Abstract
Multiple sclerosis (MS) is a major cause of disability in young adults. Following an unknown trigger (or triggers), the immune system attacks the myelin sheath surrounding axons, leading to progressive nerve cell death. Antibodies and small-molecule drugs directed against B cells have demonstrated good efficacy in slowing progression of the disease. This review focusses on small-molecule drugs that can affect B-cell biology and may have utility in disease management. The risk genes for MS are examined from the drug target perspective. Existing small-molecule therapies for MS with B-cell actions together with new drugs in development are described. The potential for experimental molecules with B-cell effects is also considered. Small molecules can have diverse actions on B cells and be cytotoxic, anti-inflammatory and anti-viral. The current B cell–directed therapies often kill B-cell subsets, which can be effective but lead to side effects and toxicity. A deeper understanding of B-cell biology and the effect on MS disease should lead to new drugs with better selectivity, efficacy, and an improved safety profile. Small-molecule drugs, once the patent term has expired, provide a uniquely sustainable form of healthcare.
Keywords: Multiple sclerosis, MS, B cells, small molecule drugs, sustainable healthcare
Introduction
Multiple sclerosis (MS) affects over two million people worldwide (National Multiple Sclerosis Society, https://www.nationalmssociety.org/). The disease is characterised by an initial autoimmune-driven, inflammatory phase followed by immune-mediated attack on the myelin surrounding nerve axons. Focal cortical lesions may develop in distinct locations. Eventually, the ensuing damage results in progressive nerve loss and increasing disability evident as disrupted motor function, visual disturbances, and bladder problems 1. As disease onset is often reported in young adults, its course can affect people for most of their adult lives. MS is considered to present in several forms: relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), primary progressive MS (PPMS) and progressive-relapsing MS (PRMS). Diagnosis of the disease is complex; there is no single test for MS, and multiple criteria, including magnetic resonance imaging (MRI) scans are used 2.
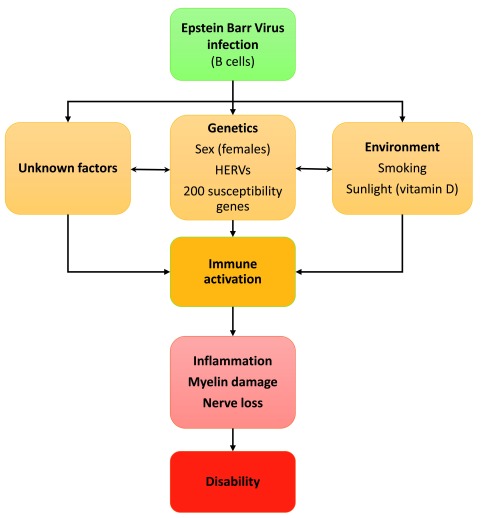
The causes of the disease are multifactorial, with infectious, genetic and environmental factors, such as lack of sunlight (through vitamin D), playing a role ( Figure 1) 3. Large genome-wide association studies (GWASs) have gradually allowed the risk genes associated with MS to be elucidated. Up to now, genetics alone has not proven useful in diagnosis 4, but the identified risk genes have been informative about the mechanism and contributors to the disease and may be of aid in predicting severity 5. Among the more than 200 risk genes, most with a link to immune function, identified as susceptibility factors, the strongest associations are with the HLA-DRB1 (human leucocyte antigen) locus in the major histocompatibility complex (MHC) 4.
Figure 1. Causes and progression of multiple sclerosis (MS).
Several studies now indicate that Epstein–Barr virus infection is necessary (but not causal) for MS to develop. Genetic factors may explain 50% of MS susceptibility whereas environmental factors together with unknowns may combine to trigger immune activation and the subsequent destruction of myelin and oligodendrocytes. This eventually leads to axonal damage and nerve cell death resulting in disability. HERV, human endogenous retrovirus.
Although multiple types of immune cells have been implicated in the pathology of MS [4], the role of B cells has recently come to the fore 6; notable clinical successes for agents which target B cells, such as CD20-targeted antibodies, rituximab, ocrelizumab and ofatumumab, are reported. In addition, an analysis of agents used to treat MS indicated that activity against a specific subset of B cells, the CD19 +CD27 + memory B cells, correlated with clinical efficacy 7– 9.
Despite this strong driver to develop new B cell–directed therapies, the current most popular animal model used to study MS-like pathologies, particularly inflammation and neurodegeneration—experimental autoimmune encephalomyelitis (EAE) in mice—does not allow an assessment of a causative role for B-cell involvement, complicating further development 10. A review of animal models unsurprisingly points to primate models, such as the marmoset, as the most representative of the human disease 11.
The recent focus on how B cells contribute to MS pathology also renews interest in the role of Epstein–Barr virus (EBV) infection in the aetiology of the disease. EBV is present in a high percentage of the human population, preferentially infects B cells, and establishes a lifelong infection in memory B cells 12. The impact of EBV in MS is controversial; some convincing recent studies indicate that infection with EBV may underlie the development of MS. Over 99% of people with MS are infected with EBV, and it has been argued that methodological differences may account for the small number of EBV-negatives 13. Although the effect of EBV has been extensively investigated in B cells and is also present in astrocytes and microglia of people with MS (pwMS) 14, the impact of EBV infection in the brain is relatively little studied. Thus, the extent and mechanism of the EBV effect remains somewhat obscure and more research is needed in this area. Numerous mechanistic links between EBV infection and MS pathology have been noted 15. Some of the most persuasive arguments are summarised in Table 1 16– 26.
Table 1. Supporting and opposing arguments for EBV involvement in MS.
| Supporting arguments | Opposing arguments (partly from 16) |
|---|---|
| • Epstein–Barr virus (EBV) infects and is latent in memory B cells, the same cell
type shown to be critical in multiple sclerosis (MS) by successful CD20 antibody and cladribine treatment. • Genome-wide association studies (GWASs) have identified a correlation between anti-EBV nuclear antigen-1 (EBNA-1) IgG titres and MS 27. Human leucocyte antigen (HLA) single-nucleotide polymorphisms showed the strongest correlation with two other genes: EVI5 and EOMES 28. • GWAS analysis reveals genetic overlap between susceptibilities to Hodgkin’s lymphoma (caused by EBV) and MS 29. • In B cells transformed into immortalised lymphoblasts by EBV infection, the vitamin D receptor (VDR) (nuclear receptor) interacts with the EBV nuclear protein EBNA3. This results in inhibition of VDR-promoted gene expression and provides a mechanistic link between vitamin D, EBV and MS 30. • EBV uses HLA DRB1 and HLA DRQ allelic variants as entry co-receptors. This is widely assumed to have immune consequences. A link between HLA variants and infectivity by EBV has not yet been made, although this has been proposed for rheumatoid arthritis 31. • Infectious mononucleosis is caused by EBV infection and is associated with an increased risk of MS 32. • EBV is found to activate the human endogenous retroviruses HERV-W/MSRV/ Syncytin-1 (human endogenous retrovirus type W/multiple sclerosis–associated retrovirus/Syncytin-1) in blood and brain cells taken from people with MS (pwMS). MSRV specifically has been reported to correlate with progression to MS 33 and with infectious mononucleosis. • A virus closely related to EBV, the γ1-herpesvirus CalHV3, infects marmosets, is resident in memory B cells, and is implicated in the marmoset experimental autoimmune encephalomyelitis model of experimental MS 34. |
• Seropositivity for pwMS infected with EBV does
not reach 100%, especially for children with early- onset MS 35. The high background of people infected with EBV clouds the interpretation. • Serum EBNA-1 antibodies are associated with MS, but no target has been found, and antibody levels are not associated with disease activity 36. • EBV seroconversion may just be a hygiene marker, and other parasites could be responsible for the development of MS 37. |
Other infections such as human herpes virus 6 16 and pinworms 17 have also been implicated in MS.
The developing picture for MS is of a disease caused by the interaction of multiple risk factors ( Figure 1), one being EBV infection. EBV could initiate changes in infected cells that lead to immune activation and a pro-inflammatory state. This alone is not sufficient to cause MS, but genetic and environmental factors then can be activated, interact and trigger the disease. Treatments defined as disease-modifying therapies (DMTs) are usually immune-modulators, other drugs treat symptoms such as limb spasticity, bladder problems, or pain. There are as yet no therapies approved to treat neurodegeneration 18. New immunomodulators have transformed the treatment of RRMS; at least 16 drugs are now licensed by the US Food and Drug Administration (FDA) ( https://www.nationalmssociety.org/Treating-MS/Medications) and these have generated a $20 billion market 19. Although the number and severity of relapses have been reduced, neurological damage still occurs from onset and the disease often continues to the progressive form. Furthermore, those patients with diagnosed PPMS have historically had no DMTs available to them.
Notably, the first drug indicated for PPMS, the anti-CD20 antibody ocrelizumab (Ocrevus ®), was approved by the FDA in 2017 20. Ocrelizumab and other anti-CD20 therapies rapidly deplete circulating B cells. Their effectiveness in patients with PPMS and RRMS points to an important role for B cells in multiple MS forms. In a phase 3 trial, ocrelizumab was associated with lessened disease activity and progression in patients with RRMS compared with interferon beta-1a 21. Along the same lines, a 2018 study compared rituximab (an anti-CD20 antibody approved for other conditions) as an initial treatment for patients with RRMS and found its clinical efficacy to be notably superior to that of several other first-line DMTs, including dimethyl fumarate, natalizumab and fingolimod 22.
These agents have been paradigm-changing in providing the first DMT for patients with PPMS, a potentially more effective first line of treatment for RRMS, as well as turning the therapeutic focus from T cells (previously thought of as the main autoimmune effector cell in MS) to B cells. The remarkable clinical evidence with these drugs clearly illustrates that B cells play a central role in MS pathogenesis. Although this is exciting, there does remain a critical need for improvements to existing treatments in RRMS and new stand-alone treatments for SPMS and PPMS that serve to minimise adverse effects and the burden of high expense.
This review focusses on small-molecule approaches to immune-related MS therapy, covering agents on the market, in clinical trials, and some new research approaches. We specifically emphasise potential B cell–targeted therapies and the effects of already-available therapies on the B-cell population because of the recent notable success of ocrelizumab and other anti-CD20 antibodies.
Small molecules have unique and beneficial properties as therapies. The advantages include the ability to access intracellular targets and penetrate the central nervous system (CNS), low cost of manufacture, ease of administration, and the option to withdraw therapy rapidly. The primary disadvantage of small molecules is their potential lack of cellular specificity. The low cost of manufacture of small molecules compared with biological or cell-based therapies is often overlooked, but with increasing healthcare demands, a path to sustainable and affordable treatments is required for the future.
Identifying the genes and proteins key to multiple sclerosis pathophysiology as potential drug targets
Large GWASs have identified multiple genes associated with MS (reviewed in 4). Of note, HLA-DRB1*15:01 has an odds ratio (OR) of about 3.5; OR is the ratio of people with a proposed disease-specific allele to those who do not have the disease, where 1 is no association and more than 1 indicates an increased risk. In total, 31 HLA genes have been found 23. Non-HLA genes have also been indicated to contribute to genetic susceptibility; in particular, a very recent communication by the International Multiple Sclerosis Genetics Consortium (IMSGC) ( Supplementary Table 1, Table 1) lists over 200 genes, showing the non-HLA-related genes 23.
