Abstract
This study concerned the controlled synthesis of periodic glycopolymers by reversible addition-fragmentation chain transfer (RAFT) copolymerization. To this end, maltose- and lactose-substituted vinyl ethers (MalVE and LacVE, respectively) and maltose-substituted maleimide (MalMI) were newly synthesized. RAFT copolymerization of MalVE and ethyl maleimide (EtMI) (monomer feed ratio: MalVE:EtMI = 1:1) afforded periodic glycopolymers (poly(MalVE-co-EtMI)) consisting of major parts of alternating structure (-(MalVE-EtMI)n-) and a small part of consecutive sequences of EtMI (–EtMI-EtMI-). Occurrence of the latter sequences was caused by the homopolymerizability of maleimide under the present polymerization condition, and the formation of the consecutive sequences of EtMI was successfully suppressed by varying the monomer feed ratio. RAFT copolymerization of LacVE and EtMI was also found to proceed and similarly yielded periodic glycopolymers (poly(LacVE-co-EtMI)). Moreover, RAFT copolymerization of LacVE and MalMI (monomer feed ratio: LacVE:MalMI = 1:1) was performed to give copolymers (poly(LacVE-co-MalMI)) having composition ratio of LacVE/MalMI ≈ 36/64. The resultant periodic glycopolymers poly(MalVE-co-EtMI) and poly(LacVE-co-EtMI) were subjected to lectin binding assay using concanavalin A and peanut agglutinin, exhibiting the glycocluster effect. Moreover, these glycopolymers obtained from the copolymerization of VE and MI were found to be non-cytotoxic.
Keywords: periodic glycopolymer, alternating glycopolymer, RAFT copolymerization, carbohydrate-substituted vinyl ether, carbohydrate-substituted maleimide, glycosaminoglycan mimics, CuAAC click reaction
1. Introduction
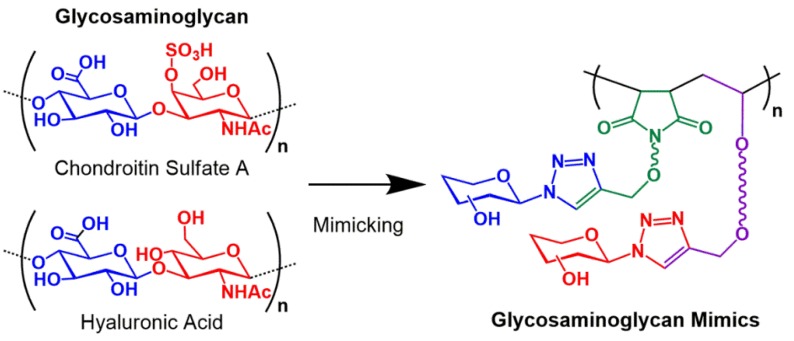
Glycosaminoglycans (GAGs) are known as physiologically active polysaccharides being ubiquitous both in the extracellular matrix and on cell surfaces, and play key roles in wide-ranging life phenomena as well as in diseases affecting mammalians [1,2,3]. From the view of structural aspect, GAGs are long, linear, unbranched, and negatively-charged polysaccharides composed of repeating disaccharide units consisting of an amino sugar and an uronic acid. In addition, major GAGs are sulfated and distinguished from each other by the disaccharides structures and various sulfation patterns [1,2,3]. Thus, GAGs are naturally occurring alternating copolymers. The synthesis of GAG mimics based on glycopolymers by both conventional and controlled polymerizations have been reported [4,5,6,7,8,9,10]. Particularly, in the last decade, with the remarkable development in precisely controlled radical polymerization such as ATRP and RAFT polymerization techniques [11,12,13,14,15,16,17], various multi-component glycopolymers with two or more carbohydrate-substituted repeating units have been synthesized and investigated as GAG mimics [5,6,7,8]. In the syntheses of GAG-mimetic glycopolymers thus far reported, the most important subjects are the incorporation of sulfated carbohydrate moieties into glycopolymer structure and regulating of the density of the sulfate groups for exhibiting physiological functions. Consequently, sequence control of the repeating units in the multi-component glycopolymers are still beyond our reach. This fact prompted us to develop a novel synthetic strategy for forming sequence-controlled glycopolymers, more specifically, glycopolymers having alternating structure. Until now, few examples have been reported about the sequence-control in glycopolymers; however, they are not alternating structure but short-block ones [18]. To achieve the alternating structure, we focused on the copolymerization of an electron-rich and an electron-deficient vinyl monomers, which have been widely recognized to afford alternating copolymers via radical polymerization mechanism [19,20,21,22,23,24,25,26]. To this end, vinyl ethers (VEs) and maleimide (MI) monomers having pendant unprotected carbohydrate moieties were newly designed by utilizing copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) click reaction between alkyne-substituted VE and MI and disaccharide azides, respectively. Here, the employed maltosyl and lactosyl azide were obtained by Shoda’s activation of unprotected sugars employing 2-chloro-1,3-dimethylimidazolinium chloride [27,28]. Disaccharide-substituted VEs and MI correspond to electron-rich and electron-deficient vinyl monomers, respectively. In addition to controlling the repeat-unit sequences, we aimed to control molecular weight and molecular weight distribution by applying RAFT copolymerization to the synthetic protocol of glycopolymers. In this study, RAFT copolymerization of maltose-substituted VE (MalVE) and ethyl maleimide (EtMI) was firstly investigated to obtain periodic glycopolymers, where MalVE unit are distributed as every other unit, and the polymerization conditions was optimized to attain a more accurate control in alternating structure. Synthesis of periodic polymers by RAFT copolymerization of naturally occurring vinyl monomers and MI derivatives were investigated by Sato and Kamigaito to yield various periodically functionalized polymers [29,30]; however, synthesis of periodically carbohydrate-functionalized polymers (periodic or alternating glycopolymers) have not yet been reported. Therefore, we demonstrated RAFT copolymerization of lactose-substituted VE (LacVE) and maltose-substituted MI (MalMI) to obtain glycopolymers periodically carrying two kinds of disaccharide residues in the pendants. This would be the first prototype of GAG-mimetic glycopolymers focusing on the sequence-control (Figure 1). In addition, specific interactions with lectins of the resultant periodic glycopolymers were examined by lectin binding assay. To confirm the potential applicability of the glycopolymers in this study as promising materials in biomedical and pharmaceutical field, cytotoxicity assessment was performed.
Figure 1.
Concept of glycosaminoglycan mimicking in this study.
