Abstract
Radiotherapy (RT), the major anti-cancer modality for more than half of cancer patients after diagnosis, has the advantage of local tumor control with relatively less systematic side effects comparing to chemotherapy. However, the efficacy of RT is limited by acquired tumor resistance leading to the risks of relapse and metastasis. To further enhance the efficacy of RT, with the renaissances of targeted immunotherapy (TIT), increasing interests are raised on RT combined with TIT including cancer vaccines, T-cell therapy, and antibody-based immune checkpoint blockers (ICB) such as anti-CTLA-4 and anti-PD1/PD-L1. In achieving a significant synergy between RT and TIT, the dynamics of radiation-induced response in tumor cells and stromal cells, especially the cross-talk between tumor cells and immune cells in the irradiated tumor microenvironment (ITME) as highlighted in recent literature are to be elucidated. The abscopal effect refereeing the RT-induced priming function outside of ITME could be compromised by the immune-suppressive factors such as CD47 and PD-L1 on tumor cells and Treg induced or enhanced in the ITME. Cell surface receptors temporally or permanently induced and bioactive elements released from dead cells could serve antigenic source (radiation-associated antigenic proteins, RAAPs) to the host and have functions in immune regulation on the tumor. This review is attempted to summarize a cluster of factors that are inducible by radiation and targetable by antibodies, or have potential to be immune regulators to synergize tumor control with RT. Further characterization of immune regulators in ITME will deepen our understanding of the interplay among immune regulators in ITME and discover new effective targets for the combined modality with RT and TIT.
Keywords: radiation therapy; irradiated tumor microenvironment, immune regulation, radiation-associated antigenic proteins, CD47, PD-1, PD-L1, HER2, tumor associated macrophage
Introduction
Both pro- and anti-tumor immune responses could be induced in tumor microenvironment under therapeutic radiation. The anti-tumor response (the abscopal effect) is observed in several human cancers treated with RT and the potential synergy for enhancing the cancer control is indicated in the combined therapy with RT and TIT 1-3. In addition to the well-defined checkpoint inhibitors PD-1 and PD-L1, exploration of other potential immune targets that are responsive to radiation in ITME will provide critical information in inventing more effective therapeutic modalities. Among the numerous IR-induced proteins, only a few of them are enhanced or induced temporally or permanently on the surface of tumor cells and could be targetable by immune approaches. Except the well-studied 'direct targets' of radiation such as DNA molecule in the nucleus, accumulating reports revealed 'indirect targets' on stromal cells in the irradiated local microenvironment as well as the cells distant to the tumor 4, 5. One of the observed indirect effects is the radiation-induced reconstruction of tumor microenvironment including release of cytokines from the irradiated cells, recruitment of antigen presenting cells, and expressions of new molecules or enhancement of original receptors at the cell membrane of tumor cells or immune cells 2, 6-9. Radiation is shown to increase the diversity of the T-cell receptor (TCR) repertoire of intratumoral T cells 10. Recent data generated by RT combined with TIT to treat metastatic cancer support the concept that the indirect effects of ionizing radiation (IR) are at least in part due to systemic antitumor immunity 11. In addition, RT with TIT shows a synergic potentiation in improving immune repertoire 12, 13 and enhances cell surface epitopes processed and presented in RT treated tumors 5. These results resuscitate the long-time holding abscopal effect which is in favor of a pro-immune and anti-cancer direction 14-16. Thus, the challenge could be further characterization and identification of such pro-immune elements in the ITME.
In the ITME, RT-associated pro-immune factors could be mixed with the radiation-mediated immune suppressors such as inhibition on M2 macrophage transformation and recruitment of immune modulator cells 17-19. It is thus suggested that the strategy of RT/TIT will meet several challenges including the absence or downregulation of major histocompatibility antigens (MHC), weak expression or presentation of antigenic proteins, and anti-inflammatory environment in irradiated tumor microenvironment 20. Such IR-associated pro-tumor immune response 21 could severely compromise the RT-induced priming immune response (abscopal effect, in the following section). Thus to enhance the tumor response to RT-TIT, the balance of anti-/pro-cancer immune regulation 2 needs to be further investigated. With the increasing interests and potential clinical benefits of RT combined with TIT, multiple level regulations observed in irradiated tumor microenvironment including tumor vasculature, immune elements, antigen visibility, stromal and immunological alternations are well discussed in a series of seminal reviews 4, 22, 23. The timing and dynamics of specific immune elements induced or enhanced in a given ITME is to be investigated. Thus, current under-focused developments are the RT-induced cell surface molecules on tumor cells that can be RT-inducible and immune-targetable as well.
Abscopal effect
Radiation-associated potential priming function in immune regulation is a long recognized phenomenon 24-26. The term abscopal effect created by RH Mole describes a relatively rare situation that “an action at a distance from the irradiated volume but within the same organism” 27. It has been observed that systemic immune modulatory factors induced by radiation have distinct immune effects on cancer cells 28. Although the exact mechanism remains to be elucidated, the abscopal effect is linked with the response of untreated lesions via a process termed “immunogenic cell death” (ICD) that helps the release of endogenous damage associated molecular patterns (DAMPs). Some of the DAMPs including calreticulin (eat me signaling), high-mobility group box 1 protein (HMGB1), and adenosine triphosphate (ATP) have been identified to play a role in activating the immune system and causing antigen presentation to T cells 4, 29, 30 (Fig. 1). For instance, the HMGB1 acts as a pro-inflammatory mediator to enhance the generation of cytokines such as TNFα, IL-1, IL-6, and IL-8 31 and tumor antigen presentation by binding to the toll-like receptor 4 (TLR4) on dendritic cells (DCs). In addition, the released DNA fragments from radiation-induced cell death are also able to stimulate the expression of interferon gene (STING) in DCs and thus to enhance DCs cross-priming 32. As low as a single dose of 0.5 Gy ionizing irradiation is shown to recruit NOS2-expressing macrophages to the tumors and increase T cell infiltration in the irradiated tumors 33, indicating a potential synergy on tumor control by RT combined with targeted immunotherapy.
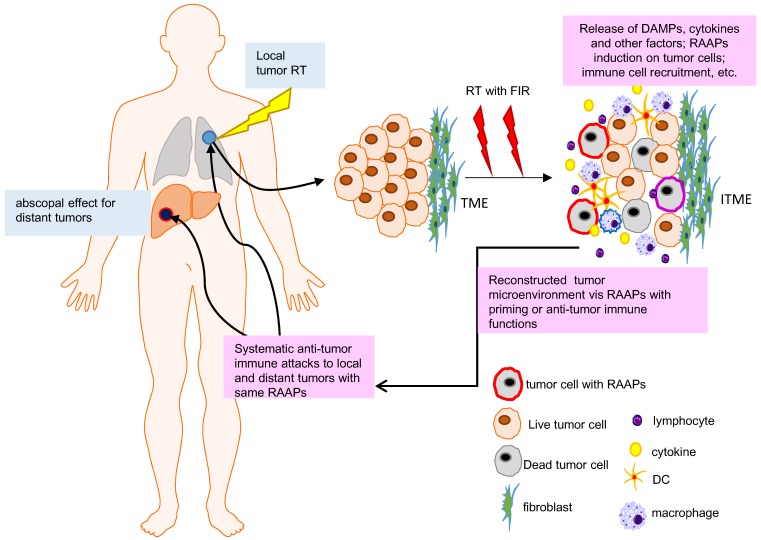
Figure 1.
Abscopal effect and RAAPs in irradiated tumor microenvironment (ITME). RT shrinks the local tumor but may also affect the distant lesions due to immunoregulation initiated by local tumor microenvironment (abscopal effect). RT can induce cell death, RAAPs (radiation-associated antigenic proteins), and immunogenic factors or cytokines, which can individually or synergistically to priming immune system via triggering the release of so called endogenous danger signals as damage-associated molecular patterns (DAMPs) and the activation of immune cells. The RAAPs and DAMPs contribute to priming of the immune system and activating immune mechanisms in a heterogenic tumor microenvironment (more information can be referred in 34).
Accumulating reports indicate that irradiated tumor cells can release an array of chemokines including CXCL16 and CXCL10 to enhance the expression of adhesion molecules E-selectin and ICAM-1 in endothelial cells and MHC, Fas, ICAM-1, and NKG2D ligands 35-41. The potential abscopal function is recently well-summarized with RT-treated 23 clinical cases in an array of human cancers 42. Another decent review has further demonstrated the abscopal effect in clinical RT treatments due to immunogenic cell death via releasing HMGB1 30 as well as tumor neoantigens (a term used to describe a patient-specific tumor antigen resulted from mutations during oncogenesis or by RT) 43, 44. During this process, radiation derived DAMPs is required for APCs (antigen-presenting cells) activation and phagocytosis 30. Additionally, IR induces NK2GD on cancer cells resulting in the promotion of natural killer cells' cytotoxic response 45. All of these actions are able to lead the visualization of tumor cells by immune system with systemic immune stimulations, which not only attracts primary tumors but is also believed to have an inhibitory function on metastatic lesions.
According to the meta-analysis by Marconi et al, the abscopal effect shows a dose-dependent pattern in response to IR 46. Although the optimum dose required for maximizing abscopal effect remains controversial 30, 47, identification of ideal RT scheme such as low or high fractionated doses may generate groundbreaking advantage in clinical practice. On the other hand, the molecular mechanism underlying abscopal effect is to be elucidated. Among the signaling pathways responsible for genotoxic stresses, the transcription factor NF-κB, a master regulator in inflammation and acute innate immune response, is well-defined to be sensitive to radiation stress and immune regulation 48, 49 and has been linked with abscopal effect 50, 51. Since the target genes of NF-κB can be induced in both tumor cells and immune cells in ITME, in the following section, NF-κB-mediated gene regulation in radiation-associated immune response is discussed.
NF-κB effector genes in radiation-associated immune response
NF-κB is a crucial transcription factor that regulates a wide scale of genes involved in both cell stress and immunity responses 8, 49, 52. NF-κB modulates effector genes involved in cellular stress responses, cytokines production, cell survival and metastasis via different homo- or hetero-dimerization of subunits RelA/p65, c-Rel, RelB, p50, and p52 53, 54. Generally, the activation of NF-κB functions in two major mechanisms, i.e., canonical and non-canonical pathways (Fig. 2). Radiation-induced NF-κB is mostly mediated via IKK-dependent canonical pathway. In addition to ROS-mediated activation of protein kinase in cell membrane receptors such as TNF-α 55, 56 and HER2 57, 58, the signals of IR-induced DNA damages in the nucleus can activate ATM/SUMO/NEMO pathway to enhance NF-κB nuclear translocation and gene transcription 59. This response is generally viewed as NF-κB-mediated DNA repair mechanism which influences the intrinsic radiation sensitivity and adaptive capacity of tumor cells in ITME such as the activation of IKKα/IKKβ/NEMO-dependent or -independent signaling pathways under the stress of genotoxicity 60. In the non-canonical pathway, RelB/p52 is activated depending upon the inducible p100 involved in lymphoid organogenesis, B-cell survival and maturation, DCs activation and bone metabolism 61.