The study builds on an earlier publication by the IMSGC detailing 97 non-HLA genes 24. The later gene set does not cover all of the earlier genes. For this review, we used the UniprotKB curated database to provide high-quality information on the gene sets ( Supplementary Table, Table 1) and use this information to relate the genes to specific cell types.
B cells
In the 2018 IMSGC set, the UniprotKB database indicates that 31 genes either are highly expressed in or have some B-cell function noted ( Supplementary Table, Table 2). Only two of these genes appear to have been drugged: CTLA4 (ipilimumab) and the cannabinoid receptor 2, CNR2 (cannabidiol).
Epstein–Barr virus
In the IMSGC gene set from 2013, four genes ( PXT1, ZMIZ1, EOMES and TRAF3) are linked to EBV. Further investigations have identified 47 EBV genes from transcriptomes of B cells and EBV cells infected at Latency III (LCLs) associated with MS 25. This study highlights the EBV-encoded cell surface protein LMP1 and its signalling pathway as a potential target for MS.
Vitamin D
Two well-recognised MS susceptibility genes— CYP27B1 and CYP24A1—control metabolism of vitamin D (1,25-dihydroxyvitamin D3) for its receptor (VDR) 3. Lack of 1,25-dihydroxyvitamin D3 is associated with several immune diseases. The synthesis of 1,25-dihydroxyvitamin D3 is enhanced by sunlight and thus provides a mechanistic basis for the observed latitude dependence of MS. Further studies highlighted ZMIZ1, ZMIZ1- AS1 (AS stands for anti-sense, signifying that the single-nucleotide polymorphism is on the anti-sense strand) and IRF8. The VDR influences the expression and function of many other genes. TAGAP (T-cell activation RhoGTPase activating protein, which has a role in Th17 differentiation) and IL2RA were also identified by the GWASs and as 1,25-dihydroxyvitamin D3 target genes in a study on CD4 + T cells 26.
In the 2017 IMSGC report on the 200-plus gene set, the authors acknowledge that CNS genes may be under-represented. They partly address this by conducting an RNA-Seq study on cortex material to provide a data set more representative of CNS genes altered by the disease pathology. Only two of the RNA-Seq genes are represented in the 2013 IMSGC GWAS set: GALC and RGS14. In the 2017 IMSGC set, RGS14 and PVR are common genes.
Astrocytes
Five genes in the GWAS sets are expressed in or linked to astrocyte function ( CLEC16A, IL22RA2, TNFRSF1A, CYP24A1 and PHGDH). Also, seven genes from these sets have a link to neurodegeneration excluding that of MS ( GALC, PITPNM2, DIKKL1, SLC2A4RG, FCRL1 and PHDGH). One other gene links to neurodegeneration ( NPEPPS); this gene codes for an aminopeptidase which may regulate neuropeptide activity and tau levels 38, 39.
Oligodendrocytes
Three genes— OLIG3 (oligodendrocyte transcription factor), ZNF365 (DISC1-binding zinc finger protein) and BCAS—are key to oligodendrocyte function. BCAS was recently reported as being present in MS lesions 40; mice lacking this gene display hypomyelination 41. The interaction database STRING links BCAS1 to QKI, a gene thought to play a major role in oligodendrocyte differentiation and myelination 42.
Intracellular organelles
Mitochondria. Mitochondrial dysfunction is thought to play a role in neurodegeneration 43. In the GWAS and RNA-Seq studies, 23 genes, including the CYP24A1 vitamin D susceptibility gene, link to mitochondria. Several others are linked to oxidation reduction and the electron transport chain: COXM1, WWOX, PRDX5, IPYR2, CYB and ALDH2 ( Supplementary Table, Table 6). Previous studies highlight the electron transport chain as a dysregulated pathway in MS lesions, although the differently expressed genes listed in these studies ( Supplementary Table, Table 7) differ from those reported in the GWAS studies. Only two genes— CYTB (cytochrome b [complex III subunit 3, mitochondrially encoded]) and CASQ1 (calsequestrin-1 [calmitine])—were reported by both the IMSG RNA-Seq 2017 study and the 2012 study. Cytochrome b is, of course, intimately involved in the generation of the proton gradient that ultimately produces ATP. Defects in the electron transport chain are also linked to reactive oxygen species (ROS) generation. Calsequestrin-1 is a calcium binding protein in muscle. Deletion of the gene in mice or mutations in the human gene or both cause muscle atrophy and mitochondrial dysfunction 44. Defects in this pathway underscore the rationale for anti-oxidant approaches, such as Nrf-2 activation (dimethylfumarate) 45 or permeability pore inhibition via mitochondrial cyclophilin D 46.
The picture is broadly reflective of MS pathology. However, very few of these genes have been pursued as drug targets and even fewer taken into clinical trials. A recent study attempted to concatenate all of the GWAS data and has produced two more restricted gene lists for pwMS versus control and pwMS undergoing treatment. This study identified interferon-gamma signalling as the major pathway involved 47. Importantly, the multiple genes associated with B-cell function/dysfunction and EBV support a role in MS pathology.
Immune cells: small-molecule drugs
In Table 2, a method of assessing the likely CNS penetration of a compound is given where there are no published data. The scoring method used is the multiparameter optimisation method 48.
Table 2. Multiple sclerosis (MS)-relevant small molecules with activity against immune cells.
| Name | Structure | Target/Mode of action | Relevance to MS | Central nervous system penetration |
|---|---|---|---|---|
| Bortezomib |

|
Proteasome | No studies reported | Does not cross the blood–brain
barrier (BBB) 68 aMultiparameter optimisation (MPO) = 2.8 |
| Carfilzomib |
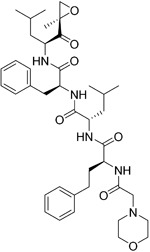
|
Proteasome | No studies reported | Does not cross the BBB
69
MPO = 1.0 |
| Ibrutinib |

|
B-cell progenitor (Bruton) tyrosine
kinase is highly expressed in immature B cells, monocytes and natural killer (NK) cells but not T cells. |
No development reported | In mice, Ibrutinib rapidly crosses
the BBB (in 0.29 hours). Brain-to- plasma ratio average for ibrutinib was found to reach 0.7 70. MPO = 2.5 |
| Evobrutinib |

|
B-cell progenitor (Bruton) tyrosine
kinase |
Positive phase IIB results were reported in MS
(7 March 2018). |
Capability to pass the BBB has not
been assessed. MPO = 2.0 |
| PRN2246
Possible structure shown |
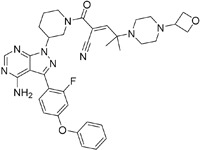
|
B-cell progenitor (Bruton) tyrosine
kinase |
Sanofi is developing a Principia MS candidate 71. | A recent press release confirmed
that PRN2246 crosses the BBB in humans and achieves therapeutic levels in the cerebral spinal fluid 72. MPO = 3.4 |
| Idelalisib |
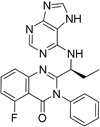
|
PI3Kδ is widely expressed in
immune cells. |
No development reported | Does not cross a healthy BBB
73
MPO = 2.9 |
| CP-25 paeoniflorin-6′-
O-benzene sulfonate |

|
BAFF | No development reported | A recent tissue distribution and
excretion study in rats suggests that CP-25 can pass through the BBB with significant differences seen between rat genders 74. MPO = 2.0 |
| Cladribine |
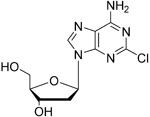
|
Adenosine deaminase | Positive results in phase III CLARITY study
75–
77
Positive results in phase III CLARITY extension study 2 Positive results in phase III ORACLE MS study 78, 79 Positive results in phase II ONWARD study adding oral cladribine to interferon beta-1a treatment 80 Long-term follow-up of safety currently active (PREMIERE) US Food and Drug Administration (FDA) accepted New Drug Application resubmission in July 2018 (Melao, FDA to Review EMD Serono’s New Request for Approval of Cladribine for Treating Relapsing MS, 2018 https:// multiplesclerosisnewstoday.com/2018/07/31/ fda-accepts-resubmitted-nda-cladribine-tablets- treatment-relapsing-ms/">https://multiplesclerosisnewstoday.com/2018/07/31/fda-accepts-resubmitted-nda-cladribine-tablets-treatment-relapsing-ms/). |
Cladribine concentration in the
cerebrospinal fluid (CSF) is about 25% of the plasma concentration at dose rates between 0.17 and 2.5 mg/m 2 per hour intravenously in patients without known meningeal disease 54. MPO = 3.3 |
| Maribavir |
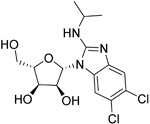
|
Potential Epstein–Barr virus (EBV)
treatment |
No studies reported | Maribavir does not cross
the BBB in rats. In monkeys, maribavir levels in the brain, CSF and vitreous humour range from 4-20%, 1-2% and <1% of corresponding plasma concentration, respectively 81. MPO = 3.4 |
| KAY-2-41 |
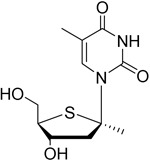
|
DNA polymerase in EBV-infected
cells |
No studies reported | Capability to pass the BBB has not
been assessed. MPO = 4.2 |
| KAY-39-149 |
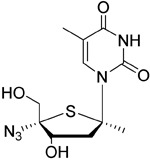
|
DNA polymerase in EBV-infected
cells |
No studies reported | Capability to pass the BBB has not
been assessed. MPO = 3.2 |
| HPMP-5-azaC |
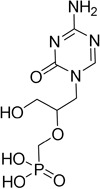
|
DNA polymerase | No studies reported | Capability to pass the BBB has not
been assessed. MPO = 3.0 |
| Glatiramer acetate
(random peptides of the following amino acids L-Glutamic Acid peptide with L-Alanine L-Lysine L-Tyrosine Acetate Salt) |
Alters antigen-presenting cell (APC)
function, potentially B cells |
FDA approved in 1997 as Copaxone for
relapsing-remitting MS (RRMS) and clinically isolated syndrome (CIS). Two generic forms were approved in 2017. |
Owing to its high polarity and
hydrophilic nature, glatiramer acetate does not penetrate the BBB 82. |
|
| Dimethyl fumarate
( Tecfidera) |
|
Antigen-presenting and cytokine-
producing functions of B cells |
FDA approved in 2013 for RRMS | Metabolite monomethyl
fumarate (MMF), the primary metabolite observed after oral dimethylfumarate (DMF) dosing, crossed the BBB. MMF exposure was measured 30 min after a single oral dose of DMF (100 mg/kg) in wild- type mice. The highest concentrations were in plasma (307.2–54.79 µM), and exposures of about 100 µM were observed between peripheral organs and brain 83. MPO = 5.0 |
| Fostamatinib |
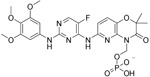
|
Protein tyrosine kinase syk (spleen
tyrosine kinase) |
No development reported | Capability to pass the BBB has not
been assessed. MPO = 2.4 |
| Fingolimod
Requires phosphorylation for activation |
|
Sphingosine-1-phosphate receptor
(S1P 1/3/4/5) |
FDA approved in 2011 for RRMS
Approved for children and adolescents with RRMS in 2018. Phase IV study comparing fingolimod with dimethyl fumarate is currently recruiting. |
FTY720 has been shown to
