Airway epithelial cells (AEC) and airway macrophages (AM) represent major targets of influenza A virus (IAV) infection in the lung, yet the two cell types respond very differently to IAV infection. We have used RNA sequencing to define the host transcriptional responses in each cell type under steady-state conditions as well as following IAV infection. To do this, different cell subsets isolated from the lungs of mock- and IAV-infected mice were subjected to RNA sequencing. Under steady-state conditions, AM and AEC express distinct transcriptional activities, consistent with distinct physiological roles in the airways. Not surprisingly, these cells also exhibited major differences in transcriptional responses following IAV infection. These studies shed light on how the different transcriptional architectures of airway cells from two different lineages drive transcriptional responses to IAV infection.
KEYWORDS: RNA sequencing, epithelial cell, influenza, lung, macrophage, mouse model
ABSTRACT
Airway epithelial cells and macrophages differ markedly in their responses to influenza A virus (IAV) infection. To investigate transcriptional responses underlying these differences, purified subsets of type II airway epithelial cells (ATII) and alveolar macrophages (AM) recovered from the lungs of mock- or IAV-infected mice at 9 h postinfection were subjected to RNA sequencing. This time point was chosen to allow for characterization of cell types first infected with the virus inoculum, prior to multicycle virus replication and the infiltration of inflammatory cells into the airways. In the absence of infection, AM predominantly expressed genes related to immunity, whereas ATII expressed genes consistent with their physiological roles in the lung. Following IAV infection, AM almost exclusively activated cell-intrinsic antiviral pathways that were dependent on interferon (IFN) regulatory factor 3/7 (IRF3/7) and/or type I IFN signaling. In contrast, IAV-infected ATII activated a broader range of physiological responses, including cell-intrinsic antiviral pathways, which were both independent of and dependent on IRF3/7 and/or type I IFN. These data suggest that transcriptional profiles hardwired during development are a major determinant underlying the different responses of ATII and AM to IAV infection.
IMPORTANCE Airway epithelial cells (AEC) and airway macrophages (AM) represent major targets of influenza A virus (IAV) infection in the lung, yet the two cell types respond very differently to IAV infection. We have used RNA sequencing to define the host transcriptional responses in each cell type under steady-state conditions as well as following IAV infection. To do this, different cell subsets isolated from the lungs of mock- and IAV-infected mice were subjected to RNA sequencing. Under steady-state conditions, AM and AEC express distinct transcriptional activities, consistent with distinct physiological roles in the airways. Not surprisingly, these cells also exhibited major differences in transcriptional responses following IAV infection. These studies shed light on how the different transcriptional architectures of airway cells from two different lineages drive transcriptional responses to IAV infection.
INTRODUCTION
In humans, infection with seasonal influenza A virus (IAV) is generally restricted to the respiratory tract. A range of different cell types reside in the airways and represent targets for IAV infection. For example, the apical epithelial surface of the upper airways (nasal, trachea, bronchi, and bronchioles) is largely comprised of ciliated cells, goblet cells, and club cells in various proportions. In the distal lung, the alveoli are composed of two distinct subsets of airway epithelial cells (AEC), alveolar type I cells (ATI) and alveolar type II cells (ATII). Infection of AEC generally results in productive virus replication, thereby promoting virus amplification and spread. Immune cell populations, including alveolar macrophages (AM) and interstitial macrophages (IM) in the lung, are also susceptible to IAV infection. In contrast to AEC, ex vivo studies indicate that macrophages tend to be less permissive or nonpermissive to productive replication by seasonal IAV (reviewed in reference 1).
In addition to differences in their abilities to support virus replication, AEC and airway macrophages sense and respond differently to seasonal IAV infection. For example, AEC and AM differ in regard to the linkages of sialic acid that predominate on the cell surface (2, 3) as well as in the expression of C-type lectin receptors (4, 5), both of which can impact susceptibility to infection by a particular IAV. AEC and macrophages also produce distinct patterns of inflammatory mediators in response to seasonal IAV in vitro (6, 7). Understanding the transcriptional signatures of AEC and AM under steady-state conditions, as well as following IAV infection, will provide insight regarding differences in their abilities to sense and respond to IAV infections.
Here, hemagglutinin-positive (HA+) AEC and immune cell subsets isolated ex vivo from the distal lungs of IAV-infected mice, as well as the corresponding cell subsets from mock-infected animals, were subjected to cell sorting and RNA sequencing (RNA-seq). AM and ATII represent major targets of IAV infection in the lung and express distinct transcriptional activities under steady-state conditions, consistent with distinct physiological roles. Not surprisingly, AM and ATII also exhibited major differences in transcriptional responses following IAV infection. We propose that lineage-specific transcriptional architecture drives the distinct physiological functions of AM and ATII in the lungs under steady-state conditions. This, in turn, is a major factor determining the distinct transcriptional and functional responses of each cell type to IAV infection.
RESULTS
Identification of parenchymal and immune cell subsets in the lungs of mock- or IAV-infected mice.
After intranasal mock or IAV infection, single-cell suspensions were prepared from distal lung at 9 h postinfection (p.i.) and examined by flow cytometry for expression of cell surface markers and IAV HA. This time point was chosen to allow for characterization of cell types first infected with the virus inoculum, prior to multicycle virus replication and the infiltration of inflammatory cells into the airways. Selection of cells that express HA at the cell surface enabled analysis of transcriptional responses from cells at a late stage in the virus replication cycle (i.e., those that have translated the HA gene segment and transported the protein to the cell surface), thereby reducing transcriptional noise from uninfected bystander cells in IAV-infected lungs. Cell suspensions were treated with bacterial sialidase prior to analysis to remove any cell-associated virions that might represent the residual virus inoculum.
Prior to RNA-seq library preparation, we characterized immune and parenchymal cell subsets in the distal lungs of mock- and IAV-infected mice. In the immune cell compartment, we identified CD24+ and CD24− monocytes, neutrophils, AM, IM, CD103+ dendritic cells (DC), and CD11b+ DC (Fig. 1A). AM represented the highest proportion of virus-infected immune cells, as determined by expression of cell surface HA (Fig. 1C, left panels).
FIG 1.
Identification of parenchymal and immune cell subsets in the lungs of mock- or IAV-infected mice at 9 h p.i. Mice were infected via the intranasal route with IAV strain PR8 or mock infected with allantoic fluid from uninfected eggs diluted in virus diluent. At 9 h p.i., mice were killed, single-cell suspensions were prepared from the distal lung, and cells were stained with different mAb as described in Materials and Methods. (A and B) Expression of cell surface viral HA on different populations of immune cells (CD45.2+), including CD24+ and CD24− monocytes (Gr1+ Mar1−), neutrophils (Gr1hi Mar1−), AM (Mar1− Gr1− CD64+ MerTK+ CD11c+ CD11b−), IM (Mar1− Gr1− CD64+ MerTK+ CD11c+/− CD11b+), CD103+ DC (Mar1− Gr1− CD64− MerTK− CD11c+ CD24+ IA/IE+ CD11b−), and CD11b+ DC (Mar1− Gr1− CD64− MerTK− CD11c+ CD24+ IA/IE+ CD103−) (A), or parenchymal cells (CD45.2− CD146− CD31− CD326+), including ciliated cells (CD55+ CD104+ IA/IE− CD24+ Sca1+/−), club cells (CD55− CD104− IA/IE− CD24− Sca1−), and ATII (CD55− CD104− IA/IE+ CD24− Sca1−) (B). PI, propidium iodide; IA/IE, major histocompatibility complex class II; MerTK, MER proto-oncogene tyrosine kinase. Expressions of viral HA on the cell surface of immune and parenchymal cells from mock-infected animals are also shown. (C) Percentage of HA+ cells in different cell subsets expressed as a percentage of the total viable cells in distal lung cell suspensions. Data show the means ± standard deviations (SD) from three independent experiments (n = 3 to 5 mice per experiment).
