Sustained remission of HIV infection is prevented by a persistent reservoir of latently infected cells capable of reinitiating systemic infection and viremia. To evaluate strategies to reactivate and deplete this reservoir, we developed and characterized a new humanized mouse model consisting of highly immunodeficient mice intrasplenically injected with peripheral blood mononuclear cells from long-term ART-suppressed HIV-infected donors. Reactivation and dissemination of HIV infection was visualized in the mouse spleens in parallel with the onset of viremia. The applicability of this model for evaluating reservoir depletion treatments was demonstrated by establishing, through delayed time to viremia and phylogenetic analysis of plasma virus, that treatment of these humanized mice with a broadly neutralizing antibody, 10-1074, depleted the patient-derived population of latently infected cells. This mouse model represents a new in vivo approach for the preclinical evaluation of new HIV cure strategies.
KEYWORDS: HIV, latent infection, reservoir
ABSTRACT
Curing HIV infection has been thwarted by the persistent reservoir of latently infected CD4+ T cells, which reinitiate systemic infection after antiretroviral therapy (ART) interruption. To evaluate reservoir depletion strategies, we developed a novel preclinical in vivo model consisting of immunodeficient mice intrasplenically injected with peripheral blood mononuclear cells (PBMC) from long-term ART-suppressed HIV-infected donors. In the absence of ART, these mice developed rebound viremia which, 2 weeks after PBMC injection, was 1,000-fold higher (mean = 9,229,281 HIV copies/ml) in mice injected intrasplenically than in mice injected intraperitoneally (mean = 6,838 HIV copies/ml) or intravenously (mean = 591 HIV copies/ml). One week after intrasplenic PBMC injection, in situ hybridization of the spleen demonstrated extensive disseminated HIV infection, likely initiated from in vivo-reactivated primary latently infected cells. The time to viremia was delayed significantly by treatment with a broadly neutralizing antibody, 10-1074, compared to treatment with 10-1074-FcRnull, suggesting that 10-1074 mobilized Fc-mediated effector mechanisms to deplete the replication-competent reservoir. This was supported by phylogenetic analysis of Env sequences from viral-outgrowth cultures and untreated, 10-1074-treated, or 10-1074-FcRnull-treated mice. The predominant sequence cluster detected in viral-outgrowth cultures and untreated mouse plasma was significantly reduced in the plasma of 10-1074-treated mice, whereas two new clusters emerged that were not detected in viral-outgrowth cultures or plasma from untreated mice. These new clusters lacked mutations associated with 10-1074 resistance. Taken together, these data indicated that 10-1074 treatment depletes the reservoir of latently infected cells harboring replication competent HIV. Furthermore, this mouse model represents a new in vivo approach for the preclinical evaluation of new HIV cure strategies.
IMPORTANCE Sustained remission of HIV infection is prevented by a persistent reservoir of latently infected cells capable of reinitiating systemic infection and viremia. To evaluate strategies to reactivate and deplete this reservoir, we developed and characterized a new humanized mouse model consisting of highly immunodeficient mice intrasplenically injected with peripheral blood mononuclear cells from long-term ART-suppressed HIV-infected donors. Reactivation and dissemination of HIV infection was visualized in the mouse spleens in parallel with the onset of viremia. The applicability of this model for evaluating reservoir depletion treatments was demonstrated by establishing, through delayed time to viremia and phylogenetic analysis of plasma virus, that treatment of these humanized mice with a broadly neutralizing antibody, 10-1074, depleted the patient-derived population of latently infected cells. This mouse model represents a new in vivo approach for the preclinical evaluation of new HIV cure strategies.
INTRODUCTION
Despite years of antiretroviral therapy (ART), a very long-lived population of HIV-infected CD4+ memory T cells harboring transcriptionally inactive HIV proviruses persists and rapidly reintroduces systemic HIV infection within weeks after the cessation of ART (1–3). The effectiveness of treatments to reduce the latent reservoir (LR) can be evaluated by using the in vitro quantitative viral outgrowth assay (QVOA), the standard assay used to quantify the LR in infected individuals, to compare pretreatment and posttreatment LRs (4, 5). However, the predicative value of the QVOA to document a treatment-induced reduction in the LR is limited by its capacity to measure only a fraction of the population of replication-competent proviruses in the LR (1). Consequently, the most definitive approach for evaluating successful LR depletion is to demonstrate significant delay or absence of viral rebound after analytical treatment interruption (ATI) (6). Strategies such as “shock and kill” are being evaluated for their capacity to deplete the LR by reactivating and eliminating latently infected cells and thereby inducing sustained remission, which may lead to a functional cure of HIV-infected individuals (7). However, these approaches have so far been minimally effective in clinical trials, likely because only a fraction of latently infected cells are reactivated by the latency reversing agents (LRAs) used (8, 9), and the anti-HIV immune response in the treated individuals was incapable of eliminating the reactivated cells (10–14).
One preclinical model used to evaluate the efficacy of new and more effective shock and kill strategies was NOD-Rag1null IL2rgnull mice transplanted with human hematopoietic stem cells (HSCs) derived from fetal liver which, after becoming populated with human T cells, are infected with HIV and then treated with a short ART course or a combination of three broadly neutralizing antibodies (bNAbs) to suppress viremia (15). However, after only a short course of ART, the reservoirs generated in that humanized mouse model are most likely labile with a half-life of days to weeks rather than the more stable latent reservoir of chronic infection, which has a half-life of approximately 4 years (16). While newer bone marrow, liver, thymus (BLT)-humanized mouse models constructed using graft-versus-host-resistant C57BL/6 Rag2−/− γc−/− CD47–/– (TKO) mice treated with ART for 18 weeks may provide an improved model for studying HIV latency (17), results from studies using this mouse model may not predict the effectiveness of strategies to activate and deplete the stable LR of patients treated with suppressive ART for several years. In addition, these humanized mouse models infected with HIV do not permit the molecular and functional characterization of the HIV produced by reactivated latently infected cells present in long-term HIV-infected individuals.
In vitro evaluation of latency reversing agents using latently infected CD4+ T cells from ART-suppressed HIV-infected patients are more predictive of the in vivo efficacy of these strategies than studies performed using latently HIV-infected T cell lines or ex vivo generated latently infected T cells (18). Consequently, mice populated with CD4+ T cells from ART-suppressed HIV-infected patients would be an attractive preclinical model to evaluate the efficacy of strategies to deplete the LR. Previous studies have reported that viremia can be detected after PBMC or CD4+ T cells from HIV-infected individuals with undetectable viral loads were intraperitoneally injected into highly immunodeficient NOD-SCID-IL-2Rγ−/− (NSG) mice (19) or humanized mice reconstituted with human T cells after transplantation with human CD34+ HSCs (20). This approach, termed a murine viral outgrowth assay (MVOA), was proposed as a more sensitive viral outgrowth assay to detect replication competent HIV in CD4+ T cells from HIV-positive individuals from whom virus was not detectable by standard QVOA. We postulated that we could apply a divergent strategy to develop a new preclinical mouse model which rapidly develops viremia to directly evaluate the impact of treatments to deplete the LR. We posited that this model would also enable us to investigate mechanisms of in vivo reservoir activation and depletion by performing phenotypic and sequence analysis of plasma virus and visualizing at a cellular level the reactivation of the LR and dissemination of infection within the lymphoid tissues. We hypothesized that direct introduction of the peripheral blood mononuclear cells (PBMC) from ART-suppressed donors into the spleen would permit more rapid and robust viremia than the intraperitoneal route used in the MVOA models. The intrasplenic route would also provide a lymphoid environment wherein the reactivated latently and productively infected CD4+ T cells would interact with NK cells and other effector cells present in the injected PBMC, enabling us to evaluate the capacity of these effector cells to mobilize FcR-mediated responses, such as antibody-dependent cellular cytotoxicity (ADCC), to deplete the reservoir.
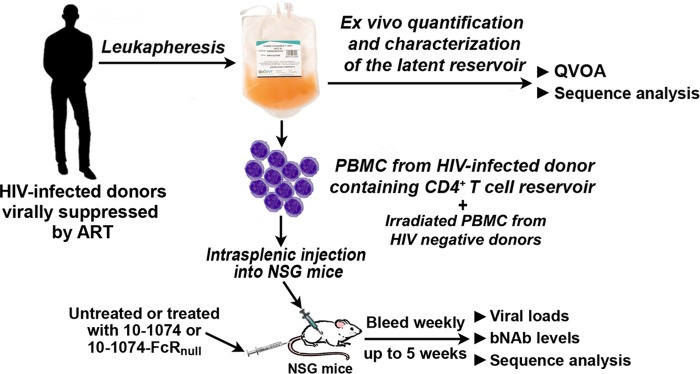
This proposed strategy of intrasplenically injecting NSG mice with PBMC obtained from HIV-infected individuals virally suppressed by long-term ART, which we termed Splenic-injected Primary HIV-Infected Reservoir (SPHIR) mice, built upon our previous experience using a humanized mouse model consisting of NSG mice intrasplenically injected with activated human PBMC and HIV to study NK cell-mediated inhibition of acute HIV infection (21, 22). We demonstrate here that the SPHIR mouse spleens provide a lymphoid environment wherein latently infected cells from an ART-suppressed donor were rapidly reactivated to produce replication-competent virus which rapidly disseminated infection to human T cells in the spleen which led to viremia within 1 week of intrasplenic injection. The emergence of viremia after intrasplenic injection of PBMC from ART-suppressed donors in mice not treated with ART mirrors the course of treatment interruption in virally suppressed HIV-infected individuals (23). Furthermore, we observed that treatment of the SPHIR-mice with a bNAb, 10-1074, significantly delayed the time to the development of viremia (time-to-viremia) and depleted the dominant population of HIV emerging from the reactivated LR, indicating that bNAb treatment could deplete the LR through Fc-mediated effector mechanisms.
