Abstract
Patient: Male, 60
Final Diagnosis: Hyponatremia
Symptoms: Alcohol intoxication
Medication: —
Clinical Procedure: —
Specialty: Neurosurgery
Objective:
Unusual clinical course
Background:
Development of syndrome of inappropriate antidiuretic hormone secretion or cerebral salt wasting has been commonly noted in post-traumatic brain injury, and this condition may lead to hyponatremia resulting in cerebral edema and possible cerebral herniation. However, the predominant topographic pattern of edema from hyponatremia has not been well documented. Unlike numerous reports on hyponatremia and vasospasm following aneurysmal subarachnoid hemorrhage, the data for traumatic brain injury patient are still limited. We report on a rare patient with malignant middle cerebral artery infarction as a result of hyponatremia following traumatic brain injury.
Case Report:
A 60-year-old Native American male with significant past medical history of alcoholism, hypertension, and hemorrhagic stroke presented to the emergency department by emergency medical service after he was struck by a vehicle in a hit-and-run incident. The patient sustained multiple abrasions, and he had elevated alcohol levels. His initial Glasgow Coma Score (GCS) was 14 with a confused conversation (V4). Computer tomography (CT) of the head showed 5 mm thickness acute subdural and subarachnoid hemorrhage of right frontal, temporal, and parietal areas, with 3 mm midline shift at the level of foramen of Monro. Traumatic brain injury conservative treatment was initiated as well as alcoholic withdrawal protocols in the intensive care unit. Patient initially improved neurologically despite low sodium levels. He recouped to fully conscious, with a GCS score of 15, at 24 hours after admission. On day 9, he was found unresponsive with a head CT showed malignant right middle cerebral artery infarction, resulted in 15 mm subfalcine herniation. The patient passed away 48 hours later, as patient’s family declined further intervention.
Conclusions:
The management and prevention of post-traumatic vasospasm may be complicated even in asymptomatic and neurologically intact patients. Close neurological monitoring and prevention protocols are important in activating appropriate management.
MeSH Keywords: Brain Injuries; Hyponatremia; Subarachnoid Hemorrhage, Traumatic; Vasospasm, Intracranial
Background
Syndrome of inappropriate antidiuretic hormone secretion (SIADH) and cerebral salt wasting (CSW) leading to hyponatremia are common following neurologic insults such as traumatic brain injury (TBI) [1,2]. Diagnosis of hyponatremia due to either etiology must be precise because there is different patho-physiology and different treatments for SIADH and CSW [2,3]. The mechanism behind SIADH is the overproduction of anti-diuretic hormones, which acts on the V2 receptors located in the late distal tubules and collecting ducts, which leads to an increase in free water reabsorption [3]. As for CSW, there may be an overproduction of natriuretic peptides resulting in vasodilation, an increase in glomerular filtration rate due to afferent arteriole dilation and efferent arteriole constriction, and overall natriuresis and decreased plasma sodium levels [3]. Although both condition vary in their mechanisms and treatments, they both result in hyponatremia and potential cerebral edema and herniation. Physician must be aware of cerebral edema and herniation due to prolonged hyponatremia and demyelination from rapid correction of hyponatremia, especially in alcoholic patient [4]. Literature has demonstrated diffuse cerebral edema from uncorrected hyponatremia, but not the specific herniation location or arterial infarction location [5–7].
Besides the development of hyponatremia post-TBI post-traumatic arterial vasospasms (PTV) in the presence of traumatic subarachnoid hemorrhage may also occur but has not been extensively researched [8]. Unlike vasospasms in the presence of aneurysmal subarachnoid hemorrhage, which is characterized by neurological deterioration and often found on routine surveillance imaging, the routine surveillance for PTV is not often performed [8]. Therefore, there is an increase in morbidity and mortality as there is no-to-limited specific treatment guideline or clinical prevention for PTV. In this report, we present a case of a patient who developed malignant middle cerebral artery infarction as a result of hyponatremia and vasospasm after TBI.
