradoxical arthritis should be considered as a possible diagnosis in patients under anti-TNF treatment.
Footnotes
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Matsuoka K, Kobayashi T, Ueno F. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53(3):305–353. doi: 10.1007/s00535-018-1439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandinelli F, Manetti M, Ibba-Manneschi L. Occult spondyloarthritis in inflammatory bowel disease. Clin Rheumatol. 2016;35(2):281–289. doi: 10.1007/s10067-015-3074-z. [DOI] [PubMed] [Google Scholar]
- 3.Voulgari P V. Rheumatological manifestations in inflammatory bowel disease. Ann Gastroenterol. 2011;24(3):173–180. [PMC free article] [PubMed] [Google Scholar]
- 4.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Wright V. Seronegative polyarthritis: a unified concept. Arthritis Rheum. 1978;21(6):619–633. doi: 10.1002/art.1780210603. [DOI] [PubMed] [Google Scholar]
- 6.Dougados M, van der Linden S, Juhlin R. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34(10):1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 7.De Vlam K, Mielants H,, Cuvelier C,, De Keyser F, Veys EM, De Vos M. Spondyloarthropathy is underestimated in inflammatory bowel disease: prevalence and HLA association. J Rheumatol. 2000;27(12):2860–2865. [PubMed] [Google Scholar]
- 8.Olivieri I, Cantini F, Castiglione F. Italian Expert Panel on the management of patients with coexisting spondyloarthritis and inflammatory bowel disease. Autoimmun Rev. 2014;13(8):822–830. doi: 10.1016/j.autrev.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Peluso R, Di Minno MN, Iervolino S. Enteropathic Spondyloarthritis: From Diagnosis to Treatment. Clin Dev Immunol. 2013;2013:1–12. doi: 10.1155/2013/631408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vavricka SR, Brun L, Ballabeni P. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106(1):110–119. doi: 10.1038/ajg.2010.343. [DOI] [PubMed] [Google Scholar]
- 11.Manguso F, Sanges M, Staiano T. Cigarette smoking and appendectomy are risk factors for extraintestinal manifestations in ulcerative colitis. Am J Gastroenterol. 2004;99(2):327–334. doi: 10.1111/j.1572-0241.2004.04039.x. [DOI] [PubMed] [Google Scholar]
- 12.Ossum AM, Palm Ø, Lunder AK. Ankylosing Spondylitis and Axial Spondyloarthritis in Patients With Long-term Inflammatory Bowel Disease: Results From 20 Years of Follow-up in the IBSEN Study. J Crohn’s Colitis. 2018;12(1):96–104. doi: 10.1093/ecco-jcc/jjx126. [DOI] [PubMed] [Google Scholar]
- 13.Speca S, Dubuquoy L. Chronic bowel inflammation and inflammatory joint disease: Pathophysiology. Joint Bone Spine. 2017;84(4):417–420. doi: 10.1016/j.jbspin.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 15.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384(6605):134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 16.Akkoç N, Yarkan H, Kenar G, Khan MA. Ankylosing Spondylitis: HLA-B27-Positive Versus HLA-B27-Negative Disease. Curr Rheumatol Rep. 2017;19(5):26. doi: 10.1007/s11926-017-0654-8. [DOI] [PubMed] [Google Scholar]
- 17.Salvarani C, Fries W. Clinical features and epidemiology of spondyloarthritides associated with inflammatory bowel disease. World J Gastroenterol. 2009;15(20):2449–2455. doi: 10.3748/wjg.15.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Ding J, Wang M, Zhou W, Feng M, Guan W. Clinical features of Crohn disease concomitant with ankylosing spondylitis. Medicine (Baltimore) 2016;95(28):e4267. doi: 10.1097/MD.0000000000004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez-Navarro C. López de Castro JA. ERAP1 in ankylosing spondylitis: genetics, biology and pathogenetic role. Curr Opin Rheumatol. 2013;25(4):419–425. doi: 10.1097/BOR.0b013e328362042f. [DOI] [PubMed] [Google Scholar]
