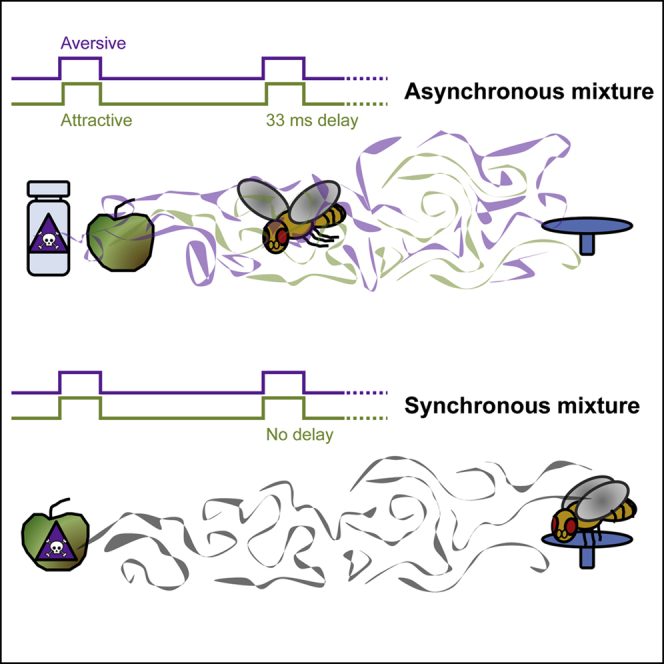
Summary
Odorants of behaviorally relevant objects (e.g., food sources) intermingle with those from other sources. Therefore to determine whether an odor source is good or bad—without actually visiting it—animals first need to segregate the odorants from different sources. To do so, animals could use temporal stimulus cues, because odorants from one source exhibit correlated fluctuations, whereas odorants from different sources are less correlated. However, the behaviorally relevant timescales of temporal stimulus cues for odor source segregation remain unclear. Using behavioral experiments with free-flying flies, we show that (1) odorant onset asynchrony increases flies' attraction to a mixture of two odorants with opposing innate or learned valence and (2) attraction does not increase when the attractive odorant arrives first. These data suggest that flies can use stimulus onset asynchrony for odor source segregation and imply temporally precise neural mechanisms for encoding odors and for segregating them into distinct objects.
Subject Areas: Biological Sciences, Entomology, Behavioral Neuroscience
Graphical Abstract
Highlights
-
•
Flies can detect whether two mixed odorants arrive synchronously or asynchronously
-
•
This temporal sensitivity occurs for odorants with innate and learned valences
-
•
Flies' behavior suggests use of odor onset asynchrony for odor source segregation
Biological Sciences; Entomology; Behavioral Neuroscience
Introduction
A natural scene is composed of simple stimuli, such as color, brightness, and movement of visual objects. In addition, it consists of relational stimuli that reflect the spatial and temporal correlations of those items that belong to the same object (e.g., the correlated movements of a person's body parts that allow us to segregate the person from the crowd). The mechanisms of how sensory systems use relational stimuli for object recognition are well understood in vision and audition. For example, humans use differences in stimulus onsets of a few tens of milliseconds to segregate visual objects from a background (Usher and Donnelly, 1998) or to segregate concurrent sounds from different sources (Hukin and Darwin, 1995). In contrast to vision and audition, olfaction research has mainly focused on simple stimuli, such as chemical identity, concentration, and dynamics of odorants (Galizia, 2014, Uchida et al., 2014), but it is largely unknown how the olfactory system processes relational stimuli that underlie olfactory object recognition.
Olfactory object recognition involves recognizing whether different odorants originate from the same or different sources (odor source segregation) (Hopfield, 1991). Odor source segregation can, in theory, be achieved from afar by analyzing the spatial distribution of odor plumes. The spatial distribution of odor plumes in the atmosphere is determined by the diffusion of odorant molecules and by the wind (Celani et al., 2014, Murlis et al., 2000). Because wind generally is turbulent, odor plumes are fragmented into filaments (similar to a plume of cigarette smoke). Because wind can transport odorant molecules much faster than diffusion, different odorants from the same source will largely stay together and will form a plume with relatively stable odorant concentration proportions (homogeneous plume). In contrast, odorants from different sources will be mixed by turbulent convection and will form a plume with variable odorant concentration proportions (heterogeneous plume).
Accordingly, plume heterogeneity enables animals to segregate odor sources (slugs [Hopfield and Gelperin, 1989], insects [Andersson et al., 2011, Baker et al., 1998, Saha et al., 2013, Szyszka et al., 2012], crabs [Weissburg et al., 2012]). But how do they do it? Animals could use temporal sampling for segregation, because in heterogeneous (multi-source) plumes the odorants from different sources exhibit less correlated fluctuations than in homogeneous (single-source) plumes (Celani et al., 2014, Hopfield, 1991). Thus, animals could use odorant onset asynchrony to detect that two odorants originate from different sources (Baker et al., 1998). Flying insects are particularly well adapted for detecting fine-scale temporal differences in odorant onset. Flying moths, for example, can segregate two odorants from two sources that are just 1 mm apart (Baker et al., 1998) and honey bees can use 6-ms short differences in odorant arrival for odor source segregation (Szyszka et al., 2012). Moreover, insects' rapid ligand-gated ionotropic olfactory receptors (Sato et al., 2008, Wicher et al., 2008) allow rapid and temporally precise odorant transduction (Egea-Weiss et al., 2018, Schuckel et al., 2008, Szyszka et al., 2014) and olfactory neurons are sensitive to stimulus onset asynchrony in the range of a few to tens of milliseconds (Broome et al., 2006, Meyer and Galizia, 2012, Nikonov and Leal, 2002, Saha et al., 2013, Stierle et al., 2013). However, the neural mechanisms of odor source segregation are still unknown.
Here we studied the capability of the fruit fly Drosophila melanogaster to use temporal stimulus cues for odor source segregation. We chose Drosophila because its genetic tractability will facilitate the determination of the causal relationship between behavioral odor source segregation and neural activity. We found that flies can detect a short difference in the arrival of two odorants (onset asynchrony of 33 ms). Odorant onset asynchrony increases flies' attraction to binary mixtures of odorants with opposing valence, suggesting that flies can use stimulus onset asynchrony for odor source segregation.
Results
To test whether flies can use stimulus onset asynchrony for odor source segregation, we measured flies' attraction toward synchronous (to mimic one odor source) and asynchronous (to mimic two odor sources) binary mixtures of attractive and aversive odorants in a wind tunnel (Figure 1A). In each experimental trial a single fly walked into the wind tunnel through a tube, which ended on top of a take-off platform (Figure 1B). The air flow at the take-off platform had a speed of 40 cm/s. Odorant stimuli were applied with a custom-made stimulator (Raiser et al., 2016) that was located outside of the wind tunnel to prevent turbulences and to allow for temporally precise odorant stimuli. We tracked flies' flights in 3D and measured their odor attraction using odorants with either innate or conditioned valence (original data are available in Data S1). After each experimental trial we removed and discarded the fly.
