Highlights
-
•
We aimed to detect EEG changes induced by meditative interventions in PTRD subjects.
-
•
PTRD subjects exhibited increased gamma activity in the IPL relative to controls.
-
•
Changes of delta activity in the precuneus correlated with changes of the QOL scale.
Keywords: EEG, Meditation, eLORETA, PTRD, Gamma band, Delta band
Abstract
Objective
Neurophysiological changes related to meditation have recently attracted scientific attention. We aimed to detect changes in electroencephalography (EEG) parameters induced by a meditative intervention in subjects with post-traumatic residual disability (PTRD), which has been confirmed for effectiveness and safety in a previous study. This will allow us to estimate the objective effect of this intervention at the neurophysiological level.
Methods
Ten subjects with PTRD were recruited and underwent psychological assessment and EEG recordings before and after the meditative intervention. Furthermore, 10 additional subjects were recruited as normal controls. Source current density as an EEG parameter was estimated by exact Low Resolution Electromagnetic Tomography (eLORETA). Comparisons of source current density in PTRD subjects after the meditative intervention with normal controls were investigated. Additionally, we compared source current density in PTRD subjects between before and after meditative intervention. Correlations between psychological assessments and source current density were also explored.
Results
After meditative intervention, PTRD subjects exhibited increased gamma activity in the left inferior parietal lobule relative to normal controls. In addition, changes of delta activity in the right precuneus correlated with changes in the psychological score on role physical item, one of the quality of life scales reflecting the work or daily difficulty due to physical problems.
Conclusions
These results show that the meditative intervention used in this study produces neurophysiological changes, in particular the modulation of oscillatory activity of the brain.
Significance
Our meditative interventions might induce the neurophysiological changes associated with the improvement of psychological symptoms in the PTRD subjects.
1. Introduction
Meditation refers to a set of various and specific methods of attentional engagement (Cahn et al., 2010). Recently, Buddhist and Yoga meditation practices have been applied not only for their original spiritual purpose but also for their capacity to improve health-related problems (Thomas et al., 2014). We previously reported the effectiveness of complementary and alternative medicine (CAM) including Yoga meditation in subjects with post-traumatic residual disability (PTRD) (Hayashi et al., 2013). In this previous study we defined subjects with PTRD as individuals who had difficulty in social lives by residual physical disabilities due to a severe disaster or accident in spite of undergoing standard medical treatment over one year. Standard medical treatment over one year had not improved their symptoms, thus, they were regarded as a group of patients that were resistant for general medical treatment. Patients with physical and mental distress derived from traumas of accidents or disasters tend to suffer from prolonged distress even if they undergo general medical treatments. These patients usually have difficulty to be cured by general medical therapy and some of them ask for help with CAM therapies. However, such CAM therapies have not been sufficiently verified for effectiveness and safety in patients with post-traumatic distress. Thus, we conducted a previous study on meditation in this kind of patients and confirmed the effectiveness (e.g., improvement in psychological symptoms) and safety of this intervention. This previous study, however, investigated psychological symptoms based on self-administered questionnaire and had the limitation of objectivity when evaluating the status of the patients (Hayashi et al., 2013).
Electroencephalography (EEG) has attracted a great deal of scientific attention as an objective measure to assess biological changes induced by meditation (Lutz et al., 2004, Tei et al., 2009, Cahn et al., 2010, Thomas et al., 2014, Faber et al., 2015, DeLosAngeles et al., 2016, Braboszcz et al., 2017, Kang et al., 2018, Lee et al., 2018, Schoenberg et al., 2018). Early studies on expert meditators reported enhanced alpha power as state and trait features associated with meditative practices (Wallace, 1970, Kasamatsu and Hirai, 1966). Later studies failed to replicate these findings (Cahn et al., 2010). Recent studies suggest neurophysiological findings associated with meditation in different frequency bands. For instance, significant enhanced gamma power has been demonstrated in a study of expert Buddhist meditators (Lutz et al., 2004). Another study also proved that a group of expert Western meditators exhibited significantly enhanced gamma power in a “mindfulness” meditation compared to a mind-wandering condition (Cahn et al., 2010). On the other hands, some researchers observed decreases of low gamma (25–48 Hz), beta (13–25 Hz) power and increases in frontal midline and temporo-parietal theta power during deeper absorptions of Buddhist concentrative meditation (DeLosAngeles et al., 2016).
In addition to gamma frequency band, delta activity has drawn attention in neurophysiological studies of meditation in terms of mental “detachment”. The meditative condition has a property of a detached observation from ongoing experience. Meditators acquire this attitude through exercise of the intent not to analyze (not trying to explain), not to judge (good, bad, right, wrong), and not to expect anything (Cardoso et al., 2004). This reduced reactivity and engagement in observed experiences is the so called “detachment” (Tei et al., 2009). Increased frontal delta activity in experienced Zen and Qi-Gong meditators suggest significant interactions between meditative practice and this frequency band (Tei et al., 2009, Faber et al., 2008). Enhanced frontal delta activity in experienced meditators may reflect a functional inhibition of appraisal systems consistent with a “detachment” in analysis, judgment, and expectation (Tei et al., 2009).
