Abstract
Purpose of review
Glucocorticoid therapy is currently the most widely used treatment for Duchenne muscular dystrophy (DMD), having consistently shown to prolong ambulation by 2 years, reduce the frequency of scoliosis, and improve cardiorespiratory function. Among the most frequent side effects of glucocorticoids are fractures due to osteoporosis, linear growth retardation or arrest, and pubertal delay, the subjects of this review.
Recent findings
The diagnosis of osteoporosis has shifted in recent years away from a bone mineral density-centric to a fracture-focused approach, with particular emphasis on early vertebral fracture identification (one of the key triggers for osteoporosis intervention). Delayed puberty should be addressed in an age-appropriate manner, with numerous options available for sex steroid replacement. Growth impairment, however, is a more challenging complication of glucocorticoid-treated DMD, one that is most likely best addressed through growth-sparing therapies that target the dystrophinopathy.
Summary
With glucocorticoid prescription an increasingly prevalent component of DMD care, early attention to management of osteoporosis and delayed puberty are important components of multidisciplinary and anticipatory care. The treatment of short stature remains controversial, with no accepted therapy currently available to over-ride the toxic effects of glucocorticoids on the growth axis.
Keywords: bisphosphonates, bone mineral density, Duchenne muscular dystrophy, growth, growth hormone, osteoporosis, puberty, testosterone, fractures
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder, occurring in 1 of 5 000 live male births [1]. Loss-of-function mutations in dystrophin cause progressive degeneration of skeletal and cardiac muscle so that untreated patients typically lose ambulation by 12 years of age, and succumb to respiratory insufficiency in the late second and third decades [2]. There is no cure for DMD; the only treatment that has consistently shown benefit is glucocorticoid therapy [3■■]. Glucocorticoids slow disease progression, prolong ambulation by 2 years [4,5], reduce the frequency of scoliosis, and improve cardiorespiratory function [6–9]. Many patients with DMD now live well into their 30 s on long-term glucocorticoids. Despite their benefits, glucocorticoids nevertheless hold a narrow therapeutic window, resulting in frequent side effects affecting numerous organ systems. Many boys with glucocorticoid-treated DMD develop a Cushingoid appearance, testament to the high and prolonged doses that are used to treat this condition.
Among the most frequent side effects of glucocorticoids, including those that are the most disconcerting to patients and families, are fractures due to osteoporosis, linear growth retardation or arrest, and pubertal delay. With multidisciplinary and anticipatory care, osteoporosis and pubertal delay can be effectively managed. Growth impairment, however, is a more challenging complication of glucocorticoid-treated DMD, one that will most likely only be addressed through growth-sparing therapies that specifically target the dystrophinopathy. In this review, we discuss what is known about growth, pubertal delay, and osteoporosis in glucocorticoid-treated DMD, including best practices for diagnosis, monitoring, and treatment, and the doors that are open for improved approaches in the future.
SHORT STATURE
Reduced growth and short stature are common clinical manifestations of DMD even in the absence of glucocorticoid therapy. Natural history studies performed in glucocorticoid-naïve DMD populations have shown that slow growth is apparent early in the disease course, and that final adult height is reduced by approximately one standard deviation (SD) score relative to the healthy average [2,10–12,13■]. Growth failure is magnified by the high glucocorticoid doses used to treat DMD, and is one of the most commonly reported glucocorticoid-related side effects [14■■]. Short stature can be emotionally distressing to patients, to the point that there are concerns it may contribute to diminished quality of life [15] and affect child and caregiver decisions regarding choice of glucocorticoid regimen and adherence to therapy [16■]. A representative growth curve of a boy with glucocorticoid-treated DMD and growth failure is shown in Fig. 1.
FIGURE 1.

A representative growth curve of a boy with glucocorticoid-treated Duchenne muscular dystrophy (DMD) and growth failure.
