Abstract
Background:
The aim of our study was to identify the association between Human papillomavirus (HPV) positive rate and smoking in lung cancer (LC) patients. Meanwhile, to analyze differences among gender, age differences on HPV infection rate in LC patients.
Methods:
We performed a systematic literature search through PubMed, Wan Fang, China National Knowledge Infrastructure (CNKI), MEDLINE, EMBASE (OVID), and Web of Science databases from 1991–2017, and we searched these keywords such as “lung cancer”, “HPV”, “smoking”, and “human papillomavirus”. Review Manager 5.3 software was used to analyze. An estimate of the odds ratio (OR) with 95% confidence intervals (CI) was calculated.
Results:
In China, a statistical significance was observed between HPV positive rate and smoking in LC patients (OR=2.34, 95%CI: 1.76–3.09, P<0.001; I2 =25%). However, after stratified by region, no significance was observed in other regions, gender, and age.
Conclusion:
HPV infections are associated with smoking in LC patients. The association between HPV infection and smoking in LC patients may relate to different regions. There were no differences between gender and age among HPV infection rates in LC patients. To identify the etiology of smoking, HPV, and LC, a further experimental research needs to be conducted.
Keywords: Human papillomavirus, Smoking, Meta-analysis, Gender, Age
Introduction
Lung cancer (LC) is the most common cause of morbidity and mortality around the world. According to the statistical data (1), there were 1.8 million new cases and 1.6 million deaths in 2012. Now the pathogenesis of LC is inconsistent. Although smoking is one of the major factors in the development of LC, about 25% of patients with LC are non-smoker. Hence, the occurrence of LC has many potential risk factors, such as the occupational exposure of asbestos and radon, environmental pollution, biological carcinogenic factors and so on. With the detection of human papillomavirus in LC, people have paid attention to the viral infection which is the carcinogenic factor of LC.
Human papillomavirus (HPV), a small and naked deoxyribonucleic acid (DNA) virus, consisting of double-stranded circular DNA, is believed to be an important factor contributing to the etiology of certain benign and malignant lesions in humans. HPV infections are associated with up to 35% of oropharyngeal cancers (2). In recent years, with the rapid development of molecular biology, increasing evidence suggests that HPV may play an important role in the development of LC. Since 1980, HPV infection may relate to the development of LC. People began to pay attention to the association between LC and HPV infection and its possible carcinogenic mechanism (3). However, the evidence on effect of HPV on the development of LC is still inconsistent. HPV infection increased LC risk (4), and HPV16/18 infection increased the risk of lung squamous cell carcinoma. This meta-analysis suggested that HPV infection is an important factor in the prognosis of LC. However, this study did not report the association between HPV and clinical features in LC patients, such as smoking status, age, region, gender and so on. Hence, we aimed to study the association between HPV and smoking, gender, and age in LC patients.
Methods
Search strategy
We performed a systematic literature study with multiple strategies: 1) electronic database searches, such as PubMed, Wan Fang, China National Knowledge Infrastructure (CNKI), MEDLINE, EMBASE (OVID), and Web of Science, and keywords such as “lung cancer,” “HPV,” “smoking,” and “human papillomavirus,” were used; 2) request for articles to researchers; 3) review of reference sections of articles obtained from searches. Studies matched with the selection criteria and available from 1991–2017, were included in the analysis. This meta-analysis was performed in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (5).
Study selection and inclusion criteria
Studies were selected if they met the following criteria: 1) they were case-control, cross-sectional or cohort studies comparing HPV infection in lung tissue among LC patients and non-cancer controls; 2) research involved smoking; 3) they provided information needed to calculate odds ratio (OR) with 95% confidence intervals. We excluded studies in which the subject population were not LC patients. Duplicate studies, reviews, local reports, conference abstracts, and presentations were excluded. When an overlap of patients was found in several studies, only the study with the largest sample size and detailed information was included. Two co-authors (Rui Z, Ling C) independently extracted relevant studies following the inclusion criteria (6). Disagreements were resolved through discussion in a panel meeting (6). The characteristics of the records included in the meta-analysis are shown in Table 1.
