Abstract
Background:
Our aim in this research was to establish whether integrating an electronically generated calculation and report on the Drug Burden Index (DBI) in the Home Medicines Review (HMR) setting is an accurate, feasible and useful risk assessment tool to assess risk of anticholinergic and sedative medications; and to establish whether the intervention of DBI together with HMR is associated with a reduced use of anticholinergic and sedative medications in older community-dwelling adults in Australia.
Methods:
An interventional feasibility study was conducted. Accredited clinical pharmacists (APs) were recruited to participate. Each AP was educated on implementation of the DBI into HMR practice and given access to the DBI Calculator© web-based software to generate the DBI report for inclusion in HMR reports for general practitioners (GPs). APs recruited patients (⩾65 years) who were referred to them for HMRs. Patients were sent a letter about their DBI exposure, and a prompt to visit their GP to discuss their medication management options. GPs, APs and patients were asked to evaluate the feasibility and utility of the DBI report. A medication inventory was collected from patients at the time of the HMR and at 3 months to determine whether the intervention affected deprescribing of medications with anticholinergic and sedative effects.
Results:
Regarding the feasibility of the DBI report as a risk assessment tool within HMR, 89% of APs and 67% of GPs agreed that it would be feasible. The DBI Calculator© was potentially inaccurate, as 26% of DBI scores were underestimated and 7% were overestimated (at baseline). At 3 months, the median (interquartile range) DBI for patients (n = 100) significantly decreased from 0.82 (0–1.33) to 0.67 (0–1.29) (p = 0.014). Additionally, of patients with a DBI > 0 (n = 66), 36.4% had their DBI score decrease, and 6.1% had a score increase.
Conclusion:
This study demonstrated that integration of the DBI Calculator© into HMR is a feasible and useful method to prompt deprescribing of anticholinergic and sedative medications in older adults. There is potential for the accuracy of the web-based platform to be improved.
Registration of trial:
Name: Feasibility study of the Drug Burden Index with Home Medicines Review.
Website: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368523
Trial ID: ACTRN 12615000539538.
Keywords: deprescribing, Drug Burden Index, intervention, older adults, polypharmacy
Introduction
Among people aged over 65 years, multimorbidity (two or more chronic diseases)1,2 is present in 75–88% of people, and more than 40% in this age group have polypharmacy (use five or more medications).3,4 Polypharmacy is associated with poor clinical outcomes such as mortality, falls, disability and frailty.5 Medications with anticholinergic and sedative properties are frequently used by older adults and are associated with adverse drug events (ADEs), including poor physical and cognitive function and neuropsychiatric ADEs, leading to increased hospitalization and nursing home admissions. In some cases, these medications may be inappropriately prescribed, contribute to polypharmacy and be targeted for deprescribing.6–9
The Drug Burden Index (DBI) is a pharmacological measure of the cumulative exposure to anticholinergic and sedative medications. The DBI was developed to estimate the risk of these medications to physical and cognitive functional impairment in older adults.10,11 Increasing DBI has been associated with poorer physical function, falls, frailty, hospitalization and mortality in older adults.12,13 To date, two interventional studies have been conducted in the primary care setting specifically aiming to reduce total anticholinergic and sedative medication use, measured by DBI.14,15 The study by Gnjidic and colleagues, which used a letter and phone-call intervention directed towards general practitioners (GPs), used DBI to prompt consideration of cessation or dose reduction of anticholinergic and sedative medications. This study observed a nonsignificant reduction of DBI in 32% of patients in the intervention group, and 19% of patients in the control group.14 In 2018, Van der Meer and colleagues conducted a randomized controlled trial (single blind) where patients randomized into the intervention arm received a pharmacist-led medication review that focused on anticholinergic and sedative medications. This study found no difference in the proportion of patients with a ⩾0.5-point decrease in DBI scores between the intervention and control groups.15
In Australia, the Home Medicines Review (HMR) service aims to aid in the reduction of preventable drug-related hospital admissions and improve the quality use of medicines in older adults.16 The HMR service is a government-funded community-based collaborative service between patients, GPs and accredited clinical pharmacists (APs). An AP, a pharmacist that has undergone specialized training to undertake HMRs, receives a referral for a patient from the GP, conducts an interview with the patient in the home to gain a comprehensive medication profile, and documents medication review findings and recommendations in a report for the GP, which can then be used to formulate the patient’s medication management plan.17 Observational studies have demonstrated the impact of medication review in reducing the number of prescribed medications and drug-related problems, including reducing DBI scores.18,19 However, there is limited evidence supporting the benefit of medication reviews on clinical outcomes.20 Studies observing associations between pharmacist recommendations for medications with anticholinergic and sedative effects and important clinical outcomes in older adults are limited to pilot studies or studies of single drug classes.21–24
Calculation of the DBI requires knowledge of anticholinergic and sedative properties of medications, their minimum efficacious doses, and consideration of medications with the same ingredients taken in different formulations together.11 In previous studies, we found that approximately 18% of pharmacists were unable to accurately calculate the DBI for a hypothetical patient and therefore the utility of the DBI as a clinical risk assessment tool may require software to facilitate the calculation and its interpretation.25,26 A recent Cochrane review suggested that it is unclear whether interventions using computerized clinical decision support systems (CCDSS) may improve appropriate polypharmacy, and further studies are required.27 The HMR service model provides an ideal study setting to investigate the accuracy, feasibility and utility of an electronic intervention of implementing DBI as a risk assessment tool.
Therefore, in a population of older community-dwelling adults (⩾65 years) receiving the HMR service in Australia, the study objectives were to:
(1) establish whether addition of an electronically generated calculation and report on DBI in the HMR setting is an accurate, feasible and useful risk assessment tool to assess risk of anticholinergic and sedative medications; and
(2) establish whether the intervention of the DBI together with HMR is associated with a reduced use of anticholinergic and sedative medications.