Recently, several investigations on EEG activity associated with meditation have been conducted using exact Low Resolution Electromagnetic Tomography (eLORETA) (Thomas et al., 2014, Tei et al., 2009, Milz et al., 2014). eLORETA is a three-dimensional, discrete, linear, and weighted minimal norm inverse solution method (Pascual-Marqui et al., 2011). Its unique property was described in detail in our previous study (Hata et al., 2016, Kitaura et al., 2017). eLORETA has shown to hold improved localization properties in the presence of noise, and in multiple source situations (Pascual-Marqui et al., 2011).
We have already reported the effectiveness (e.g., improvement of psychological symptoms) and safety of our meditative intervention (Hayashi et al., 2013). However, as mentioned above, our previous study was partially insufficient in terms of objective estimation. In the present study, we aimed to identify static EEG activities, not instantaneous neurophysiological activities, induced by meditative intervention in PTRD subjects using eLORETA. We also evaluated the correlations between EEG parameters and clinical assessment to identify the brain regions related to psychological changes, thus providing new possible neurophysiological evidence of effectiveness of meditative interventions.
2. Methods
2.1. Subjects
The subjects with PTRD were recruited from the web pages of the Department of Integrative Medicine, Osaka University Graduate School of Medicine. The PTRD subjects, resistant on general medical therapy, were recruited and intervened by meditative therapy in this study. These subjects met the following criteria: (1) physical distress stemming from accidental injury in the past year, (2) age of 20 years or over, and (3) the subjects gave written informed consent to participate in the study. In addition, we established the following exclusion criteria: (1) subjects with psychiatric or cognitive disorders, such as major depression, bipolar disorder, schizophrenia, and dementia, (2) subjects that underwent intensive medical treatment, or, medically unstable subjects even under general medical treatment, (3) patients who were considered to have a poor prognosis, (4) subjects without ability of reading and writing in Japanese (Hayashi et al., 2015). Through the screening process, 10 PTRD subjects were enrolled in this study. Prior to the enrollment, we acquired the patients’ referral document and agreement for entry in this study from the doctors in charge, screening the subjects in terms of physical or mental diseases. When the subjects were considered to require intensive medical treatment, we advised them to undergo general medical treatment. In this case, once the patients were treated and became stable, they were re-enrolled in this study.
Prior to the intervention, the participants were interviewed for the details of physical impediment and accidental injuries and assessed by psychological questionnaires. Psychological questionnaires included the Visual Analog Scale for physical and mental distress, Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al., 2001, Muramatsu and Kamijima, 2009) to assess depressive state, the Impact of Event Scale-Revised (IES-R) (Weiss, 2004, Asukai et al., 2002) to assess traumatic distress, and 36-item short-form (SF-36) (Fukuhara et al., 1998a, Fukuhara et al., 1998b) for evaluation of quality of life (QOL). PHQ-9 is utilized for screening the patients with major depression, and not less than 10 points in this scale reflect suspicious major depression. IES-R is the scale for screening the patients with PTSD, and 25 or more points suggests a suspicion of PTSD. SF-36 consists of 8 QOL items and exhibits standardized score with 50 for the average score and 10 for the standard deviation. Role physical scores are included in SF-36 and they reflect the difficulty or limitation in work or daily activity due to physical problems in one month before measurement. From week-1 to week-8 they received the meditative intervention (described below) once a week and were carefully instructed to practice the meditation in daily life. Finally at week-9, the psychological questionnaires were performed again on all subjects. With regard to neurophysiological assessments, EEG recordings were recorded at week-1 and week-9 in all subjects for evaluation of the effect of the intervention.
In addition, 10 participants without history of mental illness and cognitive dysfunction were recruited as normal controls for comparing the EEG data between groups. One EEG recording was conducted in the each healthy subject.
Prior to the enrollment, we explained to all subjects about the use of their clinical data for this research and written informed consent was obtained. Our study protocol was approved by the ethical committee of Osaka University Hospital and our study was conducted in accordance with relevant guidelines, regulations, and the declaration of Helsinki.
2.2. Meditative intervention
All participants were instructed to practice the Asana pose, isometric exercise with softly exhaling, as training for focusing consciousness about their bodies, by certified Yoga therapists of Japan Yoga Therapy Society at every visit, providing more attention into following meditation. After the Asana pose, they were instructed to practice meditation based on the 24 min audio instruction. Then they were also trained to meditate by audio instructions of compact disk in order to meditate by their own self in their home every day. The 24-min audio instructions included the body-scan method, which is the meditative method for focused consciousness about the specific body parts, the breathing method with one side nose or both side nose, and the So-ham method, which is the meditative breathing method of deeply inhalation with the word “So” and slowly exhalation with the word “Ham”. The certified Yoga therapists checked the mindfulness meditation level of each participant and instructed them based on their proficiency level of meditation in each session time. We measured the Mindful Attention Awareness Scale (MAAS) (Brown and Ryan, 2003), which assesses a core characteristic of mindfulness, in order to verify the meditation quality.