Table 1:
Characteristics of the 19 eligible studies in this meta-analysis
| Author | Year | Region | HPV(+) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ag <50 | Age≥50 | Age<60 | Age≥60 | Smoking | No Smoking | Male | Female | |||
| Zhou et al. ( 16 ) | 2015 | China | 8/16 | 22/47 | 20/38 | 10/25 | 19/43 | 11/20 | ||
| Zhou et al. ( 15 ) | 2015 | China | 6/15 | 19/45 | 18/43 | 7/17 | 19/46 | 6/14 | ||
| Xiong et al. ( 14 ) | 2016 | China | 3/43 | 4/40 | 6/44 | 1/39 | 6/53 | 1/30 | ||
| Zhang et al. ( 13 ) | 2012 | China | 8/23 | 9/20 | 9/20 | 8/23 | 13/30 | 4/13 | ||
| Chen et al. (12) | 2005 | China | 43/85 | 18/41 | 42/91 | 19/35 | ||||
| Liu et al.(11) | 2007 | China | 6/21 | 29/68 | 26/64 | 9/25 | 28/69 | 7/20 | ||
| Zhang et al. (24) | 2016 | China | 3/21 | 5/30 | 6/28 | 2/23 | 5/29 | 3/22 | ||
| S.A.Nadji et al. (8) | 2007 | Iran | 5/12 | 28/114 | 28/108 | 5/21 | 27/104 | 6/25 | ||
| E.Sarchianaki et al. (9) | 2014 | Greece | 8/38 | 0/7 | 17/91 | 2/9 | ||||
| Yang et al. (10) | 2006 | China | 12/33 | 11/40 | 14/39 | 9/34 | ||||
| Zhou et al. (23) | 2014 | China | 15/22 | 2/14 | 15/26 | 2/12 | ||||
| Jiang er al. (22) | 2005 | China | 18/38 | 3/22 | 17/43 | 4/17 | ||||
| Yuan et al. (19) | 2006 | China | 6/17 | 25/59 | 26/50 | 5/26 | 24/59 | 7/17 | ||
| Zhen et al. (21) | 2015 | China | 2/6 | 15/37 | 12/22 | 4/21 | 12/30 | 4/13 | ||
| Huang et al. (20) | 2006 | China | 8/15 | 11/29 | 15/26 | 4/18 | 13/34 | 6/10 | ||
| Wang et al. (18) | 2005 | China | 6/12 | 12/30 | 15/26 | 3/16 | 14/32 | 4/14 | ||
| Kong et al. (25) | 2009 | China | 18/33 | 2/14 | 15/35 | 5/12 | ||||
| Yan et al. (17) | 2009 | China | 31/57 | 4/11 | ||||||
| E.Argyri et al. (26) | 2017 | Greece | 2/66 | 0/1 | 1/58 | 1/9 | ||||
Data extraction
Screening of the title and abstract was performed independently in the first step, and disagreement was resolved by discussion. Full-text review was retrieved and then detailed evaluation was followed (6). All data extraction were conducted independently and checked by two authors, disagreements being resolved by discussion.
Statistical analysis
Odds ratios (ORs) with corresponding 95% CIs were calculated if there were sufficient data. Heterogeneity of these studies was evaluated using the P-value and the I2 statistic (7). If I2<50%, a fixed-effect model was used to evaluate inter-study heterogeneity; otherwise, a random-effect model was used. All statistical analysis was carried out with the use of Review Manager 5.3 (Cochrane). Moreover, P-values less than 0.05 were considered statistically significant. All statistical tests were two-sided.
Eligible studies
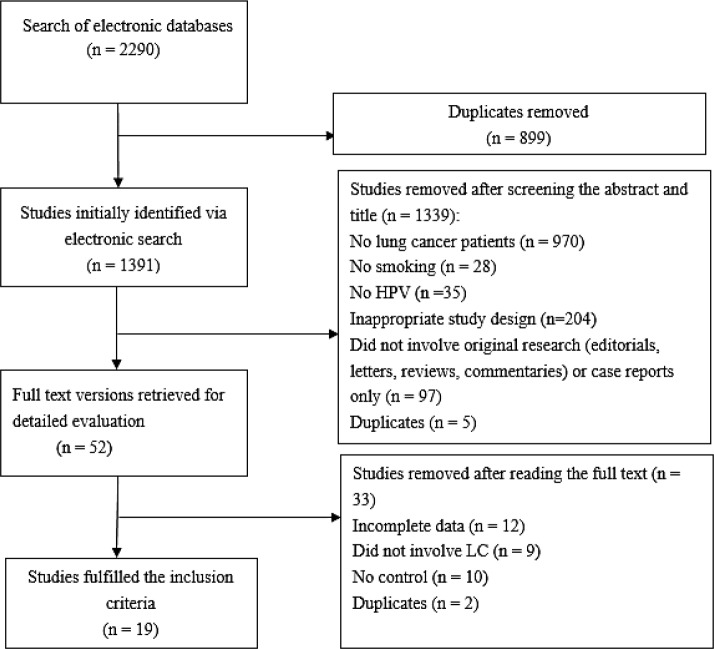
This study of the electronic databases revealed 2290 studies, of which 899 overlapped among different search categories. The search strategy in Fig. 1 as the QUOROM statement flowchart in which the detailed procedure of reference identification along with information regarding exclusion criteria applied at different stages of the selection is described. After screening the title and abstract of the 1391 unique references, 1339 were excluded, and 52 studies were required for further assessment. After screening full-text reviews, 33 studies excluded, while only 19 (8–26) articles fulfilled the predefined inclusion criteria and were selected to be involved in the analysis, eligible studies in this meta-analysis.