cross the BBB in rats, dogs and humans. Tissue-to-blood partition coefficient values in rats were 22.9 for lymph nodes, 27.1 for brain, and 15.7 for thymus. MPO = 2.8 |
| Amiselimod |
|
S1P 1 | Positive results in phase II MOMENTUM study for
RRMS 84 |
Capability to pass the BBB has not
been assessed. MPO = 2.8 |
| Siponimod |
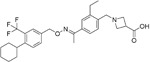
|
S1P 1/ S1P 5 | Positive results in phase II trial for RRMS
84
Mechanistic phase III trial completed Efficacy and safety phase III EXPAND trial is currently active for patients with secondary progressive MS (SPMS). |
Brain distribution was examined
using an iodine-labelled analogue of siponimod, [ 123I]MS565. Intravenous injections gave concentrations in the rhesus monkey brain of 0.008 to 0.014 %ID/mL at around 24 hours 85. MPO = 2.3 |
| Ozanimod |
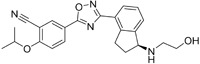
|
S1P 1/ S1P 5 | Positive results in phase II RADIANCE study
86
Positive results in phase III SUNBEAM study 87 |
Ozanimod effectively crosses the
BBB and demonstrated a high volume of distribution in both mouse and rat species, 10 and 13 L/kg, with a brain-to-blood ratio of 10:1 and 16:1, respectively 88. MPO = 3.7 |
| Ceralifimod |
|
S1P 1/ S1P 5 | Positive results in phase II and phase II extension
for RRMS (DreaMS) 89 Merck is not pursuing phase III development. |
Capability to pass the BBB has not
been assessed. MPO = 3.0 |
| Ponesimod |
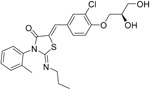
|
S1P 1 | Positive results in phase II
90
Phase II extension is active. Phase III POINT study is currently recruiting. |
Capability to pass the BBB has not
been assessed. MPO = 1.9 |
| GSK2018682 |

|
S1P 1/ S1P 5 | Phase I trial completed | Capability to pass the BBB has not
been assessed. MPO = 3.1 |
| Laquinimod |

|
APC/nuclear factor-kappa B (NF-κB)
pathway /Inhibits cytokines and lymphocyte migration into the central nervous system |
Phase III trial completed for RRMS | The laquinimod concentration in the
cerebrum, cerebellum and spinal cord reached about 7% to 8% in relation to the blood concentration at the time point of maximum concentration (2 hours post-dose) in healthy rats with intact BBB 91. MPO = 5.3 |
| Teriflunomide |
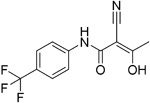
|
Pyrimidine synthesis inhibitor | FDA approved in 2012 for RRMS
Cochrane review, only 14 mg/day delays disability 92 Positive results in phase III TOWER study for RRMS 93 Active phase III study for patients with first clinical episode was suggestive of MS (TERICIS). |
Only limited penetration across the
BBB (brain-to-blood ratio ≤2%). MPO = 5.4 |
| Leflunomide |
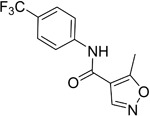
|
Pyrimidine synthesis inhibitor | No trials reported | MPO = 5.7
Metabolised to teriflunomide as detailed above |
| Mitoxantrone |

|
Topoisomerase II inhibitor/Induces
B-cell death |
FDA approved in 2000 for SPMS, progressive-
relapsing MS (PRMS) and worsening RRMS but not primary progressive MS (PPMS) |
Does not cross the BBB but may
penetrate brain tumours 94 MPO = 3.3 |
| Tofacitinib |
|
Inhibitor of the enzyme Janus kinase 1
(JAK1) and Janus kinase 3 (JAK 3) |
No development reported | Shows limited distribution across
the BBB with a cerebral-to-systemic blood ratio of 0.05, as assessed by either peak plasma concentration (C max) or area under the curve 95. MPO = 4.8 |
| Mycophenolic acid |
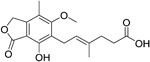
|
IMPDH2 | Clinical trial completed 61 | Capability to pass the BBB has not
been assessed. MPO = 5.3 |
aMPO is assessed using the published method.
Adenosine deaminase: clues to the mode of action of cladribine (Mavenclad)
Adenosine deaminase is often highlighted in discussions on the mechanism of action of cladribine as the active metabolite of the drug is unable to be broken down by this enzyme 49. Children with adenosine deaminase deficiency have severe combined immunodeficiency disease characterised by reduced B and T lymphocyte counts 50. The lack of adenosine deaminase leads to an increase in dNTP levels ( Figure 2), which can lead to cytotoxicity by a number of different mechanisms. dNTPs are selectively toxic towards T and B cells 51 and this susceptibility has been rationalised by examining the levels of the enzymes involved in maintaining NTP levels. Lymphocytes contain low levels of the NTP catabolic enzyme 5′-nucleotidase which favours high NTP levels in the absence of adenosine deaminase 52.
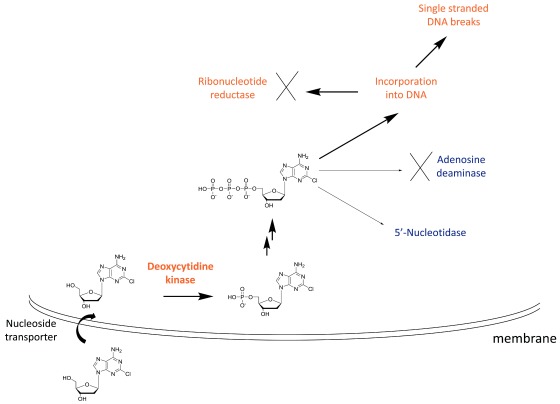
Figure 2. Mechanism of action of cladribine.
Cladribine is taken up into cells by nucleoside transporters and then is phosphorylated to the mono-phosphate (the rate-limiting step) by deoxycytidine kinase, highly expressed in lymphocytes. Subsequent phosphorylation steps produce the active species, the triphosphate. The triphosphate cannot be efficiently degraded by adenosine deaminase, and 5′-nucleotidase has low expression in lymphocytes. This leads to high levels of the cladribine triphosphate, which is toxic to cells by a number of mechanisms, including incorporation into DNA leading to single-stranded breaks.
In the case of cladribine action, the drug enters the cell via nucleoside transporters and is successively phosphorylated to the triphosphate ( Figure 2). The rate-limiting step for this process is the initial phosphorylation by cytidine kinase, an enzyme that is highly expressed in lymphocytes ( Figure 2) 53. Owing to the inability of cladribine triphosphate to be broken down by adenosine deaminase and the low levels of 5′ nucleotidase in lymphocytes, this active metabolite incorporates into DNA, leading to single-stranded DNA breaks and ribonucleotide reductase inhibition. This is thought to be the major cause of lymphocyte toxicity. Other mechanisms include disruption of DNA repair and an epigenetic effect.
Cladribine has medium oral absorption (37%–51% bioavailable) with a half-life of 5.7 to 19.7 hours 54. The drug achieves very good levels in cerebrospinal fluid (25% of plasma levels) and is metabolised mostly in the blood (in contrast to most drugs) 55. The testing and registration of cladribine for MS have a long and tortuous history 56 culminating in the CLARITY (Cladribine Tablets Treating Multiple Sclerosis Orally) phase 3 57 and CLARITY extension 2 study. The drug is efficacious in reducing relapses following short courses of treatment (4–5 days) at 3.5 or 5.25 mg/kg with reductions in relapse rate of 57.6% and 54.5% versus placebo, respectively. Side effects such as lymphopenia and infection were predictable in line with an agent that depletes B and T cells. Cladribine is licensed for hairy cell leukaemia and B-cell chronic lymphocytic leukaemia. In 2017, it was approved for MS in Europe 58 and is marketed by Merck KGaA (EMD Serono in the US) as Mavenclad. Merck filed for FDA approval on 30 July 2018.
Inosine-5′-monophosphate dehydrogenases (IMPDH1 and IMPDH2)
IMPDH1 and IMPDH2 catalyse the conversion of inosine 5-phosphate to xanthosine 5-phosphate. IMPDH2 is the rate-limiting enzyme for guanosine biosynthesis 59, and there are similarities with the mechanism of cladribine above. Inhibitors of these enzymes are generally immune-suppressive. Mycophenolate mofetil is an old drug which has recently shown some clinical benefit in MS 60, 61. It is a non-selective inhibitor of IMPDH1 and 2 and has Ki values of 40 and 10 nM, respectively 62. The mechanism of immune suppression has been characterised in systemic lupus erythematosus 63 as slowing B-cell proliferation and plasmablast formation. The lack of selectivity for mycophenolate and other older drugs has led to a search for more selective inhibitors. Sanglifehrin is a natural product with immune-suppressive properties that only recently have been characterised as working through inhibition of IMPDH2 64. The spiroketal moiety of sanglifehrin is responsible for this activity, forming a ternary complex between IMPDH2, sanglifehrin, and cyclophilin A. Sappanone is a covalent and selective inhibitor of IMPDH2 over IMPDH1, binding to the Cys140 on the protein 65, and this type of compound may offer promise for an effective treatment with fewer side effects.
Dihydroorotate dehydrogenase inhibitors: teriflunomide
Dihydroorotate dehydrogenase (DHODH) is a critical mitochondrial enzyme involved in the de novo biosynthesis of pyrimidines. Inhibiting this enzyme limits the available pyrimidine reserve needed for the increased proliferation of T and B lymphocytes seen in patients with MS 66, 67. This lowers the inflammatory response to auto-antigens by decreasing the number of activated T and B cells available to cross the blood–brain barrier into the CNS.
Teriflunomide acts primarily as a non-competitive and selective inhibitor of DHODH and is approved for the treatment of relapsing forms of MS (RRMS). The registration and approval of teriflunomide were due to the success of three randomised placebo-controlled trials in relapsing MS, which demonstrated that daily oral dosing of 7 to 14 mg is effective versus placebo in three key measures: relapses, disability progression and brain lesions. Clinical trial III (Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis, or TOPIC) showed that 72% of patients remained relapse-free on teriflunomide 14 mg versus 62% with placebo 96. A recent study on the effect of teriflunomide on different immune cell subpopulations in patients with MS indicated that, while teriflunomide significantly reduces absolute counts of total CD19 + B cells and mature and regulatory B-cell subsets, it affects T-cell numbers to a noticeably lesser extent and shows no detectable effect on natural killer (NK) cell numbers 97. However, the reduction in memory B cells is modest, and a more recent study suggests that when teriflunomide is used as a second-line treatment, relapse rates of patients with RRMS were increased compared with those who switched to dimethyl fumarate 98. Oral bioavailability of teriflunomide is about 100%, and peak plasma levels are achieved within 1–2 hours of ingestion 99. Teriflunomide is the active metabolite of leflunomide ( Table 2) 100, 101. Further data on teriflunomide were presented at the 2018 European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) meeting to indicate a reduction in T-cell receptor (TCR) repertoire diversity in patients with RRMS. Metabolic analysis of T cells of patients showed increased metabolic potential over controls; the authors consider that teriflunomide can improve energy production in T cells via dihydroorotate inhibition 102.
