MCPyV is the first polyomavirus directly implicated in the development of an aggressive human cancer, Merkel cell carcinoma (MCC). Although MCPyV is constantly shed from healthy skin, the MCC incidence increases among aging and immunocompromised individuals. To date, the events connecting initial MCPyV infection and subsequent transformation still remain elusive. MCPyV differs from other known polyomaviruses concerning its cell tropism, entry receptor requirements, and infection kinetics. In this study, we examined the cellular requirements for endocytic entry as well as the subcellular localization of incoming virus particles. A thorough understanding of the determinants of the infectious entry pathway and the specific biological niche will benefit prevention of virus-derived cancers such as MCC.
KEYWORDS: MCPyV, endocytosis, polyomavirus, virus entry, virus-host interaction
ABSTRACT
Merkel cell polyomavirus (MCPyV) is a small, nonenveloped tumor virus associated with an aggressive form of skin cancer, Merkel cell carcinoma (MCC). MCPyV infections are highly prevalent in the human population, with MCPyV virions being continuously shed from human skin. However, the precise host cell tropism(s) of MCPyV remains unclear: MCPyV is able to replicate within a subset of dermal fibroblasts, but MCPyV DNA has also been detected in a variety of other tissues. However, MCPyV appears different from other polyomaviruses, as it requires sulfated polysaccharides, such as heparan sulfates and/or chondroitin sulfates, for initial attachment. Like other polyomaviruses, MCPyV engages sialic acid as a (co)receptor. To explore the infectious entry process of MCPyV, we analyzed the cell biological determinants of MCPyV entry into A549 cells, a highly transducible lung carcinoma cell line, in comparison to well-studied simian virus 40 and a number of other viruses. Our results indicate that MCPyV enters cells via caveolar/lipid raft-mediated endocytosis but not macropinocytosis, clathrin-mediated endocytosis, or glycosphingolipid-enriched carriers. The viruses were internalized in small endocytic pits that led the virus to endosomes and from there to the endoplasmic reticulum (ER). Similar to other polyomaviruses, trafficking required microtubular transport, acidification of endosomes, and a functional redox environment. To our surprise, the virus was found to acquire a membrane envelope within endosomes, a phenomenon not reported for other viruses. Only minor amounts of viruses reached the ER, while the majority was retained in endosomal compartments, suggesting that endosome-to-ER trafficking is a bottleneck during infectious entry.
IMPORTANCE MCPyV is the first polyomavirus directly implicated in the development of an aggressive human cancer, Merkel cell carcinoma (MCC). Although MCPyV is constantly shed from healthy skin, the MCC incidence increases among aging and immunocompromised individuals. To date, the events connecting initial MCPyV infection and subsequent transformation still remain elusive. MCPyV differs from other known polyomaviruses concerning its cell tropism, entry receptor requirements, and infection kinetics. In this study, we examined the cellular requirements for endocytic entry as well as the subcellular localization of incoming virus particles. A thorough understanding of the determinants of the infectious entry pathway and the specific biological niche will benefit prevention of virus-derived cancers such as MCC.
INTRODUCTION
Polyomaviruses (PyVs) are small, nonenveloped, double-stranded DNA (dsDNA) viruses with a diameter of 45 to 50 nm. The icosahedral (T=7) capsids consist of 72 homopentameric capsomers of the major capsid protein VP1 with the minor capsid proteins VP2 and VP3 located within a cavity underneath the VP1 pentamers. The PyV capsid harbors a chromatinized, circular dsDNA genome of about 5 kb (1, 2). Well-studied PyVs, such as simian virus 40 (SV40) and murine PyV (mPyV), possess a broad cell tropism and can transform cells in vitro and in animals (3, 4). Of the human polyomaviruses, JC and BK viruses are the best studied (5, 6). JC and BK viruses were initially identified in brain and urine samples, respectively (7, 8). Initial infection with these viruses occurs early in life and typically leads to persistent infections that are typically benign (9–11). Upon immunosuppression, however, persistent JC and BK virus infections may lead to severe diseases, such as progressive multifocal leukoencephalopathy or PyV-associated nephropathy, with potentially fatal outcomes (3, 4).
In 2008, Feng and colleagues identified Merkel cell polyomavirus (MCPyV) in a rare form of skin cancer known as Merkel cell carcinoma (MCC) (12). MCC is an aggressive cancer with increasing incidence (13, 14), which is most likely to develop in immunocompromised and elderly populations upon prolonged UV exposure (15, 16). About 80% of MCCs are positive for MCPyV DNA integrated into the host genome (12). As for most PyVs, MCPyV infections are widespread and predominantly asymptomatic. In fact, MCPyV is continuously shed from healthy skin, with a prevalence of 60% to 80% (17–19). However, MCPyV DNA has also been isolated from respiratory, urine, and blood samples (20), and the range of tissues in which persistent infection can be established is thus still unclear. The presence of integrated MCPyV DNA in MCC cells is thought to cause cancer through the continuous expression of the transforming large T (LT) and small T (sT) antigens. Integration of the viral DNA into the host cell genome is coupled to truncation of the LT C-terminal domain, which is important for viral genome replication and can induce p53 activity, triggering cell cycle arrest (21, 22). The viability of MCC cells depends on the expression of LT and/or sT, as pan-T knockdown in MCC-derived cells leads to cell death (23, 24). Importantly, the cellular origin of MCC is still under debate. A recent report suggests that dermal fibroblasts are target cells for productive infection, whereas Merkel cells are not permissive for virus entry or productive infection (25, 26). Thus, it remains unclear exactly which events give rise to MCC.
Since cell culture systems to produce sufficient quantities of infectious MCPyV are not readily available, MCPyV vectors, so-called pseudoviruses (PsVs), are important tools to study entry. As MCPyV does not contain detectable levels of VP3 (27), PsVs consist of VP1/VP2-only capsids that harbor a reporter plasmid (e.g., coding for enhanced green fluorescent protein [EGFP] or luciferase). Expression of the reporter allows easy readout for successful entry, i.e., delivery of the viral DNA to the site of transcription and replication (28). In an effort to identify MCPyV-permissive cell lines and to better understand the tissue tropism of MCPyV, the tumor cell library NCI-60 was screened for transducibility and the ability to support virus replication with MCPyV PsV and native virions, respectively (29). Of those, A549 cells, a non-small-cell lung cancer cell line, showed robust transducibility with MCPyV PsV (28).
Since MCPyV is an emerging virus, little is known about the basic biology of the virus, in particular how initial infection occurs. Initial studies on the mechanism of MCPyV infection addressed the cell surface interactions and cellular tropism of MCPyV (25, 28, 30). MCPyV relies on binding sulfated glycosaminoglycans (GAGs) for initial attachment, similar to papillomaviruses (28). However, it also requires interaction with carbohydrates containing a linear sialic acid (Sia) motif, i.e., Neu5Acα2-3Gal, like other PyVs (28, 30, 31).
Different viruses utilize distinct preexisting cellular endocytosis pathways for infectious entry (32). These endocytic pathways are characterized by a specific set of cellular factors and regulators facilitating endocytic vesicle formation. This set of cellular factors is used to distinguish different pathways. Well-studied endocytic pathways include clathrin-mediated endocytosis (CME), caveolar/lipid raft-mediated endocytosis, and macropinocytosis (33), which are essential for receptor-mediated endocytosis, turnover of plasma membrane receptors, and fluid-phase uptake. To facilitate safe delivery and release of the viral genome to and at the site of replication, viruses are routed through specific intracellular target organelles, such as endosomes, the Golgi apparatus, or the endoplasmic reticulum (ER).
Host cell entry of most PyVs occurs by caveolar/lipid raft-mediated endocytosis (34–37), whereas JC virus, for example, uses CME (38). After endocytosis, virions are routed through the endosomal pathway to be delivered to the ER (39). There, a chaperone- and disulfide isomerase-mediated uncoating step occurs, whereupon the modified particles are translocated into the cytosol by the ER-associated degradation machinery (40–43). In the present study, we aimed to identify the cellular requirements for virus entry into host cells to understand similarities to and differences from other PyVs. For this, we used small-compound inhibitors of decisive cellular factors/processes during MCPyV infection. As functional controls, we employed well-studied viruses, including SV40, human papillomavirus 16 (HPV16), influenza A virus (IAV), or the alphavirus Semliki Forest virus (SFV) (36, 44–47). In addition, we characterized the entry route of MCPyV by ultrathin-section transmission electron microscopy (TEM). Our results support a model in which MCPyV enters A549 cells by caveolar/lipid raft-dependent endocytosis and where viruses are routed through the endosomal pathway to the ER. To our surprise, we found that MCPyV acquired a lipid membrane within endosomes. This membrane was absent once the virus located in the ER.
(This article was submitted to an online preprint archive [48].)
RESULTS
MCPyV PsV cell binding and infection depend on sulfated glycosaminoglycans.
To study the infectious entry requirements of MCPyV, we tested the susceptibility of the keratinocyte-derived cell lines HeLa and HaCaT in comparison to A549 cells. Using PsV, we found that neither HeLa nor HaCaT cells showed green fluorescent protein (GFP) expression at 72 h postinfection (p.i.) and are thus poorly permissive for MCPyV entry (Fig. 1A). We therefore decided to use A549 cells for our study, which allow for infectious internalization, as previously described (29).
FIG 1.