Case Report
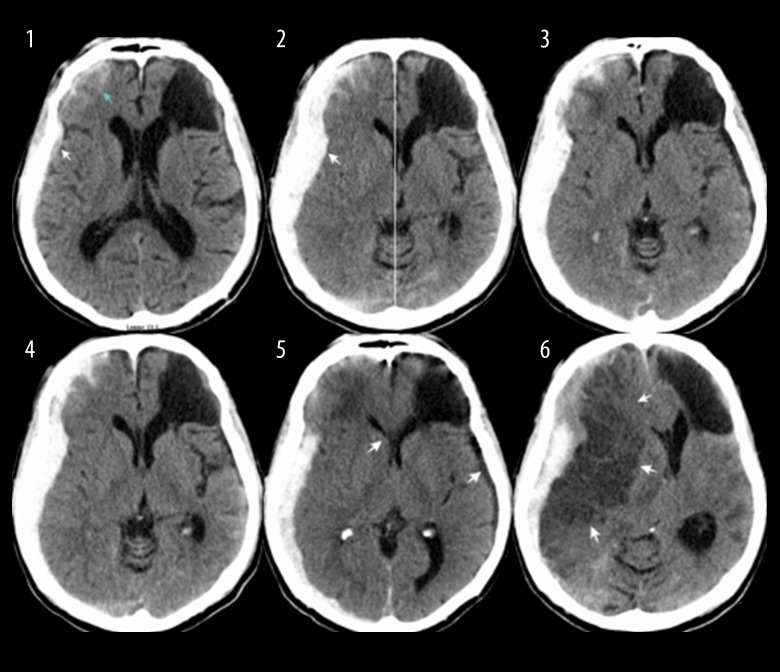
The patient was a 60-year-old Native American male who presented to the emergency room (ER) by emergency medical service (EMS) ambulance at Southeastern Regional Medical Center (SRMC) after being hit by a car. There were no witnesses to describe the impact, but he was found down, at the curbside, in a 25 MPH speed limited area. He was awake, with an alcohol smell by the time EMS arrived. His initial Glasgow Coma Score (GCS) score was 14 (V4 for confusion). The full examination revealed abrasion wound at right forehead, left thigh, left elbow, and both knees. Computer tomography (CT) of the head showed acute subdural and subarachnoid hemorrhage of right frontal, temporal, and parietal area 5 mm maximal thickness with 3 mm midline shift at the foramen of Monro (Figure 1-2). There was an area of left frontal encephalomalacia, which appeared stable compare to the previous scan 2 years earlier (Figure 1-1). His initial laboratory values were normal except his blood alcohol was 339. Electronic medical records showed his past medical history included type II diabetes, hypertension, cardiac arrhythmia, coronary artery disease, depression, gastro-esophageal reflux, and alcoholism. He does not take any home medications due to poor compliance. His family confirmed a history of remote brain bleed from trauma more than 3 years ago. He had a negative carotid ultrasound study from outside hospital more than 3 years ago. He was baseline neurologically intact and reportedly drank alcohol almost every day. CT neck, chest, and abdomen were negative. The initial diagnosis was acute subdural hematoma and alcoholic intoxication. The decision was made to manage the subdural hematoma conservatively. He was admitted to the intensive care unit (ICU) with an hourly neurological status examination. Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol was initiated, vitamin K and B12 were given. Hypertonic saline therapy was given for 5 days, followed by fluid restriction protocol and oral salt supplements. The spot EEG (electroencephalogram) was negative for ictal or inter-ictal activity. Two interval CT head during his ICU stay appeared stable (Figure 1–3, 1–4). He was transferred to the regular unit after he emerged from the withdrawal stage at post trauma day 6. He was neurologically intact and continued doing well despite a low sodium and osmolarity level. At day 9, he was last seen normal, sitting in his recliner and having dinner before he was found unresponsive 2 hours later. His nurse found him lying on the floor with his right side down. He was marginally able to maintain his airway without any vomitus content around him. Neither carotid nor radial pulse was palpated during an initial primary survey. Cardiopulmonary resuscitation was promptly initiated with a cervical collar in place. At the secondary survey after hemodynamic stabilization, his pupils were 5 mm fixed on the right, and 3 mm slightly reactive on the left. His GCS score at that point was 5T (E1M4Vt). His best neurological exam was withdrawn from pain on the right arm. Seizure could not be ruled out due to lack of video monitoring in his room. His sodium was 117 mmol/L from the blood drawn during CPR (cardiopulmonary resuscitation). Stat CT head showed right middle cerebral artery infarction with subfalcine herniation, compared to CT scan a day prior (Figures 1–5, 1–6, 2). Repeated neurological examination after he returned from the CT suite revealed devastating GCS score of 3T. His pupils were both fixed and dilated. Only cough and gag reflex were presented. The family decided not to pursue any further treatment, and he passed away 48 hours later. No autopsy was performed.
