Abstract
Glucose-6-phosphate dehydrogenase (G6PDH or G6PD) is the key regulatory enzyme in the oxidative pentose phosphate pathway (OPPP). The cytosolic isoforms including G6PD5 and G6PD6 account for the major part of the G6PD total activity in plant cells. Here, we characterized the Arabidopsis single null mutant g6pd5 and g6pd6 and double mutant g6pd5/6. Compared to wild type, the mutant seeds showed a reduced germination rate and root elongation under salt stress. The seeds and seedlings lacking G6PD5 and G6PD6 accumulate more reactive oxygen species (ROS) than the wild type under salt stress. Cytosolic G6PD (cy-G6PD) affected the expression of NADPH oxidases and the G6PD enzymatic activities in the mutant atrbohD/F, in which the NADPH oxidases genes are disrupted by T-DNA insertion and generation of ROS is inhibited, were lower than that in the wild type. The NADPH level in mutants was decreased under salt stress. In addition, we found that G6PD5 and G6PD6 affected the activities and transcript levels of various antioxidant enzymes in response to salt stress, especially the ascorbate peroxidase and glutathione reductase. Exogenous application of ascorbate acid and glutathione rescued the seed and root phenotype of g6pd5/6 under salt stress. Interestingly, the cytosolic G6PD negatively modulated the NaCl-blocked primary root growth under salt stress in the root meristem and elongation zone.
Keywords: germination, glucose-6-phosphate dehydrogenase, NaCl, NADPH oxidases, reactive oxygen species, root system architecture
Introduction
The oxidative pentose phosphate pathway (OPPP) is the major pathway of the production of NADPH, which is used for biosyntheses and redox balance in plant cells (Esposito et al., 2003; Kruger and von Schaewen, 2003; Hutchings et al., 2005; Cardi et al., 2011). The main regulatory step of OPPP is catalyzed by glucose-6-phosphate dehydrogenase (G6PDH or G6PD). The majority of NADPH in the cytoplasm is produced by G6PD and 6-phosphogluconate dehydrogenase (Huan et al., 2014). Arabidopsis genome-wide analysis indicates the presence of two cytosolic (cy-G6PD) and four plastidial (pla-G6PD) isoforms (Wakao and Benning, 2005). The cy-G6PD includes G6PD5 and G6PD6. Based on the difference in amino acid sequence, the pla-G6PD can be divided into P1, P2, and P0 type: P1 mainly exists in the chloroplast (G6PD1); P2 mainly exists in plastids and some non-oxygen cells (G6PD2, G6PD3), P0 is a non-functional enzyme (G6PD4) (Wakao and Benning, 2005). Many studies have indicated that G6PD plays an important role in plants to cope with stresses, including salinity and drought (Meyer et al., 2011; Liu et al., 2013; Huan et al., 2014; Wang et al., 2016). Certainly, salinity is a major environmental restriction for the growth of agricultural crops and negatively affects plant productivity (Hasegawa et al., 2000; Zhu, 2001; Dal Santo et al., 2012).
Salinity brings about water deficit and ion stress, which cause destabilization of cell membranes, inhibition of essential enzymes, overproduction of reactive oxygen species (ROS), and decrease in nutrient supply (Hasegawa et al., 2000; Dal Santo et al., 2012). ROS regulate many biological processes including seed germination and root growth in plants (Kwak et al., 2006; Dunand et al., 2007). It has been documented that ROS are produced through both enzymatic and non-enzymatic reactions in plants (Apel and Hirt, 2004; Ma et al., 2012). ROS directly originate from two ROS-generating NADPH oxidases, impairing stress inhibition of primary root elongation in Arabidopsis (Kwak et al., 2006; Jiao et al., 2013). However, continuously increased levels of ROS exceed cellular antioxidant capacity, thus are toxic to cells and affect all cellular biomolecules (Niforou et al., 2014; Jia et al., 2016). In Arabidopsis genome, there are 10 NADPH-oxidase catalytic subunit genes (AtrbohA-J) (Marino et al., 2012). NADPH oxidase controls shoot branching and reproductive organ development in tomato, and is required for pollen tube growth in tobacco (Sagi et al., 2004; Potocky et al., 2007). NADPH oxidases require NADPH to generate superoxide, which can be dismutated subsequently to hydrogen peroxide (Stampfl et al., 2016). In maize, ROS derived from NADPH oxidase is necessary for normal root growth (Liszkay et al., 2004). In bacteria, studies provide experimental evidence for a role of NADPH oxidase-derived ROS in establishing a relationship with pattern-triggered immunity in Arabidopsis (Stampfl et al., 2016). Such oxidative bursts are usually accompanied by transient oxidation of the cytosol (decreased NADPH levels) that triggers redox signaling and activation of the OPPP (Landi et al., 2016; Stampfl et al., 2016; Wang et al., 2016).
Plants can minimize the effects of salinity stress by removing excess ROS via increasing antioxidant enzyme activities (Yang et al., 2015; Landi et al., 2016). More recently, it is reported that G6PD plays a primary role during stress response by providing more NADPH for the antioxidant systems favoring ROS scavenging functions (Dal Santo et al., 2012; Landi et al., 2016). G6PD functions on modulating reduced glutathione levels in reed callus (Wang et al., 2008), establishing tolerance of red kidney bean roots to salt stress (Liu et al., 2007), and upregulating plasma membrane (PM) H+-ATPase activity, which results in the enhanced K+/Na+ ratio (Li et al., 2011).
In Arabidopsis, non-dormant seeds produce significant ROS during imbibition (Leymarie et al., 2012; Chen et al., 2014a). Seed germination and root growth are critical phases in the plant life cycle (Chen et al., 2014a; Wang et al., 2014). The ability of seeds to properly germinate depends on its oxidative status (Rajjou et al., 2012; Chen et al., 2014a). Over-accumulation of ROS causes oxidative damages to cellular components (Bailly et al., 2008; Parkhey et al., 2012). In plants, some hydrogen peroxide-scavenging substances protect seeds and roots from excessive oxidative damages, for example, ascorbate (Asc) and reduced glutathione (GSH) (Dal Santo et al., 2012; Chen et al., 2014a). GSH and Asc detoxify H2O2 mainly through the ascorbate-glutathione cycle, which is the most effective way to scavenge H2O2 in plants (Noctor and Foyer, 1998; Wang et al., 2016).
Based on the above studies, although the relationship between G6PDs and salt stress have been elucidated (Wang et al., 2008), function of G6PDs depends upon the developmental stage, organ/tissue, and species. In this work, we used the genetic and molecular approaches to study the function of cy-G6PDs. We characterized the function of G6PD5 and G6PD6, which show enhanced tolerance to salt stress during seed germination and root growth, and functional interaction and synergism between G6PD and GSH during salt stress. We revealed a novel interplay between rbohD/F, ROS, ascorbate peroxidase (APX), and glutathione reductase (GR).
Materials and Methods
Plant Materials and Growth Conditions
Arabidopsis thaliana Col-0 was used as the WT plant. The T-DNA insertion mutants g6pd5 (CS804669) and g6pd6 (SALK_016157C) were purchased from the Arabidopsis Biological Resource Center1. The T-DNA in the g6pd5 mutant is inserted in the coding region of At3g27300, and in the g6pd6 mutant it is inserted in the coding region of At5g40760. The overexpression plants of G6PD5 (OE#1, OE#9) and G6PD6 (OE#17, OE#21) were generated by transforming the G6PD5- or G6PD6-containing constructs into WT. The double mutant g6pd5/6 was generated by crossing g6pd5 with g6pd6, followed by screening the F2 progeny for homozygosity at both loci by PCR genotyping. atrbohD1 (CS9555), atrbohF1 (CS9557), atrbohD1/F1 (CS9558) were obtained from the Arabidopsis Biological Resource Center. Seeds were sterilized with 1.5% NaClO for 15 min, washed with sterile water for three times, placed at 4°C for 2–4 days and then planted on the half-strength Murashige and Skoog (½ MS) medium (pH 5.8) containing 1% sucrose and 0.8% agar at 23°C under 100–120 μmol photons ⋅ m-2 ⋅ s-1 with a 16 h/8 h light/dark photoperiod in the growth room.
Phenotypic Analysis and Statistics
In all assays, WT, g6pd5, g6pd6, g6pd5/6, OE#1, OE#9, OE#17, and OE#21 seeds (approximately 50 seeds for each replicate. For root elongation measurements, 15 seeds were used per replicate) were surface-sterilized. The seeds were sown on ½ MS medium with or without different concentration of NaCl and then incubated at 23°C with a 16 h/8 h light/dark photoperiod. The number of planted and germinated seeds was recorded 5 days after planting on the medium. Radicle emergence of >1 mm indicated seed germination. Three replicates were used for each treatment. Five-day-old seedlings with roots 1–1.5 cm long were transferred from agar plates containing ½ MS medium onto a new agar medium supplemented with different concentrations of NaCl. Increases in root length were measured after 3 days of treatment (Rosado et al., 2006; Nan et al., 2014). The length of the primary roots was measured with NIH Image software (Image J, version 1.43).
