The manipulation and engineering of microbiomes could lead to improved human health, environmental sustainability, and agricultural productivity. However, microbiomes have proven difficult to alter in predictable ways, and their emergent properties are poorly understood. The history of biology has demonstrated the power of model systems to understand complex problems such as gene expression or development. Therefore, a defined and genetically tractable model community would be useful to dissect microbiome assembly, maintenance, and processes. We have developed a tractable model rhizosphere microbiome, designated THOR, containing Pseudomonas koreensis, Flavobacterium johnsoniae, and Bacillus cereus, which represent three dominant phyla in the rhizosphere, as well as in soil and the mammalian gut. The model community demonstrates emergent properties, and the members are amenable to genetic dissection. We propose that THOR will be a useful model for investigations of community-level interactions.
KEYWORDS: Bacillus cereus, Flavobacterium johnsoniae, Pseudomonas koreensis, biofilm, colony expansion, emergent properties, inhibitory network, model community, rhizosphere
ABSTRACT
The quest to manipulate microbiomes has intensified, but many microbial communities have proven to be recalcitrant to sustained change. Developing model communities amenable to genetic dissection will underpin successful strategies for shaping microbiomes by advancing an understanding of community interactions. We developed a model community with representatives from three dominant rhizosphere taxa, the Firmicutes, Proteobacteria, and Bacteroidetes. We chose Bacillus cereus as a model rhizosphere firmicute and characterized 20 other candidates, including “hitchhikers” that coisolated with B. cereus from the rhizosphere. Pairwise analysis produced a hierarchical interstrain-competition network. We chose two hitchhikers, Pseudomonas koreensis from the top tier of the competition network and Flavobacterium johnsoniae from the bottom of the network, to represent the Proteobacteria and Bacteroidetes, respectively. The model community has several emergent properties, induction of dendritic expansion of B. cereus colonies by either of the other members, and production of more robust biofilms by the three members together than individually. Moreover, P. koreensis produces a novel family of alkaloid antibiotics that inhibit growth of F. johnsoniae, and production is inhibited by B. cereus. We designate this community THOR, because the members are the hitchhikers of the rhizosphere. The genetic, genomic, and biochemical tools available for dissection of THOR provide the means to achieve a new level of understanding of microbial community behavior.
INTRODUCTION
Modern understanding of microbiomes has been accompanied by recognition of their vast complexity, which complicates their study and manipulation. Powerful -omics approaches that profile community features such as genomes, metabolites, and transcripts have illuminated the richness of many communities (1). These global portraits of complex communities have been complemented by genetic and biochemical dissection of much simpler communities and, in particular, binary interactions of one bacterial species with one host, such as bacterial symbionts of legume roots (2) and squid light organs (3). The study of these systems has elucidated the pathways regulating interactions among bacteria and between bacteria and their environments.
The explosion of understanding of two-species interactions has generated a scientific thirst for more tools to attain mechanistic understanding of multispecies community behaviors. Model systems consisting of more than one microbial member include communities in flies (4, 5), the medicinal leech (6), and engineered systems (7). Key among the traits that demand more mechanistic studies are the components of community assembly and robustness, which is the ability to resist and recover from change (8, 9). Understanding these traits has particular value today, as many researchers aim to modify microbial communities to achieve outcomes to improve human health, environmental sustainability, and agricultural productivity.
Over the last century, the challenge to alter microbial communities in predictable ways has stymied microbiologists. Examples include attempts to change the human gut microbiome by ingestion of yogurt or probiotics (10) or to alter plant microbiomes with inundative application of disease-suppressive microorganisms (11). Few treatments have induced long-term changes due to intrinsic community robustness.
Communities express emergent properties, which are traits that cannot be predicted from the individual members (12). For example, metabolic exchange between yeast and Acetobacter spp. yields a mixture of volatile compounds attractive to Drosophila hosts that was not produced by either microbe in pure culture (13). Such higher-order or emergent properties of communities (14) might explain the difficulty in manipulating them. In addition, many communities contain functional redundancy that is likely to contribute to robustness.
Classical genetic analysis, defined as the isolation and study of mutants, in model organisms has advanced understanding of processes such as gene regulation in Escherichia coli and the mouse and development in flies and nematodes, but such reductionist genetic approaches are inaccessible in most microbial community-level analyses. A genetically tractable model system for studying microbial community assembly and robustness has the potential to transform our understanding of community processes. The scientific community recognizes the power of model systems in relation to microbial communities (15–17), and many groups have started to address this call (18–20). Importantly, common microbiome principles will only emerge with a diverse set of models to interrogate. Toward this end, we developed a model system involving three bacterial species, Pseudomonas koreensis, Flavobacterium johnsoniae, and Bacillus cereus, which interact under field and laboratory conditions, are amenable to genetic analysis, and represent three major phyla in microbiomes on plant roots and in the human gut.
RESULTS
Source of community members.
We sought a model community that is simple and contains genetically tractable species that likely interact under natural conditions, both competitively and cooperatively. We drew upon our previous work demonstrating the peculiar tendency of B. cereus to carry “hitchhikers” (21) when the bacterium is isolated from soybean roots. These biological hitchhikers are cryptic bacteria that become visible in culture only after apparently pure cultures of B. cereus are maintained at 4°C for several weeks (22). It is important to note that biological hitchhiking involves a physical association and is distinct from genetic hitchhiking which selects for neutral alleles (23). Most hitchhikers are members of the Bacteroidetes phylum, with a small proportion from the Proteobacteria and Actinomycetes (22). We selected 21 candidates that include B. cereus UW85, 12 hitchhikers, and eight other isolates from the roots that harbored the hitchhikers (Fig. 1B and C). These isolates represented the four dominant phyla present in the rhizosphere, Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria (24).
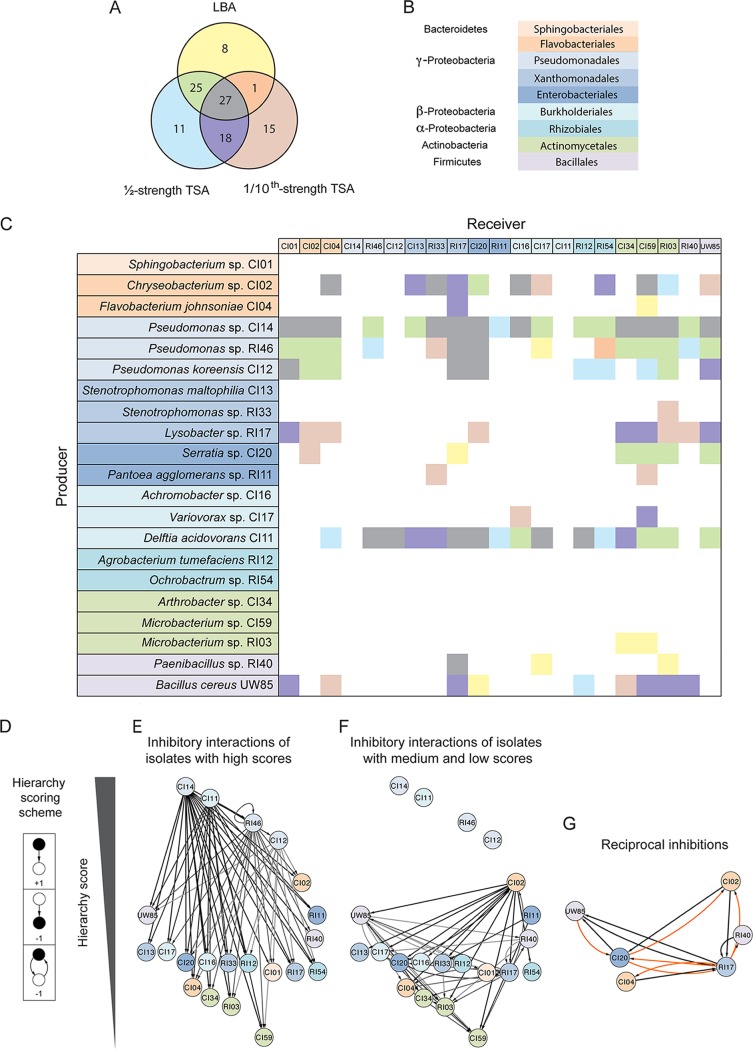
FIG 1.