Figure 1.
Subsets1 to 6: Computed tomography (CT) head scans. CT head at different time interval showing the development of subdural and subarachnoid hemorrhage of right frontal, temporal, and parietal area with midline shift at the foramen of Monro. The images also show prior, stable left frontal encephalomalacia.
Discussion
Hyponatremia during hospital admission is associated with increased risk of mortality [5,6,9]. In spontaneous intracerebral hemorrhage patient, Kuramatsu et al. reported a prevalence of hyponatremia on admission of 15.6% in 464 patients with an odd ratio of 2.2 predictor of hospital mortality [7]. However, compared to patients with TBI, Lohani et al. found 27.2% prevalence of hyponatremia in 33 TBI patients, and a greater number of TBI patients had elevated central venous pressure (CVP) indicating that the hyponatremia was more likely due to SIADH than CSW [1]. Cole et al., similarly, found that the development of SIADH was more frequent than CSW following TBI [2]. SIADH and CSW must be distinguished before initiate treatment because SIADH is associated with volume-expansion with normal-to-elevated CVP, whereas CSW is associated with volume-contraction with low CVP [2,3]. Fraser et al. summarized a detailed algorithm differentiating between SIADH and CSW, and emphasized the detrimental neurological consequences due to each condition [3]. Diringer et al. suggested electrolyte free water restriction in awake and cooperative patients given the self-limited nature of SIADH [10]. More importantly, slow correction of hyponatremia is crucial due to the risk of osmotic demyelination [2]. On the other hand, untreated cerebral edema can cause irreversible neurological damage [4]. At present, no consensus has been made regarding medication treatment for SIADH. Rabinstein et al. recommended fludrocortisone given its greater mineralocorticoids with less glucocorticoid effect compare to hydrocortisone [11]. A newer agent, antidiuretic hormone arginine vasopressin (AVP) receptor antagonist, has been proposed in chronic SIADH, but has not yet become a standard treatment [10].
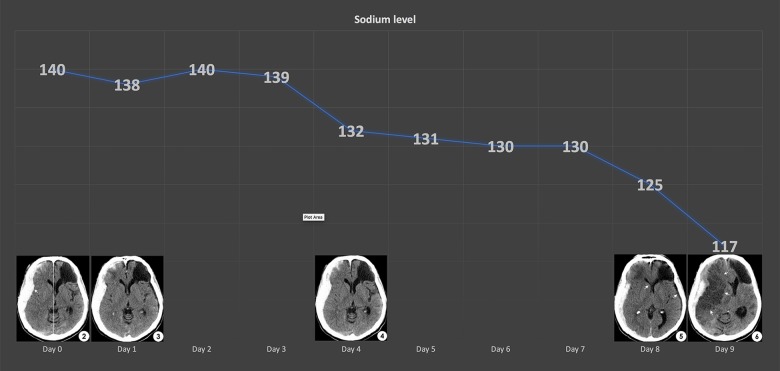
Complications from a sudden drop in sodium level include seizures, permanent brain damage, coma, respiratory arrest, cerebral edema, herniation, and death [2]. Carpenter et al. reported a child with a sudden decrease in serum sodium that was associated with reversible brain edema [12]. Donati-Genet et al. reported a child with TBI, who developed a seizure as a result of acute hyponatremia (sodium levels of 119 mmol/L) [13]. In our case, we indirectly determined the extracellular fluid volume by using calculated serum osmolality and hematocrit level. The decreasing hematocrit level, normal serum osmolality, and urine sodium less than 40 mmol/L are all characteristics of SIADH-induced hyponatremia. We monitored his sodium levels in a 4 hours interval for the first 4 days, and then 6 hours interval for 2 days, before changing it to daily draw. After emerging from the withdrawal period at day 6, he continued to improve neurologically despite decreased sodium level. Neither symptoms nor evidence of brain edema were present on serial CT brain scans from day 6 to day 8. Retrospectively, these test results gave us a false sense of security because on day 9, a malignant middle cerebral artery infarction occurred when the sodium level suddenly drop from 125 to 117 mmol/L.