2. Materials and Methods
2.1. Chemicals and Reagents
D(+)-maltose monohydrate (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan, 98.0%), D(+)-lactose monohydrate (FUJIFILM Wako Pure Chemical Corporation), sodium azide (FUJIFILM Wako Pure Chemical Corporation, 98.0%), 2-chloro-1,3-dimethylimidazolinium chloride (DMC; FUJIFILM Wako Pure Chemical Corporation, 97.0%), N,N-diisopropyl ethylamine (DIPEA; TCI, Tokyo, Japan, 98.0%), copper (II) sulfate pentahydrate (FUJIFILM Wako Pure Chemical Corporation, 99.5%), l-ascorbic acid sodium salt (AscNa; FUJIFILM Wako Pure Chemical Corporation, 98.0%), [(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan, 97.0%), N-ethylmaleimide (EtMI; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan, 98%), and 2,2′-azobis-[2-(2-imidazolin-2-yl)-propane] dihydrochlroride (VA-044; FUJIFILM Wako Pure Chemical Corporation, 97%) were used as received. Pre-wetted dialysis tubing with MWCO = 1 KD (Spectra/Por 7; nominal flat width: 18 mm, diameter 11.5 mm, volume: 1.1 mL/cm, length: 10 m) (Spectrum, Rancho Dominguez, CA, USA) was performed in a 1 L beaker by changing distilled water four or five times over a period of 24 h. Fluorescein isothiocyanate (FITC)-labeled Concanavalin A (Con A) from Canavalia ensiformis and FITC-labeled peanut agglutinin (PNA) from Arachis hypogaea were purchased from J-Oil Mills. Inc. (Tokyo, Japan) All other reagents were commercially available and used without further purification. Synthesis of N-propargylmaleimide was carried out according to the procedure reported in the literature [31]. Maltosyl azide (MalN3) and lactosyl azide (LacN3) were prepared according to the literature [27,28]. A chain transfer agent 2-(benzylsulfanylthiocarbonylsulfanyl) ethanol (BTSE) was synthesized using 2-mercaptoethanol, carbon disulfide, and benzyl bromide according to the procedure reported in the literature [32]. Synthesis of 3-[2-(2-vinyloxyethoxy)-ethoxy]-propyne (VEEP) was carried out by a reaction of 2-(vinyloxyethoxy)-ethanol with propargyl bromide in the presence of KOH in DMSO at room temperature for 40 h [33].
2.2. Methods
1H and 13C NMR spectra were recorded at 25 °C on a Bruker model AC-500 spectrometer (Bruker, Billerica, MA, USA), operating at 500 and 125 MHz, respectively, where chemical shifts (δ in ppm) were determined with respect to non-deuterated solvent residues as internal standards. Analytical size exclusion chromatography (SEC) was performed in 0.2 mol·L−1 NaNO3 aqueous solution at 40 °C, using 7.8 mm × 300 mm gel columns (TOSOH TSKgel α–3000 × 3) on a JASCO model PU2089 (JASCO, Hachioji, Japan) equipped with a UV-2075 variable-wavelength UV-vis detector (JASCO) and an RI-2031 RI detector (JASCO). The number-average molecular weight (Mn) and polydispersity ratio (Mw/Mn) were calculated from the chromatographs with respect to poly(ethylene glycol)s standards (Scientific Polymer Products, Inc., Ontario, NY, USA); Mn = 590−11,900 g/mol, Mw/Mn = 1.05–1.11). Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry was performed on a BRUKER model AutoFlex III MALDI-TOF/TOF (Bruker) using 2,5-dihydroxybenzoic acid as a matrix. Fluorescence emission spectra were recorded on a JASCO Type FP-6500 spectrometer (JASCO).
2.3. Synthesis of MalVE
To MalN3 (570 mg, 1.6 mmol) was added the solution of CuSO4·5H2O (39 mg, 0.15 mmol) and AscNa (61 mg, 0.31 mmol) in H2O (14 mL), then the solution of TBTA (82 mg, 0.15 mmol) and VEEP (340 mg, 2.0 mmol) in DMF (14 mL) was added, and the mixture was stirred for 19 h at 25 °C. After concentration of the reaction mixture under reduced pressure, the product was purified by silica gel column chromatography (H2O/acetonitrile = 1/6, v/v) and then stirred with metal scavenger (SiliaMetS® Imidazole, Silicycle Inc., Québec, QC, Canada)420 mg, 5 equiv. for Cu) overnight at 25 °C. After removing of metal scavenger by filtration, the filtrate was concentrated under reduced pressure, and the residue was subject to column chromatography (H2O/acetonitrile = 1/9, v/v) on SiO2, followed by freeze-dried, to allow isolation of MalVE as a white powder (530 mg, 1.0 mmol, 65%). 1H NMR (500 MHz, D2O): δ (ppm) 8.29 (s, 1H, triazole), 6.52 (dd, J1 = 14.3 Hz, J2 = 6.9 Hz, 1H, CH2=CH), 5.80 (d, 1H, J = 8.6 Hz, H1), 5.59 (d, 1H, J = 3.8 Hz, H1′), 4.34 (dd, J1 = 14.4 Hz, J2 = 2.2 Hz, 1H, CH2=CH), 4.16 (dd, J1 = 6.9 Hz, J2 = 2.2 Hz, 1H, CH2=CH), 4.1–3.3 (m, 20H); 13C NMR (125 MHz, D2O) δ (ppm) 151.9, 143.9, 123.1, 100.9, 87.2, 87.0, 79.1, 78.0, 76.6, 73.6, 73.3, 72.4, 71.6, 69.9, 69.8, 69.1, 68.9, 67.3, 63.5, 60.8, 60.3.
2.4. Synthesis of LacVE
To LacN3 (570 mg, 1.6 mmol) was added the solution of CuSO4·5H2O (39 mg, 0.15 mmol) and AscNa (61 mg, 0.31 mmol) in H2O (14 mL), then the solution of TBTA (82 mg, 0.15 mmol) and VEEP (340 mg, 2.0 mmol) in DMF (14 mL) was added, and the mixture was stirred for 19 h at 25 °C. After concentration of the reaction mixture under reduced pressure, the product was purified by silica gel column chromatography (H2O/acetonitrile = 1/6, v/v) and then stirred with metal scavenger (SiliaMetS® Imidazole, 420 mg, 5 equiv. for Cu) overnight at 25 °C. After removing of metal scavenger by filtration, the filtrate was concentrated under reduced pressure, the residue was subject to column chromatography (H2O/acetonitrile = 1/9, v/v) on SiO2, followed by freeze-dried, to allow isolation of LacVE as a white powder (530 mg, 1.0 mmol, 65%).1H NMR (500 MHz, D2O): δ (ppm) 8.29 (s, 1H, triazole), 6.51 (dd, J1 = 14.3 Hz, J2 = 6.9 Hz, 1H, CH2=CH), 5.80 (d, 1H, J = 9.3 Hz, H1), 4.52 (d, 1H, J = 7.8 Hz, H1′), 4.35 (dd, J1 = 14.4 Hz, J2 = 2.2 Hz, 1H, CH2=CH), 4.16 (dd, J1 = 6.9 Hz, J2 = 2.2 Hz, 1H, CH2=CH), 4.1–3.3 (m, 20H); 13C NMR (125 MHz, D2O) δ (ppm) 151.2, 144.3, 124.3, 102.9, 88.1, 87.3, 77.7, 77.3, 75.4, 74.5, 72.5, 72.0, 71.0, 69.5, 68.9, 68.6, 67.3, 63.0, 61.1, 59.7.