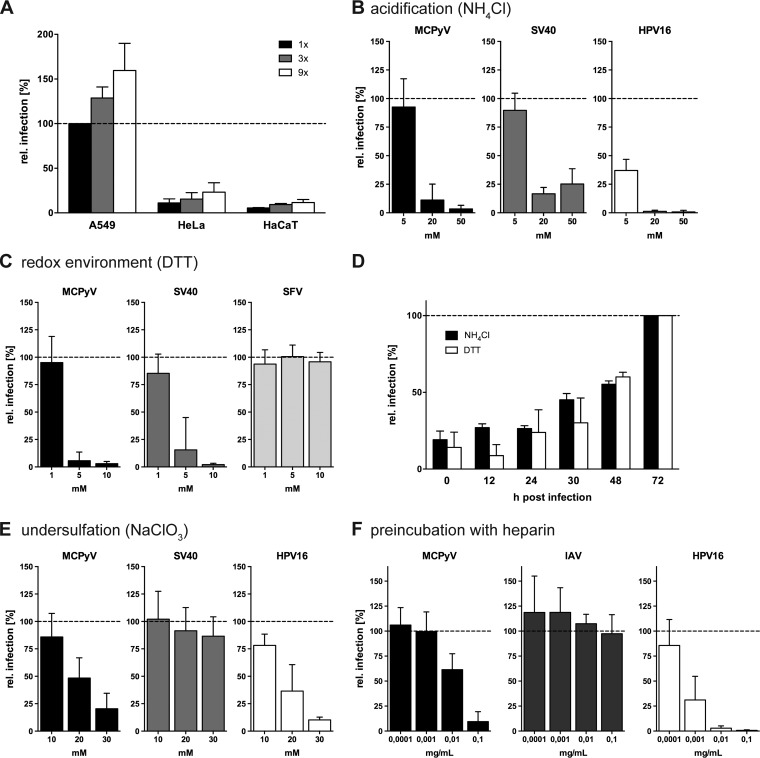
MCPyV infection is slow and asynchronous and relies on interaction with sulfated glycans. (A) A549, HeLa, or HaCaT cells were infected with increasing amounts of MCPyV PsVs. Depicted are relative infection values (percent) related to the MCPyV amount yielding 20% absolute infection in A549 cells ± standard deviations (SD) (1× = 80 ng VP1). (B) A549, CV-1, or HeLa cells were infected with MCPyV, SV40, or HPV16, respectively, in the presence of the indicated concentrations of NH4Cl. (C) A549, CV-1, or BHK cells were infected with MCPyV, SV40, or SFV, respectively, in the presence of the indicated concentrations of DTT. (D) A549 cells were infected with MCPyV for a total of 72 h, while 10 mM NH4Cl or 5 mM DTT was added at the indicated hours p.i. (E) A549, CV-1, or HeLa cells were infected with MCPyV, SV40, or HPV16, respectively, upon pretreatment with the indicated concentrations of NaClO3 for 16 h prior to infection. (F) A549 cells were infected with MCPyV or IAV, and HeLa cells were infected with HPV16 virions, which were treated with the indicated concentrations of heparin for 1 h prior to infection. Depicted in panels B to F are average relative infection values compared to those of untreated controls ± SD from at least 3 independent experiments.
As an initial strategy to elucidate the cellular route(s) of entry, perturbation of cellular processes followed by infection is key to identifying factors/organelles facilitating MCPyV entry. The time course of MCPyV infectious entry into A549 cells was rather slow and plateaued after 72 h p.i. (data not shown). Since indirect effects occur often after prolonged cellular perturbation, it is important to minimize such effects (49). For this, we compared small interfering RNA (siRNA)-mediated knockdown, expression of dominant negative mutants, and small-compound inhibition (data not shown). The efficacy and reliability of siRNA-mediated knockdown and expression of dominant mutants turned out to be questionable, as judged by infection with control viruses, so we turned exclusively to small-compound inhibition.
We first tested whether inhibition of acidification of endosomal compartments with the weak base ammonium chloride (NH4Cl) perturbed MCPyV infection (50). In the presence of NH4Cl, MCPyV infection was blocked in a dose-dependent fashion (Fig. 1B), similar to infection with SV40 and HPV16, which served as positive controls (47, 51). This indicates the requirement for low pH in endosomal compartments during infectious internalization of MCPyV.
SV40 is directed to the ER for uncoating and subsequent translocation to the cytosol using ER-resident disulfide oxidoreductases and the ER-associated degradation machinery (40). Dithiothreitol (DTT) is a powerful reducing agent and thus perturbs the cellular redox environment and disulfide oxidoreductase functions (52). Accordingly, DTT treatment arrests SV40 in the ER (40). We tested whether MCPyV was also sensitive to DTT treatment. MCPyV and SV40 infectivities were clearly reduced in the presence of DTT, whereas entry of SFV, which does not require a redox-driven ER uncoating step (44), was unaffected (Fig. 1C).
Next, the ability of NH4Cl or DTT to block MCPyV infection in relation to the time course of entry was tested. For this, the drugs were added at different times p.i. NH4Cl and DTT exhibited similar propensities to block MCPyV infection, where infectivity was inhibited upon early addition and increased with additions at later time points (Fig. 1D). The increase at later time points indicates that the virus had passed the step blocked by the drugs. At 30 h p.i., about 50% of MCPyV had passed the acid-activated step, whereas the redox-dependent step appeared to occur slightly later (Fig. 1D). The time courses indicate in addition a rather slow and asynchronous entry process akin to that of HPV16 (Fig. 1D) (47).
Prolonged treatment of cells with drugs that block endocytosis and trafficking can have indirect or cytotoxic effects. To address this problem, we employed an inhibitor swap approach in which cells were transiently treated with a drug of interest followed by replacement by another that blocks entry at a later stage, such as acid activation in endosomes or redox-mediated uncoating in the ER (47, 49). We therefore exchanged the inhibitor-containing inoculum for viruses with slow entry kinetics (MCPyV, HPV16, and SV40) to medium containing either NH4Cl (MCPyV and HPV16) or DTT (SV40) according to their entry kinetics, i.e., 30 h p.i. for MCPyV, 12 h p.i. for HPV16, and 10 h p.i. for SV40. Moreover, several different viruses served as positive and negative controls to assess the efficacy of treatment as well as the potential for pleiotropic drug effects.
Initially, as a proof of feasibility, the requirement for sulfated GAGs was tested. MCPyV attachment to cells generally depends on such GAGs (28, 30, 31). Therefore, treatment with sodium chlorate (NaClO3) was used to induce the production of undersulfated GAGs (53). Treatment of A549 cells with 10, 20, or 30 mM NaClO3 for 16 h prior to and throughout infection with MCPyV resulted in a dose-dependent reduction in infectivity (Fig. 1E). HPV16 infectivity was similarly reduced, as expected (54). In contrast, infection with SV40 was unaffected, suggesting different requirements for the two PyVs (Fig. 1E). In addition, we tested the inhibitory effect of heparin, a highly sulfated GAG, on MCPyV infection. In line with previous results (28), preincubation of MCPyV pseudovirions with heparin efficiently blocked infection, only slightly less efficiently than the positive control, HPV16 (Fig. 1F). IAV was used as a negative control, as IAV infection depends on interaction with sialic acids (55) and is independent of GAG binding. Heparin did not affect IAV infectivity (Fig. 1F). Taken together, the results confirm that MCPyV infection requires interactions with sulfated cell surface GAGs.
Inhibitory profile of MCPyV endocytosis.
After confirming data from previous reports, we set out to characterize the endocytic pathway employed by MCPyV. First, CME was blocked with the small-molecule inhibitor pitstop2, which interacts with the clathrin N-terminal domain and thereby interferes with assembly of the clathrin coat (56). Infection with MCPyV was unaffected by treatment with pitstop2 (Fig. 2A), similar to SV40, which enters cells by caveolar/lipid raft-mediated endocytosis (34, 36, 51). As expected, infection with SFV, which enters cells by CME (44, 57), was efficiently reduced. Thus, MCPyV entry occurs independently of CME.
FIG 2.
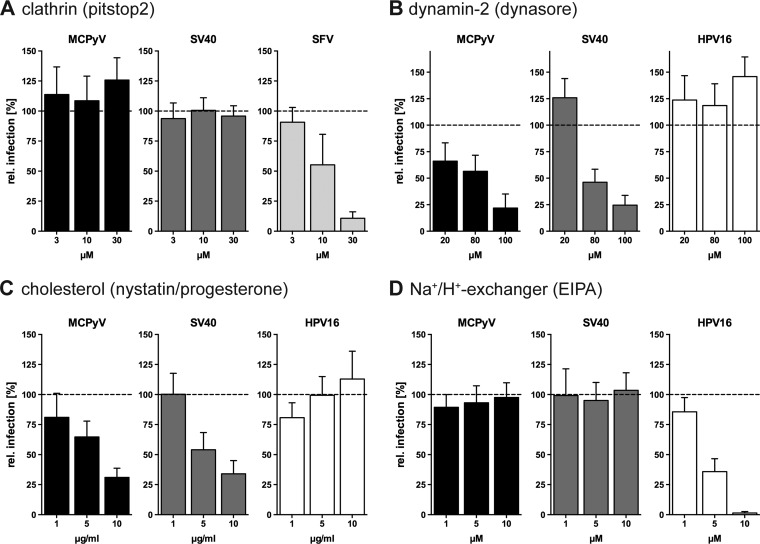
MCPyV infection is dynamin and cholesterol dependent but independent from clathrin and Na+/H+ exchangers. (A) A549, CV-1, or BHK cells were infected with MCPyV, SV40, or SFV, respectively, in the presence of the indicated concentrations of pitstop2. (B to D) A549, CV-1, or HeLa cells were infected with MCPyV, SV40, or HPV16, respectively, in the presence of the indicated concentrations of dynasore (B), nystatin-progesterone (C), and EIPA (D). Depicted are percentages of infection values relative to those of solvent-treated controls ± SD from at least 3 independent experiments.
Another important regulator of several endocytic pathways is dynamin-2 (Dyn2). The large GTPase regulates scission of endocytic pits in several endocytic pathways, i.e., CME, caveolar/lipid raft-mediated endocytosis, interleukin-2 (IL-2) endocytosis, and phagocytosis (32). To study the potential Dyn2 involvement in MCPyV uptake, dynasore (an inhibitor of dynamin GTPase activity) was employed (58). SV40 served as a positive control as it is known to depend on Dyn2 activity (59). Infection by MCPyV was blocked dose dependently by dynasore to a residual level of 22% ± 13%, similar to SV40 (Fig. 2B). As a negative control, HPV16 was used. HPV16 enters host cells by a clathrin-, caveolin-, cholesterol-, and dynamin-independent but actin-dependent endocytic pathway (47). As expected, HPV16 infection remained unperturbed or even increased after dynasore treatment (Fig. 2B). This indicates that MCPyV is endocytosed by a Dyn2-dependent pathway similarly to SV40 but distinct from HPV16 endocytosis.
To further ascertain that MCPyV enters cells by a similar endocytic mechanism as that of SV40, we perturbed cholesterol-dependent caveolar/lipid raft-mediated endocytosis. To this end, we treated cells with nystatin and progesterone, which sequester cholesterol and prevent cholesterol synthesis, respectively (34, 59–62). As expected, infections with MCPyV and SV40 were inhibited upon perturbation of cholesterol to residual levels of 31% ± 8% and 34% ± 11%, respectively (Fig. 2C), whereas HPV16 infection remained unaffected, as described previously (47). This suggests that MCPyV infection, like SV40 infection, requires cholesterol-rich membrane domains (34).