The degree of growth failure has varied widely among reports and appears to be influenced by duration of glucocorticoid therapy, dosing regimen (daily versus intermittent), and specific agent (prednisone vs. deflazacort). At one center, 40 boys treated with daily deflazacort for an average of 5.5 years were shorter by an average of 10 cm at 18 years of age compared to a matched glucocorticoid-naïve group [8]. Another center reported that the prevalence of short stature (height <3rd percentile) climbed from less than 10% of the 97 boy cohort prior to glucocorticoid initiation, to 72% following 8.5 years of daily glucocorticoid therapy [16■]. Both observational and clinical trial data have shown that daily glucocorticoid regimens resulted in greater growth impairment compared to intermittent dosing [17■,18]. A natural history study that analyzed the clinical outcomes of subjects on 14 different glucocorticoid regimens found that growth delay was more commonly reported in subjects on deflazacort compared to prednisone [19].
Mechanisms of impaired growth
The mechanisms underlying DMD-related growth failure are unclear. Both circulating levels of growth factors and skeletal maturation during childhood have been normal [10,20]. Studies that reported the growth hormone (GH) response to provocative testing were not in line with a primary defect in GH secretion, although mild deficits in peak GH levels were observed in some subjects [21,22]. Emerging data suggest a possible link between the dystrophin genotype and growth. Two recent studies reported that growth was more severely impaired in subjects with mutations in the distal segment of the dystrophin gene, mutations that that were predicted to alter the expression of the Dp71 isoform [12,13■]. The mechanism by which altered Dp71 expression may contribute to growth failure is not known, as this isoform is not present in fully differentiated skeletal muscle [23].
The growth suppressive effects of glucocorticoids are complex and multifactorial [24]. The primary detrimental effect of glucocorticoid exposure on growth appears to be growth plate toxicity. Glucocorticoids are suspected to impair growth plate activity via inhibition of chondrocyte and osteoblast differentiation [25], induction of chondrocyte and osteoblast apoptosis [26], and disruption in the local production of paracrine hormones including insulin-like growth factor 1 (IGF-1) and C-type natriuretic peptide [27,28]. The GH–IGF-1 axis is also disrupted by glucocorticoid excess, characterized by impaired GH secretion and increased peripheral resistance to both GH and IGF-1 [29,30]. Disrupted GH secretion has been shown in patients with glucocorticoid-treated DMD, as evidenced by a study in which 45% of subjects had peak stimulated GH levels ≤7 ng/ml [31].
Treatment of short stature
The use of recombinant human (rh)GH to treat short stature in DMD is controversial. The anticipated benefit of exogenous GH in the setting of ongoing glucocorticoid-mediated growth plate toxicity is unclear and there are theoretical concerns that rapid growth or tall stature may worsen muscle function in DMD [32,33]. Insulin resistance is common in boys with DMD [16■,34], and could progress with rhGH therapy. There have been no randomized, controlled trials evaluating the safety and efficacy of rhGH to promote growth in DMD. The largest report of rhGH included 39 boys with DMD on daily glucocorticoid therapy [31]. Growth velocity increased from 1.3 cm/year pretreatment to 5.2 cm/year over 12 months. No detrimental effects on neuromuscular function were observed; however, suspected rhGH-related side effects included insulin resistance in two boys and increased intracranial hypertension in one boy. rhGH was reported to be well tolerated in an RCT investigating its use to improve cardiac output; however, growth outcomes were not reported [35].
The published literature suggests significant variability in the use of rhGH in clinical practice. A review of data from 683 boys with DMD living in five regions of the United States reported that 1.9% of subjects had been treated with rhGH [36■■]. This is in contrast to a single-center report where 37% of subjects on daily glucocorticoid therapy had received rhGH [16■]. In the absence of robust safety and efficacy data, current guidelines do not recommend the routine use of rhGH in the DMD population [37■■]. The recommendations do note, however, that growth should be monitored closely until attainment of final height, and they allow for rhGH therapy to be considered on a case-by-case basis [37■■,38].
DELAYED PUBERTY
Hypogonadotropic hypogonadism is another common endocrine abnormality encountered in the management of DMD. Observational studies have consistently reported that the majority of adolescents with DMD on daily high-dose glucocorticoid therapy have delayed or absent puberty [31,39,40]. Unlike growth, pubertal development does not appear to be affected in the absence of glucocorticoid use. Similar to short stature, delayed pubertal maturation may adversely affect self-esteem and impair quality of life during adolescence. Prolonged testosterone deficiency may additionally have negative implications for bone strength, cardiovascular health, and cognitive development, although these associations have not been formally investigated in DMD.
