Although ubiquitination has been shown to play important roles in the HCV life cycle, the roles of deubiquitinating enzymes (DUBs), which cleave ubiquitin chains from their substrates, in HCV propagation have not been investigated. Here, we identified USP15 as a DUB regulating HCV propagation. USP15 showed no interaction with viral proteins and no participation in innate immune responses. Deficiency of USP15 in Huh7 cells resulted in suppression of the translation of HCV RNA and reduction in the amounts of lipid droplets, and the addition of fatty acids partially restored the production of infectious HCV particles. These data suggest that USP15 participates in HCV propagation in hepatic cells through the regulation of viral RNA translation and lipid metabolism.
KEYWORDS: deubiquitination, HCV, innate immunity, lipid, translation
ABSTRACT
Hepatitis C virus (HCV) utilizes cellular factors for efficient propagation. Ubiquitin is covalently conjugated to the substrate to alter its stability or to modulate signal transduction. In this study, we examined the importance of ubiquitination for HCV propagation. We found that inhibition of deubiquitinating enzymes (DUBs) or overexpression of nonspecific DUBs impaired HCV replication, suggesting that ubiquitination regulates HCV replication. To identify specific DUBs involved in HCV propagation, we set up RNA interference (RNAi) screening against DUBs and successfully identified ubiquitin-specific protease 15 (USP15) as a novel host factor for HCV propagation. Our studies showed that USP15 is involved in translation of HCV RNA and production of infectious HCV particles. In addition, deficiency of USP15 in human hepatic cell lines (Huh7 and Hep3B/miR-122 cells) but not in a nonhepatic cell line (293T cells) impaired HCV propagation, suggesting that USP15 participates in HCV propagation through the regulation of hepatocyte-specific functions. Moreover, we showed that loss of USP15 had no effect on innate immune responses in vitro and in vivo. We also found that USP15-deficient Huh7 cells showed reductions in the amounts of lipid droplets (LDs), and the addition of palmitic acids restored the production of infectious HCV particles. Taken together, these data suggest that USP15 participates in HCV propagation by regulating the translation of HCV RNA and the formation of LDs.
IMPORTANCE Although ubiquitination has been shown to play important roles in the HCV life cycle, the roles of deubiquitinating enzymes (DUBs), which cleave ubiquitin chains from their substrates, in HCV propagation have not been investigated. Here, we identified USP15 as a DUB regulating HCV propagation. USP15 showed no interaction with viral proteins and no participation in innate immune responses. Deficiency of USP15 in Huh7 cells resulted in suppression of the translation of HCV RNA and reduction in the amounts of lipid droplets, and the addition of fatty acids partially restored the production of infectious HCV particles. These data suggest that USP15 participates in HCV propagation in hepatic cells through the regulation of viral RNA translation and lipid metabolism.
INTRODUCTION
Hepatitis C virus (HCV) belongs to the Flaviviridae family and possesses a single-stranded positive-sense RNA genome (1). Viral RNA is translated to a precursor polyprotein, which is cleaved into 10 viral proteins by host and viral proteases. Among the HCV proteins, the core, E1, and E2 proteins form viral particles, and nonstructural protein 3 (NS3), NS4A, NS4B, NS5A, and NS5B are responsible for HCV RNA replication. NS2 protein cleaves the junction between NS2 and NS3, and p7 has been shown to exhibit ion channel activity (1). HCV infection leads to chronic infection and eventually induces steatosis, cirrhosis, and hepatocellular carcinoma (2). HCV core protein localizes with many cellular components, such as the nucleus, endoplasmic reticulum (ER), lipid droplets (LDs), lipid rafts, and mitochondria (3–7). On the other hand, HCV infection epidemiologically correlates with extrahepatic manifestations (EHMs), such as type 2 diabetes, mixed cryoglobulinemia, and non-Hodgkin lymphoma (8). Liver-specific HCV core transgenic (CoreTG) mice develop insulin resistance, steatosis, and hepatocellular carcinoma (9, 10), suggesting that HCV core protein plays a role in liver diseases and EHMs.
Efficient propagation of HCV requires several cellular factors, such as miR-122, a liver-specific microRNA that binds to two sites of HCV RNA to facilitate HCV replication (11, 12), and protein complexes of molecular chaperones and cochaperones, such as heat shock proteins, cyclophilin A, FK506-binding protein 8 (FKBP8), and FKBP6 (13–15). In addition, phosphatidylinositol-4-kinase alpha/beta-mediated phosphatidylinositol-4-phosphate is required to construct the appropriate membrane structure for HCV replication (16–18), and components of lipoproteins, such as apolipoprotein E (APOE) and APOB, play important roles in the maturation of HCV particles (19–21). Lipid rafts, LDs, and their associated proteins are also involved in HCV replication (22–24). Therefore, HCV utilizes various cellular organelles and host factors to facilitate efficient propagation.
Ubiquitination is a posttranslational modification that regulates cellular homeostasis. The HCV core protein was reported to be ubiquitinated by E6-associated protein (E6AP) to suppress viral particle formation (25). Blockage of the cleavage of core protein by signal peptide peptidase (SPP) has been shown to induce the ubiquitination of core protein by translocation in renal carcinoma on chromosome 8 (TRC8) to suppress the induction of ER stress in cultured cells (26). Zinc mesoporphyrin (ZnMP) has been reported to induce the degradation of NS5A via ubiquitination (27). It was also reported that interferon-stimulated gene 12a (ISG12a) induced by HCV infection ubiquitinates and degrades NS5A by S-phase kinase-associated protein 2 (SKP2) (28). NS5B was shown to interact with human homolog 1 of protein linking integrin-associated protein and cytoskeleton (hPLICs) to promote proteasomal degradation (29). In addition, HCV infection has been shown to induce the ubiquitination of Parkin to promote mitophagy (30, 31) and regulate the ubiquitination of retinoic acid-inducible gene I (RIG-I) through the ISG15/protein kinase R (PKR) pathway (32). These data suggest that ubiquitination participates in various steps of the HCV life cycle.
In this study, we found that treatment with an inhibitor of deubiquitinating enzymes (DUBs) or overexpression of nonspecific DUBs impaired HCV replication, suggesting that ubiquitination is important for HCV propagation. RNA interference (RNAi)-mediated screening targeting DUB genes identified ubiquitin-specific protease 15 (USP15) as a novel host factor that participates in HCV replication. Translation of HCV RNA was significantly impaired in USP15-deficient Huh7 (USP15KOHuh7) cells. Deficiency of USP15 in hepatic but not in nonhepatic cell lines significantly reduced the propagation of HCV. Unlike in previous reports, we found that USP15 was not involved in RIG-I-mediated innate immune responses in vitro and in vivo. In addition, we found that the expression of sterol regulatory element-binding protein 1c (SREBP-1c), a master regulator of fatty acid synthesis and LDs, was suppressed in USP15KOHuh7 cells. USP15 was localized on LDs, and the addition of fatty acids restored the production of infectious HCV particles in USP15KOHuh7 cells. Taken together, these data suggest that USP15 is a crucial host factor for HCV propagation in hepatic cells through the regulation of viral RNA translation and lipid metabolism.
RESULTS
Ubiquitination and deubiquitination are required for HCV propagation.
To examine the importance of ubiquitination during HCV replication, HCV replicon cells were treated with PR-619, a nonspecific DUB inhibitor. Intracellular HCV RNA was significantly reduced by treatment with PR-619, without obvious cytotoxicity (Fig. 1A), suggesting that inhibition of DUBs impaired HCV replication. OTUD7B, OTUB1, and OTUD1 are DUBs which specifically cleave the K11-, K48-, and K63-linked ubiquitin chains from nonspecific target proteins, respectively (33–35). Immunofluorescence microscopic observation of Huh7 cells overexpressing OTUD7B, OTUB1, and OTUD1 revealed the cytoplasmic localization of these DUBs, which suggested that all DUBs cleave ubiquitin chains of the target proteins in the cytoplasm (Fig. 1B and C). To examine which types of ubiquitination are involved in HCV replication, Huh7 cells overexpressing DUBs were infected with HCV. Overexpression of all DUBs in Huh7 cells impaired HCV propagation (Fig. 1D and E), suggesting that K11-, K48-, and K63-linked ubiquitination are required for HCV propagation. Although it still remained unclear whether OTUD7B, OTUB1, or OTUD1 participates in the propagation of HCV, our data suggest that ubiquitination and deubiquitination are required for efficient HCV propagation.
FIG 1.
Ubiquitination is important for HCV replication. (A) HCV replicon (9-13) cells were treated with PR-619, a nonselective DUB inhibitor, or dimethyl sulfoxide (DMSO) for 24 h, and cell viability and intracellular HCV RNA levels were determined by PI staining and qRT-PCR, respectively. (B) Expression of FLAG-tagged OTUD7B, OTUB1, and OTUD1 in Huh7 cells was detected by Western blotting using anti-FLAG antibody. (C) Subcellular localization of OTUD7B, OTUB1, or OTUD1 overexpression in Huh7 cells was observed by confocal microscopy. Each DUB (green) or nucleus (blue) was stained with anti-FLAG antibody and DAPI, respectively. (D) Huh7 cells expressing the indicated DUBs were infected with HCV at an MOI of 3. After 4 days postinfection, the culture supernatants were collected, and infectious HCV titers in the culture supernatants were determined by a focus-forming assay. WT, wild type; FFU, focus-forming units. (E) HCV was inoculated into Huh7 cells expressing the indicated DUBs at an MOI of 3 and stained with anti-NS5A antibody at 4 days postinfection. *, P < 0.05; **, P < 0.01.
RNAi screening to determine DUBs involved in HCV propagation.
Because treatment with a DUB inhibitor and overexpression of nonspecific DUBs suppressed HCV replication, we next tried to identify specific DUBs involved in HCV replication by RNAi-based screening (Fig. 2A). The family of DUBs consists of approximately 100 genes. We established 61 stable Huh7.5.1 cell lines, each of which expressed a short hairpin RNA (shRNA) against one of the DUBs (step 1) (Fig. 2A) and selected 30 of the cell lines that exhibited at least a 40% reduction of DUB expression (step 2) (Fig. 2A). We then inoculated HCV into the 30 cell lines and quantified the intracellular HCV RNA levels after 4 days (step 3) (Fig. 2A). We found that the cell lines harboring an shRNA against USP15 exhibited the most efficient reduction of HCV RNA in our screening (Fig. 2B). Next, we confirmed that USP15 expression was reduced in Huh7.5.1 cells expressing shRNA against USP15 (shUSP15) compared to Huh7.5.1 cells expressing control shRNA against LacZ (shLacZ) (Fig. 2C). In addition, the level of intracellular HCV RNA in Huh7.5.1 cells expressing shUSP15 upon infection with HCV was significantly lower than that in Huh7.5.1 cells expressing shLacZ (Fig. 2D). These data suggest that USP15 is involved in the propagation of HCV.