Network analysis of inhibitory interactions among rhizosphere isolates. (A) Venn diagram of the inhibitory interactions identified between the isolates as determined by the presence of zones of inhibition in three media: Luria-Bertani agar (LBA), ½-strength tryptic soy agar (TSA), and 1/10-strength TSA. (B) Colors indicate phylogeny of isolates used in the inhibitory matrix and network. (C) Inhibitory interaction matrix between B. cereus UW85 and hitchhiker isolates in three media. Potential producers, which are isolates tested for the ability to inhibit others, are on the y axis, and receivers, which are isolates tested for inhibition by others, are on the x axis. There are two different color codes used in panel C. One indicates the phylogeny of isolates in the row and column title, and the second one for the matrix results using the colors scheme shown in the Venn diagram corresponding to the medium in which the interaction appears. (D) Hierarchy scoring scheme used to organize the isolates in the hierarchy interaction network, in which black is the focal point. (E and F) Inhibitory interaction network organized by hierarchy score. (E) Inhibitory interactions generated from the isolates with high hierarchy scores. (F) Inhibitory interactions generated from the isolates with medium and low hierarchy scores. (G) Reciprocal inhibitory interactions observed in the inhibitory network. Orange indicates interactions observed in only 1 medium, and black indicates interactions observed in 2 or 3 media.
Higher-order organization structures in an inhibitory interaction network of rhizosphere isolates.
Competition plays an important role in microbial communities (25). To identify competitive interference interactions in our collection of 21 rhizosphere isolates, we evaluated them in pairs for inhibition of the other members in three different media. Of 105 inhibitory interactions detected, 71 interactions (68%) were conserved under at least two growth conditions, and 27 interactions were conserved under all three conditions (Fig. 1A). From the 105 inhibitory interactions observed among the rhizosphere bacteria, we constructed a matrix (Fig. 1C). The strains display a high degree of interaction, indicated by high connectance (value of C = 0.24, which represents the fraction of all possible interactions or number of interactions/number of species2). On average, each isolate interacted with six other isolates as either the source or target of inhibition. The interaction matrix appears to be producer determined with a negative sender-receiver asymmetry value (Q) of −0.31 (26). This suggests that the structure of the inhibitory networks is more controlled by the producers and their secreted antibiotics than by the tester strains. Connectance values reported previously for diverse food-web structures are between 0.026 and 0.315 (27), and the strains tested in this study had a value of 0.24, indicating high connectance.
To detect higher-order community organization mediated by inhibitory interactions, we used a hierarchy-scoring scheme in which the ability to inhibit was given a positive score and sensitivity generated a negative score (Fig. 1D). We observed inhibitory hierarchical interactions in which the top isolates of the network inhibit isolates that receive a medium score, and in turn, these medium-scoring isolates inhibit isolates that receive a lower score (Fig. 1E and F).
The four isolates that received the highest score, Delftia acidovorans and three Pseudomonas spp., were responsible for 58 (55%) of the inhibitory interactions (Fig. 1E). Nonhierarchical interactions were infrequent (6%), and these were largely reciprocal interactions between six isolates with middle and low scores, such as Lysobacter sp. strain RI17 (Fig. 1G), which is inhibited by five isolates under almost all conditions. Reciprocal inhibition by the Lysobacter sp. against these strains appeared predominantly under the lower-nutrient condition (1/10-strength tryptic soy agar [TSA]).
P. koreensis isolate inhibits members of the Bacteroidetes in root exudates.
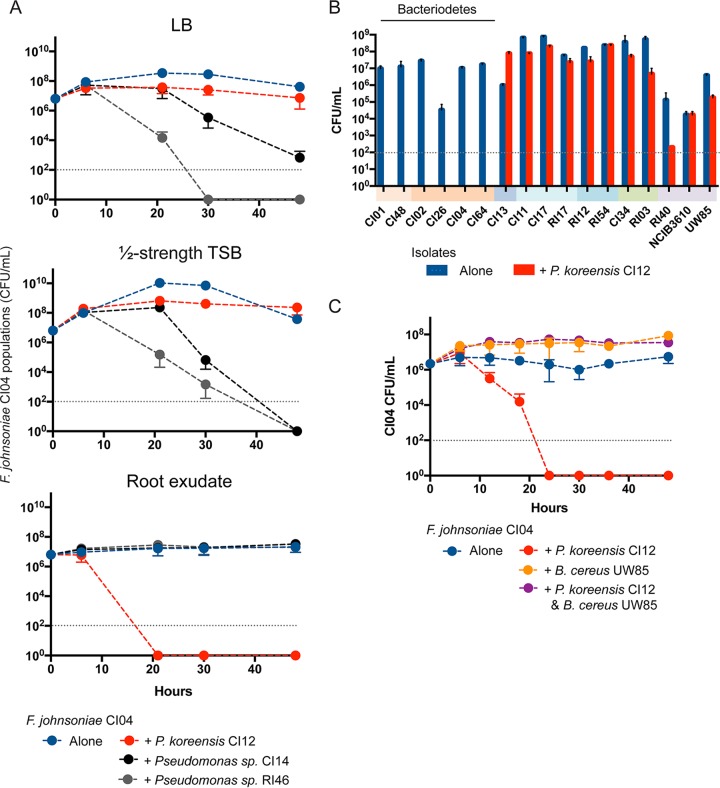
Three of the strains inhibited most of the other isolates, placing them at the top of the inhibitory network. These strains were all members of the Pseudomonas fluorescens complex. They are the only members of the collection that consistently inhibit Bacteroidetes isolates, which is the most abundant phylum among the coisolates of B. cereus, making the inhibitory activities of these P. fluorescens members of particular interest. In addition, members of the P. fluorescens group have been shown to alter the structure of microbial communities on roots, making the inhibition of Bacteroidetes of interest to understand communities (28). To determine whether the three Pseudomonas strains similarly inhibit members of the Bacteroidetes, we tested them against F. johnsoniae CI04. Pseudomonas sp. CI14 and Pseudomonas sp. RI46 inhibited F. johnsoniae CI04 growth in standard medium and P. koreensis CI12 inhibited F. johnsoniae CI04 only in root exudate (Fig. 2A). Against a panel of diverse rhizosphere isolates grown in root exudate, P. koreensis CI12 inhibited primarily Bacteroidetes strains (Fig. 2B).
FIG 2.