Treatment of delayed puberty
Testosterone replacement is specifically recommended for the treatment of glucocorticoid-induced hypogonadotropic hypogonadism in adults [41] and has been shown to increase lean body mass in adults with other forms of muscular dystrophy [42], but has not been well studied in DMD. There have been no RCTs investigating the safety and efficacy of testosterone for any indication in patients with DMD [43■], and published clinical experience is currently limited to a single center review of 14 boys treated with testosterone for pubertal delay [44]. In that cohort, growth velocity increased and therapy was reported to be well liked by subjects; side effects were minor and consistent with known effects of testosterone [44].
Current guidelines recommend that all boys with DMD undergo close monitoring for pubertal development and that testosterone therapy should be initiated by age 14 years in those with confirmed hypogonadism [37■■]. This recommendation was based upon the wealth of clinical experience supporting the use of testosterone to manage hypogonadism in other populations, and the perception of an international expert panel of a favorable benefit-to-risk ratio in boys with DMD. There were insufficient data to support the choice of one testosterone preparation over another; however, it was recommended that therapy be started at a low dose and increased slowly to adult levels over a few years. Currently it is not known whether testosterone requirements will be lifelong in some patients, or whether a short course of testosterone during adolescence will be the bridge to normal endogenous production through adulthood. The recommended clinical approach to growth failure and delayed puberty in boys with DMD is outlined in Fig. 2.
FIGURE 2.
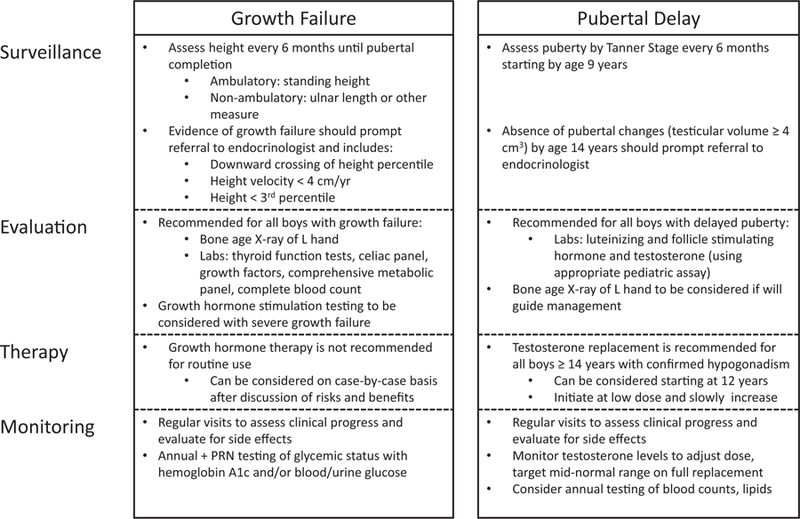
The recommended clinical approach to growth failure and delayed puberty in boys with DMD.
SKELETAL HEALTH AND OSTEOPOROSIS
Bone health monitoring and diagnosis of osteoporosis in Duchenne muscular dystrophy
Up to 60% of boys with DMD will have low-trauma extremity fractures even without glucocorticoid therapy [16■,17■,45–47,48■], a risk that is doubled when receiving glucocorticoid treatment [46]. In addition, as many as 50% of boys with glucocorticoid-treated DMD will have symptomatic vertebral fractures [46,49■]; the true vertebral fracture prevalence may be even higher, because vertebral fractures are frequently asymptomatic, especially in their earlier phases of development. As a result, vertebral fractures frequently go undetected in the absence of monitoring with periodic spine radiographs [48■,50–53]. In children, including in boys with DMD, vertebral fractures are most frequent in the mid-thoracic region and the upper lumbar spine [54,55]. Lumbar fractures are an additional osteoporosis feature in DMD [55], hypothesized to result from the exaggerated lumbar lordosis typical of this condition.