Fig. 1:
Search strategy
We identified 19 studies around the world, 16 studies of which were from China, and 3 studies from Greece and Iran. Studies contained 1245 samples. All studies have reported prevalence of HPV, patients’ demography, gender differences in HPV positive rate, HPV detection methods, HPV positive rate in smoking LC patients, and other significant information.
Ethics approval
The study’s protocol and data collection procedure were approved by the Institute of Public Health and Management. No individual data are used; only group data are reported. Thus, consent is not applicable.
Results
Association between HPV positive rate and smoking status of LC patients
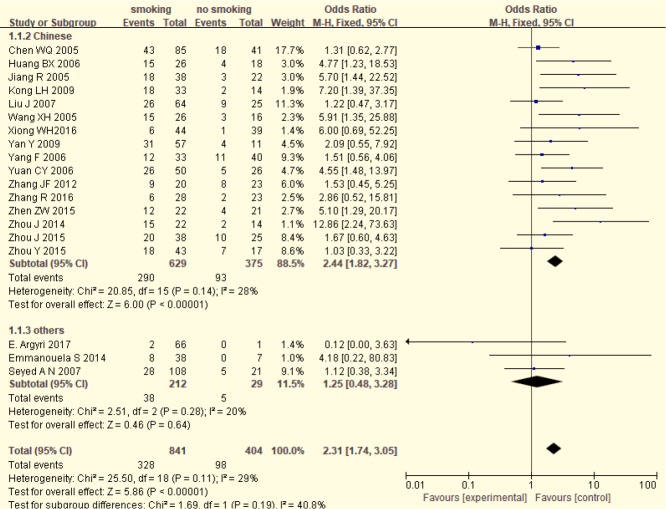
HPV positive rate in smoking LC patients was higher than that in non-smoking LC patients (39.00% VS. 24.26%). Fig. 2 shows a forest plot of the overall association between HPV positive rate and smoking status of LC patients. A statistical significance was observed between HPV positive rate and smoking status of LC patients (OR=2.31, 95%CI: 1.74–3.05, P<0.001; I2 =29%). In addition, after stratified by region, significance was also detected in 16 Chinese studies (OR=2.44, 95%CI: 1.82–3.27, P<0.001; I2 =28%). However, no significance was showed in 3 other studies (OR=1.25, 95%CI: 0.48–3.28, P=0.64; I2 =20%).
Fig. 2:
The relationship between HPV positive rate and smoking in LC patients
Gender differences of HPV positive rate in patients with LC
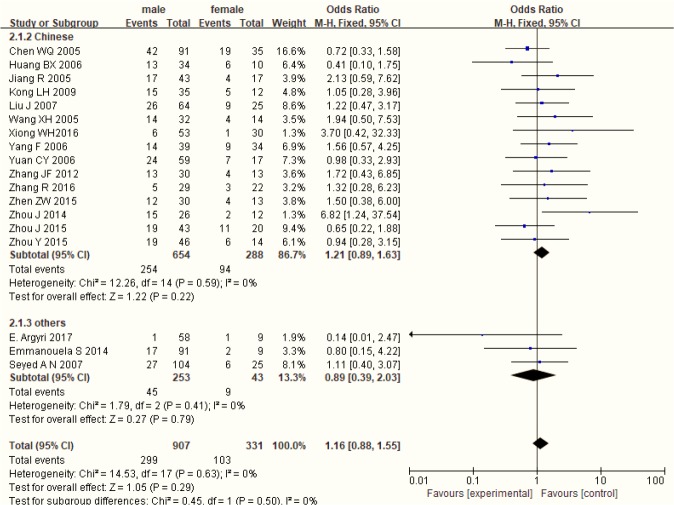
Fig. 3 shows a forest plot of the gender differences of HPV positive rate in LC patients, stratified by region. No statistical significance was observed between gender and HPV in LC patients (OR=1.16, 95%CI: 0.88–1.55, P=0.29; I2 =0%), also in different region.