2.3. EEG recordings and data processing
All EEG recordings were conducted with digital 19-channel EEG equipment (EEG-1000/EEG-1200, Nihon Kohden, Inc., Tokyo, Japan). The scalp electrodes were arranged according to the International 10–20 system (i.e., Fp1, Fp2, F7, F3, Fz F4, F8, T7, C3, Cz, C4, T8, P7, P3, Pz, P4, P8, O1, O2). The impedance of these electrodes was maintained below 5 kΩ. Linked ear references, sampling rate of 500 Hz, and filtered frequency band between 0.53 Hz and 60 Hz were applied in this study. Eyes open and closed states and meditative condition were recorded with careful vigilance control.
Then we acquired 120 s of artifact-free eyes-closed EEG data from all subjects’ recordings and divided them into 2-s fragments off-line. Thus, 60 artifact-free epochs of 2 s-fragments (stemmed from 120 s data) were explored in all subjects. All EEG data were visually inspected by trained and certified electroencephalographers. The EEG data with artifact components, such as eye blinking, muscle artifact and signs of drowsiness, were rejected by visual inspection. We investigated only eyes-closed and awake EEG components in order to properly estimate the brain function in genuine resting state. EEG data were assessed with the LORETA-KEY software.
EEG recordings were conducted for about 40 min, including eyes-closed state for 20 min, and meditative state for 20 min (body-scan, breathing meditation with one side nose, with both side noses, and So-ham meditation for 5 min, respectively).
2.4. EEG source localization estimated by eLORETA
We explored the cortical distribution of current density calculated by eLORETA with eyes-closed and awake EEG components. The brain templates of eLORETA and electrodes coordinates were determined according to the Montreal Neurological Institute average MRI brain map (MNI152) (Mazziotta et al., 2001). The solution space was restricted in the cortical gray matter endowed with 6239 voxels of 5 × 5 × 5 mm space resolution. The reliability and efficiency of eLORETA tomography for investigating brain activity was validated in several scalp EEG studies which compared different modalities, such as structural MRI (Worrell et al., 2000), fMRI (Mulert et al., 2004, Vitacco et al., 2002), PET (Dierks et al., 2000), and intracranial EEG (Zumsteg et al., 2006).
Sixty epochs of artifact-free 2-s EEG fragments in all subjects were processed by eLORETA for current density analysis in each five frequency bands: delta (2–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), gamma (30–60 Hz).
3. Statistical analyses
We applied statistical nonparametric mapping method (SnPM) (Holmes et al., 1996) implemented in eLORETA software, exploring current density. Between-group difference of source localization in each frequency band was estimated by voxel-by-voxel independent F-ratio-tests derived from log-transformed current density power. In the resulting three-dimensional statistical mapping, cortical voxels with significant differences were identified by means of a nonparametric permutation/randomization procedure (i.e., based on the Fisher’s permutation method, with the threshold set at the 5% probability level), comparing the mean source power in each voxel and the distribution in the permutated values. By evaluating the empirical probability distribution of the “maximal-statistics” in the null hypothesis, permutation and randomization tests have demonstrated to be effective in controlling the Type I error in neuroimaging studies (Nichols and Holmes, 2002, Winkler et al., 2016). eLORETA software conduct 5000 data randomizations to determine the critical probability threshold of the evaluated log F-ratio values with correction in multiple comparisons among all voxels in each frequencies, independently of Gaussianity. The availability of SnPM for LORETA analyses has been validated in several studies (Pascual-Marqui et al., 1999, Anderer et al., 1998). Detailed description of the nonparametric randomization has been reported in previous studies (Nichols and Holmes, 2002, Winkler et al., 2016).
To assess the association between the EEG parameter (i.e., current density) and neuropsychological test, we explored the correlations in regression analysis with eLORETA software. The critical probability threshold of r-values was assessed with nonparametric randomization (Nichols and Holmes, 2002, Winkler et al., 2016).
In this study we explored the associations between cortical voxels in each frequency band (delta, theta, alpha, beta, gamma) and three clinical items (PHQ-9, IES-R, and SF-36. SF-36 contains 8 QOL subscale items, including role-physical item) based on rigorous corrections for multiple comparisons (Nichols and Holmes, 2002).
4. Results
4.1. Demographic and clinical characteristics
No participants dropped out in this study. We confirmed the significant improvement in the Mindful Attention Awareness Scale (MAAS) (pre-intervention: 66.4 ± 11.7, post-intervention: 73.5 ± 12.7, p = 0.003), thus, verifying the quality of meditation of each participants in this intervention. Clinical characteristics of the PTRD subjects are shown in Table 1. About half of the participants in this study were victims of disasters. Table 2 shows the demographic and clinical data of all subjects who had difficulty in social lives by residual physical disabilities from past trauma. No statistical differences were seen in gender and age between the study groups. Only participants with PTRD were surveyed about distress or psychological trauma.
Table 1.