Vertebral fractures are more severe at first identification if boys have not been monitored with periodic spine radiographs during the asymptomatic phase [48■]. To identify the earliest signs of vertebral fractures, recent guidance encourages periodic lateral thoraco-lumbar spine radiographs starting around the time of diagnosis or glucocorticoid initiation, followed by spine radiographs every 1 to 2 years for those on glucocorticoids, and every 2 to 3 years for glucocorticoid-naïve boys or those who have stopped glucocorticoid therapy [56■■]. Spine x-rays should be carried out sooner if there is back pain or a decline in spine bone mineral density (BMD) Z-score by at least 0.5 SD in a 12-month period. For vertebral fracture detection, the Genant semiquantitative method is now recognized as a valid method in children, given numerous pediatric studies showing that the Genant method for vertebral fracture identification is associated with biologically logical vertebral fracture predictors including a history of prior vertebral fractures, low or declining spine BMD Z-scores, and back pain [52,53,57]. Overall, these recommendations are in line with the move away in recent years from a BMD-centric to a more fracture-focused diagnostic approach to osteoporosis in children [58■,59].
Vertebral fractures can occur as early as 6 months following glucocorticoid initiation (on average between 2–3 years) in DMD when monitored with routine spine radiographs from the time of glucocorticoid initiation [48■]. Untreated, vertebral fractures are linked to chronic back pain and spine deformity [48■], whereas leg fractures can cause permanent, premature loss of ambulation and challenges in daily care for those in wheelchairs [45]. Death because of fat embolism syndrome after long bone injuries has also been reported [60,61]. Osteoporosis treatment with bisphosphonates has been linked to survival [62], an observation which provides further rationale for effective osteoporosis diagnosis, surveillance, and treatment strategies. Because vertebral fractures typically do not undergo reshaping in the DMD setting, the importance of early identification and treatment in order to prevent the ‘vertebral fracture cascade’ is further underscored [56■■].
Studies have shown that total body BMD Z-scores decline early in the course of the disease [63■], whereas spine BMD declines more significantly once ambulation is lost [63■]; both are adversely impacted by glucocorticoid exposure [55]. With solidification of glucocorticoid exposure in the care plan of patients with DMD, attention has now turned to the relative effects of different glucocorticoid regimens on various organ systems, including bone strength and growth. Crabtree et al. [17■] recently showed that there was a trend for boys on daily glucocorticoids to retain ambulation longer than boys on intermittent glucocorticoids, but at the cost of more frequent vertebral fractures, increases in body mass index Z-scores and reductions in height Z-scores.
In view of these collective observations, the international consortium of experts in DMD and skeletal health that informed the recent Centres for Disease Control (CDC) guidances concluded that bone morbidity was sufficiently high in DMD to warrant early identification and intervention, and that trials are now needed to investigate strategies for the prevention of first-ever fractures in pediatric DMD [56■■]. As a first step, boys with DMD are diagnosed with osteoporosis in the presence of at least one low-trauma long bone or vertebral fracture, which in turn triggers consideration for osteoporosis therapy [56■■]. Given the high risk of fractures in DMD, the international CDC consortium concluded it was not necessary to await multiple long bone fractures by a certain age before diagnosing osteoporosis, in contrast to the suggestion by the International Society for Clinical Densitometry that two or more long bone fractures by age 10 or three or more long bone fractures by age 19 are required before diagnosing a child with osteoporosis [59].
Prevention and treatment of osteoporosis: standards of care and emerging therapy
To date, there are no drug trials that have been adequately powered to assess the prevention of first-fractures in DMD, despite the need. Therefore, the management of osteoporosis follows principles of secondary prevention that are applicable to any child with risk factors for bone fragility, including timely identification of vertebral fractures with periodic spine radiographs and integration of bone pain and fracture histories into routine care [58■]. With this approach, boys will be referred to an osteoporosis expert at the first sign of low-trauma fractures in order to discuss initiation of osteoporosis treatment. Osteoporosis therapy falls into two broad categories—antiresorptive and anabolic treatment.
