Abstract
The gut microbiota plays the important role to support the immunological tolerance. To study a metabolic activity of the intestinal microflora the 44 patients suffering from bronchial asthma and 17 healthy volunteers were tested. The short-chain fatty acids (SCFA) spectrum was determined using the method of gas-liquid chromatographic analysis. We have detected a significant decrease the total content of the fatty acids in the feces (P<0.001), the absolute concentrations of the individual acids (acetate, propionate, butyrate; P<0.001) and the total content of isoacids (P<0.001) for all of the patients with bronchial asthma in the study, regardless of the phenotype. The anaerobic type of the SCFA spectrum was detected for the majority (83%) of the patients tested. The aerobic type of the SCFA metabolic profile was detected for another part (17%) of the patients tested. Being detected the parameters change could play the principal role as for the bronchial asthma course as to support the organism sensibilisation.
Key words: Short-chain fatty acids, Gut microbiota, Bronchial asthma, Immunomodulation
Introduction
The intestinal microbiota is individual and constant in general. It participates in a variety of physiological functions of a human body, including immune, digestive and genetic as well as in the regulation of cholesterol metabolism, etc. A realizing of the main effects of microflora is carried out through the participation of microorganisms’ vital activity products.
In general, the gut microbiota is individual and constant. The microbiota has been involved in the variety physiological functions of a human body, including immune, digestive and genetic functions as well as in the regulation of cholesterol metabolism, etc. The intestinal microflora influence is based on the participation of the vital activity products of microorganisms in the processes marked above.
The main metabolites of the intestinal microflora are presented by the short chain fatty acids (monocarboxylic acid), dicarboxylic acids (succinic acid), hydroxy acids (lactic), amino acids (β-alanine, γ- aminobutyric acid, glutamic acid, ε- aminocaproic), cyclic nucleotide (cAMP, cGMP) and gases (H2, CO2, CH4, NH3, NO).1-3
The short-chain fatty acids (SCFAs) are formed by the saccharolytic microflora in the colon. SCFAs are involved in the energy supply process to the epithelium, in a proliferation and differentiation regulation of the epithelial cells, at the local and systemic immunity activation. These acids have maintained the ion exchange and ones influence on the intestinal motility and antibacterial effect. SCFAs serve as the substrates for lipo- and gluconeogenesis and other.2,3 Short-chain fatty acids (including isomers) are acetate, propionate, butyrate, isobutyrate, isovaleric, valeric, isocaproic, and caproic acids.4,5 Any SCFAs have being formed by bacteria of the certain type during the substrate fermentation, so that makes to evaluate the functional activity of specific representatives of the microflora being as the important parameter of the homeostasis of the intestinal biotope. Short-chain fatty acids (including isomers) involved are acetate, propionate, butyrate, isobutyrate, isovaleric, valeric, isocaproic, and caproic acids.4,5
The potential roles of the SCFAs in the immune system regulation and in the development of the allergic diseases (bronchial asthma, atopic dermatitis, food allergies) were discussed in the published researches (Figure 1). There were considered the next mechanisms of their interaction with the antigen structure recognition receptors (the PPR-pattern recognition receptors), G-protein receptors of the polymorph nuclear neutrophils and macrophages - GPR41 (FFAR3), GPR43 (FFAR2), GPR109A, the nuclear transcription factor and the tumor necrosis factor in the studies.5-7 The violation of the intestinal microflora composition has led to a shifting of the SCFAs forming process towards to the T-helper type 2 affecting on the bronchopulmonary system state.6-10 A. Trompette et al. have found the SCFAs level decrease had led to increase of the allergic reactions from the respiratory tract side in the tested mice, which were fed with low fiber food in the diet.5 Gallacher, et al. have demonstrated that SCFA stimulates the CD4 + Foxp3 regulatory cells, the proinflammatory cytokines formation is reduced and T cells can return to the phenotype of T-helper type 1.6 The bacterial colonization of the sterile mice leads to the stimulation of the secretory IgA and CD4 + T cells reducing the IgE level.7
Figure 1.