Methodology
Study design
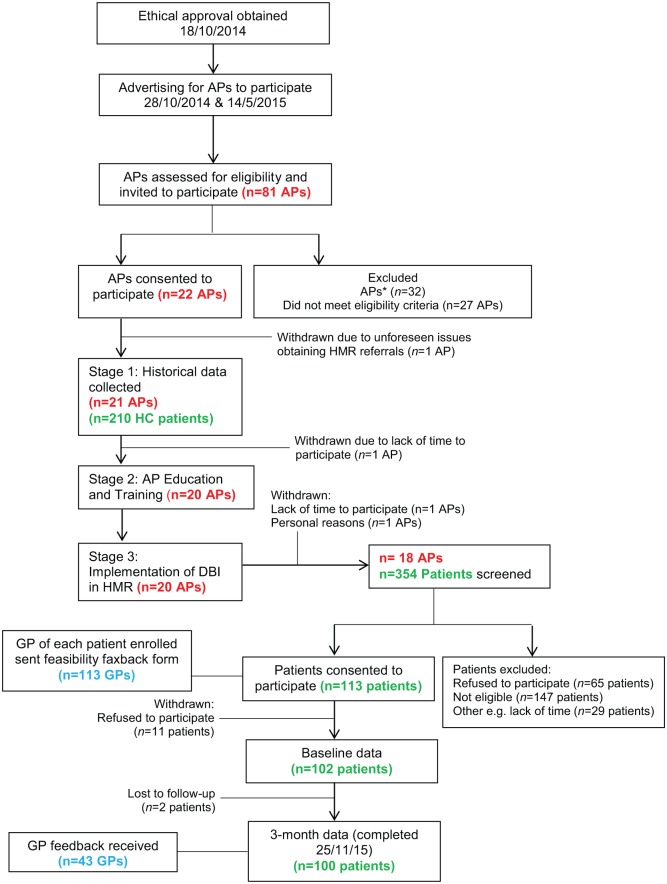
This interventional feasibility study using historical controls, investigated the accuracy, feasibility and usefulness of reporting the DBI in the HMR setting (Figure 1). The study was conducted between October 2014 and November 2015. The protocol for this study was retrospectively registered with the Australian New Zealand Clinical Trials Registry (ANZCTR): ACTRN12615000539538. All study participants gave written, informed consent. Ethical approval was obtained from the Executive of the Human Research Ethics Committee of the University of Sydney, Australia (2014/648).
Figure 1.
Flow of accredited clinical pharmacists (APs), patients and general practitioners (GPs) through the feasibility study.
*APs who initiated interest in participating in the study, but did not respond to the investigators upon receiving further information about the study.
DBI, Drug Burden Index; HC, historical control; HMR, Home Medicines Review.
Stage 1: retrospective cohort (historical data collection)
APs were recruited by advertising in professional organization electronic newsletters and were screened according to eligibility criteria (Supplementary Table 1). Stage 1 required each AP participant enrolled in the study to provide anonymous data on their 10 most recent HMRs, by extracting data from their previous HMR referrals and reports, to complete a historical data collection sheet. These data were used as the historical control dataset for this study. The data provided by APs included sociodemographics and a medication inventory for their patients. The inclusion criteria for patients in the historical control dataset were: (a) that they received the HMR service; and (b) aged ⩾ 65 years.
Stage 2: education and training
Stage 2 of the study involved education and training of AP participants on the implementation of the DBI into the HMR service. Conducted by an accredited clinical pharmacist (LKO) via a webinar, the education and training supported the development of the pharmacists’ clinical skills and provided the training to partake in stage 3. This included instructions on how to determine patient eligibility and to obtain patient consent for participation in the study. Each AP received study materials such as data collection sheets, patient/carer participant consent forms, patient education materials, patient surveys, and pharmacist reading and education materials.
Stage 3: prospective cohort (intervention data collection)
Summary of calculating DBI
The DBI for every patient was calculated according to the formula:
| Equation 1. |
where B indicates burden, AC indicates anticholinergic medications and S indicates sedative medications. With the assumption that anticholinergic and sedative effects of different medications are additive, similar to a linear model of pharmacological effect, the DBI was calculated using the following formula:
| Equation 2. |
where D is the daily dose, and δ is the minimum recommended daily dose as listed by the medication product information approved by the Therapeutic Goods Administration of Australia.10, 12 The approved medication product information was also used to identify medications with anticholinergic or sedative pharmacological effects. Medications with both anticholinergic and sedative effects were classified as anticholinergic. Complementary medications and medications that were prescribed ‘when required’ (PRN) were excluded from DBI calculations.11
The DBI Calculator© and report
The DBI Calculator© is a reliable CCDSS that has been developed to calculate and report on DBI of older patients taking multiple medications.25 The DBI Calculator© report was redesigned after receiving feedback from focus groups and interviews consisting of GPs, APs and specialist physicians in a previous study.25 The patient’s medication list with DBI score for each medication and the patient’s total DBI and risk level were retained. Alterations to the design included a detailed explanation of the DBI, an introduction of a scale, and columns to allow APs and GPs to comment on the use of the medications (Supplementary Figure 1).
Implementation of the DBI report into HMR
Each AP enrolled in the study was responsible for inviting their HMR patients to participate in the project (Figure 1). Patients were screened by the APs according to patient participant eligibility criteria (Supplementary Table 2) and all potential patient participants were provided with verbal and written information about the study. In cases where patients were unable to provide written informed consent but were still eligible and willing (assented) to participate in the study, consent was obtained from the patient’s carer. The HMR service continued independently of whether patients chose to participate in the study.
Upon completion of the patient interview and at the time of writing the HMR report for the referring GP, APs used the DBI Calculator© to generate a DBI report specific for the patient to attach to the HMR report. Within the HMR report to the referring GP, pharmacists also included a reference to the attached DBI report.