Next, a potential role for macropinocytosis was assessed. The Na+/H+ exchanger regulates macropinocytosis by controlling submembranous pH, which in turn regulates the formation of membrane protrusions (63). Ethylisopropylamiloride (EIPA)-mediated inhibition of the Na+/H+ exchanger (64) is a classical treatment to interfere with macropinocytosis. This treatment affected neither MCPyV nor SV40 infection (Fig. 2D), whereas HPV16 infection was efficiently blocked, as expected (47). Thus, MCPyV endocytosis occurs independently of the Na+/H+ exchanger and, consequently, also of macropinocytosis or related pathways.
In summary, MCPyV entry required cholesterol-rich membranes and dynamin but did not require clathrin or macropinocytic pathways. These observations are most consistent with an entry pathway that employs caveolar/lipid raft-mediated endocytosis.
Regulation of MCPyV endocytosis depends on actin dynamics, Rho-like GTPases, and signaling via Tyr kinases and phosphatases PP1 and PP2A/B.
Next, we addressed functional regulators involved in endocytic processes with respect to their role in MCPyV entry.
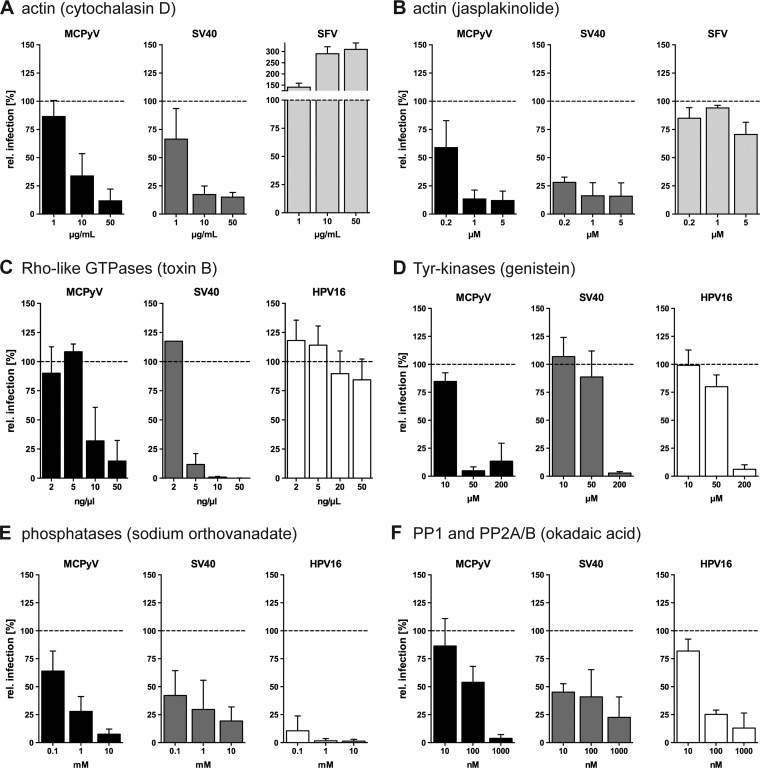
Actin polymerization is required for most endocytic pathways employed by viruses, where formation of protrusions, intracellular transport, or vesicle scission is facilitated (32, 47). Cytochalasin D and jasplakinolide inhibit actin dynamics by blocking either polymerization or depolymerization, respectively (65, 66). Both cytochalasin D and jasplakinolide efficiently blocked MCPyV and SV40 infection (Fig. 3A and B), where the inhibitory effect of jasplakinolide was less pronounced than for SV40. In contrast, CME-mediated uptake of SFV was unaffected by stabilization of actin filaments (Fig. 3B) and increased upon actin depolymerization (Fig. 3A).
FIG 3.
MCPyV infection is dependent on actin, Rho GTPases, Tyr kinases, and cellular phosphatases. (A and B) A549, CV-1, or BHK cells were infected with MCPyV, SV40, or SFV, respectively, in the presence of the indicated concentrations of cytochalasin D (A) or jasplakinolide (B). (C to F) A549, CV-1, or HeLa cells were infected with MCPyV, SV40, or HPV16, respectively, in the presence of the indicated concentrations of toxin B (C), genistein (D), sodium orthovanadate (E), or okadaic acid (F). Depicted are percentages of infection values relative to those of solvent-treated controls ± SD from at least 3 independent experiments.
Actin polymerization is most often regulated by the activity of pathway-specific Rho-like GTPases (32, 67, 68). Toxin B from Clostridium difficile is a broad inhibitor of all Rho-like GTPases (69) and was hence used to assess the relevance of Rho GTPases for infection by MCPyV and SV40. As a negative control, we used HPV16 infection, which was not perturbed by treatment with toxin B, as expected (Fig. 3C) (47). Toxin B reduced MCPyV and SV40 infections to 15% ± 18% and to 0.1% ± 0.1% residual infections, respectively (Fig. 3C). From this, we conclude that Rho-like GTPases likely mediate actin-dependent steps during MCPyV endocytosis (Fig. 3C). Analogous to SV40, the key step may be actin-dependent closure and fission of endocytic vesicles from the plasma membrane (59).
Ligand-induced activation of tyrosine (Tyr) kinases regulates the activation of several endocytic pathways, such as macropinocytosis and caveolar/lipid raft-mediated endocytosis, whereas CME and other pathways are Tyr kinase independent (32, 68). Genistein, a broad and efficient inhibitor of Tyr kinases (70), was used during infection with MCPyV, SV40, and HPV16. As expected for HPV16 and SV40, infection was blocked upon treatment with 200 μM genistein (Fig. 3D). Interestingly, MCPyV infection was already sensitive to 50 μM genistein treatment (Fig. 3D), suggesting a strong requirement for Tyr kinases, potentially at multiple levels.
Next, cellular phosphatases were inhibited with the broadly active agent sodium orthovanadate, a competitive inhibitor of all phosphatases (71, 72). Entries of MCPyV, SV40, and HPV16 were all strongly inhibited in the presence of orthovanadate (Fig. 3E). Okadaic acid, a more specific inhibitor of phosphatase families PP1, PP2A, and PP2B (73), also inhibited MCPyV, SV40, and HPV16 (Fig. 3F).
In summary, MCPyV entry is likely facilitated by dynamic actin rearrangement that is regulated by Rho-like GTPases as well as by the activity of Tyr kinases and PP1 and/or PP2A/B phosphatases.
Intracellular trafficking of MCPyV requires endosomal acidification, functional microtubular dynamics, and an intact redox environment.
Treatment with NH4Cl reduced MCPyV infection, indicating an acid activation step (Fig. 1B). To test whether this acid-activated step occurred in endosomes, bafilomycin A1, an inhibitor of the endosomal proton pump V-ATPase, was used (74). Bafilomycin A1, like NH4Cl, inhibited the infectivity of MCPyV, SV40, and HPV16 in a dose-dependent fashion (Fig. 4A). Thus, MCPyV depends on low endosomal pH for either acid activation or trafficking within maturing endosomes.
FIG 4.
MCPyV infection requires endosomal acidification, functional microtubular dynamics, and an intact redox environment. (A to C) A549, CV-1, or HeLa cells were infected with MCPyV, SV40, or HPV16, respectively, in the presence of the indicated concentrations of bafilomycin A (A), nocodazole (B), and brefeldin A (C). (D) A549, BHK, or HeLa cells were infected with MCPyV, SFV, or HPV16, respectively, in the presence of the indicated concentrations of aphidicolin. Depicted are percentages of infection values relative to those of solvent-treated controls ± SD from at least 3 independent experiments.
Most intracellular transport occurs along microtubules (75). To assess microtubule involvement in MCPyV infection, depolymerization of microtubules by treatment with the polymerization blocker nocodazole was used (76). As reported previously, SV40 and HPV16 entries require the integrity of microtubules (Fig. 4B). Similarly, MCPyV infection strongly depended on intact microtubular dynamics (Fig. 4B). This suggests that MCPyV requires microtubules for transport of vesicular compartments or of the virus itself during host cell entry.
To study the involvement of processes of intracellular transport from the ER to the Golgi apparatus in MCPyV entry, we used brefeldin A, which eventually leads to Golgi collapse into the ER (77). Upon perturbation with brefeldin A, infections with MCPyV, SV40, and HPV16 were blocked (Fig. 4C). This indicates that infection with these viruses requires the functional integrity of the secretory ER/Golgi compartments.
Another hint for an involvement of the ER during infectious internalization can be drawn from the perturbation of the cellular redox environment by DTT. At the concentrations used, DTT interferes mostly with the formation of disulfide bonds during folding in the ER but not with existing disulfide bonds in folded proteins (52). Since MCPyV infection was blocked by DTT (Fig. 1C), it is reasonable to assume that MCPyV, like SV40, requires an ER step for host cell entry, possibly for uncoating and translocation into the cytosol.
After escape from the ER, SV40 is thought to be imported into the nucleus via the nuclear pore complexes (78). In contrast, HPV16 requires cell cycle progression, i.e., nuclear envelope breakdown, for nuclear entry (79, 80). To address whether MCPyV infection depends on mitotic activity for nuclear entry, we blocked cells in S phase using aphidicolin, an inhibitor of DNA polymerases alpha and delta (81). MCPyV entry was efficiently blocked by aphidicolin to a similar extent as for HPV16 (Fig. 4D). The RNA virus SFV replicates in the cytoplasm and therefore served as a negative control (82). SFV infection was not affected by aphidicolin treatment (Fig. 4D), as expected. These results indicate that MCPyV infection may depend on the mitotic activity of the host cells.
MCPyV is internalized into small, noncoated vesicles.