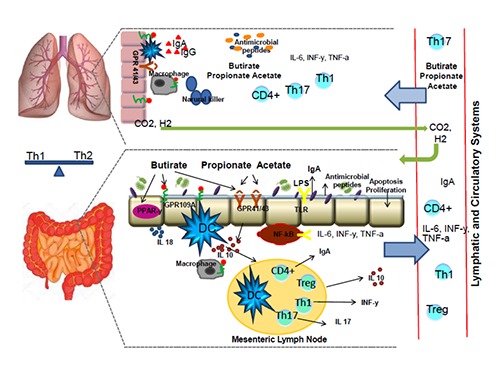
A model of the short-chain fatty acids (SCFAs) regulatory influence on the lung immunology. Butyrate, propionate and acetate connect to G-protein receptors GPR 43 and GPR 41. Butyrate also interacts with GPR 109A and peroxisome proliferation activating receptors (PPAR-γ). All of the SCFAs influence on the function of the nuclear transcription factor (NFkB), on the dendritic cells (DC) thereby modulating the activity of Treg lymphocytes and various regulatory cytokines (IL-10, TGF-α, INF-γ, IL-6). A circulation of the regulatory cytokines and SCFAs provides their transportation to the lungs through the bloody and lymphatic systems, where they are involved in the immune and anti-inflammatory reactions, thereby providing the gut-lungs axis connection. The SCFAs quantity decreasing contributes to the activation of Th-2 cells with an increasing of the specific IgE level and also the levels of the mast cells, basophils, eosinophils. Subsequently, these reasons will have led to the bronchial hyper reactivity and asthma development.
The aim of our research was to study the SCFA’s content and profile in feces of the patients with bronchial asthma in compare with the healthy individuals.
Materials and Methods
This cross-sectional prospective study was run from January 2017 to March 2018. The study was approved by the clinical research ethics committee and was conducted in accordance with the Declaration of Helsinki. The aim of the study had been explained to the potential participants and the signed consents were obtained from the patients before their enrolment in the study. The 44 patients with bronchial asthma in the acute stage were included in the study. The patients tested had taken not any antibacterial drugs, proand prebiotics, proton pump inhibitors and hypoglycemic agents for the previous 3 months. All of the patients were undergone the standardized range of the clinical and laboratory studies, there were included the blood, sputum and urine tests, the biochemical blood tests, the parameters of the immunoglobulins (A, G, E classes) level, the C-reactive protein tests, X-ray of the lungs, the respiratory function tests. The control group was consisted from the 17 healthy volunteers.
The SCFA spectrums of the feces samples were determined using the method of gas-liquid chromatographic analysis in according to the standard medical technique for the SCFA determination in various biological substrates with the gas-liquid chromatography. The study was carried out on the gas chromatograph “Chromos GH- 1000 with a flame ionization detector”. For all of the participants were calculated the absolute and relative content of the acetate (C2), propionate (C3) and butyrate (C4) acids, which have make the main contribution to the total acids pool with the C2 – C4 acid profile, the isoacids level and ratio of isoacids to the unbranched chain acids (isoCn / Cn). The values of the anaerobic index (AI = (C3 + C4) / C2) were calculated in the study also. The index represents the redox potential of the intraluminal medium.
Statistical processing and analysis of the results were carried out using the program Statistica 10 (StatSoft Inc., USA). The parametric and non-parametric statistical methods were used. The mean and standard deviations were determined with the Mann-Whitney test by evaluating of the p- parameter significance. The significance level was considered as acceptable at P≤0.05.
Results
The patients tested were divided into the groups depended on the phenotype of the disease (allergic (AA) and non-allergic (NA) asthma).
The first group was consisted from the 24 patients with allergic asthma (AA) (mean age was 37.5±10.4 yrs., anamnesis duration was 11.6±8.9 yrs., 12 females and 12 males).
The second group was consisted from the 20 individuals were suffered from the non-allergic form (NA) of bronchial asthma (mean age was 38.2±12.6 yrs., anamnesis duration was 10.2±5.8 yrs., 10 females and 10 males).
The third group was consisted from the 17 healthy volunteers (mean age was 37.6±9.5 yrs., 9 females and 8 males).
All of the groups were comparable in the age (P>0.05), sex (P>0.05), the first and the second groups were also comparable in the anamnesis duration (P>0.05) and disease severity (P>0.05).
It was detected a significant decrease the total content of fatty acids in the feces tested and the absolute concentrations of the individual acids, also the total content of isoacids for all of the patients with bronchial asthma regardless of the phenotype of the disease. The results obtained are in Table 1 and Figure 2. There were obtained not any statistically significant differences in the level and spectrum of SCFA because of the disease phenotype in our study. The three types of changes were identified in the unbranched chain fatty acid profile (C2-C4) (Figure 3).
Table 1.