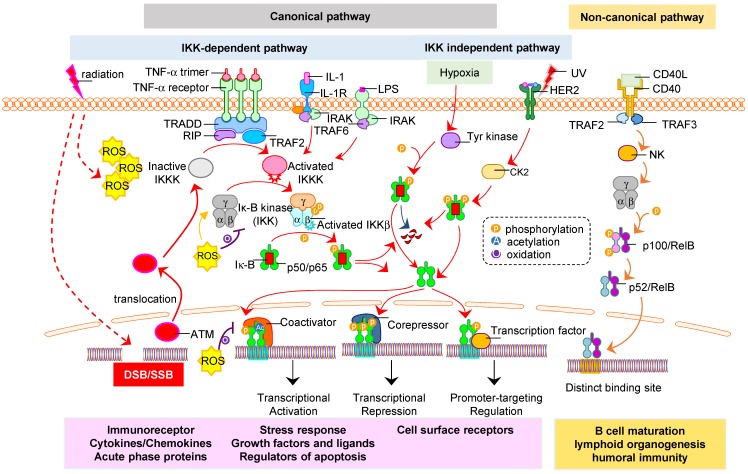
Figure 2.
Activation of NF-κB in ITME. Radiation can induce NF-κB activation in both tumor and stromal cells including immune cells in ITME. The subunits RelA (p65), c-Rel, RelB, p105/p50, and p100/p52 can form active homo- or hetero- dimeric complex and activated by the canonical and non-canonical pathway 62, 63. Radiation-induced NF-κB is mainly via canonical pathway that is divided into two categories: IKK-dependent or IKK-independent. The IκB is degraded upon phosphorylation by IKK which relocates NF-κB dimer (p50/p65) to nucleus for transcription of radiation-target genes including RAAPs such as CD47, HER2, and PD-1/PD-L1. UV or hypoxia activates NF-κB via IKK-independent phosphorylation of IκB in which RelB/p52 is activated via metabolism of p100. On the other hand, radiation-induced DNA damage activates nuclear ATM that also enhances NF-κB via regulation of IKK activity 8, 9, 64. The reactive oxygen species (ROS) produced by radiation triggers the NF-κB signaling in cytoplasm by IKK complex phosphorylation 65, while inhibits NF-κB signaling via oxidation of IKKɣ in cytoplasm or oxidation of p50 in nucleus. The canonical pathway impacts various biological processes, including immune response, inflammation, and cell growth and survival. The non-canonical pathway regulates important biological functions, including lymphoid organogenesis, B-cell maturation and DC activation.
A unique role of NF-κB in the ITME is indicated by its gene regulating functions in both tumor cells and immune cells 66. NF-κB is activated not only by IR-induced nuclear DNA damage via ATM in the stressed tumor cells, but also by IR-induced cytokines 8, 67. Radiation increased inflammation in tumor by activating the NF-κB pathway induces expressions of pro-inflammatory cytokines. The translocation of NF-κB complex to the nucleus through both canonical and non-canonical pathways induces various pro-inflammatory gene expression including TNFα, IL-6, IL-1α and IL-1β. Such inflammatory response coupled with potential release of RAAPs may enhance DCs maturation and prime tumor specific T cells 68. In doubly deficient p50/RelA or p50/c-Rel mice, the development and function of DCs were significantly impaired and IL-12 expression was abolished, suggesting that DC development is linked with the canonical NF-κB pathway 69. Sauter et al demonstrated that necrotic tumor cells were necessary to mature DCs that then primed CD8+ T cells 70. Hou et al demonstrated that impaired canonical NF-κB pathway could also reduce RT efficacy, indicating the canonical NF-κB-induced immune response in ITME. The same report also suggested that the non-canonical pathway in DC cells activated by STING pathway inhibited the release of IFN and blockage of p52-RelB nuclear translocation contributed to radiotherapy enhancement 71. HMGB1 that is released from necrotic cells is shown to induce DCs maturation via an NF-κB pathway. A tumor-promoting role for NF-κB activation is also indicated in TAMs (tumor-associated macrophages) in mouse tumor model. Since IR induces cell death via apoptosis and/or necrosis, it is reasonably believed that IR helps to prime anti-tumor immunity via antigenic proteins released by cancer cells and increase antigen uptake by DCs, which enhances DC maturation. However, these results also implicate that due to huge effector gene profile affected by NF-κB regulation in tumor and normal cells in the ITME, NF-κB itself may not be an ideal immune target in cancer treatment to synchronize RT-mediated cancer treatment. NF-κB pathways may be used as a tool to identify specific tumor antigenic proteins especially the antigenic membrane proteins such as CD47 and HER2 (refer to below) as well as neoantigens that can be applied for the combined therapy of RT with TIT. Moreover, elucidation of the specific clusters of genes of NF-κB responsible for control of pro- and anti-tumor immunity in both tumor and immune cells in the ITME will add significant insights on radiation-associated immune regulation in solid tumor.
Radiation induced immune-targetable elements
Radiation-induced tumor cell death has been shown to induce the release of tumor associated antigens and the “danger signals”, including HSPs, HMGB1, and calreticulin, which can induce DCs maturation and differentiation 72. However, it is argued that although such released antigenic proteins may potentially prime and activate immune cells, they may not be highly effective if such antigenic proteins are not efficiently presented on the surface of therapy-surviving tumor cells. Therefore, radiation-induced antigenic proteins in the surviving tumor cells especially the highly tittering antigenic proteins from cancer stem cells or stem-like cancer cells in the recurrent and metastatic lesions need to be further elucidated. As discussed above, recent studies have revealed significant alterations on the surface of cells (including both tumor and tumor-related cells). The usefulness of such antigenic proteins termed as RAAPs in this review will depend on their unique feature of differentially expressed on tumor cells rather than normal cells in an irradiated solid tumor 73. Further identification of this cluster of RAAPs in irradiated tumor microenvironment especially on the basis of individual tumors may improve the specific tumor targeting and increase 'indirect killing' effect of radiation. Below, we will summarize a short list of the radiation-induced antigenic proteins and cellular factors.
HLA
Human leukocyte antigen (HLA) is a well-studied complex of genes encoding the major histocompatibility complex (MHC) that locates on cell surface for fundamental immune regulation. MHC plays a key role in the acquired immune regulation to identify foreign molecules and defines histocompatibility. MHC is able to bind to antigens and displays them on the cell surface for recognition by the appropriate T-cells. MHC consists of two classes with a large scale of different alleles allowing them to be highly adaptive to varied epitopes. MHC class 1 (with subfamilies of classical ones-A, B, C and non-classical ones-E, F, G) present antigens from inside of cells (including self-antigens or integrated antigens of pathogens such as viruses), whereas, MHC class 2 (with subfamilies of DR, DP, and DQ) present antigens from outside of cells. All of the MHC molecules are sensitive to the genotoxic stress induced by radiation with either up- or down-regulating their expression 74. In cancer cells, MHC expression is generally down-regulated which is related to the ability of escaping from immune surveillance. The Class 1 molecules of MHC are reduced in tumor cells 75, 76 which reduces the chance to be recognized by active immune cells. This class is known to be induced by IR via radiation induced interferon (IFN type 1) release. However, the molecules in MHC Class 2 along with its subfamilies show different radiation responses 77, 78.
HLA-G, from the non-classical category of MHC class 1, is upregulated in cancer cells 79 and suppressed via radiation due to its sensitivity to RT, indicating a potential biomarker and target for tumor imaging and radiosensitization 80. HLA-G differs from the classical groups of HLA based on its function of suppressing immune cells via binding their Ig-like receptors (such as ILT-4 ILT-2, KIR2DL4 to monocytes, B and T cells and natural killers). Similarly, HLA-E is also found to be enhanced in cancer cells and reduced under radiation 80. Gallegos et al speculated that this downregulation of HLA-E is a secondary event to the downregulation of HLA-G which indicates that HLA-G may be required for stabilizing HLA-E 74. In addition, HLA-G enhances the apoptosis of immune cells and down regulates chemokine receptors on immune effector cells. Therefore radiation mediated down-regulation of these HLAs in cancer cells are potential immune regulators for enhancing the functions of immune cells in the ITME.
Immune checkpoint proteins
CTLA-4: CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), a member of the immunoglobulin superfamily, has a critical function in tumorigenesis process and CTLA-4 blockade combined with RT is shown to enhance survival in a murine glioma model 81 and in human cancers 82, 83. The potential synergy between RT and TIT with anti-CTLA-4 is recently well-summarized 83, 84. CTLA-4 is predominantly expressed on Treg cells 85 and thus is termed as immune checkpoint inhibitors together with PD-1/PD-L1. Classically, T cells are activated via APC presentation with MHC as a primary signal and CD80/86 as a secondary costimulatory signal. These surface molecules are known to be interacted with TCRs and CD28 on T cells thus activating the immune response. However, increased expression of CTLA-4 is shown to compete with CD28 86, 87. Because CTLA-4 has greater affinity than CD28, its high expression on cancer cells inhibits immune effector cells activation via increased CTLA4-CD80/86 binding. In addition, CTLA-4 is known to regulate integrin dependent motility of immune active cells 88 and prevent T cells to form long term interactions with APC 89. This inhibitory environment may push the immune modulatory cells to release inhibitory molecules such as TGF-β 90 or IL-10 91, escalating the immune suppressor microenvironment. When such effect on Treg cells is blocked, activation of T cells and diversity in TCR repertoire is enhanced 92.
PD-1/PD-L1: The other radiation-associated immune key checkpoint proteins are PD-1 and PD-L1 which act in later phase of immune suppression process (compared to CTLA-4) 93 and are important for peripheral tolerance. Their interaction causes inhibition of secretion of TNFα and IFN-ɣ, and activation of TGF-β secretion 94. The final effect is expectedly in favor of immunosuppression. Additionally, PD-1 /PD-L1 is thought to have a role in 'adaptive suppression' of T cell activation 95. Like CTLA-4, it pushes T cells to reduce their TCR expression, to reprogram their methylation level of their genome, to become allergic to locally expressed antigens, and to incline to apoptosis as well in in T cell exhaustion 96. More interestingly, Prima et al hypothesized that COX2-mediated PGE2 production pathway might modulate PD-L1 expression on myeloid derived suppressor cells. To prove that, they utilized pharmacologic inhibitors for COX2 and showed a reduced production of PGE2 together with reduced expression of PD-L1 97.
Blocking of these immune checkpoint proteins aims to 'wake up' the cytotoxic cells and abolish their exhaustion. It also causes the clonal expansion of T cells that will be expanded to a diversity of TCR repertoire 92. Thus, RT with anti-PD-1/PD-L1 strategy is superior to single therapy since blocking therapies increases the power of 'in situ vaccine effect' therefore it creates more antigenic stimulation related cytotoxicity 84. It is thus believed that IR-mediated cell death augments tumor-specific immunity. Deng et al revealed that IR combined with anti-PD-L1 enhanced T cell-mediated tumor cytotoxicity by enhancing the interplay IR and inhibition of PD-L1/PD-1 98. Hu et al reviewed the overall response rate of immune therapy in breast cancers (19%) and the combination therapy significantly enhanced the response rate 99. Pike et al investigated the overall survival outcomes for several types of cancers after combination therapy with PD-1 inhibition plus RT and their results indicate a prolonged survival compared to traditional single therapies 100. Several questions remain to be addressed in such combined therapies, which include the dynamics of PD-1/PD-L1 expression in irradiated tumor cells and immune cells, and the timing the RT that can eliminate a bulk of tumor cells lack of PD-1 expression before anti-PD-1/PD-L1 therapy.