The current standard of care for the first-line treatment of fragility fractures in childhood including DMD is intravenous (IV) bisphosphonate therapy (pamidronate, zoledronic acid, and neridronate). Neridronate is only available and approved for use in children with osteogenesis imperfecta in Italy, whereas zoledronic acid and pamidronate are prescribed off-label the world over) [58■]. Oral bisphosphonate therapy during childhood is not recommended because of data from controlled trials in pediatric osteogenesis imperfecta showing improvements in the height of vertebral bodies with IV bisphosphonate therapy [64–66], but not with oral agents [67–69]. These observations are likely influenced by the relatively low bioavailability of oral agents [70]. The lack of improvement in vertebral height with oral bisphosphonates is highly relevant to DMD where the frequency of vertebral fractures is high. Degrees of vertebral body reshaping following IV bisphosphonate therapy have been described in pediatric DMD [55], a phenomenon which is growth-dependent and thereby limited by both the patient’s growth velocity and residual growth potential. Early treatment of vertebral fractures is paramount to maintaining the structural integrity of vertebral bodies as far as possible in DMD, because intervention that starts once there are more advanced stages of vertebral collapse may be unable to prevent further loss of vertebral height in some cases [55]. Bisphosphonates should be prescribed by an expert knowledgeable in the potential side effects and their management, contraindications and appropriate dosing regimens, all of which have been reviewed in detail elsewhere [56■■,58■,71■■]. The overall approach to bisphosphonate administration in DMD is summarized in Fig. 3.
FIGURE 3.

The approach to the diagnosis and treatment of osteoporosis in boys with Duchenne muscular dystrophy (DMD). Reproduced with permission from [56].
Denosumab is a newer antiresorptive agent that targets RANKL to prevent the activation of RANK, thereby inhibiting bone resorption [72]. Denosumab is approved in Canada and the United States for postmenopausal women and osteoporotic men with a high risk for fracture, and for adults with glucocorticoid-induced osteoporosis [73]. Denosumab is particularly interesting in DMD, given observations that RANKL activation is implicated in the inflammatory pathway arising from the dystrophinopathy [74■].
To date, denosumab has been used on compassionate grounds in children with osteogenesis imperfecta [75,76], giant cell tumors [77], aneurysmal bone cysts [78], fibrous dysplasia [79], and in a 13-year-old boy with DMD [80■]. Although lumbar spine BMD increased significantly following 18 months of denosumab and the drug was well tolerated in this boy [80■], this study raises concern about one of the known precautions of denosumab – break-through re-activation of bone turnover markers on six monthly denosumab. As a monoclonal antibody, denosumab’s effect is much shorter than bisphosphonates, with cessation resulting in rebound of bone turnover markers, declines in BMD, and even increases in vertebral fractures (the rebound phenomenon) in women with postmenopausal osteoporosis [81,82■]. These observations appear to result from re-activation of osteoclasts in the face of denosumab’s waning effect. Osteoclast re-activation has even been clinically evident between serial denosumab doses in children with osteogenesis imperfecta, resulting in overt hypercalcemia and hypercalciuria [83■]. The rebound phenomenon is an important consideration for any patient embarking on the use of denosumab. Currently off-label for the treatment of glucocorticoid-induced osteoporosis in children, denosumab requires further study in the DMD setting.
Teriparatide – recombinant human PTH (1–34) – is approved by the Food and Drug Administration for initial treatment of women with postmenopausal osteoporosis who are at high risk of fracture or who have failed prior osteoporosis therapy, and for glucocorticoid-associated osteoporosis [84]. DMD is a compelling clinical scenario for osteoanabolic therapy such as teriparatide, since bone turnover on trabecular surfaces is reduced in boys with DMD even prior to bisphosphonate therapy, falling dramatically to 10% of the healthy average after 2 years of pamidronate or zoledronic acid when given to treat painful vertebral fractures [55,85■]. Unfortunately, this anabolic agent has a black box warning against its use in children because of the observation of osteosarcoma in growing rats treated with doses three to 50 times higher than the adult human equivalent and for much longer durations [86]. A recent case report of teriparatide in a 20-year-old male with DMD described complete resolution of vertebral fracture-induced back pain following 6 months of therapy, along with increases in lumbar spine BMD, in bone biomarkers and improved quality of life [87■]. It should be noted, however, that the effect of PTH on bone appears to be blunted in adults when administered following bisphosphonate therapy [88], an observation which may be a limiting factor in adults with DMD who received bisphosphonates as children. Like denosumab, teriparatide warrants further study in DMD, in this case restricted to the postpediatric population.