The content values of the short-chain fatty acids for the groups examined.
| Parameters | Normal | AA | NA | P |
|---|---|---|---|---|
| Common content, mg/g | 10.33±0.63 | 2.56±1.15 | 2.94±1.23 | P<0.001 |
| Acetate (C2), mg/g | 5.87± 0.22 | 1.31± 0.59 | 1.41±0.59 | P<0.001 |
| Propionate (C3), mg/g | 1.77±0.08 | 0.56±0.33 | 0.64±0.36 | P<0.001 |
| Butyrate (C4), mg/g | 1.73±0.09 | 0.39±0.23 | 0.57±0.23 | P<0.001 |
| Isoacids (Cn), mg/g | 0.62±0.36 | 0.31±0.18 | 0.19±0.05 | P<0.001 |
In comparing with The Mann-Whitney test: AA (allergic asthma) and healthy individuals; NA (non-allergic asthma) and healthy individuals.
Figure 2.
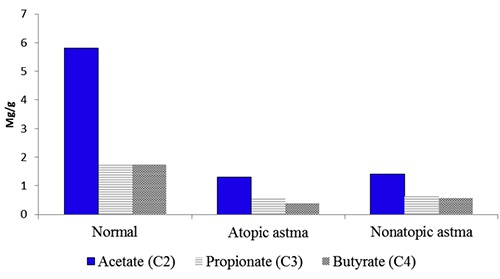
The total content and spectrum of the short-chain fatty acids in the feces samples for the groups studied. Acetate: normal (5,8), Atopic astma (1,31), nonatopic astma (1,41); propionate: normal (1,77), Atopic astma (0,56), nonatopic astma (0,64); butyrate: normal (1,73), atopic astma (0,39), nonatopic astma (0,57).
Figure 3.
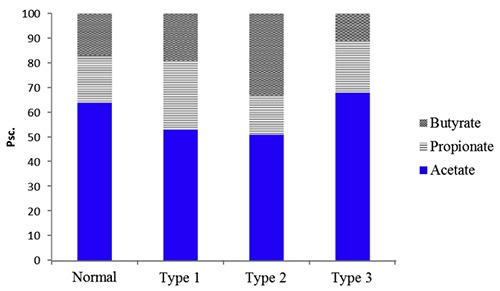
The SCFA spectrum with different metabolic profiles is in the patients with bronchial asthma. Acetate: normal (64), type 1(53), type 2(51), type 3(68); propionate: normal (19), type 1(28), type 2(16), type 3(21); butyrate: normal (17), type 1(19), type 2(33), type 3(11).
The first type was characterized by the relative content decreasing of the acetate (C2) acid and increasing of the propionate (C3) and butyrate (C4) acids proportion. These changes were detected for 59% (26 individuals) of the examined patients and it was testified in favor the activity reducing of the aerobic unit of the microorganisms (E. coli, Strepto-and Staphylococci). The activity increasing of the conditionally pathogenic flora and strict anaerobes (Bacteroides, Eubacteria, Fusobacteria, Coprococci and another) were noted simultaneously. So, the anaerobic type of the SCFA spectrum was detected in the majority of the patients tested.
The second type of the changes has shown a decreasing of the of acetate acid (C2) and propionate acid (C3) proportion with an increasing of the butyrate acid (C4) content. This SCFA spectrum was detected for 25% of the patients observed (11 individuals). The low concentration of the propionic acid could be explained by a decreasing of the activity and abundance of the anaerobic flora in the intestine and perhaps by the propionate absorption increasing. This type of the metabolic profile is anaerobic in general.
Regarding to the third type of the changes, the acetate (C2) and propionate (C3) acids proportions have been prevailed while the oil (C4) acid proportion was decreased. This spectrum of the acids was detected for 16% (7 individuals) of the examined patients and its composition was closer to the aerobic type of the metabolic profile.
The increasing of the acetic acid proportion could be associated with the aerobic activation of microorganisms, the pathogenic and conditionally pathogenic representatives of the intestinal microflora (Enterobacter spp., Citrobacter spp., Escherichia coli, Corynebacterium spp., Streptococcus faecalis, Staphylococcus spp., Pseudomonas spp., Bacillus spp., Campylobacter, etc.).
Aerobes activation in the colon is associated with the inhibition of the anaerobic population (Lactobacterium, Bifidobacterium, Peptostreptococcus, Eubacteria, and Fusobacterium) in the patients on preserving the propionic acid bacteria, ones are the main producers of propionic acid.
The proteolytic activity of microorganisms was evaluated by the total amount of the C4 – C6 (IsoCn) isoacids concentrations and value of the proportions the C4 – C6 isoacids to all of the unbranched C4 – C6 acids (IsoCn / Cn).
