Abstract
Introduction
This study aims to investigate the potential effects of regional hyperthermia combined with chemotherapy (RHCT) as a treatment strategy for advanced gastric cancer (AGC).
Method
118 AGC patients were randomly divided into treatment plans with chemotherapy (CT) alone or with RHCT. The prognostic value of clinicopathologic characteristics was assessed in terms of overall survival of AGC patients.
Results
The disease control rate was determined to be 70.9% and 46.0% for the RHCT and CT group, respectively (P = 0.006). The median survival was determined to be 23.5 months for the RHCT group and 14.0 months for the CT group (P = 0.010). The 3-year survival rate for the RHCT group was 11.4% and 0% for the CT group (P = 0.018). No difference in grade 3 or 4 adverse events was observed between the two groups (P > 0.05). Multivariate analysis showed that hyperthermia, disease stage, Glasgow prognostic score, and abdominal metastasis were closely associated with the prognosis of these AGC patients.
Conclusion
The study suggests that combination treatment with RHCT for AGC has clinical potential for both short- and long-term curative effects without compromising toxicity.
Keywords: Hyperthermia, Chemotherapy, Advanced gastric cancer, Prognosis
1. Introduction
Gastric cancer is one of the world’s leading malignant cancers, ranking the second highest in morbidity and fourth in highest mortality. More than 950,000 patients are newly diagnosed every year [1], and epidemiologic studies have showed a high incidence in Asia [2]. In clinical practice, most patients were diagnosed at an advanced stage, with a median survival less than 12 months [3]. Chemotherapy has been used for treating gastric cancer since the 1980s, and the most commonly used anticancer drugs are fluorouracil, platinum, anthracycline, paclitaxel, camptothecin. However, the clinical outcomes with use of these chemotherapy drugs remains unsatisfactory [4, 5, 6].
Hyperthermia is a therapeutic procedure used to raise the temperature of a region of the body affected by cancer. It not only acts directly on the tumor target but has also shown synergistic effects on chemotherapy [7, 8, 9]. However, it is not clear whether regional hyperthermia combined with with chemotherapy (RHCT) could have clinical value for the treatment of AGC. In fact, several clinical studies have evaluated the therapeutic potential of RHCT, but the results remained discrepant. In this study, we investigated clinical outcomes of RHCT in a clinical trial with a total of 118 patients, and the results showed that RHCT could benefit AGC patients receiving traditional chemotherapy.
2. Patients and methods
2.1. Study Population
This randomized, single-center, open-label, phase 2 trial was carried out between May 2012 and June 2016. Eligible patients with pathologically confirmed stage III-IV gastric cancer according to the 7th edition of the American Joint Committee on Cancer staging criteria were enrolled in this trial. All patients showed no obvious liver, kidney, or heart disease, with a Karnofsky score ≥70 points. Eligible patients were randomly allocated to treatment groups receiving RHCT or CT, and no significant contraindications to hyperthermia were observed in these patients during the clinical treatment.
All patients provided written, informed consent before the study. The study was approved by the Scientific Research Board of Zhejiang Xiaoshan Hospital, and the clinical procedure for each patient was closely managed in accordance with the Declaration of Helsinki, local laws, and applicable regulatory requirements.
2.2. Procedures
Patients in the CT group received oral S-1, 80 mg/m2 , for 14 consecutive days, and 130 mg/m2 of oxaliplatin was delivered intravenously on day 1. The regimen was repeated every 3 weeks. The entire treatment process was carried out for at least 3 cycles. Patients in the RHCT group received the same chemotherapy regimen and simultaneously received abdominal regional hyperthermia twice a week from the start to the end of the chemotherapy. Computed tomographic data for each patient was inputted into a computer in order to determine where the heating treatment should focus. Heat was provided with a heating device (NRL-002 type, Jilin Maida Co., Jilin, China) that uses 36.40 MHz waves as the heat source. The input power on this device is 2000 W, with an output power that ranges from 600–800 W. Heat was applied around the navel, with an effective heating area greater than 25 cm in diameter. Thermometer probes were placed in the abdominal cavity via abdominocentesis and on the surface of the skin in the hyperthermia area of the rectum. The intra-abdominal temperature was expected to prevail. During hyperthermia, the temperature was monitored in real time. In general, the tumor center temperature rose to the target range in 10-15 min, T90 was 42~43 °C, and the heating lasted for 60 minutes.