We also identified parenchymal cells subsets (Fig. 1B), including type II AEC (ATII) (CD326++ CD55− CD104− IA/IE+ CD24− Sca1−), club cells (CD326++ CD55− CD104− IA/IE− CD24− Sca1−), and ciliated cells (CC) (CD326++ CD55+ CD104+ IA/IE− CD24+ Sca1−/+), which were confirmed via quantitative PCR (qPCR) using previously reported gene transcripts (8–11). Ciliated cells expressed both Scgb1a1 and Foxj1, club cells expressed very high levels of Scgb1a1, and ATII expressed Sftpa1, Sftpb, Sftpc, and Sftpd (data not shown). Despite pooling lung cell suspensions from 3 to 5 IAV-infected mice, very few HA+ ATI cells were detected (data not shown), indicating that these cells may not survive the cell collection methods or may lose surface expression of appropriate markers after cell collection and/or viral infection (12). ATII cells represented the highest proportion of virus-infected parenchymal cells, as determined by expression of cell surface HA (Fig. 1C, right panels).
HA+ ATII and AM isolated from IAV-infected mice express similar levels of viral gene transcripts.
We performed cell enrichment followed by cell sorting to obtain purified immune and parenchymal cell subsets for RNA-seq. For these studies, we used a few key cell surface markers, rather than the full panel of phenotypic markers used in Fig. 1, to identify and isolate both immune and parenchymal cell subsets from lung samples. Sufficient numbers of purified HA+ AM and CD103+ DC (immune cell subset), as well as ATII and CC (parenchymal cell subset), were isolated from IAV-infected animals. The corresponding cell populations were isolated and analyzed from mock-infected animals. Representative flow cytometry plots of cell populations collected for RNA-seq are shown (Fig. 2A). The presence of viral transcripts in HA+ cell populations was verified by alignment of RNA-seq expression data to the A/Puerto Rico/8/34 (PR8) genome retrieved from the NCBI gene database. HA+ AM and ATII contained viral gene transcripts at similar proportions, and these were much higher than those detected in CD103+ DC or CC (Fig. 2B), consistent with more-extensive virus replication in AM and ATII. Next, we examined the expression of the eight viral gene segment transcripts in HA+ ATII and AM. While no significant differences were observed in the expression of 7 out of 8 viral genes, the viral NS gene was expressed at significantly higher levels (log2-fold change of 0.7) in HA+ AM (Fig. 2C).
FIG 2.
HA+ ATII and AM isolated from IAV-infected mice express similar levels of viral gene transcripts. Single-cell suspensions prepared from distal lungs of mock- or IAV-infected mice were enriched, stained, sorted, and then subjected to RNA-seq. (A) Representative flow cytometry plots showing the gating strategy to isolate ATII, CC, AM, and CD103+ DC from IAV-infected (HA+) and mock-infected (HA−) mice by fluorescence-activated cell sorter (FACS) analysis for RNA-seq. MHCII, major histocompatibility complex class II. (B) Viral transcript counts by RNA-seq in ATII, AM, CC, and CD103+ DC from IAV-infected (HA+) and mock-infected (HA−) mice are shown as a percentage of total host transcript counts. RNA-seq reads were aligned to gene transcripts of IAV strain PR8, and counts of viral transcripts were determined. (C) Viral transcripts were aligned to the PR8 genome, and log2-fold changes, 95% confidence intervals, and false discovery rates (FDR) are shown. Data were pooled from four independent experiments (n = 3 to 5 mice per experiment). ***, P < 0.001; n.s, not significant.
IAV-infected HA+ ATII cells modulate expression of a larger number of genes and more-diverse biological functions than HA+ AM.
Having confirmed similar levels of viral transcripts in HA+ ATII and AM, we asked if the intrinsic cellular responses to IAV infection differed between these cell types. Comparison of AM and ATII from mock- or IAV-infected mice demonstrated that ATII were more transcriptionally responsive to IAV infection, showing differential expression of >2,000 genes, compared to only ∼1,000 genes in HA+ AM (Fig. 3A). HA+ ATII showed similar numbers of upregulated (1,375) and downregulated (1,292) genes relative to ATII from mock-infected mice (ATII mock). In contrast, HA+ AM showed approximately twice as many upregulated (687) as downregulated (330) genes compared to AM mock.
FIG 3.

IAV-infected HA+ ATII cells modulate expression of a larger number of genes and more-diverse biological functions than HA+ AM. (A) Numbers of differentially expressed genes significantly up- or downregulated in HA+ ATII and AM compared to the corresponding cell subsets from mock-infected animals. (B) GO terms for the differentially expressed genes significantly enriched (fold enrichment of >1; P value of <0.05) in HA+ ATII and HA+ AM were retrieved from the Panther database. The number of enriched GO terms (top panel) and fold enrichment (bottom panel) are shown in the bar graph and scatterplot, respectively. For fold enrichment, statistical significance was determined by one-way analysis of variance (ANOVA) with Bonferroni’s correction. ***, P < 0.0001; n.s, not significant. (C) Keywords related to function were identified in enriched GO terms (fold enrichment of >1), and the numbers of times that particular keywords occurred (i.e., frequency) were tabulated and are represented in a heat map. Data were pooled from four independent experiments (n = 3 to 5 mice per experiment).
To investigate which cellular functions were represented in the differentially expressed (DE) genes, significantly enriched Gene Ontology (GO) biological process terms for these genes were retrieved from Panther DB (see Table S1 in the supplemental material). Compared to the corresponding cell populations from mock-infected mice, HA+ ATII cells upregulated a diverse range of cellular functions (416 enriched GO terms) compared to HA+ AM (192 enriched GO terms) (Fig. 3B, top panel). However, the average fold enrichment of these functions was significantly higher in HA+ AM than in HA+ ATII (Fig. 3B, bottom panel). Few cellular functions were enriched in downregulated genes of HA+ ATII (69 GO terms) and HA+ AM (11 GO terms), and the average fold enrichment was not significantly different between HA+ ATII and HA+ AM.
Next, overrepresented GO terms in genes modulated in HA+ AM or HA+ ATII were categorized into functions based on keywords identified within the GO terms (Fig. 3C and Table S1). In HA+ AM, GO terms in upregulated genes that were significantly overrepresented were associated with intrinsic immunity, cell death, signaling, and translation/protein related, whereas GO terms related to a diverse range of cellular functions (i.e., cellular immunity, transcription, cell cycle, and cell adhesion/motility, etc.) were significantly overrepresented in HA+ ATII. In the downregulated genes, GO terms enriched in HA+ ATII included transport, localization, translation/protein related, lipids, and endoplasmic reticulum (ER)/Golgi, while very few GO terms were overrepresented in downregulated genes from HA+ AM (Fig. 3C).
IAV-infected HA+ AM predominantly upregulate genes associated with antiviral activity, whereas HA+ ATII upregulate genes associated with a broad spectrum of cellular functions.
Because common cellular functions were upregulated during IAV infection in both cell types, we asked if these responses were unique to or shared by each cell type. First, we examined upregulated (413) genes unique to HA+ AM (Fig. 4A). The most enriched GO term in “HA+ AM only” was the response to virus (Fig. 4B and Table S2). Next, we examined the 274 common genes that were upregulated in both HA+ AM and HA+ ATII (AM and ATII shared). The most enriched GO terms of the shared upregulated genes included response to type I interferon (IFN), IFN-α, and IFN-β; response to virus; and regulation of viral genome replication (Fig. 4C and Table S2). The functions of the 1,101 genes uniquely upregulated following IAV infection in HA+ ATII showed significant enrichment of GO terms involved in broad cellular functions, including positive regulation of leukocyte apoptotic process, regulation of granulocyte chemotaxis, ribosome biogenesis, and cilium movement, among others (Fig. 4D and Table S2). Pathway analysis of the shared and unique genes further revealed a central theme in that all genes upregulated by HA+ AM interacted to promote intrinsic immunity, either with or without transcriptional functions. In contrast, the gene interactions in HA+ ATII were much more diverse and included transcriptional and RNA-related responses that were both related and not related to intrinsic immunity. Of note, HA+ ATII shared the conserved network of intrinsic immunity genes identified in HA+ AM (Fig. 5).