2.5. Synthesis of MalMI
To MalN3 (400 mg, 1.0 mmol) was added the solution of CuSO4·5H2O (27 mg, 0.10 mmol) and AscNa (44 mg, 0.15 mmol) in H2O (10 mL), then the solution of TBTA (59 mg, 0.10 mmol) and N-propargylmaleimide (180 mg, 1.3 mmol) in DMF (10 mL) was added, and the mixture was stirred for 15 h at 25 °C. After concentration of the reaction mixture under reduced pressure, the residue was subject to column chromatography (H2O/acetonitrile = 1/6, v/v) on SiO2, (H2O/acetonitrile = 1/3, v/v) on Al2O3, and (H2O/acetonitrile = 1/9, v/v) on SiO2, followed by freeze-dried, to allow isolation of MalMI as a white powder (190 mg, 0.35 mmol, 35%). 1H NMR (500 MHz, D2O): δ (ppm) 8.23 (s, 1H, triazole), 6.90 (s, 2H, maleimide), 5.75 (d, 1H, J = 8.6 Hz, H1), 5.49 (d, 1H, J = 3.8 Hz, H1′), 4.00–3.27 (m, 14H); 13C NMR (125 MHz, D2O) δ (ppm) 172.2, 143.1, 134.6, 123.5, 99.6, 87.3, 77.4, 76.3, 75.7, 72.8, 72.7, 72.1, 71.7, 69.3, 60.5, 60.4, 32.1.
2.6. Copolymerization of MalVE and EtMI under Conventional Radical Polymerization Conditions
Conventional radical copolymerization of MalVE and EtMI was carried out with VA-044 as an initiator. To a solution of MalVE (38 mg, 70 μmol) and EtMI (8.8 mg, 70 μmol) in H2O (0.16 mL) and acetonitrile (0.12 mL) was added VA-044 (0.5 mg, 1.4 μmol) in a glass tube ([MalVE]0/[EtMI]0/[VA-044]0 = 50/50/1). The resulting solution was degassed by three freeze–pump–thaw cycles, then the glass tube was sealed under vacuum, heated at 60 °C for 1 h and quenched by rapid cooling. The reaction mixture was analyzed by SEC and 1H NMR spectroscopy. The products were purified by dialysis against distilled water and freeze-dried to give copolymer.
2.7. Copolymerization of MalVE and EtMI under RAFT Polymerization Conditions
RAFT copolymerization of MalVE and EtMI was carried out with BTSE as a chain transfer agent and VA-044 as an initiator. To a solution of MalVE (220 mg, 410 μmol), EtMI (51 mg, 410 μmol) and BTSE (2.0 mg, 8.2 μmol) in H2O (0.97 mL) and acetonitrile (0.72 mL) was added VA-044 (2.6 mg, 8.2 μmol) in a glass tube ([MalVE]0/[EtMI]0/[VA-044]0/[BTSE]0 = 50/50/1/1). The resulting solution was degassed by three freeze–pump–thaw cycles, then the glass tube was sealed under vacuum, heated at 60 °C for 15–120 min and quenched by rapid cooling. The reaction mixture was analyzed by SEC and 1H NMR spectroscopy. The products were purified by dialysis against distilled water and freeze-dried to give copolymer.
2.8. Copolymerization of LacVE and EtMI under RAFT Polymerization Conditions
RAFT copolymerization of LacVE and EtMI was carried out with BTSE as a chain transfer agent and VA-044 as an initiator. To a solution of LacVE (81 mg, 150 μmol), EtMI (19 mg, 150 μmol) and BTSE (0.7 mg, 3.0 μmol) in H2O (0.34 mL) and acetonitrile (0.26 mL) was added VA-044 (1.0 mg, 3.0 μmol) in a glass tube ([LacVE]0/[EtMI]0/[VA-044]0/[BTSE]0 = 50/50/1/1). The resulting solution was degassed by three freeze–pump–thaw cycles, then the glass tube was sealed under vacuum, heated at 60 °C for 10–80 min and quenched by rapid cooling. The reaction mixture was analyzed by SEC and 1H NMR spectroscopy. The products were purified by dialysis against distilled water and freeze-dried to give copolymer.
2.9. Copolymerization of LacVE and MalMI under RAFT Polymerization Conditions
RAFT copolymerization of LacVE and MalMI was carried out with BTSE as a chain transfer agent and VA-044 as an initiator. To a solution of LacVE (60 mg, 110 μmol), MalMI (56 mg, 110 μmol) and BTSE (0.6 mg, 2 μmol) in H2O (0.65 mL) and acetonitrile (0.50 mL) was added VA-044 (0.4 mg, 1 μmol) in a glass tube ([LacVE]0/[MalMI]0/[VA-044]0/[BTSE]0 = 50/50/0.5/1). The resulting solution was degassed by three freeze–pump–thaw cycles, then the glass tube was sealed under vacuum, heated at 60 °C for 20–240 min and quenched by rapid cooling. The reaction mixture was analyzed by SEC and 1H NMR spectroscopy. The products were purified by dialysis against distilled water and freeze-dried to give copolymer.
2.10. Lectin Binding Assay
A Tris-HCl buffer solution (0.1 M, pH 7.5, including 1 mM MnCl2, 1 mM CaCl2, and 10 mM NaCl) of FITC-labeled lectin (final conc. of Con A; 2 μM, PNA; 8 μM) was added to a copolymer buffer solution. The resulting solution was incubated at room temperature for 9 h in the dark. After centrifugation, the fluorescence intensity of the supernatant was analyzed by a fluorescence spectrophotometer (λex; 495 nm, λem; 518 nm). The association constant was calculated using the Steck–Wallack equation [34].
2.11. Cytotoxicity Assessment
Mouse mesenchymal stem cell line (D1 cell, ATCC® Number: CRL-12424™, cell passage 4) was seeded at a cell density of 9000 cells/cm2 with the control medium (Dulbecco’s modified eagle’s medium with 10% fetal bovine serum and 1% antibiotics) and cultured overnight to attach the cells. The cells were treated with the control medium (negative control group) or the media with poly(MalVE-co-EtMI) and poly(MalMI-co-LacVE) separately for an additional four days. The concentrations of each polymer varied from 0 to 100 μg·mL−1. WST-8 (Cell Counting Kit, Donjindo, Kumamoto, Japan) was used to assess the cell cytotoxicity in accordance with the manufacturer’s instruction. The absorbance was measured with microplate reader (SpectraMax M5; Molecular Devices, San Jose, CA, USA) at a wavelength of 450 nm. BellCurve (Social Survey Research Information Co., Ltd., Tokyo, Japan) was used for the statistical analysis. Statistical significance was evaluated using a one-way analysis of variance (ANOVA), followed by a Tukey–Kramer test. All results are presented as the mean ± standard deviation (SD) (n = 5).