FIG 2.
RNAi screening to identify specific DUBs involved in HCV propagation. (A) Schematic representation of the experimental procedure for our RNAi screening. Huh7.5.1 cells were infected with retroviruses expressing shRNA targeting DUBs (shDUBs). To validate the knockdown of each DUB gene in Huh7.5.1 cell lines expressing shDUBs, qRT-PCR was performed by using the primer sets shown in Table 1. shDUB Huh7.5.1 cell lines that exhibited a >40% reduction in the expression of their specific DUB were selected for further screening. DUB knockdown Huh7.5.1 cells were infected with HCV at an MOI of 0.5, and intracellular HCV RNA levels were quantified after 4 days. (B) The levels of intracellular HCV RNAs at 4 days postinfection were determined by qRT-PCR as a relative value against the GAPDH mRNA level in cells. Data are presented as relative values compared to those in Huh7.5.1 cells expressing shRNA against the LacZ gene. (C) The expression level of USP15 in Huh7.5.1 cells and those expressing shRNA targeting either LacZ (shLacZ) or USP15 (shUSP15) was quantified by qRT-PCR. (D) The intracellular HCV RNA levels in Huh7.5.1 cells and those expressing either shLacZ or shUSP15 upon infection with HCV at an MOI of 0.5 were determined by qRT-PCR at the indicated time points. dpi, days postinfection.
Deficiency of USP15 impairs HCV propagation.
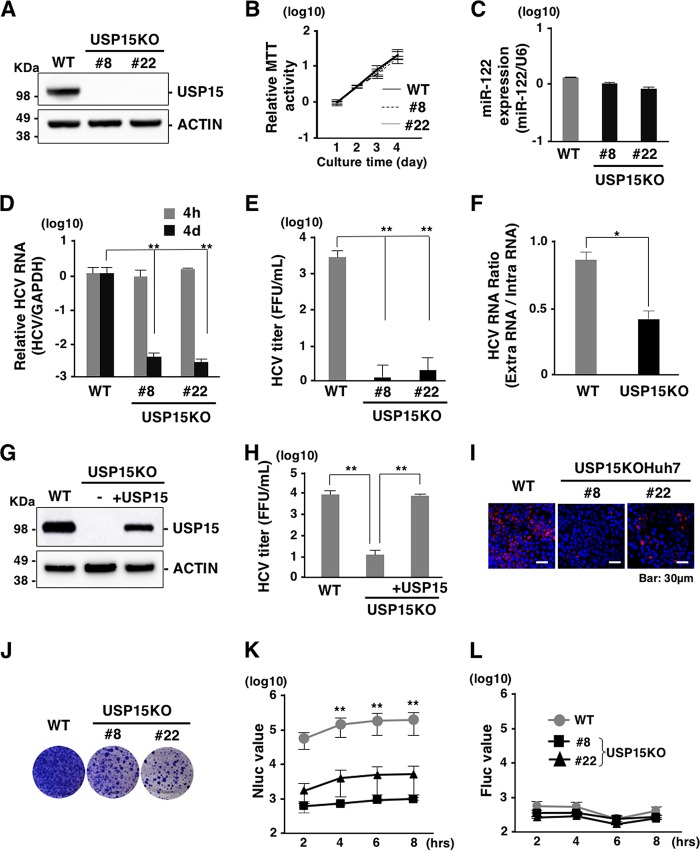
To further confirm the effect of USP15 on HCV propagation, we established two USP15 knockout Huh7 cell lines (USP15KOHuh7 clones 8 and 22) (Fig. 3A) using the CRISPR/Cas9 system. The cell growth (Fig. 3B) and expression of miR-122, a determinant of HCV propagation in hepatocytes (Fig. 3C), of the USP15KOHuh7 cell lines were comparable to those of parental Huh7 cells. To evaluate the effect of USP15 deficiency on HCV propagation, Huh7 and USP15KOHuh7 cells were infected with HCV. Intracellular HCV RNA levels (Fig. 3D), the infectious titer in the culture supernatants (Fig. 3E), and the ratio of extra- to intracellular HCV RNAs (Fig. 3F) at 4 days postinfection were decreased in USP15KOHuh7 cells. Exogenous expression of USP15 in USP15KOHuh7 cells restored the production of infectious HCV particles (Fig. 3G and H). These data suggest that USP15 is involved in HCV propagation. In addition, Huh7 and USP15KOHuh7 cells were transfected with an HCV subgenomic replicon RNA by electroporation, and colony formation was determined. Deficiency of USP15 decreased the expression of HCV protein (Fig. 3I) and the numbers of colonies compared to those in Huh7 cells (Fig. 3J). To further examine the roles of USP15 in translation of HCV RNA, an in vitro-transcribed HCV subgenomic RNA (sgRNA) possessing mutations in the active sites of RNA-dependent RNA polymerase of NS5B and a secreted form of NanoLuc (NLuc) as a reporter (pSGR-NLuc-JFH1GND) was transduced into Huh7 and USP15KOHuh7 cells by electroporation. NLuc activities were significantly suppressed in the USP15KOHuh7 cell lines (clones 8 and 22) compared with those in parental Huh7 cells (Fig. 3K). On the other hand, the activity of cap-dependent translation exhibited no significant difference between Huh7 and USP15KOHuh7 cells (Fig. 3L). Collectively, these results suggest that USP15 participates in HCV propagation through at least two distinct pathways, production of infectious particles and translation of viral RNA.
FIG 3.
Deficiency of USP15 impairs HCV propagation. (A) USP15-deficient Huh7 (USP15KOHuh7) cells were generated using a CRISPR/Cas9 system. The expression of USP15 was confirmed by immunoblotting. We successfully established two independent USP15KOHuh7 clones. (B) Wild-type (WT) and USP15KOHuh7 cells were seeded onto 24-well plates and incubated for 4 days. The cell growth of each cell line was analyzed by using an MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay kit (Nacalai Tesque, Kyoto, Japan) according to the manufacturer’s protocol. (C) The expression of miR-122 in WT and USP15KOHuh7 cells was quantified by qRT-PCR. U6 RNA was used as the internal control. (D) WT and USP15KOHuh7 cells were infected with HCV at an MOI of 3. After 4 h or 4 days postinfection, the intracellular HCV RNA level was quantified by qRT-PCR using GAPDH mRNA as the internal control. (E) WT and USP15KOHuh7 cells were infected with HCV at an MOI of 3. After 4 days postinfection, infectious titers in the culture supernatants were determined by a focus-forming assay. (F) WT and USP15KOHuh7 cells were infected with HCV at an MOI of 3. After 4 days postinfection, extracellular and intracellular HCV RNA levels were quantified by qRT-PCR. The ratios between intracellular and extracellular HCV RNA levels were calculated as HCV RNA rates. (G) USP15KOHuh7 cells were transfected with a plasmid expressing FLAG-USP15, and USP15KOHuh7 cells expressing FLAG-USP15 were established. The expression of USP15 was confirmed by immunoblotting. (H) USP15KOHuh7 cells, cell lines with restored USP15, and Huh7 cells were infected with HCV. After 4 days postinfection, infectious titers in the culture supernatants were determined by a focus-forming assay. (I) HCV was inoculated into Huh7 cells or USP15KOHuh7 cells at an MOI of 3 and stained with anti-NS5A antibody at 4 days postinfection. (J) In vitro-transcribed HCV subgenomic replicon RNA (pSGR-JFH1) was electroporated into WT and USP15KOHuh7 cells, and the cells were incubated for 3 weeks in the presence of 1 mg/ml of G418. Colonies were visualized by Giemsa staining. (K) In vitro-transcribed HCV subgenomic replicon RNA (pSGR-NLuc-JFH1GND) was electroporated into WT and USP15KOHuh7 cells, and the activity of NLuc in culture supernatants was monitored. (L) In vitro-transcribed capped-FLuc RNA was electroporated into parental and USP15KOHuh7 cells, and the activity of FLuc in electroporated cells was monitored.
USP15 supports HCV propagation in a hepatocyte-specific manner.
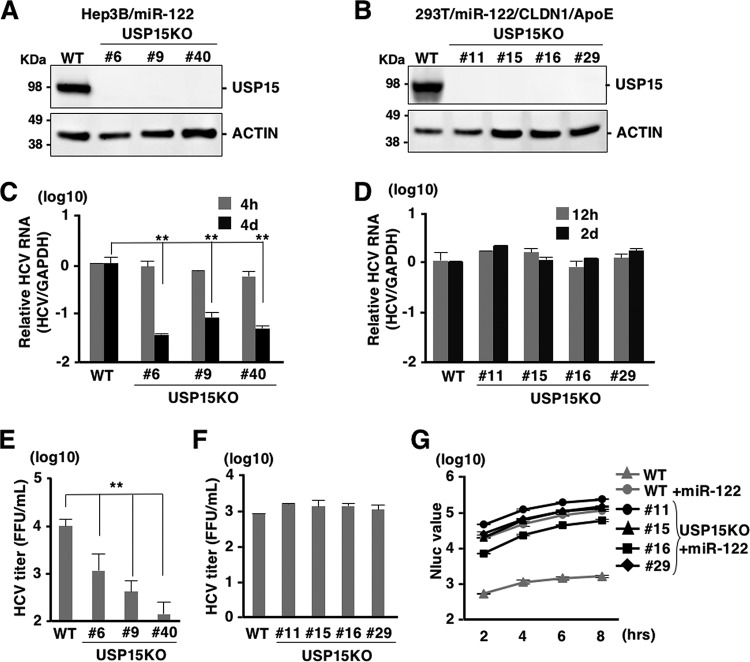
Not only Huh7 cells but also Hep3B cells expressing miR-122 (Hep3B/miR-122), HepG2 cells expressing CD81 (HepG2/CD81), and 293T cells expressing miR-122, Claudin 1 (CLDN1), and APOE (293T/miR-122/CLDN1/APOE) have been shown to permit HCV propagation (36–38). To examine the effects of USP15 on HCV propagation in cell lines other than Huh7 cells, we also established USP15KO cell lines in Hep3B/miR-122 and 293T/miR-122/CLDN1/APOE cells (Fig. 4A and B). USP15KO and parental Hep3B/miR-122 cells were infected with HCV, and the level of intracellular HCV RNA was determined at 4 h and 4 days postinfection. Although intracellular HCV RNA levels were comparable in USP15KO and parental Hep3B/miR-122 cells at 4 h postinfection, they were significantly reduced in USP15KO cells at 4 days postinfection (Fig. 4C). Infectious titers in the culture supernatant at 4 days postinfection were also reduced in USP15KO cells (Fig. 4E). These data are consistent with those for Huh7 cells (Fig. 3D and E). In contrast, intracellular HCV RNA levels in USP15KO and parental 293T/miR-122/CLDN1/APOE cells at 12 h and 2 days postinfection (Fig. 4D) and infectious titers in the culture supernatants at 2 days postinfection (Fig. 4F) were comparable, suggesting that USP15 is required for propagation of HCV in hepatic cell lines (Huh7 and Hep3B cells) but not in a nonhepatic cell line (293T cells). To examine the effect of USP15 in 293T cells on the translation of viral RNA, subgenomic HCV RNA of SGR-NLuc-JFH1GND was electroporated into USP15KO and parental 293T cells expressing miR-122. Although the expression of miR-122 in parental 293T cells significantly enhanced the translation efficiency of HCV RNA, NLuc activities were comparable among USP15KO and parental 293T cells expressing miR-122 (Fig. 4G), suggesting that translation of HCV RNA is not dependent on USP15 in 293T cells. It has been proposed that HCV utilized a liver-specific function for efficient propagation (1). Therefore, our data suggest that USP15 participates in HCV propagation in an miR-122-independent and hepatocyte-specific manner.