CONCLUSION AND FUTURE DIRECTIONS
Although glucocorticoids are the current mainstay of DMD treatment, this class of drugs nevertheless hold a narrow therapeutic window, a fact that behooves prescribing clinicians to carefully monitor and treat myriad glucocorticoid-related side effects. Although the treatment of delayed puberty and osteoporosis in DMD are now formally integrated into routine clinical care [71■■,89■■], short stature in this setting remains an unresolved issue, one that is likely best-targeted by glucocorticoid-sparing therapies [89■■]. Further studies are needed to determine the optimal timing of pubertal induction for those with delayed physical maturation, the relative benefits and risks of growth-promoting therapy to mitigate glucocorticoid-induced short stature, and the prevention of first-fractures in DMD.
KEY POINTS.
Through multidisciplinary and anticipatory care, osteoporosis and delayed puberty due to glucocorticoid therapy in DMD can be effectively managed.
The diagnosis of osteoporosis in DMD has shifted away from a bone mineral density-centric to a fracture-focused approach. Vertebral fractures are key signs of bone fragility that, like low-trauma long bone fractures, signal a need for osteoporosis treatment.
Monitoring for vertebral fractures should begin around the time of diagnosis, and no later than the time of glucocorticoid initiation.
Intravenous rather than oral bisphosphonates are preferred as first-line treatment for osteoporosis in DMD, given the lack of evidence for efficacy with oral agents at the fracture-prone spine in numerous clinical trails targeting pediatric osteoporotic diseases.
While delayed puberty can be effectively managed with sex steroid replacement, to date the treatment of short stature remains controversial, with no accepted therapy currently available to over-ride the toxic effects of glucocorticoids on the growth axis.
Acknowledgements
L.M.W. is supported by a Research Chair from the University of Ottawa.
Financial support and sponsorship
None.
Footnotes
Conflicts of interest
D.R.W. has been a consultant to Marathon Pharmaceuticals. L.M.W. has been a consultant to and has participated in clinical trials with Novartis Pharmaceuticals and Amgen Inc.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Emery AE. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Disord 1991; 1:19–29. [DOI] [PubMed] [Google Scholar]
- 2.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil 1995; 74:S70–S92. [DOI] [PubMed] [Google Scholar]
- 3.■■.Matthews E, Brassington R, Kuntzer T, et al. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev 2016; CD003725.Summary of the current evidence available to support the use of glucocorticoids for the treatment of DMD
- 4.Manzur AY, Kuntzer T, Pike M, Swan AV. Glucocorticoid corticosteroids for Duchenne muscular dystrophy (Review). The Cochrane Library 2009; (1):1–91. [DOI] [PubMed] [Google Scholar]
- 5.Eagle M, Bourke J, Bullock R, et al. Managing Duchenne muscular dystrophy—the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscular Disord 2007; 17:470–475. [DOI] [PubMed] [Google Scholar]
- 6.Alman BA, Raza SN, Biggar WD. Steroid treatment and the development of scoliosis in males with Duchenne muscular dystrophy. J Bone Joint Surg 2004; 86-A:519–524. [DOI] [PubMed] [Google Scholar]
- 7.Biggar WD, Gingras M, Fehlings DL, et al. Deflazacort treatment of Duchenne muscular dystrophy. J Pediatr 2001; 138:45–50. [DOI] [PubMed] [Google Scholar]
- 8.Biggar WD, Harris VA, Eliasoph L, Alman B. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscular Disord 2006; 16:249–255. [DOI] [PubMed] [Google Scholar]