CD47: In addition to above checkpoint proteins, CD47 (cluster of differentiation 47), is becoming one of key immune targets in cancer immunotherapy. CD47 is an anti-phagocytic signal in cancer cells to evade from TAMs for immune escaping 101. Originally identified in β3 integrin-mediated signaling on leukocytes 102, CD47 interacts with thrombospondin-1, signal regulatory protein-alpha (SIRPα), leading to a different cellular functions 103-109. The interaction between CD47 on tumor cells and SIRP-α on immune effector cells generates a "don't eat me" signal to suppress the immune response 110. CD47 ligates SIRPα expressed on macrophages and DCs and initiates a dephosphorylation cascade to inhibit phagocytosis 111-113. Consistently, CD47-/- cells are removed fast when they are adoptively transferred to the congeneric wild-type mice 114. Jaiswal et al and Majeti et al showed that CD47 in leukemic stem cells (LSCs) can inhibit macrophage activity that is linked with the growth advantage of LSCs 115, 116.
CD47 is widely expressed at low level in normal tissues and highly expressed on multiple tumors, including hematological and solid cancers 117-121. Considering the important inhibitory role of CD47 in phagocytosis of tumor cells, it is thus viewed as one of potential immune target in anti-cancer immunotherapy. Initially, most studies focused on the direct killing effect of anti-human CD47 122-124. Subsequently, its indirect functions in regulating phagocytosis or tumor death were noticed. Recently, CD11b activation which is necessary for macrophage activation for tumor cell phagocytosis is found to be actively involved in anti-tumor innate immunity 125. Combining CD47 blockade with irradiation significantly affected tumor growth and enhanced tumor sensitivity to irradiation in syngeneic immunocompetent mouse 126. Transcription of CD47 can be enhanced by IR 127 and inhibition of CD47 signaling conferred a survival advantage to irradiated normal tissue via promoting viability and proliferative capacity in an autophagy-dependent manner 128. Liu et al found that the therapeutic effects of CD47 blockage depended on DCs but not macrophage cross-priming of T cell responses in syngeneic immunocompetent mouse. Chemotherapy might impact the efficacy of anti-CD47 treatment by suppressing T-cell memory response 129. Currently, about eight phase I clinical trials and two phase II clinical trials are evaluating the effects by blocking CD47-SIRPα pathway in various cancer types. Combined treatments of humanized monoclonal anti-CD47 antibody Hu5F9-G4 and chemotherapy were administered to patients with solid tumors that are not fit for conventional therapies 130, 131. However, the clinical responses could not be confirmed probably due to the small numbers of patients recruited 132.
The enhanced expression of CD47 in the tumor microenvironment could severely hamper tumor radiosensitivity 126, 133. Betancur et al showed that CD47 were remarkably enhanced in HER2 positive breast cancer HCC1954 and luminal A MCF7. NF-κB and PPARα were identified as two transcription factors that bound with super-enhancer of CD47 and regulated protein expression 134. Our lab discovered a co-expression pattern of CD47 and HER2 in a series of radioresistant breast cancer cell lines that survive from fractionated doses of IR 135. Since both promoters of CD47 and HER2 contain NF-κB binding motif, a possible co-expression pattern between these two RAAPs is proposed (Fig. 3, and unpublished data). More recently CD47 was found have another co-expression model in breast cancer as well. Cook et al revealed that glucose regulated protein 78 (GRP78) may correlate with the expression of CD47. Thus, targeting GRP78 significantly altered CD47 expression, indicating UPR involvement in CD47 regulation 136, 137. These two RT-induced membrane proteins should be considered as the candidates for dual immune targets in the RT combined therapy.
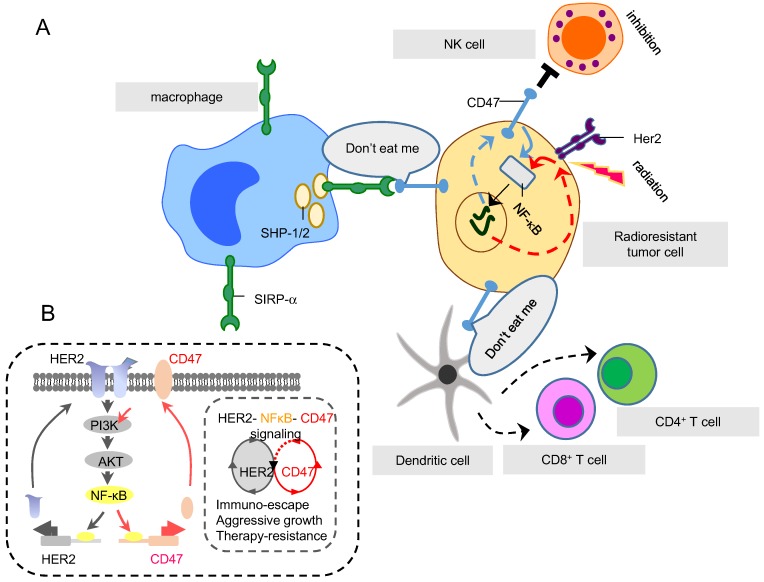
Figure 3.
Two RAAPs, CD47 and HER2, regulated by NF-κB in radioresistant cells. (A) SIRP-α is an inhibitory receptor expressed on immune cells including macrophages, DCs and neutrophils. SIRP-α binds CD47 (generating do-not-eat me signaling on tumor cells) to recruit inhibitory molecules, such as SHP-1 and SHP-2, preventing the activation of immune myeloid cells, in particular macrophages, so to shield tumor cells from macrophage-mediated phagocytosis. (B) Both HER2 and CD47 are RAAPs inducible in breast cancer cells by radiation via NF-κB-mediated transactivation. CD47 provides the immune-shielding capacity to escape the immune surveillance which together with HER2-mediated intrinsic pro-survival networks, contributes to the overall tumor aggressive phenotype and radioresistance. Therefore, immune blockage of CD47 and HER2 may be able to maximize macrophage-mediated phagocytosis on tumor cells that survived RT.
Other immune regulators
A large scale of molecules can alter their expressions under radiation and is assumed to be involved in immune regulation (a selected list is shown in Table 1). Most of such molecules are derived from ER with the accelerated production under IR and they directly modulate cell survival. Below, we list some of stress-associated molecules as well as non-ER derived 'danger signal' molecules that show potential targetable application.
Table 1.
Selected RT-related immune regulators induced by radiation
| Molecule | Function | Immune and/or tumor cells | Behavior under IR | References |
|---|---|---|---|---|
| HMGB1 | Danger signal, enhancing tumor immunogenicity | both immune and tumor cells | Inducible by IR | 138, 139 |
| GRP78 | Immune regulation and signal transduction | both immune and tumor cells | Radioresistance | 140 |
| HLA | Reduced expression to escape (except HLA-G and HLA-E) | Immune cells | Induced | 40, 141, 142 |
| CTLA-4 | Immunosuppression | activated T cells | Induced under low doses, diminished under high doses | 143-145 |
| PD-1/PD-L1 | Immunosuppression | T cells/tumor cells | Induced | 98, 146, 147 |
| CD47 | “Don't eat me” signaling | tumor cells | Induced (in survived cells) | 109, 148 |
| Ox40 | Support for immune effector cells and APC | immune cells | Induced | 149-152 |
HMGB1: HMGB1 (25 kDa molecular weight) is an intra-nuclear protein regulating gene transcription by binding chromosomal proteins or interacting with several transcription factors 153. Although HMGB1 physiologically enhances immune activation and motility through TLR4 activation 154, several studies show that HMGB1 is linked with poor prognosis probably due to its interaction with myeloid differentiation factor 88 and TLR4 154-156. He et al found that HMGB1 which helped tumor cell proliferation was released into the medium in Hela, HT29, HT116 cells treated with 10 Gy IR 157. However, the priming function of induced HMGB1 is suggested to translocate to cytosol after acetylation or phosphorylation and secreted to extracellular compartment in passive or active way. HMGB1 secretion is induced by interferons (IFNs) in acetylated or phosphorylated type to extracellular compartment. HMGB1 can be released from active immune cells. For instance, activated DCs secrete HMGB1 before maturation and the extracellular HMGB1 induces a feedback signaling for the maturation of DCs and activation of T cells. As to passively secretion, it is released by dead cells or dying cells, such as RT induced cell death. It has been shown that HMGB1 level is enhanced in the tumor microenvironments with increased tumor antigen-specific T-cells in patients with esophageal cancer treated by chemoradiotherapy 138 and the release of HMGB1 is proportional to the radiation doses delivered by carbon-ion beam irradiation 139.
GRP78 (glucose regulated protein 78, or termed immunoglobulin heavy chain binding protein, BIP): a member of heat-shock protein 70 (HSP70) family located in endoplasmic reticulum (ER) lumen, plays a key role in the UPR and is linked with tumor resistance and prognosis 158, 159. Although known in regulating protein folding stress 160, GRP78 is shown to be involved in a wide array of stress conditions including glucose depletion, low oxygen levels, high levels of oxidant molecules 161 as well as in DNA-damage response 162. GRP78 is able to regulate lipid metabolism related to drug sensitivity and anti-tumor immunity in breast cancer 137. GRP78 and MTJ-1 a transmembrane protein are identified in the macrophage plasma membrane lysates, indicating GRP78 can be relocated to cell surface to participate in immune regulation and signal transduction 163, 164. GRP78 is shown to be involved in hepatitis C virus (HCV) replication and Toll-like receptor-3 (TLR3)-initiated antiviral state 165. The safety of TIT using anti-GRP78 antibody PAT-SM6 has been tested in mouse model for treatment of relapsed or refractory multiple myeloma 166. Although such tests should be further evaluated, TIT with GRP78 antibody was demonstrated to inhibit CD47 and enhance tumor infiltration of immune cells to increase an anti-tumor microenvironment. The correlations between GRP78 and radiation is demonstrated by the observation that expression of GRP78 is related to the radiosensitivity of brain endothelial cells 140 and expression of GRP78 is enhanced in breast cancer stem cells (BCSCs) and responsible for self-renewal and radioresistance of BCSCs 167. TIT with anti-GRP78 antibody showed extraordinary inhibition on the aggressive phenotype of GBM and NSCLC cells due to inhibition of PI3K/Akt/mTOR pathway, and tumor inhibition was enhanced by RT combined with TIT of GRP78 in tumor model of NSCLC and GBM 168.
CD134 (Ox40): This molecule, mainly expressed on activated CD4 and CD8 T cells, supports the activation of immune effector and antigen presenting cells and promotes them to elongate their survival time and release cytokine 169. Additionally CD134 suppresses the differentiation and activity of Treg 170. Moran et al arranged series of experiments by using both CD134 agonists and antagonists plus with anti-immune checkpoint protein antibodies. The findings were encouraging for the further clinical usage of CD134 agonists because of its significant anticancer, pro-immune effects 171. Combination of CD134 with radiation in lung cancer model resulted in an overall survival rate of 80% at 100 days compared to 0% in mice treated with either modality alone 172. Similarly, surgical removal of 10-14 day sarcoma resulted in 50% local tumor recurrence whereas anti-CD134 delivered at the time of the operation eliminated local recurrence in 100% of mice. In addition anti-CD134 with surgery and radiation led to a survival rate of 50% at 70 days 173. These two studies indicate that CD134 is a promising immune target and anti-CD134 combined with RT has the priority for clinical trials.