Intervention patient participants were provided questionnaires (at baseline, i.e. during HMR interview and 3 months after the HMR) to complete and return. The questionnaires included sociodemographic questions (collected at baseline), Beliefs about Medicines Questionnaire (BMQ)-specific questionnaire (collected at baseline), and questions about the feasibility and utility of the patient version of the DBI report (collected at 3 months; Table 1).28 The BMQ specific was used to measure the perspectives of intervention patients on use of their multiple medications (polypharmacy). The BMQ specific comprises two scales: one assessing patients’ beliefs about the necessity of their medications for maintaining present and future health (necessity scale) and the other assessing their concerns about the potential adverse consequences of using their medications (concerns scale). All the questions are rated on a five-point Likert scale. The mean [standard deviation (SD)] of responses to the statements for each scale were calculated and reported. Higher means (midpoint of 3) indicate stronger beliefs in the concepts represented by the scale.
Table 1.
Summary of information gathered from patients in the prospective (intervention) arm of the study.
| Data | Details | Prospective (intervention) | |
|---|---|---|---|
| Baseline (0 months) | Follow up (3 months) | ||
| Sociodemographic | Age, sex, ethnicity, locality, education status, marital status, BMI | ✓ | |
| Medication Inventory | (1) Prescription and nonprescription medications (2) Pharmacist findings and recommendations (3) BMQ specific |
✓ ✓ ✓ |
✓ |
BMI, body mass index; BMQ, Beliefs about Medicines Questionnaire28; HMR, Home Medicines Review.
For each intervention patient recruited into the study, a faxback feasibility survey was provided to the patient’s referring GP. At the end of patient recruitment, APs were also provided with a feasibility survey (Table 2).
Table 2.
Information collected about the feasibility of the DBI with Home Medicines Review (HMR) from patients, accredited clinical pharmacists and general practitioners.
| Feasibility/usefulness questions | Patients | Accredited pharmacists | General practitioners |
|---|---|---|---|
| ‘Did you receive the DBI report?’ (Y/N) | ✓ | ✓ | |
| ‘What did you think of the DBI report?’ (very useful, somewhat useful, not very useful or not useful at all) |
✓ | ✓ | ✓ |
| ‘Was the report easy to read and understand?’ (Y/N) | ✓ | ✓ | ✓ |
| ‘What feedback did you receive about the DBI report from the doctors?’ (no feedback, very useful, somewhat useful, not very useful, not useful at all) |
✓ | ||
| ‘Did you take it to the doctor and talk to him/her about it?’ (Y/N) | ✓ | ||
| ‘Was the DBI tool valuable for your practice?’ (Y/N) | ✓ | ✓ | |
| ‘Do you think the DBI report is feasible to provide a medication risk assessment with the HMR process?’ (Y/N) | ✓ | ✓ | |
| ‘Did you use the information on this report for decision making?’ (Y/N) | ✓ |
DBI, Drug Burden Index; Y/N, yes/no.
Pharmacist demographic data
APs provided data regarding their sociodemographics, including age, sex, pharmacy qualification, years of practice and locality of practice (measured by the Pharmacy Access/Remoteness Index of Australia which provides a standardized measure of the physical and professional remoteness of pharmacies throughout Australia29).
Patient medication assessment
A medication inventory was provided by APs for all historical control (stage 1) and intervention (stage 3) patients. The name, dose and frequency of administration of all prescribed and over-the-counter medications at the time of the HMR were recorded on data collection sheets. The number of regular (prescribed or over the counter), PRN (as required) and complementary medications were recorded for each patient. Medications were coded according to the Anatomical Therapeutic Chemical (ATC) classification system. The DBI was measured using the previously published equation and methods.10,14 For historical control patients, the DBI was calculated manually and for intervention patients, DBI was measured manually and autogenerated using the DBI Calculator©.
Statistical analyses
All data were transferred from data collection sheets into computer databases. The collected data were analysed using IBM® Statistical Package for Social Sciences Statistics (SPSS® Version 21, Armonk, NY, USA).
To analyse the feasibility of implementing the DBI into HMR, descriptive statistics were used to report the patient, AP and GP responses to questions on feasibility and usefulness (Table 2). The written feedback provided by the APs about the implementation of the intervention was qualitatively analysed by content analysis. To analyse the accuracy of the DBI Calculator©, DBI scores calculated manually were compared with DBI scores autogenerated with the web-based platform for the intervention patient dataset. A Pearson’s correlation was used to determine the relationship between autogenerated and manually calculated DBI scores.
The Kolmogorov–Smirnov test and histogram were used to test the distribution of the DBI in the historical and intervention patient populations. As DBI was not normally distributed, the Mann–Whitney nonparametric test for continuous variables and χ2 test for categorical variables were used to compare characteristics between groups.
To analyse the effect of the DBI and HMR intervention on deprescribing anticholinergic and sedative medications in older adults, cross-sectional analyses were conducted to compare proportions of patients whose DBI increased, decreased or remained unchanged in the intervention group at baseline and 3 months.
Descriptive characteristics were summarized using means, SD [or median and interquartile ranges (IQR) for data not normally distributed] and proportions. The p value of less than 0.05 was set as statistically significant. Any missing data (not affecting the primary or secondary outcomes) were excluded from analyses.
Results
Study participation rates
As of June 2015, there were 26,179 general registered pharmacists in Australia, of whom 2396 were trained and practising APs. There were two rounds of advertising for APs to participate in this study (October 2014 and May 2015). Advertising for participation in this study reached an estimated 3196 pharmacists; 81 pharmacists enquired about the study (willingness to participate 2.5%) and were assessed for eligibility. Of these, 22 pharmacists were eligible and consented to participate in the study. Following stage 3 of the study, four pharmacists withdrew for various reasons (Figure 1). Of the 22 APs originally recruited, 21 provided data on their 10 most recent eligible HMR patients, resulting in a total of 210 historical controls. During the intervention stage, 18 APs screened a total of 354 HMR patients for eligibility and recruited 113 patients (recruitment rate 31.9%) into the study. Upon follow up, 13 patients withdrew (11.5% lost to follow up) resulting in a total of 100 patients in the intervention stage (Figure 1).
