Highlights
-
•
New pathogenic familial SMARCA4 variant, c.3081+1G>T.
-
•
Prophylactic surgery in healthy carrier of germline SMARCA4 mutation.
-
•
Long term hormone therapy in a 13-year-old girl.
Keywords: SMARCA4 gene; BRG1 protein; Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT); Prophylactic oophorectomy
1. Introduction
Small cell carcinoma of the ovary of hypercalcemic type (SCCOHT) is a rare malignant neoplasm with fewer than 500 cases reported (Callegaro-Filho et al., 2016). SCCOHT mainly occurs in girls and young women with a median age of 24 years (Young et al., 1994). Most patients have advanced disease at the time of diagnosis, but even when diagnosed at stage I, the prognosis is poor, with 5-year survival of 30–55% (Foulkes et al., 2016; Witkowski et al., 2016). Half of all patients with SCCOHT die within the first year of diagnosis.
Recent studies have shown that SCCOHT is caused by inherited and acquired mutations in the SMARCA4 gene (located at 19p13.2) (Witkowski et al., 2014). The gene encodes the BRG1 protein that participates in SWI/SNF chromatin remodeling and functions as a gene activator or repressor (Fukumoto et al., 2018). The protein is lost in a large majority of SMARCA4 mutated tumors and the immunohistochemical demonstration of protein loss is a valuable tool for pathologists in the diagnosis of SCCOHT (Witkowski et al., 2014; Bailey et al., 2015; Clarke et al., 2016).
At this time there is no definitely effective treatment for SCCOHT and no targeted therapies. For familial cases, there is no consensus regarding the usefulness of screening or modalities, with serial pelvic ultrasound and serum calcium level measurements sometimes used. Furthermore, there is no evidence that early detection of the disease has any effect on outcome. Therefore, prophylactic removal of the ovaries is the only viable prevention option for genetic carriers of SMARCA4 mutation.
2. Case report
We report the case of a healthy 13 year-old pubertal Caucasian girl with a family history of SCCOHT. Her mother was diagnosed with SCCOHT at age 24 and died at 26. Her maternal aunt was diagnosed with the same disease at age 16 and died a year later. Both the mother and maternal aunt (sisters) were diagnosed with SCCOHT prior to the identification of the SMARCA4 mutations as the cause of the disease. The sisters died without having blood derivatives saved; their tumor paraffin blocks were eliminated from pathology archives after 10 years and thus are not available for further testing.
The patient underwent genetic counseling at the Department of Genetics at Doernbecher Children's Hospital, Oregon Health & Science University. Genetic analysis of SMARCA4 gene revealed a likely pathogenic variant, c.3081+1G>T. By the history, she inherited the mutation from her mother, and therefore maternal grandparents were tested for SMARCA4 mutation. The maternal grandfather, a 66-year-old healthy male, was found to carry the same mutation, confirming that the variant was maternally inherited. The maternal grandmother had normal SMARCA4 gene.
The patient had a normal abdominal ultrasound and normal serum calcium level (9.3 mg/dL). The benefits and risks of prophylactic surgery was discussed at a multidisciplinary tumor board including gynecologic oncologists, a geneticist, and a pediatric reproductive endocrinologist. The timing and the implications of prophylactic laparoscopic bilateral salpingo-oophorectomy were discussed with the patient and her family. The patient subsequently underwent bilateral salpingo-oophorectomy at the age 13. She received hormone replacement therapy with estrogen and progesterone after surgery. The ovaries and tubes were serially sectioned and examined in total pathologically. The ovaries and tubes were histologically normal. The constitutional DNA, RNA, and protein were obtained at the time of surgery and stored.
The expression of SMARCA4 (BRG1) protein was assessed immunohistochemically. Four-μm sections were cut from both ovaries and slides were stained with BRG1 antibody (Abcam; pretreatment ER2 20 min; dilution 1:100). Standard immunohistochemical methods were employed, including appropriate positive and negative tissue controls. A Bondmax Leica immunostainer was used with diaminobenzidene (DAB) as the chromogen.