TLRs: TLRs (toll-like receptors) are both cytosolic and membranous molecules which recognize pathogens by their pathogen associated molecular patterns and danger signals released from damaged cells. Due to their unique capacity of regulating the immune cascade in pro-inflammatory response, their potential application in TIT has been suggested 174 as well as in combined therapy with RT 175, 176. Accumulating preclinical evidence showed that agonists of TLRs, especially TLR3, 4, 7/8 and 9 agonists, efficiently promote tumor-targeting immune responses initiated by anticancer immunotherapy. By providing essential requirements for stimulating innate and adaptive immunity as well as their effects on the tumor microenvironment, local application of TLR agonist as single agent exhibited effectively to eradicate tumors. There are two FDA-approved TLR agonists used as cancer monotherapy, bacillus Calmette-Guerin (BCG) and imiquimod. Apetoh et al reported that breast cancer patients with a TLR4 loss-of-function allele relapse more quickly after radio- or chemo-therapy than those carrying the normal TLR4 allele. Further studies illustrated that activation of DCs during radio- or chemo- therapy required TLR4-mediated signaling 177. Intravenous administration of the TLR7 agonist, R848 with RT, can inhibit T-and B-cell lymphoma in animal model; and led to a tumor-specific memory which is believed to prevent tumor recurrence 178.
CRT: CRT (Calreticulin), a danger-sensor protein derived from endoplasmic reticulum, is able to translocate to the cell membrane after radiation where it generates "eat me" signals for tumor associated macrophages and lymphocytes 30, 179. CRT viewed as an immunogenic signature associated with low-dose radiation should be categorized in the cluster of RAAPs and can interact with APCs to support IR induced ICD 136. Surface expression of CRT was enhanced by radiation in an array of human cancer cells including prostate, breast, and lung 29 to promote APCs and T cell-mediated tumor cell killing 180.
Other radiation related immune regulators
Different non-immune and immune cells are present in irradiated solid tumors; some are thought to be pro-cancer like Treg, M2 macrophages, myeloid derived suppressor cells, neutrophils (N2 type), basophils, B lymphocytes, mast cells and some others on the contrary such as M1 macrophages, antigen presenting cells (DCs), CD8+ T lymphocytes or natural killer cells. Most importantly, these immune active cells are able to communicate and cooperate in the ITME to release cytokines and even non-coding transferrable genome parts 181. Some of such cellular and non-cellular factors are described below.
Extracellular matrix (ECM): ECM plays a key role in intercellular communication, sequestration of various factors, cellular adhesion and migration. As content, it has various types of proteins (collagen, elastin, laminin etc.), proteoglycans (keratin sulfate, heparin sulfate etc.) and liquid. Collagen and fibronectin are known to be related to tissue strength while proteoglycans support the sequestration of growth factors and cytokine providing 182, 183. To be specific to cancer, extracellular matrix is showed to create a scaffold which guides tumor cells in their migration process and chemotaxis 184. Moreover, cross-linking of proteins in ECM is surprisingly showed to support tumor cells to invade their local environment easier 185. It was also projected by recent studies that ECM affected anti-tumor therapy efficacy by its interstitial fluid pressure 186, desmoplastic stroma and tissue rigidity 187.
With local tumor RT, ECM can be remodeled in various ways, actually in favor of pro-tumor forces 188 which is believed to link with DNA damage, cell death and hypoxia 189, 190 as well as different factors released (such as VEGF, CSF-1, SDF-1 etc.) from both local host cells and tumor cells. These factors may attract various cells like TAMs or CAFs which have the capability to alter the microenvironment matrix by their products and even with their own cellular structures. Hypoxia, on the other hand causes acidification in stroma (whether it is induced by IR or not) due to accumulation of lactic acid released from cancer cells under highly-glycolytic conditions. Although it is unclear low pH status could be a unflavored factor for priming immune system, low pH is shown to alter cellular behaviors such as alteration of transcription factor expressions or increased invasiveness and migration 191. Since HIF1 (hypoxia induced factor 1) is a well-defined activator of VEGF 192, 193, HIF1 may shape tumor vascular support for survival and RT-resistance. These tumor associated vessels showed enhanced permeability with increased turbulent flow and increased numbers of pericytes 194 that can enhance tumor invasion/metastasis via reduction of P- and E-selectin, V-CAM, I-CAM, which are related to binding and homing for immune cells. Therefore, pericytes could be a potential target to enhance immune response under RT.
Tumor cells are able to adhere to structural components of ECM leading to enhancement of cellular polarity and EMT (epithelial-mesenchymal transformation). This might be due to interaction of collagen, fibrin and/or laminin with integrin on the cell membrane via pathways such as FAK, PI3K/Akt/Stat3 and NF-κB 195, 196. These all molecules along with their capability to cellular polarization may allow tumor cells to be apoptosis-resistant to chemotherapy and radiotherapy 183, 197. Another way that ECM may cause anti-tumor therapy resistance is extracellular vehicles (EVs) such as the case in endothelial-derived EVs via noncoding RNA activators for Stat1 and/or decoy for antibody treatment 198. Such EVs could be potential immune targets to block tumor acquired radioresistance.
DNA exonuclease Trex1: The TREX1 gene in mammalian cells encodes a dominant DNA exonuclease TREX1 enzyme that functions to degrade DNA 199, 200. Mutations in the TREX1 gene are linked with a group of autoimmune diseases 201. The DNA fragments accumulated in the cytoplasm of the irradiated cells can help cells to release interferon-β following activation of the DNA sensor cGAS and its downstream effector STING which is able to prime CD8+T cells for initiating the anti-tumor effect. Interestingly, TREX1 is identified to be inducible in irradiated cancer cells and the IR-induced TREX1 reduces the immunogenicity of irradiated cancer cells by degrading the DNA fragments accumulated in the cytoplasm 202, 203. Such TREX1-mediated suppressive function on interferon-β has the potential of being an effective approach to regulate tumor immunogenicity in the combined therapy of RT and TIT.
Cancer-associated fibroblasts (CAFs): Fibroblasts are the most common cell type in connective tissue and prevail in tumor tissues. They are normally found in a quiescent form with the potential to be transformed reversibly into their activated form under stress conditions such as wound healing. The CAFs are an irreversible state of fibroblasts found in tumor microenvironment and linked to several actions in tumor progression. A noteworthy skill of CAFs is that they are extremely resistant to radiotherapy 204. The CAFs are believed to be the sufficient source of VEGF-α as well as other growth factors and extracellular matrix proteins to support reorganization and vascularization of tumor microenvironment 205, 206. The CAFs also have pro-inflammatory characteristics 207 and are found to interact with local tumor cells and immune cells.
The communication between tumor cells and CAFs also promotes the release of TGF-α that is able to remodel the microenvironment in favor of invasion and metastasis 208. However tumor cells not only crosstalk with CAFs or APCs but also communicate with TAMs as well. This cross-talking is carried out with several different signaling pathways such as EGF, CSF-1, and Wnt 209. And this often concludes with increased release of proteases like cathepsin 210 to promote further invasion and progression. Another combination in this crosstalk concept is realized with endothelial vesicles which again provide further organization and might play role in APC recruitment 211. However the studies to prove the importance of CAFs and their crosstalk model revealed controversial findings. Some studies indicate increased aggressiveness in depleted stroma 212 while the others show a togetherness of low stromal index and better prognosis via improved drug delivery.
Other active immune cells
Macrophages are one of the main immune active cells involved in almost all inflammatory situations including ITME. Macrophages either promote inflammation and chaos (M1 macrophages) or push cells to act for tissue healing and fibrosis in the affected area (M2 macrophages).TAMs are found to be recruited to tumor microenvironment via CCL2 213, 214. The chemokine CCL2 (also termed monocyte chemoattractant molecule-1, MCP-1) can recruit CCR2-expressing monocytes to tumor microenvironment where the monocytes are able to differentiate into TAMs and dendritic cells 215, 216. Since these 2 subtypes of macrophages are functionally different, their products and activated signaling pathways are varied. Via NF-ĸB, STAT11 and IRF 217, 218 activator signals, M1s uses CXCL9 and CXCL10 to recruit immune effector cells. In contrast, M2s secrete CCL5, CCL17, CCL20, CCL22 to recruit immune modulator cells like Tregs via IRF4, STAT6, c-Myc, PRAR signaling 219. Although the functions of TAMs on tumor cells are still in debate, increasing results support the pro-tumor effects. Via NF-κB signaling, TAMs promote EMT 220 (a well-known radioresistant state of cells), local invasion, intra- and extravasation (by neovascularization) 221, 222, seeding and growth at distant sides; together indicating their relationship with increased metastasis risk. However, in addition above mentioned TIT via anti-CD47 which enhances macrophage-mediated phagocytosis on tumor cells, macrophages in the metastatic tumors are found to secrete granulin that activates local myofibroblasts and leads to fibrosis via periostin secretion 223-225. This mechanism is thought to be a protective role of organism to prevent further invasions and metastasis.
In addition, TAMs are capable of recruiting (via CCL5, CCL20, CCL22) and activating Treg (via IL-10 226 for TGF-β production 84 and inducing the checkpoint proteins and ARG1 227 and NOS to suppress effector T cells, which is thought to promote immune suppression in tumor microenvironment 228, 229. Pinto et al investigated the relationship between TAMs and cancer cells in co-cultures of different cancer cell lines. Although their results showed different responses for different cancer cell lines, the overall output from their study was that TAMs were definitely in contact with cancer cells and they even might regulate metabolism, gene expression and cytokine production under IR and overall radiation response of cancer cells 230. Fortunately, IR shifts the pro-tumor effects predominance to anti-tumor side. IR pushes the transformation M2 to M1 231 and production of NO 232. However the alone application of IR generally is not sufficient to overcome this strong predominance. That's one of the reasons why IR becomes much more effective when combined with immune-therapeutics.
Dendritic cells: Tumor infiltration of DCs and several other immune effector cells is carried out via several cytokines such as CXCL16, TNFα, IL-1β, IL-6 secreted by IR induction of tumor cells 233. Attracted APCs, however, might play a little different role on the contrary of generally known antigen presentation process. Current studies indicated that irradiated APCs play an immune suppressor role via down regulating its immune activator (T cell polarizator) IL-12 and IL-23 cytokines. Moreover to this, in tumor microenvironment, the communication between CAFs and DCs (like COX2 dependent PGE2, released from fibroblasts) provides DCs to maintain their immune suppressor activity through promoting further IL-23 synthesis despite IR application. In the presence of IL-1β and TNFα, IL-23 is found to be a T cell polarizing factor that accelerates CD4+ cells to transform into Th17 cells resulting in an immune modulator environment. In addition, the DCs are showed to maintain their presence and abilities even under the genotoxic stress of IR (with some modifications), indicating their strong adaption under IR condition 204. Further elucidation will be in need on the mechanistic insights of cross-talk between DCs and tumor cells, especially the communication between DCs and radiation-surviving tumor cells.