FIG 4.
IAV-infected HA+ AM predominantly upregulate genes associated with antiviral activity, whereas HA+ ATII upregulate genes associated with diverse cellular functions. (A) Numbers of differentially expressed genes significantly upregulated (compared to mock counterparts) that are unique to HA+ AM (AM only) or HA+ ATII (ATII only) or shared (AM and ATII shared) are shown. (B to D) GO terms for differentially expressed genes that were significantly enriched in AM only (B), AM and ATII shared (C), and ATII only (D) were retrieved from the Panther database. GO terms for upregulated genes are presented as scatterplots of fold enrichment versus the significance of the particular GO term. The size of each data point corresponds to the number of genes in the GO term.
FIG 5.
Pathway analysis (STRING database) was performed for upregulated genes (relative to mock) that were unique to HA+ AM (AM only) or HA+ ATII (ATII only), that were common to HA+ AM and ATII (AM and ATII shared), or that represented all upregulated genes from AM (total AM). Nodes related to the functional categories “intrinsic immunity,” “transcription,” and “intrinsic immunity and transcription” representing their respective GO terms are shown.
Next, we correlated the expression levels of unique genes upregulated in HA+ ATII to those in mock and HA+ AM (Fig. 6A). We also determined the number of genes differentially expressed in HA+ ATII that were not detected in mock/HA+ AM or in mock ATII (Fig. 6B) according to summarized classifications in Fig. 3C. Positive correlations between HA+ ATII and mock/HA+ AM populations indicated that irrespective of infection, AM express many of the genes that were differentially upregulated in HA+ ATII. Furthermore, most of these genes were also expressed in both AM populations; hence, while these genes were uniquely upregulated in HA+ ATII, their expression per se was by no means unique to ATII. Based on these findings, we conclude that there is no major deficit in the ability of AM to express genes associated with a diverse array of cellular functions and immune responses that are uniquely upregulated following IAV infection of ATII cells.
FIG 6.
Irrespective of infection, AM constitutively express most of the genes that ATII upregulate in response to IAV infection. (A) Expression levels (log2 counts per million [CPM]) of unique genes that were significantly upregulated in HA+ ATII were compared to expression levels of the same genes in either mock AM (left panels) (red symbols) or HA+ AM (right panels) (blue symbols) and are represented in scatterplots. Correlation was performed using the Pearson correlation method. Total unique HA+ ATII genes (i) and their functional categories “translation and protein related” (ii), “transcription and RNA related” (iii), “cellular immunity” (iv), and “intrinsic immunity” (v) are shown in different scatterplots. (B) The numbers of genes expressed in HA+ ATII that were not detected by RNA-seq in mock/HA+ AM or in mock ATII are shown as bar graphs.
In the absence of IAV infection, transcriptional profiles correlate with the unique biological functions of different lung cell subsets.
Transcriptional and functional differences between AM and ATII in response to IAV could be attributed to their lineage. To address this, we examined transcriptional responses in cells from immune (CD103+ DC) and parenchymal (CC) cell lineages. CD103+ DC are crucial in priming T cell responses against influenza (13), whereas a major function of CC is to limit the entry of particulate matter into the distal lung (14). Principal-component (PC) analysis of RNA-seq data showed transcriptional variance among the different cell types (i.e., AM, ATII, CD103, and CC), based on their cell lineage (Fig. 7A). The transcriptional profiles of AM were more similar to those of CD103+ DC than to those of either ATII or CC (Fig. 7B), consistent with their shared immune cell origin.
FIG 7.
In the absence of infection, transcriptional profiles correlate with the unique biological functions of different lung cell subsets. (A) Principal-component analysis of AM, CD103+ DC, ATII, and ciliated cells (CC) derived from mock- and IAV-infected mice from four independent experiments. For each cell type, mock and HA+ cells are represented by light- and dark-shaded symbols, respectively. (B) The similarities between mock and HA+ populations of AM, CD103+ DC, ATII, and CC were calculated using mutual information analysis and are represented in a heat map, whereby high mutual information indicates that the gene profiles between the cell types are highly correlated. Nats, natural unit of information. (C) The numbers of differentially expressed genes that are unique to and shared between mock AM, CD103+ DC, ATII, and CC from C57BL/6 mice are shown in a Venn diagram. (D) Enriched Panther Slim GO biological process terms were retrieved for differentially expressed genes unique to mock AM, CD103+ DC, ATII, and CC from C57BL/6 mice. Fold enrichment and significance of the enriched GO terms are presented as a scatterplot, including data for each of the four cell types. Note that the size of individual data points corresponds to the number of genes in the GO term.
We compared mock ATII, AM, CC, and CD103 cells to each other in a pairwise comparison, and differentially expressed genes unique to or shared between them were retrieved. Enrichment of GO terms for unique cell type-specific genes were then identified (Fig. 7C). Based on transcriptional analyses, the enriched GO terms corresponded to the known biological functions associated with each lung cell subset (Fig. 7D). For example, gene expression in AM was predominantly associated with immune functions, including immune system process (e.g., Toll-like receptor signaling pathway and pattern recognition receptor signaling pathway), immune response, and macrophage activation (Table S3). Enriched GO terms in CD103+ DC were also associated with immune functions, including immune system process, antigen processing and presentation, and intracellular signal transduction. Different GO terms were enriched in the epithelial cell subsets ATII (e.g., lipid metabolic process) and CC (e.g., cellular component movement) (Table S3). These data confirm that constitutive transcriptional responses in the immune and parenchymal cell subsets examined correlate with their known physiological functions in the airways.
Differences in the regulatory pathways modulating responses of AM and ATII to IAV infection.
Genes involved in the same functional pathways are often regulated by common transcription factors (TFs). We sought to identify the transcription factors that upregulated gene transcripts following IAV infection and to determine if the distinct functional responses of AM and ATII to infection extended to their regulatory circuitry. We used the predictive algorithm CiiiDER (see Materials and Methods) to predict the transcription factors involved in each cell type. As shown in Fig. 8A, IAV-infected HA+ AM and ATII shared significant enrichment of IFN regulatory factor 7 (IRF7), IRF1, IRF9, STAT1, STAT2, IRF2, IRF8, and other inflammatory TF binding sites (TFBSs) compared to their corresponding mock counterparts. Examination of all genes upregulated in HA+ AM and HA+ ATII also predicted that IRF7, IRF1, STAT1, and STAT2 could be differentially enriched between AM and ATII. Therefore, we applied this algorithm to the unique genes upregulated in HA+ ATII compared to those upregulated in HA+ AM and plotted the significantly enriched predicted transcription factors to the expression levels in HA+ ATII (Fig. 8B, top panel) and HA+ AM (Fig. 8B, bottom panel) detected by RNA-seq. Indeed, we found that IRF1, IRF2, IRF7, IRF8, IRF9, STAT1, and STAT2 were significantly enriched in upregulated genes unique to HA+ AM, whereas REL and NF-κB1 were enriched in upregulated genes unique to HA+ ATII cells.
FIG 8.