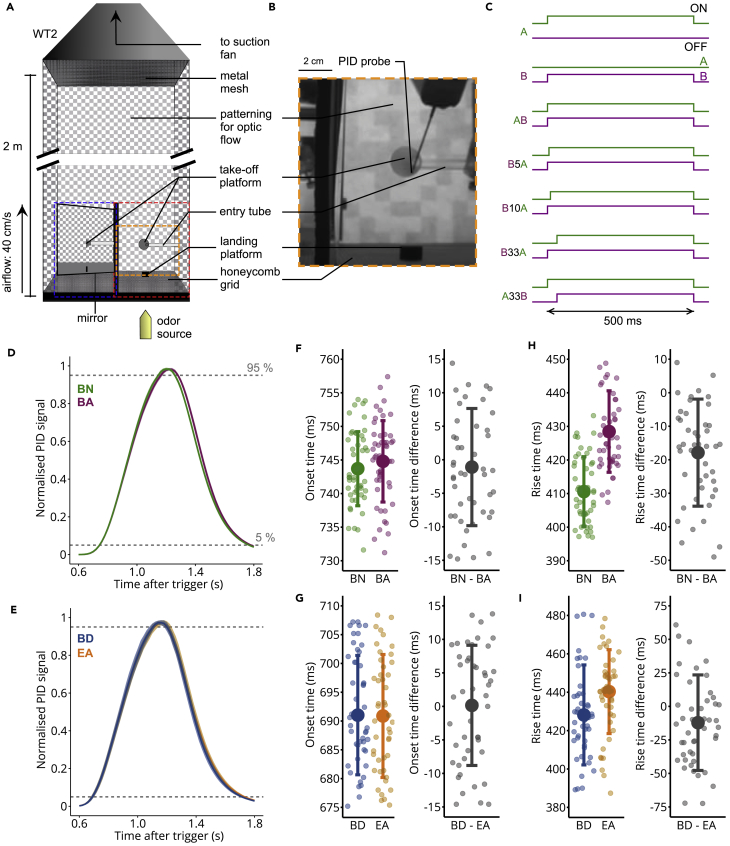
Figure 1.
Delivering Temporally Precise Olfactory Stimuli in a Wind Tunnel
(A) Diagram of a wind tunnel 2 (WT 2). Red and blue dashed boxes indicate the captured x-y and z-y planes, respectively. The olfactory stimulator was placed outside the wind tunnel to minimize turbulences. The orange box outlines the image in (B).
(B) The layout of WT 2, showing the position where the odorant concentrations were recorded using a PID.
(C) Valve states for creating odorant pulses for the different stimuli. The attractive odorant A and aversive odorant B are represented in green and magenta, respectively. When asynchronous mixtures were presented, the first odorant was always given for 500 ms, and the following odorant with an onset delay. Both odorants had the same offset time. Pulses were repeated every 2 s.
(D) PID recordings of pulsed stimuli for the odorant pair with innate valence 2-butanone (BN, green) and butanal (BA, magenta) (mean and SD over 50 pulses). Valves opened for 500 ms. Each PID signal was normalized to the maximum concentration reached.
(E) Same as (D) for the odorant pair with conditioned valence 2,3-butanedione (BD, blue) and ethyl acetate (EA, orange), averaged over 50 pulses.
(F) Left: Onset time (time taken to reach 5% of maximum concentration after valve trigger) for BN and BA (mean and SD over 50 pulses). Individual points represent the onsets for each pulse. Right: Onset time difference between pairs of successive BN and BA pulses (mean and SD over 50 pulses).
(G) Same as (F) for BD and EA.
(H) Left: Rise time (time taken to reach 95% of maximum concentration from the 5% onset time) for BN and BA (mean and SD over 50 pulses). Individual points represent the rise times for each pulse. Right: Mean rise time difference between pairs of successive BN and BA pulses (mean and SD over 50 pulses).
(I) Same as (H) for BD and EA.
See also Figure S1.
The pairs of odorants with innate valence used were 2-butanone (BN) and butanal (BA) and BN and benzaldehyde (BZ). For the conditioned odorants, we used 2,3-butanedione (BD) and ethyl acetate (EA). We chose these odorants based on their innate valences measured in tethered flying flies (Badel et al., 2016), in which BN was attractive, whereas BA and BZ were aversive, and BD and EA were behaviorally neutral (note that in previous studies EA and BD were attractive in walking paradigms; Rodrigues and Siddiqi, 1978, Steck et al., 2012). To mimic homogeneous odorant plumes from one source we presented both odorants as a synchronous mixture (no onset delay between odorants). To mimic heterogeneous odorant plumes from different sources we presented both odorants as asynchronous mixtures (with 5- to 33-ms delays between odorant onsets) (Figure 1C). We used different wind tunnels (WT 1 and WT 2) and different arrangements of the landing platforms in an attempt to optimize experimental conditions (see Transparent Methods). However, we found no clear differences in flies' performance, indicating that the results are robust and do not depend on specific arrangements of the wind tunnels. To eliminate between-session variability, all data shown in a given panel of a figure were collected during the same experimental sessions. Accordingly, data points should be compared within panels, but not between panels.
Tracking of Temporally Well-Controlled Odorant Stimuli in the Wind Tunnel
We determined the temporal precision of stimulus delivery by measuring the stimulus dynamics with a photoionization detector (PID) (Figures 1D–1I and S1A–S1C). The inlet of the PID was placed at the surface of the take-off platform (Figure 1B). Each odorant was presented 50 times in three separate experimental sessions for the three odorant pairs BN/BA, BN/BZ, or BD/EA. The onset times (time it took from valve opening to reach 5% of the maximum PID signal) were temporally precise across trials, with standard deviations ranging between 6 ms (BN, BA) and 10 ms (BD, EA) (Figures 1F, 1G, and S1B).
The onset times were similar for all odorant pairs (BN/BA, BN: 744 ± 6 ms, BA: 745 ± 6 ms; BN/BZ, BN: 750 ± 7 ms, BZ: 756 ± 7 ms; BD/EA, BD: 691 ± 10 ms, EA: 691 ± 10 ms; mean ± SD). The rise times (time it took to reach from 5% to 95% of the maximum PID signal) were also similar for the odorant pair BN/BA (BN, 411 ± 10 ms; BA, 428 ± 12 ms; mean ± SD) and for the odorant pair BD/EA (BD, 428 ms ± 26 ms; EA, 440 ms ± 21 ms), but less similar for the odorant pair BN/BZ (BN, 400 ± 12 ms; BZ, 444 ms ± 9 ms) (Figures 1H, 1I, and S1C). The differences in stimulus dynamics could be explained by the difference in the molecular mass between odorants, as stimulus dynamics gets slower with increasing molecular mass (in g/mol, BN, 72; BA, 72; BD, 86; EA, 88; BZ, 106) (Martelli et al., 2013, Raiser et al., 2016). Note, that part of the stimulus onset variability reflects the variability of the PID measurement itself and the actual stimulus dynamics may be less variable.