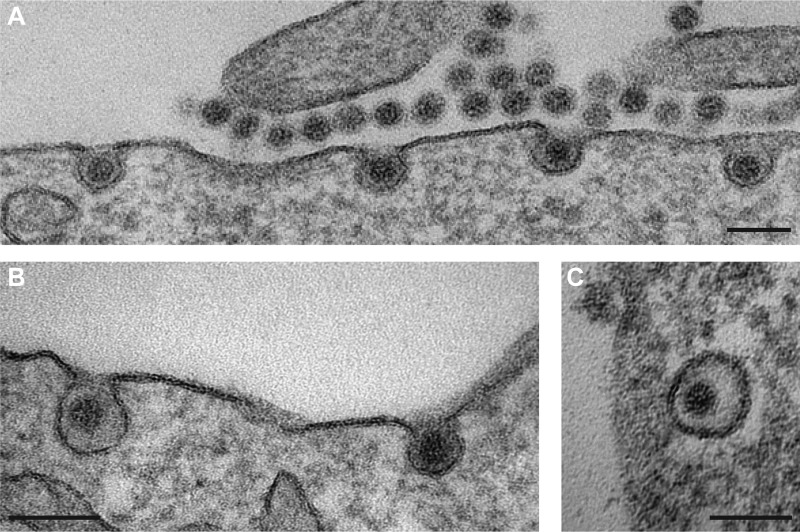
Our inhibition studies indicate that MCPyV and SV40 use similar pathways to infect cells. To confirm this notion, we examined virion trafficking using TEM. Localization of virus particles was assessed 2, 8, 16, 24, and 48 h p.i. Interestingly, the virus localized to several specific cellular compartments at each time point, in line with asynchronous internalization and trafficking of the MCPyV particles. Virus particles were readily detectable bound to the cell surface (Fig. 5A). In addition, they were found within small, inward-budding membrane invaginations without any visible protein coat (Fig. 5A and B). Interestingly, virions resided in two distinct but similarly frequent populations of invaginations, where frequency was estimated based on the number of invaginations of 10 cells. In one class of invaginations (n = 27), the plasma membrane appeared to make close contact with the virion surface, while the second class of invaginations exhibited 10-nm ± 8-nm distances between the membrane and the virion surface (n = 19). The first class of invaginations is reminiscent of previous observations for SV40, which is known to make close contact with the sialylated head groups of plasma membrane glycolipids (83, 84). The latter class of invaginations suggests possible interactions between the virion and a glycoprotein receptor with a large ectodomain, such as a heparan sulfate proteoglycan (HSPG). In addition, MCPyV virions were found within intracellular vesicles without an apparent protein coat (Fig. 5C). These data, and the absence of virus in large, coated, or tubular invaginations, are consistent with MCPyV uptake by caveolar/lipid raft-mediated endocytosis rather than uptake by macropinocytosis, CME, or glycosphingolipid-enriched carriers (61, 84–86).
FIG 5.
MCPyV is taken up into tight-fitting inward-budding pits (A and B) and into small, uncoated vesicles (C). A549 cells were infected with MCPyV particles for 2 to 24 h before fixation with glutaraldehyde. Cells were processed for TEM according to standard procedures. Images of early entry events were taken. Bars, 100 nm.
MCPyV traffics via the endolysosomal route to the ER.
After initial uptake into endocytic vesicles, MCPyV particles were found in early endosomal compartments and later accumulated in late endosomal/lysosomal structures (Fig. 6A and B). Other polyomaviruses, such as SV40, JC PyV, and BK PyV, are then trafficked to the ER via Golgi- and non-Golgi routes. In the ER, oxidoreductases and chaperones mediate partial disassembly of the virions (uncoating) and transfer of partially disassembled virions into the cytosol (40, 84, 87–89). MCPyV was undetectable in the Golgi apparatus (Fig. 6D), the cytosol, or the nucleus. Similar to other PyVs, MCPyV was observed in the lumen of the ER but only to a minor extent (Fig. 6C).
FIG 6.
MCPyV travels through the endolysosomal system to the ER, omitting the Golgi apparatus. A549 cells were infected with MCPyV particles for 2 to 24 h before fixation with glutaraldehyde. Cells were processed for TEM according to standard procedures. (A to C) Virus particles were found in endosomal compartments (A and B) and in the ER (C). (D) MCPyV was absent from the Golgi apparatus. Bars, 100 nm.
Taken together, the electron microscopy (EM) results thus support the conclusion that MCPyV and SV40 use similar infectious entry pathways.
MCPyV acquires a lipid envelope in endosomal compartments.
In addition to the observations described above, we surprisingly found MCPyV particles with what appeared to be a double-layer lipid membrane in endosomal compartments (Fig. 7A and B). This membrane was tightly associated with the particles. These enveloped particles were observed only in endosomes but not in virus preparations, during binding to the plasma membrane, nor within endocytic pits or vesicles close to the plasma membrane (compare Fig. 5 and 8). The enveloped virions were easily detectable in maturing and late endosomes as well as endolysosomes, similar to nonenveloped particles. In fact, both enveloped and nonenveloped particles were detectable side by side in the same endosomes (Fig. 7C, enveloped black arrows versus nonenveloped white arrow). This suggested that the process of acquiring a lipid envelope occurs during viral passage through the endosomal pathway rather than in distinct, specialized organelles. It is unclear whether the enveloped particles are part of the productive infectious entry pathway or are instead a dead end for the virus. Possibly, this phenotype reflects a new mode of antiviral defense or a mechanism to evade endosomal degradation.
FIG 7.
MCPyV virions acquire a membrane envelope during cell entry (A to C). A549 cells were infected with wild-type MCPyV particles before fixation with glutaraldehyde. Cells were processed for TEM according to standard procedures. Images of virus particles in intracellular compartments were taken after 8 h and 16 h p.i. Note the envelope around the virus particles in the representative endosomal compartments. In panel C, note that enveloped particles (black arrows) are found side by side with nonenveloped virions (white arrow) in the same organelle. Bars, 100 nm; inset bar, 50 nm.
FIG 8.
MCPyV sialic acid binding mutants are taken up into A549 cells but fail to mediate infection. (A, Bi, and Ci) MCPyV wild-type and mutant particles were analyzed by electron microscopy after negative staining. Depicted are representative images of virions. Bars, 50 nm. (Bii and iii and Cii and iii) A549 cells were infected with W76A and Y81V MCPyV particles for 2 to 24 h before fixation with glutaraldehyde. Cells were processed for TEM according to standard procedures. Images of virus particles in endocytic pits and in intracellular compartments were taken after 8 h p.i. (ii) and 16 h p.i. (iii), respectively. Bars, 100 nm for panels ii and 200 nm for panels iii. (D) Totals of 60 ng (1×), 300 ng (5×), and 600 ng (10×) of MCPyV wild-type and W76A and Y81V mutant particles were used in infection assays with A549 cells for 72 h.
The role of sialic acid for MCPyV entry.
Previous studies showed that mutations in the sialic acid binding pocket of MCPyV VP1 rendered the particle noninfectious but did not perturb binding to cells (30). Hence, sialic acid interaction is essential for infectious entry, whereas initial attachment occurs mainly by GAG engagement. Since we observed MCPyV particles in endocytic pits in two distinct populations that may reflect binding to gangliosides and HSPGs, two sialic acid binding site mutants (W76A and Y81V) were monitored using EM. The mutant PsVs assembled without visible defects (Fig. 8A, Fig. 8Bi, and Fig. 8Ci) and showed similar size distributions (44 ± 3 nm for the wild type [wt] versus 43 ± 3 nm for W76A and versus 44 ± 3 nm for Y81V). Notably, the mutants were unable to mediate infection in A549 cells (Fig. 8D), as expected (30). To verify the desired glycan binding abilities of the sialic acid mutants, we conducted saturation transfer difference-nuclear magnetic resonance (STD-NMR) experiments to probe for binding of sialylated oligosaccharides and GAG oligosaccharides to MCPyV wt and mutant virus-like particles (VLPs). STD-NMR makes use of energy transfer from proteins to their ligands upon binding and thus allows determination of binding specificities by comparing the changes in the resonance frequencies of the ligand molecules in association with wild-type or mutant viruses (30, 90). Here, 3′ sialyllactose (SL) and a chemically well-defined heparan sulfate (HS) pentasaccharide, Arixtra (Ax), were chosen as a minimal sialylated MCPyV ligand and a short GAG oligosaccharide, respectively (Fig. 9A and B). VLPs themselves are not visible in standard 1H NMR spectra because of the excessive line width broadening associated with their high molecular weight. 1H NMR spectra of the MCPyV VLP preparation therefore contain only proton resonances belonging to buffer components and small-molecule impurities associated with the VLPs after overnight dialysis into D2O buffer (Fig. 9Ai). In contrast, 1H NMR spectra of glycan receptor proxies, such as 3′ SL or the GAG Arixtra, exhibit the full proton resonance set of the respective oligosaccharide (Fig. 9Av and Fig. 9Biv, respectively). For the STD-NMR spectra, VLPs and oligosaccharides were mixed, but instead of standard 1H spectra, STD-NMR spectra were recorded with selective excitation of the VLPs, meaning that oligosaccharide resonances should be observed only if magnetization transfer from the VLPs to the oligosaccharide takes place, i.e., if a complex is formed. This was the case for wt VLPs and in part for Y81V VLPs with 3′ SL (Fig. 9Aii and iii, respectively) but not for W76A VLPs (Fig. 9Aiv). All observed resonances for W76A VLPs were already present in the 1H NMR spectra of the VLPs (Fig. 9Ai), with the exception of a very weak sialic acid methyl group signal (Sia-CH3), which indicated no binding. For wt VLPs and Y81V VLPs, individual resonances from the Sia and galactose (Gal) moieties in 3′ SL were observed in the STD-NMR spectra (highlighted in Fig. 9Aii and iii). Therefore, both the Sia and Gal (but not the glucose) rings of 3′ SL must be in close proximity to the sialic acid binding site in MCPyV VLPs. In contrast, in the STD-NMR experiment with Arixtra, the W76A VLPs yielded spectra with the full set of Arixtra proton resonances, just like the wt VLPs (Fig. 9Bi and ii). From these experiments, we can conclude that the five sugar rings that make up the Arixtra compound are all bound by the VLPs and that the impairment of the sialic acid binding site by the W76A mutation does not impact the GAG binding site.
FIG 9.