3. Results and Discussion
3.1. Comparison of Copolymerization of MalVE and EtMI with and without RAFT Agent
Synthesis of 3-[2-(2-vinyloxyethoxy)-ethoxy]-propyne (VEEP) was carried out by a reaction of 2-(vinyloxyethoxy)-ethanol with propargyl bromide in the presence of KOH in DMSO at room temperature for 40 h [33]. Maltosyl azide and lactosyl azide were prepared according to the literature [27,28]. Maltose- or lactose-substituted vinyl ethers (MalVE and LacVE) were synthesized by CuAAC reactions of an alkyne-containing VE (VEEP) with the corresponding disaccharide azides. Maltose-substituted maleimide (MalMI) was synthesized in a similar way to the VE versions instead of using N-propargyl MI. The formation of these disaccharide-substituted vinyl monomers was confirmed by 1H and 13C NMR spectroscopy (Figure S1).
Copolymerization of MalVE and EtMI (feed ratio: MalVE:EtMI = 1:1) was performed in H2O/acetonitrile (4/3, v/v) at 60 °C with 2,2′-azobis-[2-(2-imidazolin-2-yl)propane] dihydrochloride (VA-044) in the presence or absence of BTSE as the RAFT agent (a chain transfer agent widely used in RAFT polymerization [13,14,15]) ([MalVE]0/[EtMI]0/[VA-044]0/[BTSE]0 = 50/50/1/1 or 50/50/1/0, [MalVE]0 + [EtMI]0 = 15 wt %). Size exclusion chromatography (SEC) analysis showed both the polymers obtained with or without BTSE were unimodal but clearly different in molecular weight (MW) and molecular weight distribution (MWD). The polymer obtained with BTSE possesses lower MW and narrower MWD compared that without BTSE (polymer (with BTSE): Mn = 3900, Mw = 5800, Mw/Mn = 1.51; polymer (without BTSE): Mn = 13,000, Mw = 21,000, Mw/Mn = 2.15) (Figure S2). These results suggest the possibility of controlled copolymerization by RAFT process for the copolymerization of MalVE and EtMI with BTSE.
3.2. RAFT Copolymerization of MalVE and EtMI
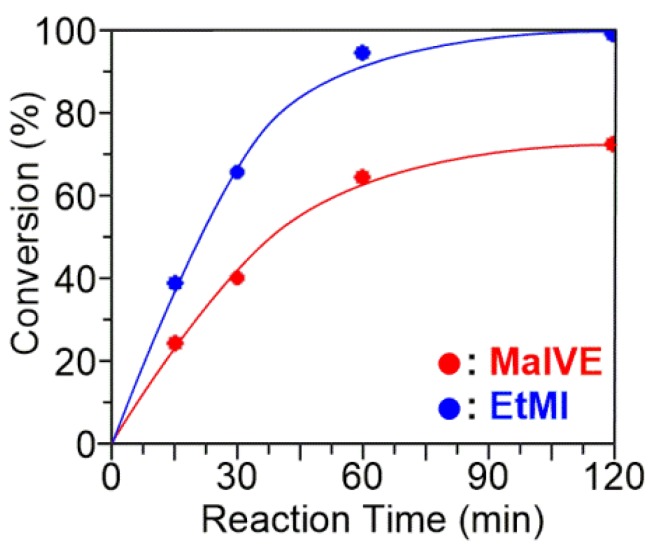
Considering the result of the preliminary experiment, RAFT copolymerization of MalVE and EtMI with BTSE was investigated in detail ([MalVE]0/[EtMI]0/[VA-044]0/[BTSE]0 = 50/50/1/1, [MalVE]0 + [EtMI]0 = 15 wt %). As shown in Figure 2, the copolymerization smoothly proceeded and completed within 2 h. Because only EtMI is capable of homopolymerizing in the RAFT-based radical polymerization process, the conversion of MalVE reached saturation at around 70% while quantitative consumption of EtMI was observed. SEC traces of the obtained copolymers were unimodal and shifted to higher molecular region as the polymerization proceeded with keeping Mw/Mn values of ca. 1.5 or below (Figure 3a,b). Figure 3b shows the Mn and Mw/Mn of the resultant copolymers obtained from the RAFT copolymerization of MalVE and EtMI at various conversions. The observed Mn values (the filled circles in Figure 3b) determined by the peak intensity ratio of the terminal phenyl protons and protons of MalVE and EtMI repeating units (Figure 4) were found to increase in direct proportion to the monomer conversion and were also in good agreement with the calculated values (the solid line in Figure 3b), supporting the chain lengths of the resultant copolymers poly(MalVE-co-EtMI) were well controlled by the RAFT copolymerization. The Mn values of the obtained copolymers (the filled squares in Figure 3b), which were measured by poly(ethylene glycol) (PEG)-calibrated SEC, were much smaller than the calculated values, which is due to the significant difference in hydrodynamic volume of the bulky disaccharide-carrying glycopolymers with respect to the PEG standards. Figure 4 shows the 1H NMR spectrum of poly(MalVE-co-EtMI), where all key signals assignable to the maltose moiety (the protons at C-1 and C-1′) and linkage triazole protons of MalVE units, methyl protons of EtMI units, and the terminal phenyl protons at α-end from BTSE were clearly observed. The composition ratio and degree of polymerization (DPn) of poly(MalVE-co-EtMI) were estimated using Equations (1)–(5):
| Composition ratio of MalVE (%) = [AHa/(AHa + AHb/3)] × 100% | (1) |
| Composition ratio of EtMI (%) = [(AHb/3)/(AHa + AHb/3)] × 100% | (2) |
| DPn of MalVE = [AHa/(APh/5)] | (3) |
| DPn of EtMI = [(AHb/3]/(APh/5)] | (4) |
| Mn NMR = 244.4 + 537.5 × (DPn of MalVE) + 125.1 × (DPn of EtMI) | (5) |
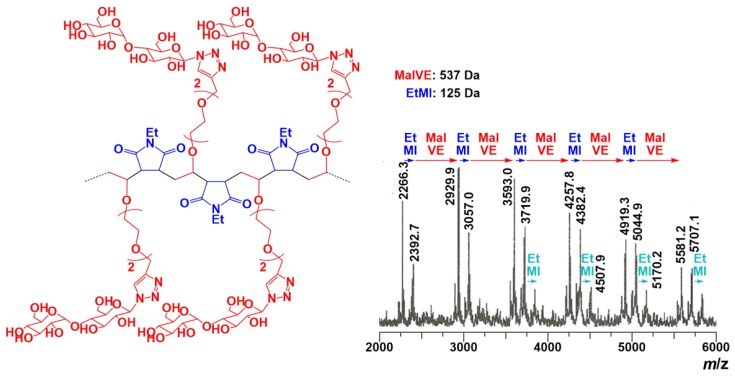
where AHa, AHb and APh represent the relative peak areas of the triazole proton (peak Ha), the methylene protons (peak Hb) and aromatic protons (peak Ph), respectively. Results show that the composition ratio was calculated to be MalVE:EtMI = 42:58 for the copolymer obtained for 120 min reaction. Results of the RAFT copolymerization at MalVE:EtMI = 1:1 feed ratio are summarized in Table 1. An equimolar mixture of MalVE and EtMI was polymerized but the obtained copolymers were found to be almost constant in composition ratio of MalVE/EtMI ≈ 40/60 irrespective of the monomer conversion. This is because only EtMI possesses homopolymerization ability. As a result, the RAFT copolymerization in this study afforded a novel glycopolymer having nearly periodic structure, where major parts possess alternating structure (-(MalVE-EtMI)n-) accompanied by a small part of consecutive sequence of EtMI (–EtMI-EtMI-). The predicted structure was supported by MALDI-TOF-MS analysis in the presence of 2,5-dihydroxybenzoic acid (2,5-DHB) as the matrix and NaCl as the ionizing agent (Figure 5). The spectrum shows a dominant series of the alternating sequence (-(MalVE-EtMI)n-) in which each peak is separated by intervals of 125 Da for EtMI and 537 Da for MalVE, accompanied by a minor series of the consecutive sequence of EtMI ((–EtMI-EtMI-)). Based on the composition ratio of the copolymers (MalVE/EtMI ≈ 40/60) determined by 1H NMR analysis (Table 1), the content of the alternating sequences (-(MalVE-EtMI)n-) in one polymer chain is evaluated to be ca. 80% on average. To improve the extent of control in the alternating structure of the copolymers, copolymerization was examined by varying the initial monomer feed ratio [MalVE]0/([MalVE]0 + [EtMI]0) in the range from 0.1 to 0.85. As shown in Figure 6, the copolymer composition ratios slightly changed depending on the monomer feed ratio, consequently almost alternating structure was attained using an excess amount of MalVE ([MalVE]0/([MalVE]0 + [EtMI]0) = 0.85) for copolymerization. The monomer reactivity ratio was fitted by a penultimate model, as shown in Figure 6 [24]. In this model, there are four parameters for the monomer reactivity ratios, r11, r12, r21, and r22 (Scheme S1). Considering the consecutive addition of VE moiety hardly occurs under the radical polymerization condition, we assumed that r11 and r21 were zero, the r12 and r22 were thus calculated to be 0.90 and 0.20, respectively, by the Kelen–Tüdõs method for the penultimate model. In Figure 6, the plateau region above 0.67 of MalVE content in feed indicated the glycopolymers (poly(MalVE-co-EtMI)) consisting of almost alternating structure.