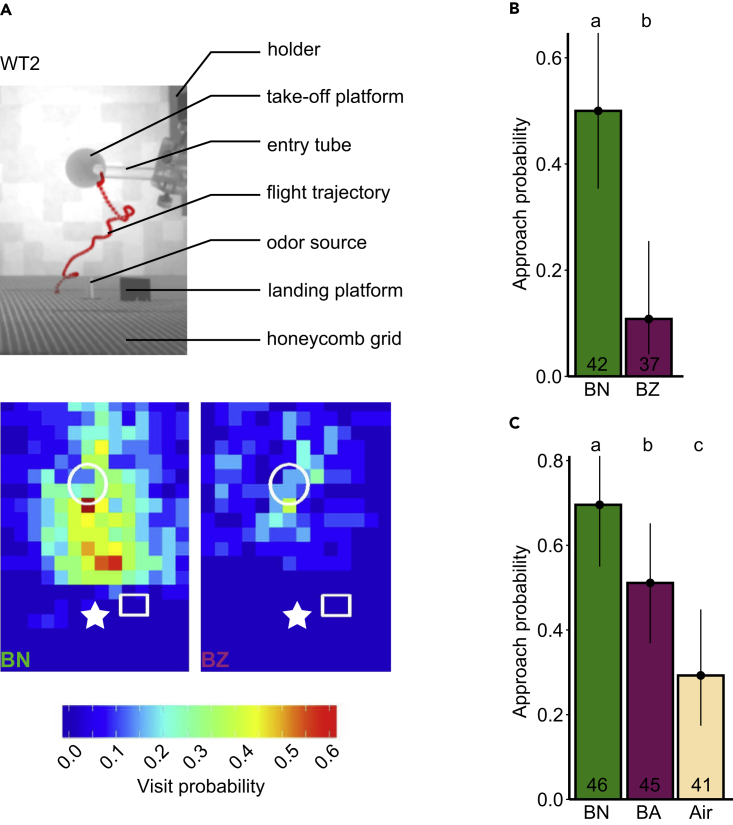
To visualize how flies explored space based on the odorant experience, we projected their flight trajectories on a plane and calculated the probability across flies to visit a particular pixel (visit probability, Figure 2A). When presented with the innately attractive odorant BN, flies were more likely to fly toward the target (which was either the actual odor source or a black platform near the odor source, see Transparent Methods) compared with the innately aversive odorant BZ. To assess the approach to the target, we counted the number of flies that reached halfway between the center of the take-off platform and the target (3.1 cm [117 pixels] for WT 1 and 2.7 cm [71 pixels] for WT 2) and calculated the approach probability by dividing this number by the total number of flies. Flies flew closer toward the target when stimulated with an attractive odorant than with an aversive odorant or a control air stimulus (Air) (p(BN > BZ)> 0.999 in Figure 2B; p(BN > BA) = 0.962, p(BN > Air)> 0.999 in Figure 2C; all statistical significances are given as Bayesian probabilities; see Transparent Methods and Table S2 for a comparison with frequentist statistics). However, in contrast to previous studies (van Breugel et al., 2018; Budick and Dickinson, 2006, Houot et al., 2017, Saxena et al., 2018), flies rarely landed at or near the target. This discrepancy might reflect the fact that, in contrast to these previous studies, our odorant delivery device was outside the wind tunnel. Positioning the odor delivery device inside the wind tunnel creates turbulences, and these turbulences could possibly provide localization cues for the fly to land. In contrast, our wind tunnel setting might mimic an odor source at a distance.
Figure 2.
Odor Tracking in the Wind Tunnel
(A) Top: Flight trajectory of a flying fly (red) in the wind tunnel during stimulation with BN. Bottom: Visit probability map equivalent to top image for BN and BZ (set 1). Each bin represents 20 × 20 pixels in the image, corresponding to 7.6 × 7.6 mm at the height of the landing platform. Each bin shows the mean binary value across flies. The take-off platform (white circle), landing platform (white rectangle), and odor source (white star) are indicated for position reference. n = 24 and 20 for BN and BZ, respectively.
(B) Approach probability to cross the half distance between take-off platform and landing platform for BN and BZ. Bars represent the mean. Vertical lines represent the 95% credible intervals. The lower-case letters represent significantly different responses for the different odorants; this applies for all figures. Numbers in bars indicate the number of flies; this applies for all figures.
(C) Same as in (B) but for BN, butanal (BA), and a blank air stimulus (Air).
See also Figures S1 and S2.
The percentage of flies that started flying ranged between 85% and 96% for the attractive odorant BN, ranged from 68% to 84% for the aversive odorants BZ or BA, and was 71% for the blank air control (Table S1). The average latency to flight ranged from 10–27 s, corresponding to 5–13 odorant pulses before taking off (Table S1, Figures S1H, S1J, S2D, and S2E). There was no consistent connection between the valence of an odorant and the latency to flight (e.g., latency to flight was longer for BZ than for BN (Figure S1H), but there was no difference for BN and BA (Figure S1J).
Attraction toward Asynchronous Mixtures of Odorants with Differing Innate Valence
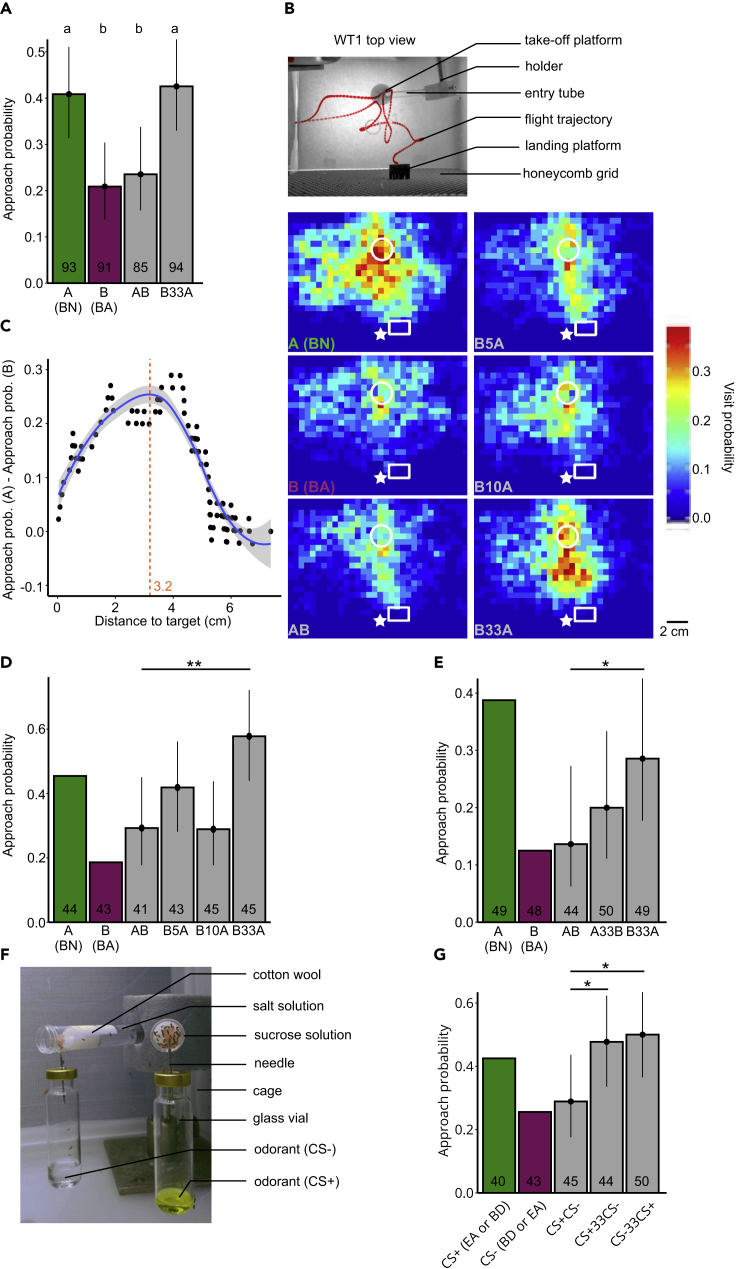
To test whether flies can detect stimulus onset asynchrony, we presented the attractive odorant BN (A) and the aversive odorant BA (B) either as single odorants combined in a synchronous mixture (AB) or in asynchronous mixtures in which B preceded A by 33 ms (B33A) (Figure 3A). Note that we used the odorant pair BN/BA to test the effect of stimulus onset asynchrony rather than BN/BZ because the differences in stimulus dynamics between BN and BZ make this odorant pair unsuitable for generating synchronous mixtures (Figure S1).
Figure 3.