MCPyV sialic acid binding mutants are capable of GAG binding. (A) STD-NMR difference spectra of MCPyV VLPs with 3′ sialyllactose (SL) and 1H reference spectra. The chemical formula of 3′ SL serves as an overview of the general assignment of the residues. Black/gray boxes visualize residues of origin for the peaks in the NMR spectra. NMR spectra are shown from top to bottom. (i) MCPyV wt VLP 1H reference spectrum. (ii) STD-NMR spectrum of wt VLPs with 3′ SL exhibiting saturation transfer from the capsid to 3′ SL. Note the resonance peaks from the indicated residues of 3′ SL (black/gray boxes). (iii) Same spectrum for Y81V mutant VLPs. (iv) Same spectrum with W76A mutant VLPs showing no magnetization transfer to 3′ SL. Note the absence of resonance peaks from 3′ SL. (v) 3′-SL 1H spectrum for comparison to the STD-NMR spectra in panels ii to iv. Small-molecule impurities are observed in the VLP preparation (i and iv), some of which are buffer components (sharp resonances with asterisks); others are unidentified molecules that are likely associated with the capsids (broad resonances with asterisks; the peak broadening suggests slow molecular tumbling, i.e., association with the VLPs). HDO signals were truncated for the sake of visibility. (B) STD-NMR difference spectra of MCPyV VLPs with a GAG pentasaccharide (Arixtra [Ax]) and 1H reference spectra. The chemical formula of Ax serves as an overview of the general assignment of the residues. Black/gray boxes visualize residues of origin for the peaks in the NMR spectra. NMR spectra are shown from top to bottom. (i) VLP 1H reference spectrum. (ii) STD-NMR spectrum of wt VLPs with an Ax pentasaccharide exhibiting saturation transfer from the capsid to the GAG. (iii) Same spectrum with W76A mutant VLPs. (iv) Ax 1H spectrum for comparison. Note that identical saturation transfer to the GAG is observed for the wt and the W76A mutant that does not bind to 3′ SL (Fig. 9Bii versus Biii versus 9Aiv). HDO signals were truncated for the sake of visibility. Abbreviations: Sia, sialic acid; Neu5Ac, N-acetylneuraminic acid; (α2-3), α2-3 glycosidic bond; Gal, galactose; (β1-4), β1-4 glycosidic bond; Glc, glucose; Ac, acetyl group; CH3, methyl group; ax, axial; eq, equatorial; GlcNS6S, 2-deoxy-2-sulfamido-α-d-glucopyranosyl-6-O-sulfate; GlcA, glucuronic acid; GlcNS3,6S, 3-O-sulfated glucosamine; IdoA2S, 2-O-sulfo-α-l-iduronic acid; GlcNS3,6SOMe, 3-O-sulfated glucosamine methylester; red, reduced.
EM analyses showed that the W76A and Y81V mutants readily bound to A549 cells, presumably through interactions with HSPGs (Fig. 8Bii and Fig. 8Cii) (30). Interestingly, both mutants were exclusively observed in the second class of invaginations (i.e., invaginations with a >5-nm gap between the plasma membrane and the virion surface) (Fig. 8Bii and Fig. 8Cii). This observation supports the concept that the first class of invaginations are formed through short-range contacts between the virion and sialylated glycolipids (which the mutants fail to bind), while the second class of invaginations involves interactions with bulkier HSPGs.
DISCUSSION
MCPyV is the first human PyV clearly linked to the development of a specific cancer (12). Initial studies on the principal mechanism of infection confirmed the requirement of sulfated glycosaminoglycans for attachment and sialylated glycans for infectious uptake into host cells, which may, in fact, reflect a common mechanism for several PyVs (28, 30, 31, 91). To extend our knowledge of the mechanism of initial infection, we addressed additional cellular requirements and routes of virus entry. Our evidence from inhibitor and morphological studies indicates that MCPyV infects cells asynchronously via a caveolar/lipid raft-dependent endocytic pathway that is similar to that used by SV40. After internalization, virus particles were routed to endolysosomal compartments and the ER. Our preliminary evidence suggests that entry of MCPyV depends on progression of the cell cycle, potentially for the nuclear entry step.
The initial step of virus entry is binding to target cells. Previous work suggested that the interaction with sulfated GAGs is required for MCPyV binding, whereas sialic acids have a postattachment role (28). We confirmed that heparin, a highly sulfated GAG, was able to compete for cell surface GAGs, thus prohibiting binding and infection of A549 cells. In addition, we addressed the role of GAGs and sialic acids concerning their influence on endocytic uptake. Interestingly, we observed two populations of MCPyV particles on the plasma membrane and in endocytic pits differing in their distances to the limiting membrane, possibly representing the different binding receptor species. In principle, these could reflect binding to proteinaceous receptors with a large ectodomain (wide pits) or gangliosides (sialylated lipids and tight pits), as shown for JC virus uptake via sialylated glycoproteins (92) and SV40 uptake by ganglioside interaction (93). Alternatively, the proteinaceous receptor may constitute HSPGs.
To further unravel the individual roles of sialylated glycans and GAGs during MCPyV entry, two sialic acid binding site mutants (W76A and Y81V) (30) were subjected to a STD-NMR-based binding assay. These studies confirmed that binding to sialylated glycans was abolished for W76A but not for Y81V, whereas either mutant retained binding of the HS pentasaccharide. This finding indicates that the binding sites for sialic acid and sulfated GAGs do not overlap and are independent of each other. The most striking difference between wild-type MCPyV and the sialic acid binding site mutants in the TEM experiments was that the mutants were exclusively detected in endocytic pits, with large distances between viral particles and plasma membrane layers. The most likely interpretation of these observations is thus that the large distance reflects binding to a proteinaceous receptor containing sulfated GAGs, such as HSPGs, whereas tightly fitted binding to the plasma membrane reflects ganglioside engagement. Since uptake of HSPGs has been suggested to occur primarily through flotillin- and dynamin-dependent endocytosis (94, 95), MCPyV internalization in wider pits via HSPGs seems distinct from the caveolar/lipid raft-mediated internalization required for infection. It thus appears that MCPyV internalization via HSPGs is a dead end. While W76A and Y81V MCPyVs were readily observable in endosomes, they were not detected in the ER. Hence, the W76A and Y81V mutations likely rendered the particles noninfectious, as they were unable to engage sialic acid-containing gangliosides efficiently and thus were not taken up by an infectious pathway that routes the virus to the ER.
Since the sialic acid binding mutants were found in endosomes but not the ER, the interaction with sialic acid receptors is crucial for routing the virus to the ER. This is in line with previous work indicating that branched sialylated glycosphingolipids target murine polyomavirus (mPyV) and SV40 from endolysosomes to the ER (36, 51, 83, 96). The role of HSPGs remains less clear. It may be that MCPyV interacts with sulfated GAGs simply because they may serve as an initial high-affinity attachment factor, which facilitates later interaction with low-affinity glycosphingolipids. An alternative mechanism may be the induction of a conformational change in the virus capsid upon GAG binding that may facilitate uncoating and transfer to the secondary receptor, as has been described for HPV16, a virus with a similar tissue tropism (54, 97). However, such GAG-induced conformational changes have no precedent among other PyVs. Of note, JC PyV can utilize two distinct pathways for infectious internalization, where one depends on interaction with sulfated GAGs and the other relies on sialic acid binding; this effect could depend on the host cell type (91). As the niche(s) for MCPyV infection remains only partially understood, the role of HSPGs in MCPyV entry may thus depend on specific cell types that are being infected in vivo.
Receptor engagement leads to the endocytosis of MCPyV. In line with our initial hypothesis that infectious entry of MCPyV may follow a path similar to that of SV40, our inhibitory experiments showed that infectious entry was independent of clathrin and the activity of Na+/H+ exchangers but required dynamin and cholesterol as well as actin dynamics. Since MCPyV particles were not found in coated pits or membrane protrusions, entry does not involve CME or macropinocytosis. The combined requirements for dynamins, cholesterol, and actin dynamics indicated endocytosis by caveolar/lipid raft-mediated endocytosis. Dynamins are also involved in phagocytosis and endocytosis of interleukin-2 (IL-2), both of which also require actin and lipid rafts/cholesterol (32). Phagocytosis occurs only in specialized cells, and it is characterized by long outward protrusions that close around large cargos (98). Thus, an involvement of phagocytosis can be excluded, similar to macropinocytosis. IL-2 endocytosis, on the other hand, occurs into small, inward-budding vesicles, which are mostly formed at the base of an outward protrusion (99). Such initial pits containing MCPyV can be found in the electron micrographs; however, in the majority of cases, MCPyV is taken up into small vesicles from flat membrane regions, which makes IL-2-like endocytosis seem unlikely.
Caveolar/lipid raft-mediated endocytosis routes cargos into endosomal compartments (51, 100). We found MCPyV particles in high abundances in endosomal compartments resembling endosomes, lysosomes, and multivesicular and lamellar bodies. We also observed small numbers of MCPyV virions in the ER. Retrograde trafficking from endosomes to the ER is a general mechanism employed by PyVs. The low frequency of ER-localized MCPyV virions is similar to previous observations of mPyV entry (96) and hints at a bottleneck for trafficking from endosomes to the ER. Murine PyV as well as JC and BK PyVs have been shown to require acidic-pH environments in the endolysosomal compartment, presumably for a conformational change facilitating membrane penetration after translocation into the ER by an as-yet-unknown mechanism (89, 101, 102). For MCPyV, acidification in the endolysosomal system may serve a similar role.
MCPyV infection is sensitive to DTT treatment (Fig. 1C) and may therefore be dependent on the redox environment within the ER. In analogy to SV40, uncoating and membrane translocation to the cytoplasm may occur in the ER with the help of the ERAD machinery and cytoplasmic chaperones (39, 40).
As a final step, the viral genome must be delivered to the nucleus, which is the site of early gene expression and replication for PyV. Interestingly, we found that MCPyV infection was sensitive to cell cycle block in S phase, which indicates that mitosis is required for completion of MCPyV entry. Previous work on HPV16 identified its dependence on mitotic activity of the target cells, which allows delivery of the viral genome to the nucleus upon nuclear envelope breakdown (80, 103). However, SV40 has been described to enter the nucleus of interphase cells through the nuclear pore complex by making use of nuclear localization signals in the viral capsid proteins after ERAD-dependent partial disassembly (40, 104–106). It thus remains unclear why MCPyV target cells actively progress through the cell cycle. Nevertheless, our findings are in line with the importance of WNT signaling during infection of dermal fibroblasts (25).
To our surprise, we regularly observed enveloped particles in endosomal compartments. It remains unclear when and how these virions acquire a membranous envelope, whether this reflects an antiviral mechanism, or whether it is part of the infectious-entry mechanisms. It is conceivable that MCPyV uses the ESCRT machinery that generates intraluminal vesicles (ILVs) in multivesicular bodies (107). Alternatively, it may wrap itself in a membrane within the late endosomal/lysosomal compartment. The membrane could theoretically shield the virus from hydrolases and may give it the time to persist until infection can be completed, e.g., by progression through the cell cycle. Alternatively, these enveloped virus particles might arise through an unknown antiviral mechanism. Future studies will address the relevance of such enveloped particles for the infectious route.