Coculture of rhizosphere isolates with Pseudomonas spp. and B. cereus UW85. (A) Competition experiments between F. johnsoniae CI04 and either Pseudomonas sp. CI14, Pseudomonas sp. RI46, or P. koreensis CI12 in three media, Luria-Bertani broth (LB), ½-strength tryptic soy broth (TSB), and soybean root exudate. (B) Rhizosphere isolates were grown alone or in coculture with P. koreensis CI12 in soybean root exudate. Colored bars under x axis indicate phylogenetic groups as in Fig. 1. (C) F. johnsoniae CI04 grown alone, in coculture with P. koreensis CI12 or B. cereus UW85, and in triple culture with P. koreensis CI12 and B. cereus UW85. Gray dotted line, limit of detection.
B. cereus protects F. johnsoniae from P. koreensis by modulating levels of koreenceine metabolites.
To explore the ecology of B. cereus and its hitchhikers, P. koreensis CI12 and F. johnsoniae CI04, we added B. cereus UW85 to the coculture of the hitchhikers. Unpredictably, B. cereus UW85 enabled the growth of F. johnsoniae CI04 in coculture with P. koreensis CI12 (Fig. 2C) without affecting the growth of P. koreensis CI12 (Fig. S1). F. johnsoniae protection is dependent upon B. cereus arrival at stationary phase (Fig. S2). B. subtilis NCIB3160, a well-studied spore-forming bacterium, also protected F. johnsoniae in a stationary-phase-dependent manner (Fig. S2). Among three B. subtilis NCIB3160 mutants affected in transcriptional regulators related to stationary phase (29), only the mutant affected in spo0H, which controls the early transition from exponential to stationary phase, was unable to protect F. johnsoniae from P. koreensis (Fig. 3). An spo0H mutant in B. cereus UW85 also did not protect F. johnsoniae from P. koreensis (Fig. 3).
FIG 3.
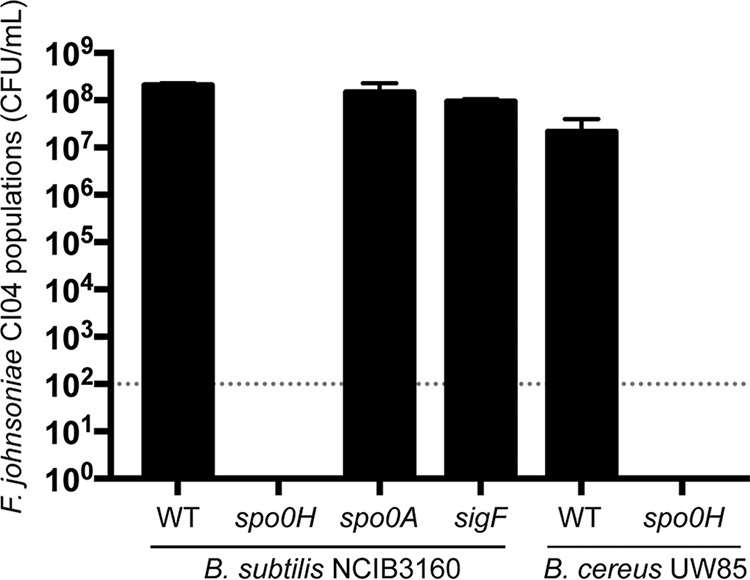
Effect of Bacillus spp. on populations of F. johnsoniae in the presence of P. koreensis. Triple culture was of P. koreensis CI12, F. johnsoniae CI04, and either B. subtilis NCIB3160 or B. cereus UW85, or their mutants. Gray dotted line, limit of detection.
P. koreensis CI12 population dynamics. P. koreensis CI12 grown alone, in coculture with F. johnsoniae CI04 or B. cereus UW85, and in triple culture with F. johnsoniae CI04 and B. cereus UW85. Download FIG S1, TIF file, 25.7 MB (25.7MB, tif) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Correlation between Bacillus spp. spore density and F. johnsoniae protection in triple culture. (A and B) Triple culture of F. johnsoniae CI04 and P. koreensis CI12 with either B. cereus UW85 (A) or B. subtilis NCIB3160 (B). The cell density of the Bacillus inoculum is on the x axis. Bacillus spore densities and F. johnsoniae CI04 cell densities in the triple culture at 30 h are on the y axis. Gray dotted line, limit of detection. Download FIG S2, TIF file, 34.7 MB (34.7MB, tif) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We recently described a P. koreensis family of bacterial alkaloids, koreenceine A, B, and C, that influence the growth of F. johnsoniae in root exudates (30). Cell filtrates of cocultures of P. koreensis and F. johnsoniae inhibit F. johnsoniae growth, whereas filtrates of P. koreensis cultured alone or with both F. johnsoniae and B. cereus do not inhibit F. johnsoniae growth (Table 1). The levels of koreenceine A, B, and C were higher when P. koreensis was cocultured with F. johnsoniae than when it was cultured alone (Table 1). The addition of B. cereus to cocultures of P. koreensis and F. johnsoniae severely reduced the accumulation of koreenceine A and C, with a minor effect on the level of koreenceine B. The B. cereus Δspo0H mutant did not protect F. johnsoniae in triple culture, nor did it reduce the accumulation of koreenceine A and C as substantially as did wild type B. cereus (Table 1). We propose that B. cereus protects F. johnsoniae by selectively reducing the levels of koreenceine A and C accumulated by P. koreensis.
TABLE 1.
Accumulation of koreenceine A, B, and C in coculture with F. johnsoniae or B. cereusa
| Source of cell extracts | F. johnsoniae growth (107 CFU/ml) | Accumulation (relative ion counts × 105) of: |
||
|---|---|---|---|---|
Koreenceine A 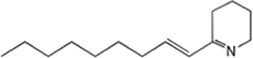
|
Koreenceine B 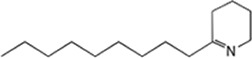
|
Koreenceine C 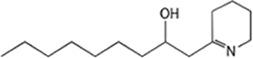
|
||
| P. koreensis | 1.4 ± 0.4 | 3.6 ± 0.4 | 62.9 ± 5.5 | 3.2 ± 0.4 |
| P. koreensis with F. johnsoniae | ND | 75.9 ± 11.4 | 275.9 ± 17.9 | 35.3 ± 6.5 |
| P. koreensis with F. johnsoniae and B. cereus WT | 6.3 ± 1.2 | 3.1 ± 0.4 | 167.6 ± 17.3 | 0.7 ± 0.2 |
| P. koreensis with F. johnsoniae and B. cereus spo0H | ND | 31.5 ± 9.8 | 310.1 ± 54.1 | 7.4 ± 2.6 |
Koreenceine A, B, and C concentrations are expressed as ion counts from LC/HR-ESI-QTOF-MS analysis from cell filtrates of cultures of P. koreensis CI12 grown alone, with F. johnsoniae CI04, F. johnsoniae CI04 and B. cereus UW85 wild type, or with F. johnsoniae CI04 and B. cereus UW85 spo0H. F. johnsoniae CI04 population growth in the corresponding conditions in CFU per milliliter. ND, not detected.