Figure 2.
Time–monomer conversion curves for the RAFT copolymerization of MalVE and EtMI with BTSE.
Figure 3.
(a) SEC curves of poly(MalVE-co-EtMI) using 0.2 mol·L−1 NaNO3 aq. as the eluent; and (b) experimentally observed Mn and Mw/Mn value of poly(MalVE-co-EtMI) plotted against theoretical Mn of poly(MalVE-co-EtMI). Filled circles and squares correspond to the Mn data obtained by 1H NMR and SEC, respectively.
Figure 4.
1H NMR spectrum of poly(MalVE-co-EtMI) in D2O.
Table 1.
RAFT copolymerization of MalVE and EtMI 1.
| Reaction Time (min) | Conversion (%) 2 | M n SEC 3 | M n NMR 2 | Mw/Mn3 | Composition Ratio (%) 1 | ||
|---|---|---|---|---|---|---|---|
| MalVE | EtMI | MalVE | EtMI | ||||
| 15 | 24 | 39 | 2100 | 9600 | 1.34 | 41 | 59 |
| 30 | 40 | 66 | 3100 | 14,000 | 1.46 | 42 | 58 |
| 60 | 65 | 95 | 4200 | 24,000 | 1.49 | 40 | 60 |
| 120 | 73 | 100 | 4400 | 27,000 | 1.53 | 42 | 58 |
1 Polymerization conditions: H2O/acetonitrile = 4/3 (v/v), 60 °C, [MalVE]0 + [EtMI]0 = 15 wt %, [MalVE]0/[EtMI]0/[VA-044]0/[BTSE]0 = 50/50/1/1. 2 Determined by 1H NMR. 3 Estimated by PEG-calibrated SEC.
Figure 5.
MALDI-TOF-MS spectrum of poly(MalVE-co-EtMI).
Figure 6.

Copolymer composition curve for the copolymerization of MalVE (M1) and EtMI (M2). The dotted line in plot was fitted by the Kelen–Tüdõs method, assuming that the values of r11 and r21 are 0.
3.3. RAFT Copolymerization of LacVE and EtMI
RAFT copolymerization of LacVE and EtMI was also investigated under similar conditions as those of MalVE and EtMI ([LacVE]0/[EtMI]0/[VA-044]0/[BTSE]0 = 50/50/1/1, [LacVE]0 + [EtMI]0 = 15 wt %). The RAFT copolymerization proceeded smoothly without an induction period, and EtMI was quantitatively consumed and the conversion of LacVE reached over 85% within 80 min (Figure S3). The results of the RAFT copolymerization of LacVE and EtMI are summarized in Table 2. Throughout the polymerization, all SEC chromatograms of the obtained copolymers were unimodal and their polydispersity indices were maintained at around 1.5 or below (Figure S4). As in the case of the combination of MalVE and EtMI, due to the homopolymerizability of EtMI, the copolymerization at LacVE:EtMI = 1:1 feed ratio results in the formation of copolymers being almost constant in composition ratio of LacVE/EtMI ≈ 40/60 irrespective of the monomer conversion. These results indicate the BTSE-mediated copolymerization of LacVE and EtMI proceeded in a controlled fashion.
Table 2.
RAFT copolymerization of LacVE and EtMI 1.
| Reaction Time (min) | Conversion (%) 2 | M n SEC 3 | M n NMR 2 | Mw/Mn3 | Composition Ratio (%) 1 | ||
|---|---|---|---|---|---|---|---|
| LacVE | EtMI | LacVE | EtMI | ||||
| 10 | 28 | 37 | 3600 | 10,000 | 1.46 | 43 | 57 |
| 20 | 49 | 66 | 4600 | 22,000 | 1.49 | 42 | 58 |
| 40 | 75 | 92 | 6200 | 29,000 | 1.53 | 43 | 57 |
| 80 | 87 | 100 | 6600 | 28,000 | 1.51 | 43 | 57 |
1 Polymerization conditions: H2O/acetonitrile = 4/3 (v/v), 60 °C, [LacVE]0 + [EtMI]0 = 15 wt %, [LacVE]0/[EtMI]0/[VA-044]0/[BTSE]0 = 50/50/1/1. 2 Determined by 1H NMR. 3 Estimated by PEG-calibrated SEC.