Vasospasm is a well-known complication following TBI with an incidence of 25–40% [14,15]. Similar to aneurysmal subarachnoid hemorrhage, Taneda et al. reported that the vasospasm occurred on day 4 to day 16 and a correlation between the traumatic subarachnoid hemorrhage location and vasospastic artery [15]. Chu et al. reported that vasospasm after mild TBI with minimal focal subarachnoid hemorrhage occurred at day 10, and then resolved by day 21 [16]. Oertel et al. demonstrated 45.2% post-traumatic vasospasm at day 2 after a TBI event, which resolved within 5 days [17]. They also found a higher incidence of PTV in younger patients, those with low GCS score, and preference for anterior circulation location [17]. Early vasospasm started on day 2, and peaked on day 4–5 has been reported [18]. Vasospasm as early as 12 hours after traumatic incidence was reported in severe patient with GCS <6 [19]. In addition to the location of blood, mechanical force can also cause vasospasm by inducing endothelin hypersensitivity and prolong hypercontractility [20]. Perrein et al. found direct vasoactive agent from an injured brain parenchyma [21]. Armonda et al. conducted a wartime study, and found that blast injury was a potential cause of vasospasm, which may be successfully treated by endovascular balloon angioplasty [22]. Unlike vasospasm after aneurysmal subarachnoid hemorrhage, the outcome in TBI patients is still under debate. Lee et al. found that the presence of PTV was correlated with increased bad prognosis, while Steiger et al. found no negative relationship between post-traumatic vaso-spasm and outcome [23,24]. Sander et al. compared transcranial Doppler ultrasonography (TCD) flow velocity, and found similarity among traumatic and aneurysmal subarachnoid hemorrhage, but varying final outcomes [25]. In this case, we suspected vasospasm as an etiology of middle cerebral artery infarction for a number of reasons. First, the onset on day 9 correlated with post-traumatic vasospasm chronology, and second, the laterality of right middle cerebral artery infarction due to right traumatic subarachnoid hemorrhage (Figure 2). Third, the focal artery pathology favored vasospasm rather than diffuse edema from hyponatremia. Although literature confirmed the relationship between hyponatremia and vasospasm following aneurysm subarachnoid hemorrhage via brain natriuresis protein [26–30], the mechanism in TBI cases is still less defined. Nonetheless, providing the reasons discussed, concordant with sodium level, we believed this affirmed the relationship between hyponatremia and vasospasm in our case.
Figure 2.
Graph of sodium levels and corresponding computed tomography (CT) head scans at various time frame. As the sodium levels decreased, risk of cerebral edema increased, and between day 8 and day 9, the sudden drop from 125 to 117 mmol/L demonstrated a subfalcine herniation on the STAT CT head scan.
We confessed the limitations in our rural community hospital. Finite specialist coverage and technology limited us from investigating all the possibilities in a timely manner. Nonetheless, these facts ought not to mandate patient transfer, nor make physicians less competent. Servadei et al. suggested certain post-acute care guidelines and emphasized the significant of TBI study in the rural community where TBI is an endemic disease [31]. We, as rural area physicians, agree that there should be specific guidelines set in place, more emphasis in TBI studies including PTV in rural communities, and that each step of care should be critically linked to other steps and dictate the final outcome [31].
Conclusions
Specific location of hyponatremia-induced cerebral herniation remains unclear, but the potential development of diffuse edema and herniation due to rapid, uncorrected hyponatremia is undeniable, and therefore, prompt surveillance and treatment of the underlying cause is advised with special caution in slowly correcting the sodium levels to prevent osmotic pontine demyelination. Although studies have reported that there is an increased risk of PTV in younger patients with low GCS score, outliers or exception may not follow the characteristic patient prolife, however, at the same time, routine surveillance and prevention of all TBI patient may not be cost-effective, and may expose the patient to unnecessary radiation and anxiety. Nonetheless, continued collection and accumulation of atypical presentation of PTV in TBI patients may help us narrow the chronology and ultimately assist us in treatment and prevention of malignant arterial infarction in TBI patients with normal presentation.