- 20.Danoy P, Pryce K, Hadler J. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn’s disease. PLoS Genet. 2010;6(12):e1001195. doi: 10.1371/journal.pgen.1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricart E, Panaccione R, Loftus EV. Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2004;10(3):207–214. doi: 10.1097/00054725-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129(3):827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Braun J, Sieper J. Building consensus on nomenclature and disease classification for ankylosing spondylitis: results and discussion of a questionnaire prepared for the International Workshop on New Treatment Strategies in Ankylosing Spondylitis. Ann Rheum Dis. 2002;61(3):61–67. doi: 10.1136/ard.61.suppl_3.iii61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudwaleit M, van der Heijde D, Landewé R. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70(1):25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 25.Harbord M, Annese V, Vavricka SR. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(3):239–254. doi: 10.1093/ecco-jcc/jjv213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spekhorst LM, Oldenburg B, van Bodegraven AA. Prevalence of- and risk factors for work disability in Dutch patients with inflammatory bowel disease. World J Gastroenterol. 2017;23(46):8182–8192. doi: 10.3748/wjg.v23.i46.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut. 1998;42(3):387–391. doi: 10.1136/gut.42.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheth T, Pitchumoni CS, Das KM. Musculoskeletal manifestations in inflammatory bowel disease: a revisit in search of immunopathophysiological mechanisms. J Clin Gastroenterol. 2014;48(4):308–317. doi: 10.1097/MCG.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Reyna TS. Martínez-Reyes C, Yamamoto-Furusho JK. Rheumatic manifestations of inflammatory bowel disease. World J Gastroenterol. 2009;15(44):5517–5524. doi: 10.3748/wjg.15.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantini F, Niccoli L, Nannini C. Case-control Study on Dactylitis, Enthesitis, and Anterior Uveitis in Spondyloarthritis Associated with Inflammatory Bowel Diseases: Role of Coexistent Psoriasis. J Rheumatol. 2017;44(9):1341–1346. doi: 10.3899/jrheum.161518. [DOI] [PubMed] [Google Scholar]
- 31.Watad A, Cuthbert RJ, Amital H, McGonagle D. Enthesitis: Much More Than Focal Insertion Point Inflammation. Curr Rheumatol Rep. 2018;20(7):41. doi: 10.1007/s11926-018-0751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert RGW, Bakker PAC, van der Heijde D. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis. 2016;75(11):1958–1963. doi: 10.1136/annrheumdis-2015-208642. [DOI] [PubMed] [Google Scholar]
- 33.Gandjbakhch F, Terslev L, Joshua F. Ultrasound in the evaluation of enthesitis: status and perspectives. Arthritis Res Ther. 2011;13(6):R188. doi: 10.1186/ar3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivieri I, Barozzi L, Favaro L. Dactylitis in patients with seronegative spondylarthropathy. Assessment by ultrasonography and magnetic resonance imaging. Arthritis Rheum. 1996;39(9):1524–1528. doi: 10.1002/art.1780390912. [DOI] [PubMed] [Google Scholar]
- 35.Sudoł-Szopińska I, Jurik AG, Eshed I. Recommendations of the ESSR Arthritis Subcommittee for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases. Semin Musculoskelet Radiol. 2015;19(4):396–411. doi: 10.1055/s-0035-1564696. [DOI] [PubMed] [Google Scholar]
- 36.Schueller-Weidekamm C, Mascarenhas V, Sudol-Szopinska I. Imaging and Interpretation of Axial Spondylarthritis: The Radiologist’s Perspective - Consensus of the Arthritis Subcommittee of the ESSR. Semin Musculoskelet Radiol. 2014;18(03):265–279. doi: 10.1055/s-0034-1375569. [DOI] [PubMed] [Google Scholar]
- 37.Rudwaleit M, Jurik AG, Hermann K-GA. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68(10):1520–1527. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- 38.Sudoł-Szopińska I, Jurik A, Eshed I. Recommendations of the ESSR Arthritis Subcommittee for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases. Semin Musculoskelet Radiol. 2015;19(04):396–411. doi: 10.1055/s-0035-1564696. [DOI] [PubMed] [Google Scholar]
- 39.Puhakka KB, Melsen F, Jurik AG, Boel LW, Vesterby A, Egund N. MR imaging of the normal sacroiliac joint with correlation to histology. Skeletal Radiol. 2004;33(1):15–28. doi: 10.1007/s00256-003-0691-4. [DOI] [PubMed] [Google Scholar]
- 40.Weber U, Lambert RGW, Østergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: An international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 2010;62(10):3048–3058. doi: 10.1002/art.27571. [DOI] [PubMed] [Google Scholar]