Epstein–Barr virus as a target
Should EBV be established as the trigger for MS or as a contributor to the pathology, then specific EBV anti-virals might also offer a route to treatment. A small study using autologous EBV-specific T-cell therapy has been reported 103 noting promising clinical improvement. Unfortunately, however, the state of EBV anti-viral chemotherapy is poor 104.
Maribavir, a nucleoside anti-viral, has been investigated in detail for inhibition of EBV replication 105. This drug initially stalled in development for cytomegalovirus (CMV) but achieved success in the treatment of haemopoietic stem cell transplant and solid organ transplant patients 106. It has recently been granted breakthrough status by the FDA. It is, however, not currently being developed for EBV 107. Maribavir works through the EBV protein kinase BGLF4 105 rather than through a standard nucleoside target. Phosphonate anti-viral nucleosides, including HPMP-5-azaC, have shown EBV efficacy in vitro and in animal models 108. Herpes viruses may incorporate their own viral thymidine kinase and this is responsible for the high selectivity and low toxicity of agents such as acyclovir. Although viral EBV thymidine kinase has restricted specificity compared with other herpes virus thymidine kinases 109, some nucleosides such as KAY-2-41 and KAY-39-149 show high activity against EBV 110. No development of these agents is reported. The similarity between the mode of action of cladribine and that of anti-viral nucleosides is obvious, but there is currently no report of the activity (if any) of cladribine against EBV-infected cells. The development of EBV vaccines is problematic 111, but efforts are continuing and some recent success was observed in animals 112.
Proteasome inhibitors bortezomib and carfilzomib
Bortezomib is a proteasome inhibitor used for the treatment of multiple myeloma and mantle cell lymphoma, two B cell–associated cancers. Bortezomib preferentially affects plasma cell differentiation and survival through its action on the nuclear factor-kappa B (NF-κB) pathway. This pathway is also key in inflammatory and autoimmune diseases 113. The proteasome is also directly involved with processing of MHC class I peptides 114. Following B-cell activation, B cells become more susceptible to proteasome inhibition 115. Thus, bortezomib has some selectivity in inducing apoptosis in activated B cells (plasma cells). Bortezomib has been trialled in the autoimmune diseases refractory systemic lupus erythematosus and neuromyelitis optica spectrum disorder 116 and demonstrated some efficacy 117. A negative aspect of bortezomib is the reported chemotherapy-induced peripheral neuropathy. This is caused by dysregulation of sphingosine-1-phosphate receptor-1 in astrocytes, which can be treated with fingolimod 118. Carfilzomib is a recently approved proteasome inhibitor for lymphomas. A recent study showed that carfilzomib together with other proteasome inhibitors had an effect on activated B cells and on naïve and—importantly from an MS perspective—memory B cells 119. There appears to be potential for the use of proteasome inhibitors in MS, particularly if their action on different subtypes of B cells becomes better understood and if the side effects are minimised.
Bruton’s (B-cell) tyrosine kinase inhibitors
Bruton’s (B-cell) tyrosine kinase (BTK) is an essential kinase for the maturation of B cells together with phosphatidylinositol 3-kinase isoform p110delta (PI3Kδ). This pathway is important for autoimmune diseases and B-cell malignancies. In MS, BTK inhibitors show potential for highly specific removal of B cells and potential to diminish autoantibody release in rheumatoid arthritis models 120. The first covalent BTK inhibitor to be marketed was ibrutinib, a covalent tyrosine kinase inhibitor with activity against BTK and now licensed for the B-cell malignancies mantle cell lymphoma, del17p chronic lymphocytic leukaemia 121, and Waldenström’s macroglobulinemia 122. Although no development of ibrutinib for MS has been reported, two other inhibitors have reached development: evobrutinib (Merck KGgA) has reported positive phase IIB data, and PRN2246 (Principia/Sanofi) is still at an earlier stage 123. Quite recently, at the 2018 Congress of the ECTRIMS, Merck presented clinical data using evobrutinib in patients with RRMS and illustrated positive results by reduction of T1 +Gd lesions compared with placebo, justifying future clinical studies 124.
PI3Kδ inhibitors
As indicated above, PI3Kδ inhibitors can prevent the maturation of B cells and therefore are candidates for MS therapy. In contrast to BTK, this protein is also expressed in T cells. The expression of this enzyme is not restricted to B cells and thus could exhibit off-target effects. The gene PIK3R1—phosphatidylinositol 3-kinase regulatory subunit alpha (PI3-kinase regulatory subunit alpha)—has been listed as a priority gene from an interaction map analysis of GWAS data 23. PIK3R1 is linked to a primary immunodeficiency resulting in low or absent circulating B cells 125. So far, there have been no reports of development of idelalisib or other PI3Kδ inhibitors for MS. A black box warning has been issued for idelalisib which may hamper its use 126.
B cell–activating factor and tumour necrosis factor alpha regulators
B cell–activating factor (BAFF) is a member of the tumour necrosis factor (TNF) family of receptors 127 and is a key survival factor for B-cell subsets encoded by the TNFSF13B gene. A secreted cytokine, it is a ligand for three TNF receptors: TACI, BCMS and BAFF-R. The interaction between BAFF and BAFF-R activates the canonical NF-κB signalling pathway for B-cell survival and activation. The association of BAFF with MS has been known since the early 2000s; BAFF is upregulated in astrocytes within MS lesions 128. A recent study demonstrated an association of a variant of TNFSF13B (BAFF-var) with MS 129. The same study showed no involvement of BAFF-var with multiple T-cell traits, consistent with an effect on B cells and particularly on memory B cells. Biological therapies that target this cytokine are available. This gene appears to be a rare example of one that is associated with the disease by genetics and has also been drugged. The actions of this cytokine and others on B cells and on the course of the disease have not been fully elucidated. Atacicept—a fusion protein of BAFF and a proliferation-inducing ligand (APRIL)—worsens MS, an effect ascribed to an increase in memory B cells 7.
CP-25 is a derivative of the natural product paeoniflorin 130, which is able to inhibit B-cell proliferation in a similar manner to rituximab (CD20 B-cell targeting antibody) and etanercept (TNF receptor chimera) and also downregulate the expression of BAFF-R on B cells. The mechanism of these effects is not reported 131. It will be interesting to note the progression of this compound and ones with a similar mode of action. The effect of CP-25 on B-cell subsets was more modest than that of rituximab 131.
Dimethylfumarate: Tecfidera
Dimethylfumarate (DMF) is an old drug originally used to treat psoriasis. Rapid metabolism converts DMF to monomethylfumarate, the main species in circulation. This compound can react with glutathione to trigger an anti-oxidant response. The main mechanism of action is thought to be via activation of the transcription factor Nrf2, thus inducing a protective effect on cells 45, although different targets of DMF have been observed 132. Multiple actions on the immune system in pwMS have been noted 133, including a potentially important decrease in CD19 +CD27 + memory B cells 134. This may in fact be a main mechanism of action due to the dramatic effects observed with other B cell–targeted therapies.
Janus kinase inhibitors
Janus kinases (JAKs) are downstream effectors of cytokine receptors and thus are potentially useful in MS given the importance of cytokines in driving MS. Although JAK inhibitors, such as tofacitinib, are in trials for many autoimmune diseases, MS is not currently a disease indication for inhibitors of this type 135. The JAK2 gene is listed as a priority gene from the protein interaction analysis in the recent GWAS 23.
Sphingosine-1-phosphate receptor inhibitors
Fingolimod is a prodrug that is activated by phosphorylation with sphingosine kinases. The phosphate form can then bind and initially activate receptors sphingosine-1-phosphate 1 (S1P1), S1P3, S1P4 and S1P5 136. The action on the S1P1 receptor is thought to mediate as a functional antagonism with removal of the receptor from the membrane and proteasomal degradation. The S1P1 receptor is essential for lymphocyte trafficking from lymph nodes. Although the mechanism was thought to be primarily through T cells, studies also point to an effect on astrocytes and microglia 137 and this may be via the S1P3 receptor 138. Despite increasing serum levels of BAFF in pwMS, fingolimod did not activate memory B cells or plasma cells 139. The overall beneficial effects of fingolimod in MS are tempered by side effects 140 and have stimulated the development of more selective drugs such as siponimod ponesimod and ozanimod 136. Only detailed clinical analysis will establish the relative effectiveness of these compounds, particularly as they must now compete with the emerging B cell–directed therapies.
Aryl hydrocarbon receptor
Laquinimod, a quinolone-3-carboxamide derivative, is an innovative oral anti-inflammatory drug developed for the treatment of RRMS, PPMS and Huntington’s disease. Preclinical studies have shown that laquinimod reduces inflammatory cell infiltrates in the CNS. Furthermore, laquinimod suppresses clinical signs in EAE models and decreases the formation of meningeal B-cell aggregates in EAE mice 141– 143. Three phase 3 studies evaluating the efficacy and safety of laquinimod 0.6 mg as a treatment for RRMS have been conducted. The first study (ALLEGRO, or Assessment of Oral Laquinimod in Preventing Progression in Multiple Sclerosis) showed statistically significant differences between laquinimod and placebo in reducing the risk of sustained disability progression and rate of MRI-measured brain volume loss 144. The second study (BRAVO) did not reach the primary endpoint of the trial but indicated a significant reduction in disability progression and brain atrophy 145. CONCERTO (The Efficacy and Safety and Tolerability of Laquinimod in Subjects With Relapsing Remitting Multiple Sclerosis) was the third study to be completed, and it failed to meet its primary endpoint of a difference between patients receiving laquinimod 0.6 mg/day and those receiving equivalent placebo in confirmed 3-month disease progression; however, it did demonstrate a significant effect on reducing brain volume loss and clinical relapses 146.
The therapeutic effect of laquinimod was recently found to be dependent on aryl hydrocarbon receptor (AhR) activation and has been shown to induce several genes downstream linked with the AhR pathway. Among these genes were CYP1A1 and AHRR, showing the highest average fold change in both naïve and EAE mice 147. AhR activation via laquinimod alters the phenotype of antigen-presenting cells and autoreactive T and B cells, reducing the humoral response associated with MS 143, 148.