Potential clinic trials
Accumulating clinical and experimental data support a potential synergy of RT with TIT 5, 234. Radiation induced or enhanced concomitant immunity on different tumors has been tested in animal models 4, 235. The “optimal RT doses” to enhance tumor immunogenicity is to be further optimized especially based on different tumors and individual patients. Dewan et al observed that RT with fractionated doses combined with anti-CTLA4 treatment not only delayed the growth of primary tumors but induced abscopal effect with enhanced CD8+ T cells in mouse breast and colon cancer models 144. Local RT combined with immune targeting by anti-PD-1 or anti-PD-L1 antibody also demonstrate the benefits in activation of the cytotoxic T-cells with improved tumor control and animal survival 98. Surface GRP78 is closely related to tumor radiotherapy response and cancer recurrence. Targeting surface GRP78 by peptide or antibody strengthens the reaction of tumor to radiotherapy 168, 236. In addition, the IFNγ produced by CD8+ T cells is shown to enhance PD-L1 expression in tumor cells after RT with fractionated ionizing radiation (FIR) 234, 237. Enhanced tumor responses and survival benefits are also detected in mouse with intracranial gliomas treated by anti-PD-1 combined with stereotactic RT 146. All of these animal models have generated supportive data for clinical trials of RT combined with TIT.
In a pioneering clinical trial, local RT was conducted with the regimen of 35 Gy in 10 fractions to one metastatic site along with concurrent GM-CSF (125 μg/ml subcutaneously injected daily for 2 weeks, starting during the second week of radiotherapy). This trail identified the abscopal effects in 11 of 41 patients. The mortality rate in patients absent of abscopal effects was more than twice than patients with it 238. In the current era of PD1/PD-L1, increasing reports have indicated the encouraging synergy of RT with checkpoint blockade 239. Many such trials are underway and data are going to be accumulated soon 240. However, it was found that RT/anti-PD-1 therapy was effective only on RT-naïve tumors and anti-PD-1 did not generate anti-tumor efficacy in RT-relapsed tumors 241, indicating additional factors against systematic immune surveillance could be induced in RT-surviving tumor such as RT-resistant cancer stem cells, which is to be further elucidated.
The ideal situation is that we could define a RT regimen that augments the immunogenicity of tumor cells without activation of tumor immune suppressing factors such as PD-1/PD-L1 and CD47 in ITME. Optimization of such RT doses and selection of an effective immune target for TIT could be a challenge, especially with the variations in RT responses to individual TME. However, with the twilight of more clinical studies being evaluated, increasing numbers of studies and clinical trials have demonstrated that hypo-fractionated regimen of RT before TIT could enhance the efficacy of tumor control 144, 234 with increased TAA (tumor-associated antigen) production, thus augmenting immune stimulation. However there are some obstacles and controversial speculations about the timing for RT delivery. It is suggested that the effectiveness of RT may be limited if it is applied before TIT since the predominance of immune suppressive forces in the untreated TME may eliminate appropriate induction of TAAs 214. On the other hand, if it is applied after immune therapy, it might destroy the local immune effective cells which are recruited by applied immune therapy. Although facing such dilemma, the common consensus signposts that the hypo-fractionated regimen should be strong enough to induce tumor immunity but not to damage local immune cells 242, 243. Regimens such as 5Gy x 4, 8 Gy x 3 are effective approaches applied with the immune checkpoint blockade 144. Among the immune targets in clinical trials that can be potentially combined with RT, immune checkpoint protein inhibitors are currently popularly studied. Other immune effectors against tumor cells such as soluble immune activator including interleukins like IL-3, immune cells colony stimulant factors like GM-CSF, inducers and growth factors for specific cell types like Flt3-L for DCs 244 are also being investigated.
Conclusion and Perspectives
With the renaissance of targeted immunotherapy especially checkpoint inhibitors being approved by FDA, antibody-based ICBs such as atni-CTLA-4 and anti-PD1/PD-L1 have created impressive clinical impacts on treatment of an array of human cancers including lung cancer, melanoma, glioblastoma and other solid tumors. These exciting results encouraged further investigations on potential synergy of TIT with RT. In Clinicaltrials.gov, increasing trials have been registered: “CTLA-4/radiotherapy” (67 studies); “PD-1/radiotherapy” (182 studies); “PD-L1/radiotherapy” (186 studies); “CD47/ radiotherapy” (1 study). These primary trials reveal that combination of RT with TIT may improve clinical outcomes compared with either treatment alone. For instance, in treatment of melanoma patients with brain metastases, the 3-year survival rate for patients treated with ipilimumab (targeting CTLA-4) and stereotactic radiosurgery was 50% while for patients without treatment of ipilimumab was 39% 245. Prolonged survival and metastases control were also observed in patients with PD1-targeting inhibitor OPDIVO combined with RT 147. Moreover, overall survival in the combined treatment group (RT combined ipilimumab or anti-PD-1) was reported to be superior to single treatment group 246, 247. As such, in the eve of a coming era of combined modalities of RT with increasing immune targets, a potential significant enhancement in cancer control could be expected.
However, although RT/TIT combined therapy is promising, current clinical studies are mainly limited to CTLA-4 or PD-1 targeting inhibitors. The fact that many patients show no response to combined treatment and some responders develop acquired resistance after initial response indicating that the RAAPs discussed in this review need to be explored. Most importantly, the mechanistic insights of radiation induced anti-tumor response (priming function) requires further elucidated. Elements induced in ITME including CXCL16, CTLA-4, CD47, HER2, PD-1 and PD-L1 in line with many other potential immune suppressors may enhance the immune-escaping capacity of tumor cells, which may severally compromise the radiation-induced priming function. The induced immune suppressors on the other hand provide targetable molecules for TIT or for combined RT/TIT. The potential clinical benefits should be expected from further targeting these different immune-associated elements to increase the tumor immunogenicity and the priming function. The ideal situation would be that an RT regimen could be optimized to enhance the priming function rather than immune suppressive factor in ITEM and thus will benefit with TIT. For example, the radiation-surviving tumor cells including cancer stem cells that may be effectively eliminated by TIT following RT if such fraction of tumor cells express the proper immunologic molecules on the cell surface such as PD-1/PD-L1 248. More experimental results and clinical data are expected to reveal additional new insights of immune regulators in the ITME. These results will be necessary to deepen our understanding of radiation-associated immune regulation and guide to invent more effective targets in RT combined with targeted immunotherapy.
Acknowledgments
We apologize to the authors whose publications could not be included in this review due to limited space. The authors thank Dr. Mansoor Ahmed at NCI, NIH and Dr. Andrew Vaughan at UC Davis Cancer Center for their critical reviewing the manuscript. This work was supported by National Cancer Institute Grants RO1 CA213830 (to JJL). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U. S. C. Section 1734 solely to indicate this fact.
Author contributions
O.M.O., and J.J.L. conceived the idea for the review. O.M.O., L.Z., and J.J.L. wrote the manuscript. L.Z. generated the figure panels. L.Z., and J.J.L edited the final version of the manuscript.
References
- 1.Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol. 2015;12:527–540. doi: 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13:516–524. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marciscano AE, Walker JM, McGee HM, Kim MM, Kunos CA, Monjazeb AM. et al. Incorporating Radiation Oncology into Immunotherapy: proceedings from the ASTRO-SITC-NCI immunotherapy workshop. J Immunother Cancer. 2018;6:6. doi: 10.1186/s40425-018-0317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diegeler S, Hellweg CE. Intercellular Communication of Tumor Cells and Immune Cells after Exposure to Different Ionizing Radiation Qualities. Front Immunol. 2017;8:664. doi: 10.3389/fimmu.2017.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demaria S, Coleman CN, Formenti SC. Radiotherapy: Changing the Game in Immunotherapy. Trends Cancer. 2016;2:286–294. doi: 10.1016/j.trecan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed KM, Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 2008;44:1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 10.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halvorsen AR, Helland A, Fleischer T, Haug KM, Grenaker Alnaes GI, Nebdal D. et al. Differential DNA methylation analysis of breast cancer reveals the impact of immune signaling in radiation therapy. Int J Cancer. 2014;135:2085–2095. doi: 10.1002/ijc.28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludgate CM. Optimizing cancer treatments to induce an acute immune response: radiation Abscopal effects, PAMPs, and DAMPs. Clin Cancer Res. 2012;18:4522–4525. doi: 10.1158/1078-0432.CCR-12-1175. [DOI] [PubMed] [Google Scholar]
- 14.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bockel S, Antoni D, Deutsch E, Mornex F. [Immunotherapy and radiotherapy] Cancer Radiother. 2017;21:244–255. doi: 10.1016/j.canrad.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A, Bode B, Wenger RH, Lehmann K, Sartori AA, Moch H. et al. gamma-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS One. 2011;6:e28217. doi: 10.1371/journal.pone.0028217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P. et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 18.Chiang CS, Fu SY, Wang SC, Yu CF, Chen FH, Lin CM. et al. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2:89. doi: 10.3389/fonc.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Teng F, Kong L, Meng X, Yang J, Yu J. Radiotherapy combined with immune checkpoint blockade immunotherapy: Achievements and challenges. Cancer Lett. 2015;365:23–29. doi: 10.1016/j.canlet.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25:2559–2572. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W. et al. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front Pharmacol. 2018;9:185. doi: 10.3389/fphar.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E. et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L. et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975;48:863–866. doi: 10.1259/0007-1285-48-574-863. [DOI] [PubMed] [Google Scholar]
- 26.Raventos A. An abscopal effect of x-ray upon mouse spleen weight. Radiat Res. 1954;1:381–387. [PubMed] [Google Scholar]
- 27.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 28.Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer. 2016;40:10–24. doi: 10.1016/j.currproblcancer.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu ZI, McArthur HL, Ho AY. The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Curr Breast Cancer Rep. 2017;9:45–51. doi: 10.1007/s12609-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H. et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A. et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N. et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Holzel M, Bovier A, Tuting T. Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer. 2013;13:365–376. doi: 10.1038/nrc3498. [DOI] [PubMed] [Google Scholar]
- 35.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN. et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 36.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 37.Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996;56:5150–5155. [PubMed] [Google Scholar]