RESULTS
Intrasplenic injection into NSG mice enables rapid in vivo reactivation of PBMC from ART-suppressed HIV-infected donors and robust dissemination of HIV infection.
To evaluate the efficacy of treatments to deplete the LR and prevent the recrudescence of infection, we developed an in vivo model to recapitulate the earliest stages of LR activation and recurrence of viremia observed after treatment interruption of HIV-infected individuals. We hypothesized that direct intrasplenic injection of PBMC from HIV-infected individuals into the lymphoid tissue environment of the mouse spleen would more rapidly reactivate latently HIV-infected cells present in the PBMC and cause more robust productive infection as quantified by plasma viremia, than injection by the intraperitoneal or intravenous route. To investigate this, we compared the timing and amplitude of viremia after NSG mice (5 mice/group) were either intrasplenically, intraperitoneally, or intravenously injected with PBMC from an HIV-infected donor (OM5001) who was virally suppressed by ART for >100 months and has a high LR, as measured by QVOA, of 10 infectious units/106 (IUPM) CD4+ T cells (Table 1). We monitored the reactivation of the latently HIV-infected cells and production of infectious HIV by quantifying viremia in the mouse plasma weekly as performed in clinical treatment interruption studies. By 2 weeks after injection, viremia was detected in all five of the intrasplenically injected mice compared to four of five intraperitoneally injected mice and one of five intravenously injected mice. The level of viremia was significantly higher (mean = 9,229,281 HIV copies/ml) in the intrasplenically injected mice than in the intraperitoneally injected mice (P = 0.034, mean = 6,838 HIV copies/ml) and the intravenously injected mice (P = 0.029, mean = 591 HIV copies/ml) (Fig. 1). The more robust viremia after intrasplenic injection may be due to the presence of a greater concentration of human CD4+ T cells in the densely packed structure of the spleen enabling increased cell-to-cell HIV transmission after intrasplenic injection compared to other injection routes and to the more direct communication of the spleen with the bloodstream, which may facilitate more efficient and increased export into the circulatory system of HIV produced by infected cells in the spleen.
TABLE 1.
Characteristics of study participantsa
| Participant ID | Age (yr) | Gender | ART regimen | Duration of viral load <50 copies/ml (mo) | Time to initiation of ART (mo) | IUPM |
|---|---|---|---|---|---|---|
| OM5001 | 43 | M | DTG/FDC/TDF | >100 | 14 | 10.5 |
| B004 | 63 | F | FTC/TDF/EFV | >20 | – | 2.8 |
| OM5334 | 32 | M | EVG/FTC/COBI/TAF/RPV | 32 | 2 | 1.7 |
| OM5148 | 47 | M | NVP/KVX | 105 | 57 | 1.0 |
The age, gender, ART regimen (ABC, abacavir; COBI, cobicistat; DTG, dolutegravir; EFV, efavirenz; EVG, elvitegravir; FTC, emtricitabine; KVX, abacavir/3TC; NVP, nevirapine; RAL, raltegravir; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate), time viremia was suppressed, and time from diagnosis to initiation of ART are listed for each donor. In addition, the latent replication-competent HIV reservoir in the PBMC of each donor was quantified by QVOA and is reported as infectious units/million CD4+ T cells (IUPM). –, unknown.
FIG 1.
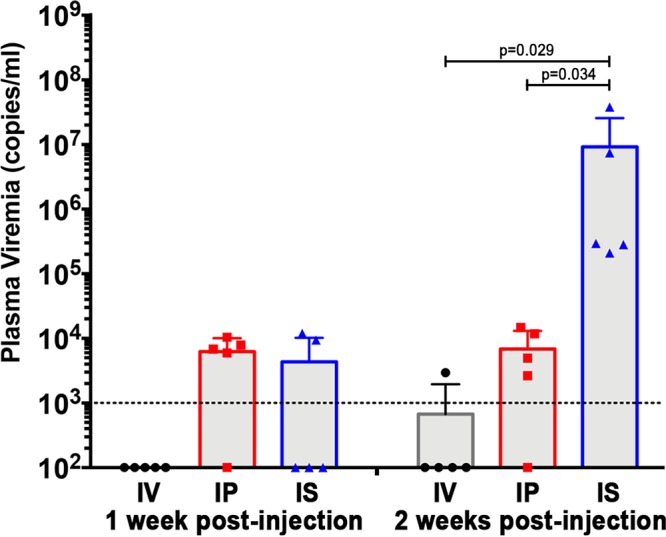
Comparison of the onset of viremia after intrasplenic, intraperitoneal, and intravenous injection of HIV+ donor PBMC into NSG mice. The viral loads in the plasma of NSG mice (n = 5 mice/group) 1 week and 2 weeks after coinjection of PBMC from an ART-suppressed HIV+ donor (30 × 106 cells) and irradiated allogeneic PBMC (6 × 106 cells) by either intrasplenic, intraperitoneal, or intravenous injection. The data are presented as a dot plot graph of the viral load for each mouse, and the group means ± the standard deviations (SD) for each group and time point are shown. The dotted line represents the detection limit of 1,000 copies/ml.
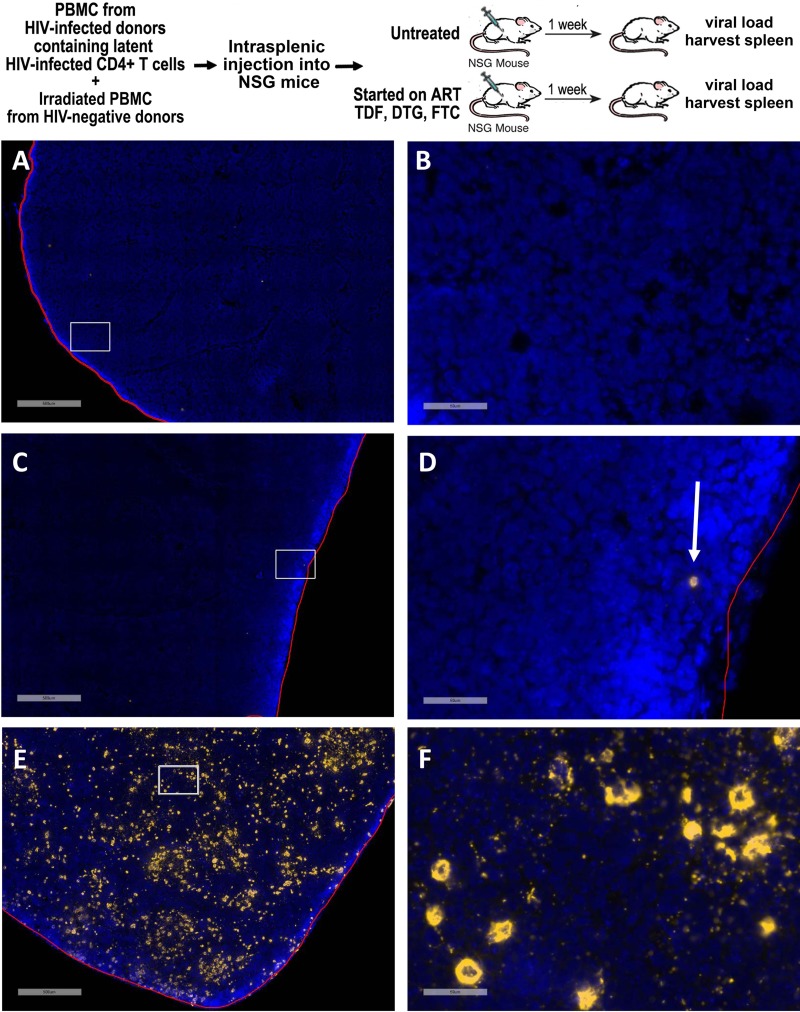
We further evaluated the rapid reactivation of the latently infected cells onset of viremia by using RNAscope in situ hybridization to visualize HIV-RNA+ cells in the spleen (24, 25) 1 week after intrasplenic injection of NSG mice with PBMC from an ART-suppressed HIV-infected donor with a high reservoir. Because suppressive ART should prevent the infection of new cells, we postulated that HIV RNA+ T cells visualized in spleens from SPHIR mice treated with suppressive ART would represent reactivated latently HIV-infected T cells, while HIV RNA+ T cells in untreated SPHIR mice would also include the population of CD4+ T cells infected by HIV produced by the reactivated latently HIV-infected cells. HIV RNA+ cells were not detected in a spleen harvested from a negative-control NSG mouse, demonstrating the specificity of the HIV-specific RNAscope in situ hybridization (Fig. 2A and B). No viremia was detected in the ART-treated NSG mice 1 week after intrasplenic injection of PBMC from an ART-suppressed HIV-infected donor, while rare HIV RNA+ cells (0.04 HIV RNA+ cells/mm2) were visualized in its spleen, likely representing reactivated latently infected cells. (Fig. 2C and D). In contrast to the ART-treated mice, marked viremia (394,330 HIV RNA copies/ml) was detected in an untreated mouse 1 week after intrasplenic injection with PBMC from the same ART-suppressed HIV+ donor, and the number of HIV RNA+ cells in the spleen was ∼3,000-fold higher (121 HIV RNA+ cells/mm2) (Fig. 2E and F). This likely represents the additional CD4+ T cells rapidly infected by HIV produced by the reactivated latently HIV-infected cells in the absence of ART suppression.
FIG 2.