Characteristics of the accredited pharmacists
All APs had a Bachelor of Pharmacy qualification. The majority (70%) of APs had more than 15 years of experience working as a pharmacist, with 75% conducting >100 HMRs per year. APs were mainly practising in community pharmacy practice (55%) and practised in highly accessible areas in Australia (85%; Supplementary Table 3).
Characteristics of the patients
Across the historical control and intervention groups, the mean age (SD), proportion of male and female participants, marital status and body mass index were comparable. There was a significantly higher proportion of patients in the intervention patients group who lived in accessible areas compared with the historical patients (p = 0.037) (Supplementary Table 4). The mean (SD) of the BMQ-specific necessity and concerns questions were 3.4 (0.5) and 3.1 (0.6), respectively, indicating patients believed that their multiple medications were necessary and were slightly concerned about the adverse consequences about their medications.
Feasibility and utility of the DBI in Home Medicines Review
During stage 3, all pharmacists were able to produce a DBI report for their patients at baseline (n = 113). Twenty-seven patients and one GP reported not receiving the patient version of the DBI letter and DBI report, respectively. Within the HMR reports written by APs and sent to GPs (at 3 months, n = 100), 92 (92%) of reports contained a reference to the attached DBI report. When comparing the accuracy of the manual (considered gold standard, n = 100) and autogenerated DBI calculations (n = 97) for the intervention patients at baseline, the autogenerated DBI calculations did not match the manual calculations for 33 (33%) of the patients. Of the 33%, 26% of the autogenerated DBI calculations were underestimated and 7% were overestimated. At baseline, the mean (SD) autogenerated DBI was 0.81 (0.9), compared with manual DBI calculations 0.92 (1.0; r = 0.95, p < 0.01). At 3 months, the mean (SD) automated DBI was 0.71 (0.9), compared with manual DBI calculations 0.83 (0.9; r = 0.96, p < 0.01).
The feasibility and utility of the DBI report or letter are presented in Table 3. Most patients (81%), APs (89%) and GPs (83%) found the DBI report very or somewhat useful. Across all three groups, most participants found the DBI report easy to read and understand. Regarding assessing the DBI report as a risk assessment tool within the HMR service as part of usual care, 89% of APs and 67% of GPs agreed that it would be feasible. Furthermore, 57% of GPs used the information in the DBI report for their decision making. GPs found the DBI report more useful and feasible, and used the information for decision making when patients’ baseline DBI > 0 compared with patients with DBI = 0 (Table 4). Overall, although APs found that the DBI report was useful and complemented the HMR report, APs reported some issues with the website and report in practice (Table 5).
Table 3.
Responses to feasibility and utility questions from patients, APs and GPs who received/accessed the DBI report or letter.
| Feasibility/usefulness questions | Intervention patients (n = 73) | Accredited pharmacists (n = 18) | General practitioners (n = 42) |
|---|---|---|---|
| (1) ‘What did you think of the DBI report?’ n (%) | |||
| • Very useful/somewhat useful | 59 (80.8%) | 16 (88.9%) | 35 (83.3%) |
| • Not very useful/not useful at all | 12 (16.4%) | 2 (11.1%) | 7 (16.7%) |
| • Missing data | 2 (2.7%) | ||
| (2) ‘Was the report easy to read and understand?’ n (%) | |||
| Yes | 65 (89.0%) | 15 (83.3%) | 33 (78.6%) |
| No | 5 (6.8%) | 2 (11.1%) | 0 |
| Missing data | 3 (4.1%) | 1 (5.6%) | 9 (21.4%) |
| (3) ‘What feedback did you receive about the DBI report from the doctors?’ n (%) | |||
| • No Feedback | 14 (77.8%) | ||
| • Somewhat Useful | – | 3 (16.7%) | – |
| • Not Useful at all | 1 (5.6%) | ||
| (4) ‘Did you take it to the doctor and talk to him about it?’ n (%) | |||
| Yes | 50 (68.5%) | – | – |
| No | 22 (30.1%) | ||
| Missing data | 1 (1.4%) | ||
| (5) ‘Was the DBI tool valuable for your practice?’ n (%) | |||
| Yes | – | See Table 4 | 24 (57.1%) |
| No | 10 (23.8%) | ||
| Missing data | 8 (19.0%) | ||
| (6) ‘Do you think the DBI report is feasible to provide a medication risk assessment with the HMR process?’ n (%) | |||
| Yes | 16 (88.9%) | 28 (66.7%) | |
| No | 1 (5.6%) | 6 (14.3%) | |
| Missing data | 8 (19.0%) | ||
| (7) ‘Did you use the information on this report for decision making?’ n (%) | |||
| Yes | – | See Table 5 | 24 (57.1%) |
| No | 10 (23.8%) | ||
| Missing data | 8 (19.0%) |
AP, accredited clinical pharmacist; DBI, Drug Burden Index; GP, general practitioner.
Table 4.
Feasibility and utility of the DBI report among patients and GPs according to baseline DBI score.
| Intervention patient’s baseline DBI = 0 (n = 34) |
Intervention patient’s baseline DBI > 0 (n = 66) |
|
|---|---|---|
| GPs | ||
| (1) ‘What did you think of the DBI report?’ n (%) | ||
| • Very useful/ somewhat useful | 8 | 21 |
| • Not very useful/ not useful at all | 2 | 4 |
| • Missing data | 24 | 41 |
| (6) ‘Do you think the DBI report is feasible to provide a medication risk assessment with the HMR process?’ n (%) | ||
| Yes | 8 | 16 |
| No | 0 | 4 |
| Missing data | 26 | 46 |
| (7) ‘Did you use the information on this report for decision making?’ n (%) | ||
| Yes | 6 | 19 |
| No | 4 | 6 |
| Missing data | 24 | 41 |
| Patients | ||
| ‘What did you think of the DBI report?’ n (%) | ||
| • Very useful/ somewhat useful | 23 | 36 |
| • Not very useful/not useful at all | 2 | 10 |
| • Missing data | 9 | 20 |
DBI, Drug Burden Index; GP, general practitioner.