Topoisomerase II inhibitors: mitoxantrone
Topoisomerase II is a nuclear enzyme that modifies the topology of DNA by catalysing the transient breaking and rejoining of the phosphodiester backbone 149. Topoisomerase II inhibitors block the action of these DNA enzymes, interrupting their catalytic cycle, and are thought to give rise to the presence of protein-associated double-strand breaks, which may be lethal to a cell 150, 151. Two forms of topoisomerase II exist, possibly products of a gene duplication event, topoisomerase II α and β. Mitoxantrone, a synthetic antineoplastic anthracenedione, was shown to target both topoisomerase II α and β and consequently interfere with DNA repair 152. By inhibiting DNA replication and DNA-dependent RNA synthesis, mitoxantrone makes the cell incapable of dividing, thus suppressing the proliferation of autoreactive T cells, B cells, macrophages and other antigen-presenting cells that mediate myelin degradation 153. Mitoxantrone was initially proven to be effective in EAE 154. In addition, it has proven efficacy in the treatment of worsening RRMS, SPMS and PRMS as assessed in three controlled clinical trials 155– 157. However, the risks of severe adverse events of mitoxantrone are similar to those seen with other anthracyclines—myelosuppression and cardiotoxicity—and it has seen decreased use because of the introduction of newer therapies such as fingolimod 158.
Glatiramer acetate
Glatiramer acetate (Copaxone) is a simple but random polymer of the amino acids Ala, Glu, Lys and Tyr 159. Originally conceived to mimic basic myelin, the drug is an immunomodulator and shows activity in the EAE animal model. A Cochrane review concluded that it had no effect on disease progression but showed some efficacy in “relapse related clinical outcomes” 160. In common with effects of other agents, its effects were thought to be T cell–related, but recent studies have pointed to a correlation with B-cell activity 161.
Discussion
The study of the genetics associated with MS continues to improve our understanding of its mechanism, further implicating B cells and EBV in MS pathogenesis. However, an indication of the limitation of the genetic approach and the importance of other factors is that genetic susceptibility has thus far not proven useful in diagnosis. Even individuals with a high genetic susceptibility are unlikely to develop the disease 4. The ideal scenario for genetic studies is that a key target that triggers the disease or is crucial in the pathology will emerge. This idea is, of course, best illustrated by the anti-cancer drug Gleevec, which targets the bcr-abl kinase. However, even with monogenic diseases, such as Huntington’s, therapies can be slow to appear 162. For Alzheimer’s disease, there have been several discoveries linking particular genes and proteins to the disease but this has not produced any therapies to date. Complex multifactorial diseases such as MS would seem to be unlikely candidates for a magic bullet resulting from genetic studies.
The effectiveness of the new B cell–targeted agents, such as the CD20 antibodies and cladribine, has placed B-cell therapies at the centre of MS therapy. These therapies, however, have a non-optimal therapeutic index, and investigation of alternative B-cell agents is warranted. With cheap and effective small molecules such as cladribine already available, new agents need to demonstrate significantly improved side-effect profiles. This review concentrated on the promise of new small-molecule agents as B cell–directed drugs. Clearly, agents such as the BTK inhibitor evobrutinib have already shown some potential in clinical trials 123.
Accumulating evidence places EBV as central to the disease, but no effective control of EBV has been achieved as yet. The only advanced therapy likely to have EBV efficacy, maribavir, has not been trialled for EBV. Maribavir is effective in human CMV therapy, and a trial in MS would be justified. Maribavir shows good CNS penetration, allowing it to access CNS cells such as astrocytes that are known to be infected with EBV. If EBV is responsible only for activation of the immune system, then EBV-specific treatment will have an effect similar to that of current immune therapies; however, if significant EBV infection affects other cells such as astrocytes, then a more pronounced effect should be evident. While debate continues on the role of EBV in MS, it will be difficult to address this question without an effective EBV therapy. B-cell therapies remove only the latent form and do not eliminate the virus elsewhere in the body. The marmoset model is perhaps superior to rodent models as a way to evaluate MS therapies; testing anti-EBV therapies would require that the CalHV3 virus show a sensitivity similar to that of EBV to the anti-viral agent.
Treatment options for patients with MS have improved enormously over the last few decades; however, current therapies are expensive and ultimately do not prevent disease progression. Small molecules represent a more affordable and sustainable class of drugs that are favourable for MS in particular because of higher blood–brain barrier penetration. Here, we have discussed small molecules that target B cells, and EBV-infected B cells, and are currently being pursued or warrant future investigation in the context of MS. However, few of the genes and pathways targeted affect MS susceptibility genes that are implicated in the mechanism of the disease. The noteworthy progress of anti-CD20 antibodies and cladribine has brought the role of B cells in MS pathology to the forefront of current research. This opens the door to numerous exciting possibilities to develop unique, affordable and easily maintainable therapies.
Methodology
Gene and protein data were downloaded from UniprotKB, a curated high-quality database. Gene lists were inputted to https://www.uniprot.org/uploadlists/, and the data were downloaded in tab format and saved in Excel ( Supplementary Table). Selected gene data were supplemented with PubMed searches. Gene lists were taken from the following sources: Patsopoulos 2017 23, Beecham 2013 24 (both IMSGC), Fischer 43, Sevastou 163 (RNA-Seq mouse EAE) and EBV genes Afrasiabi 2018 25.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
David R. Booth, Centre for Immunology and Allergy Research, Westmead Institute for Medical Research, University of Sydney, Westmead, NSW, Australia
Bruce V Taylor, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia
Funding Statement
AG was funded by a grant from the National MS Society – Fast Forward group (FF-1602-07939). KT was funded by a PhRMA Foundation Predoctoral fellowship in Pharmacology.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
Supplementary material
Supplementary Table 1 - Gene lists
References
- 1. Jacques FH: Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2015;84:963 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 2. Thompson AJ, Banwell BL, Barkhof F, et al. : Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Booth DR, Ding N, Parnell GP, et al. : Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun. 2016;17(4):213–219. 10.1038/gene.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parnell GP, Booth DR: The Multiple Sclerosis (MS) Genetic Risk Factors Indicate both Acquired and Innate Immune Cell Subsets Contribute to MS Pathogenesis and Identify Novel Therapeutic Opportunities. Front Immunol. 2017;8:425. 10.3389/fimmu.2017.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hilven K, Patsopoulos NA, Dubois B, et al. : Burden of risk variants correlates with phenotype of multiple sclerosis. Mult Scler. 2015;21(13):1670–1680. 10.1177/1352458514568174 [DOI] [PubMed] [Google Scholar]
- 6. Lehmann-Horn K, Kinzel S, Weber MS: Deciphering the Role of B Cells in Multiple Sclerosis-Towards Specific Targeting of Pathogenic Function. Int J Mol Sci. 2017;18(10): pii: E2048. 10.3390/ijms18102048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Baker D, Marta M, Pryce G, et al. : Memory B Cells are Major Targets for Effective Immunotherapy in Relapsing Multiple Sclerosis. EBioMedicine. 2017;16:41–50. 10.1016/j.ebiom.2017.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Rahmanzadeh R, Weber MS, Brück W, et al. : B cells in multiple sclerosis therapy-A comprehensive review. Acta Neurol Scand. 2018;137(6):544–556. 10.1111/ane.12915 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Bose T: Role of Immunological Memory Cells as a Therapeutic Target in Multiple Sclerosis. Brain Sci. 2017;7(11): pii: E148 10.3390/brainsci7110148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sefia E, Pryce G, Meier UC, et al. : Depletion of CD20 B cells fails to inhibit relapsing mouse experimental autoimmune encephalomyelitis. Mult Scler Relat Disord. 2017;14:46–50. 10.1016/j.msard.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 11. Bjelobaba I, Begovic-Kupresanin V, Pekovic S, et al. : Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J Neurosci Res. 2018;96(6):1021–1042. 10.1002/jnr.24224 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Lassmann H, Niedobitek G, Aloisi F, et al. : Epstein-Barr virus in the multiple sclerosis brain: a controversial issue--report on a focused workshop held in the Centre for Brain Research of the Medical University of Vienna, Austria. Brain. 2011;134(Pt 9):2772–2786. 10.1093/brain/awr197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pakpoor J, Ramagopalan SV: Epstein-Barr virus is a necessary causative agent in the pathogenesis of multiple sclerosis: yes. Mult Scler J. 2013;19(13):1690–1691. 10.1177/1352458513506505 [DOI] [PubMed] [Google Scholar]
- 14. Hassani A, Corboy JR, Al-Salami S, et al. : Epstein-Barr virus is present in the brain of most cases of multiple sclerosis and may engage more than just B cells. PLoS One. 2018;13(2):e0192109. 10.1371/journal.pone.0192109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Pender MP: Preventing and curing multiple sclerosis by controlling Epstein-Barr virus infection. Autoimmun Rev. 2009;8(7):563–568. 10.1016/j.autrev.2009.01.017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Mentis AA, Dardiotis E, Grigoriadis N, et al. : Viruses and endogenous retroviruses in multiple sclerosis: From correlation to causation. Acta Neurol Scand. 2017;136(6):606–616. 10.1111/ane.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kearns PKA, Casey HA, Leach JP: Hypothesis: Multiple sclerosis is caused by three-hits, strictly in order, in genetically susceptible persons. Mult Scler Relat Disord. 2018;24:157–174. 10.1016/j.msard.2018.06.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Giovannoni G, Cutter G, Sormani MP, et al. : Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult Scler Relat Disord. 2017;12:70–78. 10.1016/j.msard.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 19. Faissner S, Gold R: Efficacy and Safety of the Newer Multiple Sclerosis Drugs Approved Since 2010. CNS Drugs. 2018;32(3):269–287. 10.1007/s40263-018-0488-6 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Mulero P, Midaglia L, Montalban X: Ocrelizumab: a new milestone in multiple sclerosis therapy. Ther Adv Neurol Disord. 2018;11:1756286418773025. 10.1177/1756286418773025 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Hauser SL, Bar-Or A, Comi G, et al. : Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. 2017;376(3):221–234. 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Granqvist M, Boremalm M, Poorghobad A, et al. : Comparative Effectiveness of Rituximab and Other Initial Treatment Choices for Multiple Sclerosis. JAMA Neurol. 2018;75(3):320–327. 10.1001/jamaneurol.2017.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Patsopoulos N, Baranzini SE, Santaniello A, et al. : The Multiple Sclerosis Genomic Map: Role of peripheral immune cells and resident microglia in susceptibility. bioRxiv. 2017. 10.1101/143933 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. International Multiple Sclerosis Genetics Consortium (IMSGC), . Beecham AH, Patsopoulos NA, et al. : Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45(11):1353–1360. 10.1038/ng.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Afrasiabi A, Parnell GP, Fewings N, et al. : Genetic evidence that the latency III stage of Epstein-Barr Virus infection is a therapeutic target for Multiple Sclerosis. bioRxiv. 2018;390609 10.1101/390609 [DOI] [Google Scholar]