Demographic and clinical data of participants with post-traumatic residual disabilities.
| Trauma | Gender | Age | Type of injury |
|---|---|---|---|
| Disaster | Female | 46 | Fracture of the right scapula, Bruise of whole body, Crush syndrome |
| Disaster | Female | 68 | Injury of the left lung, Fracture of the left rib, Laceration of forehead, PTSD |
| Disaster | Female | 72 | Dystonia of body trunk, PTSD |
| Disaster | Female | 40 | Fracture of the pelvis, fracture of the rib, sprain fracture of the right astragalus |
| Accident | Female | 72 | Bursting fracture of the lumbar vertebra |
| Accident | Male | 64 | Aortic dissection |
| Accident | Male | 53 | Cervical spine injury |
| Accident | Male | 68 | Bruise of the lumbar vertebra |
| Accident | Female | 50 | Fracture of the right carpus |
| Accident | Male | 43 | Paresis of the right fibular nerve |
PTSD; Post Traumatic Stress Disorder.
Table 2.
Demographic and clinical data of all participants.
| Subjects | Normal controls | |
|---|---|---|
| N (female) | 10(6) | 10(6) |
| Age | 57.5 ± 12.4 | 56.8 ± 4.5 |
| IES-R(pre-intervention) | 17.4 ± 11.2 | |
| IES-R(post-intervention) | 19.3 ± 12.6 | |
| PHQ-9(pre-intervention) | 8.4 ± 4.9 | |
| PHQ-9(post-intervention) | 6.1 ± 4.1 | |
| Role physical (pre-intervention) |
29.1 ± 17.2 | |
| Role physical (post-intervention) |
29.5 ± 15.1 |
IES-R; The Impact of Event Scale – Revised. PHQ-9; Patient Health Questionnaire-9.
4.2. Source localization analyses
Fig. 1 illustrates the averaged eLORETA cortical solutions of the PTRD subjects before and after the intervention, and that of the normal controls in each frequency band. The highest current density values were found in alpha band in all three groups. The highest alpha current density values were of 1.15 (Amp/m2) before intervention, 1.48 (Amp/m2) after intervention, and 1.12 (Amp/m2) in controls. There was a similar distribution of alpha cortical sources with maximal current density over the parieto-occipital areas in all groups.
Fig. 1.
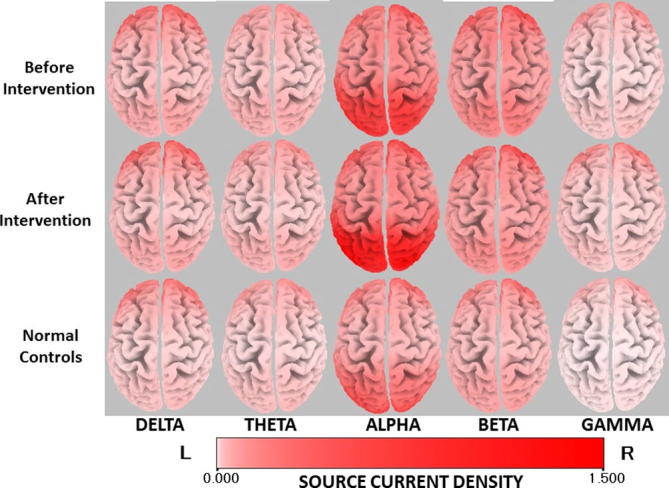
Averaged eLORETA current density in each frequency band of the participants before and after intervention, and normal controls. The red cortical area indicates the location with higher current density.
The second highest current density values were seen in beta band in all study groups: 0.94 (Amp/m2) before intervention, 0.98 (Amp/m2) after intervention, and 0.72 (Amp/m2) in normal controls.
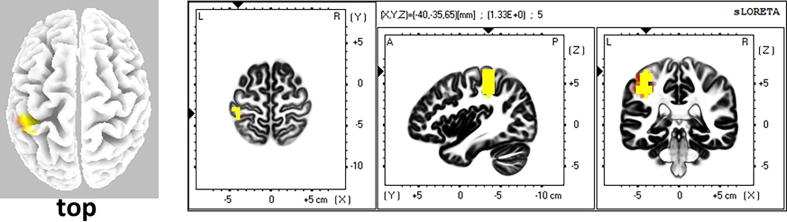
Statistical analysis showed no significant differences between PTRD subjects before and after intervention, and between controls and before intervention in any frequency band. However, after intervention PTRD subjects exhibited significantly higher current density values in the left inferior parietal lobule (IPL) in gamma band relative to the normal controls (Log F ratio = 1.30, p < 0.05). (Fig. 2).
Fig. 2.
Comparison of current density in gamma band between the participants after intervention and normal controls. The yellow area shows the significantly higher current density in the subjects after intervention (Log F ratio = 1.30, p < 0.05). The area is located around the left inferior parietal lobule.
In the supplementary analysis we explored the comparison between resting and meditation (body-scan, breathing meditation with one side nose, with both side nose, and So-ham meditation, respectively) EEG of 120 s of artifact-free data in each frequency band. However, these investigations did not yield any significant difference.
4.3. Correlations between psychological measures and EEG activity
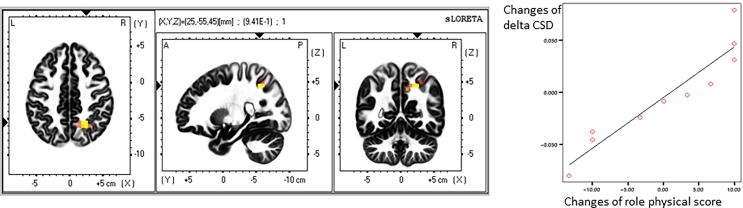
Correlations between psychological measures and EEG activity (i.e., current density) were investigated in all frequency bands. Fig. 3 maps the location with significantly positive correlation between role physical scores and changes in delta band current density caused by the meditative intervention. The area with significant correlation is located around the right precuneus (r = 0.941, corrected p = 0.0136).