FIG 4.
Cell type-specific reduction of HCV propagation in USP15KO cells. (A) USP15KOHep3B/miR-122 cells (clones 6, 9, and 40) were generated using the CRISPR/Cas9 system. The expression of USP15 was confirmed by immunoblotting. (B) USP15KO293T cells (clones 11, 15, 16, and 29) were also generated using the CRISPR/Cas9 system and subjected to immunoblotting to confirm USP15 expression. (C) USP15KOHep3B/miR-122 and parental Hep3B/miR-122 cells were infected with HCV at an MOI of 3. After 4 h or 4 days postinfection, intracellular HCV RNA was quantified by qRT-PCR using GAPDH mRNA as the internal control. (D) Parental and USP15KO293T cells were lentivirally transduced with miR-122, CLDN1, and APOE and then infected with HCV (MOI = 10) at 2 days posttransduction. After 12 h or 2 days postinfection, intracellular HCV RNA levels were quantified by qRT-PCR. (E) Infectious titers of parental and USP15KOHep3B/miR-122 cells in the culture supernatants were determined by a focus-forming assay at 4 days postinfection. (F) Infectious titers of parental and USP15KO293T cells in the culture supernatants were determined by a focus-forming assay at 2 days postinfection. (G) In vitro-transcribed subgenomic HCV replicon RNA (SGR-NLuc-JFH1GND) was electroporated into parental and USP15KO293T cells expressing miR-122, and the activity of NLuc in the culture supernatants was determined.
USP15 is not involved in innate immune responses.
USP15 has been reported to be a DUB targeting tripartite motif-containing protein 25 (TRIM25) and to positively regulate RIG-I-mediated innate immune responses (39). TRIM25 has been shown to conjugate the K63-linked ubiquitin chains to RIG-I to facilitate downstream signaling pathways (39). USP15 has been reported to remove the K48-linked ubiquitin chains of TRIM25 mediated by the linear ubiquitin assembly complex (LUBAC) to protect TRIM25 from proteasomal degradation (39). On the other hand, USP15 has been shown to target RIG-I and to remove the K63-linked ubiquitin chains from RIG-I (40). RIG-I senses viral RNAs such as those of Japanese encephalitis virus (JEV) and vascular stomatitis virus (VSV) and activates downstream molecules to activate innate immune responses (41). In contrast, encephalomyocarditis virus (EMCV) is recognized by melanoma differentiation-associated gene 5 (MDA5) rather than RIG-I (41). To investigate the involvement of USP15 in RNA virus infection, parental and USP15KOHuh7 cells were infected with JEV, VSV, and EMCV. Although intracellular JEV RNA levels were comparable between parental and USP15KO cells (Fig. 5A), infectious titers in the culture supernatants at 2 days postinfection were slightly decreased in USP15KOHuh7 cells (Fig. 5B). In contrast, viral titers of VSV and EMCV were comparable between parental and USP15KOHuh7 cells (Fig. 5C and D). These data suggest that USP15 plays a small role in the propagation of JEV but is not involved in the propagation of VSV and EMCV.
FIG 5.

USP15 is partially involved in propagation of JEV but not VSV or EMCV. (A) WT or USP15KOHuh7 cells were infected with JEV at an MOI of 3. The intracellular JEV RNA level was quantified by qRT-PCR at each time point. (B) WT or USP15KOHuh7 cells were injected with JEV at an MOI of 3 and incubated for 2 days. Infectious JEV titers in the culture supernatants were determined by a focus-forming assay. (C) WT and USP15KOHuh7 cells were infected with VSV at an MOI of 3. Infectious VSV titers in the culture supernatants were determined by a plaque-forming assay at the indicated time points. (D) WT and USP15KOHuh7 cells were infected with EMCV at an MOI of 1. Infectious EMCV titers in the culture supernatants were determined by a plaque-forming assay at the indicated time points. hpi, hours postinfection.
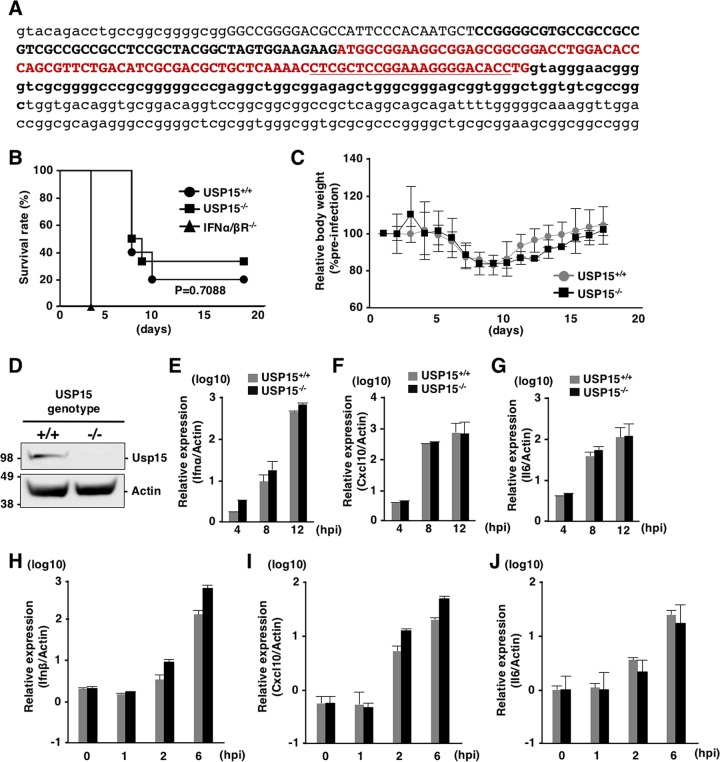
To further assess the involvement of USP15 in RIG-I-mediated antiviral effects in vivo, we generated USP15−/− mice possessing a deletion of 223 bp in the Usp15 genomic locus by using the CRISPR/Cas9 system (Fig. 6A). The USP15−/− mice were fertile and visually normal, as reported previously (42). We intranasally challenged USP15−/−, USP15+/+, and IFNα/βR−/− mice with a lethal dose of VSV and monitored the survival rates and body weights. Deficiency of USP15 had no significant effect on the survival of mice against VSV infection, while IFNα/βR−/− mice showed high sensitivity to VSV challenge (Fig. 6B), and the changes of body weight were comparable between USP15+/+ and USP15−/− mice (Fig. 6C), suggesting that USP15 does not participate in survival after VSV challenge. In addition, mouse embryonic fibroblasts (MEFs) prepared from USP15+/+ and USP15−/− mice (Fig. 6D) were infected with VSV, and the induction of ISGs was monitored. The inductions of Ifnα, Cxcl10, and Il6 were comparable between the two MEFs (Fig. 6E to G). Finally, USP15+/+ MEFs and USP15−/− MEFs were transfected with poly(I·C). Sensing of poly(I·C) has been shown to be length dependent. High- and low-molecular-weight poly(I·C) are recognized by MDA5 and RIG-I, respectively (43), and the production of mRNAs of Ifnβ, Cxcl10, and Il6 in USP15+/+ and USP15−/− MEFs upon transfection with low-molecular-weight poly(I·C) exhibited no difference (Fig. 6H to J). Collectively, these results suggest that USP15 does not participate in the induction of innate immune responses in vitro and in vivo.
FIG 6.
USP15 is not involved in innate immune responses in vivo. (A) Generation of USP15−/− mice. Lowercase letters indicate parts of introns, and capital letters indicate exon 1. The red sequence is the open reading frame of exon 1 of USP15. Sequences of the guide RNA targeting USP15 are underlined. USP15−/− mice possessed the 223-nucleotide deletion shown in boldface type. (B and C) USP15+/+ (n = 10), USP15−/− (n = 6), and IFNα/βR−/− (n = 5) mice (16 to 17 weeks old) were intranasally infected with VSV (4 × 106 PFU), and their survival rates (B) and body weights (C) were monitored daily. Body weight changes at each time point are indicated as values relative to the body weights of mice at 1 day postinfection. (D) MEFs were prepared from USP15−/− and USP15+/+ mice. The expression of USP15 was confirmed by immunoblotting. (E to G) MEFs infected with VSV at an MOI of 1 were collected at each time point, and the mRNAs of Ifnα (E), Cxcl10 (F), and Il6 (G) were quantified by qRT-PCR. (H to J) MEFs transfected with low-molecular-weight poly(I·C) (2 μg/ml) were collected at each time point, and the mRNA levels of Ifnβ (H), Cxcl10 (I), and Il6 (J) were determined by qRT-PCR.
USP15 participates in lipid metabolism to facilitate HCV propagation.