3.4. RAFT Copolymerization of LacVE and MalMI
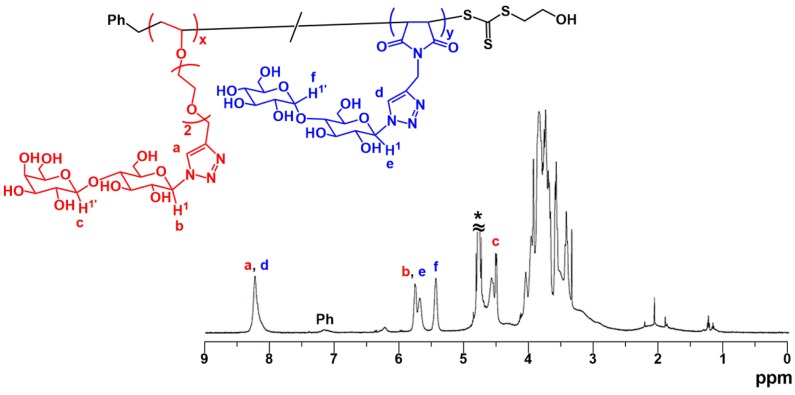
The goal of our research was the synthesis of alternating glycopolymers as glycosaminoglycan mimics, where two kinds of carbohydrate-substituted vinyl monomers are alternately connected with each other. In this study, we developed a new synthetic approach for the synthesis of alternating glycopolymers with controlled molecular weight and polymer chain length distribution. Based on the results of the synthesis of periodic glycopolymers mentioned above, we here demonstrated the RAFT copolymerization of two kinds of disaccharide-substituted vinyl monomers, LacVE and MalMI. RAFT copolymerization of LacVE and MalMI was carried out using BTSE and VA-044 in H2O/acetonitrile (4/3, v/v) at 60 °C ([LacVE]0/[MalMI]0/[VA-044]0/[BTSE]0 = 50/50/1/1, [LacVE]0 + [MalMI]0 = 10 wt %). The copolymerization smoothly occurred and the conversions of LacVE and MalMI reached 67% and 81%, respectively, after a period of 4 h (Figure S5). Unlike the complete consumption in the copolymerization using EtMI as a comonomer, MalMI was not quantitatively consumed, probably due to the steric hindrance of the disaccharide-substituted pendant structure. The polymerization results are summarized in Table 3. SEC analysis indicated all the chromatograms of the copolymers were unimodal (Figure S6) and their Mw/Mn values were around 1.5 or below. Figure 7 depicts 1H MMR spectrum in D2O of the obtained copolymer poly(LacVE-co-MalMI). Along with the linkage triazole proton (Peaks a and d), two pairs of characteristic proton signals at C-1 and C-1′ of the disaccharide moieties (Peaks e and f for MalMI unit, and Peaks b and c for LacVE unit) were clearly observed. Moreover, aromatic protons assignable to the benzyl moiety at α-end, which originated from BTSE, were observed. Based on the integrated intensities of the key signals, the composition ratio was calculated to be LacVE/MalMI ≈ 36/64. All the copolymers obtained at various conversions possessed similar compositions. In comparison with the composition ratios of poly(MalVE-co-EtMI) and poly(LacVE-co-EtMI) (VE/MI ≈ 40/60), the ratios are not significantly different from that of poly(LacVE-co-MalMI), showing the sequence regulation in the copolymer synthesis by RAFT copolymerization may be affected only by the reactivity the vinyl moieties not by the steric hindrance of the pendant carbohydrate residues. Therefore, the target alternating glycopolymers are expected to be synthesized by the RAFT copolymerization of an excess use of carbohydrate-substituted VE.
Table 3.
RAFT copolymerization of LacVE and MalMI 1.
| Reaction Time (min) | Conversion (%) 2 | M n 3 | Mw/Mn3 | Composition Ratio (%) 2 | ||
|---|---|---|---|---|---|---|
| LacVE | MalMI | LacVE | MalMI | |||
| 20 | 3 | 22 | 4700 | 1.40 | 36 | 64 |
| 30 | 18 | 34 | 5000 | 1.48 | 35 | 65 |
| 60 | 30 | 49 | 5800 | 1.52 | 36 | 64 |
| 120 | 61 | 74 | 5900 | 1.50 | 36 | 64 |
| 240 | 67 | 81 | 6000 | 1.47 | 36 | 64 |
1 Polymerization conditions: H2O/acetonitrile = 4/3 (v/v), 60 °C, [LacVE]0 + [MalMI]0 = 10 wt %, [LacVE]0/[MalMI]0/[VA-044]0/[BTSE]0 = 50/50/1/1. 2 Determined by 1H NMR. 3 Estimated by PEG-calibrated SEC.
Figure 7.
1H NMR spectrum of poly(LacVE-co-MalMI) in D2O.
3.5. Lectin Binding Assay
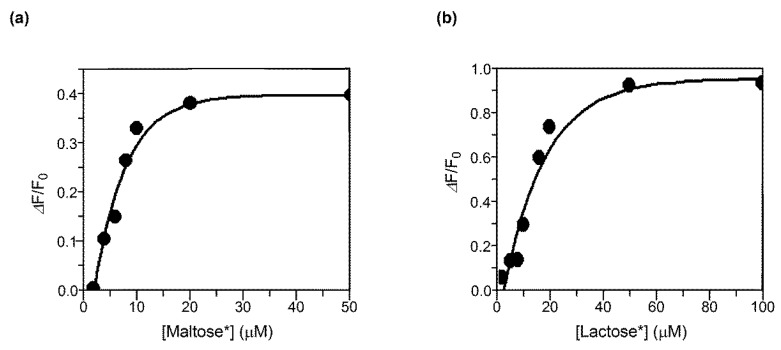
The binding property of copolymers poly(MalVE-co-EtMI) and poly(LacVE-co-EtMI) were investigated, and the association constants (Ka) for the lectin–saccharide interaction were estimated using fluorescein-labeled lectins (Figure 8). The addition of copolymer to the buffer solution of FITC-labeled lectin resulted in decreasing the intensity of fluorescence due to multivalent binding of α-glucoside residue in maltose with Con A and of β-galactoside residue in lactose with PNA, respectively. No glycopolymer–lectin binding was observed when non-corresponding lectin was added to each copolymer solution in the turbidity test (Figure S7). The values of Ka were 1.3 × 105 M−1 (poly(MalVE-co-EtMI) and Con A) and 5.0 × 104 M−1 (poly(LacVE-co-EtMI) and PNA), respectively. The Ka value of free saccharide with corresponding lectin is reported in the order of 103 M−1 [35]. Therefore, these results indicate that the periodic glycopolymers poly(MalVE-co-EtMI) and poly(LacVE-co-EtMI) strongly and specifically interacted with corresponding lectin in aqueous condition due to the multivalency of saccharide in glycopolymers.
Figure 8.
Quenching of the fluorescence intensity of: (a) FITC-labeled Con A by the addition of poly(MalVE-co-EtMI); and (b) FITC-labeled PNA by the addition of poly(LacVE-co-EtMI). [Maltose*] and [Lactose*] correspond to the concentration of the pendant maltose moiety in the poly(MalVE-co-EtMI) and the pendant lactose moiety in the poly(LacVE-co-EtMI), respectively.
3.6. In Vitro Cytotoxicity
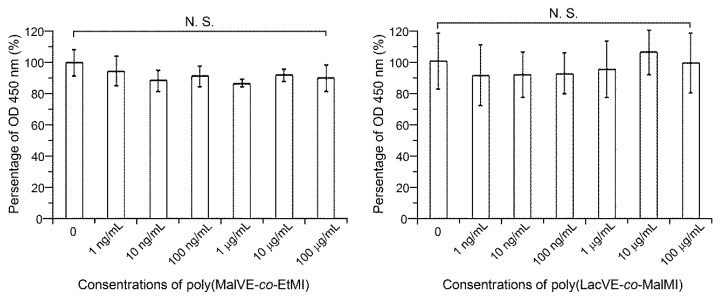
Cytotoxicity studies (Figure 9) show that there was no significant difference in cellular viability when D1 cells were treated with the media including poly(MalVE-co-EtMI) or poly(LacVE-co-MalMI) in comparison with that of medium without polymers. The results indicate that both polymers may potentially cause no or poor cytotoxicity for the cells, when concentrations are below 100 μg mL−1.