Fig. 3.
Correlation between changes of electric neuronal activity (current density in Amp/m2) in delta band and scores in role physical associated with the meditative intervention. In the left figure the yellow area shows the significantly positive correlation between changes of current density in delta band and scores in role physical in the subjects with post-traumatic residual disabilities associated with the meditative intervention. The area is located around the right precuneus. In the right graph the correlation is shown between changes of current density in delta band and scores in role physical in the right precuneus (r = 0.941, corrected p = 0.0136).
5. Discussion
In this study, we explored changes in EEG activity (i.e., current density) induced by meditative intervention and the association between the EEG activity and psychological measures in PTRD subjects.
Although no significant differences were observed in direct comparison between meditative and resting state in the PTRD subjects, we found significantly higher gamma current density in the left IPL of PTRD subjects after intervention compared with normal controls (Fig. 2) which was not found before intervention compared with normal subjects. Of course, the interpretation of our results may have limitations, but we suggest that they are consistent with previous reports of increased gamma band power in EEG studies associated with meditation (Lutz et al., 2004, Thomas et al., 2014, Braboszcz et al., 2017, Schoenberg et al., 2018). Of note, Cahn et al (2010) also found that experienced meditators exhibited significantly enhanced gamma power at parietal electrodes, which is in line with our results. There is substantial evidence that the lateral parietal cortex plays an important role in attention processing. Interestingly, meditation and attentional processing are generally considered to have a close link in various meditation methods (Hauswald et al., 2015), as supported by several studies demonstrating improvement in attentional task performance induced by meditative training (Hodgins and Adair, 2010, Slagter et al., 2007). From a neurophysiological viewpoint, attention processing has been reported to have a close association with high-frequency oscillatory activity. For instance, Jensen et al. (2007) suggested gamma oscillations were associated with attentional processing of working memory. Furthermore, the IPL is associated with internally oriented process and self-awareness (Fox and Raichle, 2007). Thus, the changes in gamma oscillatory activity in the IPL associated with the meditative intervention found in this study may reflect meditation-related attentional process or self-awareness.
In the above quoted literature (Hayashi et al., 2013) we demonstrated the clinical improvement (i.e., mental distress, physical distress, depressive scale) in PTRD subjects induced by our current meditative intervention. With regard to treatment for traumatic stress, treatment responders in post-traumatic stress disorder (PTSD) are reported to exhibit enhanced fMRI activation of the left IPL at baseline, prior to treatment (van Rooij et al., 2015). This suggests the left IPL activation significantly predicts treatment response. Furthermore, the IPL appears to be associated with decrease in re-experiencing symptoms. It is noteworthy that synchronicity of high frequency oscillations is considered to play an important role in synaptic plasticity (Wespatat et al., 2004), suggesting a link between high-frequency oscillations and structural changes in the human brain (Hauswald et al., 2015). Based on this argument, we can speculate that an increase in gamma activity in the left IPL after meditative intervention may be related to synaptic changes which contribute to treatment response to post-traumatic distress through a decrease in re-experiencing symptoms. Thus, the meditative intervention applied in this study might have brought PTRD subjects into a preparatory state for treatment.
Regarding our correlation results, recent investigations have found correlation between delta activity around the right precuneus and changes in the role physical score. Patients with anxiety disorders have been reported to exhibit deactivation of right precuneus in single-photon emission computed tomography (SPECT) in treatment responders (Carey et al., 2004), suggesting that reduced activity of precuneus could have association with treatment of anxiety. Results from a study using repetitive transcranial magnetic stimulation (rTMS) and SPECT, indicate that rTMS can improve role-physical problems in patients with major depressive disorders. This symptom improvement is associated with a reduced perfusion of the precuneus, a brain region involved in self-focus and self-processing (Dumas et al., 2012). Patients with depressive disorders show enhanced self-focus and link negative affect (depression, anxiety, and negative mood) to enhanced attention to the self (Mor and Winquist, 2002). Furthermore, these patients also exaggerate self-processing, namely, they assess the stimuli as strongly related to their selves (Lemogne et al., 2011). Excessive self-referential processing yield distorted interpretations for social stimuli and prolonged social fears due to maladjusted cognition regarding self (Bögels and Mansell, 2004). Dumas et al. (2012) suggested that reduced perfusion of the precuneus might have association with a reduction of self-referential processing, allowing patients to remark their own attention to their environment, not self, thus, the patients with depressive disorders exhibited the improvement of psychological symptoms. In this study, we found significant delta activities and their activities could relate to the deactivation of the concerned brain region. Reduced activities of precuneus might yield detachment for self, associated with treatment for anxiety or depression, thus producing the improvement of psychological symptoms through the medium of role physical.