In the light of our experiments and previous reports (28, 30), we propose that MCPyV attachment and internalization, at least in the model A549 cell line, are mediated by HS-type GAGs but that additional sialic acid binding is essential to route the internalized virus to the productive infection pathway of retrograde ER trafficking. However, entry into the ER seems to be a bottleneck for infections.
MATERIALS AND METHODS
Cell lines, antibodies, and reagents.
HeLa cells were obtained from the ATCC. A549 cells were a kind gift from C. Buck (NIH, National Cancer Institute, Bethesda, MD, USA). CV1 cells were a kind gift from J. Kartenbeck (DKFZ, Heidelberg, Germany). BHK Helsinki cells were a kind gift from A. Helenius (ETH Zürich, Switzerland). 293TT cells were a kind gift from J. Schiller (NIH, National Cancer Institute, Bethesda, MD, USA). Aphidicolin, EIPA, cytochalasin D, nocodazole, nystatin, NH4Cl, NaClO3, heparin, and sodium orthovanadate were obtained from Sigma-Aldrich. Bafilomycin A1, genistein, and progesterone were obtained from AppliChem. Brefeldin A, cyclosporine, jasplakinolide, and okadaic acid were obtained from Calbiochem. Dynasore was obtained from Merck. Pitstop2 was obtained from Abcam. RedDot2 was obtained from VWR.
Viruses.
MCPyV pseudoviruses (PsVs) containing a GFP reporter plasmid (MCPyV-GFP) were produced by transfection of 293TT cells with pwM2m, ph2m, and phGluc, as described previously (108, 109). In brief, 293TT cells were transfected with the indicated plasmids. After 48 h, cells were harvested and lysed. For optimal maturation, lysates were incubated for a further 24 h with 25 mM ammonium sulfate (pH 9.0) (110). MCPyV PsVs were purified on a 25% to 39% linear OptiPrep gradient (Sigma-Aldrich).
HPV16 pseudoviruses containing a GFP reporter plasmid (HPV16-GFP) were produced by transfection of 293TT cells with p16SheLL and pCIneo, as described previously (108, 109). The procedure was similar to the one for MCPyV production described above. IAV strain A/Puerto Rico/8/34 (PR8) stocks were prepared as described previously (111). In short, MDCK cells were infected, and the supernatants were collected. Virus was then purified by sucrose gradient centrifugation. SV40 and SFV were produced as described previously (40, 112).
Infection assays upon inhibitor treatment.
To most comprehensively describe the effects of small-molecule inhibitors in infection assays with MCPyV, HPV16, and SV40, inhibitor dilutions were used in a 96-well format from inhibitor master plates in parallel, where 3,000 or 10,000 A549 cells were seeded in RPMI medium (5% fetal calf serum [FCS] and 2 mM glutamine) at least 6 h prior to infection. Cells were treated with 80 μl of the following inhibitors at the indicated concentrations, diluted in RPMI (5% FCS, 2 mM glutamine) or SV40 infection medium (RPMI with 3% bovine serum albumin [BSA] and 10 mM HEPES [pH 6.8]): overnight preincubation with nystatin-progesterone, sodium chlorate, and toxin B and 30 min of preincubation with bafilomycin A1, brefeldin A, cytochalasin D, dithiothreitol, dynasore, EIPA, genistein, jasplakinolide, NH4Cl, nocodazole, okadaic acid, pitstop2, sodium orthovanadate, and wortmannin. Virus was diluted in RPMI (5% FCS, 2 mM glutamine) or SV40 infection medium, and 20 μl of the inoculum was added to each well. MCPyV samples were incubated for 30 h until the inhibitor dilutions were exchanged to RPMI containing 10 mM NH4Cl and 10 mM HEPES (pH 7.4) and incubated for a further 42 h. Samples were fixed by the addition of a final concentration of 4% paraformaldehyde (PFA) to the wells. As an exception, infections of sodium chlorate-treated cells were performed under the continued presence of the drug with daily renewal until fixation. Nuclei were stained with RedDot2. Infection was scored by analysis of GFP expression by microscopy (Zeiss Axio Observer Z1 microscope equipped with a Yokogawa CSU22 spinning-disc module and a CoolSnap HQ camera [Visitron Systems GmbH]). Infection with HPV16 was similar to that with MCPyV except that the exchange of medium to Dulbecco’s modified Eagle’s medium (DMEM) containing 10 mM NH4Cl and 10 mM HEPES (pH 7.4) occurred after 12 h postinfection and fixation was done after a total of 48 h. The SV40 inoculum was replaced by fresh DMEM containing 5 mM DTT after 10 h of incubation until fixation after 24 h. Cells were incubated with SFV for 4 h until fixation. Samples were fixed as described above. For detection of infection, SV40 and SFV samples were stained with an anti-LTag antibody (catalog number sc-20800) or SFV glycoprotein antibody (113), respectively, and an Alexa Fluor 488 (AF488)-coupled secondary antibody; subsequently, nuclei were stained with RedDot2. Infection was scored by microscopy (Zeiss Axio Observer Z1 microscope equipped with a Yokogawa CSU22 spinning-disc module and a CoolSnap HQ camera [Visitron Systems GmbH]). Cell numbers and infection indices were determined by using the MatLab script Infection Counter as described previously (40, 51). To test whether preincubation of virus particles with heparin blocked the ability to infect target cells, viruses were incubated for 1 h with heparin in 10 mM HEPES (pH 7.4) at room temperature. Subsequently, cells were infected with the inoculum for 1 h, when the medium was exchanged and cells were fixed after 72 h for MCPyV, 8 h for IAV, and 48 h for HPV16. IAV infection was assessed by scoring nuclear protein-positive cells (anti-NP, clone AA5H; Bio-Rad) and analyzed as described above.
Alternatively, the effects of select inhibitors on MCPyV and control virus infections were tested in a flow cytometry-based assay. Here, 3 × 104 A549 cells were seeded in 24-well plates, 5 × 104 HeLa cells were seeded in 12-well plates, or 2.5 × 105 BHK Helsinki cells were seeded in 12-well plates 6 to 8 h prior to treatment with aphidicolin overnight. The compound was renewed before cells were infected with MCPyV, HPV, or SFV, respectively. Aphidicolin was renewed daily until fixation of the cells at 72 h p.i. by trypsinization and the subsequent addition of 4% paraformaldehyde. The percentage of GFP-positive cells was measured by flow cytometry using a BD FACSCalibur instrument.
Electron microscopy.
For negative-staining EM of virus particles, about 8 × 106 PsVs in phosphate-buffered saline (PBS)–0.8 M NaCl were absorbed for 5 min on Formvar-coated, carbon-sputtered grids. Particles were contrasted for 7 min with 1% phosphotungstic acid. Samples were analyzed directly after drying. The sample was analyzed at 80 kV on a FEI-Tecnai 12 electron microscope (FEI, Eindhoven, Netherlands). Images of selected areas were documented with an Olympus Veleta 4k charge-coupled-device (CCD) camera.
For ultrathin-section transmission EM, virus particles were added for 2 h, 8 h, and 16 h to A549 cells before fixation with 2.5% glutaraldehyde in PBS. Samples were postfixed with 0.5% OsO4, block stained with 0.5% uranyl acetate, and, after dehydration, embedded in Epoxy resin. Sixty-nanometer ultrathin sections were cut and counterstained with uranyl acetate and lead. Images of selected areas were documented with an Olympus Veleta 4k CCD camera.
NMR spectroscopy.
NMR experiments were conducted at 283 K using a 600-MHz Bruker Avance spectrometer equipped with a TXI triple-resonance room-temperature probe head. NMR tubes with a 3-mm internal diameter (ID) and with a 200-μl sample volume were used. Each sample contained 55.6 nM MCPyV VLPs (i.e., 20 μM the major capsid protein VP1), either the wild type or mutant, and 1 mM oligosaccharide (either 3′ SL or the Arixtra GAG pentasaccharide). Prior to NMR sample preparation, VLPs were dialyzed in Slide-A-Lyzer Mini dialysis devices (Thermo Fisher Scientific) against a solution containing 150 mM NaCl and 1 mM CaCl2 (pH 6.0) in D2O. 3′ SL (Carbosynth) was added from a 40 mM stock solution prepared in pure D2O, and Arixtra was added from a 7.2 mM stock solution in 150 mM NaCl plus 1 mM CaCl2 (pH 6.0) in D2O, dialyzed from ready-to-inject syringes (Aspen). For 3′-SL-containing samples, off- and on-resonance frequencies in STD-NMR experiments were set to −30 ppm and 7.3 ppm, respectively, while −30 ppm and −0.5 ppm were used for Arixtra-containing samples, owing to the different chemical shift ranges of both glycans. The irradiation power of the selective pulses was set to 57 Hz. The saturation time was 2 s, and a total relaxation delay of 3 s was used. A 50-ms continuous-wave spin-lock pulse with a strength of 3.2 kHz was employed to suppress residual protein signals. A total number of 512 scans and a total number of 10,000 points were collected, and spectra were multiplied with a Gaussian window function prior to Fourier transformation.
MCPyV VLP preparation for NMR spectroscopy.
MCPyV VLPs were produced essentially according to a previously described protocol (114). The pwM and ph2m vectors, coding for MCPyV VP1 and VP2, respectively (27) (see www.addgene.org), were used at a 5:1 ratio for transfection of 293TT cells. OptiPrep was omitted during purification and replaced with two CsCl density gradient centrifugation steps.
ACKNOWLEDGMENTS
We thank N. Cordes and E. Weghake (Cellular Virology, Münster, Germany) for technical support during virus production and infection experiments. We also thank members of the Schelhaas laboratory for helpful comments on the manuscript.
This work was supported by funding to M.S. by the German Research Foundation (DFG EXC 1003 [partly]) and within the InfectERA initiative by funding from the Federal Ministry for Education and Research (BMBF) (031L0095A). We also acknowledge further support from the DFG to B.S.B. and M.S. (1294/3-1 and SCHE 1552/3-1, respectively, of FOR2327 ViroCarb).