Fig. 3:
Gender differences in HPV positive rate in LC patients
Age differences of HPV positive rate in patients with LC
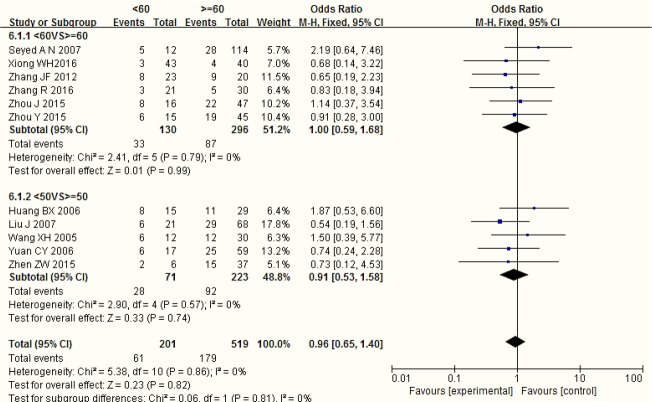
Fig. 4 shows a forest plot of the age differences of HPV positive rate in LC patients, stratified by different age group. No statistical significance was observed between age and HPV in LC patients (OR=0.96, 95%CI: 0.65–1.40, P=0.86; I2 =0%).
Fig. 4:
Age differences in HPV positive rate in patients with LC
Sensitivity analysis
To assess the sensitivity of this meta-analysis, we sequentially removed individual studies from it. The pooled ORs had good stability, and statistical significance was found by fix-effect model. The results of this meta-analysis are reliable. Moreover, funnel plot indicates that no significant publication bias exists in this review.
Discussion
HPV is thought to be a high risk of lung cancer. The HPV infection rate in smoker was higher than that in non-smoker LC patients. However, HPV infection rate was not related to smoking in the development of LC. The association between smoking and HPV infection and whether these two factors exert a synergistic effect on the development of LC are still in the dispute.
Now, HPV infection was closely related to smoking in the occurrence of LC. Smoking could directly cause HPV infection, since smoking can decrease Langhans cell which is antigen-presenting cell in epithelial tissue, then lead to immune deficiency, which is helpful to the activation of HPV and the persistence of infection (27). All HPV infection cases were moderate smokers in LC patients (28). Both HPV infection and P53 protein expression were associated with smoking, and smoking and HPV infection may have a synergic effect on the development of LC (19). However, there was no significant difference between HPV infection and smoking in LC patients (11). HPV was thought to be an environmental pollution factor, which may lead to airway injury, and eventually, lead to LC. There was no significant difference between smoking and non-smoking in HPV infection rate in LC patients, which indicated that smoking may be an independent carcinogenic factor in the development of LC, and no synergic effect with HPV infection (10, 29). Our study showed that HPV positive infection are associated with smoking in LC patients, smoking can lead to HPV infection; HPV infection and smoking have a synergic effect on the development of LC. Meanwhile, HPV is the risk factor of LC, this is consistent with previous studies.
In the study of LC and HPV infection, we found that not only smoking status but also age, and gender are significant clinical features. There were regional differences in HPV infection in non-smokers with LC that East Asia was higher than Europe (30). The HPV infection rate was similar in non-smoker and quit-smoking populations in Asia, while in Europe non-smoker was higher that quit-smoking people. The HPV infection rate of non-smoker was 68.7% in Taiwan, 60% in Korea, 23.8% in China and 12.4% in Japan (30). HPV infection is associated with different region, which was inconsistent with our study. We conducted a stratified analysis to study the regional differences. After stratified by region, a statistical significance was detected in Chinese, not in others, which suggested that in China, HPV infections are associated with smoking in LC patients. The association between HPV infection and smoking in LC patients may be related to different regions. The lack of statistical significance in other countries may be due only 2 other studies included.
The incidence rate was higher in male and in the elder. However, age and gender were always ignored as the mixed factors in the study of HPV infection and LC. Few studies reported the association between age, gender and HPV infection in LC patients. There was no association between HPV infection and gender in LC patients (31). Our study made two forest plots for gender and age differences in HPV positive rate in LC patients. There were no significant gender and age differences in HPV positive rate in LC patients, which suggested that there was no association among age, gender and HPV infection in LC patients (Figs. 3,4).