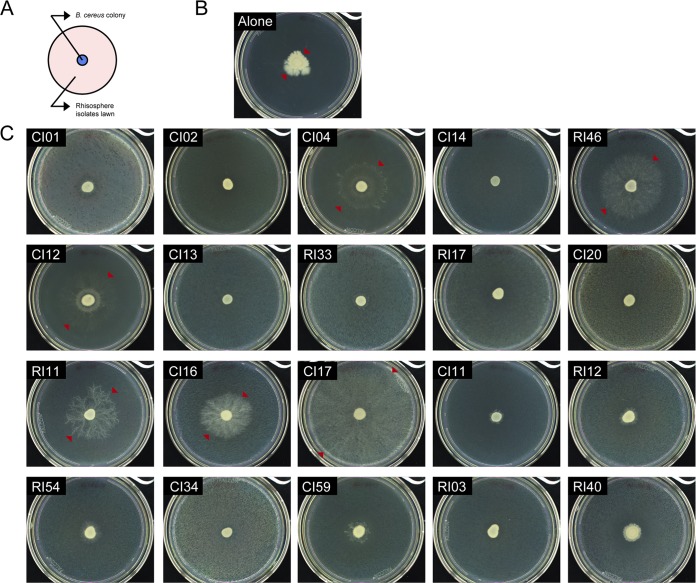
Rhizosphere isolates modulate B. cereus colony expansion.
Among 20 rhizosphere isolates, six isolates induced B. cereus patches to expand in a dendritic pattern when plated on a lawn of the corresponding isolate on 1/10-strength TSA (Fig. 4). B. cereus was the only isolate of the collection that displayed colony expansion. A similar motility pattern has been observed in B. cereus translocating in an artificial soil microcosm (31), suggesting that this motility might be important in adapting to the soil environment. In pure culture, B. cereus colonies expanded in an irregular pattern with dense branches and asymmetrical bulges, whereas on lawns of the six isolates, including P. koreensis and F. johnsoniae, expansion was greater and radially symmetrical (Fig. 4 and 5A and Table S1). Colonies of B. cereus spread into the neighboring colonies of F. johnsoniae CI04 and P. koreensis CI12 when the two strains were grown in close proximity on plates (Fig. 5B). B. cereus spread both around and across P. koreensis CI12, and across, but not around, F. johnsoniae CI04. B. cereus did not display colony expansion when in contact with or proximal to Paenibacillus sp. strain RI40 (Fig. 5).
FIG 4.
B. cereus UW85 colony expansion in the presence of the rhizosphere isolates. (A) Schematic representation of the spread-patch plates. (B) B. cereus UW85 grown alone. (C) B. cereus UW85 grown on a lawn of each member of the community. Photographs were taken after 4 days at 28°C. Arrows indicate the limits of the B. cereus colony.
FIG 5.
Effect of community members on B. cereus UW85 colony expansion. (A) B. cereus UW85 plasmid-dependent GFP strain grown alone or on a lawn of P. koreensis CI12, F. johnsoniae CI04, or Paenibacillus sp. RI40. Bright-field (BF) and GFP imaging of colonies 5 days after inoculation. (B) B. cereus UW85 GFP strain grown in close proximity to a colony of P. koreensis CI12, F. johnsoniae CI04, or Paenibacillus sp. RI40. Arrows indicate B. cereus UW85 expansion over colonies of the other isolates. Bright-field, GFP channel, and overlay of the two channels of plates after 2 days of growth (F. johnsoniae CI04) and after 5 days of growth (P. koreensis CI12 and Paenibacillus sp. RI40).
Area of B. cereus colonies is larger in the presence of either F. johnsoniae or P. koreensis. Download Table S1, DOCX file, 0.04 MB (38.9KB, docx) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
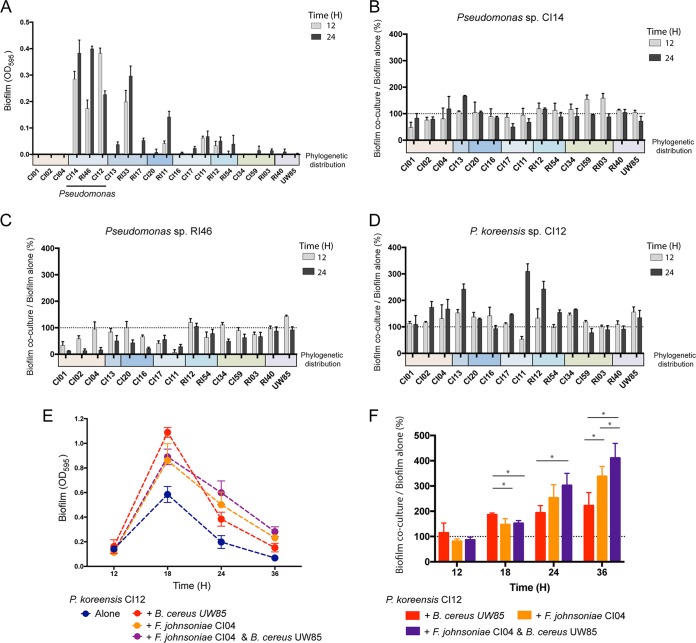
Rhizosphere isolates modulate Pseudomonas biofilm formation.
Among the hitchhikers and rhizosphere isolates, Pseudomonas sp. isolates produced the most robust biofilms (Fig. 6A). In pairwise tests, poor biofilm producers changed the behavior of isolates of Pseudomonas spp. (Fig. 6B to D). When alone, P. koreensis CI12 produced maximum biofilm at 18 h, after which the biofilm began to dissociate (Fig. S3). F. johnsoniae CI04 and B. cereus UW85 each increased the maximum biofilm formed by P. koreensis CI12 at 18 h and reduced the rate of biofilm dissociation (Fig. 6E and F). A mixture of all three strains followed the same pattern as the pairs, although the triple mixture maintained more biofilm at 36 h (P < 0.01) (Fig. 6F), suggesting that F. johnsoniae CI04 and B. cereus UW85 together sustain the P. koreensis CI12 biofilm longer than either alone.
FIG 6.
Biofilm formation by rhizosphere isolates. Biofilm was quantified by measuring the optical density at 595 nm (OD595) after staining with crystal violet. (A) Crystal violet quantification of biofilm formation for each of the 21 isolates at 12 and 24 h after inoculation. (B to D) Pseudomonas biofilm production when grown with a poor biofilm producer normalized against Pseudomonas biofilm in pure culture at 12 and 24 h, either Pseudomonas sp. RI46 (B), Pseudomonas sp. CI14 (C), or P. koreensis CI12 (D). (E) Biofilm formation by P. koreensis CI12 growing alone, in coculture with either F. johnsoniae CI04 or B. cereus UW85, and in triple culture at 12, 18, 24, and 36 h. (F) P. koreensis CI12 biofilm production when grown with two other isolates normalized against P. koreensis growth in pure culture. *, P < 0.01. Colored bars under the x axis indicate phylogenetic groups as in Fig. 1. Gray dotted line, limit of detection.
P. koreensis CI12 biofilm quantification and growth of the planktonic population. P. koreensis CI12 growth is on the left y axis, and crystal violet quantification of biofilm formation for P. koreensis CI12 is on the right y axis. Download FIG S3, TIF file, 25.6 MB (25.6MB, tif) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
We constructed a model community built upon microbes isolated from the soybean rhizosphere. We selected B. cereus as the first member of the community because of its ubiquitous distribution in soil and on roots (32) and its influence on the rhizosphere (33–36). Approximately 3% to 5% of B. cereus colonies isolated from roots carry “hitchhikers,” other species that are only visible in culture over time in cold storage (22). B. cereus and its hitchhikers are derived from the same habitat and appear to have a physically intimate association, suggesting that their interactions in culture are relevant to the natural community; therefore, the second and third candidates for the model community were chosen from among the hitchhikers.