REFERENCES
- 1.Gjoerup O, Chang Y. 2010. Update on human polyomaviruses and cancer. Adv Cancer Res 106:1–51. doi: 10.1016/S0065-230X(10)06001-X. [DOI] [PubMed] [Google Scholar]
- 2.Moens U, Calvignac-Spencer S, Lauber C, Ramqvist T, Feltkamp MCW, Daugherty MD, Verschoor EJ, Ehlers B, ICTV Report Consortium. 2017. ICTV virus taxonomy profile: Polyomaviridae. J Gen Virol 98:1159–1160. doi: 10.1099/jgv.0.000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross L. 1953. A filterable agent, recovered from Ak leukemic extracts, causing salivary gland carcinomas in C3H mice. Proc Soc Exp Biol Med 83:414–421. [DOI] [PubMed] [Google Scholar]
- 4.Eddy BE, Borman GS, Grubbs GE, Young RD. 1962. Identification of the oncogenic substance in rhesus monkey kidney cell cultures as simian virus 40. Virology 17:65–75. [DOI] [PubMed] [Google Scholar]
- 5.Chang Y, Moore PS. 2012. Merkel cell carcinoma: a virus-induced human cancer. Annu Rev Pathol 7:123–144. doi: 10.1146/annurev-pathol-011110-130227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCaprio JA, Garcea RL. 2013. A cornucopia of human polyomaviruses. Nat Rev Microbiol 11:264–276. doi: 10.1038/nrmicro2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner S, Field A, Coleman D, Hulme B. 1971. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 297:1253–1257. doi: 10.1016/S0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 8.Padgett B, Zurhein G, Walker D, Eckroade R, Dessel B. 1971. Cultivation of Papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 297:1257–1260. doi: 10.1016/S0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 9.Kean JM, Rao S, Wang M, Garcea RL. 2009. Seroepidemiology of human polyomaviruses. PLoS Pathog 5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stolt A, Sasnauskas K, Koskela P, Lehtinen M, Dillner J. 2003. Seroepidemiology of the human polyomaviruses. J Gen Virol 84:1499–1504. doi: 10.1099/vir.0.18842-0. [DOI] [PubMed] [Google Scholar]
- 11.Walker DL, Padgett BL. 1983. The epidemiology of human polyomaviruses. Prog Clin Biol Res 105:99–106. [PubMed] [Google Scholar]
- 12.Feng H, Shuda M, Chang Y, Moore PS. 2008. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgson NC. 2005. Merkel cell carcinoma: changing incidence trends. J Surg Oncol 89:1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- 14.Lemos B, Nghiem P. 2007. Merkel cell carcinoma: more deaths but still no pathway to blame. J Invest Dermatol 127:2100–2103. doi: 10.1038/sj.jid.5700925. [DOI] [PubMed] [Google Scholar]
- 15.Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. 2002. Merkel cell carcinoma and HIV infection. Lancet 359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 16.Locke FL, Rollison DE, Sondak VK. 2015. Merkel cell carcinoma and immunosuppression: what we still need to know. J Natl Cancer Inst 107:dju422. doi: 10.1093/jnci/dju422. [DOI] [PubMed] [Google Scholar]
- 17.Hampras SS, Michel A, Schmitt M, Waterboer T, Kranz L, Gheit T, Fisher K, Sondak VK, Messina J, Fenske N, Cherpelis B, Tommasino M, Pawlita M, Rollison DE. 2015. Merkel cell polyomavirus (MCV) T-antigen seroreactivity, MCV DNA in eyebrow hairs, and squamous cell carcinoma. Infect Agent Cancer 10:35. doi: 10.1186/s13027-015-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. 2010. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 7:509–515. doi: 10.1016/j.chom.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastrana DV, Pumphrey KA, Cuburu N, Schowalter RM, Buck CB. 2010. Characterization of monoclonal antibodies specific for the Merkel cell polyomavirus capsid. Virology 405:20–25. doi: 10.1016/j.virol.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spurgeon ME, Lambert PF. 2013. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology 435:118–130. doi: 10.1016/j.virol.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, Chang Y. 2008. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A 105:16272–16277. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Wang X, Diaz J, Tsang SH, Buck CB, You J. 2013. Merkel cell polyomavirus large T antigen disrupts host genomic integrity and inhibits cellular proliferation. J Virol 87:9173–9188. doi: 10.1128/JVI.01216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, Moore PS, Becker JC. 2010. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol 84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. 2011. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest 121:3623–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Yang R, Payne AS, Schowalter RM, Spurgeon ME, Lambert PF, Xu X, Buck CB, You J. 2016. Identifying the target cells and mechanisms of Merkel cell polyomavirus infection. Cell Host Microbe 19:775–787. doi: 10.1016/j.chom.2016.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Krump NA, MacDonald M, You J. 2018. Merkel cell polyomavirus infection of animal dermal fibroblasts. J Virol 92:e01610-17. doi: 10.1128/JVI.01610-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastrana DV, Tolstov YL, Becker JC, Moore PS, Chang Y, Buck CB. 2009. Quantitation of human seroresponsiveness to Merkel cell polyomavirus. PLoS Pathog 5:e1000578. doi: 10.1371/journal.ppat.1000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schowalter RM, Pastrana DV, Buck CB. 2011. Glycosaminoglycans and sialylated glycans sequentially facilitate Merkel cell polyomavirus infectious entry. PLoS Pathog 7:e1002161. doi: 10.1371/journal.ppat.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schowalter RM, Reinhold WC, Buck CB. 2012. Entry tropism of BK and Merkel cell polyomaviruses in cell culture. PLoS One 7:e42181. doi: 10.1371/journal.pone.0042181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neu U, Hengel H, Blaum BS, Schowalter RM, Macejak D, Gilbert M, Wakarchuk WW, Imamura A, Ando H, Kiso M, Arnberg N, Garcea RL, Peters T, Buck CB, Stehle T. 2012. Structures of Merkel cell polyomavirus VP1 complexes define a sialic acid binding site required for infection. PLoS Pathog 8:e1002738. doi: 10.1371/journal.ppat.1002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson KD, Garcea RL, Tsai B. 2009. Ganglioside GT1b is a putative host cell receptor for the Merkel cell polyomavirus. J Virol 83:10275–10279. doi: 10.1128/JVI.00949-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu Rev Biochem 79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 33.Doherty GJ, McMahon HT. 2009. Mechanisms of endocytosis. Annu Rev Biochem 78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 34.Anderson HA, Chen Y, Norkin LC. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell 7:1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parton RG, Richards AA. 2003. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4:724–738. [DOI] [PubMed] [Google Scholar]
- 36.Pelkmans L, Kartenbeck J, Helenius A. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol 3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 37.Norkin LC, Anderson HA, Wolfrom SA, Oppenheim A. 2002. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J Virol 76:5156–5166. doi: 10.1128/JVI.76.10.5156-5166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pho MT, Ashok A, Atwood WJ. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J Virol 74:2288–2292. doi: 10.1128/JVI.74.5.2288-2292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupzyk A, Tsai B. 2016. How polyomaviruses exploit the ERAD machinery to cause infection. Viruses 8:E242. doi: 10.3390/v8090242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schelhaas M, Malmstrom J, Pelkmans L, Haugstetter J, Ellgaard L, Grunewald K, Helenius A. 2007. Simian virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell 131:516–529. doi: 10.1016/j.cell.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 41.Inoue T, Tsai B. 2015. A nucleotide exchange factor promotes endoplasmic reticulum-to-cytosol membrane penetration of the nonenveloped virus simian virus 40. J Virol 89:4069–4079. doi: 10.1128/JVI.03552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geiger R, Andritschke D, Friebe S, Herzog F, Luisoni S, Heger T, Helenius A. 2011. BAP31 and BiP are essential for dislocation of SV40 from the endoplasmic reticulum to the cytosol. Nat Cell Biol 13:1305–1314. doi: 10.1038/ncb2339. [DOI] [PubMed] [Google Scholar]
- 43.Walczak CP, Tsai B. 2011. A PDI family network acts distinctly and coordinately with ERp29 to facilitate polyomavirus infection. J Virol 85:2386–2396. doi: 10.1128/JVI.01855-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helenius A, Kartenbeck J, Simons K, Fries E. 1980. On the entry of Semliki Forest virus into BHK-21 cells. J Cell Biol 84:404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vries E, Tscherne DM, Wienholts MJ, Cobos-Jiménez V, Scholte F, García-Sastre A, Rottier PJM, de Haan CAM. 2011. Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog 7:e1001329. doi: 10.1371/journal.ppat.1001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matlin KS, Reggio H, Helenius A, Simons K. 1981. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol 91:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schelhaas M, Shah B, Holzer M, Blattmann P, Kuhling L, Day PM, Schiller JT, Helenius A. 2012. Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLoS Pathog 8:e1002657. doi: 10.1371/journal.ppat.1002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Becker M, Dominguez M, Greune L, Soria-Martinez L, Pfleiderer MM, Schowalter R, Buck CB, Blaum BS, Schmidt MA, Schelhaas M. 2018. Infectious entry of Merkel cell polyomavirus. bioRxiv doi: 10.1101/456673. [DOI] [PMC free article] [PubMed]
- 49.Kuhling L, Schelhaas M. 2014. Systematic analysis of endocytosis by cellular perturbations. Methods Mol Biol 1174:19–46. doi: 10.1007/978-1-4939-0944-5_2. [DOI] [PubMed] [Google Scholar]
- 50.Boron WF, De Weer P. 1976. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engel S, Heger T, Mancini R, Herzog F, Kartenbeck J, Hayer A, Helenius A. 2011. Role of endosomes in simian virus 40 entry and infection. J Virol 85:4198–4211. doi: 10.1128/JVI.02179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braakman I, Helenius J, Helenius A. 1992. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J 11:1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Safaiyan F, Kolset SO, Prydz K, Gottfridsson E, Lindahl U, Salmivirta M. 1999. Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. J Biol Chem 274:36267–36273. [DOI] [PubMed] [Google Scholar]
- 54.Cerqueira C, Liu Y, Kuhling L, Chai W, Hafezi W, van Kuppevelt TH, Kuhn JE, Feizi T, Schelhaas M. 2013. Heparin increases the infectivity of human papillomavirus type 16 independent of cell surface proteoglycans and induces L1 epitope exposure. Cell Microbiol 15:1818–1836. doi: 10.1111/cmi.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC. 1988. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- 56.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Kräusslich H-G, Shupliakov O, Robinson PJ, McCluskey A, Haucke V. 2011. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146:471–484. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 57.Doxsey SJ, Brodsky FM, Blank GS, Helenius A. 1987. Inhibition of endocytosis by anti-clathrin antibodies. Cell 50:453–463. [DOI] [PubMed] [Google Scholar]