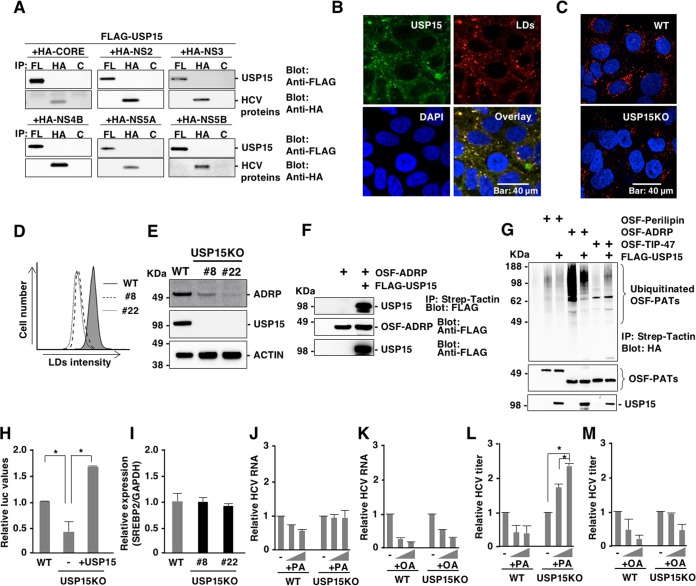
Due to a lack of information about the function of USP15 in hepatocytes, we investigated the roles of USP15 during HCV infection. First, we investigated the possibility that USP15 interacts with viral proteins. Immunoprecipitation analysis revealed no interaction of USP15 with viral proteins (Fig. 7A). Confocal microscopic observation showed that USP15 colocalized with LDs (Fig. 7B). Previous reports suggest that LDs and lipid metabolism are important for HCV replication and infectious particle formation (19, 24, 44). Therefore, we investigated the roles of USP15 in the formation of LDs. LDs in parental and USP15KOHuh7 cells were stained with HCS LipidTOX Red neutral lipid stain and observed by confocal microscopy. The amounts of LDs were significantly reduced in USP15KOHuh7 cells compared with parental Huh7 cells (Fig. 7C and D). Adipose differentiation-related protein (ADRP) is a marker for LDs (45), and expression of ADRP was significantly impaired in the USP15KOHuh7 cell lines (Fig. 7E). Next, we examined the interaction of USP15 with ADRP. Immunoprecipitation analyses revealed that USP15 specifically interacts with ADRP (Fig. 7F). ADRP is a member of the perilipin/APRP/TIP47 (PAT) family, which shares a conserved region named the PAT domain (45). We then examined the ubiquitination of PAT family proteins and deubiquitination by USP15. ADRP, but not perilipin and TIP-47, was ubiquitinated, and the expression of USP15 reduced the ubiquitination of ADRP (Fig. 7G), suggesting that ADRP is one of the substrates for USP15. LDs consist of neutral lipids, such as cholesterol esters and triglycerides (TGs), and function as storage sites for fatty acids (46). SREBP-1c and SREBP-2 are master transcriptional factors that regulate fatty acid synthesis and cholesterol synthesis, respectively (47, 48). Next, we examined the expression of these transcriptional factors in USP15KOHuh7 cells. Expression of SREBP-1c but not SREBP-2 was significantly reduced in USP15KOHuh7 cells (Fig. 7H and I), suggesting that reduction of fatty acid production participates in the suppression of HCV propagation in USP15KOHuh7 cells. To verify this possibility, palmitic acid (PA) and oleic acid (OA) were added to the culture media of parental and USP15KOHuh7 cells, and the intracellular HCV RNA level was determined 4 days after infection with HCV. Although intracellular HCV RNA levels in both parental and USP15KOHuh7 cells exhibited no significant change by the addition of fatty acids (Fig. 7J and K), infectious titers in the culture supernatants of USP15KOHuh7 cells but not those of parental Huh7 cells were significantly enhanced by the addition of PA but not OA (Fig. 7L and M). Taken together, these results suggest that USP15 participates in the regulation of lipid metabolism and facilitates the production of infectious HCV particles.
FIG 7.
USP15 controls LD formation to facilitate HCV propagation. (A) Interactions between FLAG-USP15 and HA-tagged viral proteins in 293T cells were evaluated by immunoprecipitation (IP) with antibodies to FLAG (FL), HA, and Glu-Glu (control [C]) tags. Immunoprecipitants were subjected to immunoblotting by using FLAG and HA antibodies. (B) The subcellular localization of USP15 in Huh7 cells was observed by confocal microscopy. USP15 (green), lipid droplets (red), and nuclei (blue) were stained with anti-USP15 antibody, HCS LipidTOX Red neutral lipid stains, and DAPI, respectively. (C) Lipid droplets in WT or USP15KOHuh7 cells were observed by confocal microscopy. Lipid droplets (red) and nuclei (blue) were stained with HCS LipidTOX Red neutral lipid stains and DAPI, respectively. (D) The intensity of stained LDs was quantified by FACS analysis. (E) The expression of ADRP, a marker of LDs, in parental and USP15KOHuh7 cells was detected by immunoblotting by using the indicated antibodies. (F) The interaction between FLAG-USP15 and OSF-tagged ADRP in 293T cells was evaluated by immunoprecipitation with Strep-Tactin beads. Immunoprecipitants were subjected to immunoblotting by using FLAG antibodies. (G) HA-ubiquitin and OSF-perilipin, -ADRP, or -TIP-47 were coexpressed in 293T cells with or without FLAG-USP15. Ubiquitinated proteins were purified by using Strep-Tactin beads at 2 days posttransfection. Immunoprecipitants were subjected to immunoblotting by using HA antibodies. (H) The expression levels of SREBP-1c in Huh7 cells, USP15KOHuh7 cells, and restored USP15Huh7 cells were examined by using a reporter assay. Huh7 cells, USP15KOHuh7 cells, and USP15KOHuh7 cells expressing USP15 were transfected with pGL3-Basic SREBP-1c and pRL-TK and incubated for 2 days. Luciferase activity was determined by using a dual-luciferase reporter assay system (Promega, Madison, WI). (I) The expression of SREBP2 in parental and USP15KOHuh7 cells was quantified by qRT-PCR. mRNA of GAPDH was used as the internal control. (J and L) WT and USP15KOHuh7 cells were treated with 100 or 200 μM palmitic acid (PA) during HCV infection at an MOI of 3. After 4 days postinfection, intracellular HCV RNA levels (J) or infectious HCV titers (L) in culture supernatants were determined. (K and M) Huh7 and USP15KOHuh7 cells were treated with 100 or 200 μM oleic acid (OA) during HCV infection at an MOI of 3. After 4 days postinfection, intracellular HCV RNA levels (K) or infectious HCV titers (M) in the culture supernatants were determined.
DISCUSSION
In this study, we identified USP15 as a novel host factor for HCV propagation. This is the first report to identify a specific DUB involved in HCV propagation. Our data suggest that USP15 participates in at least two steps in the HCV life cycle: translation of viral RNA and production of infectious particles. HCV RNA is translated to viral proteins through the internal ribosomal entry site (IRES) (49). Deficiency of USP15 significantly impaired the translation of viral RNA specific for the HCV IRES. In addition, the lack of USP15 showed no effect on HCV propagation in nonhepatic cells, such as 293T cells, suggesting that USP15 targets hepatocyte-specific factors to regulate the translation of HCV RNA. Fehr et al. reported that HCV translation was active in G0/G1 phase and that expression of endogenous miR-122 and AGO2 was impaired in S phase in Huh7 cells but not in HeLa cells (50), suggesting that cell type-specific regulation is involved in HCV translation and expression of miR-122. Liver-specific miR-122 binds to the two sites of HCV RNA to facilitate HCV replication (11, 51), and many reports suggest that binding of miR-122 to HCV RNA promotes the translation of HCV RNA (52, 53). Because DUB is unlikely to directly interact with miR-122, USP15 may interact with components of the miR-122/AGO2 complex. Although the activity of HCV IRES-mediated RNA translation was significantly enhanced by the expression of miR-122, as previously reported, deficiency of USP15 did not affect the enhancement of HCV IRES-mediated translation by the expression of miR-122 (Fig. 4G), suggesting that USP15 participates in HCV IRES-mediated translation through an miR-122-independent pathway. Various host factors, such as heterogeneous nuclear ribonucleoprotein L (hnRNP L), nuclear factor 90 (NF90), NF45, poly(C)-binding protein 2 (PCBP2), and insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), have been shown to participate in IRES-mediated translation and replication of HCV through the interaction between 5′ and 3′ untranslated regions (UTRs) of HCV (54–57). Further studies are needed to understand the roles of USP15 in HCV RNA translation through interaction with these host factors.
LDs are cellular organelles for the storage of TGs and have a single membrane (58). Once cells require energy, stored TGs undergo hydrolysis to produce fatty acids through the activation of lipolytic pathways. A number of LD-associated proteins have been identified. Members of the perilipin family, which includes perilipin, ADRP, TIP-47, S3-12, and myocardial lipid droplet protein (MLDP)/oxidative tissue-enriched PAT protein (OXPAT)/lipid storage droplet protein 5 (LSDP5), are major regulators of LD homeostasis (59, 60). Among them, HCV NS5A interacts with TIP-47 to facilitate HCV replication (61). In addition, HCV core protein has been shown to displace ADRP from the LD surface (62) and to contribute to efficient virus assembly through interaction with LDs (63). In this study, we show that USP15 colocalizes with LDs in Huh7 cells without any interaction with HCV proteins, suggesting that USP15 interacts with LD-associated proteins to regulate LD formation and participates in the production of infectious HCV particles. We showed that a lack of USP15 reduces the amounts of LDs in Huh7 cells. It is known that nonhepatic cells, such as 293T cells, possess small amounts of LDs, and in 293T cells, LDs and LD-related fatty acids are not involved in the propagation of HCV. This might be one of the reasons why HCV propagation in USP15KO293T cells is comparable to that in parental cells. We also showed that expression of SREBP-1c was impaired in USP15KOHuh7 cells. It was demonstrated that HCV core protein enhances the binding of liver X receptor α (LXRα) and retinoid X receptor α (RXRα) to the LXR response element during HCV infection (64, 65). These reports indicate that HCV core protein plays crucial roles in the modulation of lipid metabolism during HCV infection. However, immunoprecipitation analyses showed that USP15 does not interact with HCV core protein, suggesting that USP15 regulates lipid metabolism independent of the interaction with HCV core protein.