Stimulus Onset Asynchrony Makes a Mixture of Odorants with Differing Valences More Attractive
(A) Approach probabilities (determined by the half distance threshold) for the single odorants BN (A), BA (B), their synchronous mixture (AB), and their asynchronous mixture (B33A). Bars represent the mean. Vertical lines represent the 95% credible intervals. The lower-case letters represent significantly different responses to the odorant treatments (this dataset is pooled from experiments shown in D and E).
(B) Top: Flight trajectory of a flying fly (red) in the wind tunnel during stimulation with BN (A). Bottom: Visit probability maps for A (BN) and B (BA) and the synchronous (AB) and asynchronous (B5A, B10A, B33A) mixtures. The take-off platform (white circle), landing platform (white rectangle), and odor source (white star) are indicated for position reference. n = 44, 43, 41, 43, 45, and 45 for A, B, AB, B5A, B10A and B33A, respectively.
(C) Thresholding method that uses the distance that separates flies' approach probabilities for A and B best (maximized A-B difference threshold). Each point represents the proportion of A-stimulated flies that approached the target by the given minimum distance to the target minus the proportion of B-stimulated flies. The blue trend line was fitted using locally weighted scatterplot smoothing. The gray area indicates the 95% confidence interval. The distance at the peak of the trend line was defined as threshold (orange dashed line and value).
(D) Approach probabilities (determined by the maximized A-B difference thresholding method) of the experiment to test flies' limit to detect onset asynchrony. Stars represent significantly different responses between AB and the other mixtures. As A and B are used to determine the threshold, they were not included in the statistical analysis.
(E) Approach probabilities (maximized A-B difference thresholding method) of the experiment to test the effect of odorant order in asynchronous mixtures.
(F) Conditioning setup in which flies were left for autonomous differential conditioning. Flies can freely fly in the cage and enter the odorized tubes containing cotton wool soaked either with aversive salt solution or attractive sucrose solution.
(G) Approach probability for odorant mixtures with conditioned valences (maximized A-B difference thresholding). Odorants BD and EA were used equally as often as the CS+ and CS−.
See also Figures S2 and S3.
Flies showed a higher approach probability for A compared with B (p(A > B) = 0.998) or with the synchronous mixture AB (p(A > AB) = 0.993) (Figure 3A). Moreover, flies showed a higher approach probability for the asynchronous mixture B33A compared with synchronous mixture AB (p(B33A > AB) = 0.996) or with the aversive odorant B (p(B33A > B)> 0.999). This shows that flies perceive the synchronous mixture AB and the asynchronous mixture B33A differently, with the onset asynchrony making the mixture more attractive.
To test whether flies are sensitive for shorter onset asynchronies we applied synchronous and asynchronous mixtures that started with B and with onset times differing by 5, 10, or 33 ms (B5A, B10A, B33A) (Figure 3B). Flies presented with A showed more activity in general, along with a higher visit probability near the target compared with flies presented with B. Flies showed a similar visit probability map for the synchronous mixture AB as for B. However, when stimulated with the asynchronous mixtures B33A, flies showed more activity near the target compared with AB and B.
To make the quantification of flies' approach behavior less arbitrary and to account for the fact that flies distributed differently in the two different wind tunnels and experimental sets, we calculated an approach area that segregated flies' approach probabilities for the attractive odorant A and the aversive odorant B the most (Figures 3C and S1–S3). We determined this area for each experimental set separately (see Transparent Methods). Note that this method maximizes the differences in approach probability between odorants A and B by design. Therefore we refrain from comparing flies' approach probabilities for A or B and restrict the comparisons to the mixtures.
The flies' responses to the mixtures depended on the timing between B and A (Figure 3D). For an onset asynchrony of 33 ms (B33A), flies were attracted to the target and scored a higher approach probability than for the synchronous mixture AB (p(B33A > AB) = 0.995), similar to that of A alone. However, for onset asynchronies of 5 or 10 ms (B10A), flies' approach probabilities were not different from the approach probability for AB (p(B5A > AB) = 0.894, p(B10A > AB) = 0.500).
Next, we wanted to discern whether the order in which odorants are presented in a mixture affects how a fly perceives the mixture. We used the same paradigm and odorants as before and stimulated flies with the synchronous mixture AB and the asynchronous mixtures A33B (A precedes B) and B33A (B precedes A) (Figures 3E and S2). In this paradigm, flies showed a lower approach probability to the synchronous mixture AB than to the asynchronous mixture B33A (p(B33A > AB) = 0.957)), confirming our previous result that B33A is perceived differently to AB, and is perceived by the fly as more attractive. However, the approach probability for the asynchronous mixture A33B was not significantly different from the approach probability for AB (p(A33B > AB) = 0.793)).
These data show that flies can discriminate between the synchronous mixture AB and asynchronous mixture B33A, supporting the hypothesis that flies can use stimulus onset asynchrony to segregate the attractive component A from the mixture of A and B even if they never encountered A alone (in B33A, B started before A and A ended at the same time as B).
Attraction toward Asynchronous Mixtures of Odorants with Differing Learned Valence
Finally, we wanted to determine whether flies' capability to discriminate between synchronous and asynchronous mixtures only works for odorants with differing innate valence, or whether it also works for odorants with differing learned valences. To address this question, we used an autonomous differential conditioning paradigm and paired one odorant (positively conditioned stimulus, CS+) with a 1 M sucrose solution and another odorant (negatively conditioned stimulus, CS−) with a saturated NaCl solution (Figure 3F). We used the odorants EA and BD equally often for CS+ and CS−. Thus CS+ and CS− only differ with regard to the learned valences, devoid of odorant-specific innate valences.
Also in this experiment, flies discriminated between synchronous and asynchronous mixtures and showed lower approach probabilities to the synchronous mixture of CS+ and CS− (CS+CS−) than to the asynchronous mixture CS+33CS− or CS−33CS+ (p(CS+33CS− > CS+CS−) = 0.965, p(CS−33CS+ > CS+CS−) = 0.981) (Figures 3G and S3). Together, these findings support the hypothesis that flies can use stimulus onset asynchrony to segregate odorants with both learned and innate valences from mixtures.
Discussion
We asked whether Drosophila can use stimulus onset asynchrony to segregate an attractive target odorant from a mixture with an aversive odorant. We found that flies show stronger attraction to asynchronous mixtures (mimicking two odorant sources) than to synchronous mixtures (mimicking one source). These results suggest that the fly's olfactory system can use temporal stimulus cues for olfactory object recognition and odor source segregation.
Stimulus Cues for Odor Source Segregation
Insects, crabs, and slugs can segregate an attractive target odorant from an aversive odorant depending on whether both odorants originate from the same source (forming a homogeneous plume) or from different sources (forming a heterogeneous plume) (Andersson et al., 2011, Baker et al., 1998, Hopfield and Gelperin, 1989, Weissburg et al., 2012). To segregate two odorants from different sources, animals could in theory (1) use temporal sampling to detect time differences in odorant arrival, (2) use spatial sampling to detect the spatial heterogeneity of odorant concentrations along or between olfactory organs, or (3) recognize the target odorant during bouts of its pure, unmixed presence.