Visualization of latency reactivation and spread of infection in the SPHIR mouse spleens. The experimental protocol is summarized in the top panel. OM5001 SPHIR mice generated by intrasplenic injection of NSG mice with OM5001 PBMC (30 × 106) and irradiated allogeneic PMBC (6 × 106) were either treated with daily intraperitoneal injections of tenofovir disoproxil fumarate, emtricitabine, and dolutegravir (n = 2 mice) or left untreated (n = 1 mouse). One week later, spleen sections were analyzed by using RNAscope for HIV RNA expression (gold) and counterstained with DAPI (blue). (A to F) Representative images of a control NSG mouse spleen at ×4 magnification (A), with the highlighted box shown at ×40 magnification (B); an ART-treated OM5001 SPHIR mouse spleen at ×4 magnification (C), with the highlighted box shown at ×40 magnification and with an HIV RNA-positive cell indicated by an arrow (D); and an untreated OM5001 NSG mouse spleen at ×4 magnification (E), with the highlighted box shown at ×40 magnification (F). Scale bars: A, C, and E, 500 μm; B, D, and F, 50 μm.
Treatment with the bNAb 10-1074 depletes the HIV latent reservoir by mobilizing Fc-mediated effector responses.
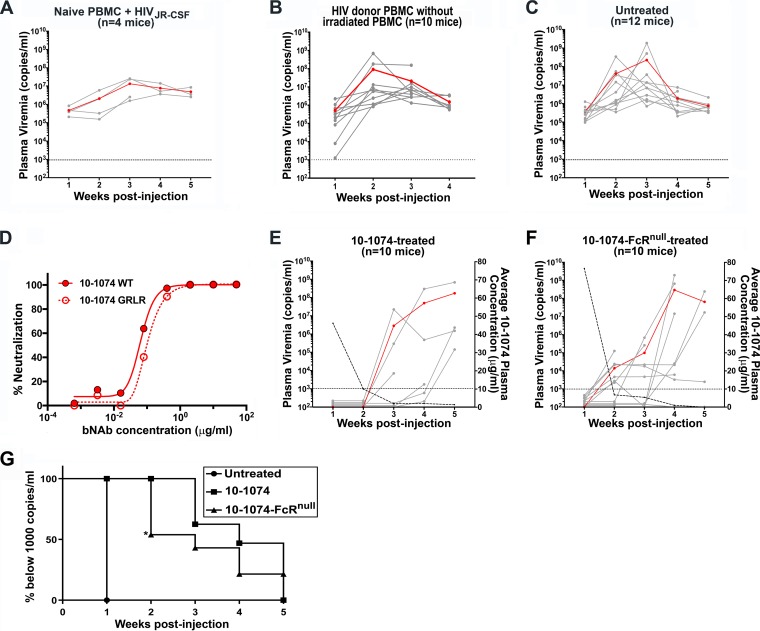
Studies in humanized mice, macaques, and HIV-infected patients have indicated that treatment with bNAb may reduce the size of the LR, as indicated by absent or delayed rebound viremia after treatment interruption (15, 26–30). The reduced capacity of FcRnull bNAbs to delay viral rebound in humanized mice infected with HIV indicated that Fc-mediated effector mechanisms are utilized by bNAbs to reduce the LR size (15). The human monoclonal antibody 10-1074, which targets the V3 supersite of HIV Env, one of the most potent bNAbs isolated, suppressed viremia in HIV-infected individuals and can mediate potent in vitro ADCC activity (31, 32). As outlined in the experimental protocol (Fig. 3), we postulated that the SPHIR mice would provide a preclinical model to determine whether mobilization of Fc-mediated effector responses by bNAb treatment is capable of targeting and eliminating the reactivated replication-competent latently infected T cells present in HIV-infected individuals. To control for donor and temporal variability, PBMC were obtained from donor OM5001 by leukapheresis, divided into aliquots, and frozen to provide sufficient PBMC for the injection of multiple mice with PBMC from the same donor obtained at the same time. As a positive control for the development of viremia after intrasplenic injection of HIV-infected PBMC, NSG mice were intrasplenically injected with PBMC from an HIV-negative donor, a mix of irradiated allogeneic PBMC, and HIVJR-CSF generated from an HIVJR-CSF infectious molecular plasmid (33). By 1 week after intrasplenic injection, pronounced viremia, which ranged from 105 to 106 HIV RNA copies/ml, was detected in all four NSG mice intrasplenically coinjected with HIV-naive PBMC and HIVJR-CSF (Fig. 4A). Irradiated PBMC are routinely added to in vitro QVOA assays to stimulate HIV production by latently infected cells (5). To optimize the SPHIR model, we investigated the effect of intrasplenic coinjection of irradiated PBMC from HIV-negative donors with PBMC from HIV-infected donor OM5001 on reactivation of latently infected cells and the development of viremia. We saw viremia at 1 week in all of the NSG mice (n = 10 mice) intrasplenically injected with OM5001 donor PBMC alone (Fig. 4B) and in all of the NSG mice (n = 12 mice) intrasplenically coinjected with OM5001 donor PBMC and with irradiated HIV-negative PBMC (Fig. 4C). However, because the level of viremia in two of the mice intrasplenically injected only with OM5001 donor PBMC was <10,000 copies/ml and because the levels of viremia 1 week after intrasplenic injection were more consistent when the NSG mice were also coinjected with irradiated HIV-negative PBMC, we chose to coinject irradiated HIV-negative PBMC with the HIV donor PBMC for subsequent experiments.
FIG 3.
Experimental protocol for construction of SPHIR mice and treatment studies. PBMC from HIV-infected donors virally suppressed by ART (<50 copies/ml) for >20 months were obtained by leukapheresis and intrasplenically injected into NSG mice with irradiated allogeneic PBMC from HIV-naive donors. Some mice were intravenously injected with bNAb 10-1074 or bNAb 10-1074-FcRnull at the time of intrasplenic injection (0.5 mg/mouse). The mice were bled weekly for viral load quantification and sequence analysis.
FIG 4.
Construction of SPHIR mice and treatment with 10-1074 or 10-1074-FcRnull. (A) Plasma HIV RNA levels (copies/ml) in individual NSG mice were measured at weekly intervals after intrasplenic coinjection of irradiated allogeneic PBMC (6 × 106 cells) and 30 × 106 of HIV-naive PBMC and HIVJR-CSF (∼1 × 106 IU/3 × 107 PBMC). (B and C) NSG mice were intrasplenically injected with OM5001 PBMC (30 × 106) with either no added irradiated allogeneic PMBCs (B) or coinjected with irradiated allogeneic PMBCs (6 × 106) from three HIV-naive donors (C). HIV loads in the plasma were measured in weekly bleeds and are indicated by solid gray lines, with the geometric mean of plasma viremia indicated by a red line (scale on the left axis). The dotted line represents detection limit of 1,000 copies/ml. (D) The neutralization activity of 10-1074 and 10-1074-FcRnull was evaluated by ex vivo neutralization of infection of TZM-bl cells with a 10-1074-sensitive subtype B virus, X2278, with the indicated concentrations of 10-1074 and 10-1074-FcRnull. (E and F) Mice were intrasplenically coinjected with OM5001 PBMC (30 × 106) with irradiated allogeneic PMBC (6 × 106) from three HIV-naive donors and intravenously treated with one dose (0.5 mg) of either unmodified 10-1074 (E) or 10-1074-FcRnull (F). The mice were bled weekly to measure HIV loads in the plasma and the 10-1074 or 10-1074-FcRnull plasma concentrations. The plasma viral loads of individual mice are indicated by solid gray lines, with the geometric mean of plasma viremia indicated by a red line (scale on left axis), and the dotted line represents detection limit of 1,000 copies/ml. The average plasma antibody concentration in the mice is indicated by the dashed line (scale on the right axis). (G) Kaplan-Meier plots summarizing the time-to-viremia data. The percentage of mice with undetectable viremia after intrasplenic injection with OM5001 PBMC and irradiated allogeneic PMBC with no treatment (C) or treated with 10-1074 (E) or 10-1074-FcRnull (F) is displayed on the y axis, and the time when viremia was first detected is displayed on the x axis. The indicated level of statistical significance (*) at week 2 comparing 10-1074-treated and 10-1074-FcRnull-treated mice was P = 0.0195 and was determined by a log-rank test and chi-square analysis with df = 1 for comparing the survival probability between two groups.
To evaluate whether bNAb treatment would mobilize Fc-mediated effector responses to reduce the population of reactivated replication-competent latently infected T cells, we constructed SPHIR mice by intrasplenic coinjection of OM5001 donor PBMC with irradiated HIV-negative PBMC as shown in Fig. 4C, and groups of SPHIR mice were treated either by intravenous injection of either bNAb 10-1074 or bNAb 10-1074 carrying the G236R/L328R Fc region mutations (10-1074-FcRnull) which abrogates its Fc-mediated effector functions but not its neutralizing activity (Fig. 4D). While all of the untreated OM5001 SPHIR-mice (12 of 12 mice) displayed viremia at 1 week after PBMC injection (Fig. 4C and G), significantly lower numbers of OM5001 SPHIR-mice treated with 10-1074 displayed viremia 1 week (0 of 10 mice, P < 0.001), 2 weeks (0 of 9 mice, P < 0.001), 3 weeks (3 of 8 mice, P = 0.005), and 4 weeks (3 of 6 mice, P = 0.036) after intrasplenic injection (Fig. 4E and G). Despite the presence of equivalent levels of 10-1074 or 10-1074-FcRnull in the serum, we observed a significant difference between treatment with 10-1074 and 10-1074-FcRnull. Although none of the OM5001 SPHIR mice (0 of 9 mice) displayed viremia until 3 weeks after 10-1074 treatment, viremia occurred in 46% (6 of 13) of OM5001 SPHIR mice 2 weeks after 10-1074-FcRnull treatment (Fig. 4F and G). The significantly more delayed time-to-viremia observed after 10-1074 treatment compared to after 10-1074-FcRnull treatment (P = 0.0195) suggested that 10-1074 treatment reduced the population of replication-competent latently infected cells in the OM5001 SPHIR mice through Fc-mediated effector mechanisms.