2.3. Outcomes
The primary endpoint of this clinical trial was the proportion of patients who achieved an objective response rate (ORR, the percentage of patients with complete response [CR] or partial response [PR]), and the proportion of patients who achieved a disease control rate (DCR, including the percentage of patients with the best overall response of CR, PR, or stable disease[SD]). The secondary key endpoint is overall survival (OS) (time from randomization to death from any cause). Other secondary endpoints include therapy safety.
2.4. Follow-up
Adverse events (AE) were assessed throughout the study and were documented with guidelines of the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Severity of all AEs was graded with these criteria. Tumors were assessed by computed tomography or magnetic resonance imaging scan every 6 weeks for the first 48 weeks, then subsequently every 12 weeks until the disease progressed objectively or other cancer treatment was started. Brain imaging and bone scans were performed when they were clinically needed. All patients were followed up until the data was analyzed or patients died. Follow-up time ranged from 3 to 60 months, with an average of (36.0 ± 3.2) months.
2.5. Statistical analysis
Statistical analyses and data visualization were performed with IBM SPSS version 22.0 (IBM SPSS, Inc., Chicago, IL, USA) and Graph Pad Prism, Version 6.01 (Graph Pad Software Inc., San Diego, CA, USA). The Chi-square test was used for the comparison of enumeration data. The Kaplan-Meier method was used for univariate analysis. P values less than 0.05 were considered significant. All of the tests were performed two-sided.
3. Results
3.1. Patient characteristics
One hundred-twenty patients with gastric cancer were screened. After exclusion of 2 ineligible patients, a total of 118 patients were enrolled in this study (Figure 1). The patients were randomly assigned to the RHCT (n=55) or CT group (n=63; Table 1). The median age of enrolled patients was 62.5 years (28 to 92 years), with 87 (73.7%) male patients and 31(26.3%) female patients; of these patients, 60 patients were diagnosed with stage III cancer, and 58 patients with stage IV cancer (49.2%).Patient characteristics of each group are illustrated in Table 1.
Figure 1.
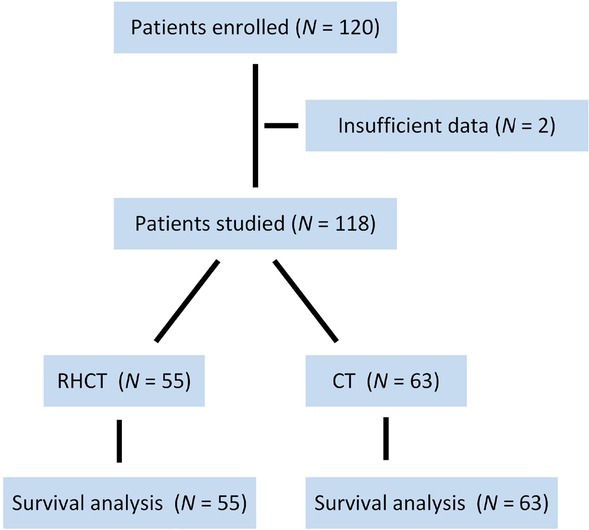
The scheme of this study. RHCT regional hyperthermia with chemotherapy; CT chemotherapy
Table 1.
Clinical characteristics (n=118)
| Total | RHCT | CT | P value | |
|---|---|---|---|---|
| Age, years | 0.236 | |||
| Median | 62.5 | 61 | 64 | |
| Range | 28-92 | 32-86 | 28-92 | |
| ≥60 | 69 | 29 | 40 | |
| <60 | 49 | 26 | 23 | |
| Gender | 0.516 | |||
| Male | 87 | 39 | 48 | |
| Female | 31 | 16 | 15 | |
| Pathology | 0.068 | |||
| Non-SRC | 107 | 47 | 60 | |
| SRC | 11 | 8 | 3 | |
| Curative surgery | 0.909 | |||
| Yes | 38 | 18 | 20 | |
| No | 80 | 37 | 43 | |
| Tumor Stage | 0.646 | |||
| III | 36 | 32 | 34 | |
| IV | 52 | 23 | 29 | |
| Metastasis | 0.732 | |||
| Abdomen | 71 | 34 | 37 | |
| Non-abdomen | 47 | 21 | 26 |
RHCT regional hyperthermia with chemotherapy, CT chemotherapy alone, SRC signet-ring cell carcinoma, abdomen metastasis in abdomen or abdominal organ
3.2. Treatment efficacy
The DCR in the RHCT group was 70.9% (39/55 cases), which was superior to that of the CT group (46.0%, 29/63 cases) with statistical significance (P = 0.006) (Table 2). The OS of the RHCT group was 16.2 months on average, with the median OS determined to be 23.5 months. The median OS for the CT group was 9.0 months (95%CI: 6.1 to 11.9 months), while the median OS for RHCT was 14 months (95%CI: 8.7 to 19.3 months). Statistical significance was observed for the OS of these two groups (P = 0.010) (Figure 2).