Our data suggest that flies can segregate a target odorant from an asynchronous mixture without ever encountering the target odorant in its unmixed form (in BΔtA, the target odorant A is always mixed with B, because A starts after and ends with B) and that odor source segregation is no better when the target odorant A arrives first. Therefore, in our experiments flies must have used spatial or temporal sampling for odor source segregation. Theoretically, flies could have used spatial sampling if they orient non-parallel to the wind direction. Then, in the case of an asynchronous mixture BΔtA, the downwind antenna could encounter odorant B, whereas the upwind antenna already encounters the mixture AB. However, this spatial difference in odorant input across both antennae will last for 1 ms at most, given that both antennae span around 0.4 mm and that the odorant stimulus moves at 40 cm/s. Thus the spatial cue across antennae has a much shorter duration (1 ms at most) than the temporal cue provided by the stimulus (33 ms). It is therefore likely that flies used temporal sampling for detecting the odorant onset asynchrony. The capability to segregate odor sources based on odorant onset asynchrony could allow flies to ignore bad objects (e.g., a spoiled food source where food and detrimental odorants originate from the same source) and to find a good object in a patch of bad objects (e.g., food and detrimental odorants originate from different sources) without actually visiting the source.
The odor source segregation paradigms that previous studies and our study used were odor recognition tasks in which the target odor either had an innate (Andersson et al., 2011, Baker et al., 1998, Weissburg et al., 2012) or a learned valence (Hopfield and Gelperin, 1989, Saha et al., 2013, Szyszka et al., 2012). Therefore the neural process of odor source segregation could occur during the encoding of odor identity or during the encoding of innate or learned odor valence. The small necessary odorant onset asynchrony for odor source segregation (33 ms in our study) poses temporal constraints on the precision of the neural code for odorant identity or valence, which we will discuss next.
Temporal Precision of the Neural Code for Odorant Identity
The temporal precision with which odorant identity can be encoded is limited by the temporal precision of stimulus-evoked action potentials (spikes) (Jeanne and Wilson, 2015). In Drosophila, the temporal precision of olfactory receptor neurons is high and the timing of the first odor-evoked spikes jitters with a standard deviation of down to 0.2 ms (Egea-Weiss et al., 2018). Olfactory receptor neurons of a given type (neurons that express the same olfactory receptor) coalesce in distinct glomeruli of the antennal lobe where they converge onto projection neurons. Compared with olfactory receptor neurons, projection neuron responses show less trial-to-trial variability and faster dynamics (Bhandawat et al., 2007). The high temporal precision and high spike rates of odor-evoked responses in Drosophila olfactory receptor neurons (de Bruyne et al., 1999, Egea-Weiss et al., 2018, Martelli et al., 2013) and projection neurons (Bhandawat et al., 2007, Wilson et al., 2004) would allow for rapid and temporally precise encoding of odorant identity. For example, odorant identity could be encoded within a few tens of milliseconds by reading out the increase in spike rates across the earliest responding neurons only (Krofczik et al., 2009, Nawrot, 2012), or by reading out the differences in response latencies across neurons (Brill et al., 2013, Egea-Weiss et al., 2018, Krofczik et al., 2009, Martelli et al., 2013, Müller et al., 2002, Paoli et al., 2018).
In Drosophila, there is evidence that the spike rates across the earliest responding neurons, and not the differences in response latencies, encode behaviorally relevant odorant identity information: downstream neurons in the mushroom body (Kenyon cells) are insensitive to response latency differences between projection neurons in the range of tens of milliseconds (Gruntman and Turner, 2013). We therefore propose that behaviorally relevant odorant identity information for odor source segregation is encoded in the spike rates across the earliest responding projection neurons. This hypothesis is supported by the fact that downstream Kenyon cells generally have short integration time windows and respond to odorant onsets in a temporally precise manner (Demmer and Kloppenburg, 2009, Farkhooi et al., 2013, Ito et al., 2008, Perez-Orive, 2002, Szyszka et al., 2005, Turner et al., 2008).
Neural Responses to Synchronous and Asynchronous Odorant Mixtures
What is the neural correlate of odor source segregation based on odorant onset asynchrony? In the antennal lobe, synchronous odorant mixtures often induce neural activity patterns that lack part of the component information (synthetic mixture representation) (Deisig et al., 2006, Krofczik et al., 2009, Meyer and Galizia, 2012, Münch et al., 2013, Münch and Galizia, 2017, Silbering and Galizia, 2007). In contrast, asynchronous mixtures induce spatiotemporal activity patterns across projection neurons that partly match those evoked by the individual odorants (analytic mixture representation), and this asynchrony-induced shift from synthetic to more analytic mixture processing could support odor source segregation (Broome et al., 2006, Saha et al., 2013, Stierle et al., 2013) (for a modeling approach see Nowotny et al., 2013). However, the first arriving odorant often dominates the neural response to the asynchronous mixture, but such dominance of the first arriving odorant neither occurs in behavioral experiments in honey bees (Szyszka et al., 2012) nor in flies (this study). We therefore conclude that an asynchrony-induced shift from synthetic to a more analytic mixture representation in the antennal lobe cannot fully explain the behavioral odor source segregation observed in flies.
Alternatively, neural responses to both synchronous and asynchronous mixtures could contain sufficient analytical odorant information to allow for recognizing a target odorant. This hypothesis is supported by the observation that projection neurons that respond strongly to a single odorant generally also respond to its mixture with another odorant (Broome et al., 2006, Deisig et al., 2010, Saha et al., 2013, Silbering and Galizia, 2007, Stierle et al., 2013). Likewise, Kenyon cells that respond to a single odorant generally also respond to its mixture with another odorant (Campbell et al., 2013, Honegger et al., 2011, Shen et al., 2013). In addition, there is behavioral evidence for analytical processing of synchronous mixtures in Drosophila, as flies' responses to two odorants can add up linearly when presented as a synchronous mixture (Badel et al., 2016, Thoma et al., 2014). Moreover, flies fail in biconditional discrimination or negative patterning, tasks that require synthetic mixture processing (Young et al., 2011).
We therefore propose that (1) synchronous and asynchronous mixtures of odorants A and B activate a largely overlapping population of odorant-identity-encoding neurons (projection neurons and Kenyon cells), (2) this population includes both the A- and the B-activated neurons, and (3) odorant-identity-encoding neurons preserve the onset times of odorants A and B.
Neural Encoding of Odorant Valence and Odor Source Segregation
Stimulus-onset-asynchrony-induced timing difference between the A- and the B-activated projection neurons and Kenyon cells could be detected during the process of odorant recognition, i.e., during the transformation of the odorant identity code into a valence code. In insects, the innate odorant valence is encoded by lateral horn neurons (Jeanne et al., 2018, Jefferis et al., 2007, Roussel et al., 2014, Strutz et al., 2014), whereas learned odorant valence is encoded by mushroom body output neurons (Aso et al., 2014, Hige et al., 2015, Strube-Bloss et al., 2011). Both lateral horn neurons and mushroom body output neurons could preserve the temporal differences in odorant onsets: lateral horn neurons (in Drosophila) allow faster odorant detection than projection neurons (Jeanne and Wilson, 2015). Likewise, mushroom body output neurons (in honey bees) respond on average more rapidly to odorants than projection neurons (Strube-Bloss et al., 2012) and spike-timing-dependent plasticity (in locusts) enhances the synchronization of co-activated mushroom body output neurons (Cassenaer and Laurent, 2007).
Therefore, whether odorants A and B originate from one or two sources could be detected by coincidence-detecting neurons that receive input from valence-encoding lateral horn neurons or mushroom body output neurons. Those coincidence-detecting neurons would respond to synchronous input from the A- and B- activated valence-encoding neurons (A and B come from one source) but not to asynchronous input (A and B come from different sources). Coincidence detection could, for example, be mediated by N-methyl-D-aspartate (NMDA) glutamate receptors (Mayer et al., 1984). The existence of glutamatergic neurons and NMDA receptors in both the lateral horn and in the mushroom body (Sinakevitch et al., 2010), and glutamatergic valence-encoding mushroom body output neurons in Drosophila (Aso et al., 2014, Owald et al., 2015), is consistent with this hypothetical mechanism.