Consequently, HPV infection may relate to smoking and region in LC patients, but not to gender and age. The detection rate may alter with different detection methods in different study. For lack of sample size, there were some limitations in our study. Therefore, a large sample of study was needed to investigate the synergistic effect on smoking and HPV infection in LC, also mechanism needed. So our study suggested that smoking may be a risk factor of HPV infection in LC patients, and it promotes the development of LC. Moreover, region is associated with HPV infection rate in smoking LC patients, age but gender are not.
Some limitations exist in our meta-analysis, 16 studies from China, while 2 other studies, which may lead to regional bias. HPV gene type may have an association with smoking in LC. However there was only one study involves HPV gene type, therefore, we are unable to analyze the association between HPV gene type and smoking in LC. Although our study suggested that HPV infections are associated with smoking in LC patients, we do not know the certain etiology of smoking, HPV, and LC. Smoking increased HPV infection, which in turn caused LC, however the exact mechanism required further experimental research.
Conclusion
The forest plot shows that HPV infections are associated with smoking in LC patients. Unfortunately, most of our involved studies are from China, which may lead to regional bias. After stratified by age and gender, no statistical differences were observed in our study. Hence, smoking may be a risk factor of HPV infection in LC patients, gender and age may not relate to HPV infection in LC patients.
Ethical consideration
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We thank all the participants of the study. We would like to especially thank Ge Li for his contribution in the search strategy and statistical analysis.
Footnotes
Funding
None.
Conflict of interest
All authors declare that they have no competing interests.
References
- 1. Guo LW, Liu SZ, Zhang SK, et al. (2017). Human papillomavirus infection as a prognostic marker for lung adenocarcinoma: a systematic review and meta-analysis. Oncotarget, 8( 21): 34507– 34515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grulich AE, Jin F, Conway EL, et al. (2010). Cancers attributable to human papillomavirus infection. Sex Health, 7( 3): 244– 252. [DOI] [PubMed] [Google Scholar]
- 3. Syrjanen KJ. (1980). Epithelial lesions suggestive of a condylomatous origin found closely associated with invasive bronchial squamous cell carcinomas. Respiration, 40( 3): 150– 160. [DOI] [PubMed] [Google Scholar]
- 4. Zhai K, Ding J, Shi H. (2015). HPV and lung cancer risk: a meta-analysis. J Clin Virol, 63: 84– 90. [DOI] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, et al. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 6( 7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liberati A, Altman DG, Tetzlaff J, et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ, 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins JP, Thompson SG. (2002). Quantifying heterogeneity in a meta-analysis. Stat Med, 21( 11): 1539– 58. [DOI] [PubMed] [Google Scholar]
- 8. Nadji SA, Mokhtari-Azad T, Mahmoodi M, et al. (2007). Relationship between lung cancer and human papillomavirus in north of Iran, Mazandaran province. Cancer Lett, 248( 1) 41– 46. [DOI] [PubMed] [Google Scholar]
- 9. Sarchianaki E, Derdas SP, Ntaoukakis M, et al. (2014). Detection and genotype analysis of human papillomavirus in non-small cell lung cancer patients. Tumour Biol, 35( 4): 3203– 9. [DOI] [PubMed] [Google Scholar]
- 10. Fei Y, Yang J, Hsieh WC, et al. (2006). Different Human Papillomavirus 16/18 Infection in Chinese Non-Small Cell Lung Cancer Patients Living in Wuhan, China. Jpn J Clin Oncol, 36( 5) 274– 279. [DOI] [PubMed] [Google Scholar]
- 11. Liu J, Wu CH, Lu Y, et al. (2007). The correlation of HPV infection with the expression of epidermal growth factor receptor and vascular endothelial growth factor in non- small cell lung cancer. Tumor, 27( 10): 821– 824. [Google Scholar]