Figure 9.
Cytotoxicity studies for the obtained glycopolymers. The mouse mesenchymal stem cell line (D1 cell) was treated with/without poly(MalVE-co-EtMI) or poly(LacVE-co-MalMI) for four days. The data were normalized to the control group without polymers. The results are presented as percentages (control group: 100%). Data represent the mean with standard deviation (n = 5 per group). N.S., not significant.
4. Conclusions
In this study, we demonstrated that the RAFT copolymerization of a combination of carbohydrate-substituted VE (as an electron-rich vinyl monomer) and EtMI (as an electron-deficient vinyl monomer) is a promising synthetic approach for yielding novel type of periodic glycopolymers. Moreover, almost alternating sequence control in copolymers can be achieved by regulating the initial monomer feed ratio. It should be emphasized that alternating glycopolymers as glycosaminoglycan mimics, in which two kinds of saccharide moieties are alternately incorporated in every pendant, can be designed by the use of a combination of carbohydrate-containing VE and MI monomers. Another possible advantage of the present synthetic strategy is that it would be capable of affording mimics of non-naturally occurring polysaccharides with alternating structure, which may possess novel physiological and/or pharmaceutical functions. RAFT copolymerization of various combinations of carbohydrate-substituted VE and MI derivatives are in progress and will be reported elsewhere.
Acknowledgments
We are indebted to Maruzen Petrochemical Co. (Tokyo, Japan) for donating 2-hydoxyethyl vinyl ether.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4360/11/1/70/s1, Figure S1: 1H and 13C NMR spectra of vinyl monomers (MalVE, LacVE and MalMI), Figure S2: SEC curves of poly(MalVE-co-EtMI) obtained in the radical copolymerization with and without RAFT agent, Scheme S1: Schematics of radical copolymerization of MalVE (M1) and EtMI (M2), Figure S3: time–conversion curves for the RAFT copolymerization of LacVE and EtMI with BTSE, Figure S4: experimentally observed Mn and Mw/Mn value of poly(LacVE-co-EtMI) plotted against theoretical Mn of poly(LacVE-co-EtMI), Figure S5: time–conversion curves for the RAFT copolymerization of LacVE and MalMI with BTSE, Figure S6: SEC curves of poly(LacVE-co-MalMI), Figure S7: Photography of poly(MalVE-co-EtMI) and poly(LacVE-co-EtMI) solution before and after the addition of FITC-unlabeled Con A or PNA.
Author Contributions
Y.H., T.T., J.M., and M.M. conceived the project, designed the experiments, directed the research, and wrote the manuscript. T.O. and Y.Y. performed the experiments. J.Z. performed the cytotoxicity assessment experiments. All authors discussed the results and edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jackson R.L., Busch S.J., Cardin A.D. Glycosaminoglycans: Molecular Properties, Protein Interactions, and Role in Physiological Processes. Physiol. Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- 2.Hileman R.E., Fromm J.R., Weiler J.M., Linhardt R.J. Glycosaminoglycan—Protein interactions: Definition of consensus sites in glycosaminoglycan binding proteins. BioEssays. 1998;20:156–167. doi: 10.1002/(SICI)1521-1878(199802)20:2<156::AID-BIES8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Yabe T., Maeda N. Histochemical Analysis of Heparan Sulfate 3-O-Sulfotransferase Expression in Mouse Brain. In: Balagurunathan K., Nakato H., Desai U., editors. Glycosaminoglycans: Methods in Molecular Biology. Volume 1229. Humana Press; New York, NY, USA: 2015. pp. 377–387. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q., Chen G., Chen H. Chemical synthesis of glycosaminoglycan-mimetic polymers. Polym. Chem. 2018 doi: 10.1039/C8PY01338A. in press. [DOI] [Google Scholar]
- 5.Miura Y., Mizuno H. Interaction Analyses of Amyloid β Peptide (1–40) with Glycosaminoglycan Model Polymers. Bull. Chem. Soc. Jpn. 2010;83:1004–1009. doi: 10.1246/bcsj.20100094. [DOI] [Google Scholar]
- 6.Oh Y.I., Sheng G.J., Chang S.-K., Hsieh-Wilson L.C. Tailored Glycopolymers as Anticoagulant Heparin Mimetics. Angew. Chem. Int. Ed. 2013;52:11796–11799. doi: 10.1002/anie.201306968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheng G.J., Oh Y.I., Chang S.-K., Hsieh-Wilson L.C. Tunable Heparan Sulfate Mimetics for Modulating Chemokine Activity. J. Am. Chem. Soc. 2013;135:10898–10901. doi: 10.1021/ja4027727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M., Lyu Z., Chen G., Wang H., Yuan Y., Ding K., Yu Q., Yuan L., Chen H. A new avenue to the synthesis of GAG-mimicking polymers highly promoting neural differentiation of embryonic stem cells. Chem. Commun. 2015;51:15434–15437. doi: 10.1039/C5CC06944K. [DOI] [PubMed] [Google Scholar]
- 9.Sun X.-L., Grande D., Baskaran S., Hanson S.R., Chaikof E.L. Glycosaminoglycan Mimetic Biomaterials. 4. Synthesis of Sulfated Lactose-Based Glycopolymers That Exhibit Anticoagulant Activity. Biomacromolecules. 2002;3:1065–1070. doi: 10.1021/bm025561s. [DOI] [PubMed] [Google Scholar]
- 10.Baskaran S., Grande D., Sun X.-L., Yayon A., Chaikof E.L. Glycosaminoglycan-Mimetic Biomaterials. 3. Glycopolymers Prepared from Alkene-Derivatized Mono- and Disaccharide-Based Glycomonomers. Bioconjugate Chem. 2002;13:1309–1313. doi: 10.1021/bc0255485. [DOI] [PubMed] [Google Scholar]
- 11.Matyjaszewski K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules. 2012;45:4015–4039. doi: 10.1021/ma3001719. [DOI] [Google Scholar]
- 12.Tsarevsky N.V., Matyjaszewski K. “Green” Atom Transfer Radical Polymerization: From Process Design to Preparation of Well-Defined Environmentally Friendly Polymeric Materials. Chem. Rev. 2007;107:2270–2299. doi: 10.1021/cr050947p. [DOI] [PubMed] [Google Scholar]
- 13.Moad G., Rizzardo E., Thang S.H. Living Radical Polymerization by the RAFT Process—A First Update. Aust. J. Chem. 2006;59:669–692. doi: 10.1071/CH06250. [DOI] [Google Scholar]