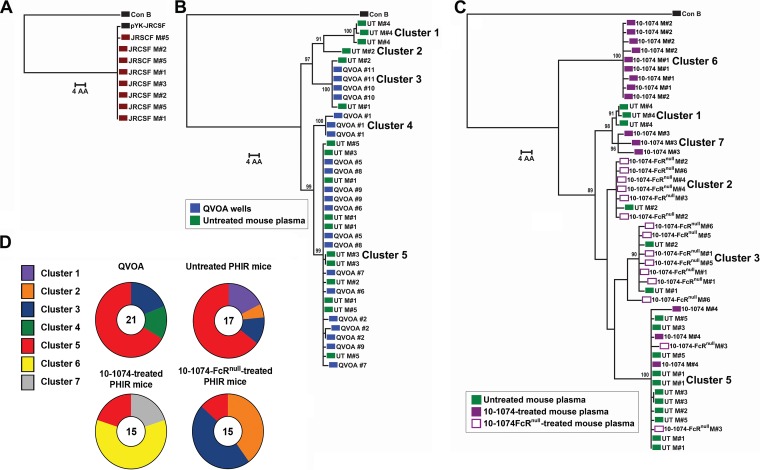
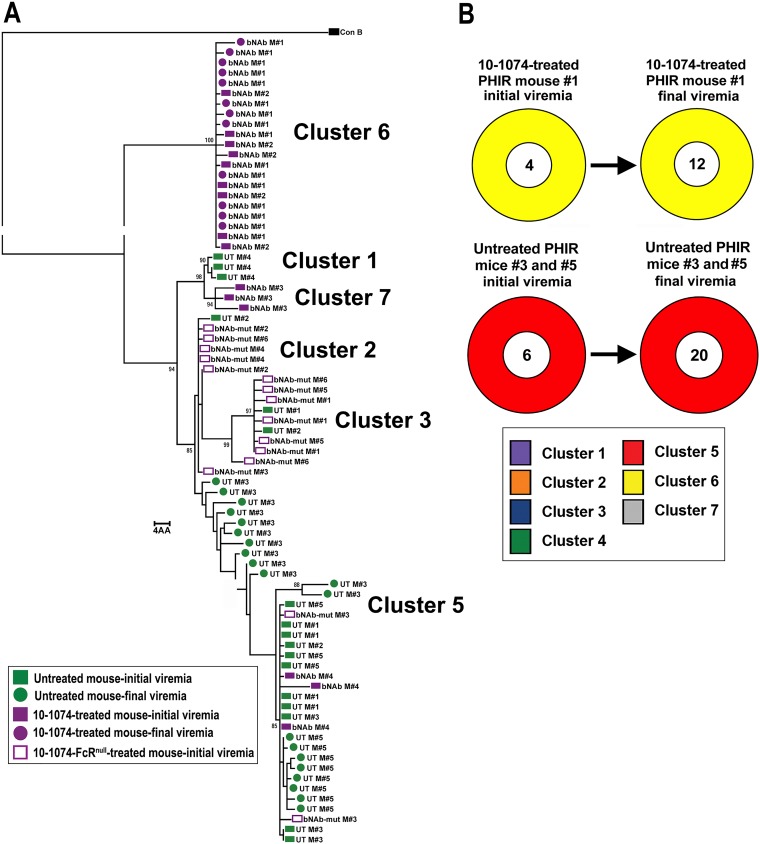
We further evaluated the impact of 10-1074 treatment on the population of replication-competent latently infected cells in the OM5001 SPHIR mice by comparing the Env sequences of viral RNA extracted from the untreated and treated mouse plasma and viral outgrowth culture wells as determined by single genome sequencing (SGS). Phylogenetic analysis of individual Env sequences of plasma viral RNA from NSG mice 1 week after intrasplenic injection with HIV-naive donor PBMC and clonal HIVJR-CSF (Fig. 4A) did not detect any significant changes in the Env sequences from the plasma of four different mice compared to the JR-CSF plasmid sequence used to produce the virus (Fig. 5A). Consequently, plasma Env sequences from the initial viremia in the SPHIR mice constructed using donor PBMC should represent the proviral sequences of the reactivated latently infected T cells producing infectious HIV. Phylogenetic analysis of Env sequences from the culture supernatant of viral outgrowth wells (n = 9 wells, two to three sequences/well) and plasma viral RNA from untreated OM5001 SPHIR-mice (n = 5 mice, three to five sequences/mouse) 1 week after intrasplenic injection demonstrated that ≥65% of the sequences from both sources were grouped in Cluster 5 (Fig. 5B and D). Two unique sequence clusters, clusters 1 and 2, each detected in the plasma of an OM5001 SPHIR mouse, were not detected in viral outgrowth wells while sequence cluster 4 was only detected a single viral outgrowth well. The fraction of cluster 5 Env sequences in the plasma from 10-1074-treated (n = 4 mice, three to five sequences/mouse) and 10-1074-FcRnull-treated (n = 6 mice, two to three sequences/mouse) OM5001 SPHIR mice from the weekly bleed when viremia was first detected was significantly reduced compared to the viral outgrowth wells (P = 0.008 and P = 0.002, respectively) and plasma from the untreated OM5001 SPHIR mice (P = 0.016 and P = 0.005, respectively) (Fig. 5C and D). Of great interest was the predominance in the plasma of 10-1074-treated mice of two new sequence clusters, cluster 6 in two treated SPHIR mice and cluster 7 in a third treated SPHIR mouse (Fig. 5C and D), which were not detected in any of the QVOA wells or in the plasma of untreated or 10-1074-FcRnull-treated OM5001 SPHIR mice. It is very unlikely that this is due to the selection of 10-1074-resistant escape variants because 10-1074 bNAbs are completely dependent upon an intact potential N-linked glycosylation site at position N332 for neutralization activities (34), and all of these plasma virus sequences continued to contain the potential N-linked glycosylation site at position N332 and an intact 324G(D/N)IR327 motif (Fig. 6) associated with sensitivity to 10-1074 (32). We performed Env sequence analysis on the virus in the plasma of one 10-1074-treated SPHIR mouse (mouse 1) 2 weeks after the initial viremia at week 3 and of two untreated PHIR mice (mouse 3 and mouse 5) 4 weeks after the onset of viremia at week 1. The plasma of the 10-1074-treated mouse continued to contain only cluster 6 sequences 2 weeks later, and the plasma of the untreated mouse continued to contain only cluster 5 sequences 4 weeks later, with some sequence changes observed likely due to mutations introduced during multiple rounds of replication (Fig. 7).
FIG 5.
Phylogenetic Env trees on initial viremia sequences. A maximum-likelihood phylogenetic tree of gp160 Env protein sequences aligned to consensus subtype B sequence and midpoint rooted for visualization is shown. Bootstrap values over 85 for dominant branches are presented. (A) Comparison to the pYK-JRCSF plasmid sequence of eight plasma sequences from four mice 1 week after injection with HIV-naive PBMC and clonal HIV-1JR-CSF. (B) Comparison of 21 sequences from nine OM5001 QVOA culture wells to 17 sequences from the plasma of five untreated SPHIR mice obtained 1 week after intrasplenic injection of OM5001 PBMC (UT). (C) Comparison of the 17 HIV plasma sequences from the untreated OM5001-SPHIR mice (UT) shown in panel B to 15 HIV plasma sequences from four 10-1074-treated OM5001-SPHIR mice (10-1074) and 15 HIV plasma sequences from six 10-1074-FcRnull-treated OM5001-SPHIR mice (10-1074-FcRnull) obtained from the first weekly bleed with detectible viremia. Each colored symbol indicates one sequence, and all are labeled by the individual QVOA well number or mouse number. (D) Fraction of sequence from each cluster determined by phylogenetic analysis (see panels B and C) from QVOA culture wells and plasma from untreated, 10-1074-treated, or 10-1074-FcRnull-treated OM5001 SPHIR mice. The number in the center of each ring indicates the total number of sequences analyzed.
FIG 6.
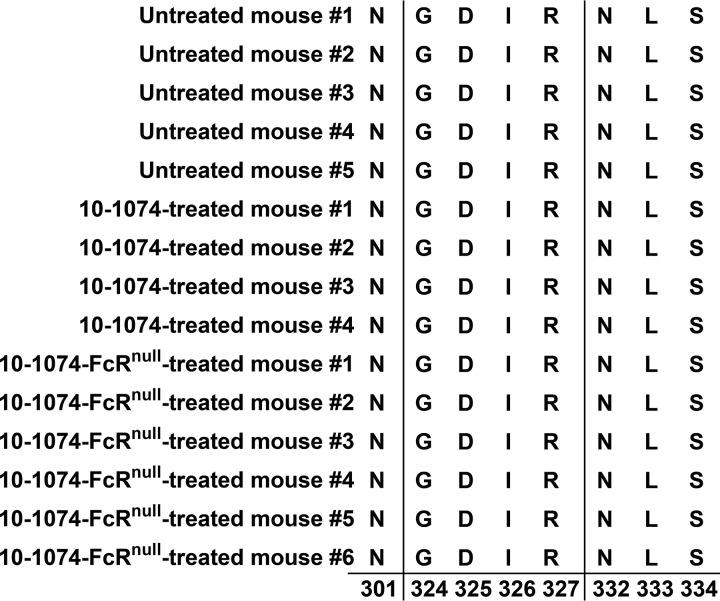
Absence of mutations in the V3 Env region associated with 10-1074 resistance. Amino acid alignment of HxB2 Env positions 301, 324 to 327, and 332 to 334 of SGS sequences from RNA extracted from circulating HIV at the initial detection of viremia in the plasma of untreated (n = 5 mice), 10-1074-treated (n = 4 mice), and 10-1074null-treated OM5001 PHIR mice (n = 6 mice). These include the potential N-linked glycosylation sites at N301 and at N332 and the 324G(D/N)IR327 motif at the base of the V3 loop.