- 12. Chen WQ, Qi HW, Wu CG, et al. (2005). The Studies on Human Papillomavirus Infection in Squamous Cancer of the Lung. Chin J Clin Oncol, 32( 17): 966– 968. [Google Scholar]
- 13. Zhang JF, Yang QL, Huang L, et al. (2012). The relationship between human papillomavirus type 16 infection and nonsmall cell lung cancer. Chin Clin Oncol, 17( 3): 233– 236. [Google Scholar]
- 14. Xiong WM, He F, Xiao RD, et al. (2016). Association between human papillomavirus an lung cancer. Zhonghua Liu Xing Bing Xue Za Zhi, 37( 12): 1658– 1661. [DOI] [PubMed] [Google Scholar]
- 15. Zhou Y, Zhang H, Xie YH, et al. (2015). Expression of P53 and hypoxia-inducible factor-1α in human papillomavirus infection and its correlation with non-small cell lung cancer. Chin J Gerontology, 35(2): 335– 337. [Google Scholar]
- 16. Zhou J, You J, Hong ZP, et al. (2015). Analysis on the HPV16/18 infection and gene polymorphism of p53codon72 in hospitalized patients with lung cancer in Yunnan Province. Chin J Cancer Prevention Treatment, 22( 1): 19– 22. [Google Scholar]
- 17. Yan Y, Yang AM, Hu SK, et al. (2009). Correlation of HPV-16/18 infection of human papillomavirus with lung squamous cell carcinomas in Western China. Oncol Rep, 21( 6): 1627– 32. [DOI] [PubMed] [Google Scholar]
- 18. Wang XH, Wu CH, Huang BX, et al. (2005). Relationship among HPV infection, FHIT protein expression and non-small cell lung cancer. Chin J Histochemistry and Cytochemistry, 14( 4): 375– 380. [Google Scholar]
- 19. Yuan CY, Wu CH, Guo JL, et al. (2006). Infection of HPV and Its Relationship with Expression of P53 and Survivin in NSCLC. Acta Med Univ Sci Technol Huazhong, 35( 6): 740– 743. [Google Scholar]
- 20. Huang BX, Wu CH, Wang XH, et al. (2006). Detection of High Risk Papillomavirus Infection in Non-small Cell Lung Cancers. Acta Med Univ Sci Technol Huazhong, 35( 4): 436– 439. [Google Scholar]
- 21. Zhen ZW, Wu J, Yang HL. (2015). Significance of the expression of human papilloma virus proto-oncogene proteins E6E7 in non-small-cell lung cancer in Hakka region. Chin J Diagnostics (Electronic Edition), 3( 1): 7– 11. [Google Scholar]
- 22. Jiang R, Wu CH, Zheng LD, et al. (2005). Relationship between HPV Infection and Non-small Cell Lung Cancer. Acta Med Univ Sci Technol Huazhong, 34( 2): 141– 144. [Google Scholar]
- 23. Zhou J, Hong ZP, Sun XH, et al. (2014). Study on relationship between human papillomavirus infection and non-small cell lung cancer in Yunan area . Chin J Nosocomiol, 24 (22): 5470– 5472. [Google Scholar]
- 24. Zhang R. Infection of high risk HPV and its relationship with the expression of P53 protein primary lung cancer [master thesis]. Third Affiliated hospital, Xinjiang Medical University, China; 2016. [Google Scholar]
- 25. Kong HL. A study on the correlation between expression of E2F-1, Survivin protein and HPV infection in NSCLC [master thesis]. Huazhong University of Science and Technology, China; 2009. [Google Scholar]
- 26. Argyri E, Tsimplaki E, Marketos C, et al. (2017). Investigating the role of human papilloma-virus in lung cancer. Papillomavirus Res, 3: 7– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burger MP, Hollema H, Gouw AS, et al. (1993). Cigarette smoking and human papillomavirus in patients with reported cervical cytological abnormality. BMJ, 306( 6880): 749– 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kinoshita I, Dosaka Akita H, Shindoh M, et al. (1995). Human papillomavirus type 18 DNA and E6–E7 mRNA are detected in squamous cell carcinoma and adenocarcinoma of the lung. Br J Cancer, 7( 12): 344– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ragin C, Obikoya-Malomo M, Kim S, et al. (2014). HPV-associated lung cancers: an international pooled analysis. Carcinogenesis, 35( 6): 1267– 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasegawa Y, Ando M, Kubo A, et al. (2014). Human papilloma virus in non-small cell lung cancer in never smokers: A systematic review of the literature. Lung Cancer, 83( 1): 8– 13. [DOI] [PubMed] [Google Scholar]
- 31. Boyle P, Leon ME, Maisonneuve P, et al. (2003). Cancer control in women. Update 2003. Int J Gynaecol Obstet, 83 Suppl 1: 179– 202. [DOI] [PubMed] [Google Scholar]