Differences in the regulatory pathways modulating responses of AM and ATII to IAV infection. (A) CiiiDER was used to predict the transcription factors that bound to all upregulated genes expressed in HA+ AM and ATII compared to the corresponding cell types from mock-infected animals. Enrichment (log2 transcription factor enrichment) and the average log2 proportion bound of predicted transcription factors are plotted. The size of each data point represents the −log10 P value. (B) Transcription factors that bound to upregulated genes unique to HA+ ATII (top panel) compared to the upregulated genes unique to HA+ AM (bottom panel) were predicted using CiiiDER. Predicted transcription factors were matched to the corresponding transcription factors identified by RNA-seq. The RNA-seq expression data (log2-fold change) were then plotted against the transcription factor enrichment (log2 enrichment). The size of the circle represents the −log10 P value. The fill color represents the false discovery rate (log10 FDR) of the RNA-seq expression data.
The transcriptional response of AM to IAV infection is largely dependent on IRF3/7 and IFNAR2.
IRF3/7 and IFN signaling are well-established regulators of intrinsic immunity and antiviral responses (15). Furthermore, our prediction yielded transcription factors linked to IRF and IFN pathways and suggested that AM and ATII utilized these pathways to different extents in response to IAV infection. Therefore, transcriptional responses were compared in AM and ATII subsets isolated from C57BL/6 mice, along with those isolated from IFNAR2−/− or IRF3/7−/− mice. In the absence of virus infection, ATII and AM from IRF3/7−/− and IFNAR2−/− mice showed few differences in gene expression (20 to 100 genes) compared to the corresponding cell subset from C57BL/6 mice (data not shown). Irrespective of the mouse strain, ATII and AM clustered with their respective counterparts, indicating that the absence of IFNAR2 or IRF3/7 did not result in a major change in global gene expression in the absence of infection (data not shown). It should be noted that at this time, HA+ ATII and AM isolated from infected IRF3/7−/− and IFNAR2−/− mice showed slightly elevated levels of viral transcripts compared to those from C57BL/6 mice (mean counts of all virus/all mouse gene transcripts ± standard errors [SE] for ATII of 116.95% ± 4.41% for C57BL/6, 136.01% ± 4.94% for IFNAR2−/−, and 149.29% ± 14.64% for IRF3/7−/− mice; mean counts ± SE for AM of 113.17% ± 6.07% for C57BL/6, 137.31% ± 3.29% for IFNAR2−/−, and 142.5% ± 7.64% for IRF3/7−/− mice).
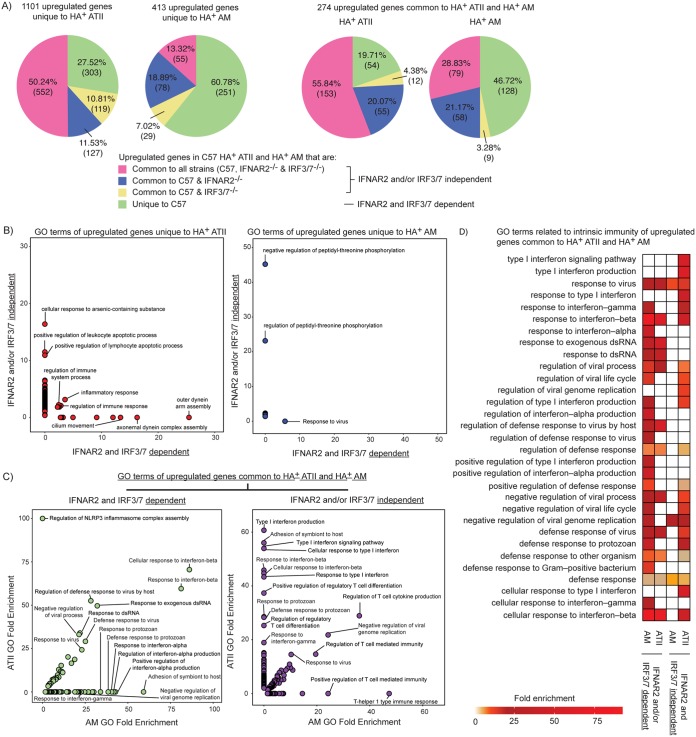
Next, we identified genes upregulated in response to IAV in lung cells from C57BL/6, IRF3/7−/−, and IFNAR2−/− mice. If upregulated genes were common to all mouse strains, or a combination of C57BL/6 and one knockout strain, we classified these genes to be independent of IRF3/7 and/or IFNAR2 regulation. If upregulated genes were unique to cells from C57BL/6 mice, they were classified as being dependent on IRF3/7 and IFNAR2 regulation. In IAV-infected HA+ ATII, 27.5% of unique and 19.7% of shared upregulated genes were dependent on either IRF3/7 or IFNAR2 (Fig. 9A). For IAV-infected HA+ AM, 60.8% of unique and 46.2% of shared upregulated genes were IRF3/7 and IFNAR2 dependent. This is consistent with our prediction that AM utilize type I IFN and IRF pathways to a greater degree than ATII in response to IAV.
FIG 9.
The transcriptional response of AM to IAV infection is largely dependent on IRF3/7 and IFNAR2. (A) Genes upregulated in only HA+ AM or HA+ ATII or upregulated in both cell types from C57BL/6 mice were matched to the genes from the corresponding cell types in IRF3/7−/− and IFNAR2−/− mice. Genes identified in cells from all three strains, or from IRF3/7 and C57BL/6 or from IFNAR2 and C57BL/6 mice, were classified as “IFNAR2 and/or IRF3/7 independent.” Genes identified only in C57BL/6 mice were classified as “IFNAR2 and IRF3/7 dependent.” Numbers and percentages of these genes are plotted in pie charts. (B) Significantly enriched GO terms from upregulated genes unique to HA+ ATII (left panel) and HA+ AM (right panel) were plotted based on whether the genes were independent of or dependent on IFNAR2 and IRF3/7. (C) Significantly enriched GO terms from upregulated genes common to HA+ ATII and HA+ AM were plotted based on whether gene upregulation was independent of or dependent on IFNAR2 and IRF3/7. dsRNA, double-stranded RNA. (D) GO terms related to the functional category “intrinsic immunity” were identified from the GO terms of upregulated genes common to HA+ ATII and HA+ AM. The fold enrichments of different GO terms are represented in a heat map.
We also examined the functional responses of the genes in relation to their dependence on IFNAR2 and IRF3/7. First, we examined the significantly enriched GO terms in upregulated genes that were unique to IAV-infected HA+ ATII or AM. Unique IRF3/7- and IFNAR2-dependent genes upregulated in HA+ ATII were significantly enriched in dynein-, microtubule-, and cilium-related GO terms (Fig. 9B, left panel). The only term that was significantly enriched in the unique IRF3/7- and IFNAR2-dependent genes expressed by HA+ AM was “response to virus” (Fig. 9B, right panel).
We then examined GO terms that were significantly enriched in upregulated genes common to IAV-infected HA+ ATII and AM. The majority of IRF3/7- and IFNAR2-dependent GO terms were significantly enriched in the upregulated shared genes related to cell-intrinsic immune responses (Fig. 9C, left panel). Of interest, regulation of the NLRP3 inflammasome complex was an IRF3/7- and IFNAR2-dependent function enriched in HA+ ATII but not HA+ AM. In contrast, enrichment of the IRF3/7- and IFNAR2-dependent terms IFN-α and IFN-α related was observed in HA+ AM but not HA+ ATII (Fig. 9C, left panel).
Among the GO terms enriched in the upregulated shared genes, regulation of T cell responses was independent of IRF3/7 and/or IFNAR2 in both cell types (Fig. 9C, right panel). ATII also activated type I IFN, IFN-β, and IFN-β-related responses in an IFNAR2- and/or IRF3/7-independent manner, and this was not observed in AM. To examine how ATII and AM differ in their dependence on IFNAR2 and IRF3/7 to activate intrinsic immunity, we retrieved the GO terms related to intrinsic immunity, as shown in Fig. 3. Intrinsic immunity responses were largely dependent on IFNAR2 and/or IRF3/7 in both HA+ AM and HA+ ATII (Fig. 9D). Some of the intrinsic immunity responses to IAV infection were also independent of IFNAR2 and IRF3/7 in ATII, whereas this was rarely the case in AM. Thus, activation of IRF3/7- and IFNAR2-regulated intrinsic immune pathways in response to IAV is conserved in AM and ATII. However, AM and ATII utilize these pathways differently following infection, with AM being more reliant on IRF3/7 and IFNAR2 regulation than ATII.