A decreasing of the IsoCn and IsoCn / Cn values was found in the individuals with a predominantly anaerobic spectrum of SCFA (Type 1 and Type 2) and an increasing of these parameters was detected in the patients with an aerobic spectrum of the SCFA metabolic profile in the subgroup. The data of the relative total content of isoacids, the ratio of the isoacids to acids concentrations and the anaerobic index are presented in Table 2.
Table 2.
The relative concentrations of isoacids, the isoacids to acids ratios and the anaerobic index by the SCFA profile type depending.
| Parameters | Normal | Type 1 | Type 2 | Type 3 |
|---|---|---|---|---|
| Isoacids (Cn), pcs. | 0.059±0.002 | 0.067±0.01* | 0.094±0.07* | 0.14±0.02* |
| Iso Cn/Cn, pcs. | 0.45±0.08 | 0.35±0.08* | 0.37±0.17 | 0.86±0.13** |
| Redox index, pcs. | -0.55±0.08 | -0.87±0.12** | -1.07±0.38** | -0.46±0.06 |
**P<0,01
*P<0,05 after comparing to normal.
The values of the anaerobic index represent the redox potential of the environment, differed significantly under the different metabolic types (Table 2).
The redox potential of the environment is characterized by the value of the anaerobic indexes, ones are significantly differ under the different metabolic types (Table 2). A significant shift toward the negative values of the index magnitude was observed for the anaerobic type (P<0.01 in comparing to reference values) and the shift toward a zone of the opposite values of the index magnitude was observed for the aerobic type.
Respectively, it may be confirmed by a growth of the anaerobic or aerobic bacteria populations against the background of decreasing the obligate flora activity in the connection with the inhibition of ferredoxin- contained respiratory enzymes ensuring their normal vital activity.
Discussion
The synthesis of SCFAs is very important for the various functions of the microbiota. The SCFAs make a significant contribution to provide the local and systemic organism functions, these acids serve as one of the energy sources and effect on the expression of various genes.
The SCFAs regulate the processes of proliferation and apoptosis of the epithelial cells and interact with the immune system cells.5 The low concentrations of acetic acid were observed in the feces samples of the three-month-old infants and as it was associated to a high risk of the atopy developing at further.8 The correlation of the SCFA’s and eosinophils levels and with the proinflammatory mediators production, bronchial obstruction phenomena has been demonstrated in the experimental studies.6,7
The results obtained showed a significant decreasing the absolute content of the short chain fatty acids with the number of carbon atoms C2-C4 for the patients with bronchial asthma, so it was the first sign the metabolic activity decreasing of the lactic acid flora (Bifidobacterium and Lactobacterium). One of the main roles of the intestinal microflora is a synthesis the necessary amount of the fatty acids in the right proportion. Normally, the SCFAs ratio is a stable parameter and one should be maintained in the ratio of acetate: propionate: butyrate as (60:20:18).3,8
We have detected a decreasing of the SCFA’s profile of the acetic acid, the relative content increasing of the butyrate acid and multidirectional changes the propionate acid content. It was testified by the metabolic activity increase of the anaerobic microflora for the majority (84%) of the patients with bronchial asthma. For 16% of the patients tested the predominance of acetate acid was revealed in the SCFA profile while the relative content being reduced of butyrate and propionate acids, as it was evidenced by the metabolic activity increasing of the aerobic microflora, in particular E. coli, Streptococci (Streptococcus salivarius, Str. mutans, and other strains) and Staphylococci.8 It was established the isomers of SCFA are formed as a result of the vital activity of the microorganisms in utilizing the peptides. E. coli, fecal Streptococci and Staphylococci, some bacilli are considered as the strongest proteolytic. Some types of the anaerobes (Bacteroides fragilis, Bacteroides ruminicola, and Bacteroides melaninogenicus) have had ability to proteolysis, but its activity is less expressed.6,8 Total number of the isoacids and ratio of the acids isomers to the straightchain acids had been significantly reduced (P<0.05) for the majority of the patients with bronchial asthma. In according to some opinions, these changes are associated to the synthesis impaired, the glycoproteins secretion and mucin formation, as well as with an activation of the certain types of anaerobic bacteria having extremely low ability to proteolysis.1,5 An increasing of these parameters (isoCn and isoCn / Сn) have been detected in 16% of the patients, it may indicate on a destruction of the epithelial layer of the intestinal wall. It is known, the epithelial mucus serves as a metabolic substrate for the proteolytic microflora.7 So in that case, one can speak about the proteolytic activity increased of aand anaerobic populations of the facultative and residual microorganisms.8 The values of the anaerobic index for various types of the metabolic profile changes of the acids indicate a disturbance of the microorganisms habitat contributing to the populations growth of the anaerobic (1st and 2nd type) or aerobic (3rd type) microflora. The redox potential reducing to the negative direction has confirmed the imbalance of the aerobic / anaerobic populations of the microorganisms to inhibit the vital activity of the obligate anaerobes (in activating the facultative anaerobes, in particular, Bacteroides, Propionibacterium and perhaps, the residual representatives of the (conditionally pathogenic) microflora.5-8 Being shifted towards the slightly negative values the redox potential of the intraluminal environment makes rise to the growth of the optional aerobic and residual (conditionally pathogenic) aerobic microflora.5-8 The SCFA changes revealed give evidence about the pronounced disorders in the intestinal biotope microbiocenosis and that could be considered as one of the reasons of the bronchial asthma development.