Comparison with Mammalian Olfaction
Several studies have suggested that mammals have difficulties in segregating the single odorants from mixtures (e.g., Laing and Francis, 1989), but temporal differences in odorant arrival can help odor segregation: For example, in humans, stimulus onset asynchrony in tens of milliseconds impairs the detection of the following odorant (Laing et al., 1994) and in mice a delay in tens of seconds between a background odorant and a following target odorant facilitates the detection of the target odorant (Linster et al., 2007). However, it is currently unknown whether mammals can also use odorant onset asynchronies in millisecond range for odor source segregation. The timescales of olfactory processing in mice suggest that they could detect stimulus onset asynchrony in the tens of milliseconds range and use it for odor segregation: First, mice can identify odorants rapidly (within less than 200 ms) (Abraham et al., 2004, Uchida and Mainen, 2003). Second, odor-evoked spikes in olfactory bulb neurons can be temporally precise, with an average trial-to-trial standard deviation of just 12 ms (Shusterman et al., 2011). This high spike timing precision allows rapid odor coding, for example, by reading out the differences in response latencies (Haddad et al., 2013, Junek et al., 2010, Schaefer and Margrie, 2012, Spors, 2006) or by reading out the earliest responding neurons only (Wilson et al., 2017).
The next important steps will be to determine the behaviorally relevant stimulus timescales for odor source segregation in natural environments and to conduct causal studies on the underlying neural mechanisms. Doing this both in insects and mammals offers the possibility to reveal the differences and unifying principles of olfactory object recognition.
Limitations of the Study
The finding that flies show a higher approach to the asynchronous mixture B33A (the attractive odorant A arrives 33 ms after odorant B) than to the synchronous mixture AB shows that flies can detect stimulus onset asynchrony. However, it does not allow conclusions about the perceptual differences between B33A and AB. Thus we cannot discriminate between the proposed explanations that (1) flies perceive the attractive odorant A better in B33A than in AB or (2) flies perceive odorant A equally well in B33A and AB, and the 33-ms onset asynchrony adds the information that A and B originate from different sources. The question about the perceptual differences between AB and B33A could be answered to some extent by analyzing how neural responses to AB and B33A relate the responses of A and B alone.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Stefanie Neupert for advice on the statistics and comments on the manuscript, C. Giovanni Galiza, Thomas Nowotny, and Mario Pannunzi for comments on the manuscript and Cansu Tafrali for help with the experiments. This project was funded by the Human Frontier Science Program (RGP0053/2015 to PS.).
Author Contributions
P.S. conceptualized and designed the study. Y.M.G. and A.S. performed the data collection. T.T. wrote the video processing script and provided expertise on the analysis. Y.G.M. and A.S. performed the video processing. A.S. performed the statistical analysis. P.S., Y.G.M., and A.S. wrote and edited the manuscript. P.S. supervised the study.
Declaration of Interests
The authors declare no competing interests.
Published: March 29, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.02.014.
Supplemental Information
The CSV file contains 186,182 rows. The 13 columns contain the following information per column:
1. The odorant pair used in the experiment for the attractive odorant (A) and aversive odorant (B), or the conditioned odorants CS+ and CS−.
2. The video file name for each experiment, containing the date, experimenter initials, and the odorants used.
3. The stimulus presented to the fly during that experiment.
4. The response of the fly, as to whether it flew from the platform or not. Flies that departed from the platform had their coordinates for the first 10 s of flight recorded. Those that did not fly were assigned the same coordinate for a single time point, which was the closest physical point the fly could be to the target without leaving the platform.
5. The set, determining the arrangement of the wind tunnel (see Transparent Methods).
6. The video frame that the coordinate was recorded. Each video was recorded at 90 frames per second, and lasted for 2 or 3 minutes.
7. The x coordinate of the fly.
8. The y coordinate of the fly.
9. The z coordinate of the fly.
10. The x coordinate of the center of the take-off platform.
11. The y coordinate of the center of the take-off platform.
12. The z coordinate of the center of the take-off platform.
13. The x coordinate of the middle of the target, at the edge closest to the take-off platform.
14. The y coordinate of the middle of the target, at the edge closest to the take-off platform.
15. The z coordinate of the middle of the target, at the edge closest to the take-off platform.
16. The figure in the main text that this data was used for.
References
- Abraham N.M., Spors H., Carleton A., Margrie T.W., Kuner T., Schaefer A.T. Maintaining accuracy at the expense of speed. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Andersson M.N., Binyameen M., Sadek M.M., Schlyter F. Attraction modulated by spacing of pheromone components and anti-attractants in a bark beetle and a moth. J. Chem. Ecol. 2011;37:899–911. doi: 10.1007/s10886-011-9995-3. [DOI] [PubMed] [Google Scholar]
- Aso Y., Sitaraman D., Ichinose T., Kaun K.R., Vogt K., Belliart-Guérin G., Plaçais P.Y., Robie A.A., Yamagata N., Schnaitmann C. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife. 2014;3:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badel L., Ohta K., Tsuchimoto Y., Kazama H. Decoding of context-dependent olfactory behavior in Drosophila. Neuron. 2016;91:155–167. doi: 10.1016/j.neuron.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Baker T.C., Fadamiro H.Y., Cosse A.A. Moth uses fine tuning for odour resolution. Nature. 1998;393:530. [Google Scholar]
- Bhandawat V., Olsen S.R., Gouwens N.W., Schlief M.L., Wilson R.I. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat. Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breugel F., Huda A., Dickinson M.H. Distinct activity-gated pathways mediate attraction and aversion to CO2 in Drosophila. Nature. 2018;564:420–424. doi: 10.1038/s41586-018-0732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill M.F., Rosenbaum T., Reus I., Kleineidam C.J., Nawrot M.P., Rossler W. Parallel processing via a dual olfactory pathway in the honeybee. J. Neurosci. 2013;33:2443–2456. doi: 10.1523/JNEUROSCI.4268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome B.M., Jayaraman V., Laurent G. Encoding and decoding of overlapping odor sequences. Neuron. 2006;51:467–482. doi: 10.1016/j.neuron.2006.07.018. [DOI] [PubMed] [Google Scholar]
- de Bruyne M., Clyne P.J., Carlson J.R. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J. Neurosci. 1999;19:4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budick S.A., Dickinson M.H. Free-flight responses of Drosophila melanogaster to attractive odors. J. Exp. Biol. 2006;209:3001–3017. doi: 10.1242/jeb.02305. [DOI] [PubMed] [Google Scholar]
- Campbell R.A.A., Honegger K.S., Qin H., Li W., Demir E., Turner G.C. Imaging a population code for odor identity in the Drosophila mushroom body. J. Neurosci. 2013;33:10568–10581. doi: 10.1523/JNEUROSCI.0682-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassenaer S., Laurent G. Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts. Nature. 2007;448:709–713. doi: 10.1038/nature05973. [DOI] [PubMed] [Google Scholar]