Footnotes
Conflict of Interests
None.
References:
- 1.Lohani S, Devkota UP. Hyponatremia in patients with traumatic brain injury: Etiology, incidence, and severity correlation. World Neurosurg. 2011;76(3):355–60. doi: 10.1016/j.wneu.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Cole CD, Gottfried ON, Liu JK, Couldwell WT. Hyponatremia in the neurosurgical patient: Diagnosis and management. Neurosurg Focus. 2004;16(4):E9. doi: 10.3171/foc.2004.16.4.10. [DOI] [PubMed] [Google Scholar]
- 3.Fraser J, Stieg P. Hyponatremia in the neurosurgical patient: Epidemiology, pathophysiology, diagnosis, and management. Neurosurgery. 2006;59:222–29. doi: 10.1227/01.NEU.0000223440.35642.6E. discussion 222. [DOI] [PubMed] [Google Scholar]
- 4.Adrogué HJ. Consequences of inadequate management of hyponatremia. Am J Nephrol. 2005;25(3):240–49. doi: 10.1159/000086019. [DOI] [PubMed] [Google Scholar]
- 5.Sturdik I, Adamcova M, Kollerova J, et al. Hyponatremia is an independent predictor of in-hospital mortality. Eur J Intern Med. 2014;25(4):379–82. doi: 10.1016/j.ejim.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122(9):857–65. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuramatsu J, Bobinger T, Volbers B, et al. Hyponatremia is an independent predictor of in-hospital mortality in spontaneous intracerebral hemorrhage. Stroke. 2014;45(5):1285–91. doi: 10.1161/STROKEAHA.113.004136. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mufti F, Amuluru K, Changa A, et al. Traumatic brain injury and intracranial hemorrhage-induced cerebral vasospasm: A systematic review. Neurosurg Focus. 2017;43(5):E14. doi: 10.3171/2017.8.FOCUS17431. [DOI] [PubMed] [Google Scholar]
- 9.Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD. Mortality and serum sodium: Do patients die from or with hyponatremia? Clin J Am Soc Nephrol. 2011;6(5):960–65. doi: 10.2215/CJN.10101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diringer M, Zazulia A. Hyponatremia in neurologic patients: Consequences and approaches to treatment. Neurologist. 2006;12(3):117–26. doi: 10.1097/01.nrl.0000215741.01699.77. [DOI] [PubMed] [Google Scholar]
- 11.Rabinstein A, Bruder N. Management of hyponatremia and volume contraction. Neurocrit Care. 2011;15(2):354–60. doi: 10.1007/s12028-011-9585-9. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter J, Weinstein S, Myseros J, et al. Inadvertent hyponatremia leading to acute cerebral edema and early evidence of herniation. Neurocrit Care. 2007;6(3):195–99. doi: 10.1007/s12028-007-0032-x. [DOI] [PubMed] [Google Scholar]
- 13.Donati-Genet PCM, Dubuis J, Girardin E, Rimensberger PC. Acute symptomatic hyponatremia and cerebral salt wasting after head injury: An important clinical entity. J Pediatr Surg. 2001;36(7):1094–97. doi: 10.1053/jpsu.2001.24770. [DOI] [PubMed] [Google Scholar]
- 14.Martin NA, Doberstein C, Alexander M, et al. Posttraumatic cerebral arterial spasm. J Neurotrauma. 1995;12(5):897–901. doi: 10.1089/neu.1995.12.897. [DOI] [PubMed] [Google Scholar]
- 15.Taneda M, Kataoka K, Akai F, et al. Traumatic subarachnoid hemorrhage as a predictable indicator of delayed ischemic symptoms. J Neurosurg. 1996;84(5):762–68. doi: 10.3171/jns.1996.84.5.0762. [DOI] [PubMed] [Google Scholar]
- 16.Chu L, Sharma M, Shoamanesh A. Severe cerebral vasospasm and infarction after minor head trauma. Can J Neurol Sci. 2017;44(5):618–20. doi: 10.1017/cjn.2017.200. [DOI] [PubMed] [Google Scholar]