Table 5.
Written feedback from accredited pharmacists regarding the implementation of DBI into the Home Medicines Review (HMR) service.
|
General comments
• ‘The colour format certainly made it stand out.’ (AP #07) • ‘DBI useful quantitative, i.e. GP prescribing in number of patients.’ (AP #08) • ‘Colour was great … I think the quantification of the risk is excellent and very much calculating and reporting within the report … I’ve definitely gained more confidence around recommending reduction of these medications.’ (AP #09) • ‘Good feedback (received from the GPs) but extra reading for them.’ (AP #12) • ‘I think that it is a good tool that can be very useful in HMRs, particularly if a particular patient is experiencing those side effects.’ (AP #17) • ‘The report was useful as it gave a value which included a weighting for dose. It would have been good to receive some feedback from GPs.’ (AP #18) • ‘It was good, but time consuming to set up.’ (AP #04) |
|
Barriers to implementation
Report related • ‘Was very difficult to add patient details, etc. to the (report) as I don’t have the full editing software.’ (AP #09) • ‘(The reports) were somewhat cramped. This was due to the need to incorporate research approval, etc.’ (AP #01) • ‘I think that it needs more context, it reads as being very “researchy” rather than useful. Would be great to give some example of effect size of the various stratifications, for example, 0.5–1.0 increases falls risk by 50%, hospitalization due to delirium by 30% or whatever. Don’t really see the value in including the equation.’ (AP #02) • ‘DBI useful quantitative, that is, GP prescribing in number of patients, but not sure of effectiveness in changing prescribing for individual patients.’ (AP #08) • ‘The blurb at the bottom never stayed on the first page and ended up printing on third page with a blank second page, not professional, but I spent time tweaking to try to make it fit … the report takes up quite a bit of space for an HMR report (most doctors want 2–3 pages). I’m not sure if attaching a DBI report will greatly improve prescribing around sedating and anticholinergic medications.’ (AP #09) • ‘I wondered whether “landscape” format would be better: narrower section for “what is the DBI?” explanation and more room for pharmacist and doctor comments.’ (AP #13) • ‘Could do without the calculation mechanism at top of the report. Not sure if it adds anything.’ (AP #14) Website related • ‘Website was a bit annoying to use – time consuming to enter a lot of medications.’ (AP #02) • ‘Need to improve the efficiency of the medication input into the system. If could be accessed as part of HMR software that would be great but may be better to identify the at-risk medications to the doctors (not the full lists as they can get too long).’ (AP #09) • ‘Found it difficult to incorporate into my Word document report, it changed margins, layout or original report. I had to print out DBI report and fax to GP with HMR report (could not email DBI report with HMR report).’ (AP #11) • ‘Please speed up the website.’ (AP #14) |
AP, accredited clinical pharmacist; DBI, Drug Burden Index; GP, general practitioner.
Impact of DBI report intervention on prescribing and deprescribing
The median (IQR) DBI for the historical control patients was 0.50 (0–1.00). The intervention patients had a statistically significant higher median DBI of 0.82 (0–1.33) at baseline compared with the historical control (Mann–Whitney U test, z = −2.27, p = 0.023). At 3 months, the median (IQR) DBI for intervention patients significantly decreased to 0.67 (0.00–1.29; Wilcoxon signed-rank test, z = −2.45, p = 0.014). There was an equal distribution of patients with DBI = 0 and DBI > 0 across the historical and intervention datasets. The mean number of PRN and complementary medications were significantly higher for the intervention group (Table 6).
Table 6.
Medication characteristics of historical control and intervention patient study populations.
| Characteristic | Historical (n = 210) | Intervention (n = 100) | p value | |
|---|---|---|---|---|
| Baseline | 3 months | |||
| Medications (mean ± SD) | ||||
| Regular | 8.90 ± 3.7 | 9.51 ± 3.5 | 9.47 ± 3.6 | |
| PRN (as required) | 1.85 ± 1.8 | 2.35 ± 2.1$ | 2.40 ± 2.0 | $p = 0.031 |
| Complementary | 1.47 ± 1.5 | 1.90 ± 1.6‡ | 1.90 ± 1.6 | ‡p = 0.022 |
| DBI score (median, IQR) | 0.50 (0–1.00) | 0.82 (0.00–1.33)* | 0.67 (0.00–1.29)** | *p = 0.023, **p = 0.014 |
| DBI = 0 | 77 (36.7) | 34 (34.0) | 34 (34.0) | |
| DBI > 0 | 133 (63.3) | 66 (66.0) | 66 (66.0) | |
Only statistically significant values are shown.
Statistically significant p = 0.023; Mann–Whitney U test compared with historical control. **Statistically significant p = 0.014; Wilcoxon’s signed rank test compared with baseline intervention patients.
Statistically significant p = 0.031 compared with historical control.
Statistically significant p = 0.022 compared with historical control.
DBI, Drug Burden Index; IQR, interquartile range; SD, standard deviation.
Although the proportion of intervention patients at 3 months with DBI = 0 (n = 34) and DBI > 0 (n = 66) did not change, 36.4% of intervention patients with a baseline DBI > 0 had their DBI score decrease, whilst 6.1% had a score increase. For patients with a DBI = 0 at baseline, 8.8% of patients had their DBI increase at 3 months.
The medications prescribed (prescription and over the counter) for each patient participant in the historical and intervention datasets was analysed by ATC therapeutic class (Supplementary Table 5). The most common medications prescribed contributing to the DBI included oxycodone, pregabalin and amitriptyline. In the intervention group, there was a nonsignificant reduction in the number of patients using oxycodone, amitriptyline and pregabalin.