Table 2.
Comparisons of treatment response in the two groups
| CR | PR | SD | PD | |
|---|---|---|---|---|
| RHCT | 0 | 4 | 35 | 16 |
| CT | 0 | 3 | 26 | 34 |
RHCT regional hyperthermia with chemotherapy, CT chemotherapy alone, CR complete response, PR partial response, SD stable disease, PD progression disease.
Figure 2.
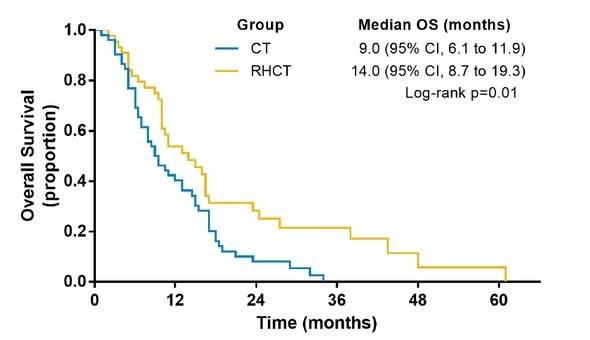
Survival curves of patients in RHCT and CT group. RHCT regional hyperthermia with chemotherapy; CT chemotherapy
3.3. Safety and tolerability
The most common AEs observed in the patients during the clinical trial were bone marrow suppression, gastrointestinal reactions, and liver/kidney damage. Of note, the incidence of AEs for patients enrolled in these two groups showed no statistical significance (P> 0.05) (Table 3).
Table 3.
Comparisons of adverse reactions in the two groups
| RHCT | CT | χ2 value | P value | |
|---|---|---|---|---|
| Bone marrow | ||||
| suppression | 22 | 21 | 1.169 | 0.974 |
| I° | ||||
| II° | 11 | 13 | ||
| III° | 5 | 6 | ||
| IV° | 0 | 1 | ||
| Gastrointestinal reactions | 16 | 12 | 1.611 | 0.469 |
| I° | ||||
| II° | 8 | 9 | ||
| III° | 1 | 3 | ||
| IV° | 0 | 0 | ||
| Liver / kidney damage | 15 | 13 | 0.038 | 0.846 |
| I° | ||||
| II° | 5 | 5 | ||
| III° | 0 | 0 | ||
| IV° | 0 | 0 |
RHCT regional hyperthermia with chemotherapy, CT chemotherapy alone
3.4. Univariate and multivariate analysis
The study indicated the treatment with RHCT had an improved therapeutic response and provided prolonged
Figure 3.
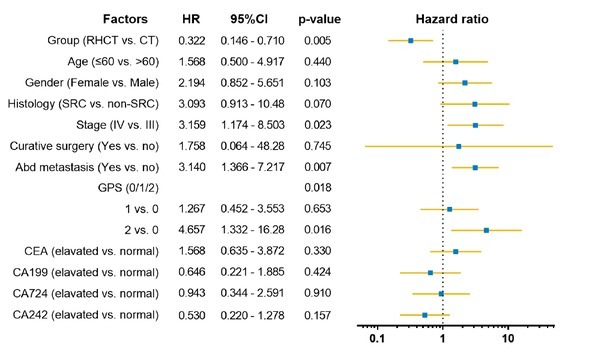
Multivariate analysis of overall survival. RHCT regional hyperthermia with chemotherapy; CT chemotherapy; SRC signetring cell carcinoma; GPS Glasgow Prognostic Score
survival for AGC patients. Clinically, several factors have been found to influence the prognosis of AGC, including tumor stage, inflammation index (Glasgow Prognostic Score, GPS), and tumor markers. In this study, we also calculated GPS according to C-reactive protein (CRP) and albumin (Alb) with criteria of score 0 indicating normal for both CRP and Alb, score 1 indicating that either CRP or Alb was abnormal, and score 2 indicating elevated CRP levels with Alb [10]. The multivariate analysis further showed that hyperthermia, disease stage, GPS, and abdominal metastasis were independent prognostic factors for these patients, as shown in Figure 2.