- 26. Berge T, Leikfoss IS, Brorson IS, et al. : The multiple sclerosis susceptibility genes TAGAP and IL2RA are regulated by vitamin D in CD4+ T cells. Genes Immun. 2016;17(2):118–127. 10.1038/gene.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Y, Zhu G, Charlesworth JC, et al. : Genetic loci for Epstein-Barr virus nuclear antigen-1 are associated with risk of multiple sclerosis. Mult Scler. 2016;22(13):1655–1664. 10.1177/1352458515626598 [DOI] [PubMed] [Google Scholar]
- 28. Parnell GP, Gatt PN, Krupa M, et al. : The autoimmune disease-associated transcription factors EOMES and TBX21 are dysregulated in multiple sclerosis and define a molecular subtype of disease. Clin Immunol. 2014;151(1):16–24. 10.1016/j.clim.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 29. Khankhanian P, Cozen W, Himmelstein DS, et al. : Meta-analysis of genome-wide association studies reveals genetic overlap between Hodgkin lymphoma and multiple sclerosis. Int J Epidemiol. 2016;45(3):728–740. 10.1093/ije/dyv364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yenamandra SP, Hellman U, Kempkes B, et al. : Epstein-Barr virus encoded EBNA-3 binds to vitamin D receptor and blocks activation of its target genes. Cell Mol Life Sci. 2010;67(24):4249–4256. 10.1007/s00018-010-0441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trier N, Izarzugaza J, Chailyan A, et al. : Human MHC-II with Shared Epitope Motifs Are Optimal Epstein-Barr Virus Glycoprotein 42 Ligands-Relation to Rheumatoid Arthritis. Int J Mol Sci. 2018;19(1): pii: E317. 10.3390/ijms19010317 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Sheik-Ali S: Infectious mononucleosis and multiple sclerosis - Updated review on associated risk. Mult Scler Relat Disord. 2017;14:56–59. 10.1016/j.msard.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 33. Dolei A: The aliens inside us: HERV-W endogenous retroviruses and multiple sclerosis. Mult Scler. 2018;24(1):42–47. 10.1177/1352458517737370 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. 't Hart BA, Jagessar SA, Haanstra K, et al. : The Primate EAE Model Points at EBV-Infected B Cells as a Preferential Therapy Target in Multiple Sclerosis. Front Immunol. 2013;4:145. 10.3389/fimmu.2013.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pakpoor J, Disanto G, Gerber JE, et al. : The risk of developing multiple sclerosis in individuals seronegative for Epstein-Barr virus: a meta-analysis. Mult Scler. 2013;19(2):162–166. 10.1177/1352458512449682 [DOI] [PubMed] [Google Scholar]
- 36. Munger KL, Fitzgerald KC, Freedman MS, et al. : No association of multiple sclerosis activity and progression with EBV or tobacco use in BENEFIT. Neurology. 2015;85(19):1694–1701. 10.1212/WNL.0000000000002099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salzer J, Myhr KM: Epstein-Barr virus is a necessary causative agent in the pathogenesis of multiple sclerosis: no. Mult Scler. 2013;19(13):1692–1693. 10.1177/1352458513507625 [DOI] [PubMed] [Google Scholar]
- 38. Kudo LC, Parfenova L, Ren G, et al. : Puromycin-sensitive aminopeptidase (PSA/NPEPPS) impedes development of neuropathology in hPSA/TAU P301L double-transgenic mice. Hum Mol Genet. 2011;20(9):1820–1833. 10.1093/hmg/ddr065 [DOI] [PubMed] [Google Scholar]
- 39. Ren G, Ma Z, Hui M, et al. : Cu, Zn-superoxide dismutase 1 (SOD1) is a novel target of Puromycin-sensitive aminopeptidase (PSA/NPEPPS): PSA/NPEPPS is a possible modifier of amyotrophic lateral sclerosis. Mol Neurodegener. 2011;6:29. 10.1186/1750-1326-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fard MK, van der Meer F, Sánchez P, et al. : BCAS1 expression defines a population of early myelinating oligodendrocytes in multiple sclerosis lesions. Sci Transl Med. 2017;9(419): pii: eaam7816. 10.1126/scitranslmed.aam7816 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Ishimoto T, Ninomiya K, Inoue R, et al. : Mice lacking BCAS1, a novel myelin-associated protein, display hypomyelination, schizophrenia-like abnormal behaviors, and upregulation of inflammatory genes in the brain. Glia. 2017;65(5):727–739. 10.1002/glia.23129 [DOI] [PubMed] [Google Scholar]
- 42. Thangaraj MP, Furber KL, Gan JK, et al. : RNA-binding Protein Quaking Stabilizes Sirt2 mRNA during Oligodendroglial Differentiation. J Biol Chem. 2017;292(13):5166–5182. 10.1074/jbc.M117.775544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fischer MT, Sharma R, Lim JL, et al. : NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135(Pt 3):886–899. 10.1093/brain/aws012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Olojo RO, Ziman AP, Hernández-Ochoa EO, et al. : Mice null for calsequestrin 1 exhibit deficits in functional performance and sarcoplasmic reticulum calcium handling. PLoS One. 2011;6(12):e27036. 10.1371/journal.pone.0027036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Q, Chuikov S, Taitano S, et al. : Dimethyl Fumarate Protects Neural Stem/Progenitor Cells and Neurons from Oxidative Damage through Nrf2-ERK1/2 MAPK Pathway. Int J Mol Sci. 2015;16(6):13885–13907. 10.3390/ijms160613885 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Warne J, Pryce G, Hill JM, et al. : Selective Inhibition of the Mitochondrial Permeability Transition Pore Protects against Neurodegeneration in Experimental Multiple Sclerosis. J Biol Chem. 2016;291(9):4356–4373. 10.1074/jbc.M115.700385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cervantes-Gracia K, Husi H: Integrative analysis of Multiple Sclerosis using a systems biology approach. Sci Rep. 2018;8(1):5633. 10.1038/s41598-018-24032-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Wager TT, Hou X, Verhoest PR, et al. : Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. Acs Chem Neurosci. 2010;1(6):435–449. 10.1021/cn100008c [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Christensen LF, Broom AD, Robins MJ, et al. : Synthesis and biological activity of selected 2,6-disubstituted-(2-deoxy- -and- -D-erythro-pentofuranosyl)purines. J Med Chem. 1972;15(7):735–9. [DOI] [PubMed] [Google Scholar]
- 50. Bradford KL, Moretti FA, Carbonaro-Sarracino DA, et al. : Adenosine Deaminase (ADA)-Deficient Severe Combined Immune Deficiency (SCID): Molecular Pathogenesis and Clinical Manifestations. J Clin Immunol. 2017;37(7):626–637. 10.1007/s10875-017-0433-3 [DOI] [PubMed] [Google Scholar]
- 51. Carson DA, Kaye J, Matsumoto S, et al. : Biochemical basis for the enhanced toxicity of deoxyribonucleosides toward malignant human T cell lines. Proc Natl Acad Sci U S A. 1979;76(5):2430–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Giovannoni G: Cladribine to Treat Relapsing Forms of Multiple Sclerosis. Neurotherapeutics. 2017;14(4):874–887. 10.1007/s13311-017-0573-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kawasaki H, Carrera CJ, Piro LD, et al. : Relationship of deoxycytidine kinase and cytoplasmic 5'-nucleotidase to the chemotherapeutic efficacy of 2-chlorodeoxyadenosine. Blood. 1993;81(3):597–601. [PubMed] [Google Scholar]
- 54. Liliemark J: The clinical pharmacokinetics of cladribine. Clin Pharmacokinet. 1997;32(2):120–131. 10.2165/00003088-199732020-00003 [DOI] [PubMed] [Google Scholar]
- 55. Scheible H, Laisney M, Wimmer E, et al. : Comparison of the in vitro and in vivo metabolism of Cladribine (Leustatin, Movectro) in animals and human. Xenobiotica. 2013;43(12):1084–1094. 10.3109/00498254.2013.791762 [DOI] [PubMed] [Google Scholar]
- 56. Leist TP, Weissert R: Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol. 2011;34(1):28–35. 10.1097/WNF.0b013e318204cd90 [DOI] [PubMed] [Google Scholar]
- 57. Giovannoni G, Comi G, Cook S, et al. : A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426. 10.1056/NEJMoa0902533 [DOI] [PubMed] [Google Scholar]
- 58. Merck: Cladribine Tablets Receives Positive CHMP Opinion for Treatment of Relapsing Forms of Multiple Sclerosis. In Mavenclad (Cladribine tablets) has received a positive opinion by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA).2017. Reference Source [Google Scholar]
- 59. Braun-Sand SB, Peetz M: Inosine monophosphate dehydrogenase as a target for antiviral, anticancer, antimicrobial and immunosuppressive therapeutics. Future Med Chem. 2010;2(1):81–92. 10.4155/fmc.09.147 [DOI] [PubMed] [Google Scholar]
- 60. Xiao Y, Huang J, Luo H, et al. : Mycophenolate mofetil for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev. 2014; (2):CD010242. 10.1002/14651858.CD010242.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Michel L, Vukusic S, De Seze J, et al. : Mycophenolate mofetil in multiple sclerosis: a multicentre retrospective study on 344 patients. J Neurol Neurosurg Psychiatry. 2014;85(3):279–283. 10.1136/jnnp-2013-305298 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Chen L, Wilson D, Jayaram HN, et al. : Dual inhibitors of inosine monophosphate dehydrogenase and histone deacetylases for cancer treatment. J Med Chem. 2007;50(26):6685–6691. 10.1021/jm070864w [DOI] [PubMed] [Google Scholar]
- 63. Eickenberg S, Mickholz E, Jung E, et al. : Mycophenolic acid counteracts B cell proliferation and plasmablast formation in patients with systemic lupus erythematosus. Arthritis Res Ther. 2012;14(3):R110. 10.1186/ar3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pua KH, Stiles DT, Sowa ME, et al. : IMPDH2 Is an Intracellular Target of the Cyclophilin A and Sanglifehrin A Complex. Cell Rep. 2017;18(2):432–442. 10.1016/j.celrep.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 65. Liao LX, Song XM, Wang LC, et al. : Highly selective inhibition of IMPDH2 provides the basis of antineuroinflammation therapy. Proc Natl Acad Sci U S A. 2017;114(29):E5986–E5994. 10.1073/pnas.1706778114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bar-Or A: Teriflunomide (Aubagio®) for the treatment of multiple sclerosis. Exp Neurol. 2014;262(Pt A):57–65. 10.1016/j.expneurol.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 67. Li L, Liu JC, Delohery T, et al. : The effects of teriflunomide on lymphocyte subpopulations in human peripheral blood mononuclear cells in vitro. J Neuroimmunol. 2013;265(1–2):82–90. 10.1016/j.jneuroim.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 68. Schwartz R, Davidson T: Pharmacology, pharmacokinetics, and practical applications of bortezomib. Oncology (Williston Park). 2004;18(14 Suppl 11):14–21. [PubMed] [Google Scholar]