Discussion
This is the first study exploring the feasibility of implementing the DBI report, generated by the DBI Calculator©, into the HMR service as a risk assessment tool to guide medication review. Most patient participants, APs and GPs considered the DBI report very useful or somewhat useful. Regarding the feasibility of the DBI report as a risk assessment tool as part of usual care within the HMR service, 89% of APs and 67% of GPs agreed that it would be feasible (Tables 3 and 4). Further analysis demonstrated that the utility of the DBI report in practice was based on the patient’s DBI score: GPs considered the DBI report more useful for patients with DBI score > 0 compared with DBI score = 0 (Table 4). This may be due to the accustomed behaviour of GPs to recognize and act on a patient’s results when the score is above or below target ranges. The APs also provided written feedback relating to the implementation of the DBI report into HMR practice (Table 5). Barriers to implementation were identified mainly relating to the practicalities and content of the report. This feedback is different from the pilot study, where 80% of AP participants agreed or strongly agreed that the generation of the DBI report was feasible and may be implemented into routine practice as part of the usual HMR service with ease.25 This feedback will be considered for future studies and demonstrates the importance of conducting feasibility studies in practice settings.30
Feasibility studies are used to determine whether an intervention is appropriate for further testing and to estimate important parameters that are required to design larger randomized control studies.31 These parameters include willingness of clinicians to recruit participants, number of eligible patients, follow-up rates, and characteristics of the proposed outcome measure.32 Feasibility studies also include testing procedures for their acceptability and are an essential step in the development and testing of an intervention, prior to a largescale evaluation.33 Regarding the feasibility of conducting this study, 2.5% of accredited pharmacists expressed interest in participating in the study, whilst only 18 pharmacists completed the study. Factors limiting the participation of APs in the research project may have included finding it difficult to recruit patients in research, and research protocols that were time consuming and not straightforward.34 For patients screened for participation in the intervention arm, there was a recruitment rate of 31.9%, and 11.5% of those recruited were lost to follow up. Key factors likely to influence patient participation were the time taken for collection of additional data in person and by phone at baseline and follow up. A systematic review investigating the factors affecting the recruitment of patients to randomized controlled trials found that poor recruitment is common, many studies fail to meet recruitment targets, and factors such as educating clinicians, and incentives to participants, may help recruitment rates.35 As patients and GPs involved in this study were not provided with incentives, this may have contributed to the low recruitment rates. Future studies should consider these factors.
Regarding the potential inaccuracy of the web-based DBI Calculator©, differences between manual and autogenerated DBI calculations (at baseline and 3 months) may have been due to data entry errors by the APs or to errors within the web-based calculator, such as discrepancies due to combination formulations or multiple drugs with the same ingredient.
At 3 months, the median (IQR) DBI for patients in the intervention arm of the study significantly decreased from 0.82 (0.00–1.33) to 0.67 (0.00–1.29) (p = 0.014; Table 6). In this study, the baseline median DBI for intervention patients was higher than previous studies, which may reflect patient selection bias by the study pharmacists.14,18,19 However, the median DBI score for patients in the historical control was 0.50, which is similar to two previous retrospective studies conducted in similar settings.18,19
Although the proportion of patients at 3 months with DBI = 0 and DBI > 0 did not change from baseline, 36.4% of patients with a DBI > 0 at baseline had their DBI score decrease, whilst 6.1% had a score increase at 3 months. This reduction in DBI score is higher than the proportion of patients who were exposed to DBI medications and received an HMR only18 and slightly higher than the proportion of patients who had a decrease in their DBI scores in the pilot randomized controlled trial.14 For the intervention patients in this study, there were more sedative than anticholinergic medications prescribed where doses were ceased or reduced that resulted in a reduction in DBI scores. A trial involving deprescribing of anticholinergic and sedative medications (using DBI) in residential aged care facilities is currently in progress in New Zealand, and the results of this study will provide the opportunity to compare and determine rates of deprescribing these medications in older adults.36
This study has determined the recruitment rate of pharmacists and patients, follow-up rates of patients, pharmacists and GPs, the feasibility of implementing the DBI report as an intervention into the HMR setting, and the characteristics of measured outcomes: for example, proportion of patients referred for an HMR with a DBI > 0, and the proportion of patients who had their DBI changed as a result of the intervention. As this study was a feasibility study, the sample size was based on numbers required to test feasibility of conducting the intervention, and to estimate the sample size for a future randomized controlled trial. This was comparable with guidance on feasibility studies and published feasibility studies.37
There were several strengths in this study. Recent evidence, based on comprehensive systematic reviews of reducing polypharmacy in older adults to improve clinical outcomes, conclude that successful studies incorporate multidisciplinary and multifactorial interventions, including patient education and clinician reminders.38,39 This study involved: (a) patients, GPs and APs receiving or generating information about patients’ DBIs; (b) patients receiving information about the benefits and harms of using anticholinergic and sedative medications; (c) the education of APs on deprescribing anticholinergic and sedative medications in practice by implementation of the DBI report into practice; and (d) GPs receiving the patient’s DBI report (acting as an educational tool and reminder). The multidisciplinary and multifactorial aspects of this study are likely to have contributed to the observed significant decrease in median DBI scores at 3 months. Previous studies using the pharmacist-led medication review model have demonstrated that recommendations provided by pharmacists in this setting are evidence based and are accepted as appropriate by GPs.40,41 Therefore, in our study, GPs would have been accepting of AP recommendations to change anticholinergic and sedative medications dependent on information provided in the HMR and DBI reports. Additionally, use of a CCDSS platform to administer the intervention is an innovative approach in research conducted involving HMRs.