USP15 is expressed in various tissues and has been shown to be involved in various cellular events. USP15 targets receptor-activated SMADs (R-SMADs) to regulate transforming growth factor β (TGF-β) signaling (66). In addition, USP15 interacts with SMAD7 and SMAD-specific E3 ubiquitin protein ligase 2 (SMURF2) and deubiquitinates the type I TGF-β receptor (67). USP15 has been reported to deubiquitinate Kelch-like ECH-associated protein 1 (Keap1), which regulates NF-E2-related factor 2 (Nrf2)-dependent antioxidant responses (68); murine double minute 2 (MDM2) (42); histone H2B (H2B) (69); Nrf1 (70); and p62 (71). USP15 has also been suggested to cleave ubiquitin chains of viral proteins, such as Nef of HIV-1 (72), HBx of hepatitis B virus (HBV) (73), and E6 protein of human papillomavirus (74). These reports indicate that USP15 participates in the development of many types of cancers, cellular homeostasis, and virus infection. However, the substrates and function of USP15 in hepatocytes have not been clarified. In this study, we suggest that ADRP is a novel substrate for USP15. Suppression of ADRP expression through loss of the interaction with USP15 might be a reason for the reduced size of LDs, because ADRP was reported to be involved in the determination of the size of LDs (75). Furthermore, we showed that the birth of USP15−/− mice followed Mendelian ratios, and the mouse pups developed normally and exhibited no obvious abnormalities, as reported previously (42). Recently, USP15 has been shown to be a DUB for TRIM25 (39) and RIG-I (40) and to be involved in the type I interferon (IFN) response in cells infected with RNA viruses. Upon infection by HCV, viral RNA is sensed by RIG-I, and type I IFN and ISGs are induced (76). We examined the involvement of USP15 in innate immune responses and propagation of various RNA viruses and revealed that USP15 does not participate in either the survival of mice or innate immune responses in MEFs. These data suggest that USP15 is dispensable for the induction of innate immune responses upon infection with RNA viruses. Further characterization of USP15−/− mice is needed in order to elucidate the physiological function of USP15, especially in hepatocytes.
Fatty acids have been shown to support HCV replication in replicon cells (77, 78). On the other hand, several recent papers showed that fatty acids inhibit HCV replication (77, 79–81). In the present study, we found that deficiency of USP15 in Huh7 cells reduced the amounts of LDs, and the addition of PA, but not of OA, partially restored the production of infectious HCV particles. We do not have any data indicating why OA did not support the production of infectious HCV. However, our data suggest that PA and its metabolites support the production of infectious HCV particles. On the other hand, we could not observe an enhancement of HCV particle production by treatment with PA in naive Huh7 cells due to the abundance of LDs. Taken together, these data suggest that USP15 participates mainly in the replication of HCV RNA and partially in the production of infectious HCV particles.
In summary, we identified USP15 as a novel host protein involved in HCV propagation. USP15 participates in HCV translation and plays a role in the production of infectious particles. Our data suggest that USP15 participates in the formation of LDs through the regulation of hepatic lipid metabolism to facilitate HCV propagation. Because USP15 possesses the enzymatic activity of a deubiquitinase and thus removes ubiquitin from substrates, in a future study, it would be of interest to identify the substrates of USP15 in hepatocytes. The hepatocyte-specific substrates of USP15 that are crucial for HCV propagation might be novel drug targets for chronic hepatitis C.
MATERIALS AND METHODS
Cell lines and viruses.
Huh7, Huh7.5.1, Hep3B/miR-122, Vero, 293T, Plat-E, and BHK-21 cells were obtained from the National Institute of Infectious Diseases and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 10 μg/ml streptomycin. HCV replicon cells (9-13) (82) were maintained in DMEM supplemented with 10% FBS, 1 mg/ml G418, 100 U/ml penicillin, and 10 μg/ml streptomycin. HCV derived from the genotype 2a JFH-1 strain mutated in E2, p7, and NS2 was prepared by serial passages in Huh7.5.1 cells, as mentioned previously (83). JEV (AT31 strain) was propagated in C6/36 cells. VSV and EMCV were propagated in BHK-21 cells.
Antibodies and reagents.
The following antibodies were used: anti-JEV NS3 monoclonal antibody (catalog number 578) (84), anti-HCV NS5A monoclonal antibody (5A27) (85), antiactin mouse monoclonal antibody (catalog number A2228; Sigma-Aldrich), horseradish peroxidase (HRP)-conjugated anti-FLAG mouse monoclonal antibody (catalog number A8592; Sigma-Aldrich), anti-USP15 monoclonal antibody (catalog number ab56900; Abcam), anti-FLAG mouse monoclonal antibody (catalog number F1804; Sigma-Aldrich), antihemagglutinin (anti-HA) monoclonal antibody (clone 16B12, catalog number MMS-101P; BioLegend), and anti-Glu-Glu antibody (catalog number MMS-115P; BioLegend). Oleic acid (OA) (catalog number O1008) and palmitic acid (PA) (catalog number P0500) were obtained from Sigma-Aldrich. PR-619 (catalog number SI9619) was purchased from LifeSensor. HCS LipidTOX Red neutral lipid stain was obtained from Thermo Fisher Scientific. Low-molecular-weight poly(I·C) was purchased from InvivoGen.
Plasmids.
The shRNAs against each DUB were obtained from the human shRNA library (TaKaRa Bio). pRSV-Rev (catalog number 12253), pMDLg/pRRE (catalog number 12251), pCMV-VSV-G (catalog number 8454), pX330 (catalog number 42230), and pCAG EGxxFP (catalog number 50716) were obtained from Addgene. Reporter plasmids pGL3-Basic SREBP1c and pRL-TK were previously used (64). Lentiviral vectors expressing miR-122, APOE, and Claudin 1 (CLDN1) were also used as described previously (19). The cDNAs of USP15, OTUB1, OTUD1, and OTUD7B, obtained from Wade Harper (catalog numbers 22570, 22551, 22553, and 22550; Addgene), were amplified by PCR and cloned into pEF FLAG pGK puro (86) or FUIPW (26) by using an In-Fusion HD cloning kit (TaKaRa Bio). HCV core, NS2, NS3, NS4B, NS5A, and NS5B were amplified by PCR and cloned into pCAGGS (87). The plasmid for expression of HA-tagged ubiquitin was used as described previously (64). The cDNAs of human perilipin, ADRP, and TIP-47 were amplified from cDNA generated from Huh7 cells and cloned into the pEF OSF (One-Strep-FLAG [OSF] tag) expression vector (88).
The sgRNAs of human USP15 (5′-CACCGCGACTATCGACTAGGTACC-3′) and mouse USP15 (5′-CACCGGTGTCCCCTTTCCGGAGCG-3′) were cloned into pX330 by using Ligation high ver. 2 (Toyobo). Genomic DNAs of Huh7 cells or mouse tails were extracted by using DirectPCR lysis reagents (Viagen Biotech) and amplified by PCR using a human USP15 primer set (forward primer 5′-CAACCACTGAGGATCCGCTCCCGGTGTCTTTTGGTTTCGA-3′ and reverse primer 5′-TGCCGATATCGAATTCCCTATCATTCGGGAAGGCCTGAGGT-3′) and a mouse USP15 primer set (forward primer 5′-CAACCACTGAGGATCCATTTGGTACAGACCTGCCGG-3′ and reverse primer 5′-TGCCGATATCGAATTCTCGGAATAATGGGGAACTTGGG-3′). They were then cloned into pCAG EGxxFP by using an In-Fusion HD cloning kit. pSGR-JFH1 was mutated in the GDD motif of NS5B to GND to generate an inactive form of RNA-dependent RNA polymerase. In addition, the cDNA of NanoLuc was replaced with the neo gene to generate pSGR-NLuc-JFH1GND. The cDNA of firefly luciferase (FLuc) was amplified and cloned into the pCMVTNT vector (Promega), and the construct was designated pCMVTNT Fluc. All plasmids used in this study were confirmed by sequencing with an ABI Prism 3130 genetic analyzer (Thermo Fisher Scientific).
RNAi screening.
Retroviruses expressing shRNAs against human DUBs were generated in Plat-E cells. Briefly, Plat-E cells (2 × 106 cells) were seeded on a 10-cm dish and incubated at 37°C for 24 h. Five micrograms of the retroviral transfer vector and 1 μg of pCMV-VSV-G were mixed with 500 μl of Opti-MEM and 40 μl of polyethylenimine (1 mg/ml; Polysciences), and the mixture was incubated for 15 min. The DNA complex was inoculated into Plat-E cells, and the culture medium was changed at 4 h posttransfection. The culture supernatants were collected at 3 days posttransfection. Huh7.5.1 cells (2 × 105 cells per well) were seeded on six-well plates and incubated for 24 h. The virus-containing culture supernatants (2 ml) and 8 μl of Polybrene (4 mg/ml; Sigma-Aldrich) were inoculated into Huh7.5.1 cells, and the mixture was centrifuged at 1,220 × g for 45 min at 32°C. Stable cell lines were selected with puromycin (1 μg/ml) at 2 days postinfection. To determine the efficiency of RNAi, the expression of each DUB was analyzed by using quantitative real-time PCR (qRT-PCR), as described below. Huh7.5.1 cells expressing shRNA were seeded on 24-well plates (3 × 104 cells per well), incubated for 24 h, and inoculated with HCV at a multiplicity of infection (MOI) of 0.5. After 2 h, the culture medium was changed, and the infected cells were incubated for 4 days. Intracellular HCV RNA was quantified by qRT-PCR.
qRT-PCR.
Total RNA was extracted by using Isogen II (Nippon Gene). Real-time PCR for HCV or JEV RNA was performed by using a TaqMan RNA-to-Ct 1-Step kit and a ViiA7 real-time PCR system (Thermo Fisher Scientific). The following primers were used: 5′-GAGTGTCGTGCAGCCTCCA-3′ and 5′-CACTCGCAAGCACCCTATCA-3′ for HCV 5′-GGGTCAAAGTCATTTCTGGTCCATA-3′ and 5′-TCCACGCTGCTCGAA-3′ for JEV, and 5′-TGTAGTTGAGGTCAATGAAGGG-3′ and 5′-ACATCGCTCAGACACCATG-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following probes were used: 5′-FAM (6-carboxyfluorescein)-CTGCGGAAC-ZEN-CGGTGAGTACAC-3′-IABkFQ for HCV, 5′-FAM-ATGACCTCG-ZEN-CTCTCCC-3′-IABkFQ for JEV, and 5′-FAM-AAGGTCGGA-ZEN-GTCAACGGATTTGGTC-3′-IABkFQ for GAPDH. Relative amounts of HCV or JEV RNA were determined by the ΔΔCT method using GAPDH as an internal control. For gene expression analysis, qRT-PCR was performed by using a Power SYBR green RNA-to-Ct 1-step kit (Thermo Fisher Scientific). The primers used in this study are summarized in Table 1.