- 14.Moad G., Rizzardo E., Thang S.H. Living Radical Polymerization by the RAFT Process—A Second Update. Aust. J. Chem. 2009;62:1402–1472. doi: 10.1071/CH09311. [DOI] [Google Scholar]
- 15.Moad G., Rizzardo E., Thang S.H. Living Radical Polymerization by the RAFT Process—A Third Update. Aust. J. Chem. 2012;65:985–1076. doi: 10.1071/CH12295. [DOI] [Google Scholar]
- 16.Sun H., Kabb C.P., Dai Y., Hill M.R., Ghiviriga I., Bapat A.P., Sumerlin B.S. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 2017;9:817–823. doi: 10.1038/nchem.2730. [DOI] [PubMed] [Google Scholar]
- 17.Sun H., Kabb C.P., Sims M.B., Sumerlin B.S. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 2018 doi: 10.1016/j.progpolymsci.2018.09.006. in press. [DOI] [Google Scholar]
- 18.Zhang Q., Collins J., Anastasaki A., Wallis R., Mitchell D.A., Becer C.R., Haddleton D.M. Sequence-Controlled Multi-Block Glycopolymers to Inhibit DC-SIGN-gp120 Binding. Angew. Chem. Int. Ed. 2013;52:4435–4439. doi: 10.1002/anie.201300068. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto A., Kubota T., Otsu T. Radical Polymerization of N-(alkyl-substituted phenyl)maleimides: Synthesis of Thermally Stable Polymers Soluble in Nonpolar Solvents. Macromolecules. 1990;23:4508–4513. doi: 10.1021/ma00223a002. [DOI] [Google Scholar]
- 20.Doi T., Akimoto A., Matsumoto A., Oki Y., Otsu T. Alternating Copolymerization of N-(alkyl-substituted phenyl)maleimides with Isobutene and Thermal Properties of the Resulting Copolymers. J. Polym. Sci. A Polym. Chem. 1996;34:2499–2505. doi: 10.1002/(SICI)1099-0518(19960915)34:12<2499::AID-POLA23>3.0.CO;2-2. [DOI] [Google Scholar]
- 21.Butler G.B. Recent Developments in Polymerization by an Alternating Intra-Intermolecular Mechanism. J. Polym. Sci. A Polym. Chem. 1996;34:913–923. doi: 10.1002/pola.1996.823. [DOI] [Google Scholar]
- 22.Matsumoto A., Hisano M., Yamamoto D., Yamamoto H., Okamura H. Synthesis of Sequence-Controlled Maleimide Copolymers and Application to the Design of Thermoresistant and Transparent Polymer Materials. Kobunshi Ronbunshu. 2015;72:243–260. doi: 10.1295/koron.2014-0088. [DOI] [Google Scholar]
- 23.Ojika M., Satoh K., Kamigaito M. BAB-random-C Monomer Sequence via Radical Terpolymerization of Limonene (A), Maleimide (B), and Methacrylate (C): Terpene Polymers with Randomly Distributed Periodic Sequences. Angew. Chem. Int. Ed. 2017;56:1789–1793. doi: 10.1002/anie.201610768. [DOI] [PubMed] [Google Scholar]
- 24.Satoh K., Matsuda M., Nagai K., Kamigaito M. AAB-Sequence Living Radical Chain Copolymerization of Naturally Occurring Limonene with Maleimide: An End-to-End Sequence-Regulated Copolymer. J. Am. Chem. Soc. 2010;132:10003–10005. doi: 10.1021/ja1042353. [DOI] [PubMed] [Google Scholar]
- 25.Nishimori K., Ouchi M., Sawamoto M. Sequence Analysis for Alternating Copolymers by MALDI-TOF-MS: Importance of Initiator Selectivity for Comonomer Pair. Macromol. Rapid Commun. 2016;37:1414–1420. doi: 10.1002/marc.201600251. [DOI] [PubMed] [Google Scholar]
- 26.Nishimori K., Sawamoto M., Ouchi M. Design of Maleimide Monomer for Higher Level of Alternating Sequence in Radical Copolymerization with Styrene. J. Polym. Sci. A Polym. Chem. 2018 doi: 10.1002/pola.29191. in press. [DOI] [Google Scholar]
- 27.Tanaka T., Nagai H., Noguchi M., Kobayashi A., Shoda S. One-step conversion of unprotected sugars to β-glycosyl azides using 2-chloroimidazolinium salt in aqueous solution. Chem. Commun. 2009:3378–3379. doi: 10.1039/b905761g. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T., Ishitani H., Miura Y., Oishi K., Takahashi T., Suzuki T., Shoda S., Kimura Y. Protecting-Group-Free Synthesis of Glycopolymers Bearing Sialyloligosaccharide and Their High Binding with the Influenza Virus. ACS Macro Lett. 2014;3:1074–1078. doi: 10.1021/mz500555x. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda M., Satoh K., Kamigaito M. Periodically Functionalized and Grafted Copolymers via 1:2-Sequence-Regulated Radical Copolymerization of Naturally Occurring Functional Limonene and Maleimide Derivatives. Macromolecules. 2013;46:5473–5482. doi: 10.1021/ma401021d. [DOI] [Google Scholar]
- 30.Matsuda M., Satoh K., Kamigaito M. 1:2-Sequence-Regulated Radical Copolymerization of Naturally Occurring Terpenes with Maleimide Derivatives in Fluorinated Alcohol. J. Polym. Sci. A Polym. Chem. 2013;51:1774–1785. doi: 10.1002/pola.26556. [DOI] [Google Scholar]
- 31.Stolz R.M., Northrop B.H. Experimental and Theoretical Studies of Selective Thiol–Ene and Thiol–Yne Click Reactions Involving N-Substituted Maleimides. J. Org. Chem. 2013;78:8105–8116. doi: 10.1021/jo4014436. [DOI] [PubMed] [Google Scholar]
- 32.Hales M., Barner-Kowollik C., Davis T.P., Stenzel M.H. Shell-Cross-Linked Vesicles Synthesized from Block Copolymers of Poly(d,l-lactide) and Poly(N-isopropyl acrylamide) as Thermoresponsive Nanocontainers. Langmuir. 2004;20:10809–10817. doi: 10.1021/la0484016. [DOI] [PubMed] [Google Scholar]
- 33.Tan N.M., Mori R., Tanaka T., Motoyanagi J., Minoda M. Living cationic polymerization of a vinyl ether with an unprotected pendant alkynyl group and their use for the protecting group-free synthesis of macromonomer-type glycopolymers via CuAAC with maltosyl azides. J. Polym. Sci. A Polym. Chem. 2019 doi: 10.1002/pola.29309. in press. [DOI] [Google Scholar]
- 34.Furuike T., Nishi N., Tokura S., Nishimura S.-I. Synthetic Glycoconjugates. 6. Preparation and Biochemical Evaluation of Novel Cluster-Type Glycopolymers Containing Gal β(1→4)GlcNAc (N-Acetyllactosamine) Residue. Macromolecules. 1995;28:7241–7247. doi: 10.1021/ma00125a029. [DOI] [Google Scholar]
- 35.Miura Y., Ikeda T., Kobayashi K. Chemoenzymatically Synthesized Glycoconjugate Polymers. Biomacromolecues. 2003;4:410–415. doi: 10.1021/bm025714b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.