The second candidate was F. johnsoniae, a member of the Bacteroidetes, the most abundant group of hitchhikers. B. cereus enables F. johnsoniae to grow in soybean and alfalfa root exudate by providing fragments of peptidoglycan as a carbon source (22). The third candidate for the model community was P. koreensis, which inhibits the growth of F. johnsoniae in soybean root exudates but not when B. cereus is present. Other interactions among these three species include modulation of B. cereus colony expansion by F. johnsoniae and P. koreensis and enhancement of P. koreensis biofilm formation by B. cereus and F. johnsoniae. These three candidates are genetically tractable, their genomes have been sequenced (21, 37, 38), they represent three phyla that dominate the rhizosphere and other host-associated communities (39–44), and they display both competitive and cooperative interactions. We designate the model community containing B. cereus, F. johnsoniae, and P. koreensis “THOR” to indicate that the members are the hitchhikers of the rhizosphere.
THOR has two emergent properties, colony expansion and biofilm formation, that are increased by the complete community and could not be predicted from the behavior of the individuals. Each is observed with several combinations of community members, demonstrating functional redundancy in the system. B. cereus colony expansion likely reflects other bacteria affecting B. cereus motility. To our knowledge, this is the first report to indicate that B. cereus motility is influenced by social interactions. Motility influenced by social interactions is a rapidly growing field of research that has already demonstrated diverse mechanisms by which bacteria modulate motility in other species, including diffusible metabolites and cell-to-cell contact (45, 46). The B. cereus dendritic growth patterns described here have been associated with sliding motility in semisolid agar under low-nutrient conditions (47) and in artificial soil microcosms (31). Sliding is a passive appendage-independent translocation mechanism mediated by expansive forces of a growing colony accelerated by biosurfactants (48). Future experiments will determine whether B. cereus colony expansion over a bacterial lawn is mediated by sliding and whether the inducing bacteria enable this motility with biosurfactants.
Biofilm produced by P. koreensis CI12 was augmented and sustained by other THOR members. P. koreensis CI12 is a member of the P. fluorescens complex, which contains many members that promote growth and suppress disease of plants, processes often dependent upon biofilm formation (49). The community modulation of P. koreensis CI12 biofilm formation and persistence could be a strategy to maintain bacteria in the rhizosphere. The social interactions among the members of the THOR model community provide an experimental system to probe mechanisms of P. koreensis biofilm assembly and disassembly and its role in P. koreensis lifestyle within a rhizosphere community.
In addition to having emergent community properties, THOR members interact in several ways that are common in communities. B. cereus increases F. johnsoniae growth through nutritional enhancement (22) and protects it from growth inhibition by P. koreensis, illustrating how pairwise interactions can be modulated by other members of the community, a phenomenon observed previously in synthetic communities (50, 51). Growth interference and enhancement have been considered rare in naturally occurring communities because these behaviors often cannot be predicted from pairwise interactions (52). Our results further reinforce the importance of community modulation of behaviors observed in pairwise studies.
THOR was constructed from a collection of 21 rhizosphere isolates that share several properties, such as high interactivity and higher-order organization mediated by antagonistic interactions, observed in other communities containing isolates of a single genus (26, 53, 54). We show that these properties can originate from phylogenetically diverse bacteria. We propose that the high microbial diversity detected in soil and rhizosphere communities could be in part achieved by hierarchical inhibition coupled with modulation of inhibition.
A genetically tractable community with defined composition in a controlled environment offers the opportunity to dissect the mechanisms by which communities are established, function, and maintain their integrity in the face of perturbation. Rigorous testing of the numerous mechanistic hypotheses about community behavior that have been generated by -omics analyses requires systems in which variables can be isolated. This is offered in genetically tractable systems in which the functions of individual genes can be established through mutant analysis, and the impact of environmental factors can be studied by manipulating each variable. Therefore, model systems that can be fully dissected need to be part of the arsenal of tools to advance microbial ecology to a new platform of experimental power and causal inference.
We present THOR as a simple multiphylum genetically tractable system with diverse community characteristics, some of which are the result of emergent properties. To capture the impact of multi-organism interactions and emergent properties, communities need to be studied as single genetic entities. Metagenomics introduced the concept of the community as the unit of study for genomes; similarly, “metagenetic” analysis will apply genetic analysis at the community level for mechanistic understanding (55). Such understanding will be key to designing interventions to achieve outcomes in the health of humans, the environment, and the agroecosystem.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
B. cereus UW85 and 20 coisolates and rhizosphere isolates were reported previously (22) (Table S2). B. subtilis NCIB3160 wild-type (WT) and spo0A, spo0H, and sigF mutant strains were a gift from Roberto Kolter at Harvard University. Bacterial strains were propagated on 1/10-strength tryptic soy agar (TSA) and grown in liquid culture in 1/2-strength tryptic soy broth (TSB) at 28°C with vigorous shaking. Bacillus spores were quantified by plating on 1/10-strength TSA after heating at 80°C for 10 min.
Strains used in this study. Download Table S2, DOCX file, 0.10 MB (98.8KB, docx) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Production of root exudates.
Soybean seeds were surface disinfected with 6% sodium hypochlorite for 10 min, washed with sterilized deionized water, transferred to water agar plates, and allowed to germinate for 3 days in the dark at 25°C. Seedlings were grown in a hydroponic system using modified Hoagland’s plant growth solution (56). Root exudate was collected after 10 days of plant growth in a chamber (12-h photoperiod, 25°C), filter sterilized, and stored at −20°C. An amino acid mix of equal parts alanine, aspartate, leucine, serine, threonine, and valine was added to the root exudate at a final concentration of 6 mM.
Generating an inhibitory interaction network between rhizosphere bacteria.
The presence or absence of inhibitory interactions between strains in our collection was evaluated following a modified spread-patch method. Strains were grown individually for 20 h. One-milliliter aliquots of cultures of each strain were centrifuged (6,000 × g, 6 min) and resuspended in 1 ml of the same medium (undiluted cultures), and a 1:100 dilution of each strain was prepared in the same medium (diluted culture). Inhibitory interactions were evaluated in three different medium plates, Luria-Bertani agar, 1/2-strength TSA, and 1/10-strength TSA. Plates were spread with 100 μl of the diluted cultures and spotted with 10 μl of the undiluted cultures, with four strains per plate. Plates were then incubated at 28°C and inspected for zones of inhibition after 2 days. A network of inhibitory interactions was then generated using the inhibitory interaction matrix that summarizes the detected interactions under the three conditions evaluated, where each node of the network represents one of the bacterial strains and each edge represents growth inhibition of the target. A simple hierarchy scoring was created to assign hierarchy levels based on Wright and Vetsigian (54). Each strain was assigned one “reward” point for inhibiting another strain and one “penalty” point for each strain that inhibited it. One penalty point was also assigned for reciprocal interactions. Networks were visualized using Cytoscape software (57). The sender-receiver asymmetry (Q) was calculated from the inhibitory interaction matrix, as reported by Vetsigian et al. (26).
Competition assays in liquid culture.