Confocal Microscopy
Propidium iodide (PI) fluorescence was used to visualize the cells in root tips. Seedling roots were stained with PI (Molecular Probes, Sigma, United States) according to the method described by Mei et al. (2012). Roots were incubated with 10 μg/ml PI for 5–10 min at 23°C in the dark and then washed three times with ddH2O. The roots were then imaged under a confocal microscope (Olympus FV 1000; excitation 488 nm, emission 570–650 nm).
Histochemical Staining and Assay of H2O2 Content
We used 2,7-dichlorodihydrofluorescein diacetate (H2DCF, Molecular Probes) to detect hydrogen peroxide (H2O2) accumulation in seeds and roots. Seeds of 12 h and roots of 5 days seedlings were treated with 20 μM H2DCF for 5 min, and fluorescence was monitored under a fluorescence microscope (Olympus FV 1000, excitation 488 nm and emission 500–550 nm).
For H2O2 content measurement, 10-day-old seedlings were soaked with in 150 mM NaCl solution for 12 h. Seedlings (0.3 g) were homogenized with 2 ml of 0.1% (w/v) TCA, then centrifuged at 10,000 ×g for 20 min at 4°C. The supernatant (0.5 ml) was mixed with 1 ml of 1 M potassium iodide for 1 h in the presence of 0.5 ml of 0.1 M Tris-HCl (pH 7.6). The absorbance was read at 390 nm and H2O2 content was determined using a standard curve.
Antioxidant Enzyme and Activities of G6PD Assays
Ten-day-old seedlings were soaked in 150 mM NaCl solution for 12 h. After treatment, the enzymes extraction and activity determination of G6PD and antioxidant enzymes were carried out according to the method of Liu et al. (2007) and Wang et al. (2008). Briefly, crude enzymes were extracted in extraction buffer containing 50 mM Hepes-Tris (pH 7.8), 1 mM EDTA, and 3 mM MgCl2. The homogenate was then centrifuged at 12,000 ×g for 20 min at 4°C. The supernatant was used to determine enzyme activity.
For ascorbate peroxidase (APX) activity, the reagent was composed of 0.1 mM EDTA-Na2 and 0.3 mM ascorbate. The enzyme extract (100 μl) and 1 ml reagent were mixed in cuvette in the presence of 20 μl of 9 mM H2O2. The absorbance at 290 nm was recorded for 1min.
For catalase (CAT) activity, the experiment group contained 1 ml of 15 mM H2O2 and 100 μl of enzyme extract. The change of absorbance at 240 nm was recorded.
For glutathione reductase (GR) activity, 0.52 mM Tris–HCl (pH 7.5), 6 μM EDTA, 2 mM GSSG, 4 mM NADPHNa4, and crude enzyme (100 μl) were mixed into 3 ml. GR activity was measured at 340 nm for the initial 3 min of the reaction at 25°C.
For peroxidase (POD) activity, the enzyme extract (25 μl) was mixed with 1 ml of 20 mM guaiacol in the presence of 20 μl of H2O2 for 3 min. The change of absorbance at 470 nm was record.
Superoxide dismutase (SOD) activity was measured in test tube. Reaction solution contained 2 ml of 39 mM methionine solution, 2 ml of 0.225 mM nitroblue tetrazolium, 1 ml of 0.6 mM EDTA-Na2 and 1 ml of 0.012 mM riboflavin. One tube was incubated in the light for 30 min, and the other tube was incubated in dark for 30 min. After 30 min the absorbance at 560 nm was recorded using a spectrophotometer.
For G6PD activity assay, the reagent was composed of 50 mM Hepes-Tris (pH 7.8), 1 mM EDTA, and 3 mM MgCl2. The G6PD activity was analyzed by detecting NADPH formation at 340 nm in the presence of 0.5 mM D-glucose-6-phosphate disodium salt (Sigma) and 0.5 mM NADPNa2. To distinguish the activity of cytosolic G6PD isoforms, 1,4-dithiothreitol (DTT) was added into the reaction mixture.
Determination of Glutathione Content, NADPH and NADP+ Content
Ten-day-old seedlings were soaked in 150 mM NaCl solution for 12 h. After treatment, the glutathione content was measured using the GSH content determination kit (Cat# BC1170, Solarbio, China). Glutathione can react with 5,5′-dithiobis-2-nitrobenoic acid (DTNB) to produce 2-nitro-5-fluorenyl benzoic acid and glutathione disulfide (GSSG). 2-nitro-5-mercaptobenzoic acid is a yellow product with maximal light absorbance at 412 nm.
NADPH and NADP+ were detected through NADPH and NADP+ determination kit (Cat# BC1100, Solarbio, China). NADP+ and NADPH were extracted from the samples using acidic and basic extracts, respectively. NADPH reduces the oxidized thiazole blue (MTT) to formazan by the hydrogen transfer of phenazine methyl sulfate (PMS), and the absorbance at 570 nm was detected to determine the NADPH content. The NADP+ content was determined by reducing NADP+ to NADPH using glucose-6-phosphate dehydrogenase.
Activities of NADPH Oxidase Assays
Ten-day-old seedlings were soaked in 150 mM NaCl solution for 12 h. After treatment, the activities of NADPH oxidase were evaluated according to the method of Wang et al. (2016).
Western Blot Analysis
Ten-day-old seedlings were soaked in 150 mM NaCl solution for 12 h. After treatment, the protein extraction, SDS-PAGE and subsequent western blot analysis were carried out according to the method of Wang et al. (2008). About 50 μg proteins were solubilized and separated on 12% acrylamide gels (Bio-Rad Mini protein II apparatus). After electrophoresis, the separated proteins were transferred to a polyvinylidene difiuoride membrane, and the membrane was blocked for 90 min with 5% non-fat milk in 0.5% (w/v) Tween 20, 10 mM Tris–HCl (pH 8.0), and 150 mM NaCl. At present, we do not have specific antibodies for different G6PD isoforms. The antibody of G6PD (Sigma) is a polyclonal antibody, which only detects the total protein levels of G6PD. Subsequently, the polyclonal G6PD antibody was added and incubated overnight with the membrane. After washing, alkaline phosphatase-coupled secondary antibody was added and incubated for 2 h. The chemiluminescence was determined with the Pro-light horseradish peroxidase kit (PA112, Tiangen, China). The western blotting images were caught by Tanon-5200 Chemiluminescent Imaging System (Tanon, China).
Quantitative Real-Time PCR Analysis
Total RNA was extracted with Trizol (TaKaRa) from shoots and roots. RNA was treated with RNase-free DNase (Transgen, China). First-strand cDNA was synthesized with the PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa, Transgen, China). Quantitative real-time PCR was performed using the SYBR PrimeScript RT-PCR Kit (Perfect Real Time; TaKaRa). PCR was performed using a CFX 96 Real-Time system (Bio-Rad, Hercules, CA, United States) with the following standard cycling conditions: 95°C for 10 s, followed by 40 cycles of 95°C for 5 s, and 60°C for 30 s. Primer sequences used in the study was shown in Supplementary Table S1. The cycle threshold 2(-ΔΔC(T))-based method was used for relative quantitation of gene expression. Expression levels of genes were normalized to Actin2.
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: ACTIN2 (AT3G18780), G6PD5 (AT3G27300), G6PD6 (AT5G40760), G6PD1 (AT5G35790), G6PD2 (AT5G13110), G6PD3 (AT1G24280), G6PD4 (AT1G09420), AtrbohD (AT5G47910), AtrbohF (AT1G64060), APX1 (At1G07890), SOD1 (At1G08830), POD1 (At1G67960), CAT1 (At1G20630), GR2 (At3G54660).
Generation of the G6PD5 and G6PD6 Overexpressing Lines
Arabidopsis full-length G6PD5 or G6PD6 cDNA was obtained using reverse transcription PCR, cloned into the pENTR-TOPO cloning vector (Invitrogen) and sequenced. After the LR reaction, G6PD5 or G6PD6 cDNA was inserted into the pGWB2 vector driven by the 35S promoter; this vector was named pGWB2-G6PD5 or pGWB2-G6PD6. Transformed plants were selected on hygromycin-containing medium. Plants of the second generation after transformation were used for the experiments. The empty pGWB5 vector (the ccdb gene was substituted by a nonsense segment with a termination codon) was also transferred into WT and used as control plants.
Statistical Analysis
Each experiment was repeated at least three times. Values were expressed as mean ± SE. The data were statistically analyzed using SPSS version 17.0. All comparisons were carried out with one way analysis of variance (ANOVA) followed by Duncan’s multiple range test for independent samples. In all cases, the confidence coefficient was set at P < 0.05.
Results
Expression Analyses of Cytosolic G6PD
To study the underlying role of cytosolic G6PD in Arabidopsis, we obtained T-DNA insertion mutants from the Arabidopsis Biological Resource Center (Supplementary Figure S1A). The results of quantitative real-time PCR and RT-PCR revealed that both g6pd5 or g6pd6 are loss-of function null mutants because the G6PD5 or G6PD6 transcript level in corresponding mutant was hardly detected (Supplementary Figures S1B,D). In order to further clarify the function of G6PD5 and G6PD6, we generated overexpression lines of G6PD5 (OE#1 and OE#9) and G6PD6 (OE#17 and OE#21). All overexpression lines showed elevated expression levels of G6PD5 (4- and 13-fold increase for OE#1 and OE#9, respectively) or G6PD6 (6- and 19-fold increase for OE#17 and OE#21, respectively) (Supplementary Figures S1C,D). Homozygous transgenic plants (5OE#9 and 6OE#21) were chosen for further analysis.