FIG 7.
Phylogenetic Env trees comparing initial viremia to final viremia sequences. A maximum-likelihood phylogenetic tree of gp160 Env protein sequences aligned to consensus subtype B sequence and midpoint rooted for visualization is shown. Bootstraps over 85 for dominant branches are also shown. (A) Comparison of the 17 HIV plasma sequences from five untreated OM5001-SPHIR mice at initial viremia (UT), 20 HIV plasma sequences from two untreated OM5001-SPHIR mice at final viremia (UT M#3 and M#5), 15 HIV plasma sequences from four 10-1074-treated OM5001-SPHIR mice at initial viremia (bNAb), 12 HIV plasma sequences from one 10-1074-treated OM5001-SPHIR mouse (bNAb M#1) at final viremia, and 15 HIV plasma sequences from six 10-1074-FcRnull-treated OM5001-SPHIR mice (bNAb-mut). Each colored symbol indicates one sequence, and all are labeled by the individual QVOA well number or mouse number. Initial bleeds are indicated by a square and final bleed by a circle. (B) Comparison of the fraction of sequence from each cluster determined by phylogenetic analysis from untreated M#3 and M#5 initial viremia to final viremia and from 10-1074-treated M#1 initial viremia to final viremia. The number in the center of each ring indicates the total number of sequences analyzed.
More prolonged time-to-viremia after intrasplenic injection of PBMC from other ART-suppressed HIV-infected donors with lower QVOA titers than donor OM5001.
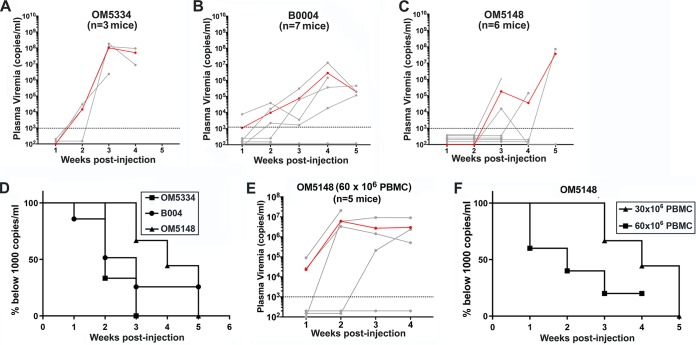
As described above, the rapid onset of viremia within 1 week of intrasplenic injection of PBMC from donor like OM5001 facilitates the in vivo evaluation of the efficacy of treatments such as bNAbs to deplete the LR reduction by quantifying the delay in the time-to-viremia. However, evaluation of the in vivo capacity of candidate LRA to reactivate latently infected T cells by accelerating the time-to-viremia would require identifying ART-suppressed donors who display a delayed time-to-viremia compared to donor OM5001 (QVOA titer = 10.5 IUPM). We compared the time-to-viremia after intrasplenic injection of PBMC from three additional HIV-infected donors who were virally suppressed by ART for more than 20 months. The clinical laboratory and clinical data for these donors and donor OM5001 are provided in Table 1. Their PBMC contained a population of replication-competent latently infected cells that ranged from 1 to 2.8 IUPM, as determined by QVOA. SPHIR mice generated with PBMC from each of these three ART-suppressed HIV-infected donors developed pronounced viremia, but at different periods (Fig. 8). The median time-to-viremia varied among the donors, ranging from 2 weeks for donor OM5334 with a QVOA titer of 1.7 IUPM (Fig. 8A and D), 3 weeks for donor B0004 with a QVOA titer of 2.8 IUPM (Fig. 8B and D), and 4 weeks for OM5148 with a QVOA titer of 1.0 IUPM (Fig. 8C and D). The median time-to-viremia could be accelerated by doubling the number of PBMC from donor OM5148 intrasplenically injected into NSG mice from 4.5 to 2 weeks. While none of the NSG mice intrasplenically injected with 30 × 106 OM5148 PBMC developed viremia by 2 weeks after injection (Fig. 8C and F), after intrasplenic injection of twice the number of OM5148 PBMC (60 × 106 cells), two of the five mice were viremic by 1 week after injection and three of five mice were viremic by 2 weeks after injection (Fig. 8E and F). We postulate that SPHIR mice constructed with PBMC from donors such as OM5148 would display an accelerated time-to-viremia after in vivo activation of the latently infected cells by in vivo LRA treatment and could be used for the in vivo evaluation of candidate LRA.
FIG 8.
In vivo reactivation of latently HIV-infected donor cells results in plasma viremia. Plasma HIV RNA levels (copies/ml) in individual NSG mice were measured at weekly intervals after intrasplenic coinjection of irradiated allogeneic PBMC (6 × 106 cells) and 30 × 106 of OM5334 donor PBMC (A), B004 donor PBMC (B), or OM5148 donor PBMC (C). The geometric mean plasma viremia (red line) and the detection limit of 1,000 copies/ml (dotted line) are shown. (D) Kaplan-Meier plots summarizing the time-to-viremia data. The percentages of mice with undetectable viremia (<1,000 copies/ml) after intrasplenic injection with 30 × 106 PBMC from either donor OM5334, B004, or OM5148 shown in panels A, B, and C are displayed on the y axis, and the times when viremia was first detected are displayed on the x axis. (E) The viral loads in the plasma of NSG mice after intrasplenic coinjection of OM5148 PBMC (60 × 106 cells) and irradiated allogeneic PBMC (12 × 106 cells). The red line indicates the geometric mean of plasma viremia. The dotted line represents detection limit of 1,000 copies/ml. (F) Kaplan-Meier plots summarizing the time-to-viremia data. The percentages of mice with undetectable viremia (<1,000 copies/ml) after intrasplenic injection with OM5148 PBMC (60 × 106 cells) shown in panel E compared to OM5148 PBMC (30 × 106 cells) shown in panel C are displayed on the y axis, and the times when viremia was first detected are displayed on the x axis.
DISCUSSION
It has been proposed that the extremely stable latent HIV reservoir can be depleted by reactivation with LRA treatment which exposes the latently infected cells to elimination by endogenous or augmented HIV-specific immune responses (3, 7, 35, 36). However, treatment of HIV-infected individuals with LRAs such as histone deacetylase inhibitors had minimal impact on the latent reservoir size (37–40). The most definitive method to establish functional elimination the LR is to demonstrate that viral rebound does not occur after ATI (6). HIV-infected individuals treated with the bNAb 3BNC117 displayed delayed viremia after ATI compared to historical controls, suggesting that bNAb treatment may reduce the LR in HIV-infected patients (29). However, routine use of ATI as an endpoint in clinical trials is complicated by the potential deleterious effects of resurgent viremia, as well as the requirement for close monitoring by frequent blood draws to enable the rapid reinitiation of ART after the detection of recurrent viremia (41). In addition, comparing the delay in the time-to-viremia by treated individuals to historical controls to determine the effectiveness of LR depletion treatments does not consider the potential variation in the time-to-viremia among infected individuals due to multiple factors uniquely affecting the biological behavior of their LR. The limitation of evaluating therapeutic responses using comparisons to the time-to-viremia after ATI in historical controls is borne out by the large fraction of placebo-treated individuals who display sustained viral suppression and delayed viral rebound after ATI, which would otherwise have been attributed to the effect of treatment (42). We postulated that the SPHIR mice, populated with PBMC containing latently infected cells obtained from ART-suppressed HIV-infected individuals, would recapitulate the outcome of ATI and enable us to compare the time-to-viremia in untreated and treated SPHIR mice, populated with PBMC from the same donor, as an indicator of LR activation, depletion, and treatment efficacy. While graft-versus-host disease (GVHD), indicated by weight loss (>15%), hunched posture, fur loss, reduced mobility, and tachypnea, has been described as a prevalent sequela after intravenous injection of human PBMC into NSG mice (43), we did not observe these symptoms in the SPHIR mice. There are several possible reasons why we did not see GVHD in these mice, including the introduction of the human PBMC into the mice by intrasplenic injection rather than intraperitoneal or intravenous injection, completion of the experiments by 5 weeks after injection prior to the potential onset of GVHD, and because the capacity of the injected PBMC from HIV-infected donors to mediate GVHD may be reduced by their underlying immunodeficient state, which is further compromised by the active HIV replication and CD4+ T cell depletion occurring after intrasplenic injection into the mice. We directly compared the time-to-viremia and rebound virus sequences of untreated, 10-1074-treated, and 10-1074-FcRnull-treated SPHIR mice generated using PBMC from the same ART-suppressed HIV-infected individual, OM5001. In contrast to a time-to-viremia of 1 week observed in all of the 12 untreated OM5001 SPHIR-mice, the time-to-viremia in all of the 10-1074-treated mice was delayed to 3 to 5 weeks after injection. We hypothesized that while 10-1074 was suppressing viremia in the first week due to its neutralizing activity, the continued delay in viremia after the reduction of 10-1074 serum concentrations below the levels which effectively suppress in vivo viremia (mean, 23.7 μg/ml) (32), was due to 10-1074 mobilization of Fc-mediated effector mechanisms to eliminate reactivated latently infected cells. This conclusion was supported by the significantly more rapid time-to-viremia we observed for OM5001 SPHIR mice treated with the 10-1074-FcRnull bNAb (Fig. 4E and F), which has neutralizing activity equivalent to the unmodified 10-1074 but is unable to induce FcR-mediated effector mechanisms such as ADCC (44). These findings are consistent with a previous observation that rebound viremia was only delayed in humanized mice infected with HIV after treatment with the unmodified bNAbs but not after treatment with the FcRnull bNAbs (15). Phylogenetic analysis provided further support for 10-1074-mediated depletion of reactivated latently infected cells. We identified the predominance of identical Env sequences in the plasma from multiple untreated OM5001 donor SPHIR mice and multiple QVOA culture wells we termed cluster 5, which is compatible with previous reports describing the clonal expansion of latently HIV-infected CD4+ T cell clones without their initiating active viral replication (45–47). 