In contrast to ATII, the transcriptional profile of AM is poised to perform antiviral defense functions.
Thus far, our data showed that AM displayed an IRF3/7- and IFNAR2-dependent transcriptional response focused on defense and antiviral functions in the presence of IAV infection, and this distinct response is likely driven by the physiological function of AM as immune cells. To examine this further, expression profiles of defense genes in mock-infected AM and ATII from C57BL/6 mice were tabulated and compared to those of genes from IAV-infected AM and ATII (Fig. 10A). Mock-infected AM (204 genes) expressed nearly twice as many defense genes as mock-infected ATII (116 genes). When we then examined the same defense genes in IAV-infected cells, we found that ATII upregulated nearly twice as many (27.94%) defense genes that were expressed only in mock-infected AM (Fig. 10A, left panel, green shading) as infected AM, which upregulated 13.79% (right panel, yellow shading) of the defense genes expressed only in mock-infected ATII.
FIG 10.
In contrast to ATII, the transcriptional profile of AM is poised to perform antiviral defense functions. (A) The numbers of defense genes expressed constitutively in mock AM and ATII are subcategorized into those that were upregulated significantly after infection in (i) both HA+ AM and ATII, (ii) only HA+ AM, (iii) only HA+ ATII, or (iv) neither cell type. (B) Constitutive expressions of specific genes related to cell lineage, inflammation, and defense are plotted on scatterplots. Color demarcates which specific genes were highly expressed in mock AM, mock ATII, or neither cell type in the absence of infection. (C) Similar to panel B, but color demarcates which specific genes were significantly upregulated in (i) both HA+ AM and ATII, (ii) only HA+ AM, (iii) only HA+ ATII, or (iv) neither cell type, after infection.
We examined some of these defense genes, together with known lineage-specific genes that regulate the development of AM and ATII, as well as genes encoding particular phenotypic markers. The transcription factors PU.1 and peroxisome proliferator-activated receptor gamma (PPARg) regulate AM development (16), while TTF1, ETV5, Foxa1, and Foxa2 play similar roles for ATII (17). In accordance with data from previous studies, AM and ATII constitutively expressed their corresponding lineage-specific master regulators (AM, Spi1/PU.1 Pparg; ATII, Etv5, Foxa1, Foxa2, and Nkx2-1/TTF1) and phenotypic genes (AM, Mertk and Itgax/CD11c; ATII, Sftpa1/b/c/d and CD74) in the absence of infection (Fig. 10B). Mock-infected AM also expressed significantly higher levels of antiviral and inflammatory molecules (Tnf and Isg15), pathogen-associated molecular pattern (PAMP) and cytokine receptors (Tlr3, Tlr7, Dhx58 [LGP2], Mavs, Ripk1, Myd88, Il12rb2, Ifngr1, and Tnfrsf1b), and transcription factors (Stat1, Nfkb1, Rel, Cebpb, Irf8, and Irf4) than did mock-infected ATII (Fig. 10B). In the presence of IAV, most immunity-related genes were not upregulated in infected AM (Fig. 10C). In contrast, IAV-infected ATII upregulated Tnf, Il12br2, Rel, Nfkb1, and Tnfrsf1b, while upregulation of Isg15, Tlr3, Dhx58, and Stat1 was observed in both infected AM and ATII.
DISCUSSION
AM and ATII represent major targets of IAV infection in the lung. Under steady-state conditions, AM and ATII express distinct transcriptional activities, consistent with distinct physiological roles in the airways. Not surprisingly, AM and ATII also exhibited major differences in transcriptional responses following IAV infection.
Many studies have focused on defining host responses in bulk transcripts from tissues derived from IAV-infected animals, although few have addressed responses in specific subsets of airway cells (18). In addition, immortalized cell lines and/or primary cultures have been widely used to investigate transcriptional responses of airway epithelial and immune cells following in vitro infection with IAV. However, culture conditions are likely to have a major impact on transcriptional and other cellular responses, particularly in AM, which may be one factor contributing to differences noted between some studies (18). Isolation of lung cells from mock- versus IAV-infected mice therefore allows for accurate insight regarding transcriptional profiles of specific cell subsets ex vivo, although enzymatic digestion and cell sorting will no doubt have some impact on these responses. For example, the experimental approaches utilized were not appropriate to isolate sufficient numbers of ATI, a major epithelial subset in the distal lung that also supports productive replication by seasonal IAV (12).
Several recent studies have used different approaches to gain insight regarding the transcriptional responses in airway cells in response to influenza virus infection. Elegant experiments using single-cycle reporter viruses and mRNA-seq in CD45− CD31− “bulk” AEC isolated from IAV-infected mice revealed that uninfected AEC as well as AEC with low and high levels of virus replication all expressed unique IFN-stimulated gene (ISG) signatures (19). Studies reported by Hancock et al. focused on transcriptional responses in bulk ATII, but not other cell types, that were directly infected (defined by expression of green fluorescent protein [GFP]) or bystander cells isolated from mice 3 days after infection with PR8 or from uninfected mice (20). Another report used single-cell RNA sequencing to define a large degree of heterogeneity in the transcriptional responses of lung tissue cells isolated from IAV-infected mice 2 days after intranasal infection (21). The studies described here take a different approach, focusing on HA+ cells (i.e., late stage of virus infection only) at 8 to 10 h postinfection (i.e., prior to secondary spread in the airways) and exclude the contribution of uninfected bystander cells in the infected lung as well as cells supporting the early stages of virus infection and/or infected cells suppressing HA expression at the cell surface. Furthermore, our studies compare responses in specific AEC and immune cell subsets, whereas Hancock et al. examined only ATII (20). Moreover, the single-cell analyses reported by Steuerman et al. grouped cell populations more broadly into airway epithelial cells and cells of the mononuclear phagocyte system (DC, monocytes, and macrophages) (21). Of interest, few epithelial cells were captured during the single-cell analysis, even though lung epithelial cells represent the main targets of IAV infection (22). Furthermore, in single-cell analyses, “infected cells” were defined based on viral mRNA load, which is likely to include cells containing virus as a result of infectious or noninfectious virus entry as well as cells supporting only early stages of virus replication. This definition, combined with secondary spread in the airways, may account for the high prevalence (∼20 to 60%) of infection in each of the nine major cell types examined at day 2 postinfection (21), which is in contrast to the much lower prevalence of HA+ infected cells observed at 8 to 10 h in our studies. Thus, our use of defined parameters to examine transcriptional responses in specific lung cell subsets complements the unbiased approaches reported using single-cell RNA sequencing.