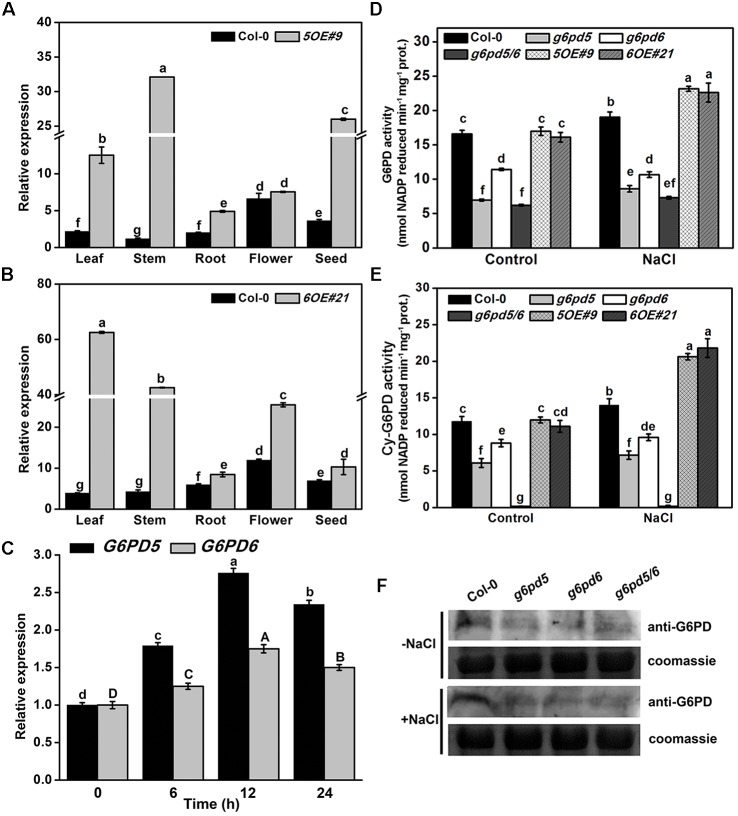
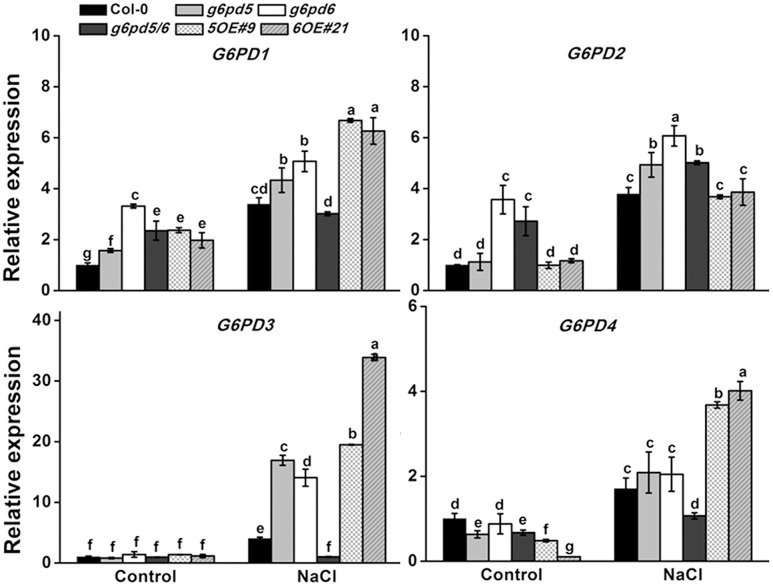
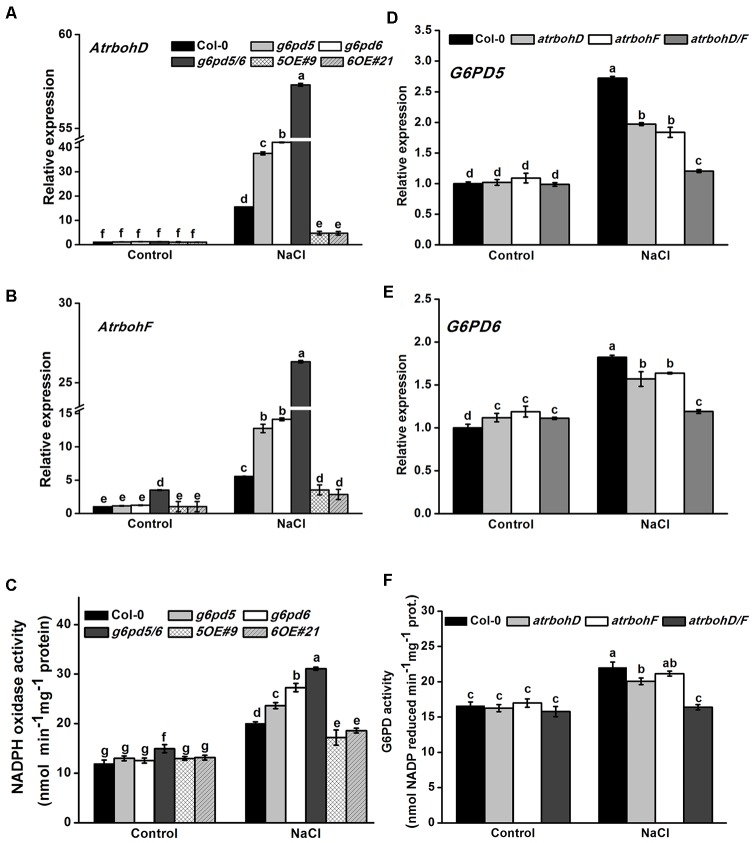
We also found that different G6PD family genes have different expression patterns (Supplementary Figure S2 and Figure 1A,B). The high expression level of cytoplasmic G6PD (G6PD5 and G6PD6) was observed in all organs examined. The total G6PD enzymatic activities in cy-G6PD loss-of-function mutants were much lower than that in WT seedlings, especially in g6pd5 and g6pd5/6 (Figure 1D). Similarly, the activities of cy-G6PD were lower in mutants than in WT (Figure 1E). It was noteworthy that cy-G6PD activity was the main factor in the enhanced total G6PD activity under salt stress, which was responsible for approximately 71% of the total G6PD activity. The western blot results were consistent with the G6PD enzymatic activities in seedlings (Figure 1F). Interestingly, under the normal condition, the expression of G6PD1 and G6PD2 in the g6pd6 or g6pd5/6 mutants was higher than that in WT. The expression of G6PD3 in the mutants was similar to WT, whereas the expression of G6PD4 in the mutants was lower than that in WT with the exception of g6pd6. Under salt stress, in single mutants, the expression of G6PD1, G6PD2, and G6PD3 was higher than that in WT, while G6PD4 had no obvious difference compared to WT. In the double mutant, the expression of the G6PD1 had no obvious difference compared to WT, while G6PD2 expression was higher than that in WT. The expression of G6PD3 and G6PD4 was lower than that in WT (Figure 2).
FIGURE 1.
The analysis of cy-G6PD in Arabidopsis seedlings with or without salt treatment. (A,B) The qRT-PCR analysis of G6PD5 (WT and 5OE#9) and G6PD6 (WT and 6OE#21) expression in Arabidopsis different organs. (C) Relative transcript levels of G6PD5 and G6PD6 in wild-type (Col-0) seedlings with the 150 mM NaCl treatment. Uppercase letters represent the error analysis of G6PD6, and lowercase letters represent the error analysis of G6PD5. (D,E) The activities of G6PD or cy-G6PD in Arabidopsis WT and mutants exposed to salt treatment. (F) Western blot analysis of G6PD expression in Arabidopsis. In this experiment, 150 mM NaCl was used for treatment. The Coomassie Brilliant Blue-stained gel was present to show that an equal amount of proteins was loaded in all lanes. Data are mean ± SE of three independent experiments, bars with different letters are significantly different at the level of P < 0.05. The experiment was repeated three with similar results.
FIGURE 2.
The qRT-PCR analysis of G6PDs expression in WT and cy-G6PD mutants. The transcript levels were normalized to Actin2 gene expression. Results are averages ± SE (n = 3), bars with different letters are significantly different at the level of P < 0.05. All experiments were repeated at least three times with similar results.