TABLE 1.
Primer set list for real-time PCR
Cell viability assay.
Huh7 (9-13) cells were seeded on 24-well plates (5 × 104 cells per well) and incubated at 37°C for 24 h. PR-619 (0.5 μM) was added, and the cells were incubated for an additional 24 h. Supernatants containing cells and adherent cells were collected and stained with 5 μg/ml of propidium iodide (PI) (catalog number P4170; Sigma-Aldrich). Cell viability was determined by flow cytometry analyses (BD) using FlowJo software (FlowJo).
Generation of USP15 knockout Huh7, Hep3B, and 293T cells.
Gene knockout Huh7, Hep3B/miR-122, and 293T cells were generated by using the CRISPR/Cas9 system as previously described (89). Briefly, cells were transfected with pX330 and pCAG EGxxFP and incubated for 1 week. Green fluorescent protein (GFP)-positive cells were sorted by fluorescence-activated cell sorter (FACS) analysis and formed single colonies. Gene deficiency was confirmed by sequencing and Western blotting.
In vitro transcription, RNA transfection, and colony formation.
The plasmid pSGR-JFH1 was linearized with XbaI and transcribed in vitro by using a MEGAscript T7 kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. pCMVTNT Fluc was linearized with BamHI and transcribed in vitro by using an mMessage mMachine T7 Ultra transcription kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. The in vitro-transcribed RNA (10 μg) was electroporated into Huh7 cells (5 × 106 cells) under conditions of 270 V and 950 μF using a Gene Pulser apparatus (Bio-Rad). For the transient experiments, cells were added into 10 ml of culture medium and plated on 12-well plates. For long-term colony formation, electroporated cells were plated in DMEM containing 10% FBS. The medium was replaced with fresh DMEM containing 10% FBS and 1 mg/ml G418 at 24 h postelectroporation. Colonies were visualized by staining with Giemsa (Merck) at 3 weeks postelectroporation.
Immunofluorescence staining.
Huh7 cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS). Cells were washed with PBS and permeabilized with 0.2% Triton X-100 in PBS for 15 min. After washing with PBS, the fixed cells were incubated with anti-NS5A mouse monoclonal antibody or anti-USP15 mouse monoclonal antibody at room temperature for 1 h. After washing, cells were incubated with Alexa Fluor 488-conjugated anti-mouse antibody and HCS LipidTOX Red neutral lipid stain diluted with 2% FBS in PBS at room temperature for 1 h. The stained cells were covered with Prolong Gold AntiFade reagent with DAPI (4′,6-diamidino-2-phenylindole) (Thermo Fisher Scientific) and observed by FluoView FV1000 confocal microscopy (Olympus).
Immunoprecipitation and immunoblotting.
Cells were lysed with lysis buffer consisting of 20 mM Tris-HCl (pH 7.4), 135 mM NaCl, 1% Triton X-100, 1% glycerol, and protease inhibitor cocktail tablets (Roche); incubated for 30 min at 4°C; and subjected to centrifugation at 14,000 × g for 15 min at 4°C. The supernatants were boiled at 95°C for 5 min, and the remaining supernatants were then incubated with anti-FLAG, -HA, or -Glu-Glu antibodies at 4°C for 90 min. After incubation with protein G-Sepharose 4B (GE Healthcare) at 4°C for 90 min, the beads were washed five times with lysis buffer and boiled at 95°C for 5 min. For ubiquitination assays, supernatants were incubated with Strep-Tactin Sepharose (IBM GmbH) for 90 min and washed with lysis buffer three times, and the precipitants were then boiled in sample buffer at 95°C for 5 min. The proteins were resolved by SDS-PAGE (Novex gels; Invitrogen), transferred onto nitrocellulose membranes (iBlot; Life Technologies), blocked with PBS containing 0.05% Tween 20 and 5% skim milk, incubated with primary antibody at 4°C for 12 h, and then incubated with HRP-conjugated secondary antibody at room temperature for 1 h. The immune complexes were visualized with Super Signal West Femto substrate (Pierce) and detected by using an LAS-3000 image analyzer system (Fujifilm).
Virus titration.
Viral titers of HCV and JEV were determined by a focus-forming assay as described previously (26). Viral titers of VSV and EMCV were quantified by a plaque-forming assay using BHK-21 cells.
Generation of USP15−/− mice.
USP15−/− mice were generated as previously described, using a C57BL/6N genetic background (89). pX330 containing sgRNA against the mouse Usp15 gene was injected into mouse zygotes and transplanted into pseudopregnant female mice. The obtained mice (F1) were crossed with wild-type mice and F2 mice, and their DNA sequences were analyzed using the primers 5′-ATTTGGTACAGACCTGCCGG-3′ (forward) and 5′-TCGGAATAATGGGGAACTTGGG-3′ (reverse).
Ethics statement.
All animal experiments were approved by the Institutional Committee of Laboratory Animal Experimentation (Research Institute for Microbial Diseases, Osaka University) (project number H27-06-0).
VSV infection in vivo.
USP15+/+, USP15−/−, and IFNα/βR−/− mice (16 to 17 weeks old) were intranasally infected with VSV (4 × 106 PFU). Their survival rates and body weights were monitored.
Reporter assay.
Huh7 cells, USP15KOHuh7 cells, and USP15KOHuh7 cells expressing USP15 were transfected with pGL3-Basic SREBP-1c and pRL-TK and incubated for 2 days. Luciferase activity was determined by using a dual-luciferase reporter assay system (Promega).
Treatment with palmitic acid and oleic acid.
PA and OA were dissolved in ethanol. Dissolved fatty acids were mixed with 10% fatty acid-free bovine serum albumin (BSA) (Sigma-Aldrich) to make a complex of fatty acid and BSA. Huh7 or USP15KOHuh7 cells were infected with HCV and treated with PA or OA for 4 days.
Statistical analysis.
All experiments were performed in triplicate with at least 3 independent experiments. All data represent the means ± standard deviations (SD) from independent experiments. The statistical analyses were performed using GraphPad Prism software. Significant differences were determined using Student’s t test and are indicated with asterisks (*, P < 0.05) and double asterisks (**, P < 0.01) in each figure. Significant differences of in vivo survival data were determined using a log rank test and are indicated with asterisks (*, P < 0.05) and double asterisks (**, P < 0.01) in each figure.
ACKNOWLEDGMENTS
We are grateful to M. Tomiyama for secretarial work and M. Ishibashi for technical assistance. We also thank D. C. S. Huang, R. Bartenschlager, F. Chisari, and T. Wakita for providing experimental materials. The Core Instrumentation Facility of Osaka University conducted the FACS sorting.
This work was supported by the Program for Basic and Clinical Research on Hepatitis from the Japan Agency for Medical Research and Development (AMED) (JP18fk0210006h0003, JP17fk0210305h0003, JP18fk0210010h0003, JP18fk0210009h0503, JP17fk0210304h0003, JP18fk0210040h0001, and JP18fk0108036h0002); by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (JP16H06432, JP16H06429, JP16K21723, JP15H04736, and JP16K19139); and by the Research Program on Emerging and Re-emerging Infectious Diseases (17fk0108109h0001).
Toru Okamoto and Yoshiharu Matsuura designed the research; Shinji Kusakabe, Toru Okamoto, and Tatsuya Suzuki performed most of the experiments. Yukari Sugiyama, Saori Haga, Kanako Horike, Makoto Tokunaga, Junki Hirano, Zhang He, David Virya Chen, Hanako Ishiga, Yasumasa Komoda, Chikako Ono, Takasuke Fukuhara, Tomohisa Tanaka, Kohji Moriishi, Moto Fukai, Sachiyo Yoshio, and Tetsuro Suzuki assisted with the experiments; Masahiro Yamamoto, Masahito Ikawa, Takashi Satoh, Shizuo Akira, Tomohisa Tanaka, Kohji Moriishi, Moto Fukai, Akinobu Taketomi, Sachiyo Yoshio, Tatsuya Kanto, and Tetsuro Suzuki analyzed and interpreted experiments; Masahiro Yamamoto provided the RNAi library; Masahito Ikawa generated USP15−/− mice; Takashi Satoh and Shizuo Akira provided IFNα/βR−/− mice and assisted with the experiments; and Toru Okamoto and Yoshiharu Matsuura obtained research grants and wrote the manuscript.