There were several potential limitations in this study. Due to the study design, not all GPs responded to the feasibility questions, resulting in a very poor response rate. Therefore, we were unable to obtain representative usability or feasibility data from the end users of the DBI Report. Additionally, pharmacists volunteered to be in this study by responding to advertising and were not specifically recruited. This may have attracted pharmacists who were knowledgeable and keen to be involved in medication management of older adults resulting in selection bias in the study. A high proportion of pharmacists recruited in this study (85%) practised in highly accessible (urban) areas in Australia. In 2012, 92% of employed pharmacists worked in major cities or inner regional centres, therefore the high feasibility as demonstrated by the opinions of healthcare practitioners may not be generalizable to other settings.42 There was also no consistency in the content or style of HMR reports written by pharmacists, with highly variable levels of detail in HMR reports sent to GPs. This may have contributed to the variability in GP uptake of recommendations relating to anticholinergic and sedative medications, and performance bias to the study. Future studies should analyse recommendation acceptance rates to assess the effectiveness of the intervention. Due to the retrospective nature of the historical control dataset and the difficulty of obtaining patient medication plans, direct comparisons could not be made on the effect of implementing the DBI report in the HMR service. Technical improvements to the DBI Calculator© may also improve the accuracy of reporting the DBI to GPs, such as upgrading the website and accuracy of the medications used to calculate DBI scores, especially with combination formulations.
Conclusion
Overall, implementation of the DBI report generated by the DBI Calculator© into the HMR service was feasible and useful, as reported by older adults, APs and GPs. The median (IQR) DBI of intervention patients was reduced from 0.82 (0.00–1.33) to 0.67 (0.00–1.29) (p = 0.014), demonstrating the deprescribing of anticholinergic and sedative medications in this patient cohort. Of those patients with a DBI > 0 at baseline (n = 66), the DBI score was reduced in 36.4% of patients at 3 months.
The role of the DBI Calculator© in practice would be to raise awareness and understanding of the cumulative risk of an older patient’s anticholinergic and sedative medications to their physical function, minimizing but not necessarily eliminating their exposure. Future studies to evaluate the recommendations of pharmacists, opinions of treating clinicians, and clinical outcomes of patients would help elucidate the clinical appropriateness of these medications for each patient.
Supplemental Material
Supplemental material, Supplementary_131218 for Integration of an electronic Drug Burden Index risk assessment tool into Home Medicines Reviews: deprescribing anticholinergic and sedative medications by Lisa Kouladjian O’Donnell, Danijela Gnjidic, Timothy F. Chen and Sarah N. Hilmer in Therapeutic Advances in Drug Safety
Acknowledgments
This work was conducted during LKO’s PhD candidature with the support of an Australian National Health Medical Research Council (NHMRC) Primary Healthcare Scholarship. DG is supported by the Australian NHMRC Dementia Leadership Fellowship. The Drug Burden Index Calculator ©. All rights reserved 2019.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Lisa Kouladjian O’Donnell  https://orcid.org/0000-0003-0927-7295
https://orcid.org/0000-0003-0927-7295
Danijela Gnjidic  https://orcid.org/0000-0002-9404-3401
https://orcid.org/0000-0002-9404-3401
Contributor Information
Lisa Kouladjian O’Donnell, Departments of Clinical Pharmacology and Aged Care, Level 13 Kolling Building, Royal North Shore Hospital, Reserve Road, St Leonards, NSW 2065, Australia.
Danijela Gnjidic, School of Pharmacy, Faculty of Medicine and Health and Charles Perkins Centre, The University of Sydney, Sydney, NSW, Australia.
Timothy F. Chen, School of Pharmacy, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia
Sarah N. Hilmer, Departments of Clinical Pharmacology and Aged Care, Royal North Shore Hospital, Sydney, NSW, Australia NHMRC Cognitive Decline Partnership Centre, Kolling Institute of Medical Research, Sydney Medical School University of Sydney, NSW, Australia
References
- 1. Brett T, Arnold-Reed DE, Popescu A, et al. Multimorbidity in patients attending 2 Australian primary care practices. Ann Fam Med 2013; 11: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Britt HC, Harrison CM, Miller GC, et al. Prevalence and patterns of multimorbidity in Australia. Med J Australia 2008; 189: 72–77. [DOI] [PubMed] [Google Scholar]
- 3. Morgan TK, Williamson M, Pirotta M, Stewart K, Myers SP, Barnes J. A national census of medicines use: a 24-hour snapshot of Australians aged 50 years and older. Med J Australia 2012; 196: 50–3. [DOI] [PubMed] [Google Scholar]
- 4. Hilmer SN. The dilemma of polypharmacy. Aust Prescr 2008; 31: 2–3. [Google Scholar]
- 5. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012; 65: 989–995. [DOI] [PubMed] [Google Scholar]
- 6. Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging 1993; 3: 335–348. [DOI] [PubMed] [Google Scholar]
- 7. Nishtala PS, Fois RA, McLachlan AJ, et al. Anticholinergic activity of commonly prescribed medications and neuropsychiatric adverse events in older people. J Clin Pharmacol 2009; 49: 1176–1184. [DOI] [PubMed] [Google Scholar]
- 8. Bell JS, Mezrani C, Blacker N, et al. Anticholinergic and sedative medicines - prescribing considerations for people with dementia. Aust Fam Physician 2012; 41: 45–49. [PubMed] [Google Scholar]
- 9. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 10. Hilmer SN, Mager DE, Simonsick EM, et al. A Drug Burden Index to define the functional burden of medications in older people. Arch Intern Med 2007; 167: 781–787. [DOI] [PubMed] [Google Scholar]
- 11. Hilmer SN. Calculating and using the Drug Burden Index score in research and practice. Expert Rev Clin Pharmacol 2018: 1–3. [DOI] [PubMed] [Google Scholar]