In response to a common stimulus (IAV infection), very different transcriptional responses were induced in AM and ATII. For example, in the absence of infection, AM constitutively express twice the number of defense genes as ATII; however, following infection, ATII upregulate three times more defense genes than AM (Fig. 10A). These results are consistent with recent findings from Hancock et al., who reported that antiviral and IFN response genes were strongly enriched in IAV-infected ATII isolated at day 3 postinfection (20). We propose that lineage-specific transcriptional architecture drives the distinct physiological functions of AM and ATII through expression of specific master regulators and genes. Thus, AM are poised to restrict IAV infection, whereas ATII elicit delayed antiviral responses. This, in turn, is likely to contribute to differences in the functional responses of each cell type to IAV. AM develop from fetal liver monocytes, which are derived from yolk sac erythromyeloid progenitors, whereas ATII develop from distal progenitors derived from anterior foregut endodermal cells (17, 23). Lineage-specific transcription factors (PU.1 and PPARg in AM [16] and TTF1, ETV5, Foxa1, and Foxa2 in ATII [17]), as well as epigenetic modifications (24, 25), drive the expression of genes associated with the distinct physiological functions of AM (i.e., immune-related and pathogen-sensing genes, among others [26]) and ATII (i.e., lipid processing and surfactant production genes, among others [9]). In accordance with these studies, we confirmed that AM and ATII expressed the corresponding transcription factor (AM, Spi1/PU.1 Pparg; ATII, Etv5, Foxa1, Foxa2, and Nkx2-1/TTF1) and phenotypic marker (AM, Mertk and Itgax/CD11c; ATII, Sftpa1/b/c/d and CD74) genes in the absence of infection (Fig. 10B and C) and that their cellular functions are consistent with their known physiological role in the airways (Fig. 7).
Consistent with the immune functions of AM, we found antiviral and inflammatory molecules (e.g., Tnf and Isg15), PAMP and cytokine receptors (e.g., Tlr3, Tlr7, Dhx58 [LGP2], Mavs, Ripk1, Myd88, Il12rb2, Ifngr1, and Tnfrsf1b), and transcription factors (e.g., Stat1, Nfkb1, Rel, Cebpb, Irf8, and Irf4) to be constitutively expressed at higher levels in AM than in ATII (Fig. 10B and C). The higher basal expression levels of these molecules are consistent with the notion that AM are poised to detect and respond quickly to pathogens without the need for nucleosome remodeling to initiate transcription and translation of these gene products. Studies have reported that PU.1 (master regulator of AM) interacts with some of the above-mentioned molecules (C/EBP [Cebp], IRF4, IRF8, and STAT1) to form transcription complexes that drive the basal transcription of primary response genes (i.e., those that do not require de novo protein synthesis) in the absence of infection (27–30). Others have demonstrated that the type I IFN enhanceosome (i.e., NF-κΒ–IRF1/3/7 complex) drives constitutive type I IFN production through the remodeling of nucleosomes, such that upon pathogen encounter, macrophages can rapidly amplify the IFN signal through STAT1/2-IRF7/9 (15, 31, 32). Indeed, we found that AM utilized IRF3/7- and IFNAR2-induced STAT1/STAT2 more extensively than did ATII (Fig. 8 and 9). In contrast to the immune readiness of AM, ATII may require upregulation of transcription factor genes following IAV infection before synthesis of antiviral and inflammatory gene products can occur. In support of this, our results showed that ATII upregulated NF-κΒ1 (p50), Rel (c-Rel), and Stat1 following IAV infection to levels similar to those observed in uninfected AM (Fig. 8 and Fig. 10C).
It is well known that IAV and other viruses hijack cellular machinery to facilitate viral replication and the release of newly synthesized viral progeny. Among these host factors, small interfering RNA (siRNA) knockdown studies have provided evidence that genes involved in kinase-mediated signaling, nucleocytoplasmic transport, mRNA processing, apoptosis induction, translational regulation, and protein ubiquitination play roles in facilitating IAV replication (reviewed in reference 33). This is further supported by comparative transcriptomic analyses in cells exposed to infectious versus inactivated IAV (34). In our studies, ATII cells also upregulated genes associated with nucleocytoplasmic transport, RNA (including rRNA and mRNA) processing, ribonucleoprotein complex biogenesis, and protein serine/threonine kinase and mitogen-activated protein kinase (MAPK) activities in response to IAV infection, whereas AM did not (Fig. 4). In contrast, both ATII and AM expressed genes related to apoptosis and protein ubiquitination in response to IAV infection (Fig. 3 and data not shown). Together, these findings suggest that besides the relative lack of inherent immune readiness to limit IAV infection, ATII may also respond to infection by upregulating specific cellular pathways (not upregulated in AM) that facilitate or promote IAV replication.
In contrast to seasonal IAV, some highly pathogenic avian influenza (HPAI) H5N1 viruses infect and replicate productively in AM (1), indicating that HPAI can bypass the antiviral modulation inherent in AM. These findings indicate that AM express all cellular factors and machinery to support the growth of some IAV strains. Moreover, they imply that AM may constitutively express and/or induce cellular restriction factors that block seasonal IAV replication, but these are overcome, evaded, or not induced during HPAI replication in macrophages. Alternatively, HPAI may exhibit different requirements regarding cellular machinery required for effective viral replication and release from infected AM. Currently, the molecular mechanisms underlying the ability of macrophages to block productive replication of seasonal IAV are not known. However, a number of host factors, such as Isg20 (35) and Adar (36) (Fig. 10), are highly expressed in AM but not ATII, and overexpression studies have implicated their potential to restrict IAV replication, at least in particular cell lines. Complementary approaches targeting particular host factors (expressed at high levels in AM but not ATII) for knockout/silencing in macrophages and for ectopic expression in epithelial cells will be the subject of future studies to define specific host restriction factors that block IAV replication in macrophages. Similar approaches could be utilized to investigate the role of host genes with “proviral” potential against seasonal IAV that are expressed at high levels in IAV-infected ATII but not in AM.
Overall, our study sheds light on how the different transcriptional architectures of airway cells from two different lineages drive transcriptional responses to IAV infection. Differences in constitutive and inducible antiviral activities between AM and ATII appear to be predefined by transcriptional regulatory networks, and our findings suggest that this is likely driven by lineage-dependent factors rather than signal-dependent factors. Comparison of the transcriptional circuitries between cells that are permissive and nonpermissive to productive replication by seasonal IAV represents an important step toward identifying particular cellular factors that may promote or restrict virus replication.
MATERIALS AND METHODS
Mice.
C57BL/6 mice, as well as IFNAR2−/− (37) and IRF3−/−/IRF7−/− (15) mice on a C57BL/6 background, were bred and maintained in the Bioresources Facility at The Peter Doherty Institute for Infection and Immunity. IRF3−/−/IRF7−/− mice were generously provided by T. Taniguchi (University of Tokyo).
Virus and infection.
IAV strain A/Puerto Rico/8/34 (PR8) (H1N1) was propagated in the allantoic cavities of 10- to 12-day-old embryonated eggs, and titers of infectious virus were determined by plaque assays on Madin-Darby canine kidney (MDCK) cells according to standard procedures (38). Mice were anesthetized with isoflurane and infected with 106 PFU via intranasal infection in a total volume of 50 μl. Control mice were mock infected with an equivalent dilution of allantoic fluid obtained from uninfected eggs. Mice were killed at 9 h p.i. unless otherwise stated. To determine titers of infectious virus, lungs from individual mice were homogenized in phosphate-buffered saline (PBS), clarified by centrifugation, and then assessed by plaque assays on MDCK monolayers.
Ethics statement.
All experiments with mice and embryonated eggs were conducted with approval from the Biochemistry & Molecular Biology, Dental Science, Medicine, Microbiology & Immunology, and Surgery Animal Ethics Committee at the University of Melbourne in accordance with the NHMRC Australian code of practice for the care and use of animals for scientific purposes. Embryonated eggs (10 to 12 days old) were provided by Seqirus, Parkville, Australia.
Flow cytometry and cell sorting.