- 17.Oertel M, Boscardin WJ, Obrist WD, et al. Posttraumatic vasospasm: The epidemiology, severity, and time course of an underestimated phenomenon: A prospective study performed in 299 patients. J Neurosurg. 2005;103(5):812–24. doi: 10.3171/jns.2005.103.5.0812. [DOI] [PubMed] [Google Scholar]
- 18.Hadani M, Bruk B, Ram Z, et al. Transiently increased basilar artery flow velocity following severe head injury: A time course transcranial doppler study. J Neurotrauma. 1997;14(9):629–36. doi: 10.1089/neu.1997.14.629. [DOI] [PubMed] [Google Scholar]
- 19.Compton JS, Teddy PJ. Cerebral arterial vasospasm following severe head injury: A transcranial doppler study. Br J Neurosurg. 1987;1(4):435–39. doi: 10.3109/02688698708999633. [DOI] [PubMed] [Google Scholar]
- 20.Alford PW, Dabiri BE, Goss JA, et al. Blast-induced phenotypic switching in cerebral vasospasm. Proc Natl Acad Sci USA. 2011;108(31):12705–10. doi: 10.1073/pnas.1105860108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrein A, Petry L, Reis A, et al. Cerebral vasospasm after traumatic brain injury: An update. Minerva Anestesiol. 2015;81(11):1219–28. [PubMed] [Google Scholar]
- 22.Armonda RA, Bell RS, Vo AH, et al. Wartime traumatic cerebral vasospasm: Recent review of combat casualties. Neurosurgery. 2006;59(6):1215–25. doi: 10.1227/01.NEU.0000249190.46033.94. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Martin NA, Alsina G, et al. Hemodynamically significant cerebral vasospasm and outcome after head injury: A prospective study. J Neurosurg. 1997;87(2):221–33. doi: 10.3171/jns.1997.87.2.0221. [DOI] [PubMed] [Google Scholar]
- 24.Steiger HJ, Aaslid R, Stooss R, Seiler RW. Transcranial Doppler monitoring in head injury: Relations between type of injury, flow velocities, vasoreactivity, and outcome. Neurosurgery. 1994;34:79–85. discussion 85. [PubMed] [Google Scholar]
- 25.Sander D, Klingelhöfer J. Cerebral vasospasm following post-traumatic subarachnoid hemorrhage evaluated by transcranial doppler ultrasonography. J Neurol Sci. 1993;119(1):1–7. doi: 10.1016/0022-510x(93)90185-2. [DOI] [PubMed] [Google Scholar]
- 26.Morinaga K, Hayashi S, Matsumoto Y, et al. [Hyponatremia and cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage] No To Shinkei. 1992;44(7):629–32. [in Japanese] [PubMed] [Google Scholar]
- 27.McGirt MJ, Blessing R, Nimjee SM, et al. Correlation of serum brain natriuretic peptide with hyponatremia and delayed ischemic neurological deficits after subarachnoid hemorrhage. Neurosurgery. 2004;54(6):1369–74. doi: 10.1227/01.neu.0000125016.37332.50. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi AI, Suri MFK, Sung GY, et al. Prognostic significance of hypernatremia and hyponatremia among patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;50(4):749–56. doi: 10.1097/00006123-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Sviri GE, Shik V, Raz B, Soustiel JF. Role of brain natriuretic peptide in cerebral vasospasm. Acta Neurochirur. 2003;145(10):851–60. doi: 10.1007/s00701-003-0101-7. [DOI] [PubMed] [Google Scholar]
- 30.Mapa B, Taylor BE, Appelboom G, et al. Impact of hyponatremia on morbidity, mortality, and complications after aneurysmal subarachnoid hemorrhage: A systematic review. World Neurosurg. 2016;85:305–14. doi: 10.1016/j.wneu.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 31.Servadei F, Picetti E, Viaroli E, Iaccarino C. Traumatic brain injury guidelines and outcome: Please don’t forget post-acute care! World Neurosurg. 2016;90:657–58. doi: 10.1016/j.wneu.2016.02.013. [DOI] [PubMed] [Google Scholar]