- 41.McQueen F, Lassere M,, Østergaard M. Magnetic resonance imaging in psoriatic arthritis: a review of the literature. Arthritis Res Ther. 2006;8(2):207. doi: 10.1186/ar1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eshed I, Bollow M, McGonagle DG. MRI of enthesitis of the appendicular skeleton in spondyloarthritis. Ann Rheum Dis. 2007;66(12):1553–1559. doi: 10.1136/ard.2007.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SJ, Binkley N, Lubner MG, Bruce RJ, Ziemlewicz TJ, Pickhardt PJ. Opportunistic screening for osteoporosis using the sagittal reconstruction from routine abdominal CT for combined assessment of vertebral fractures and density. Osteoporos Int. 2016;27(3):1131–1136. doi: 10.1007/s00198-015-3318-4. [DOI] [PubMed] [Google Scholar]
- 44.Maldonado-Pérez MB, Castro-Laria L, Caunedo-Álvarez A. et al. Does the Antitumor Necrosis Factor-α Therapy Decrease the Vertebral Fractures Occurrence in Inflammatory Bowel Disease? J Clin Densitom. 2018. pii:S1094-6950. [DOI] [PubMed]
- 45.Szafors P, Che H, Barnetche T. Risk of fracture and low bone mineral density in adults with inflammatory bowel diseases. A systematic literature review with meta-analysis. Osteoporos Int. 2018;29(11):2389–2397. doi: 10.1007/s00198-018-4586-6. [DOI] [PubMed] [Google Scholar]
- 46.Hauzeur JP, Malaise M, Gangji V. Osteonecrosis in inflammatory bowel diseases: a review of the literature. Acta Gastroenterol Belg. 2009;72(3):327–334. [PubMed] [Google Scholar]
- 47.Klingenstein G, Levy RN, Kornbluth A, Shah AK, Present DH. Inflammatory bowel disease related osteonecrosis: report of a large series with a review of the literature. Aliment Pharmacol Ther. 2005;21(3):243–249. doi: 10.1111/j.1365-2036.2005.02231.x. [DOI] [PubMed] [Google Scholar]
- 48.Kamata N, Oshitani N, Sogawa M. Usefulness of magnetic resonance imaging for detection of asymptomatic osteonecrosis of the femoral head in patients with inflammatory bowel disease on long-term corticosteroid treatment. Scand J Gastroenterol. 2008;43(3):308–313. doi: 10.1080/00365520701676773. [DOI] [PubMed] [Google Scholar]
- 49.Agrawal K, Tripathy SK, Sen RK, Santhosh S, Bhattacharya A. Nuclear medicine imaging in osteonecrosis of hip: Old and current concepts. World J Orthop. 2017;8(10):747–753. doi: 10.5312/wjo.v8.i10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alivernini S, Pugliese D, Tolusso B. Paradoxical arthritis occurring during anti-TNF in patients with inflammatory bowel disease: histological and immunological features of a complex synovitis. RMD Open. 2018;4(1):e000667. doi: 10.1136/rmdopen-2018-000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sondag M, Verhoeven F, Guillot X. “Paradoxical” arthralgia occurring under anti-TNFα treatment for inflammatory bowel disease. Joint Bone Spine. 2018;85(1):133–134. doi: 10.1016/j.jbspin.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Scaldaferri F, Pizzoferrato M, Lopetuso LR. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol Res Pract. 2017;2017:1–11. doi: 10.1155/2017/8646495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bamba S, Sasaki M, Takaoka A. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS One. 2017;12(6):e0180036. doi: 10.1371/journal.pone.0180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryant R, Schultz C, Ooi S. Obesity in Inflammatory Bowel Disease: Gains in Adiposity despite High Prevalence of Myopenia and Osteopenia. Nutrients. 2018;10(9) doi: 10.3390/nu10091192. pii:E1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palm O, Moum B, Jahnsen J, Gran JT. Fibromyalgia and chronic widespread pain in patients with inflammatory bowel disease: a cross sectional population survey. J Rheumatol. 2001;28(3):590–594. [PubMed] [Google Scholar]
- 56.Chen J-H, Chen H-J, Kao C-H, Tseng C-H, Tsai C-H.. Is Fibromyalgia Risk Higher Among Male and Young Inflammatory Bowel Disease Patients? Evidence from a Taiwan Cohort of One Million. Pain Physician. 2018;21(3):E257–264. [PubMed] [Google Scholar]
- 57.Kitis G, Thompson H, Allan RN. Finger clubbing in inflammatory bowel disease: its prevalence and pathogenesis. Br Med J. 1979;2(6194):825–828. doi: 10.1136/bmj.2.6194.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindström C, Wramsby H,, Ostberg G.. Granulomatous arthritis in Crohn’s disease. Gut. 1972;13(4):257–259. doi: 10.1136/gut.13.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]