- 38.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 39.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO. et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS. et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–3730. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Z, Chen F, Meng F, Wei J, Liu B. MHC class II restricted neoantigen: A promising target in tumor immunotherapy. Cancer Lett. 2017;392:17–25. doi: 10.1016/j.canlet.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 44.Sethuraman SN, Ranjan A. Neoantigen activation, protein translocation and targeted drug delivery in combination with radiotherapy. Ther Deliv. 2016;7:377–385. doi: 10.4155/tde-2016-0005. [DOI] [PubMed] [Google Scholar]
- 45.Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH. et al. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. 2006;38:474–484. doi: 10.1038/emm.2006.56. [DOI] [PubMed] [Google Scholar]
- 46.Marconi R, Strolin S, Bossi G, Strigari L. A meta-analysis of the abscopal effect in preclinical models: Is the biologically effective dose a relevant physical trigger? PLoS One. 2017;12:e0171559. doi: 10.1371/journal.pone.0171559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson CB, Jagsi R. The Promise of the Abscopal Effect and the Future of Trials Combining Immunotherapy and Radiation Therapy. Int J Radiat Oncol Biol Phys. 2016;95:1254–1256. doi: 10.1016/j.ijrobp.2016.02.067. [DOI] [PubMed] [Google Scholar]
- 48.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 49.Wang T, Zhang X, Li JJ. The role of NF-kappaB in the regulation of cell stress responses. Int Immunopharmacol. 2002;2:1509–1520. doi: 10.1016/s1567-5769(02)00058-9. [DOI] [PubMed] [Google Scholar]
- 50.Aravindan S, Natarajan M, Ramraj SK, Pandian V, Khan FH, Herman TS. et al. Abscopal effect of low-LET gamma-radiation mediated through Rel protein signal transduction in a mouse model of nontargeted radiation response. Cancer Gene Ther. 2014;21:54–59. doi: 10.1038/cgt.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broekgaarden M, Kos M, Jurg FA, van Beek AA, van Gulik TM, Heger M. Inhibition of NF-kappaB in Tumor Cells Exacerbates Immune Cell Activation Following Photodynamic Therapy. Int J Mol Sci. 2015;16:19960–19977. doi: 10.3390/ijms160819960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 53.Habraken Y, Piette J. NF-kappaB activation by double-strand breaks. Biochem Pharmacol. 2006;72:1132–1141. doi: 10.1016/j.bcp.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 55.Hsuan CF, Hsu HF, Tseng WK, Lee TL, Wei YF, Hsu KL. et al. Glossogyne tenuifolia Extract Inhibits TNF-alpha-Induced Expression of Adhesion Molecules in Human Umbilical Vein Endothelial Cells via Blocking the NF-kB Signaling Pathway. Molecules. 2015;20:16908–16923. doi: 10.3390/molecules200916908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wahyudi S, Sargowo D. Green tea polyphenols inhibit oxidized LDL-induced NF-KB activation in human umbilical vein endothelial cells. Acta Med Indones. 2007;39:66–70. [PubMed] [Google Scholar]
- 57.Guo G, Wang T, Gao Q, Tamae D, Wong P, Chen T. et al. Expression of ErbB2 enhances radiation-induced NF-kappaB activation. Oncogene. 2004;23:535–545. doi: 10.1038/sj.onc.1207149. [DOI] [PubMed] [Google Scholar]
- 58.Cao N, Li S, Wang Z, Ahmed KM, Degnan ME, Fan M. et al. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res. 2009;171:9–21. doi: 10.1667/RR1472.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eckert F, Jelas I, Oehme M, Huber SM, Sonntag K, Welker C. et al. Tumor-targeted IL-12 combined with local irradiation leads to systemic tumor control via abscopal effects in vivo. Oncoimmunology. 2017;6:e1323161. doi: 10.1080/2162402X.2017.1323161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabatel H, Pirlot C, Piette J, Habraken Y. Importance of PIKKs in NF-kappaB activation by genotoxic stress. Biochem Pharmacol. 2011;82:1371–1383. doi: 10.1016/j.bcp.2011.07.105. [DOI] [PubMed] [Google Scholar]
- 61.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oeckinghaus A, Ghosh S. The NF-κB Family of Transcription Factors and Its Regulation. Cold Spring Harbor Perspectives in Biology. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 64.Huang TT, Wuerzberger-Davis SM, Wu ZH, Miyamoto S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell. 2003;115:565–576. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 65.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon PS, Bardhan K, Chen MR, Paschall AV, Lu C, Bollag RJ. et al. NF-kappaB functions as a molecular link between tumor cells and Th1/Tc1 T cells in the tumor microenvironment to exert radiation-mediated tumor suppression. Oncotarget. 2016;7:23395–23415. doi: 10.18632/oncotarget.8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung M, Zhang Y, Lee S, Dritschilo A. Correction of radiation sensitivity in ataxia telangiectasia cells by a truncated I kappa B-alpha. Science. 1995;268:1619–1621. doi: 10.1126/science.7777860. [DOI] [PubMed] [Google Scholar]
- 68.Spiotto M, Fu YX, Weichselbaum RR. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol; 2016. p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16:257–270. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 70.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou Y, Liang H, Rao E, Zheng W, Huang X, Deng L. et al. Non-canonical NF-kappaB Antagonizes STING Sensor-Mediated DNA Sensing in Radiotherapy. Immunity. 2018;49:490–503. doi: 10.1016/j.immuni.2018.07.008. e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmed MM, Hodge JW, Guha C, Bernhard EJ, Vikram B, Coleman CN. Harnessing the potential of radiation-induced immune modulation for cancer therapy. Cancer Immunol Res. 2013;1:280–284. doi: 10.1158/2326-6066.CIR-13-0141. [DOI] [PubMed] [Google Scholar]
- 73.Corso CD, Ali AN, Diaz R. Radiation-induced tumor neoantigens: imaging and therapeutic implications. Am J Cancer Res. 2011;1:390–412. [PMC free article] [PubMed] [Google Scholar]
- 74.Gallegos CE, Michelin S, Dubner D, Carosella ED. Immunomodulation of classical and non-classical HLA molecules by ionizing radiation. Cell Immunol. 2016;303:16–23. doi: 10.1016/j.cellimm.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Smahel M. PD-1/PD-L1 Blockade Therapy for Tumors with Downregulated MHC Class I Expression. Int J Mol Sci; 2017. p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perea F, Bernal M, Sanchez-Palencia A, Carretero J, Torres C, Bayarri C. et al. The absence of HLA class I expression in non-small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int J Cancer. 2017;140:888–899. doi: 10.1002/ijc.30489. [DOI] [PubMed] [Google Scholar]
- 77.Ugurel S, Uhlig D, Pfohler C, Tilgen W, Schadendorf D, Reinhold U. Down-regulation of HLA class II and costimulatory CD86/B7-2 on circulating monocytes from melanoma patients. Cancer Immunol Immunother. 2004;53:551–559. doi: 10.1007/s00262-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Speiser P, Zeillinger R, Wiltschke C, Sedlak J, Chorvath B. IL-1 alpha induced, TNF alpha mediated HLA class II (DR) antigen up-regulation in a human ductal breast carcinoma cell line ZR-75-1. Neoplasma. 1993;40:137–140. [PubMed] [Google Scholar]
- 79.Ibrahim EC, Aractingi S, Allory Y, Borrini F, Dupuy A, Duvillard P. et al. Analysis of HLA antigen expression in benign and malignant melanocytic lesions reveals that upregulation of HLA-G expression correlates with malignant transformation, high inflammatory infiltration and HLA-A1 genotype. Int J Cancer. 2004;108:243–250. doi: 10.1002/ijc.11456. [DOI] [PubMed] [Google Scholar]
- 80.Michelin S, Gallegos CE, Dubner D, Favier B, Carosella ED. Ionizing radiation modulates the surface expression of human leukocyte antigen-G in a human melanoma cell line. Hum Immunol. 2009;70:1010–1015. doi: 10.1016/j.humimm.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 81.Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C. et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33:7415–7422. doi: 10.1016/j.vaccine.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vanpouille-Box C, Formenti SC, Demaria S. Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers. Clin Cancer Res. 2018;24:259–265. doi: 10.1158/1078-0432.CCR-16-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wennerberg E, Lhuillier C, Vanpouille-Box C, Pilones KA, Garcia-Martinez E, Rudqvist NP. et al. Barriers to Radiation-Induced In Situ Tumor Vaccination. Front Immunol. 2017;8:229. doi: 10.3389/fimmu.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walker LS. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun. 2013;45:49–57. doi: 10.1016/j.jaut.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong HK, Wilson AJ, Gibson HM, Hafner MS, Hedgcock CJ, Berger CL. et al. Increased expression of CTLA-4 in malignant T-cells from patients with mycosis fungoides - cutaneous T cell lymphoma. J Invest Dermatol. 2006;126:212–219. doi: 10.1038/sj.jid.5700029. [DOI] [PubMed] [Google Scholar]
- 87.Sansom DM. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 2000;101:169–177. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhattacharyya T, Purushothaman K, Puthiyottil SS, Bhattacharjee A, Muttah G. Immunological interactions in radiotherapy-opening a new window of opportunity. Ann Transl Med. 2016;4:51. doi: 10.3978/j.issn.2305-5839.2015.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 90.Shen Y, Hu GH, Kang HY, Tang XY, Hong SL. Allergen induced Treg response in the peripheral blood mononuclear cells (PBMCs) of patients with nasal polyposis. Asian Pac J Allergy Immunol. 2014;32:300–307. doi: 10.12932/AP0469.32.4.2014. [DOI] [PubMed] [Google Scholar]
- 91.Nakanishi Y, Ikebuchi R, Chtanova T, Kusumoto Y, Okuyama H, Moriya T, Regulatory T cells with superior immunosuppressive capacity emigrate from the inflamed colon to draining lymph nodes. Mucosal Immunol; 2017. [DOI] [PubMed] [Google Scholar]
- 92.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M. et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6:238ra270. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muenst S, Soysal SD, Tzankov A, Hoeller S. The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin Ther Targets. 2015;19:201–211. doi: 10.1517/14728222.2014.980235. [DOI] [PubMed] [Google Scholar]
- 95.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsai HF, Hsu PN. Cancer immunotherapy by targeting immune checkpoints: mechanism of T cell dysfunction in cancer immunity and new therapeutic targets. J Biomed Sci. 2017;24:35. doi: 10.1186/s12929-017-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A. 2017;114:1117–1122. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu ZI, Ho AY, McArthur HL. Combined Radiation Therapy and Immune Checkpoint Blockade Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys. 2017;99:153–164. doi: 10.1016/j.ijrobp.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 100.Pike LRG, Bang A, Ott P, Balboni T, Taylor A, Catalano P. et al. Radiation and PD-1 inhibition: Favorable outcomes after brain-directed radiation. Radiother Oncol. 2017;124:98–103. doi: 10.1016/j.radonc.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 101.Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer. 2011;12:58–67. doi: 10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- 102.Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 104.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J Biol Chem. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- 105.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 106.Miyashita M, Ohnishi H, Okazawa H, Tomonaga H, Hayashi A, Fujimoto TT. et al. Promotion of neurite and filopodium formation by CD47: roles of integrins, Rac, and Cdc42. Mol Biol Cell. 2004;15:3950–3963. doi: 10.1091/mbc.E04-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reinhold MI, Lindberg FP, Kersh GJ, Allen PM, Brown EJ. Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. J Exp Med. 1997;185:1–11. doi: 10.1084/jem.185.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast Reconstr Surg. 2009;124:1880–1889. doi: 10.1097/PRS.0b013e3181bceec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M. et al. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCracken MN, Cha AC, Weissman IL. Molecular Pathways: Activating T Cells after Cancer Cell Phagocytosis from Blockade of CD47 "Don't Eat Me" Signals. Clin Cancer Res. 2015;21:3597–3601. doi: 10.1158/1078-0432.CCR-14-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van den Berg TK, van der Schoot CE. Innate immune 'self' recognition: a role for CD47-SIRPalpha interactions in hematopoietic stem cell transplantation. Trends Immunol. 2008;29:203–206. doi: 10.1016/j.it.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 113.Tsai RK, Discher DE. Inhibition of "self" engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 115.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R. et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr. et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S. et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M. et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.can-03-1708. [DOI] [PubMed] [Google Scholar]