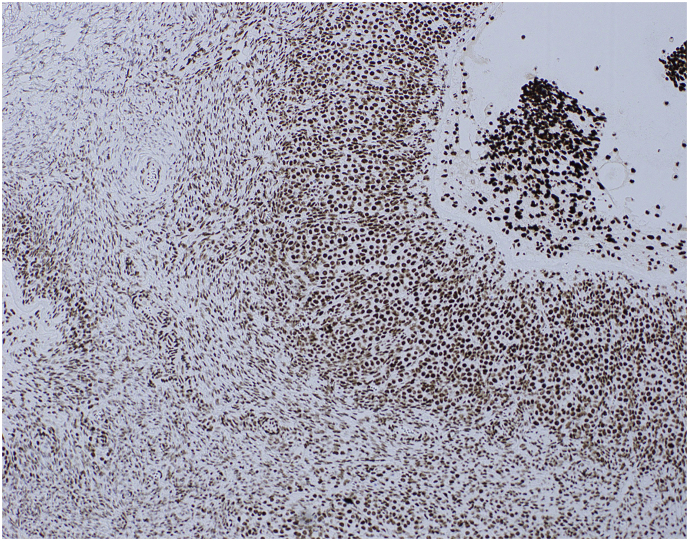
There was intact nuclear staining with SMARCA4 with diffuse immunoreactivity of the ovarian stroma, ova and the granulosa cells of the developing follicles (Fig. 1).
Fig. 1.
Retained SMARCA4 (BRG1) protein expression in one of the ovaries. There is positive nuclear staining of the granulosa cells of a developing follicle (right of photomicrograph) and the ovarian stroma (left of photomicrograph).
3. Discussion
The majority of cases of SCCOHT are due to biallelic somatic mutations in SMARCA4, but an increasing number of familial cases, attributable to a germline mutation on one allele of SMARCA4 and a somatic mutation on the other, are being identified and reported. Here we report a previously unreported germline SMARCA4 mutation (c.3081+1G>T) in four family members: an unaffected male carrier, his 2 daughters who succumbed to SCCOHT at the age of 17 and 26, and his 13 year old grand-daughter. This latter family member underwent prophylactic surgery after extensive genetic counseling. Because of the very young age of the carrier, genetic counseling included the utility of screening together with the risks, benefits and timing of risk-reducing surgery. The patient and her family expressed a clear desire to prevent the disease by surgical removal of the ovaries and tubes. The timing of surgery in this young patient was discussed and ultimately it was deemed too risky to delay it.
The screening modalities that are typically used for epithelial ovarian carcinoma, namely transvaginal ultrasound and serum CA125, are of little value in SCCOHT. Only two-thirds of affected patients have hypercalcemia (Foulkes et al., 2016). Furthermore, the value of early detection of SCCOHT is highly questionable, as the disease has only 30–55% long term survival rate even in the earliest stages (Witkowski et al., 2016).
A discussion of prophylactic surgery in female carriers of germline SMARCA4 mutations should balance the high risk of the early onset of SCCOHT (deemed high in this patient due to the significant family history, although the penetrance is unknown) against the need and risks of long-term management of hormone therapy (presented in Table 1).
Table 1.
Pros and cons of prophylactic BSO.
| Pro (in favor of early surgical intervention) | Con (against very early surgical intervention) | |
|---|---|---|
| c.3081+1G>T variant,SMARCA4 | High genetic risk of developing cancer at early age | No direct evidence of pathogenic effect of the mutation, no genetic material available from the mother or aunt |
| No screening methods | ||
| High mortality rate even in early stages | 30% long term cure in stage I | |
| No standardized or effective treatment | ||
| Psychologic consequences of living with the risk of cancer | Risk of depression and anxiety and fear of premature aging | |
| HRT available and safe | Increased risk of breast cancer with long term HRT | |
| Reproductive technique exist to maintain fertility | Freezing ovarian tissue for fertility is experimental |
Hormone replacement therapy is recommended to continue until at least age 50, with combined estrogen-progesterone to provide protective effects for cardiovascular and skeletal systems. The long-term clinical consequences of reduced androgen levels, if any, have not been studied. Exogenous androgen replacement is associated with acne and hirsuitism, and the safety of long-term androgen treatment has not been established (Speroff and Fritz, 2005). At this time, androgen replacement is not recommended.