- 12. Kouladjian L, Gnjidic D, Chen T, et al. The Drug Burden Index in older adults: theoretical and practical issues. Clin Int Aging 2014; 9: 1503–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wouters H, Van der Meer H, Taxis K. Quantification of anticholinergic and sedative drug load with the Drug Burden Index: a review of outcomes and methodological quality of studies. Eur J Clin Pharmacol 2017; 73: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gnjidic D, Le Couteur DG, Abernethy DR, et al. A pilot randomized clinical trial utilizing the Drug Burden Index to reduce exposure to anticholinergic and sedative medications in older people. Ann Pharmacother 2010; 44: 1725–1732. [DOI] [PubMed] [Google Scholar]
- 15. Van der Meer HG, Wouters H, Pont LG, et al. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open 2018; 8: e019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gowan J. Home medicine reviews and the aged. J Complement Med 2006; 5: 30–32. [Google Scholar]
- 17. Chen TF. Pharmacist-led home medicines review and residential medication management review: the Australian model. Drugs Aging 2016; 33: 199–204. [DOI] [PubMed] [Google Scholar]
- 18. Castelino RL, Hilmer SN, Bajorek BV, et al. Drug Burden Index and potentially inappropriate medications in community-dwelling older people: the impact of Home Medicines Review. Drugs Aging 2010; 27: 135–148. [DOI] [PubMed] [Google Scholar]
- 19. Nishtala PS, Hilmer SN, McLachlan AJ, et al. Impact of residential medication management reviews on Drug Burden Index in aged-care homes: a retrospective analysis. Drugs Aging 2009; 26: 677–686. [DOI] [PubMed] [Google Scholar]
- 20. Jokanovic N, Tan ECK, Van den Bosch D, et al. Clinical medication review in Australia: a systematic review. Res Social Adm Pharm. Epub ahead of print 9 July 2015. DOI: 10.1016/j.sapharm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 21. He ZK, Ball PA. Can medication management review reduce anticholinergic burden (ACB) in the elderly? Encouraging results from a theoretical model. Intern Psychogeriatr 2013; 25: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 22. West T, Pruchnicki MC, Porter K, et al. Evaluation of anticholinergic burden of medications in older adults. J Am Pharmacists Assoc 2013; 53: 496–504. [DOI] [PubMed] [Google Scholar]
- 23. Karimi S, Dharia SP, Flora DS, et al. Anticholinergic burden: clinical implications for seniors and strategies for clinicians. Consult Pharm 2012; 27: 564–582. [DOI] [PubMed] [Google Scholar]
- 24. Gould RL, Coulson MC, Patel N, et al. Interventions for reducing benzodiazepine use in older people: meta-analysis of randomised controlled trials. Br J Psychiatry 2014; 204: 98–107. [DOI] [PubMed] [Google Scholar]
- 25. Kouladjian L, Gnjidic D, Chen TF, et al. Development, validation and evaluation of an electronic pharmacological tool: the Drug Burden Index Calculator©. Res Social Adm Pharm 2016; 12: 865–875. [DOI] [PubMed] [Google Scholar]
- 26. Kouladjian L, Chen TF, Gnjidic D, et al. Education and assessment of pharmacists on the use of the Drug Burden Index in older adults using a continuing professional development education method. Am J Pharmaceut Educ 2016; 80: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patterson SM, Cadogan CA, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2014: CD008165. [DOI] [PubMed] [Google Scholar]
- 28. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999; 14: 1–24. [Google Scholar]
- 29. Pharmacy Access/Remoteness Index of Australia. Australian Population and Migration Research Centre: University of Adelaide, 2013. Available at: https://www.adelaide.edu.au/hugo-centre/services/pharia [Google Scholar]
- 30. Van Teijlingen ER, Rennie A-M, Hundley V, et al. The importance of conducting and reporting pilot studies: the example of the Scottish Births Survey. J Adv Nurs 2001; 34: 289–295. [DOI] [PubMed] [Google Scholar]
- 31. Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prevent Med 2009; 36: 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arain M, Campbell MJ, Cooper CL, et al. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 2010; 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Medical Research Council. Developing and evaluating complex interventions: new guidance. UK: Medical Research Council, 2006. [Google Scholar]
- 34. Saini B, Brillant M, Filipovska J, et al. Recruitment and retention of community pharmacists in pharmacy practice research. http://6cpa.com.au/wp-content/uploads/final-report-Community-Pharmacy-Research-Support-Centre-recruitment-and-retention-of-community-pharmacists-in-pharmacy-practice-research.pdf (accessed 12 February 2019). Sydney, Australia: Faculty of Pharmacy, University of Sydney, 2005. [Google Scholar]
- 35. Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol 2006; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ailabouni N, Mangin D, Nishtala PS. Deprescribing anticholinergic and sedative medicines: protocol for a Feasibility Trial (DEFEAT-polypharmacy) in residential aged care facilities. BMJ Open 2017; 7: e013800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol 2013; 13: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gnjidic D, Le Couteur DG, Kouladjian L, et al. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med 2012; 28: 237–253. [DOI] [PubMed] [Google Scholar]
- 39. Patterson SM, Hughes C, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2012; 5: CD008165. [DOI] [PubMed] [Google Scholar]
- 40. Castelino RL, Bajorek BV, Chen TF. Are interventions recommended by pharmacists during Home Medicines Review evidence-based? J Eval Clin Prac 2011; 17: 104–110. [DOI] [PubMed] [Google Scholar]
- 41. Krska J, Cromarty JA, Arris F, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing 2001; 30: 205–211. [DOI] [PubMed] [Google Scholar]
- 42. Australian Government. Health Workforce Australia. Australia’s Health Workforce Series-Pharmacists in Focus. 2014. Available at: http://iaha.com.au/wp-content/uploads/2014/03/HWA_Australia-Health-Workforce-Series_Pharmacists-in-focus_vF_LR.pdf (accessed 15 February 2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_131218 for Integration of an electronic Drug Burden Index risk assessment tool into Home Medicines Reviews: deprescribing anticholinergic and sedative medications by Lisa Kouladjian O’Donnell, Danijela Gnjidic, Timothy F. Chen and Sarah N. Hilmer in Therapeutic Advances in Drug Safety