An additional confirmation of that is the result of our earlier published study. As it was found in the study, the frequent detections of excess the bronchial growth syndrome in the small intestine for 67% of the patients tested with atopic asthma were associated with the high IgE titers (P<0.01), the eosinophil level (P<0.001) and in changing the forced expiratory volume in 1 sec (P<0.01). The therapy with the rifaximin and probiotic preparation (B. bifidum, B. longum, B. infantis, L. rhamnosus) has contributed to the immunological parameters improvement in decreasing the number of hospitalizations of the treated patients in the next year (p From the other side, the changes written above in the SCFA could be due to the pathogenetic mechanisms of bronchial asthma. A violation of the ventilationperfusion relations of the lungs and decrease of the CO2 and H2 elimination from the body led to the intestinal redox potential shift.11,12 The redox potential change influences on the vital activity of the obligate anaerobes and leads to the activation of the residual colon microflora and aggravation of the microbiotic disorders. The SCFA shortage aggravates the bronchopulmonary pathology and contributes to the changes progression in the intestinal flora.12
Conclusions
One of the restrictions for the experimental work was not too plenty of the patients included in the tested groups for our study. Nevertheless, having analyzed the results of our research, we can state the following: the metabolic activity changing of the gut biotope was detected for the tested patients. The violation of the intestinal microflora composition has led to a shifting of the SCFAs forming process towards to the T-helper type 2 affecting on the bronchopulmonary system state. This phenomenon is to play a principal role as in bronchial asthma development as for the organism sensibilisation supporting. Of course, to obtain more deep understanding all of the processes the new experiments should be carried out at further. The subject of this research will be the probiotics efficacy for the gut microbiota correction for the bronchial asthma treatment.
Funding Statement
Funding: none.
References
- 1.Cummings JA. Short chain fatty acids in the human colon. Gut 1981;22:763-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerda B, Perez M, Perez-Santiago J. Gut microbiota modification: another piece in the puzzle of the benefits of physical exercise in health? Front Physiol 2016;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganapathy V, Thangaraju M, Singh N. Transporters and receptors for shortchain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol. 2013;13:869-74. [DOI] [PubMed] [Google Scholar]
- 4.Berthon B, Macdonald-Wicks L, Gibson P, et al. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013;18:447-54. [DOI] [PubMed] [Google Scholar]
- 5.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014;20;159-66. [DOI] [PubMed] [Google Scholar]
- 6.Gallacher DJ, Kotecha S. Respiratory microbiome of new-born infants. Front Pediatr 2016;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkataraman A, Bassis CH, Beck J, et al. Application of a neutral community model to assess structuring of the human lung microbiome. mBio 2015;6:e02284-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrieta M. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015;7:307-12. [DOI] [PubMed] [Google Scholar]
- 9.Sun M, Wu W, Liu Z. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases J Gastroenterol 2017;52:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siavoshian S. Butyrate and trichostatin A effects on the proliferation. differentiation of humen intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut 2000;46:507-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis P, Young P, Holtrop G, et al. Diversity of human colonic butyrateproducing bacteria revealed by analysis of the butyryl-CoA: acetate CoA-transferase gene Environ Microbiol 2010;12:304-14. [DOI] [PubMed] [Google Scholar]
- 12.Potskhverashvili ND, Zolnikova ОY, Kokina NI, et al. small bowel bacterial overgrowth syndrome in patients with bronchial asthma. Russ J Gastroenterol Hepatol Coloproctol 2018;28:47-54. [Google Scholar]