4. Discussion
Gastric cancer patients have a poor prognosis as they often have AGC at the time of first diagnosis. In addition, the rate of recurrence and metastasis remain very high, even following surgery [11].
Hyperthermia is a form of physical treatment, which functions to raise the temperature of tumor-loaded tissue to 41–45 °C and is utilized as an adjunctive therapy alongside chemotherapy [7, 8, 9, 12]. Hyperthermia has been shown to strengthen anticancer effects for many types of cancers through synergistic interactions when combined with chemotherapy. Issels et al. [13] reported the clinical outcomes of an open-label, phase 3 randomized clinical trial, involving 329 patients with soft tissue sarcoma, aiming to evaluate the efficacy of regional hyperthermia plus neoadjuvant chemotherapy. They found that the addition of regional hyperthermia to chemotherapy resulted in an increased overall survival (HR = 0.73; 95% CI, 0.54-0.98; P = 0.04), as well as local progression-free survival (HR = 0.65; 95% CI, 0.49-0.86; P = 0.002). Colombo et al. [14] also reported long-term efficacy of regional hyperthermia for 83 patients with chemotherapy for bladder cancer, and results showed that the recurrence rate following RHCT was 40%, compared to 80% in the CT group, and the 10-year disease-free survival was 53% for RHCT group, while 15% for CT group (P < 0.001). These data demonstrated the efficacy of hyperthermia for bladder cancer in long-term follow-up. However, no study so far has been reported for the therapeutic potential of RHCT for AGC.
Our results showed in this study that, compared with chemotherapy alone, RHCT significantly improved both short-term effects and long-term survival, indicating that RHCT could result in a survival benefit for AGC patients. The observed AEs in patients enrolled in both groups were primarily grade 1/2 bone marrow suppression and grade 1/2 gastrointestinal reaction, with no statistical significance. We also noticed, however, one case of IV° bone marrow suppression appeared in the CT group. These observations demonstrated the clinical safety and feasibility of RHCT strategy for AGC patients.
Studies have revealed that tumor node metastasis (TNM) stage is related to the prognosis of gastric cancer. In this study, however, we found that only M stage is associated with prognosis. Clinically, the majority of patients with gastric cancer at a pathologic stage of T2 or higher have already had metastasis of the cancer to the lymph nodes or distant organs [1]. However, the patients enrolled in this study were at stage III or IV, with a higher T stage and N stage. Therefore, it is still not clear whether the T and N stage may be associated with the prognosis for gastric cancer patients with early stage of tumors when patients receive comparable RHCT and CT. Clinicopathologic features also play important roles in determining the prognosis of AGC. However, it is difficult to access these data for patients with unresectable tumors. Instead, the use of easily obtainable and simple biomarkers, including tumor markers, inflammatory factors, nutrition indexes, and coagulation factor, may provide the guidelines for selection of patients for appropriate treatment strategies. For example, inflammation associated with tumors is a critical part of the tumor microenvironment, and this inflammatory microenvironment is one of the top ten characteristics of the tumor. This demonstrates the critical role that inflammation plays in the development of the tumor. In recent years, a series of systemic inflammation indexes, including CRP, Neutrophil-to-Lymphocyte ratio, platelet to lymphocyte ratio and GPS, have been identified to reflect the body inflammation state. These indexes have gradually drawn greater attention from researchers within the field [15, 16]. Sierzega [16] summarized extensive research regarding the relationships between inflammatory indexes and the prognosis of cancer. In this study, we determined the potential prognostic value of serum biomarkers CA199, CA724 and CA242, and found no significant s correlations of these biomarkers with the clinical outcome of the AGC patients. However, multivariate analysis of this study demonstrated that GPS showed strong correlation with the prognosis of these AGC patients.