- 120.Schulenburg A, Blatt K, Cerny-Reiterer S, Sadovnik I, Herrmann H, Marian B. et al. Cancer stem cells in basic science and in translational oncology: can we translate into clinical application? J Hematol Oncol. 2015;8:16. doi: 10.1186/s13045-015-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fan D, Li Z, Zhang X, Yang Y, Yuan X, Yang M. et al. AntiCD3Fv fused to human interleukin-3 deletion variant redirected T cells against human acute myeloid leukemic stem cells. J Hematol Oncol. 2015;8:18. doi: 10.1186/s13045-015-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH. et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 2011;117:4519–4529. doi: 10.1182/blood-2010-07-296913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–2545. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 124.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS. et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schmid MC, Khan SQ, Kaneda MM, Pathria P, Shepard R, Louis TL. et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat Commun. 2018;9:5379. doi: 10.1038/s41467-018-07387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA. et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014;74:6771–6783. doi: 10.1158/0008-5472.CAN-14-0037-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Menaa C, Fan M, Lu HC, Alexandrou A, Juma S, Perks J, Li JJ. The dynamic change of CD47 expression promotes tumor burden, metastases and resistance of breast cancer cells to radiotherapy. Cancer Res. 2013;73:AACR. Poster: 4963. [Google Scholar]
- 128.Soto-Pantoja DR, Miller TW, Pendrak ML, DeGraff WG, Sullivan C, Ridnour LA. et al. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy. 2012;8:1628–1642. doi: 10.4161/auto.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA. et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L. et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS One. 2015;10:e0137345. doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Advani IF, Popplewell L, Forero-Torres A, Bartlett NL, Ghosh N, Kline JP, et al. Journal of Clinical Oncology. vol. 36; 2018. Activity and tolerabilty of the first-in-class anti-CD47 antibody Hu5F9-G4 with rituximab tolerated in relapsed/refractory non-Hodgkin lymphoma: Initial phase 1b/2 results. [Google Scholar]
- 132.Liu X, Kwon H, Li Z, Fu YX. Is CD47 an innate immune checkpoint for tumor evasion? J Hematol Oncol. 2017;10:12. doi: 10.1186/s13045-016-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miller TW, Soto-Pantoja DR, Schwartz AL, Sipes JM, DeGraff WG, Ridnour LA. et al. CD47 Receptor Globally Regulates Metabolic Pathways That Control Resistance to Ionizing Radiation. J Biol Chem. 2015;290:24858–24874. doi: 10.1074/jbc.M115.665752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Betancur PA, Abraham BJ, Yiu YY, Willingham SB, Khameneh F, Zarnegar M. et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun. 2017;8:14802. doi: 10.1038/ncomms14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duru N, Fan M, Candas D, Menaa C, Liu HC, Nantajit D. et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012;18:6634–6647. doi: 10.1158/1078-0432.CCR-12-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cook KL, Soto-Pantoja DR. "UPRegulation" of CD47 by the endoplasmic reticulum stress pathway controls anti-tumor immune responses. Biomark Res. 2017;5:26. doi: 10.1186/s40364-017-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cook KL, Soto-Pantoja DR, Clarke PA, Cruz MI, Zwart A, Warri A. et al. Endoplasmic Reticulum Stress Protein GRP78 Modulates Lipid Metabolism to Control Drug Sensitivity and Antitumor Immunity in Breast Cancer. Cancer Res. 2016;76:5657–5670. doi: 10.1158/0008-5472.CAN-15-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S. et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72:3967–3976. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- 139.Onishi M, Okonogi N, Oike T, Yoshimoto Y, Sato H, Suzuki Y. et al. High linear energy transfer carbon-ion irradiation increases the release of the immune mediator high mobility group box 1 from human cancer cells. J Radiat Res. 2018;59:541–546. doi: 10.1093/jrr/rry049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McFarland BC, Stewart J Jr, Hamza A, Nordal R, Davidson DJ, Henkin J. et al. Plasminogen kringle 5 induces apoptosis of brain microvessel endothelial cells: sensitization by radiation and requirement for GRP78 and LRP1. Cancer Res. 2009;69:5537–5545. doi: 10.1158/0008-5472.CAN-08-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Klein B, Loven D, Lurie H, Rakowsky E, Nyska A, Levin I. et al. The effect of irradiation on expression of HLA class I antigens in human brain tumors in culture. J Neurosurg. 1994;80:1074–1077. doi: 10.3171/jns.1994.80.6.1074. [DOI] [PubMed] [Google Scholar]
- 142.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8:1765–1780. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 143.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 144.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J. et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahmed KA, Stallworth DG, Kim Y, Johnstone PA, Harrison LB, Caudell JJ. et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2016;27:434–441. doi: 10.1093/annonc/mdv622. [DOI] [PubMed] [Google Scholar]
- 148.Soto-Pantoja DR, Isenberg JS, Roberts DD. Therapeutic Targeting of CD47 to Modulate Tissue Responses to Ischemia and Radiation. J Genet Syndr Gene Ther; 2011. p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev. 2008;19:253–262. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baumann R, Yousefi S, Simon D, Russmann S, Mueller C, Simon HU. Functional expression of CD134 by neutrophils. Eur J Immunol. 2004;34:2268–2275. doi: 10.1002/eji.200424863. [DOI] [PubMed] [Google Scholar]
- 151.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 152.Liu C, Lou Y, Lizee G, Qin H, Liu S, Rabinovich B. et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 154.Zhu J, Luo J, Li Y, Jia M, Wang Y, Huang Y. et al. HMGB1 induces human non-small cell lung cancer cell motility by activating integrin alphavbeta3/FAK through TLR4/NF-kappaB signaling pathway. Biochem Biophys Res Commun. 2016;480:522–527. doi: 10.1016/j.bbrc.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 155.Yue Y, Zhou T, Gao Y, Zhang Z, Li L, Liu L. et al. High mobility group box 1/toll-like receptor 4/myeloid differentiation factor 88 signaling promotes progression of gastric cancer. Tumour Biol. 2017;39:1010428317694312. doi: 10.1177/1010428317694312. [DOI] [PubMed] [Google Scholar]
- 156.Li Z, Block MS, Vierkant RA, Fogarty ZC, Winham SJ, Visscher DW. et al. The inflammatory microenvironment in epithelial ovarian cancer: a role for TLR4 and MyD88 and related proteins. Tumour Biol. 2016;37:13279–13286. doi: 10.1007/s13277-016-5163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.He S, Cheng J, Sun L, Wang Y, Wang C, Liu X. et al. HMGB1 released by irradiated tumor cells promotes living tumor cell proliferation via paracrine effect. Cell Death Dis. 2018;9:648. doi: 10.1038/s41419-018-0626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 159.Gifford JB, Hill R. GRP78 Influences Chemoresistance and Prognosis in Cancer. Curr Drug Targets. 2018;19:701–708. doi: 10.2174/1389450118666170615100918. [DOI] [PubMed] [Google Scholar]
- 160.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D. et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 161.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 162.Zhai L, Kita K, Wano C, Wu Y, Sugaya S, Suzuki N. Decreased cell survival and DNA repair capacity after UVC irradiation in association with down-regulation of GRP78/BiP in human RSa cells. Exp Cell Res. 2005;305:244–252. doi: 10.1016/j.yexcr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 163.Misra UK, Gonzalez-Gronow M, Gawdi G, Pizzo SV. The role of MTJ-1 in cell surface translocation of GRP78, a receptor for alpha 2-macroglobulin-dependent signaling. J Immunol. 2005;174:2092–2097. doi: 10.4049/jimmunol.174.4.2092. [DOI] [PubMed] [Google Scholar]
- 164.Choi HJ, Choi WS, Park JY, Prabagar MG, Kang KH, Jeon SJ. et al. SIGN-R1, a C-type lectin, binds to Bip/GRP78 and this interaction mediates the regurgitation of T-cell-independent type 2 antigen dextran through the endoplasmic reticulum. Immunobiology. 2011;216:437–446. doi: 10.1016/j.imbio.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 165.Wei D, Li NL, Zeng Y, Liu B, Kumthip K, Wang TT. et al. The Molecular Chaperone GRP78 Contributes to Toll-like Receptor 3-mediated Innate Immune Response to Hepatitis C Virus in Hepatocytes. J Biol Chem. 2016;291:12294–12309. doi: 10.1074/jbc.M115.711598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rasche L, Duell J, Castro IC, Dubljevic V, Chatterjee M, Knop S. et al. GRP78-directed immunotherapy in relapsed or refractory multiple myeloma - results from a phase 1 trial with the monoclonal immunoglobulin M antibody PAT-SM6. Haematologica. 2015;100:377–384. doi: 10.3324/haematol.2014.117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li B, Cheng XL, Yang YP, Li ZQ. GRP78 mediates radiation resistance of a stem cell-like subpopulation within the MCF-7 breast cancer cell line. Oncol Rep. 2013;30:2119–2126. doi: 10.3892/or.2013.2710. [DOI] [PubMed] [Google Scholar]
- 168.Dadey DYA, Kapoor V, Hoye K, Khudanyan A, Collins A, Thotala D. et al. Antibody Targeting GRP78 Enhances the Efficacy of Radiation Therapy in Human Glioblastoma and Non-Small Cell Lung Cancer Cell Lines and Tumor Models. Clin Cancer Res. 2017;23:2556–2564. doi: 10.1158/1078-0432.CCR-16-1935. [DOI] [PubMed] [Google Scholar]
- 169.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A. et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moran AE, Polesso F, Weinberg AD. Immunotherapy Expands and Maintains the Function of High-Affinity Tumor-Infiltrating CD8 T Cells In Situ. J Immunol. 2016;197:2509–2521. doi: 10.4049/jimmunol.1502659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yokouchi H, Yamazaki K, Chamoto K, Kikuchi E, Shinagawa N, Oizumi S. et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci. 2008;99:361–367. doi: 10.1111/j.1349-7006.2007.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gough MJ, Crittenden MR, Sarff M, Pang P, Seung SK, Vetto JT. et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother. 2010;33:798–809. doi: 10.1097/CJI.0b013e3181ee7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mikulandra M, Pavelic J, Glavan TM. Recent findings on the application of Toll-like receptors agonists in cancer therapy. Curr Med Chem; 2017. [DOI] [PubMed] [Google Scholar]
- 175.Cho JH, Lee HJ, Ko HJ, Yoon BI, Choe J, Kim KC. et al. The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget. 2017;8:24932–24948. doi: 10.18632/oncotarget.15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Scholch S, Rauber C, Weitz J, Koch M, Huber PE. TLR activation and ionizing radiation induce strong immune responses against multiple tumor entities. Oncoimmunology. 2015;4:e1042201. doi: 10.1080/2162402X.2015.1042201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 178.Dovedi SJ, Melis MH, Wilkinson RW, Adlard AL, Stratford IJ, Honeychurch J. et al. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood. 2013;121:251–259. doi: 10.1182/blood-2012-05-432393. [DOI] [PubMed] [Google Scholar]