Strains were grown individually for 16 to 20 h. A 1-ml sample was removed from each overnight culture, and the cells were washed once and resuspended in phosphate-buffered saline (PBS). Culture medium was inoculated with ∼106 F. johnsoniae cells ml−1 and ∼107 Pseudomonas sp. cells ml−1. B. cereus and B. subtilis were inoculated at densities between ∼104 cells ml−1 and ∼107 cells ml−1, depending upon the experiment. When evaluating the susceptibility of each strain to P. koreensis CI12 inhibition in root exudate, all strains except CI12 were inoculated at a final density of 1/1,000 their overnight culture density. Cultures were incubated with agitation for 2 days at 28°C. Samples were withdrawn periodically to evaluate bacterial growth by serial dilution and plating. The initial densities were determined on either LB or LB containing kanamycin (10 μg ml−1). At every other time point, Pseudomonas sp. colonies were counted on LB plates, Bacillus spp. were selected on LB plates containing polymyxin B (4 μg ml−1), and all other strains were selected on LB plates containing kanamycin (10 μg ml−1). Plates were incubated at 28°C for 2 days.
Chromosomal deletion of spo0H in B. cereus.
The gene encoding the Spo0H sigma factor was deleted using a chromosomal integration vector with a thermosensitive origin of replication that introduces the deletion with no marker. Construction of the spo0H deletion cassette was accomplished by a modified version of overlap extension (OE) PCR strategy. Fragments 1 kb upstream and 1 kb downstream of the spo0H gene were amplified using primers mut_spo0HA1/mut_spo0HA2 and mut_spo0HB1/mut_spo0HB2, respectively (Table S3). The PCR products were cloned in pENTR/d-TOPO, generating pmut_spo0H_ENTR. Primers mut_spo0HA1 and mut_spo0HB2 were designed to include a BamHI site in the 5′ region to allow transfer. The spo0H deletion construct was recovered from pmut_spo0H_ENTR using BamHI and cloned in the BamHI site of pMAD, generating pmut_spo0H_MAD in E. coli GM2929. Gene replacement was carried out in a manner similar to the method described previously (58). Briefly, pmut_spo0H_MAD was introduced into B. cereus UW85 by electroporation in 0.2-cm cuvettes with a Gene Pulser (Bio-Rad Laboratories) set at 1.2 kV. The surviving cells were cultured on 1/2-strength TSA plates with erythromycin (3 μg ml−1) and 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gal; 50 μg ml−1) at 28°C. Strains that had undergone the single recombination event were selected on plates containing erythromycin (3 μg ml−1) at 40.5°C. To select for a second crossover event, single recombinant clones were grown at 28°C in nonselective medium, diluted into fresh medium, grown at 40.5°C, and plated for single colonies on 1/2-strength TSA with X-Gal (50 μg ml−1) at 40.5°C. White colonies, which were putative double recombinants, were confirmed by PCR using mut_spo0HA1 and mut_spo0HB2 for the deletion of spo0H.
Primers used in this study. Download Table S3, DOCX file, 0.04 MB (41KB, docx) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Identification of koreenceine metabolites produced by P. koreensis CI12 in the presence of other rhizosphere members.
P. koreensis CI12 was grown alone, in coculture, or in triple culture with other rhizosphere members in root exudate at 28°C with agitation. Five milliliters of cultures was centrifuged (6,000 × g, 6 min), and supernatants were filtered using a 0.22-μm-pore-size polyethersulfone (PES) membrane filter (Millipore). The cell-free cultures were mixed with 6 ml of 2-butanol. Six milliliters of the organic phase was concentrated using a GeneVac EZ-2 Plus instrument (SP Scientific). Crude extracts were resuspended in methanol and analyzed on an Agilent 6120 single quadrupole liquid chromatography-mass spectrometry (LC/MS) system (Phenomenex Kinetex C18 column, 250 by 4.6 mm, 5 μm; flow rate, 0.7 ml min−1; mobile phase composition, H2O and acetonitrile [ACN] containing 0.1% trifluoroacetic acid [TFA]; method, 0 to 30 min, 10 to 100% ACN; hold for 5 min, 100% ACN; 1 min, 100 to 10% ACN). High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) data were obtained using an Agilent iFunnel 6550 quadrupole time of flight (Q-TOF) mass spectrometer fitted with an electrospray ionization (ESI) source coupled to an Agilent (USA) 1290 Infinity high-performance liquid chromatography (HPLC) system.
B. cereus motility assay.
B. cereus motility was evaluated using the same modified spread-patch method described above. We used B. cereus UW85/pAD123_31-26 as a green fluorescent protein (GFP) reporter (59). The images were captured using a custom Macroscope. The detector was a Canon EOS 600D Rebel T3i equipped with a Canon EFS 60-mm Macro lens. GFP was excited with a light-emitting diode (LED) using a 470/40 filter and collected with a 480/30 filter. Remote control of the camera and LED was achieved using custom software.
Microtiter plate biofilm assays.
The ability of the rhizosphere community to form a biofilm was estimated in 96-well polystyrene microtiter plates, as described previously (60), with some modifications. Briefly, strains were grown at 28°C for 20 h; cultures were centrifuged (6,000 × g, 6 min) and resuspended in sterile 10 mM NaCl to an optical density (OD) of 0.004 for Pseudomonas spp. and 0.001 for the other isolates. Cell suspensions were placed in sterile flat-bottomed microtiter plates as single species, pairs, or triple species in root exudate. Plates were covered with sterile breathable sealing film and incubated at 20°C. Cell density was determined by spectrophotometric measurement at 600 nm at the final time point (BioTek Synergy HT microplate reader). Planktonic cells were discarded, and wells were washed three times with water. Biofilms attached to the wells were stained with 0.1% crystal violet, washed three times with water, and dried. The stain was dissolved with 33% acetic acid, and its concentration was determined spectrophotometrically at 595 nm. Visualization and statistical analyses were performed with the GraphPad Prism 7 software. Differences between groups were tested for statistical significance (Student’s t test). Significance levels were set to a P value of <0.01 (*).
ACKNOWLEDGMENTS
We gratefully acknowledge Jennifer Heinritz for her assistance with microscopy.
This research was supported by the Office of the Provost at Yale University, by funding from the Wisconsin Alumni Research Foundation through the University of Wisconsin–Madison Office of the Vice Chancellor for Research and Graduate Education, and by NSF grant MCB-1243671.
Footnotes
Citation Lozano GL, Bravo JI, Garavito Diago MF, Park HB, Hurley A, Peterson SB, Stabb EV, Crawford JM, Broderick NA, Handelsman J. 2019. Introducing THOR, a model microbiome for genetic dissection of community behavior. mBio 10:e02846-18. https://doi.org/10.1128/mBio.02846-18.
Contributor Information
Gary M. Dunny, University of Minnesota Medical School.
Roberto Kolter, Harvard Medical School.
Irene Newton, Indiana University, Bloomington.