- 69. Demo SD, Kirk CJ, Aujay MA, et al. : Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67(13):6383–6391. 10.1158/0008-5472.CAN-06-4086 [DOI] [PubMed] [Google Scholar]
- 70. Goldwirt L, Beccaria K, Ple A, et al. : Ibrutinib brain distribution: a preclinical study. Cancer Chemother Pharmacol. 2018;81(4):783–789. 10.1007/s00280-018-3546-3 [DOI] [PubMed] [Google Scholar]
- 71. Relations SM: Sanofi, Principia agree to develop multiple sclerosis drug candidate.2017. Reference Source [Google Scholar]
- 72. Melao A: Potential B-cell targeting oral MS treatment, PRN2246, shows ability to reach brain in phase 1 study. In Multiple Sclerosis News Today2018. Reference Source [Google Scholar]
- 73. Janssens A: Ibrutinib and idelalisib, the B-cell receptor antagonists available for use in daily clinical practice. Belg J Hematol. 2015;6:216–224. [Google Scholar]
- 74. Zhao M, Zhou P, Yu J, et al. : The tissue distribution and excretion study of paeoniflorin-6'-O-benzene sulfonate (CP-25) in rats. Inflammopharmacology. 2018. 10.1007/s10787-018-0463-3 [DOI] [PubMed] [Google Scholar]
- 75. Terranova N, Hicking C, Dangond F, et al. : Effects of Postponing Treatment in the Second Year of Cladribine Administration: Clinical Trial Simulation Analysis of Absolute Lymphocyte Counts and Relapse Rate in Patients with Relapsing-Remitting Multiple Sclerosis. Clin Pharmacokinet. 2018. 10.1007/s40262-018-0693-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. De Stefano N, Giorgio A, Battaglini M, et al. : Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets. Mult Scler. 2018;24(2):222–226. 10.1177/1352458517690269 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Ali S, Paracha N, Cook S, et al. : Reduction in healthcare and societal resource utilization associated with cladribine tablets in patients with relapsing-remitting multiple sclerosis: analysis of economic data from the CLARITY Study. Clin Drug Investig. 2012;32(1):15–27. 10.2165/11593310-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 78. Freedman MS, Leist TP, Comi G, et al. : The efficacy of cladribine tablets in CIS patients retrospectively assigned the diagnosis of MS using modern criteria: Results from the ORACLE-MS study. Mult Scler J Exp Transl Clin. 2017;3(4): 2055217317732802. 10.1177/2055217317732802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leist TP, Comi G, Cree BA, et al. : Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol. 2014;13(3):257–267. 10.1016/S1474-4422(14)70005-5 [DOI] [PubMed] [Google Scholar]
- 80. Montalban X, Leist TP, Cohen BA, et al. : Cladribine tablets added to IFN-β in active relapsing MS: The ONWARD study. Neurol Neuroimmunol Neuroinflamm. 2018;5:e477 10.1212/NXI.0000000000000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Steininger C: Novel therapies for cytomegalovirus disease. Recent Pat Antiinfect Drug Discov. 2007;2(1):53–72. 10.2174/157489107779561634 [DOI] [PubMed] [Google Scholar]
- 82. Ziemssen T, Neuhaus O, Hohlfeld R: Risk-benefit assessment of glatiramer acetate in multiple sclerosis. Drug Saf. 2001;24(13):979–990. 10.2165/00002018-200124130-00005 [DOI] [PubMed] [Google Scholar]
- 83. Brennan MS, Matos MF, Li B, et al. : Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS One. 2015;10(3):e0120254. 10.1371/journal.pone.0120254 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Kappos L, Arnold DL, Bar-Or A, et al. : Safety and efficacy of amiselimod in relapsing multiple sclerosis (MOMENTUM): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15(11):1148–1159. 10.1016/S1474-4422(16)30192-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Tavares A, Barret O, Alagille D, et al. : Brain distribution of MS565, an imaging analogue of siponimod (BAF312), in non-human primates. Eur J Neurol. 2014;21:504 Reference Source [Google Scholar]
- 86. Cohen JA, Arnold DL, Comi G, et al. : Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):373–381. 10.1016/S1474-4422(16)00018-1 [DOI] [PubMed] [Google Scholar]
- 87. Comi G, Arnold D, Cree B, et al. : Ozanimod Demonstrates Efficacy and Safety in a Multicenter, Randomized, Double-Blind, Double-Dummy, Active-Controlled Phase 3 Trial of Relapsing Multiple Sclerosis (SUNBEAM). Neurology. 2018;90(15 Supplement). Reference Source [Google Scholar]
- 88. Scott FL, Clemons B, Brooks J, et al. : Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P 1) and receptor-5 (S1P 5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173(11):1778–1792. 10.1111/bph.13476 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Bar-Or A, Zipp F, Scaramozza M, et al. : Effect of Ceralifimod (ONO-4641), a Sphingosine-1-Phosphate Receptor-1 and -5 Agonist, on Magnetic Resonance Imaging Outcomes in Patients with Multiple Sclerosis: Interim Results from the Extension of the DreaMS Study (P3.161). Neurology. 2014;82: P3.161. [Google Scholar]
- 90. Olsson T, Boster A, Fernández Ó, et al. : Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial. J Neurol Neurosurg Psychiatry. 2014;85(11):1198–1208. 10.1136/jnnp-2013-307282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brueck W, Wegner C: Insight into the mechanism of laquinimod action. J Neurol Sci. 2011;306(1–2):173–179. 10.1016/j.jns.2011.02.019 [DOI] [PubMed] [Google Scholar]
- 92. He D, Zhang C, Zhao X, et al. : Teriflunomide for multiple sclerosis. Cochrane Database Syst Rev. 2016;3:CD009882. 10.1002/14651858.CD009882.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Confavreux C, O'Connor P, Comi G, et al. : Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(3):247–256. 10.1016/S1474-4422(13)70308-9 [DOI] [PubMed] [Google Scholar]
- 94. Green RM, Stewart DJ, Hugenholtz H, et al. : Human central nervous system and plasma pharmacology of mitoxantrone. J Neurooncol. 1988;6(1):75–83. 10.1007/BF00163544 [DOI] [PubMed] [Google Scholar]
- 95. CHMP: Assessment report EMA/CHMP/425279/2013. Xeljanz,2013. Reference Source [Google Scholar]
- 96. Miller AE, Wolinsky JS, Kappos L, et al. : Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(10):977–986. 10.1016/S1474-4422(14)70191-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Gandoglia I, Ivaldi F, Laroni A, et al. : Teriflunomide treatment reduces B cells in patients with MS. Neurol Neuroimmunol Neuroinflamm. 2017;4(6):e403. 10.1212/NXI.0000000000000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ontaneda D, Nicholas J, Carraro M, et al. : Comparative effectiveness of dimethyl fumarate versus fingolimod and teriflunomide among MS patients switching from first-generation platform therapies in the US. Mult Scler Relat Disord. 2018;27:101–111. 10.1016/j.msard.2018.09.038 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Limsakun T, Menguy-Vacheron F: Pharmacokinetics of Oral Teriflunomide, a Novel Oral Disease-Modifying Agent under Investigation for the Treatment of Multiple Sclerosis. Neurology. 2010;74:A415–A415. [Google Scholar]
- 100. Bartlett RR: Immunopharmacological profile of HWA 486, a novel isoxazol derivative--II. In vivo immunomodulating effects differ from those of cyclophosphamide, prednisolone, or cyclosporin A. Int J Immunopharmacol. 1986;8(2):199–204. 10.1016/0192-0561(86)90059-7 [DOI] [PubMed] [Google Scholar]
- 101. Aly L, Hemmer B, Korn T: From Leflunomide to Teriflunomide: Drug Development and Immunosuppressive Oral Drugs in the Treatment of Multiple Sclerosis. Curr Neuropharmacol. 2017;15(6):874–891. 10.2174/1570159X14666161208151525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Eschborni M, Klotz L, Lindner M, et al. : Teriflunomide modulates antigen-specific immune responses in an affinity-dependent fashion by inhibiting T cell metabolism. Mult Scler J. 2018;24:675–676. Reference Source [Google Scholar]
- 103. Pender MP, Csurhes PA, Smith C, et al. : Safety and clinical improvement in a phase I trial of autologous Epstein-Barr virus-specific T-cell therapy in patients with progressive multiple sclerosis. Mult Scler J. 2017;23:401 Reference Source [Google Scholar]
- 104. Pagano JS, Whitehurst CB, Andrei G: Antiviral Drugs for EBV. Cancers (Basel). 2018;10(6): pii: E197. 10.3390/cancers10060197 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Whitehurst CB, Sanders MK, Law M, et al. : Maribavir inhibits Epstein-Barr virus transcription through the EBV protein kinase. J Virol. 2013;87(9):5311–5315. 10.1128/JVI.03505-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Papanicolaou G, Silveira FP, Langston A, et al. : Maribavir for Treatment of Cytomegalovirus (CMV) Infections Resistant or Refractory to Ganciclovir or Foscarnet in Hematopoietic Stem Cell Transplant (SCT) or Solid Organ Transplant (SOT) Recipients: A Randomized, Dose-Ranging, Double-Blind, Phase 2 Study. Open Forum Infect Dis. 2016;3(suppl_1):78 10.1093/ofid/ofw194.13 [DOI] [Google Scholar]
- 107. Winston DJ, Saliba F, Blumberg E, et al. : Efficacy and Safety of Maribavir Dosed at 100 mg Orally Twice Daily for the Prevention of Cytomegalovirus Disease in Liver Transplant Recipients: A Randomized, Double-Blind, Multicenter Controlled Trial (vol 12, pg 3021, 2012). Am J Transplant. 2013;13:529. [DOI] [PubMed] [Google Scholar]
- 108. Coen N, Duraffour S, Naesens L, et al. : Evaluation of novel acyclic nucleoside phosphonates against human and animal gammaherpesviruses revealed an altered metabolism of cyclic prodrugs upon Epstein-Barr virus reactivation in P3HR-1 cells. J Virol. 2013;87(22):12422–12432. 10.1128/JVI.02231-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Coen N, Duraffour S, Topalis D, et al. : Spectrum of activity and mechanisms of resistance of various nucleoside derivatives against gammaherpesviruses. Antimicrob Agents Chemother. 2014;58(12):7312–7323. 10.1128/AAC.03957-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Coen N, Duraffour S, Haraguchi K, et al. : Antiherpesvirus activities of two novel 4'-thiothymidine derivatives, KAY-2-41 and KAH-39-149, are dependent on viral and cellular thymidine kinases. Antimicrob Agents Chemother. 2014;58(8):4328–4340. 10.1128/AAC.02825-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cohen JI: Epstein-barr virus vaccines. Clin Transl Immunology. 2015;4(1): e32. 10.1038/cti.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhao BC, Zhang X, Krummenacher C, et al. : Immunization With Fc-Based Recombinant Epstein-Barr Virus gp350 Elicits Potent Neutralizing Humoral Immune Response in a BALB/c Mice Model. Front Immunol. 2018;9:932. 10.3389/fimmu.2018.00932 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 113. Mc Guire C, Prinz M, Beyaert R, et al. : Nuclear factor kappa B (NF-κB) in multiple sclerosis pathology. Trends Mol Med. 2013;19(10):604–613. 10.1016/j.molmed.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 114. Kloetzel PM: The proteasome and MHC class I antigen processing. Biochim Biophys Acta. 2004;1695(1–3):225–233. 10.1016/j.bbamcr.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 115. Cascio P, Oliva L, Cerruti F, et al. : Dampening Ab responses using proteasome inhibitors following in vivo B cell activation. Eur J Immunol. 2008;38(3):658–667. 10.1002/eji.200737743 [DOI] [PubMed] [Google Scholar]