- 179.Malamas AS, Gameiro SR, Knudson KM, Hodge JW. Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas' sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget. 2016;7:86937–86947. doi: 10.18632/oncotarget.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 181.Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC. et al. Exosome RNA Unshielding Couples Stromal Activation to Pattern Recognition Receptor Signaling in Cancer. Cell. 2017;170:352–366. doi: 10.1016/j.cell.2017.06.031. e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 183.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kauppila S, Stenback F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. 1998;186:262–268. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 185.Kumar S, Kapoor A, Desai S, Inamdar MM, Sen S. Proteolytic and non-proteolytic regulation of collective cell invasion: tuning by ECM density and organization. Sci Rep. 2016;6:19905. doi: 10.1038/srep19905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Su C, Zhang B, Liu W, Zheng H, Sun L, Tong J. et al. High extracellular pressure promotes gastric cancer cell adhesion, invasion, migration and suppresses gastric cancer cell differentiation. Oncol Rep. 2016;36:1048–1054. doi: 10.3892/or.2016.4841. [DOI] [PubMed] [Google Scholar]
- 187.Jerrell RJ, Parekh A. Matrix rigidity differentially regulates invadopodia activity through ROCK1 and ROCK2. Biomaterials. 2016;84:119–129. doi: 10.1016/j.biomaterials.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 189.Schito L, Semenza GL. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer. 2016;2:758–770. doi: 10.1016/j.trecan.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 190.Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim Biophys Acta. 2016;1863:382–391. doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koltai T. Cancer: fundamentals behind pH targeting and the double-edged approach. Onco Targets Ther. 2016;9:6343–6360. doi: 10.2147/OTT.S115438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brat DJ, Kaur B, Van Meir EG. Genetic modulation of hypoxia induced gene expression and angiogenesis: relevance to brain tumors. Front Biosci. 2003;8:d100–116. doi: 10.2741/942. [DOI] [PubMed] [Google Scholar]
- 193.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K. et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Alexander S, Friedl P. Cancer invasion and resistance: interconnected processes of disease progression and therapy failure. Trends Mol Med. 2012;18:13–26. doi: 10.1016/j.molmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 196.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F. et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vehlow A, Storch K, Matzke D, Cordes N. Molecular Targeting of Integrins and Integrin-Associated Signaling Networks in Radiation Oncology. Recent Results Cancer Res. 2016;198:89–106. doi: 10.1007/978-3-662-49651-0_4. [DOI] [PubMed] [Google Scholar]
- 198.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M. et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A. 2011;108:15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Perrino FW, Miller H, Ealey KA. Identification of a 3'->5'-exonuclease that removes cytosine arabinoside monophosphate from 3' termini of DNA. J Biol Chem. 1994;269:16357–16363. [PubMed] [Google Scholar]
- 200.Hoss M, Robins P, Naven TJ, Pappin DJ, Sgouros J, Lindahl T. A human DNA editing enzyme homologous to the Escherichia coli DnaQ/MutD protein. EMBO J. 1999;18:3868–3875. doi: 10.1093/emboj/18.13.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Namjou B, Kothari PH, Kelly JA, Glenn SB, Ojwang JO, Adler A. et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 2011;12:270–279. doi: 10.1038/gene.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ. et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vanpouille-Box C, Formenti SC, Demaria S. TREX1 dictates the immune fate of irradiated cancer cells. Oncoimmunology. 2017;6:e1339857. doi: 10.1080/2162402X.2017.1339857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Malecka A, Wang Q, Shah S, Sutavani RV, Spendlove I, Ramage JM. et al. Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation. J Leukoc Biol. 2016;100:381–389. doi: 10.1189/jlb.3A1015-474R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers (Basel) 2015;7:2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45:229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Erez N, Glanz S, Raz Y, Avivi C, Barshack I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem Biophys Res Commun. 2013;437:397–402. doi: 10.1016/j.bbrc.2013.06.089. [DOI] [PubMed] [Google Scholar]
- 208.Nam JS, Suchar AM, Kang MJ, Stuelten CH, Tang B, Michalowska AM. et al. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor beta in a mouse model of breast cancer. Cancer Res. 2006;66:6327–6335. doi: 10.1158/0008-5472.CAN-06-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kramer N, Schmollerl J, Unger C, Nivarthi H, Rudisch A, Unterleuthner D. et al. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene. 2017;36:5460–5472. doi: 10.1038/onc.2017.144. [DOI] [PubMed] [Google Scholar]
- 210.Verbovsek U, Van Noorden CJ, Lah TT. Complexity of cancer protease biology: Cathepsin K expression and function in cancer progression. Semin Cancer Biol. 2015;35:71–84. doi: 10.1016/j.semcancer.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 211.Silva AM, Almeida MI, Teixeira JH, Maia AF, Calin GA, Barbosa MA. et al. Dendritic Cell-derived Extracellular Vesicles mediate Mesenchymal Stem/Stromal Cell recruitment. Sci Rep. 2017;7:1667. doi: 10.1038/s41598-017-01809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lehmann B, Biburger M, Bruckner C, Ipsen-Escobedo A, Gordan S, Lehmann C, Tumor location determines tissue-specific recruitment of tumor-associated macrophages and antibody-dependent immunotherapy response. Sci Immunol; 2017. p. 2. [DOI] [PubMed] [Google Scholar]
- 214.Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL. et al. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2017;23:137–148. doi: 10.1158/1078-0432.CCR-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Graves DT, Jiang YL, Williamson MJ, Valente AJ. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989;245:1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- 216.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N. et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 218.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gupta DK, Singh N, Sahu DK. TGF-beta Mediated Crosstalk Between Malignant Hepatocyte and Tumor Microenvironment in Hepatocellular Carcinoma. Cancer Growth Metastasis. 2014;7:1–8. doi: 10.4137/CGM.S14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Loinard C, Vilar J, Milliat F, Levy B, Benderitter M. Monocytes/Macrophages Mobilization Orchestrate Neovascularization after Localized Colorectal Irradiation. Radiat Res. 2017;187:549–561. doi: 10.1667/RR14398.1. [DOI] [PubMed] [Google Scholar]
- 222.Jiang S, Yang Y, Fang M, Li X, Yuan X, Yuan J. Co-evolution of tumor-associated macrophages and tumor neo-vessels during cervical cancer invasion. Oncol Lett. 2016;12:2625–2631. doi: 10.3892/ol.2016.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim CJ, Tambe Y, Mukaisho KI, Sugihara H, Kageyama S, Kawauchi A. et al. Periostin suppresses in vivo invasiveness via PDK1/Akt/mTOR signaling pathway in a mouse orthotopic model of bladder cancer. Oncol Lett. 2017;13:4276–4284. doi: 10.3892/ol.2017.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jang SY, Park SY, Lee HW, Choi YK, Park KG, Yoon GS. et al. The Combination of Periostin Overexpression and Microvascular Invasion Is Related to a Poor Prognosis for Hepatocellular Carcinoma. Gut Liver. 2016;10:948–954. doi: 10.5009/gnl15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li Z, Zhang X, Yang Y, Yang S, Dong Z, Du L. et al. Periostin expression and its prognostic value for colorectal cancer. Int J Mol Sci. 2015;16:12108–12118. doi: 10.3390/ijms160612108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA. et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 227.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 228.Guo Q, Jin Z, Yuan Y, Liu R, Xu T, Wei H. et al. New Mechanisms of Tumor-Associated Macrophages on Promoting Tumor Progression: Recent Research Advances and Potential Targets for Tumor Immunotherapy. J Immunol Res. 2016;2016:9720912. doi: 10.1155/2016/9720912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang F. et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017;8:30576–30586. doi: 10.18632/oncotarget.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pinto AT, Pinto ML, Velho S, Pinto MT, Cardoso AP, Figueira R. et al. Intricate Macrophage-Colorectal Cancer Cell Communication in Response to Radiation. PLoS One. 2016;11:e0160891. doi: 10.1371/journal.pone.0160891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Prakash H, Klug F, Nadella V, Mazumdar V, Schmitz-Winnenthal H, Umansky L. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: lesson from insulinoma. Carcinogenesis. 2016;37:301–313. doi: 10.1093/carcin/bgw007. [DOI] [PubMed] [Google Scholar]
- 232.Kung WH, Yu CF, Lee AC, Yang CD, Liu YC, Chen FH. et al. Gene expression profiling of tumor-associated macrophages after exposure to single-dose irradiation. Comput Biol Chem. 2017;69:138–146. doi: 10.1016/j.compbiolchem.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 233.Allaoui R, Bergenfelz C, Mohlin S, Hagerling C, Salari K, Werb Z. et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat Commun. 2016;7:13050. doi: 10.1038/ncomms13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R. et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin Cancer Res. 2017;23:5514–5526. doi: 10.1158/1078-0432.CCR-16-1673. [DOI] [PubMed] [Google Scholar]
- 235.Barker CA, Postow MA, Khan SA, Beal K, Parhar PK, Yamada Y. et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013;1:92–98. doi: 10.1158/2326-6066.CIR-13-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kapoor V, Dadey DY, Nguyen K, Wildman SA, Hoye K, Khudanyan A. et al. Tumor-Specific Binding of Radiolabeled PEGylated GIRLRG Peptide: A Novel Agent for Targeting Cancers. J Nucl Med. 2016;57:1991–1997. doi: 10.2967/jnumed.115.165118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 238.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M. et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 239.Kordbacheh T, Honeychurch J, Blackhall F, Faivre-Finn C, Illidge T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: building better translational research platforms. Ann Oncol. 2018;29:301–310. doi: 10.1093/annonc/mdx790. [DOI] [PubMed] [Google Scholar]
- 240.Walshaw RC, Honeychurch J, Illidge TM, Choudhury A. The anti-PD-1 era - an opportunity to enhance radiotherapy for patients with bladder cancer. Nat Rev Urol. 2018;15:251–259. doi: 10.1038/nrurol.2017.172. [DOI] [PubMed] [Google Scholar]
- 241.Herter-Sprie GS, Koyama S, Korideck H, Hai J, Deng J, Li YY. et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight. 2016;1:e87415. doi: 10.1172/jci.insight.87415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Martinov T, Fife BT. Fractionated radiotherapy combined with PD-1 pathway blockade promotes CD8 T cell-mediated tumor clearance for the treatment of advanced malignancies. Ann Transl Med. 2016;4:82. doi: 10.3978/j.issn.2305-5839.2016.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Roger A, Finet A, Boru B, Beauchet A, Mazeron JJ, Otzmeguine Y. et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. Oncoimmunology. 2018;7:e1442166. doi: 10.1080/2162402X.2018.1442166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Reynders K, De Ruysscher D. Radiotherapy and Immunotherapy: Improving Cancer Treatment through Synergy. Prog Tumor Res. 2015;42:67–78. doi: 10.1159/000437185. [DOI] [PubMed] [Google Scholar]
- 245.Tazi K, Hathaway A, Chiuzan C, Shirai K. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med. 2015;4:1–6. doi: 10.1002/cam4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Aboudaram A, Modesto A, Chaltiel L, Gomez-Roca C, Boulinguez S, Sibaud V. et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: a safe and effective combination. Melanoma Res. 2017;27:485–491. doi: 10.1097/CMR.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 247.Koller KM, Mackley HB, Liu J, Wagner H, Talamo G, Schell TD. et al. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol Ther. 2017;18:36–42. doi: 10.1080/15384047.2016.1264543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Frydenlund N, Mahalingam M. PD-L1 and immune escape: insights from melanoma and other lineage-unrelated malignancies. Hum Pathol. 2017;66:13–33. doi: 10.1016/j.humpath.2017.06.012. [DOI] [PubMed] [Google Scholar]