This is the first report of prophylactic bilateral salpingo-oophorectomy in a pubertal girl with germline SMARCA4 mutation, and only the second report of genotype-informed prophylactic surgery for prevention of SCCOHT (Berchuck et al., 2015). Berchuck et al. reported the first case of prophylactic oophorectomy in a 33 year-old mother of 3 children, a carrier of familial pathogenic c.2617–3C > T mutation. In both these cases the ovaries were histologically normal with retention of nuclear staining with SMARCA4 (BRG1). It is important to note that while in germline SMARCA4 mutation carriers both ovaries are at risk of development of SCCOHT, in the more common cases of biallelic somatic SMARCA4 mutations, the contralateral ovary is not at increased risk for SCCOHT and therefore it may be retained in stage IA disease (Berchuck et al., 2015, Figure1). It is possible that additional cases of prophylactically removed ovaries may aid in the identification of premalignant precursor lesions in SCCOHT.
As the significance of SMARCA4 mutations in SCCOHT becomes more widely appreciated, in-depth functional characterization of genes regulated by BRG1 protein in the ovary may lead to improved diagnostic markers and standardized management strategies for this rare, but aggressive cancer.
In summary, we report the case of a young pubertal female with a germline SMARCA4 mutation who underwent prophylactic salpingo-oophorectomy. As far as we are aware, this is only the second report in the literature of a patient undergoing prophylactic surgery on account of a known germline SMARCA4 mutation. We discuss the pros and cons of prophylactic oophorectomy in female patients with a known germline SMARCA4 mutation.
Author contribution
Study design: TP, WGM, WF.
IRB application and approval: TP.
Study implementation: TP, WGM, FX, DL.
Data analysis and review: TP, WGM, AK, LW, WF.
Manuscript writing and editing: TP, WGM, AK, FX, DL, LW, WF.
Acknowledgements
We thank Dr. Jing Xu, PhD, for collecting and preserving the ovarian tissue and follicles and Dr. Sonali Joshi, PhD, for propagating cell culture and preparing DNA and RNA tissue and blood.
References
- Bailey S., Murray M.J., Witkowski L. Biallelic somatic SMARCA4 mutations in small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) Pediatr. Blood Cancer. 2015;62(4):728–730. doi: 10.1002/pbc.25279. [DOI] [PubMed] [Google Scholar]
- Berchuck A., Witkowski L., Hasselblatt M., Foulkes W.D. Prophylactic oophorectomy for hereditary small cell carcinoma of the ovary, hypercalcemic type. Gynecol. Oncol. Rep. 2015;12:20–22. doi: 10.1016/j.gore.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegaro-Filho D., Gershenson D.M., Nick A.M. Small cell carcinoma of the ovary-hypercalcemic type (SCCOHT): a review of 47 cases. Gynecol. Oncol. 2016;140(1):53–57. doi: 10.1016/j.ygyno.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B.A., Witkowski L., Ton Nu T.N. Loss of SMARCA4 (BRG1) protein expression as determined by immunohistochemistry in small-cell carcinoma of the ovary, hypercalcaemic type distinguishes these tumours from their mimics. Histopathology. 2016;69(5):727–738. doi: 10.1111/his.12988. [DOI] [PubMed] [Google Scholar]
- Foulkes W.D., Gore M., McCluggage W.G. Rare non-epithelial ovarian neoplasms: pathology, genetics and treatment. Gynecol. Oncol. 2016;142(1):190–198. doi: 10.1016/j.ygyno.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Fukumoto T., Magno E., Zhang R. SWI/SNF complexes in ovarian cancer: mechanistic insights and therapeutic implications. Mol. Cancer Res. 2018;16(12):1819–1825. doi: 10.1158/1541-7786.MCR-18-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroff L., Fritz M.A. 7th ed. Lippincott Williams and Wilkins; Philadelphia: 2005. Clinical gynaecologic endocrinology and infertility. [Google Scholar]
- Witkowski L., Carrot-Zhang J., Albrecht S. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat. Genet. 2014;46(5):438–443. doi: 10.1038/ng.2931. [DOI] [PubMed] [Google Scholar]
- Witkowski L., Goudie C., Ramos P. The influence of clinical and genetic factors on patient outcome in small cell carcinoma of the ovary, hypercalcemic type. Gynecol. Oncol. 2016;141(3):454–460. doi: 10.1016/j.ygyno.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.H., Oliva E., Scully R.E. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am. J. Surg. Pathol. 1994;18(11):1102–1116. doi: 10.1097/00000478-199411000-00004. [DOI] [PubMed] [Google Scholar]