Lungs were perfused with saline before lung lobes were retrieved, and single-cell suspensions were prepared via enzymatic digestion (100 μg/ml DNase I and 0.3 mg/ml Liberase TL [Roche/Sigma-Aldrich]) and mechanical disruption (18-gauge and 20 1/2-gauge needles), followed by treatment with 100 mU/ml neuraminidase from Vibrio cholerae (Sigma-Aldrich). Following red blood cell lysis, lung cell suspensions were incubated with Fc receptor block and then stained with appropriate antibody cocktails. Antibody-stained cells were analyzed using flow cytometry (BD LSRFortessa). Antibodies against CD45.2, Mar1, CD24, CD11c, CD103, CD64, CD326, Sca1, CD104, CD146, CD31, CD55, Gr1 (all from BioLegend), MerTK (clone AF591, polyclonal goat; R&D Systems), IA/IE (eBioscience, BD Pharmingen), CD11b (BD Pharmingen), HA of IAV strain PR8 (monoclonal antibody [mAb] E1.2, from Lorena Brown, Department of Microbiology and Immunology, University of Melbourne, conjugated in-house with a Dylight488 [Dy488] or Dylight650 antibody labeling kit from ThermoFisher Scientific), donkey anti-goat IgG(H+L) Alexa Fluor 488, and propidium iodide (all from ThermoFisher Scientific) were used for staining cells. For cell sorting, antibody-stained cells were negatively enriched via biotin-streptavidin depletion (Dynabeads biotin; ThermoFisher Scientific), prior to sorting (BD FACSAria III). The cocktail used for immune depletion included antibodies against CD3e, B220, CD19, NK1.1, Thy1.2, Gr1, Ter119 (all eBioscience), and Ly6C (BioLegend), and the cocktail used for parenchymal depletion included antibodies against CD31, LYVE1, CD106, CD34, Ter119 (all eBioscience), and CD102 (BioLegend). Antibody-labeled cells were depleted using Dynabeads biotin (ThermoFisher Scientific). After depletion, the remaining cells were labeled with a fluorescence-conjugated antibody cocktail. The immunostaining cocktail included antibodies against CD45.2-PacBlue, CD11c-phycoerythrin cyanine 7 (PECy7), CD24-BV605, CD64-allophycocyanin (APC) (all from BioLegend), CD11b-APCCy7 and IA/IE-PE (BD Pharmingen), and HA PR8 E1.2-Dy488. The parenchymal staining cocktail included antibodies against CD45.2-PacBlue, T1a-PECy7, CD326-APCCy7, Sca1-BV605, CD146-peridinin-chlorophyll protein cyanine 5.5 (PerCpCy5.5) (all from BioLegend), IA/IE-PE, and HA PR8 E1.2-Dy488. Streptavidin antibodies (streptavidin-APC from eBioscience and streptavidin-PerCpCy5.5 from BioLegend) and propidium iodide were used to gate out contaminating cells not depleted in the enrichment steps and dead cells, respectively.
RNA extraction, library preparation, and sequencing.
RNA was extracted from sorted cells using an RNeasy microkit (Qiagen), reverse transcribed, and amplified using a SMART-Seq v4 ultra-low-input RNA kit for sequencing (Clontech), after which cDNA libraries were made (Nextera XT library preparation; Illumina) and sequenced with a HiSeq 2500 sequencer (Illumina).
cDNA synthesis and qPCR.
Total extracted RNA was reverse transcribed into cDNA using Superscript Vilo (ThermoFisher Scientific). Quantitative PCR (qPCR) assays were performed using TaqMan gene expression assays (6-carboxyfluorescein [FAM]) and TaqMan fast advanced master mix on the StepOnePlus real-time PCR machine (all from ThermoFisher Scientific). Relative expression was calculated using the method (39).
Bioinformatics.
RNA-seq reads were aligned to mouse genome mm10 using STAR (40), summarized by gene (mm10 annotation from Ensembl) using featureCounts (41), before normalization and differential expression testing was conducted using edgeR (42). Genes with consistently low expression levels following normalization and testing were removed using the HTSFilter package (43). Four sets of comparisons were performed: (i) for every strain of mouse and cell type, we compared samples from IAV-infected mice to their counterparts from mock-infected mice; (ii) for every strain of mouse, we compared each cell type from mock-infected mice to each of the other mock-infected cell types isolated; (iii) for every strain of mouse, we compared each cell type from IAV-infected mice to the other infected cell types isolated; and (iv) for every cell type, we compared cell types from mock-infected C57BL/6 mice to the equivalent cell types from mock-infected IFNAR2−/− and IRF3/7−/− mice. Genes with a log2-fold change of ≥1 or ≤−1 with adjusted P values of ≤0.05 were considered to be differentially expressed to significant levels.
For the IAV genome, RNA-seq reads were aligned to a synthetic genome containing the 8 gene segments of the PR8 virus retrieved from the NCBI using TopHat (44). Aligned reads were assigned to the appropriate viral segments using the featureCounts function from the Bioconductor Rsubread package (37). Differential expression analysis was performed on normalized counts in every viral segment by the total (virus and mouse) number of reads in each sample with the limma package (45), treating every viral segment as a gene.
Data mining and visualization.
Principal-component analysis was performed on CPM using XLSTAT (Addinsoft SARL, USA). Eigenvectors were then visualized with XLSTAT-3Dplot (Addinsoft SARL, USA). Differentially expressed (DE) genes that were unique or shared between cell subsets were visualized using VennDIS (46). Pathway analysis was performed using the STRING database (47). Active interaction sources were obtained from “experiments,” “databases,” “coexpression,” and “cooccurrence,” with a minimum required interaction score of 0.7 (high confidence). Only DE genes obtained from the RNA-seq data were used to query the pathway analysis. Edges show the confidence, indicating the strength of the data support. Disconnected nodes are hidden from the network.
Gene Ontology (GO) biological process terms for individual genes in the query gene set were retrieved from the Panther classification system (release 15 July 2016) (48) and the GO ontology database (release 29 July 2016). The fold enrichment and significance for GO biological process and Panther Slim GO biological process terms of every query gene set were calculated using the Panther overrepresentation test (release 15 July 2016). Graphs, heat maps, and bubble charts were visualized using the ggplot2 R package and Prism (GraphPad). Mutual information (MI) analysis was performed using the infotheo R package. Mutual information determines the dependence between the transcriptomes of two cell subsets through relating the joint distribution of gene expression probes of one subset to another with the assumption that these two subsets are independent. If both subsets share no information (and hence are independent), then MI equals zero; if both subsets are identical, then their mutual information will be similar to each other. R version 3.5.0 was used.
Transcription factor prediction was performed using CiiiDER (L. J. Gearing, H. E. Cumming, R. Chapman, A. M. Finkel, I. B. Woodhouse, K. Luu, J. A. Gould, S. C. Forster, and P. J. Hertzog, manuscript in preparation) as described above. Briefly, CiiiDER uses a Java implementation of the MATCH algorithm (50) to predict transcription factor (TF) binding sites (TFBSs) in search regions and background regions through scanning of sequences using 2016 JASPAR CORE nonredundant vertebrate position frequency matrices (51), with a deficit of 0.15 (core and matrix similarity scores of ≥0.85).
Accession number(s).
The RNA-seq data have been deposited in the NCBI GEO database under accession number GSE115904.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by project grant APP1044976 and program grant APP1071916 from the National Health and Medical Research Council (NHMRC) of Australia. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01986-18.