Phenotypic Analyses of cy-G6PD Mutants
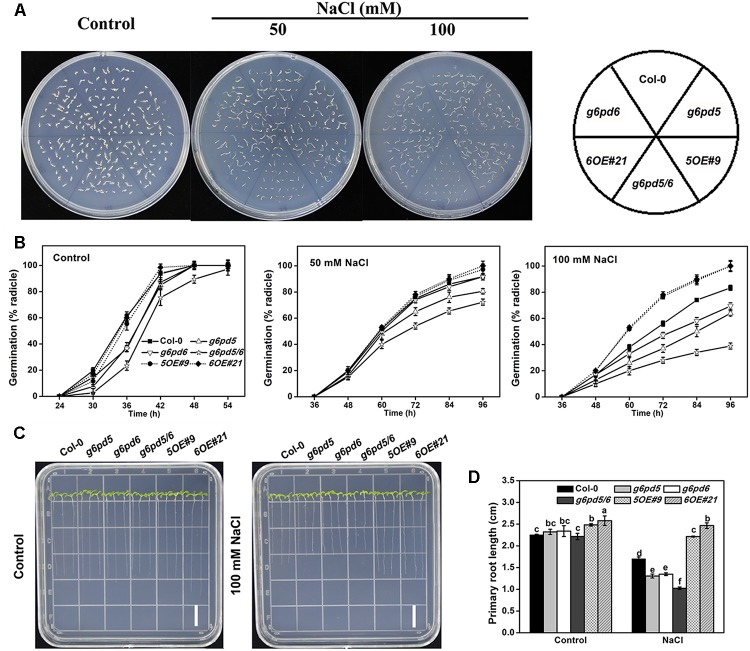
Seed germination is a critical phase in the plant life cycle. Successful execution of the germination program greatly depends on the oxidative homeostasis of seeds (Chen et al., 2014b). We evaluated the germination rates of cy-G6PD mutants under different conditions. The germination of the mutant seeds was slightly delayed compared with WT seeds but the mutant seedlings exhibited similar growth rates and plant sizes as WT (Figure 3). To determine the sensitivity of the g6pd5 or g6pd6 mutant to salt stress during seed germination and root elongation, different concentrations of NaCl (50 and 100 mM) were supplied in the medium (Figure 3). The results showed that both g6pd5 and g6pd6 single mutant exhibited slightly reduced seed germination rate (Figure 3A,B) and primary root length (Figure 3C,D) compared to WT. Because g6pd5 or g6pd6 single mutant is not significantly different from WT under salt stress, we generated the double mutant g6pd5/6 by crossing g6pd5 with g6pd6. RT-PCR results revealed that the transcripts of both G6PD5 and G6PD6 in g6pd5/6 were undetectable (Supplementary Figure S1B). Significantly, the double mutant g6pd5/6 exhibited low seed germination rate and short primary root length with increased NaCl concentrations compared to WT and single mutants, indicated the function redundancy of G6PD5 and G6PD6 (Figure 3). To determine whether the function of cy-G6PD in response to a relative higher concentration of NaCl, we analyzed the seed germination and primary root elongation under 150 mM NaCl treatment. Consistent with those in 50 or 100 mM NaCl treatment, the mutants exhibited more significant salt sensitivity compared with WT (Supplementary Figures S3A,B), whereas the length of primary roots was severely inhibited in 150 mM NaCl (Supplementary Figures S3C,D). NaCl from 50 to 150 mM promoted obvious cy-G6PD accumulation, but only 50 or 100 mM NaCl had significant effects on Arabidopsis stress tolerance.
FIGURE 3.
Seed germination and root growth of WT, g6pd5 mutant, g6pd6 mutant, g6pd5/6 mutant, G6PD5-OE, and G6PD6-OE Arabidopsis in response to NaCl stress. Seeds were germinated on ½ MS agar plates with or without various concentrations of NaCl. (A) Photographs were taken 3 days in terms of radical emergence after NaCl treatment. (B) Percentage of seed germination in WT, g6pd5 mutant, g6pd6 mutant, g6pd5/6 mutant, G6PD5-OE, and G6PD6-OE with or without different NaCl treatment. (C,D) 5-day-old seedlings were grown vertically on ½ MS agar plates supplemented with the indicated concentrations of NaCl for 3 days. Root growth was monitored and analyzed using ImageJ software. Data are reported as the average value of three replicates using >50 seeds for each genotype. One-way Duncan’s test was performed, and statistically significant differences are indicated by different lower case letters (P < 0.05). Bar, 1 cm. The experiments were repeated at least three times with similar results, and data from one representative experiment are presented.
Under normal growth conditions, slight difference in the germination and primary root growth was observed between the WT and the overexpression lines (Figure 3). However, under salt stress, the OE lines exhibited a significantly higher seed germination rate than WT (Figure 3A,B) and the root growth of OE plants was less sensitive to NaCl treatment (Figure 3C,D). These data indicate that overexpression of cy-G6PD increases salinity tolerance in Arabidopsis.
We also examined the transcript level of G6PD5 and G6PD6 in Arabidopsis seedlings under salt treatment using qRT-PCR. In accordance with our previous results, the expression of G6PD5 and G6PD6 in WT seedlings was significantly induced by salt stress (Figure 1C). In summary, G6PD5 and G6PD6 are involved in seed germination and root growth under salinity in Arabidopsis.
Response to Oxidative Damage in cy-G6PD-Overexpressing and cy-g6pd Mutant Plants
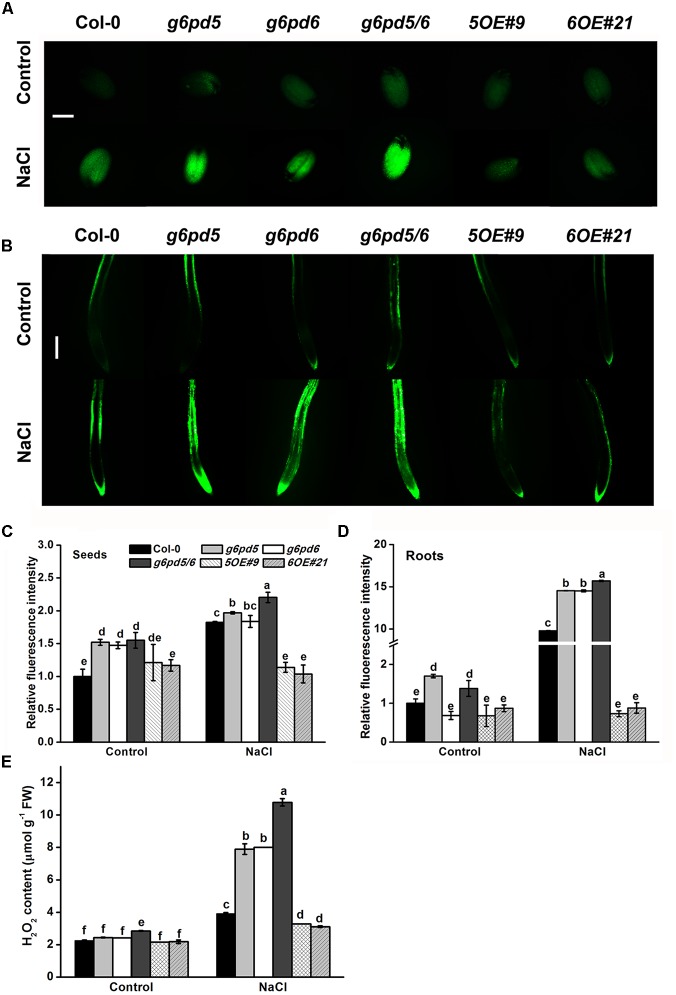
Reactive oxygen species (ROS) play a key regulatory role in the germination program under salt stress (Chen et al., 2014a). The ROS levels in cy-g6pd mutants and WT under NaCl stress were explored in this study. As shown in Figure 4, the ROS content in seeds and roots of both cy-g6pd mutant and WT was increased in response to NaCl treatment. It is noteworthy that such effects were significantly enhanced in the g6pd5/6 double mutant but attenuated in OE lines (Figure 4). Additionally, ROS content analysis revealed significantly higher levels of H2O2 in the double mutant than WT in seedlings under salt treatment, which was consistent with previous findings in seeds and roots (Figure 4E). To further dissect the role of cy-G6PD involvement in ROS signaling, exogenous H2O2 was supplied to the medium. The double mutant g6pd5/6 showed increased sensitivity to the oxidative stress, as manifested by delayed germination and retarded root elongation relative to WT (Supplementary Figures S4A,B). In contrast, OE lines exhibited reduced sensitivity to the oxidative stress (Supplementary Figures S4A,B). Moreover, exogenous application of diphenyliodonium iodide (DPI), an inhibitor for H2O2, partially rescued the root growth phenotype of g6pd5/6 (Supplementary Figure S4C). These results suggest that the oxidative level is higher in g6pd5/6 than in WT.
FIGURE 4.