- Celani A., Villermaux E., Vergassola M. Odor landscapes in turbulent environments. Phys. Rev. X. 2014;4:1–17. [Google Scholar]
- Deisig N., Giurfa M., Lachnit H., Sandoz J.C. Neural representation of olfactory mixtures in the honeybee antennal lobe. Eur. J. Neurosci. 2006;24:1161–1174. doi: 10.1111/j.1460-9568.2006.04959.x. [DOI] [PubMed] [Google Scholar]
- Deisig N., Giurfa M., Sandoz J.C. Antennal lobe processing increases separability of odor mixture representations in the honeybee. J. Neurophysiol. 2010;103:2185–2194. doi: 10.1152/jn.00342.2009. [DOI] [PubMed] [Google Scholar]
- Demmer H., Kloppenburg P. Intrinsic membrane properties and inhibitory synaptic input of kenyon cells as mechanisms for sparse coding? J. Neurophysiol. 2009;102:1538–1550. doi: 10.1152/jn.00183.2009. [DOI] [PubMed] [Google Scholar]
- Egea-Weiss A., Renner A., Kleineidam C.J., Szyszka P. High precision of spike timing across olfactory receptor neurons allows rapid odor coding in Drosophila. IScience. 2018;4:76–83. doi: 10.1016/j.isci.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkhooi F., Froese A., Muller E., Menzel R., Nawrot M.P. Cellular adaptation facilitates sparse and reliable coding in sensory pathways. PLoS Comput. Biol. 2013;9:e1003251. doi: 10.1371/journal.pcbi.1003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizia C.G. Olfactory coding in the insect brain: data and conjectures. Eur. J. Neurosci. 2014;39:1784–1795. doi: 10.1111/ejn.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruntman E., Turner G.C. Integration of the olfactory code across dendritic claws of single mushroom body neurons. Nat. Neurosci. 2013;16:1821–1829. doi: 10.1038/nn.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R., Lanjuin A., Madisen L., Zeng H., Murthy V.N., Uchida N. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat. Neurosci. 2013;16:949–957. doi: 10.1038/nn.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hige T., Aso Y., Modi M.N., Rubin G.M., Turner G.C. Heterosynaptic plasticity underlies aversive olfactory learning in Drosophila. Neuron. 2015;88:985–998. doi: 10.1016/j.neuron.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger K.S., Campbell R.A.A., Turner G.C. Cellular-resolution population imaging reveals robust sparse coding in the Drosophila mushroom body. J. Neurosci. 2011;31:11772–11785. doi: 10.1523/JNEUROSCI.1099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J.J. Olfactory computation and object perception. Proc. Natl. Acad. Sci. U S A. 1991;88:6462–6466. doi: 10.1073/pnas.88.15.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J.F., Gelperin A. Differential conditioning to a compound stimulus and its components in the terrestrial mollusc Limax maximus. Behav. Neurosci. 1989;103:329–333. [Google Scholar]
- Houot B., Gigot V., Robichon A., Ferveur J.-F. Free flight odor tracking in Drosophila: effect of wing chemosensors, sex and pheromonal gene regulation. Sci. Rep. 2017;7:40221. doi: 10.1038/srep40221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukin R.W., Darwin C.J. Comparison of the effect of onset asynchrony on auditory grouping in pitch matching and vowel identification. Percept. Psychophys. 1995;57:191–196. doi: 10.3758/bf03206505. [DOI] [PubMed] [Google Scholar]
- Ito I., Ong R.C.-Y., Raman B., Stopfer M. Sparse odor representation and olfactory learning. Nat. Neurosci. 2008;11:1177–1184. doi: 10.1038/nn.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanne J.M., Wilson R.I. Convergence, divergence, and reconvergence in a feedforward network improves neural speed and accuracy. Neuron. 2015;88:1014–1026. doi: 10.1016/j.neuron.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanne J.M., Fişek M., Wilson R.I. The organization of projections from olfactory glomeruli onto higher-order neurons. Neuron. 2018;98:1198–1213.e6. doi: 10.1016/j.neuron.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis G.S.X.E., Potter C.J., Chan A.M., Marin E.C., Rohlfing T., Maurer C.R., Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junek S., Kludt E., Wolf F., Schild D. Olfactory coding with patterns of response latencies. Neuron. 2010;67:872–884. doi: 10.1016/j.neuron.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Krofczik S., Menzel R., Nawrot M.P. Rapid odor processing in the honeybee antennal lobe network. Front. Comput. Neurosci. 2009;2:9. doi: 10.3389/neuro.10.009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing D.G., Francis G.W. The capacity of humans to identify odors in mixtures. Physiol. Behav. 1989;46:809–814. doi: 10.1016/0031-9384(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Laing D.G., Eddy A., Francis G.W., Stephens L. Evidence for the temporal processing of odor mixtures in humans. Brain Res. 1994;651:317–328. doi: 10.1016/0006-8993(94)90712-9. [DOI] [PubMed] [Google Scholar]
- Linster C., Henry L., Kadohisa M., Wilson D.A. Synaptic adaptation and odor-background segmentation. Neurobiol. Learn. Mem. 2007;87:352–360. doi: 10.1016/j.nlm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Martelli C., Carlson J.R., Emonet T. Intensity invariant dynamics and odor-specific latencies in olfactory receptor neuron response. J. Neurosci. 2013;33:6285–6297. doi: 10.1523/JNEUROSCI.0426-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M.L., Westbrook G.L., Guthrie P.B. Voltage-dependent block by Mg2+of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Meyer A., Galizia C.G. Elemental and configural olfactory coding by antennal lobe neurons of the honeybee (Apis mellifera) J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2012;198:159–171. doi: 10.1007/s00359-011-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D., Abel R., Brandt R., Zöckler M., Menzel R. Differential parallel processing of olfactory information in the honeybee, Apis mellifera L. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2002;188:359–370. doi: 10.1007/s00359-002-0310-1. [DOI] [PubMed] [Google Scholar]
- Münch D., Galizia C.G. Take time: odor coding capacity across sensory neurons increases over time in Drosophila. J. Comp. Physiol. A. 2017;203:959–972. doi: 10.1007/s00359-017-1209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch D., Schmeichel B., Silbering A.F., Galizia C.G. Weaker ligands can dominate an odor blend due to syntopic interactions. Chem. Senses. 2013;38:293–304. doi: 10.1093/chemse/bjs138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murlis J., Willis M.A., Carde R.T. Spatial and temporal structures of pheromone plumes in fields and forests. Physiol. Entomol. 2000;25:211–222. [Google Scholar]
- Nawrot M.P. Dynamics of sensory processing in the dual olfactory pathway of the honeybee. Apidologie. 2012;43:269–291. [Google Scholar]
- Nikonov A.A., Leal W.S. Peripheral coding of sex pheromone and a behavioral antagonist in the Japanese beetle, Popillia japonica. J. Chem. Ecol. 2002;28:1075–1089. doi: 10.1023/a:1015274104626. [DOI] [PubMed] [Google Scholar]
- Nowotny T., Stierle J.S., Galizia C.G., Szyszka P. Data-driven honeybee antennal lobe model suggests how stimulus-onset asynchrony can aid odour segregation. Brain Res. 2013;1536:119–134. doi: 10.1016/j.brainres.2013.05.038. [DOI] [PubMed] [Google Scholar]
- Owald D., Felsenberg J., Talbot C.B., Das G., Perisse E., Huetteroth W., Waddell S. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron. 2015;86:417–427. doi: 10.1016/j.neuron.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli M., Albi A., Zanon M., Zanini D., Antolini R., Haase A. Neuronal response latencies encode first odor identity information across subjects. J. Neurosci. 2018;38:9240–9251. doi: 10.1523/JNEUROSCI.0453-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Orive J. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Raiser G., Galizia C.G.G., Szyszka P. A high-bandwidth dual-channel olfactory stimulator for studying temporal sensitivity of olfactory processing. Chem. Senses. 2016;42:bjw114. doi: 10.1093/chemse/bjw114. [DOI] [PubMed] [Google Scholar]