Our findings should be interpreted cautiously because of the relatively small sample size. In this study the correlation between role physical scores and current density was found extremely high. This result could be caused by the inflated correlations stemmed from small sample size (Yarkoni, 2009). Further studies dealing with larger samples may confirm our results. In this study, ages of the participants covered certain spread. The previous EEG study with LORETA (Zöllig et al., 2007) suggested the different neural activity depending on age. EEG data of all participants did not include abnormal activity, such as slow waves and spikes, thus suggesting absence of major neurophysiological disturbances. However, potential minor neurophysiological effect related to age might affect our results. We found the left IPL activity with a significant difference in the comparison between participants after intervention and normal controls, not before intervention. Furthermore, no significant difference were observed in supplementary comparison between meditative and resting state in the PTRD subjects. Thus, our results had a certain limitation in interpreting that our results were induced merely by meditative intervention in participants with PTRD. The effects associated with repeated testing or passing time were not controlled in the current study design. These uncontrolled effects might affect the results, thus, cautious interpretation for our results should be needed. Another possible concern is related to the EEG analysis that was based on a relative small number of electrodes. However, EEG assessments in the real clinical scenario are usually recorded with 19 scalp electrodes. Furthermore, previous EEG studies have confirmed the accuracy of LORETA source localization using this number of scalp electrodes (De Ridder et al., 2011, Thatcher et al., 2014). Gamma activity with significant difference captured in this study could not be absolutely denied to include muscle contamination (Travis and Shear, 2010), even if we conducted rigorous artifact-rejection and statistical correction. Although ICA and band-stop filters at 60 Hz is the best way to remove artifacts (Frantzidis et al., 2014, Chriskos et al., 2018), we didn't use these artifact rejection settings but inspected visually to remove these artifacts in this study. EEG data were visually inspected for common artifacts such as eye movements and muscle activity. A further concern is that we did not assess distress or psychological trauma in the control group, but we ensured that they had no physical and/or mental disease when recruiting them for the study.
6. Conclusion
In this study we investigated changes in EEG activity associated with the meditative intervention and the correlation between EEG activity and psychological measures. Subjects with post-traumatic residual disabilities exhibited increased gamma activity in the left inferior parietal lobule relative to normal controls after intervention. In addition, changes of delta activity in the right precuneus correlated with changes in the psychological score on role physical item. These changes could associate with psychological process, leading to the improvement of psychological symptoms. The results of this study reflect neurophysiological and objective changes induced by our meditative intervention.
7. Competing financial interests
The authors declare no competing financial interests.
Author contributions
M.H., N.H., R.I., and Y.A. contributed to the experimental design, data acquisition, data analysis and interpretation, and drafting of the article. L.C, R.P.M., S.I., T.S., M.I., K.K., M.I., M.I., and T.I. assisted with experimental design as well as data analysis and interpretation. All authors examined the data and approved the final manuscript.
References
- Anderer P., Pascual-Marqui R.D., Semlitsch H.V., Saletu B. Electrical sources of P300 event-related brain potentials revealed by low resolution electromagnetic tomography: Effects of normal aging. Neuropsychobiology. 1998;37:20–27. doi: 10.1159/000026472. [DOI] [PubMed] [Google Scholar]
- Asukai N., Kato H., Kawamura N., Kim Y., Yamamoto K., Kishimoto J. Reliability and validity of the Japanese-language version of the impact of event scale-revised (IES-R-J): four studies of different traumatic events. J. Nerv. Ment. Dis. 2002;190:175–182. doi: 10.1097/00005053-200203000-00006. [DOI] [PubMed] [Google Scholar]
- Bögels S.M., Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clin. Psychol. Rev. 2004;24:827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Braboszcz C., Cahn B.R., Levy J., Fernandez M., Delorme A. Increased gamma brainwave amplitude compared to control in three different meditation traditions. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0170647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K.W., Ryan R.M. The benefits of being present: Mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Cahn B.R., Delorme A., Polich J. Occipital gamma activation during Vipassana meditation. Cogn. Process. 2010;11:39–56. doi: 10.1007/s10339-009-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso R., de Souza E., Camano L., Leite J.R. Meditation in health: an operational definition. Brain Res. Brain Res. Protoc. 2004;14:58–60. doi: 10.1016/j.brainresprot.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Carey P.D., Warwick J., Niehaus D.J., van der Linden G., van Heerden B.B., Harvey B.H. Single photon emission computed tomography (SPECT) of anxiety disorders before and after treatment with citalopram. BMC Psychiatry. 2004;4:30. doi: 10.1186/1471-244X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chriskos P., Frantzidis C.A., Gkivogkli P.T., Bamidis P.D., Kourtidou-Papadeli C. Achieving accurate automatic sleep staging on manually pre-processed EEG data through synchronization feature extraction and graph metrics. Front. Hum. Neurosci. 2018;12:110. doi: 10.3389/fnhum.2018.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLosAngeles D., Williams G., Burston J., Fitzgibbon S.P., Lewis T.W., Grummett T.S. Electroencephalographic correlates of states of concentrative meditation. Int. J. Psychophysiol. 2016;110:27–39. doi: 10.1016/j.ijpsycho.2016.09.020. [DOI] [PubMed] [Google Scholar]