The g6pd5, g6pd6, and g6pd5/6 mutant affect the ROS levels under salt stress. 1-day-old seeds and 5-day-old seedlings were grown vertically on ½ MS agar plates supplemented with the 150 mM NaCl for 12 h. (A) The levels of H2O2 were measured using the H2DCF-DA fluorochrome dyes in Arabidopsis seeds. Bar, 200 μm. (B) The levels of H2O2 were measured using the H2DCF-DA fluorochrome dyes in Arabidopsis roots. Bar, 200 μm. (C,D) Quantification of the fluorescence in Arabidopsis seeds and roots under NaCl treatment. (E) 10-day-old seedlings were grown vertically on ½ MS agar plates supplemented with the 150 mM NaCl for 12 h. Data are mean ± SE of three independent experiments, bars with different letters are significantly different at the level of P < 0.05. The experiment was repeated three with similar results.
cy-G6PD Influences the Expression of NADPH Oxidases AtrbohD and AtrbohF
Plasma membrane NADPH oxidase is considered to be an important producer of ROS, which has been shown to play a role in plant acclimation to salt stress (Ma et al., 2012; Jiao et al., 2013). In addition, the NADPH oxidases AtrbohD and AtrbohF are important in stress-inhibited primary root growth in Arabidopsis (Ma et al., 2012). To determine whether the function of cy-G6PD in response to salt stress is achieved through the NADPH oxidase signaling pathway, we analyzed the expression of NADPH oxidases genes in WT, g6pd5, g6pd6, and OE plants with or without salt treatment. As shown in Figure 5, the expression of AtrbohD and AtrbohF was markedly increased by salt treatment in all materials, and the salt-induced gene expression levels in g6pd5/6 was significantly higher than that in WT plants (Figure 5A,B). Consistently, the activity of NADPH oxidase was also higher in g6pd5/6 than in WT under salt stress (Figure 5C). These results suggest that cy-G6PD is involved in RBOH-dependent ROS production in salt-stressed seedlings. To prove the hypothesis, we examined the expression of G6PD5 and G6PD6 in NADPH oxidase mutants, atrbohD1 (CS9555), atrbohF1 (CS9557), and atrbohD1/F1 (CS9558). In these mutants, the expression of both G6PD5 and G6PD6 was lower than that in WT plants (Figure 5D,E). As expected, the G6PD enzymatic activity in atrboh loss-of-function mutants was also lower than that in WT, especially in atrbohD1/F1 (Figure 5F).
FIGURE 5.
The response of G6PD5 and G6PD6 to salt stress through NADPH oxidases signaling pathway. (A,B) Relative transcript levels of NADPH oxidases AtrbohD and AtrbohF genes in Arabidopsis seedlings with or without 150 mM NaCl treatment. (C) The activities of NADPH oxidase in Arabidopsis WT and mutants exposed to salt treatment. (D,E) Relative transcript levels of G6PD5 and G6PD6 in WT and NADPH oxidases mutant seeds (atrbohD1, atrbohF1, and atrbohD1/F1) exposed to salt treatment. The transcript levels were normalized to Actin2 gene expression. (F) The activities of G6PD in Arabidopsis WT and mutants exposed to salt treatment. Results are averages ± SE (n = 3), bars with different letters are significantly different at the level of P < 0.05. All experiments were repeated at least three times with similar results.
cy-G6PD Affects the Intracellular NADPH Levels Under Salt Stress
As a reducing power, NADPH is the substrate of the NADPH oxidase. NADPH oxidase uses NADPH to generate superoxide, which can be dismutated subsequently to hydrogen peroxide (Wang et al., 2008; Stampfl et al., 2016; Wang et al., 2016). Moreover, the NADPH/NADP+ ratio is considered a possible mechanism for G6PD regulation (Cardi et al., 2011, 2015). Thus, NADPH is a key connector between G6PD and the ROS scavenging system. Figure 6 showed that cy-G6PD affected the intracellular NADPH and NADP+ levels and the NADPH/NADP+ ratio. Consistent with the reduced cy-G6PD activity, the intracellular NADPH level and the NADPH/NADP+ ratio were significantly decreased in g6pd5/6 mutant plants exposed to salt stress. In salt-stressed cy-G6PD-overexpression plants, the NADPH level and the NADPH/NADP+ ratio were higher than that in WT (Figure 6), indicating that G6PD is important for the intracellular NADPH homeostasis.
FIGURE 6.
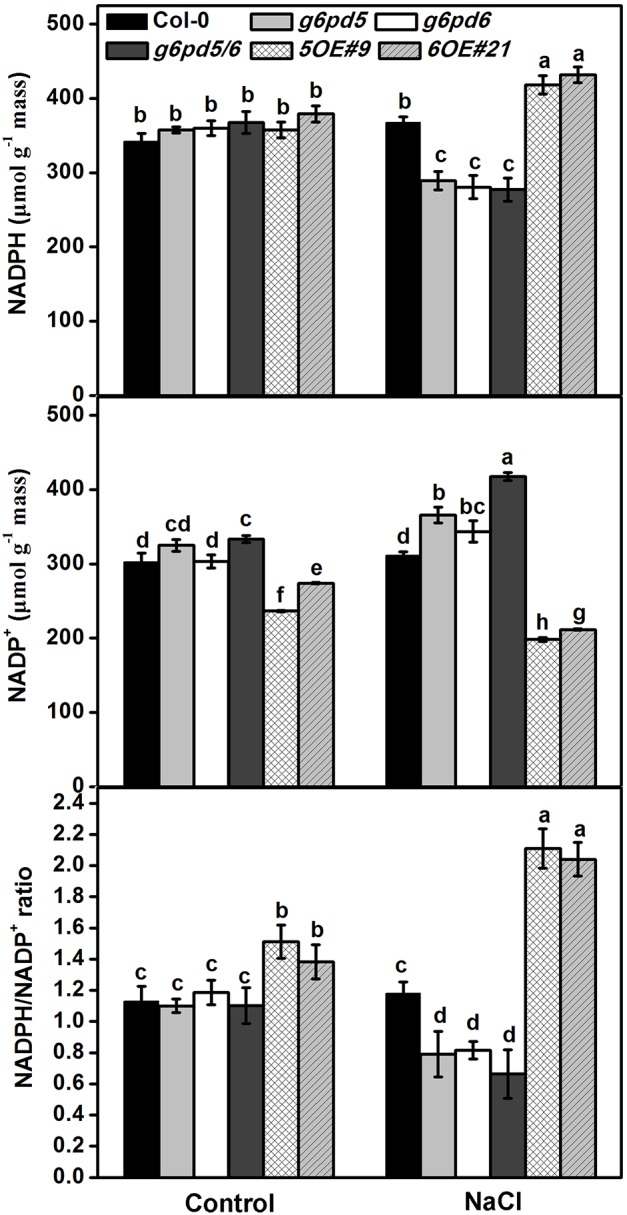
G6PD5 and G6PD6 affects NADPH content in Arabidopsis under salt stress. 10-day-old seedlings were grown vertically on ½ MS agar plates supplemented with the 150 mM NaCl for 12 h. Results are averages ± SE (n = 3), bars with different letters are significantly different at the level of P < 0.05. All experiments were repeated at least three times with similar results.
cy-G6PD Enhances the Expression of Antioxidant Responsive Genes
Antioxidant enzymes are responsive to stresses to scavenge extra ROS to maintain the balance between ROS production and scavenging (Liu et al., 2015). To investigate the effects of cy-G6PD on the expression of antioxidant enzymes, we determined the activities and expression levels of antioxidant enzymes, including APX, CAT, GR, POD, and SOD (Supplementary Figures S5A,B). The results showed that the salt stress-induced activity and expression levels of APX and GR in the g6pd5/6 mutant were significantly lower than that in WT plants (Supplementary Figures S5A,B). In contrast, the expression levels of APX and GR in OE lines were higher than that in WT (Supplementary Figures S5A,B). These results suggest that cy-G6PD involvement in the regulation of seed germination and root growth is mediated by APX and GR and that cy-G6PD enhances the capacity of plants to scavenge excessive ROS under salt stress to maintain the balance between ROS production and scavenging. The g6pd5/6 mutant is more sensitive to oxidative damages caused by salt stress because it has reduced ROS scavenging capability. These data indicate that enhanced cy-G6PD activity provides more NADPH for the antioxidant system to remove excess ROS.
cy-G6PD Enhances Glutathione Levels Under Salt Stress
Glutathione (GSH), one of the essential antioxidants and redox buffers, is involved in plant development as well as tolerance to various stresses (Wang et al., 2008). As shown in Supplementary Figure S6, the GSH content was increased by salt treatment in WT seedlings. Consistent with the role of cy-G6PD in redox regulation, the GSH level was decreased in g6pd5/6 under salt stress (Supplementary Figure S6). These results indicated that G6PD is essential for the glutathione level.
Our further analysis showed that exogenous application of ascorbate acid (ASC) or glutathione partially or fully rescued the seed germination and root growth phenotype in g6pd single and double mutants (Supplementary Figure S7). It was noteworthy that GSH was more effective than ASC (Supplementary Figure S7). In short, cy-G6PD participates in the reduction of H2O2 to H2O possibly through the glutathione peroxidase cycle or the ascorbate-glutathione cycle.