REFERENCES
- 1.Cline TD, Beck D, Bianchini E. 2017. Influenza virus replication in macrophages: balancing protection and pathogenesis. J Gen Virol 98:2401–2412. doi: 10.1099/jgv.0.000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tate MD, Brooks AG, Reading PC. 2011. Correlation between sialic acid expression and infection of murine macrophages by different strains of influenza virus. Microbes Infect 13:202–207. doi: 10.1016/j.micinf.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls JM, Bourne AJ, Chen H, Guan Y, Peiris JS. 2007. Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir Res 8:73. doi: 10.1186/1465-9921-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Londrigan SL, Tate MD, Brooks AG, Reading PC. 2012. Cell-surface receptors on macrophages and dendritic cells for attachment and entry of influenza virus. J Leukoc Biol 92:97–106. doi: 10.1189/jlb.1011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tate MD, Schilter HC, Brooks AG, Reading PC. 2011. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol 24:77–88. doi: 10.1089/vim.2010.0118. [DOI] [PubMed] [Google Scholar]
- 6.Julkunen I, Melen K, Nyqvist M, Pirhonen J, Sareneva T, Matikainen S. 2000. Inflammatory responses in influenza A virus infection. Vaccine 19:S32–S37. doi: 10.1016/S0264-410X(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 7.Sprenger H, Meyer RG, Kaufmann A, Bussfeld D, Rischkowsky E, Gemsa D. 1996. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J Exp Med 184:1191–1196. doi: 10.1084/jem.184.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQualter JL, Yuen K, Williams B, Bertoncello I. 2010. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A 107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, Quake SR. 2014. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509:371–375. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. 2012. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. 2011. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol 29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberger CM, Podyminogin RL, Askovich PS, Navarro G, Kaiser SM, Sanders CJ, McClaren JL, Tam VC, Dash P, Noonan JG, Jones BG, Surman SL, Peschon JJ, Diercks AH, Hurwitz JL, Doherty PC, Thomas PG, Aderem A. 2014. Characterization of innate responses to influenza virus infection in a novel lung type I epithelial cell model. J Gen Virol 95:350–362. doi: 10.1099/vir.0.058438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. 2008. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J Exp Med 205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilley AE, Walters MS, Shaykhiev R, Crystal RG. 2015. Cilia dysfunction in lung disease. Annu Rev Physiol 77:379–406. doi: 10.1146/annurev-physiol-021014-071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honda K, Takaoka A, Taniguchi T. 2006. Type I inteferon [sic] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Lavin Y, Mortha A, Rahman A, Merad M. 2015. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol 15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herriges M, Morrisey EE. 2014. Lung development: orchestrating the generation and regeneration of a complex organ. Development 141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell JD, Waters KM. 2017. Influenza-omics and the host response: recent advances and future prospects. Pathogens 6:E25. doi: 10.3390/pathogens6020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjaastad LE, Fay EJ, Fiege JK, Macchietto MG, Stone IA, Markman MW, Shen S, Langlois RA. 2018. Distinct antiviral signatures revealed by the magnitude and round of influenza virus replication in vivo. Proc Natl Acad Sci U S A 115:9610–9615. doi: 10.1073/pnas.1807516115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock AS, Stairiker CJ, Boesteanu AC, Monzon-Casanova E, Lukasiak S, Mueller YM, Stubbs AP, Garcia-Sastre A, Turner M, Katsikis PD. 2018. Transcriptome analysis of infected and bystander type 2 alveolar epithelial cells during influenza A virus infection reveals in vivo Wnt pathway downregulation. J Virol 92:e01325-18. doi: 10.1128/JVI.01325-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steuerman Y, Cohen M, Peshes-Yaloz N, Valadarsky L, Cohn O, David E, Frishberg A, Mayo L, Bacharach E, Amit I, Gat-Viks I. 2018. Dissection of influenza infection in vivo by single-cell RNA sequencing. Cell Syst 6:679.e4–691.e4. doi: 10.1016/j.cels.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders CJ, Vogel P, McClaren JL, Bajracharya R, Doherty PC, Thomas PG. 2013. Compromised respiratory function in lethal influenza infection is characterized by the depletion of type I alveolar epithelial cells beyond threshold levels. Am J Physiol Lung Cell Mol Physiol 304:L481–L488. doi: 10.1152/ajplung.00343.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. 2013. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marconett CN, Zhou B, Rieger ME, Selamat SA, Dubourd M, Fang X, Lynch SK, Stueve TR, Siegmund KD, Berman BP, Borok Z, Laird-Offringa IA. 2013. Integrated transcriptomic and epigenomic analysis of primary human lung epithelial cell differentiation. PLoS Genet 9:e1003513. doi: 10.1371/journal.pgen.1003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L, Jacome-Galarza CE, Handler K, Klughammer J, Kobayashi Y, Gomez-Perdiguero E, Schultze JL, Beyer M, Bock C, Geissmann F. 2016. Specification of tissue-resident macrophages during organogenesis. Science 353:aaf4238. doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escoubet-Lozach L, Benner C, Kaikkonen MU, Lozach J, Heinz S, Spann NJ, Crotti A, Stender J, Ghisletti S, Reichart D, Cheng CS, Luna R, Ludka C, Sasik R, Garcia-Bassets I, Hoffmann A, Subramaniam S, Hardiman G, Rosenfeld MG, Glass CK. 2011. Mechanisms establishing TLR4-responsive activation states of inflammatory response genes. PLoS Genet 7:e1002401. doi: 10.1371/journal.pgen.1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivashkiv LB. 2013. Epigenetic regulation of macrophage polarization and function. Trends Immunol 34:216–223. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang D, Zhang DE. 2011. Interferon-stimulated gene 15 and the protein ISGylation system. J Interferon Cytokine Res 31:119–130. doi: 10.1089/jir.2010.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langlais D, Barreiro LB, Gros P. 2016. The macrophage IRF8/IRF1 regulome is required for protection against infections and is associated with chronic inflammation. J Exp Med 213:585–603. doi: 10.1084/jem.20151764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smale ST. 2010. Selective transcription in response to an inflammatory stimulus. Cell 140:833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol 14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stertz S, Shaw ML. 2011. Uncovering the global host cell requirements for influenza virus replication via RNAi screening. Microbes Infect 13:516–525. doi: 10.1016/j.micinf.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiss GK, An MC, Bumgarner RE, Hammersmark E, Cunningham D, Katze MG. 2001. Global impact of influenza virus on cellular pathways is mediated by both replication-dependent and -independent events. J Virol 75:4321–4331. doi: 10.1128/JVI.75.9.4321-4331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu H, Li J, Yang L, Sun L, Liu W, He H. 2016. Influenza A virus-induced expression of ISG20 inhibits viral replication by interacting with nucleoprotein. Virus Genes 52:759–767. doi: 10.1007/s11262-016-1366-2. [DOI] [PubMed] [Google Scholar]
- 36.Ward SV, George CX, Welch MJ, Liou LY, Hahm B, Lewicki H, de la Torre JC, Samuel CE, Oldstone MB. 2011. RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proc Natl Acad Sci U S A 108:331–336. doi: 10.1073/pnas.1017241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fenner JE, Starr R, Cornish AL, Zhang JG, Metcalf D, Schreiber RD, Sheehan K, Hilton DJ, Alexander WS, Hertzog PJ. 2006. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol 7:33–39. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 38.Anders EM, Hartley CA, Jackson DC. 1990. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci U S A 87:4485–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 42.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rau A, Gallopin M, Celeux G, Jaffrezic F. 2013. Data-based filtering for replicated high-throughput transcriptome sequencing experiments. Bioinformatics 29:2146–2152. doi: 10.1093/bioinformatics/btt350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trapnell C, Pachter L, Salzberg SL. 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ignatchenko V, Ignatchenko A, Sinha A, Boutros PC, Kislinger T. 2015. VennDIS: a JavaFX-based Venn and Euler diagram software to generate publication quality figures. Proteomics 15:1239–1244. doi: 10.1002/pmic.201400320. [DOI] [PubMed] [Google Scholar]
- 47.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. 2015. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD. 2016. PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Res 44:D336–D342. doi: 10.1093/nar/gkv1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reference deleted.
- 50.Kel AE, Gossling E, Reuter I, Cheremushkin E, Kel-Margoulis OV, Wingender E. 2003. MATCH: a tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res 31:3576–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathelier A, Fornes O, Arenillas DJ, Chen CY, Denay G, Lee J, Shi W, Shyr C, Tan G, Worsley-Hunt R, Zhang AW, Parcy F, Lenhard B, Sandelin A, Wasserman WW. 2016. JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res 44:D110–D115. doi: 10.1093/nar/gkv1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.