10-1074 and 10-1074-FcRnull treatment significantly depleted the subpopulation of latently infected cells producing the cluster 5 sequence virus. While ≥65% of the HIV sequences from the plasma of five untreated mice (11 of 17 sequences) and the QVOA culture supernatants (14 of 21 sequences) were grouped within cluster 5, only 20% of the HIV sequences from the plasma of four mice treated with 10-1074 (3 of 15 sequences) and 13% of HIV sequences from six mice treated with 10-1074-FcRnull (2 of 15 sequences) were grouped within cluster 5 (Fig. 5D). The significant reduction of the Cluster 5 population was paralleled by the predominance of new sequence clusters, cluster 6 and cluster 7, in the plasma of 10-1074-treated mice (80%, 12 of 15 sequences) which were not detected in any of the QVOA cultures (0 of 21) or plasma from untreated SPHIR mice (0 of 17) and 10-1074-FcRnull-treated mice (0 of 15) (Fig. 5D). This is comparable to results reported after ATI in HIV-infected individuals treated with 10-1074 and 3BNC117 where the sequences detected in the rebound virus were different from those identified by in vitro Q2VOA (48). We postulate that in untreated OM5001 SPHIR mice, the population of cluster 5 sequence-producing latently infected CD4+ T cells predominated because they were more rapidly activated, possibly due to the integration of their proviruses into loci that were more transcriptionally active (49). However, rapid reactivation of these latently infected cells would also cause early expression of surface gp120, which would render these more rapidly reactivated latently infected cells more susceptible to Fc-mediated killing while 10-1074 plasma levels were still elevated in the 10-1074-treated SPHIR mice. This may account for the subsequent emergence of the cluster 6 and 7 sequence variants produced by subdominant latently infected cells. Their slower reactivation may have occurred after 10-1074 plasma levels had declined in the treated SPHIR mice to concentrations insufficient to mobilize Fc-mediated elimination, thereby enabling them to avoid Fc-mediated depletion. In the absence of 10-1074 treatment, the emergence of these subdominant clusters would be masked by the more rapid expansion and rapid replication kinetics of the other HIV variants, such as cluster 5, derived from more rapidly activated latently infected cells. It is unlikely that these variants emerged due to 10-1074 resistance because all of the plasma Env sequences from the 10-1074-treated OM5001 SPHIR-mice (15 sequences) and 10-1074-FcRnul-treated OM5001 SPHIR mice (15 sequences) expressed the motifs associated with sensitivity to 10-1074, the N-linked glycosylation site at position N332 and the 324G(D/N)IR327 motif (Fig. 6) (32).
A limitation of the SPHIR mouse model is that it contains latently infected cells derived from circulating PBMC and does not contain cells from other potential LR sites such as lymph nodes and the gut and consequently may not fully recapitulate reactivation of latent infected cells present in the lymphoid tissues (50). Nevertheless, SPHIR mice may represent an improved model for predicting the efficacy of clinical trials by recapitulating the in vivo behavior and response to therapy of the stable LR. Previous studies focused on developing a more sensitive murine viral outgrowth assay to detect replication-competent HIV in PBMC from HIV-positive individuals with undetectable viral loads by injecting PBMC from HIV-infected individuals intraperitoneally into NSG mice or humanized BLT mice (19, 20). In contrast, we designed our model to have a rapid time-to-viremia of 1 to 2 weeks after PBMC injection to investigate and evaluate the efficacy of treatments to deplete the LR and enable visualization of HIV replication in the mouse spleens. Consequently, we directly injected PBMC from ART-suppressed donors into mouse spleens rather than the intraperitoneal injection used in the MVOA models to stimulate more robust and rapid viremia. We also posited intrasplenic injection would increase the interaction between infected CD4+ T cells and NK cells and other effector cells in a lymphoid tissue to enable us to evaluate the capacity of FcR-mediated mobilization of effector cell responses, such as ADCC, to deplete the reservoir. ADCC and other FcR-mediated mechanisms may prove to be an even more important mechanism to deplete the population of latently infected T cells in the context of a recent report demonstrating the inability of HIV-specific CD8+ T cells to diminish the population of latently infected T cells capable of producing replication-competent virus (45). Furthermore, intrasplenic injection enabled us to directly visualize and quantify the number of HIV-infected T cells in the spleen by RNAscope and correlate the number of infected cells with the viral load in the plasma. These features should enable utilization of the SPHIR mouse model to evaluate the capacity of therapeutic approaches to deplete the reservoir and to utilize sequence analysis to characterize qualitatively and quantitatively the impact of HIV cure treatments on the replication-competent HIV reservoir.
We postulate that we could also evaluate the in vivo capacity of new LRA treatments to reactivate a larger fraction of the LR by demonstrating a more rapid time-to-viremia after injection of PBMC from donors that had displayed a delayed time-to-viremia of several weeks after PBMC injection (e.g., donor OM5148, Fig. 8C) and the emergence of Env sequences not detected in QVOA wells (Fig. 5D). By constructing SPHIR mice with PBMC from specific HIV-infected patients enrolled in HIV cure treatment trials, these mice may serve as avatars to determine the optimal therapeutic regimen to utilize to provide these individuals with a sustained HIV remission. This mouse model could also be used in parallel with the standard evaluation of delayed time-to-viremia after ATI in the treated patients compared to historical controls to quantify potential reduction in the LR induced by various HIV cure treatments. Groups of NSG mice could be intrasplenically injected with PBMC from the same patient collected before and after treatment; a significant delay in the time-to-viremia in mice generated from PBMCs collected after treatment combined with a phylogenetic analysis documenting changes in the plasma HIV population would indicate treatment-mediated reduction in the LR.
MATERIALS AND METHODS
Study population.
HIV-infected individuals on suppressive ART regimens were recruited from the Maple Leaf Medical Clinic (Toronto, Canada) and Whitman-Walker Health (Washington, DC) through protocols approved by the University of Toronto Institutional Review Board and the George Washington University Institutional Review Board, respectively. PBMC were obtained from these individuals by leukapheresis, and secondary use of these samples was approved by the George Washington University and Albert Einstein College of Medicine Institutional Review Boards. All subjects were adults and gave written informed consent, and their clinical and laboratory data are provided in Table 1.
Viral outgrowth assay.
QVOA assays were performed as described previously (51). Briefly, CD4+ T cells purified from donor PBMC by using the EasySep human CD4+ T cell enrichment kit (Stemcell Technologies, Vancouver, British Columbia, Canada) were plated out in serial dilutions (2, 1, 0.5, 0.2, and 0.1 million per well) into 12 wells in 24-well plates with added phytohemagglutinin (PHA; 2 μg/ml) and irradiated HIV-negative donor PBMC (2 × 106 cells/well) to reactivate the infected cells. After 2 days of culture, MOLT-4 CCR5 cells (2 × 106 cells/well) were added to amplify the HIV. After 2 weeks of culture, p24 antigen in the culture supernatant was quantified using an HIV p24 antigen enzyme-linked immunosorbent assay (ELISA) kit (Perkin-Elmer, Hopkinton, MA), and the frequency of latently infected cells capable of producing infectious HIV was calculated and expressed as IUPM, as described previously (5).
Construction of SPHIR mice.
SPHIR mice were constructed using a variation of our previously described humanized mouse model (22). PBMC isolated from HIV-infected donors (30 × 106 to 60 × 106 cells) were injected into the spleens of NSG mice (Jackson Laboratories, Bar Harbor, ME) combined with irradiated (3,000 rad) allogeneic PBMC (6 × 106 to 12 × 106 cells) from the same three HIV-negative donors. As a positive control, we intrasplenically injected PHA-activated PBMC from an HIV negative donor that were spinfected ex vivo with HIV-1JR-CSF (∼1 × 106 IU/3 × 107 PBMC) generated by transducing 293T cells with a molecular HIV clone, pYK-JRCSF (33), and irradiated allogeneic PBMC (6 × 106 cells/mouse) as described previously (22). The mice were bled weekly to monitor plasma viral load.
Plasma viral load measurements.
Total RNA was extracted from 50 to 200 μl of mouse plasma using a QIAamp MinElute virus spin kit (Qiagen, Germantown, MD) and reverse transcribed to generate cDNA using a high-capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). RT-qPCR was performed using TaqMan Universal Master Mix II, no UNG (Applied Biosystems) with added cDNA (2 μl), and the HIV gag sequence-specific primers (5′-TCAGCCCAGAAGTAATACCCATGT-3′ [sense] and 5′-CACTGTGTTTAGCATGGTGTTT-3′) and an HIV gag sequence-specific (antisense) probe (6FAM-ATTATCAGAAGGAGCCACCCCACAAGA-TAMRA) as described previously (52). Cycle threshold values were calculated using a standard curve and samples with known copy numbers of absolute HIV DNA.
Production 10-1074 and 10-1074-FcRnull antibodies.