cy-G6PD Is Required for Cell Elongation and Root Meristem Maintenance
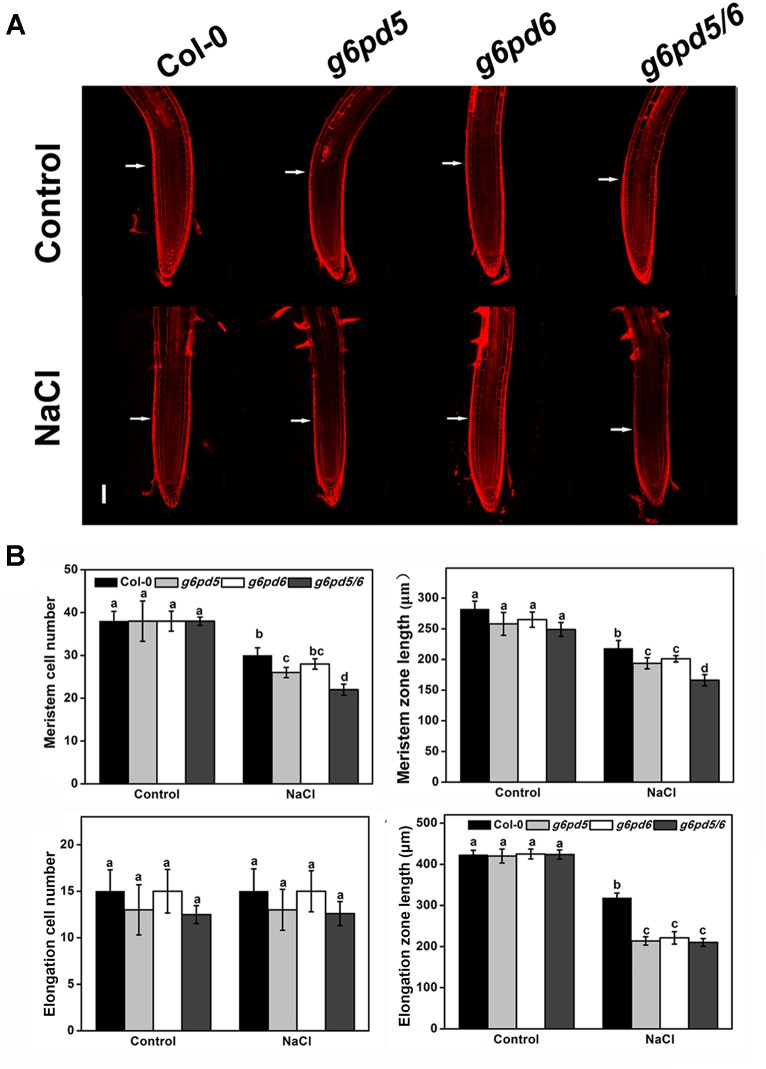
Previous studies have shown that ROS can control root elongation by loosening cell walls and inhibiting cell division (Liszkay et al., 2004; Jiao et al., 2013). To further dissect the mechanisms of cy-G6PD function in salt-repressed root growth in Arabidopsis, we measured the primary roots length in WT and cy-G6PD mutants supplied with 100 mM NaCl. The root meristem length was evaluated by determining the number of cortical cells in the region from the quiescent center (QC) to the first-elongated cell (Dello Ioio et al., 2007). The root growth of WT and mutants was similar on NaCl-free medium (Figure 7). However, g6pd mutant plants had shortened root elongation zone compared to WT after growing on NaCl-containing medium for 12 h (Figure 7A,B). These results indicate that NaCl suppresses the enlargement of the elongation zone in roots of cy-G6PD mutants relative to WT. In addition to cell elongation in the elongation zone, cell division in the root meristem zone also contributes to root growth. Therefore, we also determined the size of root apical meristem. The number of meristem cells in g6pd mutant plants was less than that in WT in the presence of NaCl, implying that cy-G6PD is required for cell division in the root meristem (Figure 7A,B).
FIGURE 7.
G6PD5 and G6PD6 regulate root meristem and elongation zone. (A) Root meristems of propidium iodide (PI)-stained images in Arabidopsis WT seedlings. The meristem zone was marked with white arrows in (A). Bars = 100 μm. (B) Root meristem cell number, meristem zone size, elongation cell number, and elogation zone size in Arabidopsis WT seedlings. The 5-day-old seedlings were treated with 100 mM NaCl for 12 h. Mean values and SE were calculated from three independent experiments (n = 20). Within each set of experiments, bars with different letters were significantly different at the 0.05 level.
Discussion
G6PDs have critical functions in plant development and stress responses (Wang et al., 2008, 2016). cy-G6PD plays a key role in plant adaptation to various stresses in several species (Dal Santo et al., 2012; Stampfl et al., 2016; Wang et al., 2016). The aim of this study was to elucidate the function and regulatory mechanism of cy-G6PD in Arabidopsis response to salt stress. The high expression level of cy-G6PD in various organs of A. thaliana suggests its important function (Supplementary Figure S2). Under normal condition, the expression of the G6PD1 and G6PD2 (both are plastidial G6PDs) in the g6pd5/6 mutants is higher than that in WT (Figure 2), suggesting that plastidial G6PDs may have function redundancy with cy-G6PDs, but this notion still needs to be further proved.
In this study, the involvement of cy-G6PD in the response of to salt stress was investigated during seed germination and root development in Arabidopsis (Figure 3). Our results showed that G6PD5 and G6PD6 play central roles in seed germination and seedling growth under unfavorable conditions. The seed germination rate of the double mutant g6pd5/6 was reduced by approximately 60% compared to WT under salt stress, implying that cy-G6PD is involved in this process (Figure 3). It was reported that the G6PD activity is increased in drought-stressed soybean seedlings and the drought-tolerant cultivar shows higher G6PD activity than the drought-sensitive cultivar (Liu et al., 2013; Wang et al., 2016). However, how stress activates the cy-G6PD activity and the regulatory roles of cy-G6PD in stress tolerance need further clarification. Thus, we characterized the g6pd5/6 mutant, which is hypersensitive to salt stress during seed germination and root elongation of seedlings. With the genetic evidence, we further determined the function of cy-G6PD in response to stress conditions. Overexpression of G6PD5 or G6PD6 results in enhanced tolerance, whereas the g6pd5/6 mutant shows attenuated tolerance compared to WT.
In addition, we demonstrated that cy-G6PD inhibited the ROS generation in the germination program under salt stress (Figure 4). ROS are the ever-present danger due to their physicochemical toxicity that they accumulate under many stress conditions (Liu et al., 2015; Wang et al., 2016). Previous reports showed that H2O2 increases the G6PD activity in red kidney bean roots and reed callus under salt stress (Wang et al., 2008; Liu et al., 2012). Furthermore, H2O2 plays a role in drought-induced increase of the total G6PD activity (Wang et al., 2016). Our results of H2O2 on G6PD5 and G6PD6 suggest that cy-G6PD is enhanced to scavenge the excessive ROS under salt stress in order to maintain the balance between ROS production and scavenging, and that the enhanced cy-G6PD activity provides more NADPH for the antioxidant system to remove excessive ROS (Figure 4).
Glutathione peroxidase cycle and ascorbate-glutathione cycle can catalyze the reduction of H2O2 to water. We investigated the role of cy-G6PD in regulating the levels of reduced form of glutathione (GSH) under salt stress and found that G6PD is involved in GSH maintenance and H2O2 accumulation (Supplementary Figures S5, S6). In plants, NADPH could be generated by ferredoxin-NADP reductase and four NADP-dehydrogenases: G6PD, 6-phosphogluconate dehydrogenase (6PGD), NADP-isocitrate dehydrogenase, and the NADP-malic enzyme (Leterrier et al., 2016). Loss-of-function of cy-G6PD dramatically decreases the intracellular NADPH level and the NADPH/NADP+ ratio under salt stress (Figure 6), suggesting that G6PD contributes to the major part of NADPH production in Arabidopsis seedlings. A similar decrease in the GSH content was observed (Supplementary Figure S6). Overexpression of cy-G6PD leads to the enhanced GSH pool and oxidative tolerance by providing more NADPH. From the above results, we concluded that cy-G6PD, the major contributor to the total G6PD activity, is the key factor for maintaining intracellular GSH and NADPH levels under salt stress; disruption of the NADPH and GSH homeostasis resulted in oxidative damages in Arabidopsis seedlings.
Understanding the roles of G6PD and NADPH oxidases will increase our knowledge of the plant ROS network in developmental and physiological challenges. As key ROS-generating enzymes, NADPH oxidases AtrbohD and AtrbohF are essential components for numerous biological processes (Chaouch et al., 2012; Jiang et al., 2013). The expression and activities of the NADPH oxidases are markedly increased by salt treatment (Figure 5). This is consistent with previous findings that an NADPH oxidase inhibitor (DPI) interfered with a defense-induced ROS burst after salt stress, and that the Arabidopsis double mutant rbohD/F exhibits decreased cy-G6PD enzymatic activities. These results suggest that cy-G6PD is involved in RBOH-dependent ROS production in salt-stressed seedlings.
In plants exposed to high salinity, G6PD contributes to ROS detoxification and the maintenance of cellular redox balance (Dal Santo et al., 2012). However, in addition to their damaging role in plants challenged by prolonged salt stress, ROS also have important signaling functions. RBOHD is involved in regulating ROS signaling in response to salinity (Miller et al., 2009, 2010; Baxter et al., 2014). cy-G6PD might also be involved in RBOH-dependent ROS production and signaling in salt-stressed plants (Stampfl et al., 2016). In this study, the expression of NADPH oxidase genes AtrbohD and AtrbohF in salt-induced g6pd5/6 seedlings is higher than that in control plants, however, expression levels of APX and GR in the g6pd5/6 mutant is significantly lower than that in WT plants. These results suggest that the high levels of ROS in g6pd5/6 plants may be sufficient to activate antioxidative defense systems. Undoubtedly, the dual role of cy-G6PD in ROS scavenging and generation in Arabidopsis still needs to be further illustrated.