REFERENCES
- 1.Paul D, Madan V, Bartenschlager R. 2014. Hepatitis C virus RNA replication and assembly: living on the fat of the land. Cell Host Microbe 16:569–579. doi: 10.1016/j.chom.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maasoumy B, Wedemeyer H. 2012. Natural history of acute and chronic hepatitis C. Best Pract Res Clin Gastroenterol 26:401–412. doi: 10.1016/j.bpg.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T, Brechot C. 1997. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci U S A 94:1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki R, Matsuura Y, Suzuki T, Ando A, Chiba J, Harada S, Saito I, Miyamura T. 1995. Nuclear localization of the truncated hepatitis C virus core protein with its hydrophobic C terminus deleted. J Gen Virol 76(Part 1):53–61. doi: 10.1099/0022-1317-76-1-53. [DOI] [PubMed] [Google Scholar]
- 5.Schwer B, Ren S, Pietschmann T, Kartenbeck J, Kaehlcke K, Bartenschlager R, Yen TSB, Ott M. 2004. Targeting of hepatitis C virus core protein to mitochondria through a novel C-terminal localization motif. J Virol 78:7958–7968. doi: 10.1128/JVI.78.15.7958-7968.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki R, Sakamoto S, Tsutsumi T, Rikimaru A, Tanaka K, Shimoike T, Moriishi K, Iwasaki T, Mizumoto K, Matsuura Y, Miyamura T, Suzuki T. 2005. Molecular determinants for subcellular localization of hepatitis C virus core protein. J Virol 79:1271–1281. doi: 10.1128/JVI.79.2.1271-1281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matto M, Rice CM, Aroeti B, Glenn JS. 2004. Hepatitis C virus core protein associates with detergent-resistant membranes distinct from classical plasma membrane rafts. J Virol 78:12047–12053. doi: 10.1128/JVI.78.21.12047-12053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galossi A, Guarisco R, Bellis L, Puoti C. 2007. Extrahepatic manifestations of chronic HCV infection. J Gastrointestin Liver Dis 16:65–73. [PubMed] [Google Scholar]
- 9.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. 1998. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med 4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 10.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. 2004. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology 126:840–848. [DOI] [PubMed] [Google Scholar]
- 11.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. 2005. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 12.Jopling CL, Schutz S, Sarnow P. 2008. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe 4:77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaul A, Stauffer S, Berger C, Pertel T, Schmitt J, Kallis S, Zayas M, Lohmann V, Luban J, Bartenschlager R. 2009. Essential role of cyclophilin A for hepatitis C virus replication and virus production and possible link to polyprotein cleavage kinetics. PLoS Pathog 5:e1000546. doi: 10.1371/journal.ppat.1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto T, Nishimura Y, Ichimura T, Suzuki K, Miyamura T, Suzuki T, Moriishi K, Matsuura Y. 2006. Hepatitis C virus RNA replication is regulated by FKBP8 and Hsp90. EMBO J 25:5015–5025. doi: 10.1038/sj.emboj.7601367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasai H, Kawakami K, Yokoe H, Yoshimura K, Matsuda M, Yasumoto J, Maekawa S, Yamashita A, Tanaka T, Ikeda M, Kato N, Okamoto T, Matsuura Y, Sakamoto N, Enomoto N, Takeda S, Fujii H, Tsubuki M, Kusunoki M, Moriishi K. 2015. Involvement of FKBP6 in hepatitis C virus replication. Sci Rep 5:16699. doi: 10.1038/srep16699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai AW, Benita Y, Peng LF, Kim S-S, Sakamoto N, Xavier RJ, Chung RT. 2009. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe 5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger KL, Cooper JD, Heaton NS, Yoon R, Oakland TE, Jordan TX, Mateu G, Grakoui A, Randall G. 2009. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc Natl Acad Sci U S A 106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, Kaderali L, Poenisch M, Blankenburg H, Hiet M-S, Longerich T, Diehl S, Ramirez F, Balla T, Rohr K, Kaul A, Buhler S, Pepperkok R, Lengauer T, Albrecht M, Eils R, Schirmacher P, Lohmann V, Bartenschlager R. 2011. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 9:32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuhara T, Wada M, Nakamura S, Ono C, Shiokawa M, Yamamoto S, Motomura T, Okamoto T, Okuzaki D, Yamamoto M, Saito I, Wakita T, Koike K, Matsuura Y. 2014. Amphipathic alpha-helices in apolipoproteins are crucial to the formation of infectious hepatitis C virus particles. PLoS Pathog 10:e1004534. doi: 10.1371/journal.ppat.1004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H, Sun F, Owen DM, Li W, Chen Y, Gale MJ, Ye J. 2007. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci U S A 104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang K-S, Jiang J, Cai Z, Luo G. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J Virol 81:13783–13793. doi: 10.1128/JVI.01091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi ST, Lee KJ, Aizaki H, Hwang SB, Lai MMC. 2003. Hepatitis C virus RNA replication occurs on a detergent-resistant membrane that cofractionates with caveolin-2. J Virol 77:4160–4168. doi: 10.1128/JVI.77.7.4160-4168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aizaki H, Lee K-J, Sung VM-H, Ishiko H, Lai MMC. 2004. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology 324:450–461. doi: 10.1016/j.virol.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 24.Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol 9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 25.Shirakura M, Murakami K, Ichimura T, Suzuki R, Shimoji T, Fukuda K, Abe K, Sato S, Fukasawa M, Yamakawa Y, Nishijima M, Moriishi K, Matsuura Y, Wakita T, Suzuki T, Howley PM, Miyamura T, Shoji I. 2007. E6AP ubiquitin ligase mediates ubiquitylation and degradation of hepatitis C virus core protein. J Virol 81:1174–1185. doi: 10.1128/JVI.01684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aizawa S, Okamoto T, Sugiyama Y, Kouwaki T, Ito A, Suzuki T, Ono C, Fukuhara T, Yamamoto M, Okochi M, Hiraga N, Imamura M, Chayama K, Suzuki R, Shoji I, Moriishi K, Moriya K, Koike K, Matsuura Y. 2017. TRC8-dependent degradation of hepatitis C virus immature core protein regulates viral propagation and pathogenesis. Nat Commun 7:11379. doi: 10.1038/ncomms11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou W, Tian Q, Zheng J, Bonkovsky HL. 2010. Zinc mesoporphyrin induces rapid proteasomal degradation of hepatitis C nonstructural 5A protein in human hepatoma cells. Gastroenterology 138:1909–1919. doi: 10.1053/j.gastro.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue B, Yang D, Wang J, Xu Y, Wang X, Qin Y, Tian R, Chen S, Xie Q, Liu N, Zhu H. 2016. ISG12a restricts hepatitis C virus infection through the ubiquitination-dependent degradation pathway. J Virol 90:6832–6845. doi: 10.1128/JVI.00352-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao L, Tu H, Shi ST, Lee K-J, Asanaka M, Hwang SB, Lai MMC. 2003. Interaction with a ubiquitin-like protein enhances the ubiquitination and degradation of hepatitis C virus RNA-dependent RNA polymerase. J Virol 77:4149–4159. doi: 10.1128/JVI.77.7.4149-4159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S-J, Syed GH, Siddiqui A. 2013. Hepatitis C virus induces the mitochondrial translocation of Parkin and subsequent mitophagy. PLoS Pathog 9:e1003285. doi: 10.1371/journal.ppat.1003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S-J, Syed GH, Khan M, Chiu W-W, Sohail MA, Gish RG, Siddiqui A. 2014. Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc Natl Acad Sci U S A 111:6413–6418. doi: 10.1073/pnas.1321114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnaud N, Dabo S, Akazawa D, Fukasawa M, Shinkai-Ouchi F, Hugon J, Wakita T, Meurs EF. 2011. Hepatitis C virus reveals a novel early control in acute immune response. PLoS Pathog 7:e1002289. doi: 10.1371/journal.ppat.1002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremm A, Freund SMV, Komander D. 2010. Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne. Nat Struct Mol Biol 17:939–947. doi: 10.1038/nsmb.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T, Yin L, Cooper EM, Lai M-Y, Dickey S, Pickart CM, Fushman D, Wilkinson KD, Cohen RE, Wolberger C. 2009. Evidence for bidentate substrate binding as the basis for the K48 linkage specificity of otubain 1. J Mol Biol 386:1011–1023. doi: 10.1016/j.jmb.2008.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mevissen TET, Hospenthal MK, Geurink PP, Elliott PR, Akutsu M, Arnaudo N, Ekkebus R, Kulathu Y, Wauer T, El Oualid F, Freund SMV, Ovaa H, Komander D. 2013. OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell 154:169–184. doi: 10.1016/j.cell.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narbus CM, Israelow B, Sourisseau M, Michta ML, Hopcraft SE, Zeiner GM, Evans MJ. 2011. HepG2 cells expressing microRNA miR-122 support the entire hepatitis C virus life cycle. J Virol 85:12087–12092. doi: 10.1128/JVI.05843-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Costa D, Turek M, Felmlee DJ, Girardi E, Pfeffer S, Long G, Bartenschlager R, Zeisel MB, Baumert TF. 2012. Reconstitution of the entire hepatitis C virus life cycle in nonhepatic cells. J Virol 86:11919–11925. doi: 10.1128/JVI.01066-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kambara H, Fukuhara T, Shiokawa M, Ono C, Ohara Y, Kamitani W, Matsuura Y. 2012. Establishment of a novel permissive cell line for the propagation of hepatitis C virus by expression of microRNA miR122. J Virol 86:1382–1393. doi: 10.1128/JVI.06242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pauli E-K, Chan YK, Davis ME, Gableske S, Wang MK, Feister KF, Gack MU. 2014. The ubiquitin-specific protease USP15 promotes RIG-I-mediated antiviral signaling by deubiquitylating TRIM25. Sci Signal 7:ra3. doi: 10.1126/scisignal.2004577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Wang D, Zhong H, Luo R, Shang M, Liu D, Chen H, Fang L, Xiao S. 2015. Ubiquitin-specific protease 15 negatively regulates virus-induced type I interferon signaling via catalytically-dependent and -independent mechanisms. Sci Rep 5:11220. doi: 10.1038/srep11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh C-S, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 42.Zou Q, Jin J, Hu H, Li HS, Romano S, Xiao Y, Nakaya M, Zhou X, Cheng X, Yang P, Lozano G, Zhu C, Watowich SS, Ullrich SE, Sun S-C. 2014. USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nat Immunol 15:562–570. doi: 10.1038/ni.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. 2008. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med 205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi ST, Polyak SJ, Tu H, Taylor DR, Gretch DR, Lai MMC. 2002. Hepatitis C virus NS5A colocalizes with the core protein on lipid droplets and interacts with apolipoproteins. Virology 292:198–210. doi: 10.1006/viro.2001.1225. [DOI] [PubMed] [Google Scholar]
- 45.Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. 1997. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38:2249–2263. [PubMed] [Google Scholar]