- De Ridder D., van der Loo E., Vanneste S., Gais S., Plazier M., Kovacs S. Theta-gamma dysrhythmia and auditory phantom perception: case report. J. Neurosurg. 2011;114:912–921. doi: 10.3171/2010.11.JNS10335. [DOI] [PubMed] [Google Scholar]
- Dierks T., Jelic V., Pascual-Marqui R.D., Wahlund L., Julin P., Linden D.E. Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intracerebral EEG-generators in Alzheimer’s disease. Clin. Neurophysiol. 2000;111:1817–1824. doi: 10.1016/s1388-2457(00)00427-2. [DOI] [PubMed] [Google Scholar]
- Dumas R., Richieri R., Guedj E., Auquier P., Lancon C., Boyer L. Improvement of health-related quality of life in depression after transcranial magnetic stimulation in a naturalistic trial is associated with decreased perfusion in precuneus. Health Qual. Life Outcomes. 2012;10:87. doi: 10.1186/1477-7525-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber P.L., Steiner M.E., Lehmann D., Pascual-Marqui R.D., Jäncke L., Esslen M. Deactivation of the medial prefrontal cortex in experienced Zen meditators. Brain Topography. 2008;20:172. [Google Scholar]
- Faber P.L., Lehmann D., Gianotti L.R., Milz P., Pascual-Marqui R.D., Held M. Zazen meditation and no-task resting EEG compared with LORETA intracortical source localization. Cogn. Process. 2015;16:87–96. doi: 10.1007/s10339-014-0637-x. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Frantzidis C.A., Vivas A.B., Tsolaki A., Klados M.A., Tsolaki M., Bamidis P.D. Functional disorganization of small-world brain networks in mild Alzheimer's Disease and amnestic Mild Cognitive Impairment: an EEG study using Relative Wavelet Entropy (RWE) Front. Aging Neurosci. 2014;6:224. doi: 10.3389/fnagi.2014.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Bito S., Green J., Hsiao A., Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 1998;51:1037–1044. doi: 10.1016/s0895-4356(98)00095-x. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Ware J.E., Jr., Kosinski M., Wada S., Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J. Clin. Epidemiol. 1998;51:1045–1053. doi: 10.1016/s0895-4356(98)00096-1. [DOI] [PubMed] [Google Scholar]
- Hata M., Kazui H., Tanaka T., Ishii R., Canuet L., Pascual-Marqui R.D. Functional connectivity assessed by resting state EEG correlates with cognitive decline of Alzheimer's disease - An eLORETA study. Clin. Neurophysiol. 2016;127:1269–1278. doi: 10.1016/j.clinph.2015.10.030. [DOI] [PubMed] [Google Scholar]
- Hauswald A., Übelacker T., Leske S., Weisz N. What it means to be Zen: marked modulations of local and interareal synchronization during open monitoring meditation. Neuroimage. 2015;108:265–273. doi: 10.1016/j.neuroimage.2014.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N., Maeda K., Yagi E., Hirai K., Tanimukai H., Ito K. Operability assessment of integrative medicine for posttraumatic residual diability. Nihon Togoiryo Gakkai Zasshi. 2013;6:65–69. (in Japanese) [Google Scholar]
- Hayashi N., Omura S., Imaida T., Shibata Y., Ishii T., Sakaue M. Operability assessment of integrative medicine for posttraumatic residual disability, the third year’s report. Nihon Togoiryo Gakkai Zasshi. 2015;8:82–88. (in Japanese) [Google Scholar]
- Hodgins H.S., Adair K.C. Attentional processes and meditation. Conscious. Cogn. 2010;19:872–878. doi: 10.1016/j.concog.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Holmes A.P., Blair R.C., Watson J.D., Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J. Cereb. Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Jensen O., Kaiser J., Lachaux J.P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kang S.S., Erbes C.R., Lamberty G.J., Thuras P., Sponheim S.R., Polusny M.A., Moran A.C., Van Voorhis A.C., Lim K.O. Transcendental meditation for veterans with post-traumatic stress disorder. Psychol Trauma. 2018 doi: 10.1037/tra0000346. (in press) [DOI] [PubMed] [Google Scholar]
- Kasamatsu A., Hirai T. An electroencephalographic study on the zen meditation (Zazen) Folia Psychiatr. Neurol. Jpn. 1966;20:315–336. doi: 10.1111/j.1440-1819.1966.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Kitaura Y., Nishida K., Yoshimura M., Mii H., Katsura K., Ueda S., Ikeda S., Pascual-Marqui R.D., Ishii R., Kinoshita T. Functional localization and effective connectivity of cortical theta and alpha oscillatory activity during an attention task. Clin. Neurophysiol. Practice. 2017;2:193–200. doi: 10.1016/j.cnp.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.J., Kulubya E., Goldin P., Goodarzi A., Girgis F. Review of the neural oscillations underlying meditation. Front. Neurosci. 2018;12:178. doi: 10.3389/fnins.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C., Gorwood P., Bergouignan L., Pélissolo A., Lehéricy S., Fossati P. Negative affectivity, self-referential processing and the cortical midline structures. Soc. Cogn. Affect. Neurosci. 