Conclusion
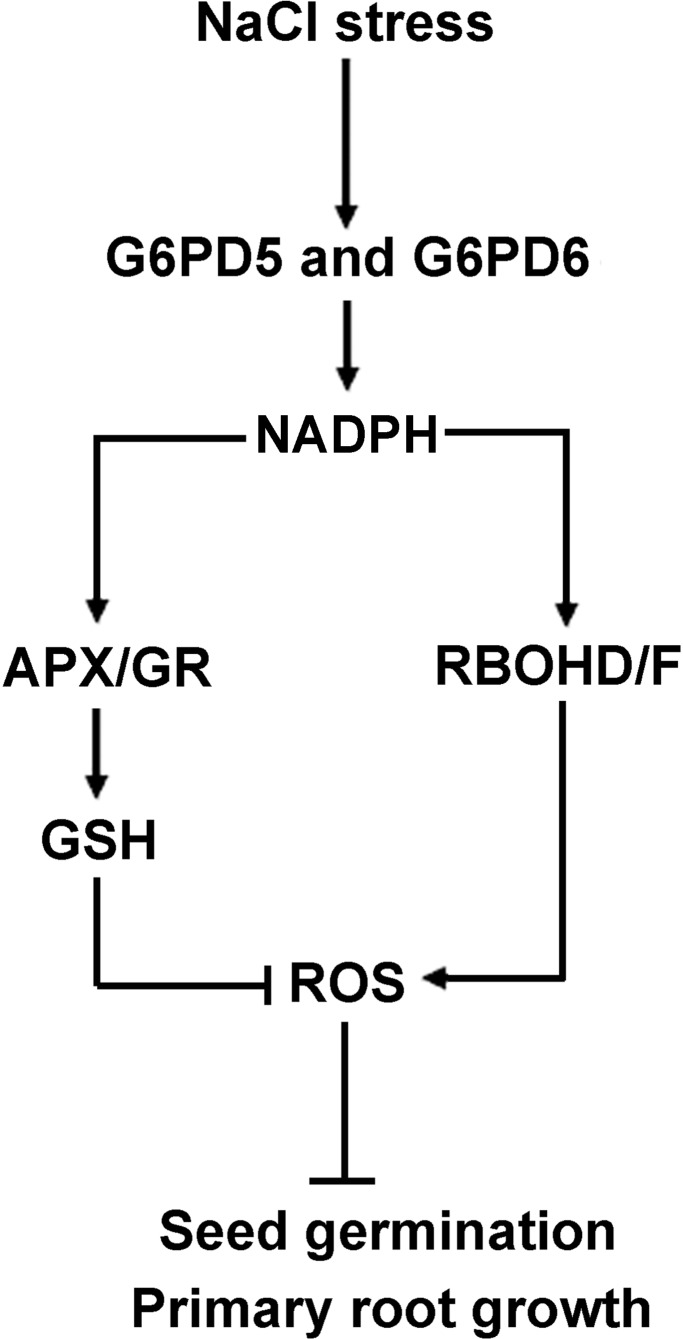
Our results showed that H2O2, NADPH, RBOHD/F, APX/GR, and GSH are required for salt-induced cy-G6PD gene function, and that the enhanced cy-G6PD plays an important role against oxidative stress by increasing the ASC and GSH levels, which in turn dampen ROS accumulation. Our findings point to a different node of this crosstalk that is activated by an increase in the cytosolic H2O2 and that is involved in dormancy, germination control, and the stress responsiveness of seeds. Based on the results presented here, we proposed a hypothetical model shown in Figure 8. In this model, salt stress induces cy-G6PD. The enhanced cy-G6PD is involved in regulating key enzymes (APX and GR) in ASC-GSH cycle by utilizing NADPH, which eventually results in the increased ASC and GSH levels. The enhanced antioxidant ability can maintain a steady-state level of H2O2 in cells, thus avoiding ROS damages. cy-G6PD is also involved in RBOH-dependent ROS production in salt-stressed seedlings. Moreover, cy-G6PD is involved in root apical meristem (RAM) maintenance through the glutathione redox-affected ROS pathway.
FIGURE 8.
Schematic illustration of a proposed model for the link between G6PD5, G6PD6, ROS, and APX-GR in Arabidopsis seed germination and root growth. In this model, arrows indicate positive regulation, bars indicate negative regulation. Salt stress induces cy-G6PD, which subsequently maintain the intracellular NADPH homeostasis, and involved in regulating key enzymes (APX and GR) in ASC-GSH cycle. The APX and GR inhibit the level of H2O2 in cells through GSH content. cy-G6PD is involved in H2O2 accumulation through applying NADPH to PM NADPH oxidase. The enhanced cy-G6PD thus control germination of Arabidopsis seeds and growth of Arabidopsis primary roots.
Author Contributions
LY, YB, XW, and WN conceived and designed the experiments. LY, NC, SW, MR, LS, and SL performed the experiments. LY analyzed the data. LY and YB wrote the manuscript. All authors reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This work was supported by the National Natural Science Foundation of China (31671595 and 31670244), Foundation of Science and Technology Program of Gansu Province (1506RJZA209), the Agricultural Biotechnology Research and Application Development Program of Gansu Province (GNSW-2016-23), the Fundamental Research Funds for the Central Universities (lzujbky-2016-80), the Foundation of Science and Technology Program of Lanzhou City (2015-3-53), the Project of Qinghai Science & Technology Department (2016-ZJ-Y01), and the Open Project of State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University (201 -KF-05).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2019.00182/full#supplementary-material
References
- Apel K., Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55 373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- Bailly C., El-Maarouf-Bouteau H., Corbineau F. (2008). From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C. R. Biol. 331 806–814. 10.1016/j.crvi.2008.07.022 [DOI] [PubMed] [Google Scholar]
- Baxter A., Mittler R., Suzuki N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65 1229–1240. 10.1093/jxb/ert375 [DOI] [PubMed] [Google Scholar]
- Cardi M., Castiglia D., Ferrara M., Guerriero G., Chiurazzi M., Esposito S. (2015). The effects of salt stress cause a diversion of basal metabolism in barley roots: possible different roles for glucose-6-phosphate dehydrogenase isoforms. Plant Physiol. Biochem. 86 44–54. 10.1016/j.plaphy.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Cardi M., Chibani K., Cafasso D., Rouhier N., Jacquot J. P., Esposito S. (2011). Abscisic acid effects on activity and expression of barley (Hordeum vulgare) plastidial glucose-6-phosphate dehydrogenase. J. Exp. Bot. 62 4013–4023. 10.1093/jxb/err100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouch S., Queval G., Noctor G. (2012). AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 69 613–627. 10.1111/j.1365-313X.2011.04816.x [DOI] [PubMed] [Google Scholar]
- Chen C., Letnik I., Hacham Y., Dobrev P., Ben-Daniel B. H., Vankova R., et al. (2014a). ASCORBATE PEROXIDASE6 protects Arabidopsis desiccating and germinating seeds stress from species mediates cross talk between reactive oxygen, acid abscisic, and auxin. Plant Physiol. 166 370–383. 10.1104/pp.114.245324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Twito S., Miller G. (2014b). New cross talk between ROS, ABA and auxin controlling seed maturation and germination unraveled in APX6 deficient Arabidopsis seeds. Plant Signal. Behav. 9:e976489. 10.4161/15592324.2014.976489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Santo S., Stampfl H., Krasensky J., Kempa S., Gibon Y., Petutschnig E., et al. (2012). Stress-induced GSK3 regulates the redox stress response by phosphorylating glucose-6-phosphate dehydrogenase in Arabidopsis. Plant Cell 24 3380–3392. 10.1105/tpc.112.101279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R., Linhares F. S., Scacchi E., Casamitjana-Martinez E., Heidstra R., Costantino P., et al. (2007). Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 17 678–682. 10.1016/j.cub.2007.02.047 [DOI] [PubMed] [Google Scholar]
- Dunand C., Crevecoeur M., Penel C. (2007). Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol. 174 332–341. 10.1111/j.1469-8137.2007.01995.x [DOI] [PubMed] [Google Scholar]
- Esposito S., Massaro G., Vona V., Di Martino Rigano V., Carfagna S. (2003). Glutamate synthesis in barley roots: the role of the plastidic glucose-6-phosphate dehydrogenase. Planta 216 639–647. 10.1007/s00425-002-0892-4 [DOI] [PubMed] [Google Scholar]
- Hasegawa P., Bressan R., Zhu J., Bohnert H. (2000). Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51 463–499. 10.1146/annurev.arplant.51.1.463 [DOI] [PubMed] [Google Scholar]
- Huan L., Xie X., Zheng Z., Sun F., Wu S., Li M., et al. (2014). Positive correlation between PSI response and oxidative pentose phosphate pathway activity during salt stress in an intertidal macroalga. Plant Cell Physiol. 55 1395–1403. 10.1093/pcp/pcu063 [DOI] [PubMed] [Google Scholar]
- Hutchings D., Rawsthorne S., Emes M. J. (2005). Fatty acid synthesis and the oxidative pentose phosphate pathway in developing embryos of oilseed rape (Brassica napus L.). J. Exp. Bot. 56 577–585. 10.1093/jxb/eri046 [DOI] [PubMed] [Google Scholar]
- Jia N., Lv T. T., Li M. X., Wei S. S., Li Y. Y., Zhao C. L., et al. (2016). The J-protein AtDjB1 is required for mitochondrial complex I activity and regulates growth and development through ROS-mediated auxin signalling. J. Exp. Bot. 67 3481–3496. 10.1093/jxb/erw171 [DOI] [PubMed] [Google Scholar]
- Jiang C., Belfield E. J., Cao Y., Smith J. A., Harberd N. P. (2013). An Arabidopsis soil-salinity-tolerance mutation confers ethylene-mediated enhancement of sodium/potassium homeostasis. Plant Cell 25 3535–3552. 10.1105/tpc.113.115659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Sun L., Song Y., Wang L., Liu L., Zhang L., et al. (2013). AtrbohD and AtrbohF positively regulate abscisic acid-inhibited primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis. J. Exp. Bot. 64 4183–4192. 10.1093/jxb/ert228 [DOI] [PubMed] [Google Scholar]
- Kruger N. J., von Schaewen A. (2003). The oxidative pentose phosphate pathway: structure and organisation. Curr. Opin. Plant Biol. 6 236–246. 10.1016/s1369-5266(03)00039-6 [DOI] [PubMed] [Google Scholar]