- Rodrigues V., Siddiqi O. Genetic analysis of chemosensory pathway. Proc. Indian Acad. Sci. Sect. B Exp. Biol. 1978;87:147–160. [Google Scholar]
- Roussel E., Carcaud J., Combe M., Giurfa M., Sandoz J.-C. Olfactory coding in the honeybee lateral horn. Curr. Biol. 2014;24:561–567. doi: 10.1016/j.cub.2014.01.063. [DOI] [PubMed] [Google Scholar]
- Saha D., Leong K., Li C., Peterson S., Siegel G., Raman B. A spatiotemporal coding mechanism for background-invariant odor recognition. Nat. Neurosci. 2013;16:1830–1839. doi: 10.1038/nn.3570. [DOI] [PubMed] [Google Scholar]
- Sato K., Pellegrino M., Nakagawa T., Nakagawa T., Vosshall L.B., Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Saxena N., Natesan D., Sane S.P. Odor source localization in complex visual environments by fruit flies. J. Exp. Biol. 2018;221:jeb172023. doi: 10.1242/jeb.172023. [DOI] [PubMed] [Google Scholar]
- Schaefer A.T., Margrie T.W. Psychophysical properties of odor processing can be quantitatively described by relative action potential latency patterns in mitral and tufted cells. Front. Syst. Neurosci. 2012;6:30. doi: 10.3389/fnsys.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckel J., Meisner S., Torkkeli P.H., French A.S. Dynamic properties of Drosophila olfactory electroantennograms. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2008;194:483–489. doi: 10.1007/s00359-008-0322-6. [DOI] [PubMed] [Google Scholar]
- Shen K., Tootoonian S., Laurent G. Encoding of mixtures in a simple olfactory system. Neuron. 2013;80:1246–1262. doi: 10.1016/j.neuron.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman R., Smear M.C., Koulakov A.A., Rinberg D. Precise olfactory responses tile the sniff cycle. Nat. Neurosci. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Silbering A.F., Galizia C.G. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J. Neurosci. 2007;27:11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinakevitch I., Grau Y., Strausfeld N.J., Birman S. Dynamics of glutamatergic signaling in the mushroom body of young adult Drosophila. Neural Dev. 2010;5:10. doi: 10.1186/1749-8104-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J. Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck K., Veit D., Grandy R., Badia S.B.I., Badia S.B.I., Mathews Z., Verschure P., Hansson B.S., Knaden M. A high-throughput behavioral paradigm for Drosophila olfaction - the Flywalk. Sci. Rep. 2012;2:361. doi: 10.1038/srep00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierle J.S., Galizia C.G., Szyszka P. Millisecond stimulus onset-asynchrony enhances information about components in an odor mixture. J. Neurosci. 2013;33:6060–6069. doi: 10.1523/JNEUROSCI.5838-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strube-Bloss M.F., Nawrot M.P., Menzel R. Mushroom body output neurons encode odor – reward associations. J. Neurosci. 2011;31:3129–3140. doi: 10.1523/JNEUROSCI.2583-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strube-Bloss M.F., Herrera-Valdez M.A., Smith B.H. Ensemble response in mushroom body output neurons of the honey bee outpaces spatiotemporal odor processing two synapses earlier in the antennal lobe. PLoS One. 2012;7:e50322. doi: 10.1371/journal.pone.0050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz A., Soelter J., Baschwitz A., Farhan A., Grabe V., Rybak J., Knaden M., Schmuker M., Hansson B.S., Sachse S. Decoding odor quality and intensity in the Drosophila brain. Elife. 2014;3:e04147. doi: 10.7554/eLife.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszka P., Ditzen M., Galkin A., Galizia C.G.G., Menzel R. Sparsening and temporal sharpening of olfactory representations in the honeybee mushroom bodies. J. Neurophysiol. 2005;94:3303–3313. doi: 10.1152/jn.00397.2005. [DOI] [PubMed] [Google Scholar]
- Szyszka P., Stierle J.S., Biergans S., Galizia C.G. The speed of smell: odor-object segregation within milliseconds. PLoS One. 2012;7:e36096. doi: 10.1371/journal.pone.0036096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszka P., Gerkin R.C., Galizia C.G.G., Smith B.H.B.H. High-speed odor transduction and pulse tracking by insect olfactory receptor neurons. Proc. Natl. Acad. Sci. U S A. 2014;111:16925–16930. doi: 10.1073/pnas.1412051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma M., Hansson B.S., Knaden M. Compound valence is conserved in binary odor mixtures in Drosophila melanogaster. J. Exp. Biol. 2014;217:3645–3655. doi: 10.1242/jeb.106591. [DOI] [PubMed] [Google Scholar]
- Turner G.C., Bazhenov M., Laurent G. Olfactory representations by Drosophila mushroom body neurons. J. Neurophysiol. 2008;99:734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- Uchida N., Mainen Z.F. Speed and accuracy of olfactory discrimination in the rat. Nat. Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Uchida N., Poo C., Haddad R. Coding and transformations in the olfactory system. Annu. Rev. Neurosci. 2014;37:363–385. doi: 10.1146/annurev-neuro-071013-013941. [DOI] [PubMed] [Google Scholar]
- Usher M., Donnelly N. Visual synchrony affects binding and segmentation in perception. Nature. 1998;394:179–182. doi: 10.1038/28166. [DOI] [PubMed] [Google Scholar]
- Weissburg M., Atkins L., Berkenkamp K., Mankin D. Dine or dash? Turbulence inhibits blue crab navigation in attractive-aversive odor plumes by altering signal structure encoded by the olfactory pathway. J. Exp. Biol. 2012;215:4175–4182. doi: 10.1242/jeb.077255. [DOI] [PubMed] [Google Scholar]
- Wicher D., Schäfer R., Bauernfeind R., Stensmyr M.C., Heller R., Heinemann S.H., Hansson B.S. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Wilson C.D., Serrano G.O., Koulakov A.A., Rinberg D. A primacy code for odor identity. Nat. Commun. 2017;8:1477. doi: 10.1038/s41467-017-01432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.I., Turner G.C., Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Young J.M., Wessnitzer J., Armstrong J.D., Webb B. Elemental and non-elemental olfactory learning in Drosophila. Neurobiol. Learn. Mem. 2011;96:339–352. doi: 10.1016/j.nlm.2011.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The CSV file contains 186,182 rows. The 13 columns contain the following information per column:
1. The odorant pair used in the experiment for the attractive odorant (A) and aversive odorant (B), or the conditioned odorants CS+ and CS−.
2. The video file name for each experiment, containing the date, experimenter initials, and the odorants used.
3. The stimulus presented to the fly during that experiment.
4. The response of the fly, as to whether it flew from the platform or not. Flies that departed from the platform had their coordinates for the first 10 s of flight recorded. Those that did not fly were assigned the same coordinate for a single time point, which was the closest physical point the fly could be to the target without leaving the platform.
5. The set, determining the arrangement of the wind tunnel (see Transparent Methods).
6. The video frame that the coordinate was recorded. Each video was recorded at 90 frames per second, and lasted for 2 or 3 minutes.
7. The x coordinate of the fly.
8. The y coordinate of the fly.
9. The z coordinate of the fly.
10. The x coordinate of the center of the take-off platform.
11. The y coordinate of the center of the take-off platform.
12. The z coordinate of the center of the take-off platform.
13. The x coordinate of the middle of the target, at the edge closest to the take-off platform.
14. The y coordinate of the middle of the target, at the edge closest to the take-off platform.
15. The z coordinate of the middle of the target, at the edge closest to the take-off platform.
16. The figure in the main text that this data was used for.