- 58.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Pelkmans L, Puntener D, Helenius A. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 60.Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. 1996. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem 271:21604–21613. [DOI] [PubMed] [Google Scholar]
- 61.Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. 1992. Caveolin, a protein component of caveolae membrane coats. Cell 68:673–682. [DOI] [PubMed] [Google Scholar]
- 62.Metherall JE, Waugh K, Li H. 1996. Progesterone inhibits cholesterol biosynthesis in cultured cells: accumulation of cholesterol precursors. J Biol Chem 271:2627–2633. doi: 10.1074/jbc.271.5.2627. [DOI] [PubMed] [Google Scholar]
- 63.Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM, Grinstein S. 2010. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol 188:547–563. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.L’Allemain G, Paris S, Pouyssegur J. 1984. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem 259:5809–5815. [PubMed] [Google Scholar]
- 65.Brown SS, Spudich JA. 1979. Cytochalasin inhibits the rate of elongation of actin filament fragments. J Cell Biol 83:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. 1994. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269:14869–14871. [PubMed] [Google Scholar]
- 67.Niedergang F, Chavrier P. 2005. Regulation of phagocytosis by Rho GTPases. Curr Top Microbiol Immunol 291:43–60. [DOI] [PubMed] [Google Scholar]
- 68.Mercer J, Helenius A. 2009. Virus entry by macropinocytosis. Nat Cell Biol 11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 69.Just I, Fritz G, Aktories K, Giry M, Popoff MR, Boquet P, Hegenbarth S, von Eichel-Streiber C. 1994. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem 269:10706–10712. [PubMed] [Google Scholar]
- 70.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262:5592–5595. [PubMed] [Google Scholar]
- 71.Gordon JA. 1991. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol 201:477–482. [DOI] [PubMed] [Google Scholar]
- 72.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. 1997. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem 272:843–851. [DOI] [PubMed] [Google Scholar]
- 73.Bialojan C, Takai A. 1988. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J 256:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoshimori T, Yamamoto A, Moriyamas Y, Futais M, Tashiroq Y. 1991. Bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 266:17707–17712. [PubMed] [Google Scholar]
- 75.Stebbings H. 1995. Microtubule-based intracellular transport of organelles. Cytoskeleton 2:113–140. [Google Scholar]
- 76.Cheung HT, Terry DS. 1980. Effects of nocodazole, a new synthetic microtubule inhibitor, on movement and spreading of mouse peritoneal macrophages. Cell Biol Int Rep 4:1125–1129. [DOI] [PubMed] [Google Scholar]
- 77.Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. 1988. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem 263:18545–18552. [PubMed] [Google Scholar]
- 78.Clever J, Yamada M, Kasamatsu H. 1991. Import of simian virus 40 virions through nuclear pore complexes. Proc Natl Acad Sci U S A 88:7333–7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pyeon D, Pearce SM, Lank SM, Ahlquist P, Lambert PF. 2009. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog 5:e1000318. doi: 10.1371/journal.ppat.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aydin I, Weber S, Snijder B, Samperio Ventayol P, Kuhbacher A, Becker M, Day PM, Schiller JT, Kann M, Pelkmans L, Helenius A, Schelhaas M. 2014. Large scale RNAi reveals the requirement of nuclear envelope breakdown for nuclear import of human papillomaviruses. PLoS Pathog 10:e1004162. doi: 10.1371/journal.ppat.1004162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spadari S, Sala F, Pedrali-Noy G. 1982. Aphidicolin: a specific inhibitor of nuclear DNA replication in eukaryotes. Trends Biochem Sci 7:29–32. doi: 10.1016/0968-0004(82)90061-5. [DOI] [Google Scholar]
- 82.Froshauer S, Kartenbeck J, Helenius A. 1988. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J Cell Biol 107:2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ewers H, Romer W, Smith AE, Bacia K, Dmitrieff S, Chai W, Mancini R, Kartenbeck J, Chambon V, Berland L, Oppenheim A, Schwarzmann G, Feizi T, Schwille P, Sens P, Helenius A, Johannes L. 2010. GM1 structure determines SV40-induced membrane invagination and infection. Nat Cell Biol 12:11–18. doi: 10.1038/ncb1999. [DOI] [PubMed] [Google Scholar]
- 84.Kartenbeck J, Stukenbrok H, Helenius A. 1989. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol 109:2721–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, Pagano RE, Hancock JF, Mayor S, Parton RG. 2005. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol 168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sabharanjak S, Sharma P, Parton RG, Mayor S. 2002. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell 2:411–423. doi: 10.1016/S1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 87.Bennett SM, Jiang M, Imperiale MJ. 2013. Role of cell-type-specific endoplasmic reticulum-associated degradation in polyomavirus trafficking. J Virol 87:8843–8852. doi: 10.1128/JVI.00664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nelson CD, Derdowski A, Maginnis MS, O’Hara BA, Atwood WJ. 2012. The VP1 subunit of JC polyomavirus recapitulates early events in viral trafficking and is a novel tool to study polyomavirus entry. Virology 428:30–40. doi: 10.1016/j.virol.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Querbes W, O’Hara BA, Williams G, Atwood WJ. 2006. Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of noncaveolar ligands. J Virol 80:9402–9413. doi: 10.1128/JVI.01086-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blaum BS, Neu U, Peters T, Stehle T. 2018. Spin ballet for sweet encounters: saturation-transfer difference NMR and X-ray crystallography complement each other in the elucidation of protein-glycan interactions. Acta Crystallogr F Struct Biol Commun 74:451–462. doi: 10.1107/S2053230X18006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geoghegan EM, Pastrana DV, Schowalter RM, Ray U, Gao W, Ho M, Pauly GT, Sigano DM, Kaynor C, Cahir-McFarland E, Combaluzier B, Grimm J, Buck CB. 2017. Infectious entry and neutralization of pathogenic JC polyomaviruses. Cell Rep 21:1169–1179. doi: 10.1016/j.celrep.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neu U, Maginnis MS, Palma AS, Stroh LJ, Nelson CD, Feizi T, Atwood WJ, Stehle T. 2010. Structure-function analysis of the human JC polyomavirus establishes the LSTc pentasaccharide as a functional receptor motif. Cell Host Microbe 8:309–319. doi: 10.1016/j.chom.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsai B, Gilbert JM, Stehle T, Lencer W, Benjamin TL, Rapoport TA. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J 22:4346–4355. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Payne CK, Jones SA, Chen C, Zhuang X. 2007. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic 8:389–401. doi: 10.1111/j.1600-0854.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarrazin S, Lamanna WC, Esko JD. 2011. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3:a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian M, Cai D, Verhey KJ, Tsai B. 2009. A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection. PLoS Pathog 5:e1000465. doi: 10.1371/journal.ppat.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cerqueira C, Samperio Ventayol P, Vogeley C, Schelhaas M. 2015. Kallikrein-8 proteolytically processes human papillomaviruses in the extracellular space to facilitate entry into host cells. J Virol 89:7038–7052. doi: 10.1128/JVI.00234-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aderem A, Underhill DM. 1999. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 99.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. 2001. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell 7:661–671. [DOI] [PubMed] [Google Scholar]
- 100.Pelkmans L, Burli T, Zerial M, Helenius A. 2004. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Liebl D, Difato F, Hornikova L, Mannova P, Stokrova J, Forstova J. 2006. Mouse polyomavirus enters early endosomes, requires their acidic pH for productive infection, and meets transferrin cargo in Rab11-positive endosomes. J Virol 80:4610–4622. doi: 10.1128/JVI.80.9.4610-4622.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eash S, Querbes W, Atwood WJ. 2004. Infection of Vero cells by BK virus is dependent on caveolae. J Virol 78:11583–11590. doi: 10.1128/JVI.78.21.11583-11590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aydin I, Villalonga-Planells R, Greune L, Bronnimann MP, Calton CM, Becker M, Lai KY, Campos SK, Schmidt MA, Schelhaas M. 2017. A central region in the minor capsid protein of papillomaviruses facilitates viral genome tethering and membrane penetration for mitotic nuclear entry. PLoS Pathog 13:e1006308. doi: 10.1371/journal.ppat.1006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakanishi A, Clever J, Yamada M, Li PP, Kasamatsu H. 1996. Association with capsid proteins promotes nuclear targeting of simian virus 40 DNA. Proc Natl Acad Sci U S A 93:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakanishi A, Shum D, Morioka H, Otsuka E, Kasamatsu H. 2002. Interaction of the Vp3 nuclear localization signal with the importin alpha 2/beta heterodimer directs nuclear entry of infecting simian virus 40. J Virol 76:9368–9377. doi: 10.1128/JVI.76.18.9368-9377.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakanishi A, Li PP, Qu Q, Jafri QH, Kasamatsu H. 2007. Molecular dissection of nuclear entry-competent SV40 during infection. Virus Res 124:226–230. doi: 10.1016/j.virusres.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hanson PI, Cashikar A. 2012. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 108.Buck CB, Pastrana DV, Lowy DR, Schiller JT. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med 119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 109.Buck CB, Thompson CD, Pang YY, Lowy DR, Schiller JT. 2005. Maturation of papillomavirus capsids. J Virol 79:2839–2846. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cardone G, Moyer AL, Cheng N, Thompson CD, Dvoretzky I, Lowy DR, Schiller JT, Steven AC, Buck CB, Trus BL. 2014. Maturation of the human papillomavirus 16 capsid. mBio 5:e01104-14. doi: 10.1128/mBio.01104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rowse M, Qiu S, Tsao J, Xian T, Khawaja S, Yamauchi Y, Yang Z, Wang G, Luo M. 2015. Characterization of potent fusion inhibitors of influenza virus. PLoS One 10:e0122536. doi: 10.1371/journal.pone.0122536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marsh M, Helenius A. 1980. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol 142:439–454. [DOI] [PubMed] [Google Scholar]
- 113.Vonderheit A, Helenius A. 2005. Rab7 associates with early endosomes to mediate sorting and transport of Semliki Forest virus to late endosomes. PLoS Biol 3:e233. doi: 10.1371/journal.pbio.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buck CB, Thompson CD. 2007. Production of papillomavirus-based gene transfer vectors. Curr Protoc Cell Biol Chapter26:Unit 26.1. doi: 10.1002/0471143030.cb2601s37. [DOI] [PubMed] [Google Scholar]