- Kwak J. M., Nguyen V., Schroeder J. I. (2006). The role of reactive oxygen species in hormonal responses. Plant Physiol. 141 323–329. 10.1104/pp.106.079004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi S., Nurcato R., De Lillo A., Lentini M., Grillo S., Esposito S. (2016). Glucose-6-phosphate dehydrogenase plays a central role in the response of tomato (Solanum lycopersicum) plants to short and long-term drought. Plant Physiol. Biochem. 105 79–89. 10.1016/j.plaphy.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Leterrier M., Barroso J. B., Valderrama R., Begara-Morales J. C., Sanchez-Calvo B., Chaki M., et al. (2016). Peroxisomal NADP-isocitrate dehydrogenase is required for Arabidopsis stomatal movement. Protoplasma 253 403–415. 10.1007/s00709-015-0819-0 [DOI] [PubMed] [Google Scholar]
- Leymarie J., Vitkauskaite G., Hoang H. H., Gendreau E., Chazoule V., Meimoun P., et al. (2012). Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 53 96–106. 10.1093/pcp/pcr129 [DOI] [PubMed] [Google Scholar]
- Li J. S., Chen G. C., Wang X. M., Zhang Y. L., Jia H. L., Bi Y. R. (2011). Glucose-6-phosphate dehydrogenase-dependent hydrogen peroxide production is involved in the regulation of plasma membrane H+-ATPase and Na+/H+ antiporter protein in salt-stressed callus from Carex moorcroftii. Physiol. Plant 141 239–250. 10.1111/j.1399-3054.2010.01429.x [DOI] [PubMed] [Google Scholar]
- Liszkay A., van der Zalm E., Schopfer P. (2004). Production of reactive oxygen intermediates O2.-, H2O2, and.OH by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 136 3114–3123; discussion 3001. 10.1104/pp.104.044784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang X., Hu Y., Hu W., Bi Y. (2013). Glucose-6-phosphate dehydrogenase plays a pivotal role in tolerance to drought stress in soybean roots. Plant Cell Rep. 32 415–429. 10.1007/s00299-012-1374-1 [DOI] [PubMed] [Google Scholar]
- Liu R., Liu Y., Ye N., Zhu G., Chen M., Jia L., et al. (2015). AtDsPTP1 acts as a negative regulator in osmotic stress signalling during Arabidopsis seed germination and seedling establishment. J. Exp. Bot. 66 1339–1353. 10.1093/jxb/eru484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wan Q., Wu R., Wang X., Wang H., Wang Z., et al. (2012). Role of hydrogen peroxide in regulating glucose-6-phosphate dehydrogenase activity under salt stress. Biol. Plant. 56 313–320. 10.1007/s10535-012-0092-4 [DOI] [Google Scholar]
- Liu Y., Wu R., Wan Q., Xie G., Bi Y. (2007). Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol. 48 511–522. 10.1093/pcp/pcm020 [DOI] [PubMed] [Google Scholar]
- Ma L., Zhang H., Sun L., Jiao Y., Zhang G., Miao C., et al. (2012). NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J. Exp. Bot. 63 305–317. 10.1093/jxb/err280 [DOI] [PubMed] [Google Scholar]
- Marino D., Dunand C., Puppo A., Pauly N. (2012). A burst of plant NADPH oxidases. Trends Plant Sci. 17 9–15. 10.1016/j.tplants.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Mei Y., Jia W. J., Chu Y. J., Xue H. W. (2012). Arabidopsis phosphatidylinositol monophosphate 5-kinase 2 is involved in root gravitropism through regulation of polar auxin transport by affecting the cycling of PIN proteins. Cell Res. 22 581–597. 10.1038/cr.2011.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Holscher C., Schwoppe C., von Schaewen A. (2011). Alternative targeting of Arabidopsis plastidic glucose-6-phosphate dehydrogenase G6PD1 involves cysteine-dependent interaction with G6PD4 in the cytosol. Plant J. 66 745–758. 10.1111/j.1365-313X.2011.04535.x [DOI] [PubMed] [Google Scholar]
- Miller G., Schlauch K., Tam R., Cortes D., Torres M. A., Shulaev V., et al. (2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2:ra45. 10.1126/scisignal.2000448 [DOI] [PubMed] [Google Scholar]
- Miller G., Suzuki N., Ciftci-Yilmaz S., Mittler R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33 453–467. 10.1111/j.1365-3040.2009.02041.x [DOI] [PubMed] [Google Scholar]
- Nan W., Wang X., Yang L., Hu Y., Wei Y., Liang X., et al. (2014). Cyclic GMP is involved in auxin signalling during Arabidopsis root growth and development. J. Exp. Bot. 65 1571–1583. 10.1093/jxb/eru019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niforou K., Cheimonidou C., Trougakos L. P. (2014). Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol. 2 323–332. 10.1016/j.redox.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G., Foyer C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 249–279. 10.1146/annurev.arplant.49.1.249 [DOI] [PubMed] [Google Scholar]
- Parkhey S., Naithani S. C., Keshavkant S. (2012). ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol. Biochem. 57 261–267. 10.1016/j.plaphy.2012.06.008 [DOI] [PubMed] [Google Scholar]
- Potocky M., Jones M. A., Bezvoda R., Smirnoff N., Zarsky V. (2007). Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 174 742–751. 10.1111/j.1469-8137.2007.02042.x [DOI] [PubMed] [Google Scholar]
- Rajjou L., Duval M., Gallardo K., Catusse J., Bally J., Job C., et al. (2012). Seed germination and vigor. Annu. Rev. Plant Biol. 63 507–533. 10.1146/annurev-arplant-042811-105550 [DOI] [PubMed] [Google Scholar]
- Rosado A., Amaya I., Valpuesta V., Cuartero J., Botella M. A., Borsani O. (2006). ABA- and ethylene-mediated responses in osmotically stressed tomato are regulated by the TSS2 and TOS1 loci. J. Exp. Bot. 57 3327–3335. 10.1093/jxb/erl094 [DOI] [PubMed] [Google Scholar]
- Sagi M., Davydov O., Orazova S., Yesbergenova Z., Ophir R., Stratmann J. W., et al. (2004). Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16 616–628. 10.1105/tpc.019398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfl H., Fritz M., Dal Santo S., Jonak C. (2016). The GSK3/shaggy-like kinase ASKalpha contributes to pattern-triggered immunity. Plant Physiol. 171 1366–1377. 10.1104/pp.15.01741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao S., Benning C. (2005). Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis. Plant J. 41 243–256. 10.1111/j.1365-313X.2004.02293.x [DOI] [PubMed] [Google Scholar]
- Wang H., Yang L., Li Y., Hou J., Huang J., Liang W. (2016). Involvement of ABA- and H2O2-dependent cytosolic glucose-6-phosphate dehydrogenase in maintaining redox homeostasis in soybean roots under drought stress. Plant Physiol. Biochem. 107 126–136. 10.1016/j.plaphy.2016.05.040 [DOI] [PubMed] [Google Scholar]
- Wang X., Ma Y., Huang C., Wan Q., Li N., Bi Y. (2008). Glucose-6-phosphate dehydrogenase plays a central role in modulating reduced glutathione levels in reed callus under salt stress. Planta 227 611–623. 10.1007/s00425-007-0643-7 [DOI] [PubMed] [Google Scholar]
- Wang Y., Law S. R., Ivanova A., van Aken O., Kubiszewski-Jakubiak S., Uggalla V., et al. (2014). The mitochondrial protein import component, TRANSLOCASE OF THE INNER MEMBRANE17-1, plays a role in defining the timing of germination in Arabidopsis. Plant Physiol. 166 1420–1435. 10.1104/pp.114.245928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Zhang L., Hao H., Zhang P., Zhu H., Cheng W., et al. (2015). Nuclear-localized AtHSPR links abscisic acid-dependent salt tolerance and antioxidant defense in Arabidopsis. Plant J. 84 1274–1294. 10.1111/tpj.13080 [DOI] [PubMed] [Google Scholar]
- Zhu J. (2001). Plant salt tolerance. Trends Plant Sci. 6 66–71. 10.1016/S1360-1385(00)01838-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.