- 9.Biggar WD, Politano L, Harris VA, et al. Deflazacort in Duchenne muscular dystrophy: a comparison of two different protocols. Neuromuscular Disord 2004; 14:476–482. [DOI] [PubMed] [Google Scholar]
- 10.Nagel BH, Mortier W, Elmlinger M, et al. Short stature in Duchenne muscular dystrophy: a study of 34 patients. Acta Paediatr 1999; 88:62–65. [PubMed] [Google Scholar]
- 11.West NA, Yang ML, Weitzenkamp DA, et al. Patterns of growth in ambulatory males with Duchenne muscular dystrophy. J Pediatr 2013; 163:1759–1763.e1751. [DOI] [PubMed] [Google Scholar]
- 12.Sarrazin E, von der Hagen M, Schara U, et al. Growth and psychomotor development of patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol 2014; 18:38–44. [DOI] [PubMed] [Google Scholar]
- 13.■.Matsumoto M, Awano H, Lee T, et al. Patients with Duchenne muscular dystrophy are significantly shorter than those with Becker muscular dystrophy, with the higher incidence of short stature in Dp71 mutated subgroup. Neuromuscul Disord 2017; 27:1023–1028.This paper discusses the natural history of stature in glucocorticoid-naïve DMD, with a link to the underlying genotype
- 14.■■.McDonald CM, Henricson EK, Abresch RT, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet 2018; 391:451–461.Large, prospective cohort study of clinical outcomes in boys with glucocorticoid-treated DMD, showing that glucocorticoid treatment is associated with reduced risks of losing meaningful mobility and upper limb function, along with a reduced risk of death
- 15.Bianchi ML, Biggar D, Bushby K, et al. Endocrine aspects of Duchenne muscular dystrophy. Neuromuscul Disord 2011; 21:298–303. [DOI] [PubMed] [Google Scholar]
- 16.■.Wong BL, Rybalsky I, Shellenbarger KC, et al. Long-term outcome of inter-disciplinary management of patients with Duchenne muscular dystrophy receiving daily glucocorticoid treatment. J Pediatr 2017; 182:296–303e291.Large, retrospective study of clinical outcomes in glucocorticoid-treated DMD, showing improved clinical statuses and manageable side effects with anticipatory and coordinated inter-disciplinary care
- 17.■.Crabtree NJ, Adams JE, Padidela R, et al. Growth, bone health & ambulatory status of boys with DMD treated with daily vs. intermittent oral glucocorticoid regimen. Bone 2018; 116:181–186.Pragmatic, longitudinal observational study comparing daily versus intermittent glucocorticoid therapy in DMD. Results showed a trend for more boys on daily glucocorticoid to remain ambulant but at the cost of more vertebral fractures, greater adiposity and markedly diminished growth. In contrast, boys on intermittent glucocorticoid had fewer vertebral fractures but with a trend for more boys to lose ambulation
- 18.Escolar DM, Hache LP, Clemens PR, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology 2011; 77:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bello L, Gordish-Dressman H, Morgenroth LP, et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology 2015; 85:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eiholzer U, Boltshauser E, Frey D, et al. Short stature: a common feature in Duchenne muscular dystrophy. Eur J Pediatr 1988; 147:602–605. [DOI] [PubMed] [Google Scholar]
- 21.Merlini L, Granata C, Ballestrazzi A, et al. Growth hormone evaluation in Duchenne muscular dystrophy. Italian J Neurol Sci 1988; 9:471–475. [DOI] [PubMed] [Google Scholar]
- 22.Zatz M, Rapaport D, Vainzof M, et al. Effect of mazindol on growth hormone levels in patients with Duchenne muscular dystrophy. Am J Med Genet 1988; 31:821–833. [DOI] [PubMed] [Google Scholar]
- 23.Tennyson CN, Dally GY, Ray PN, Worton RG. Expression of the dystrophin isoform Dp71 in differentiating human fetal myogenic cultures. Hum Mol Genet 1996; 5:1559–1566. [DOI] [PubMed] [Google Scholar]