2011;6:426–433. doi: 10.1093/scan/nsq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A., Greischar L.L., Rawlings N.B., Ricard M., Davidson R.J. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc. Natl. Acad. Sci. USA. 2004;101:16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J., Toga A., Evans A., Fox P., Lancaster J., Zilles K. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos. Trans. Roy. Soc. Lond. B Biol. Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milz P., Faber P.L., Lehmann D., Kochi K., Pascual-Marqui R.D. sLORETA intracortical lagged coherence during breath counting in meditation-naïve participants. Front. Hum. Neurosci. 2014;8:303. doi: 10.3389/fnhum.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N., Winquist J. Self-focused attention and negative affect: a meta-analysis. Psychol. Bull. 2002;128:638–662. doi: 10.1037/0033-2909.128.4.638. [DOI] [PubMed] [Google Scholar]
- Mulert C., Jäger L., Schmitt R., Bussfeld P., Pogarell O., Möller H.J. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Muramatsu K., Kamijima K. Patient Health Questionaire-9, Japanese version. Shindan To Chiryo. 2009;97:1465–1473. (in Japanese) [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;5:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui R.D., Lehmann D., Koenig T., Kochi K., Merlo M.C., Hell D. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. 1999;90:169–179. doi: 10.1016/s0925-4927(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D., Lehmann D., Koukkou M., Kochi K., Anderer P., Saletu B. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Transact. A Math. Phys. Eng. Sci. 2011;369:3768–3784. doi: 10.1098/rsta.2011.0081. [DOI] [PubMed] [Google Scholar]
- Schoenberg P.L.A., Ruf A., Churchill J., Brown D.P., Brewer J.A. Mapping complex mind states: EEG neural substrates of meditative unified compassionate awareness. Conscious. Cogn. 2018;57:41–53. doi: 10.1016/j.concog.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Slagter H.A., Lutz A., Greischar L.L., Francis A.D., Nieuwenhuis S., Davis J.M. Mental training affects distribution of limited brain resources. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei S., Faber P.L., Lehmann D., Tsujiuchi T., Kumano H., Pascual-Marqui R.D. Meditators and non-meditators: EEG source imaging during resting. Brain Topogr. 2009;22:158–165. doi: 10.1007/s10548-009-0107-4. [DOI] [PubMed] [Google Scholar]
- Thatcher R.W., North D.M., Biver C.J. LORETA EEG phase reset of the default mode network. Front. Hum. Neurosci. 2014;8:529. doi: 10.3389/fnhum.2014.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J., Jamieson G., Cohen M. Low and then high frequency oscillations of distinct right cortical networks are progressively enhanced by medium and long term Satyananda Yoga meditation practice. Front. Hum. Neurosci. 2014;8:197. doi: 10.3389/fnhum.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis F., Shear J. Focused attention, open monitoring and automatic self-transcending: Categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious. Cogn. 2010;19:1110–1118. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- van Rooij S.J., Geuze E., Kennis M., Rademaker A.R., Vink M. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology. 2015;40:667–675. doi: 10.1038/npp.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitacco D., Brandeis D., Pascual-Marqui R.D., Martin E. Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Hum. Brain Mapp. 2002;17:4–12. doi: 10.1002/hbm.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R.K. Physiological effects of transcendental meditation. Science. 1970;167:1751–1754. doi: 10.1126/science.167.3926.1751. [DOI] [PubMed] [Google Scholar]
- Weiss D.S. The impact of Event Scale-Revised. In: Wilson J.P., editor. Assessing Psychological Trauma and PTSD. second ed. The Guilford Press; 2004. pp. 168–189. [Google Scholar]
- Wespatat V., Tennigkeit F., Singer W. Phase sensitivity of synaptic modifications in oscillating cells of rat visual cortex. J. Neurosci. 2004;24:9067–9075. doi: 10.1523/JNEUROSCI.2221-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Webster M.A., Brooks J.C., Tracey I., Smith S.M., Nichols T.E. Non-parametric combination and related permutation tests for neuroimaging. Hum. Brain Mapp. 2016;37:1486–1511. doi: 10.1002/hbm.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell G.A., Lagerlund T.D., Sharbrough F.W., Brinkmann B.H., Busacker N.E. Localization of the epileptic focus by low-resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topogr. 2000;12:273–282. doi: 10.1023/a:1023407521772. [DOI] [PubMed] [Google Scholar]
- Yarkoni T. Big correlations in little studies: inflated fMRI correlations reflect low statistical power-commentary on Vul et al. Perspect. Psychol. Sci. 2009;4:294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Zöllig J., West R., Martin M., Altgassen M., Lemke U., Kliegel M. Neural correlates of prospective memory across the lifespan. Neuropsychologia. 2007;45:3299–3314. doi: 10.1016/j.neuropsychologia.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Zumsteg D., Friedman A., Wieser H.G., Wennberg R.A. Propagation of interictal discharges in temporal lobe epilepsy: correlation of spatiotemporal mapping with intracranial foramen ovale electrode recordings. Clin. Neurophysiol. 2006;117:2615–2626. doi: 10.1016/j.clinph.2006.07.319. [DOI] [PubMed] [Google Scholar]