REFERENCES
- 1.Franzosa EA, Hsu T, Sirota-Madi A, Shafquat A, Abu-Ali G, Morgan XC, Huttenhower C. 2015. Sequencing and beyond: integrating molecular “omics” for microbial community profiling. Nat Rev Microbiol 13:360–372. doi: 10.1038/nrmicro3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oldroyd GED, Murray JD, Poole PS, Downie JA. 2011. The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet 45:119–144. doi: 10.1146/annurev-genet-110410-132549. [DOI] [PubMed] [Google Scholar]
- 3.Nyholm SV, McFall-Ngai MJ. 2004. The winnowing: establishing the squid-vibrio symbiosis. Nat Rev Microbiol 2:632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]
- 4.Broderick NA, Lemaitre B. 2012. Gut-associated microbes of Drosophila melanogaster. Gut Microbes 3:307–321. doi: 10.4161/gmic.19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchon N, Broderick NA, Lemaitre B. 2013. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol 11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 6.Graf J. 2016. Lessons from digestive-tract symbioses between bacteria and invertebrates. Annu Rev Microbiol 70:375–393. doi: 10.1146/annurev-micro-091014-104258. [DOI] [PubMed] [Google Scholar]
- 7.De Roy K, Marzorati M, Van den Abbeele P, Van de Wiele T, Boon N. 2014. Synthetic microbial ecosystems: an exciting tool to understand and apply microbial communities. Environ Microbiol 16:1472–1481. doi: 10.1111/1462-2920.12343. [DOI] [PubMed] [Google Scholar]
- 8.Little AEF, Robinson CJ, Peterson SB, Raffa KF, Handelsman J. 2008. Rules of engagement: interspecies interactions that regulate microbial communities. Annu Rev Microbiol 62:375–401. doi: 10.1146/annurev.micro.030608.101423. [DOI] [PubMed] [Google Scholar]
- 9.Shade A, Peter H, Allison SD, Baho DL, Berga M, Bürgmann H, Huber DH, Langenheder S, Lennon JT, Martiny JBH, Matulich KL, Schmidt TM, Handelsman J. 2012. Fundamentals of microbial community resistance and resilience. Front Microbiol 3:417. doi: 10.3389/fmicb.2012.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A. 2017. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res 61:1600240. doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- 11.Schlatter D, Kinkel L, Thomashow L, Weller D, Paulitz T. 2017. Disease suppressive soils: new insights from the soil microbiome. Phytopathology 107:1284–1297. doi: 10.1094/PHYTO-03-17-0111-RVW. [DOI] [PubMed] [Google Scholar]
- 12.Newman DK, Banfield JF. 2002. Geomicrobiology: how molecular-scale interactions underpin biogeochemical systems. Science 296:1071–1077. doi: 10.1126/science.1010716. [DOI] [PubMed] [Google Scholar]
- 13.Fischer CN, Trautman EP, Crawford JM, Stabb EV, Handelsman J, Broderick NA. 2017. Metabolite exchange between microbiome members produces compounds that influence Drosophila behavior. Elife 6:e18855. doi: 10.7554/eLife.18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madsen JS, Sørensen SJ, Burmølle M. 2018. Bacterial social interactions and the emergence of community-intrinsic properties. Curr Opin Microbiol 42:104–109. doi: 10.1016/j.mib.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Zhalnina K, Zengler K, Newman D, Northen TR. 2018. Need for laboratory ecosystems to unravel the structures and functions of soil microbial communities mediated by chemistry. mBio 9:e01175-18. doi: 10.1128/mBio.01175-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pessotti RC, Hansen BL, Traxler MF. 2018. In search of model ecological systems for understanding specialized metabolism. mSystems 3:e00175-17. doi: 10.1128/mSystems.00175-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe BE. 2018. Using cultivated microbial communities to dissect microbiome assembly: challenges, limitations, and the path ahead. mSystems 3:e00161-17. doi: 10.1128/mSystems.00161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venturelli OS, Carr AC, Fisher G, Hsu RH, Lau R, Bowen BP, Hromada S, Northen T, Arkin AP. 2018. Deciphering microbial interactions in synthetic human gut microbiome communities. Mol Syst Biol 14:e8157. doi: 10.15252/msb.20178157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfe BE, Button JE, Santarelli M, Dutton RJ. 2014. Cheese rind communities provide tractable systems for in situ and in vitro studies of microbial diversity. Cell 158:422–433. doi: 10.1016/j.cell.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu B, Paulson JN, Zheng X, Kolter R. 2017. Simplified and representative bacterial community of maize roots. Proc Natl Acad Sci U S A 114:E2450–E2459. doi: 10.1073/pnas.1616148114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozano GL, Bravo JI, Handelsman J. 2017. Draft genome sequence of Pseudomonas koreensis CI12, a Bacillus cereus “hitchhiker” from the soybean rhizosphere. Genome Announc 5:e00570-17. doi: 10.1128/genomeA.00570-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson SB, Dunn AK, Klimowicz AK, Handelsman J. 2006. Peptidoglycan from Bacillus cereus mediates commensalism with rhizosphere bacteria from the Cytophaga-Flavobacterium group. Appl Environ Microbiol 72:5421–5427. doi: 10.1128/AEM.02928-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barton NH. 2000. Genetic hitchhiking. Philos Trans R Soc Lond B Biol Sci 355:1553–1562. doi: 10.1098/rstb.2000.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P. 2013. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 25.Foster KR, Bell T. 2012. Competition, not cooperation, dominates interactions among culturable microbial species. Curr Biol 22:1845–1850. doi: 10.1016/j.cub.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Vetsigian K, Jajoo R, Kishony R. 2011. Structure and evolution of Streptomyces interaction networks in soil and in silico. PLoS Biol 9:e1001184. doi: 10.1371/journal.pbio.1001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunne JA, Williams RJ, Martinez ND. 2002. Food-web structure and network theory: the role of connectance and size. Proc Natl Acad Sci U S A 99:12917–12922. doi: 10.1073/pnas.192407699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weller DM. 2007. Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology 97:250–256. doi: 10.1094/PHYTO-97-2-0250. [DOI] [PubMed] [Google Scholar]
- 29.Stragier P, Losick R. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet 30:297–241. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 30.Lozano GL, Park HB, Bravo JI, Armstrong EA, Denu JM, Stabb EV, Broderick NA, Crawford JM, Handelsman J. 2018. Bacterial analogs of plant piperidine alkaloids mediate microbial interactions in a rhizosphere model system. bioRxiv doi: 10.1101/499731. [DOI] [PMC free article] [PubMed]
- 31.Vilain S, Luo Y, Hildreth MB, Brozel VS. 2006. Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil. Appl Environ Microbiol 72:4970–4977. doi: 10.1128/AEM.03076-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stabb EV, Jacobson LM, Handelsman J. 1994. Zwittermicin A-producing strains of Bacillus cereus from diverse soils. Appl Environ Microbiol 60:4404–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halverson LJ, Handelsman J. 1991. Enhancement of soybean nodulation by Bacillus cereus UW85 in the field and in a growth chamber. Appl Environ Microbiol 57:2767–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert G, Clayton M, Handelsman J, Parke J. 1996. Use of cluster and discriminant analyses to compare rhizosphere bacterial communities following biological perturbation. Microb Ecol 32:123–147. [DOI] [PubMed] [Google Scholar]