Expression plasmids for 10-1074 antibody heavy and light chains were obtained from John Mascola (Vaccine Research Center, National Institutes of Health [NIH]), and we introduced the GRLR mutations (G236R;L328R) into the 1074 heavy chain gene to generate 10-1074-FcRnull, as described previously (44). 10-1074 and 10-1074-FcRnull antibodies were produced by transient transfection of 293F cells with the 10-1074 or 10-1074-FcRnull heavy-chain and light-chain plasmids and purified by affinity chromatography using HiTrap Protein A HP columns (GE Healthcare). Antibody proteins were quality controlled by running a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) protein gel and neutralization assays against a standard set of viruses, as described below.
Neutralization assays.
Neutralization of HIV infection by 10-1074 and 10-1074-FcRnull antibodies was assessed as described previously (53). Briefly, 10-1074 and 10-1074-FcRnull antibodies with a starting concentration of 50 μg/ml were serially diluted 5-fold and incubated (10 μl) with HIV pseudovirus (40 μl) in duplicate for 30 min at 37°C in 96-well clear flat-bottom black culture plates (Greiner Bio-One). TZM-bl cells were added (10,000 cells) to each well in Dulbecco modified Eagle medium containing DEAE-dextran (75 μg/ml) and incubated for 24 h at 37°C in a 5% CO2 incubator. Two days later, the cells were lysed with SpectraMax Glo Steady-Luc reporter assay (Molecular Devices, LLC, Sunnyvale, CA) reagent (100 μl), and the luminescence intensity was measured using a SpectraMax i3x multimode detection platform. Neutralization curves were calculated comparing luciferase units to a virus-only control after background subtraction and fit by nonlinear regression using the asymmetric five-parameter logistic equation in GraphPad Prism.
Antibody treatment and measurement of serum levels of 10-1074 and 10-1074-FcRnull.
SPHIR mice were intravenously injected with 0.5 mg of either 10-1074 or 10-1074-FcRnull on day 0. Plasma samples were collected weekly and plasma concentrations of 10-1074 and 10-1074-FcRnull were determined by ELISA with plates coated with HIV Env gp14089.6, and standard curves were generated using 10-1074 or 10-1074-FcRnull standards as described previously (54).
Plasma env gene amplification and phylogenetic analysis.
Plasma virus env genes were amplified using a modification of a previously described method (55, 56). cDNA was generated from plasma RNA isolated with the QIAamp viral RNA minikit (Qiagen) by reverse transcription using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) with the antisense primer envB3out (TTGCTACTTGTGATTGCTCCATG), followed by heat inactivation at 70°C for 15 min and treatment with RNase H (3 μl; Invitrogen) at 37°C for 20 min. The env genes were amplified by nested PCR using Platinum Taq high-fidelity polymerase (Invitrogen). The first round was performed using a cDNA template diluted so that PCR-positive wells were fewer than 33.3% of replicate reactions with forward and reverse primers envB5out (TAGAGCCCTGGAAGCATCCAGGAAG) and envB3out. The second PCR round was performed using 1 μl of first-round amplicon as template for 45 cycles with the forward and reverse primers envB5in (CACCTTAGGCATCTCCTATGGCAGGAAGAAG) and envB3in (GTCTCGAGATACTGCTCCCACCC). Amplicons resolved on 1% agarose gels and positive wells were Sanger sequenced by ACGT, Inc. (Wheeling, IL). After the removal of sequences that contained stop codons, large deletions, or mixed bases, the remaining Env amino acid sequences were aligned with MUSCLE in the Geneious suite version (9.1.8). Maximum-likelihood trees were generated from alignments with RAxML version 8 run on the Cyberinfrastructure for Phylogenetic Research (CIPRES) Science Gateway and visualized in MEGA.
RNAscope imaging of spleen samples.
HIV RNA-producing cells were detected in SPHIR mouse spleens by RNAscope technology (Advanced Cell Diagnostics [ACD], Newark, CA) using a similar method we used to detect SIV RNA-producing cells (57). SPHIR mouse spleens were collected, fixed in 10% buffered formalin overnight, and embedded in paraffin. Fresh-cut spleen sections (5 μm) were dewaxed in xylene, epitope unmasked by heating in citrate buffer (ACD, treatment 2), protease treated, and incubated with an HIV probe for 2 h at 40°C. The HIV probe was amplified using the RNAscope 2.5 detection kit and detected with TSA Cyanine 3 Plus (Perkin-Elmer, Waltham, MA). Sections were counterstained with DAPI (4′,6′-diamidino-2-phenylindole), mounted in Slowfad Diamond Antifade (Invitrogen), and scanned at ×4 and ×40 on an Aperio Versa 8 scanner (Leica Biosystems, Buffalo Grove, IL). The HIV RNA-producing cells were quantified using an Aperio Image Scope (version 12.3.2.5030; Leica Biosystems) with the Cellular Immunofluorescence v1 algorithm. For each spleen, three to four separate sections (each a minimum of 30 μm apart) were analyzed. The total area analyzed was a median of 119 mm2 (range, 50 to 173 mm2). The individuals performing the analysis were blinded to the treatment status of the animals.
Statistical analysis.
Comparisons of the numbers of sequences belonging to particular clusters, as well as the percentages of viremic mice at specific time points based on donor source, numbers of cells added, or treatment, were performed by using the Fisher exact test calculated by SAS v9.4 software. Comparisons of viral loads in mice injected with HIV donor PBMC by intrasplenic, intraperitoneal, or intravenous injection were performed using a Wilcoxon rank sum test. Comparison of viral loads in 10-1074- and 10-1074-FcRnull-treated SPHIR mice was done using the log-rank test. Differences were considered statistically significant when a two-sided P value was <0.05.
Study approval.
All the studies using mice and human donor cells were approved by the Institute for Animal Studies and the Institutional Review Board at Albert Einstein College of Medicine in compliance with the human and animal experimentation guidelines of the U.S. Department of Health and Human Services and adherence to the NIH Guide for the Care and Use of Laboratory Animals.
ACKNOWLEDGMENTS
This study was supported by grants from the NIH, BELIEVE (NIH grant 1UM1AI126617 to H.G., E.C., R.B.J., and R.M.L.), NIDA (DA033788 and DA044584 to H.G.), the Einstein-Rockefeller-CUNY Center for AIDS Research (P30AI124414 to H.G.), and the Charles Michael Chair in Autoimmune Diseases (to H.G.).
We are grateful to Moonseong Heo and Cuiling Wang for statistical analysis; David Hardy and Christopher Cannon from Whitman-Walker Health in Washington, DC, for providing PBMC from donor B004; James Whitney from the Beth Israel Deaconess Medical Center, Boston, MA, for providing the ART we used to treat the SPHIR mice; Dora Chan for technical support; and the NIH AIDS Reagent Program for providing HIV-1 JR-CSF Infectious Molecular Clone (pYK-JRCSF).
REFERENCES
- 1.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 3.Barouch DH, Deeks SG. 2014. Immunologic strategies for HIV-1 remission and eradication. Science 345:169–174. doi: 10.1126/science.1255512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crooks AM, Bateson R, Cope AB, Dahl NP, Griggs MK, Kuruc JD, Gay CL, Eron JJ, Margolis DM, Bosch RJ, Archin NM. 2015. Precise quantitation of the latent HIV-1 reservoir: implications for eradication strategies. J Infect Dis 212:1361–1365. doi: 10.1093/infdis/jiv218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laird GM, Eisele EE, Rabi SA, Lai J, Chioma S, Blankson JN, Siliciano JD, Siliciano RF. 2013. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog 9:e1003398. doi: 10.1371/journal.ppat.1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewin SR. 2014. Finding a cure for HIV: much work to do. Ann Intern Med 161:368–369. doi: 10.7326/M14-1573. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, Chomont N, Douek D, Lifson JD, Lo Y-R, Kuritzkes D, Margolis D, Mellors J, Persaud D, Tucker JD, Barre-Sinoussi F, Alter G, Auerbach J, Autran B, Barouch DH, Behrens G, Cavazzana M, Chen Z, Cohen ÉA, Corbelli GM, Eholié S, Eyal N, Fidler S, Garcia L, Grossman C, Henderson G, Henrich TJ, Jefferys R, Kiem H-P, McCune J, Moodley K, Newman PA, Nijhuis M, Nsubuga MS, Ott M, Palmer S, Richman D, Saez-Cirion A, Sharp M, Siliciano J, Silvestri G, Singh J, Spire B, Taylor J, Tolstrup M, Valente S, van Lunzen J, Walensky R, Wilson I, Zack J. 2016. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 22:839–850. doi: 10.1038/nm.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazkova J, Chun TW, Belay BW, Murray D, Justement JS, Funk EK, Nelson A, Hallahan CW, Moir S, Wender PA, Fauci AS. 2012. Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4+ T cells from infected individuals receiving effective antiretroviral therapy. J Infect Dis 206:765–769. doi: 10.1093/infdis/jis412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cillo AR, Sobolewski MD, Bosch RJ, Fyne E, Piatak M Jr, Coffin JM, Mellors JW. 2014. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 111:7078–7083. doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archin NM, Eron JJ, Palmer S, Hartmann-Duff A, Martinson JA, Wiegand A, Bandarenko N, Schmitz JL, Bosch RJ, Landay AL, Coffin JM, Margolis DM. 2008. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS 22:1131–1135. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G, Roney J, Watson J, Prince MH, Hoy JF, Chomont N, Fromentin R, Procopio FA, Zeidan J, Palmer S, Odevall L, Johnstone RW, Martin BP, Sinclair E, Deeks SG, Hazuda DJ, Cameron PU, Sekaly RP, Lewin SR. 2014. Activation of HIV transcription with short-course Vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Routy JP, Tremblay CL, Angel JB, Trottier B, Rouleau D, Baril JG, Harris M, Trottier S, Singer J, Chomont N, Sekaly RP, Boulassel MR. 2012. Valproic acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study. HIV Med 13:291–296. doi: 10.1111/j.1468-1293.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 13.Sagot-Lerolle N, Lamine A, Chaix ML, Boufassa F, Aboulker JP, Costagliola D, Goujard C, Pallier C, Delfraissy JF, Lambotte O, Study AE. 2008. Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS 22:1125–1129. doi: 10.1097/QAD.0b013e3282fd6ddc. [DOI] [PubMed] [Google Scholar]