- 46.Farese RVJ, Walther TC. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pai JT, Guryev O, Brown MS, Goldstein JL. 1998. Differential stimulation of cholesterol and unsaturated fatty acid biosynthesis in cells expressing individual nuclear sterol regulatory element-binding proteins. J Biol Chem 273:26138–26148. [DOI] [PubMed] [Google Scholar]
- 48.Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. 1998. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest 101:2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honda M, Ping LH, Rijnbrand RC, Amphlett E, Clarke B, Rowlands D, Lemon SM. 1996. Structural requirements for initiation of translation by internal ribosome entry within genome-length hepatitis C virus RNA. Virology 222:31–42. doi: 10.1006/viro.1996.0395. [DOI] [PubMed] [Google Scholar]
- 50.Fehr C, Conrad KD, Niepmann M. 2012. Differential stimulation of hepatitis C virus RNA translation by microRNA-122 in different cell cycle phase. Cell Cycle 11:277–285. doi: 10.4161/cc.11.2.18699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masaki T, Arend KC, Li Y, Yamane D, McGivern DR, Kato T, Wakita T, Moorman NJ, Lemon SM. 2015. miR-122 stimulates hepatitis C virus RNA synthesis by altering the balance of viral RNAs engaged in replication versus translation. Cell Host Microbe 17:217–228. doi: 10.1016/j.chom.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts APE, Lewis AP, Jopling CL. 2011. miR-122 activates hepatitis C virus translation by a specialized mechanism requiring particular RNA components. Nucleic Acids Res 39:7716–7729. doi: 10.1093/nar/gkr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimakami T, Yamane D, Jangra RK, Kempf BJ, Spaniel C, Barton DJ, Lemon SM. 2012. Stabilization of hepatitis C virus RNA by an Ago2-miR-122 complex. Proc Natl Acad Sci U S A 109:941–946. doi: 10.1073/pnas.1112263109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Masaki T, Shimakami T, Lemon SM. 2014. hnRNP L and NF90 interact with hepatitis C virus 5′-terminal untranslated RNA and promote efficient replication. J Virol 88:7199–7209. doi: 10.1128/JVI.00225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt T, Friedrich S, Golbik RP, Behrens SE. 2017. NF90-NF45 is a selective RNA chaperone that rearranges viral and cellular riboswitches: biochemical analysis of a virus host factor activity. Nucleic Acids Res 45:12441–12454. doi: 10.1093/nar/gkx931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Jeng KS, Lai MM. 2011. Poly(C)-binding protein 2 interacts with sequences required for viral replication in the hepatitis C virus (HCV) 5′ untranslated region and directs HCV RNA replication through circularizing the viral genome. J Virol 85:7954–7964. doi: 10.1128/JVI.00339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinlich S, Hüttelmaier S, Schierhorn A, Behrens SE, Ostareck-Lederer A, Ostareck DH. 2009. IGF2BP1 enhances HCV IRES-mediated translation initiation via the 3′ UTR. RNA 15:1528–1542. doi: 10.1261/rna.1578409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DiAugustine RP, Schaefer JM, Fouts JR. 1973. Hepatic lipid droplets. Isolation, morphology and composition. Biochem J 132:323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khor VK, Shen W-J, Kraemer FB. 2013. Lipid droplet metabolism. Curr Opin Clin Nutr Metab Care 16:632–637. doi: 10.1097/MCO.0b013e3283651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bickel PE, Tansey JT, Welte MA. 2009. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta 1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogt DA, Camus G, Herker E, Webster BR, Tsou C-L, Greene WC, Yen T-SB, Ott M. 2013. Lipid droplet-binding protein TIP47 regulates hepatitis C virus RNA replication through interaction with the viral NS5A protein. PLoS Pathog 9:e1003302. doi: 10.1371/journal.ppat.1003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, McLauchlan J. 2008. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner. Traffic 9:1268–1282. doi: 10.1111/j.1600-0854.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 63.Shavinskaya A, Boulant S, Penin F, McLauchlan J, Bartenschlager R. 2007. The lipid droplet binding domain of hepatitis C virus core protein is a major determinant for efficient virus assembly. J Biol Chem 282:37158–37169. doi: 10.1074/jbc.M707329200. [DOI] [PubMed] [Google Scholar]
- 64.Moriishi K, Mochizuki R, Moriya K, Miyamoto H, Mori Y, Abe T, Murata S, Tanaka K, Miyamura T, Suzuki T, Koike K, Matsuura Y. 2007. Critical role of PA28gamma in hepatitis C virus-associated steatogenesis and hepatocarcinogenesis. Proc Natl Acad Sci U S A 104:1661–1666. doi: 10.1073/pnas.0607312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsutsumi T, Suzuki T, Shimoike T, Suzuki R, Moriya K, Shintani Y, Fujie H, Matsuura Y, Koike K, Miyamura T. 2002. Interaction of hepatitis C virus core protein with retinoid X receptor alpha modulates its transcriptional activity. Hepatology 35:937–946. doi: 10.1053/jhep.2002.32470. [DOI] [PubMed] [Google Scholar]
- 66.Inui M, Manfrin A, Mamidi A, Martello G, Morsut L, Soligo S, Enzo E, Moro S, Polo S, Dupont S, Cordenonsi M, Piccolo S. 2011. USP15 is a deubiquitylating enzyme for receptor-activated SMADs. Nat Cell Biol 13:1368–1375. doi: 10.1038/ncb2346. [DOI] [PubMed] [Google Scholar]
- 67.Eichhorn PJ, Rodón L, Gonzàlez-Juncà A, Dirac A, Gili M, Martínez-Sáez E, Aura C, Barba I, Peg V, Prat A, Cuartas I, Jimenez J, García-Dorado D, Sahuquillo J, Bernards R, Baselga J, Seoane J. 2012. USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat Med 18:429–435. doi: 10.1038/nm.2619. [DOI] [PubMed] [Google Scholar]
- 68.Villeneuve NF, Tian W, Wu T, Sun Z, Lau A, Chapman E, Fang D, Zhang DD. 2013. USP15 negatively regulates Nrf2 through deubiquitination of Keap1. Mol Cell 51:68–79. doi: 10.1016/j.molcel.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Long L, Thelen JP, Furgason M, Haj-Yahya M, Brik A, Cheng D, Peng J, Yao T. 2014. The U4/U6 recycling factor SART3 has histone chaperone activity and associates with USP15 to regulate H2B deubiquitination. J Biol Chem 289:8916–8930. doi: 10.1074/jbc.M114.551754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukagai K, Waku T, Chowdhury AMMA, Kubo K, Matsumoto M, Kato H, Natsume T, Tsuruta F, Chiba T, Taniguchi H, Kobayashi A. 2016. USP15 stabilizes the transcription factor Nrf1 in the nucleus, promoting the proteasome gene expression. Biochem Biophys Res Commun 478:363–370. doi: 10.1016/j.bbrc.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 71.Jongsma MLM, Berlin I, Wijdeven RHM, Janssen L, Janssen GMC, Garstka MA, Janssen H, Mensink M, van Veelen PA, Spaapen RM, Neefjes J. 2016. An ER-associated pathway defines endosomal architecture for controlled cargo transport. Cell 166:152–166. doi: 10.1016/j.cell.2016.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pyeon D, Timani KA, Gulraiz F, He JJ, Park I-W. 2016. Function of ubiquitin (Ub) specific protease 15 (USP15) in HIV-1 replication and viral protein degradation. Virus Res 223:161–169. doi: 10.1016/j.virusres.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su Z-J, Cao J-S, Wu Y-F, Chen W-N, Lin X, Wu Y-L, Lin X. 2017. Deubiquitylation of hepatitis B virus X protein (HBx) by ubiquitin-specific peptidase 15 (USP15) increases HBx stability and its transactivation activity. Sci Rep 7:40246. doi: 10.1038/srep40246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vos RM, Altreuter J, White EA, Howley PM. 2009. The ubiquitin-specific peptidase USP15 regulates human papillomavirus type 16 E6 protein stability. J Virol 83:8885–8892. doi: 10.1128/JVI.00605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang N, Yin P, Zhou L, Li H, Zhang L. 2016. ARF1 activation dissociates ADRP from lipid droplets to promote HCV assembly. Biochem Biophys Res Commun 475:31–36. doi: 10.1016/j.bbrc.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 76.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale MJ. 2008. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kapadia SB, Chisari FV. 2005. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci U S A 102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen W, Li X-M, Li A-L, Yang G, Hu H-N. 2016. Hepatitis C virus increases free fatty acids absorption and promotes its replication via down-regulating GADD45alpha expression. Med Sci Monit 22:2347–2356. doi: 10.12659/MSM.899591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leu G-Z, Lin T-Y, Hsu JTA. 2004. Anti-HCV activities of selective polyunsaturated fatty acids. Biochem Biophys Res Commun 318:275–280. doi: 10.1016/j.bbrc.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 80.Huang H, Chen Y, Ye J. 2007. Inhibition of hepatitis C virus replication by peroxidation of arachidonate and restoration by vitamin E. Proc Natl Acad Sci U S A 104:18666–18670. doi: 10.1073/pnas.0708423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun H-Y, Lin C-C, Tsai P-J, Tsai W-J, Lee J-C, Tsao C-W, Cheng P-N, Wu I-C, Chiu Y-C, Chang T-T, Young K-C. 2017. Lipoprotein lipase liberates free fatty acids to inhibit HCV infection and prevent hepatic lipid accumulation. Cell Microbiol 19:e12673. doi: 10.1111/cmi.12673. [DOI] [PubMed] [Google Scholar]
- 82.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110–113. [DOI] [PubMed] [Google Scholar]
- 83.Russell RS, Meunier J-C, Takikawa S, Faulk K, Engle RE, Bukh J, Purcell RH, Emerson SU. 2008. Advantages of a single-cycle production assay to study cell culture-adaptive mutations of hepatitis C virus. Proc Natl Acad Sci U S A 105:4370–4375. doi: 10.1073/pnas.0800422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katoh H, Mori Y, Kambara H, Abe T, Fukuhara T, Morita E, Moriishi K, Kamitani W, Matsuura Y. 2011. Heterogeneous nuclear ribonucleoprotein A2 participates in the replication of Japanese encephalitis virus through an interaction with viral proteins and RNA. J Virol 85:10976–10988. doi: 10.1128/JVI.00846-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okamoto K, Mori Y, Komoda Y, Okamoto T, Okochi M, Takeda M, Suzuki T, Moriishi K, Matsuura Y. 2008. Intramembrane processing by signal peptide peptidase regulates the membrane localization of hepatitis C virus core protein and viral propagation. J Virol 82:8349–8361. doi: 10.1128/JVI.00306-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang DC, Cory S, Strasser A. 1997. Bcl-2, Bcl-XL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene 14:405–414. doi: 10.1038/sj.onc.1200848. [DOI] [PubMed] [Google Scholar]
- 87.Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199. [DOI] [PubMed] [Google Scholar]
- 88.Kouwaki T, Okamoto T, Ito A, Sugiyama Y, Yamashita K, Suzuki T, Kusakabe S, Hirano J, Fukuhara T, Yamashita A, Saito K, Okuzaki D, Watashi K, Sugiyama M, Yoshio S, Standley DM, Kanto T, Mizokami M, Moriishi K, Matsuura Y. 2016. Hepatocyte factor JMJD5 regulates hepatitis B virus replication through interaction with HBx. J Virol 90:3530–3542. doi: 10.1128/JVI.02776-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. 2013. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci Rep 3:3355. doi: 10.1038/srep03355. [DOI] [PMC free article] [PubMed] [Google Scholar]