- 35.Osburn RM, Milner JL, Oplinger ES, Smith RS, Handelsman J. 1995. Effect of Bacillus cereus UW85 on the yield of soybean at two field sites in Wisconsin. Plant Dis 79:551. doi: 10.1094/PD-79-0551. [DOI] [Google Scholar]
- 36.Bullied WJ, Buss TJ, Kevin Vessey J. 2002. Bacillus cereus UW85 inoculation effects on growth, nodulation, and N accumulation in grain legumes: field studies. Can J Plant Sci 82:291–298. doi: 10.4141/P01-048. [DOI] [Google Scholar]
- 37.Lozano GL, Holt J, Ravel J, Rasko DA, Thomas MG, Handelsman J. 2016. Draft genome sequence of biocontrol agent Bacillus cereus UW85. Genome Announc 4:e00910-16. doi: 10.1128/genomeA.00910-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bravo JI, Lozano GL, Handelsman J. 2017. Draft genome sequence of Flavobacterium johnsoniae CI04, an isolate from the soybean rhizosphere. Genome Announc 5:e01535-16. doi: 10.1128/genomeA.01535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delgado-Baquerizo M, Oliverio AM, Brewer TE, Benavent-González A, Eldridge DJ, Bardgett RD, Maestre FT, Singh BK, Fierer N. 2018. A global atlas of the dominant bacteria found in soil. Science 359:320–325. doi: 10.1126/science.aap9516. [DOI] [PubMed] [Google Scholar]
- 40.Rascovan N, Carbonetto B, Perrig D, Díaz M, Canciani W, Abalo M, Alloati J, González-Anta G, Vazquez MP. 2016. Integrated analysis of root microbiomes of soybean and wheat from agricultural fields. Sci Rep 6:28084. doi: 10.1038/srep28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman KSM, Thahira-Rahman J, Lakshmanaperumalsamy P, Banat IM. 2002. Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresour Technol 85:257–261. doi: 10.1016/S0960-8524(02)00119-0. [DOI] [PubMed] [Google Scholar]
- 42.Gangwar P, Alam SI, Singh L. 2011. Metabolic characterization of cold active Pseudomonas, Arthrobacter, Bacillus, and Flavobacterium spp. from Western Himalayas. Indian J Microbiol 51:70–75. doi: 10.1007/s12088-011-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.König H. 2006. Bacillus species in the intestine of termites and other soil invertebrates. J Appl Microbiol 101:620–627. doi: 10.1111/j.1365-2672.2006.02914.x. [DOI] [PubMed] [Google Scholar]
- 44.Warwick C, Arena PC, Steedman C. 2013. Health implications associated with exposure to farmed and wild sea turtles. JRSM Short Rep 4:8–7. doi: 10.1177/2042533313475574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Kyle S, Straight PD. 2018. Antibiotic stimulation of a Bacillus subtilis migratory response. mSphere 3:e00586-17. doi: 10.1128/mSphere.00586-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCully LM, Bitzer AS, Seaton SC, Smith LM, Silby MW. 2018. Social motility: interaction between two sessile soil bacteria leads to emergence of surface motility. bioRxiv doi: 10.1101/296814. [DOI] [PMC free article] [PubMed]
- 47.Hsueh YH, Somers EB, Lereclus D, Ghelardi E, Wong ACL. 2007. Biosurfactant production and surface translocation are regulated by PlcR in Bacillus cereus ATCC 14579 under low-nutrient conditions. Appl Environ Microbiol 73:7225–7231. doi: 10.1128/AEM.00690-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hölscher T, Kovács ÁT. 2017. Sliding on the surface: bacterial spreading without an active motor. Environ Microbiol 19:2537–2545. doi: 10.1111/1462-2920.13741. [DOI] [PubMed] [Google Scholar]
- 49.Danhorn T, Fuqua C. 2007. Biofilm formation by plant-associated bacteria. Annu Rev Microbiol 61:401–422. doi: 10.1146/annurev.micro.61.080706.093316. [DOI] [PubMed] [Google Scholar]
- 50.Kelsic ED, Zhao J, Vetsigian K, Kishony R. 2015. Counteraction of antibiotic production and degradation stabilizes microbial communities. Nature 521:516–519. doi: 10.1038/nature14485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narisawa N, Haruta S, Arai H, Ishii M, Igarashi Y. 2008. Coexistence of antibiotic-producing and antibiotic-sensitive bacteria in biofilms is mediated by resistant bacteria. Appl Environ Microbiol 74:3887–3894. doi: 10.1128/AEM.02497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedman J, Higgins LM, Gore J. 2017. Community structure follows simple assembly rules in microbial microcosms. Nat Ecol Evol 1:109. [DOI] [PubMed] [Google Scholar]
- 53.Pérez-Gutiérrez R-A, López-Ramírez V, Islas Á, Alcaraz LD, Hernández-González I, Olivera BCL, Santillán M, Eguiarte LE, Souza V, Travisano M, Olmedo-Alvarez G. 2013. Antagonism influences assembly of a Bacillus guild in a local community and is depicted as a food-chain network. ISME J 7:487–497. doi: 10.1038/ismej.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright ES, Vetsigian KH. 2016. Inhibitory interactions promote frequent bistability among competing bacteria. Nat Commun 7:11274. doi: 10.1038/ncomms11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Handelsman J. 2009. Metagenetics: spending our inheritance on the future. Microb Biotechnol 2:138–139. doi: 10.1111/j.1751-7915.2009.00090_8.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Circular 347. California Agricultural Experiment Station, University of California, Berkeley, Berkeley, CA. [Google Scholar]
- 57.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klimowicz AK, Benson TA, Handelsman J. 2010. A quadruple-enterotoxin-deficient mutant of Bacillus thuringiensis remains insecticidal. Microbiology 156:3575–3583. doi: 10.1099/mic.0.039925-0. [DOI] [PubMed] [Google Scholar]
- 59.Dunn AK, Handelsman J. 1999. A vector for promoter trapping in Bacillus cereus. Gene 226:297–305. [DOI] [PubMed] [Google Scholar]
- 60.Wijman JGE, de Leeuw PPLA, Moezelaar R, Zwietering MH, Abee T. 2007. Air-liquid interface biofilms of Bacillus cereus: formation, sporulation, and dispersion. Appl Environ Microbiol 73:1481–1488. doi: 10.1128/AEM.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
P. koreensis CI12 population dynamics. P. koreensis CI12 grown alone, in coculture with F. johnsoniae CI04 or B. cereus UW85, and in triple culture with F. johnsoniae CI04 and B. cereus UW85. Download FIG S1, TIF file, 25.7 MB (25.7MB, tif) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Correlation between Bacillus spp. spore density and F. johnsoniae protection in triple culture. (A and B) Triple culture of F. johnsoniae CI04 and P. koreensis CI12 with either B. cereus UW85 (A) or B. subtilis NCIB3160 (B). The cell density of the Bacillus inoculum is on the x axis. Bacillus spore densities and F. johnsoniae CI04 cell densities in the triple culture at 30 h are on the y axis. Gray dotted line, limit of detection. Download FIG S2, TIF file, 34.7 MB (34.7MB, tif) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Area of B. cereus colonies is larger in the presence of either F. johnsoniae or P. koreensis. Download Table S1, DOCX file, 0.04 MB (38.9KB, docx) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
P. koreensis CI12 biofilm quantification and growth of the planktonic population. P. koreensis CI12 growth is on the left y axis, and crystal violet quantification of biofilm formation for P. koreensis CI12 is on the right y axis. Download FIG S3, TIF file, 25.6 MB (25.6MB, tif) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains used in this study. Download Table S2, DOCX file, 0.10 MB (98.8KB, docx) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used in this study. Download Table S3, DOCX file, 0.04 MB (41KB, docx) .
Copyright © 2019 Lozano et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.