Hyperthermia generally is regarded as a potent enhancer to the effects of chemotherapy. Our study showed that RHCT could improve the therapeutic efficiency of chemotherapy for AGC, with significantly prolonged OS. Our data suggests a potential clinical impact of regional hyperthermia for adjuvant therapy of AGC, which could benefit patients with aggressive AGC.
Acknowledgments
This study was supported by grants from Key Specialist project of Hangzhou City Science and Technology Bureau (20150733Q67) to HMF.
Footnotes
Authors’ contributions: HMF, YPZ, and ZBW have been involved in drafting the article or revising it critically for important intellectual content. XYW, HW, YW, FC, YLJ, ZQJ, and YW have made substantial contributions to acquisition of data. LCZ, SLM and HMF have made substantial contributions to analysis and interpretation of data. MSL and LCZ have made substantial contributions to conception and design of this manuscript and final approval of the version to be submitted. All authors read and approved the final manuscript.
Compliance with ethical standards: All authors declare that there is no conflict of interests.
References
- 1.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 2.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50(7):1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 3.De Vita F, Giuliani F, Silvestris N, Rossetti S, Pizzolorusso A, Santabarbara G. Current status of targeted therapies in advanced gastric cancer. Expert Opin Ther Targets. 2012;2:S29–34. doi: 10.1517/14728222.2011.652616. 16 Suppl. [DOI] [PubMed] [Google Scholar]
- 4.Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47(15):2306–2314. doi: 10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15(1):78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 6.Guimbaud R, Louvet C, Ries P, Ychou M, Maillard E, Andre T. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol. 2014;32(31):3520–3526. doi: 10.1200/JCO.2013.54.1011. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Wei ZW, He YL, Schwarz RE, Smith DD, Xia GK. Efficacy of adjuvant XELOX and FOLFOX6 chemotherapy after D2 dissection for gastric cancer. World J Gastroenterol. 2013;19(21):3309–3315. doi: 10.3748/wjg.v19.i21.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oei AL, van Leeuwen CM, ten Cate R, Rodermond HM, Buist MR, Stalpers LJ. Hyperthermia Selectively Targets Human Papillomavirus in Cervical Tumors via p53-Dependent Apoptosis. Cancer Res. 2015;75(23):5120–5129. doi: 10.1158/0008-5472.CAN-15-0816. [DOI] [PubMed] [Google Scholar]
- 9.Liu T, Ye YW, Zhu AL, Yang Z, Fu Y, Wei CQ. Hyperthermia combined with 5-fluorouracil promoted apoptosis and enhanced thermotolerance in human gastric cancer cell line SGC-7901. Onco Targets Ther. 2015;8:1265–1270. doi: 10.2147/OTT.S78514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY, Cho JY. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012;83(5):292–299. doi: 10.1159/000342376. [DOI] [PubMed] [Google Scholar]
- 11.Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschoep-Lechner KE, Milani V, Berger F, Dieterle N, Abdel-Rahman S, Salat C. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia. 2013;29(1):8–16. doi: 10.3109/02656736.2012.740764. [DOI] [PubMed] [Google Scholar]
- 13.Issels RD, Lindner LH, Verweij J, Wessalowski R, Reichardt P, Wust P. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018;4(4):483–492. doi: 10.1001/jamaoncol.2017.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC) BJU Int. 2011;107(6):912–918. doi: 10.1111/j.1464-410X.2010.09654.x. [DOI] [PubMed] [Google Scholar]
- 15.Zeng YC, Chi F, Xing R, Xue M, Wu LN, Tang MY. Pre-treatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Jpn J Clin Oncol. 2016;46(2):126–131. doi: 10.1093/jjco/hyv175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierzega M, Lenart M, Rutkowska M, Surman M, Mytar B, Matyja A. Preoperative Neutrophil-Lymphocyte and Lymphocyte-Monocyte Ratios Reflect Immune Cell Population Rearrangement in Resectable Pancreatic Cancer. Ann Surg Oncol. 2017;24(3):808–815. doi: 10.1245/s10434-016-5634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