- 14.Siliciano JD, Lai J, Callender M, Pitt E, Zhang H, Margolick JB, Gallant JE, Cofrancesco J Jr, Moore RD, Gange SJ, Siliciano RF. 2007. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect DIS 195:833–836. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 15.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC. 2014. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbloom DIS, Hill AL, Laskey SB, Siliciano RF. 2017. Re-evaluating evolution in the HIV reservoir. Nature 551:E6–E9. doi: 10.1038/nature24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavender KJ, Pace C, Sutter K, Messer RJ, Pouncey DL, Cummins NW, Natesampillai S, Zheng J, Goldsmith J, Widera M, Van Dis ES, Phillips K, Race B, Dittmer U, Kukolj G, Hasenkrug KJ. 2018. An advanced BLT-humanized mouse model for extended HIV-1 cure studies. AIDS 32:1–10. doi: 10.1097/QAD.0000000000001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laird GM, Bullen CK, Rosenbloom DI, Martin AR, Hill AL, Durand CM, Siliciano JD, Siliciano RF. 2015. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest 125:1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metcalf Pate KA, Pohlmeyer CW, Walker-Sperling VE, Foote JB, Najarro KM, Cryer CG, Salgado M, Gama L, Engle EL, Shirk EN, Queen SE, Chioma S, Vermillion MS, Bullock B, Li M, Lyons CE, Adams RJ, Zink MC, Clements JE, Mankowski JL, Blankson JN. 2015. A murine viral outgrowth assay to detect residual HIV type 1 in patients with undetectable viral loads. J Infect Dis 212:1387–1396. doi: 10.1093/infdis/jiv230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlins P, Schmitt K, Remling-Mulder L, Hogan LE, Hanhauser E, Hobbs KS, Hecht F, Deeks SG, Henrich TJ, Akkina R. 2017. A humanized mouse-based HIV-1 viral outgrowth assay with higher sensitivity than in vitro qVOA in detecting latently infected cells from individuals on ART with undetectable viral loads. Virology 507:135–139. doi: 10.1016/j.virol.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seay K, Church C, Zheng JH, Deneroff K, Ochsenbauer C, Kappes JC, Liu B, Jeng EK, Wong HC, Goldstein H. 2015. In vivo activation of human NK cells by treatment with an interleukin-15 superagonist potently inhibits acute in vivo HIV-1 infection in humanized mice. J Virol 89:6264–6274. doi: 10.1128/JVI.00563-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardhi A, Wu Y, Chen W, Li W, Zhu Z, Zheng JH, Wong H, Jeng E, Jones J, Ochsenbauer C, Kappes JC, Dimitrov DS, Ying T, Goldstein H. 2017. Potent in vivo NK cell-mediated elimination of HIV-1-infected cells mobilized by a gp120-bispecific and hexavalent broadly neutralizing fusion protein. J Virol 91:e00937-17. doi: 10.1128/JVI.00937-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davey RT Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS, Lane HC. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smedley J, Turkbey B, Bernardo ML, Del Prete GQ, Estes JD, Griffiths GL, Kobayashi H, Choyke PL, Lifson JD, Keele BF. 2014. Tracking the luminal exposure and lymphatic drainage pathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission. PLoS One 9:e92830. doi: 10.1371/journal.pone.0092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M Jr, Estes JD, Lifson JD, Picker LJ. 2015. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Buning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC. 2013. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio P, Incesu RB, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, Shimeliovich I, Patel R, Burke L, Cohen YZ, Hadrigan S, Settler A, Witmer-Pack M, West AP Jr, Juelg B, Keler T, Hawthorne T, Zingman B, Gulick RM, Pfeifer N, Learn GH, Seaman MS, Bjorkman PJ, Klein F, Schlesinger SJ, Walker BD, Hahn BH, Nussenzweig MC, Caskey M. 2016. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535:556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M Jr, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, Donahue DA, Lorin V, Casartelli N, Noel N, Lambotte O, Mouquet H, Schwartz O. 2016. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, Learn GH, Cohen YZ, Lehmann C, Gillor D, Shimeliovich I, Unson-O’Brien C, Weiland D, Robles A, Kümmerle T, Wyen C, Levin R, Witmer-Pack M, Eren K, Ignacio C, Kiss S, West AP, Mouquet H, Zingman BS, Gulick RM, Keler T, Bjorkman PJ, Seaman MS, Hahn BH, Fätkenheuer G, Schlesinger SJ, Nussenzweig MC, Klein F. 2017. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med 23:185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819–822. [DOI] [PubMed] [Google Scholar]
- 34.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. 2012. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. 2009. The challenge of finding a cure for HIV infection. Science 323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 36.Deeks SG. 2012. HIV: shock and kill. Nature 487:439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 37.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. 2012. Administration of Vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N, Savic R, Roney J, Hoh R, Solomon A, Piatak M, Gorelick RJ, Lifson J, Bacchetti P, Deeks SG, Lewin SR. 2015. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2:e520–e529. doi: 10.1016/S2352-3018(15)00226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rasmussen T, Tolstrup M, Brinkmann C, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin S, Østergaard L, Søgaard O. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen TA, Lewin SR. 2016. Shocking HIV out of hiding: where are we with clinical trials of latency reversing agents? Curr Opin HIV AIDS 11:394–401. doi: 10.1097/COH.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 41.Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. 2016. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol 14:55–60. doi: 10.1038/nrmicro.2015.5. [DOI] [PubMed] [Google Scholar]
- 42.Sneller MC, Justement JS, Gittens KR, Petrone ME, Clarridge KE, Proschan MA, Kwan R, Shi V, Blazkova J, Refsland EW, Morris DE, Cohen KW, McElrath MJ, Xu R, Egan MA, Eldridge JH, Benko E, Kovacs C, Moir S, Chun TW, Fauci AS. 2017. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 419:eaan8848. doi: 10.1126/scitranslmed.aan8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali N, Flutter B, Sanchez Rodriguez R, Sharif-Paghaleh E, Barber LD, Lombardi G, Nestle FO. 2012. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rγ-null mice display a T-effector memory phenotype. PLoS One 7:e44219. doi: 10.1371/journal.pone.0044219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. 2014. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel M-R, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy J-P, Haddad EK, Sékaly R-P. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, Hughes SH. 2014. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345:179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DI, Keele BF, Ho YC, Siliciano JD, Siliciano RF. 2017. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J Exp Med 214:959–972. doi: 10.1084/jem.20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suárez I, Oliveira TY, Lorenzi JCC, Cohen YZ, Wyen C, Kümmerle T, Karagounis T, Lu C-L, Handl L, Unson-O’Brien C, Patel R, Ruping C, Schlotz M, Witmer-Pack M, Shimeliovich I, Kremer G, Thomas E, Seaton KE, Horowitz J, West AP, Bjorkman PJ, Tomaras GD, Gulick RM, Pfeifer N, Fätkenheuer G, Seaman MS, Klein F, Caskey M, Nussenzweig MC. 2018. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561:479–484. doi: 10.1038/s41586-018-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shan L, Yang HC, Rabi SA, Bravo HC, Shroff NS, Irizarry RA, Zhang H, Margolick JB, Siliciano JD, Siliciano RF. 2011. Influence of host gene transcription level and orientation on HIV-1 latency in a primary-cell model. J Virol 85:5384–5393. doi: 10.1128/JVI.02536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boritz EA, Douek DC. 2017. Perspectives on human immunodeficiency virus (HIV) cure: HIV persistence in tissue. J Infect Dis 215:S128–S133. doi: 10.1093/infdis/jix005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang SH, Ren Y, Thomas AS, Chan D, Mueller S, Ward AR, Patel S, Bollard CM, Cruz CR, Karandish S, Truong R, Macedo AB, Bosque A, Kovacs C, Benko E, Piechocka-Trocha A, Wong H, Jeng E, Nixon DF, Ho YC, Siliciano RF, Walker BD, Jones RB. 2018. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J Clin Invest 128:876–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pasternak AO, Adema KW, Bakker M, Jurriaans S, Berkhout B, Cornelissen M, Lukashov VV. 2008. Highly sensitive methods based on seminested real-time reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 unspliced and multiply spliced RNA and proviral DNA. J Clin Microbiol 46:2206–2211. doi: 10.1128/JCM.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montefiori DC. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 54.Chen W, Bardhi A, Feng Y, Wang Y, Qi Q, Li W, Zhu Z, Dyba MA, Ying T, Jiang S, Goldstein H, Dimitrov DS. 2016. Improving the CH1-CK heterodimerization and pharmacokinetics of 4Dm2m, a novel potent CD4-antibody fusion protein against HIV-1. MAbs 8:761–774. doi: 10.1080/19420862.2016.1160180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webb GM, Li S, Mwakalundwa G, Folkvord JM, Greene JM, Reed JS, Stanton JJ, Legasse AW, Hobbs T, Martin LD, Park BS, Whitney JB, Jeng EK, Wong HC, Nixon DF, Jones RB, Connick E, Skinner PJ, Sacha JB. 2018. The human IL-15 superagonist ALT-803 directs SIV-specific CD8+ T cells into B-cell follicles. Blood Adv 2:76–84. doi: 10.1182/bloodadvances.2017012971. [DOI] [PMC free article] [PubMed] [Google Scholar]