- 116. Zhang C, Tian DC, Yang CS, et al. : Safety and Efficacy of Bortezomib in Patients With Highly Relapsing Neuromyelitis Optica Spectrum Disorder. JAMA Neurol. 2017;74(8):1010–1012. 10.1001/jamaneurol.2017.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Alexander T, Sarfert R, Klotsche J, et al. : The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis. 2015;74(7):1474–1478. 10.1136/annrheumdis-2014-206016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stockstill K, Doyle TM, Yan X, et al. : Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. J Exp Med. 2018;215(5):1301–1313. 10.1084/jem.20170584 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 119. Mulder A, Heidt S, Vergunst M, et al. : Proteasome inhibition profoundly affects activated human B cells. Transplantation. 2013;95(11):1331–1337. 10.1097/TP.0b013e3182911739 [DOI] [PubMed] [Google Scholar]
- 120. Puri KD, Di Paolo JA, Gold MR: B-cell receptor signaling inhibitors for treatment of autoimmune inflammatory diseases and B-cell malignancies. Int Rev Immunol. 2013;32(4):397–427. 10.3109/08830185.2013.818140 [DOI] [PubMed] [Google Scholar]
- 121. Burger JA, Tedeschi A, Barr PM, et al. : Investigators R-. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373(25):2425–2437. 10.1056/NEJMoa1509388 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 122. Treon S, Tripsas C, Meid K, et al. : Ibrutinib in Previously Treated Patients with Waldenstrom's Macroglobulinemia Is Highly Active, Produces Durable Responses, and Is Impacted by Myd88 and Cxcr4 Mutation Status. Haematologica. 2015;100:311–311. [Google Scholar]
- 123. Merck AG: Merck Announces Positive Phase IIB Results for Evobrutinib in Relapsing Multiple Sclerosis. Merck AG;2018. Reference Source [Google Scholar]
- 124. Montalban X, Arnold DL, Weber MS, et al. : Primary analysis of a randomised, placebo-controlled, phase 2 study of the Bruton’s tyrosine kinase inhibitor evobrutinib (M2951) in patients with relapsing multiple sclerosis. In 34th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Berlin, Germany, SAGE;2018. Reference Source [Google Scholar]
- 125. Conley ME, Dobbs AK, Quintana AM, et al. : Agammaglobulinemia and absent B lineage cells in a patient lacking the p85α subunit of PI3K. J Exp Med. 2012;209(3):463–470. 10.1084/jem.20112533 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 126. Coutré SE, Barrientos JC, Brown JR, et al. : Management of adverse events associated with idelalisib treatment: expert panel opinion. Leukemia Lymphoma. 2015;56(10):2779–2786. 10.3109/10428194.2015.1022770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rickert RC, Jellusova J, Miletic AV: Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244(1):115–133. 10.1111/j.1600-065X.2011.01067.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Krumbholz M, Theil D, Derfuss T, et al. : BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. Journal of Experimental Medicine. 2005;201(2):195–200. 10.1084/jem.20041674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Steri M, Orru V, Idda ML, et al. : Overexpression of the Cytokine BAFF and Autoimmunity Risk. N Engl J Med. 2017;376(17):1615–1626. 10.1056/NEJMoa1610528 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 130. Yan D, Saito K, Ohmi Y, et al. : Paeoniflorin, a novel heat shock protein-inducing compound. Cell Stress Chaperones. 2004;9(4):378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang F, Shu JL, Li Y, et al. : CP-25, a Novel Anti-inflammatory and Immunomodulatory Drug, Inhibits the Functions of Activated Human B Cells through Regulating BAFF and TNF-alpha Signaling and Comparative Efficacy with Biological Agents. Front Pharmacol. 2017;8:933. 10.3389/fphar.2017.00933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Blewett MM, Xie JJ, Zaro BW, et al. : Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells. Sci Signal. 2016;9(445):rs10. 10.1126/scisignal.aaf7694 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 133. Mills EA, Ogrodnik MA, Plave A, et al. : Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis. Front Neurol. 2018;9:5. 10.3389/fneur.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 134. Montes Diaz G, Fraussen J, Van Wijmeersch B, et al. : Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci Rep. 2018;8(1): 8194. 10.1038/s41598-018-26519-w [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 135. Schwartz DM, Kanno Y, Villarino A, et al. : JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843–862. 10.1038/nrd.2017.201 [DOI] [PubMed] [Google Scholar]
- 136. Park SJ, Im DS: Sphingosine 1-Phosphate Receptor Modulators and Drug Discovery. Biomol Ther (Seoul). 2017;25(1):80–90. 10.4062/biomolther.2016.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Brinkmann V: FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158(5):1173–1182. 10.1111/j.1476-5381.2009.00451.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dong YF, Guo RB, Ji J, et al. : S1PR3 is essential for phosphorylated fingolimod to protect astrocytes against oxygen-glucose deprivation-induced neuroinflammation via inhibiting TLR2/4-NFκB signalling. J Cell Mol Med. 2018;22(6):3159–3166. 10.1111/jcmm.13596 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 139. Miyazaki Y, Niino M, Takahashi E, et al. : Fingolimod induces BAFF and expands circulating transitional B cells without activating memory B cells and plasma cells in multiple sclerosis. Clin Immunol. 2018;187:95–101. 10.1016/j.clim.2017.10.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 140. Yoshii F, Moriya Y, Ohnuki T, et al. : Neurological safety of fingolimod: An updated review. Clin Exp Neuroimmunol. 2017;8(3):233–243. 10.1111/cen3.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Brunmark C, Runström A, Ohlsson L, et al. : The new orally active immunoregulator laquinimod (ABR-215062) effectively inhibits development and relapses of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;130(1–2):163–172. 10.1016/S0165-5728(02)00225-4 [DOI] [PubMed] [Google Scholar]
- 142. Wegner C, Stadelmann C, Pförtner R, et al. : Laquinimod interferes with migratory capacity of T cells and reduces IL-17 levels, inflammatory demyelination and acute axonal damage in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;227(1–2):133–143. 10.1016/j.jneuroim.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 143. Varrin-Doyer M, Pekarek KL, Spencer CM, et al. : Treatment of spontaneous EAE by laquinimod reduces Tfh, B cell aggregates, and disease progression. Neurol Neuroimmunol Neuroinflamm. 2016;3(5);e272. 10.1212/NXI.0000000000000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Comi G, Jeffrey D, Kappos L, et al. : Oral Laquinimod Reduced Relapse Rate and Delayed Progression of Disability in Allegro, a Placebo-Controlled Phase III Trial for Relapsing-Remitting Multiple Sclerosis. Neurology. 2011;77:200–201. [Google Scholar]
- 145. Vollmer TL, Sorensen PS, Arnold DL, et al. : A placebo-controlled and active comparator phase III trial (BRAVO) for relapsing-remitting multiple sclerosis. Mult Scler J. 2011;17(10):S507–S508. Reference Source [Google Scholar]
- 146. Comi G, Vollmer TL, Boyko A, et al. : CONCERTO: a placebo-controlled trial of oral laquinimod in patients with relapsing-remitting multiple sclerosis. Mult Scler J. 2017;23:74–75. Reference Source [Google Scholar]
- 147. Kaye J, Piryatinsky V, Birnberg T, et al. : Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2016;113(41):E6145–E6152. 10.1073/pnas.1607843113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Toubi E, Nussbaum S, Staun-Ram E, et al. : Laquinimod modulates B cells and their regulatory effects on T cells in multiple sclerosis. J Neuroimmunol. 2012;251(1–2):45–54. 10.1016/j.jneuroim.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 149. Liu LF: DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. 10.1146/annurev.bi.58.070189.002031 [DOI] [PubMed] [Google Scholar]
- 150. Fisher GR, Patterson LH: DNA strand breakage by peroxidase-activated mitoxantrone. J Pharm Pharmacol. 1991;43(1):65–68. 10.1111/j.2042-7158.1991.tb05455.x [DOI] [PubMed] [Google Scholar]
- 151. De Isabella P, Palumbo M, Sissi C, et al. : Topoisomerase II DNA cleavage stimulation, DNA binding activity, cytotoxicity, and physico-chemical properties of 2-aza- and 2-aza-oxide-anthracenedione derivatives. Mol Pharmacol. 1995;48(1):30–38. [PubMed] [Google Scholar]
- 152. Errington F, Willmore E, Tilby MJ, et al. : Murine transgenic cells lacking DNA topoisomerase IIbeta are resistant to acridines and mitoxantrone: analysis of cytotoxicity and cleavable complex formation. Mol Pharmacol. 1999;56(6):1309–1316. 10.1124/mol.56.6.1309 [DOI] [PubMed] [Google Scholar]
- 153. Fox EJ: Mechanism of action of mitoxantrone. Neurology. 2004;63(12 Suppl 6):S15–18. 10.1212/WNL.63.12_suppl_6.S15 [DOI] [PubMed] [Google Scholar]
- 154. Ridge SC, Sloboda AE, McReynolds RA, et al. : Suppression of experimental allergic encephalomyelitis by mitoxantrone. Clin Immunol Immunopathol. 1985;35(1):35–42. 10.1016/0090-1229(85)90075-3 [DOI] [PubMed] [Google Scholar]
- 155. Edan G, Miller D, Clanet M, et al. : Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62(2):112–118. 10.1136/jnnp.62.2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Millefiorini E, Gasperini C, Pozzilli C, et al. : Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. J Neurol. 1997;244(3):153–159. 10.1007/s004150050066 [DOI] [PubMed] [Google Scholar]
- 157. Hartung HP, Gonsette R, König N, et al. : Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018–2025. 10.1016/S0140-6736(02)12023-X [DOI] [PubMed] [Google Scholar]
- 158. Hande KR: Clinical applications of anticancer drugs targeted to topoisomerase II. Biochim Biophys Acta. 1998;1400(1–3):173–184. 10.1016/S0167-4781(98)00134-1 [DOI] [PubMed] [Google Scholar]
- 159. Teitelbaum D, Arnon R, Sela M: Copolymer 1: from basic research to clinical application. Cell Mol Life Sci. 1997;53(1):24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. La Mantia L, Munari LM, Lovati R: Glatiramer acetate for multiple sclerosis. Cochrane Database Syst Rev. 2010; (5):CD004678. 10.1002/14651858.CD004678.pub2 [DOI] [PubMed] [Google Scholar]
- 161. Rovituso DM, Duffy CE, Schroeter M, et al. : The brain antigen-specific B cell response correlates with glatiramer acetate responsiveness in relapsing-remitting multiple sclerosis patients. Sci Rep. 2015;5; 14265. 10.1038/srep14265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pharmaceuticals I: New Data from IONIS-HTT Rx Phase 1/2 Study Demonstrates Correlation Between Reduction of Disease-causing Protein and Improvement in Clinical Measures of Huntington's Disease. In First drug to demonstrate lowering of mutant huntingtin, the disease-causing protein, in people with Huntington's disease. Carlsbad, CA.2018. Reference Source [Google Scholar]
- 163. Sevastou I, Pryce G, Baker D, et al. : Characterisation of Transcriptional Changes in the Spinal Cord of the Progressive Experimental Autoimmune Encephalomyelitis Biozzi ABH Mouse Model by RNA Sequencing. PLoS One. 2016;11(6): e0157754. 10.1371/journal.pone.0157754 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.