- 24.Olney RC. Mechanisms of impaired growth: effect of steroids on bone and cartilage. Horm Res 2009; 72(Suppl 1):30–35. [DOI] [PubMed] [Google Scholar]
- 25.Fujita T, Fukuyama R, Enomoto H, Komori T. Dexamethasone inhibits insulin-induced chondrogenesis of ATDC5 cells by preventing PI3K-Akt signaling and DNA binding of Runx2. J Cell Biochem 2004; 93:374–383. [DOI] [PubMed] [Google Scholar]
- 26.Chrysis D, Ritzen EM, Savendahl L. Growth retardation induced by dexamethasone is associated with increased apoptosis of the growth plate chondrocytes. J Endocrinol 2003; 176:331–337. [DOI] [PubMed] [Google Scholar]
- 27.Delany AM, Durant D, Canalis E. Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol (Baltimore, MD) 2001; 15:1781–1789. [DOI] [PubMed] [Google Scholar]
- 28.Prickett TC, Lynn AM, Barrell GK, et al. Amino-terminal proCNP: a putative marker of cartilage activity in postnatal growth. Pediatr Res 2005; 58:334–340. [DOI] [PubMed] [Google Scholar]
- 29.Wehrenberg WB, Janowski BA, Piering AW, et al. Glucocorticoids: potent inhibitors and stimulators of growth hormone secretion. Endocrinology 1990; 126:3200–3203. [DOI] [PubMed] [Google Scholar]
- 30.Jux C, Leiber K, Hugel U, et al. Dexamethasone impairs growth hormone (GH)-stimulated growth by suppression of local insulin-like growth factor (IGF)-I production and expression of GH- and IGF-I-receptor in cultured rat chondrocytes. Endocrinology 1998; 139:3296–3305. [DOI] [PubMed] [Google Scholar]
- 31.Rutter MM, Collins J, Rose SR, et al. Growth hormone treatment in boys with Duchenne muscular dystrophy and glucocorticoid-induced growth failure. Neuromuscul Disord 2012; 22:1046–1056. [DOI] [PubMed] [Google Scholar]
- 32.Zatz M, Rapaport D, Vainzof M, et al. Relation between height and clinical course in Duchenne muscular dystrophy. Am J Med Genet 1988; 29:405–410. [DOI] [PubMed] [Google Scholar]
- 33.Bodor M, McDonald CM. Why short stature is beneficial in Duchenne muscular dystrophy. Muscle Nerve 2013; 48:336–342. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Cruz M, Sanchez R, Escobar RE, et al. Evidence of insulin resistance and other metabolic alterations in boys with Duchenne or Becker muscular dystrophy. Int J Endocrinol 2015; 2015:867273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cittadini A, Ines Comi L, Longobardi S, et al. A preliminary randomized study of growth hormone administration in Becker and Duchenne muscular dystrophies. Eur Heart J 2003; 24:664–672. [DOI] [PubMed] [Google Scholar]
- 36.■■.Weber DR, Thomas S, Erickson SW, et al. Bone health and endocrine care of boys with Duchenne muscular dystrophy: data from the MD STARnet. J Neuromuscular Dis 2018; 5:497–507.A population-based cohort study using data from the Muscular Dystrophy Surveillance, Tracking and Research Network (MD STARnet) to study the frequency of boys who receive DMD care in line with current standards. A low percentage of DMD males had records of bone density scans, endocrinology evaluations, or treatment with endocrine or bone health pharmacotherapy. The authors signaled that endocrine and bone healthcare may represent an unmet need in the DMD population
- 37.■■.Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 2018; 17:251–267.Updated Centres for Disease Control guidances for the diagnosis and management of DMD, including short stature, delayed puberty (and adrenal insufficiency)
- 38.Leung DG, Germain-Lee EL, Denger BE, Wagner KR. Report on the Second Endocrine Aspects Of Duchenne Muscular Dystrophy Conference December 1–2, 2010, Baltimore, Maryland, USA. Neuromuscul Disord 2010; 21:594–601. [DOI] [PubMed] [Google Scholar]
- 39.Merlini L, Gennari M, Malaspina E, et al. Early corticosteroid treatment in 4 Duchenne muscular dystrophy patients: 14-year follow-up. Muscle Nerve 2012; 45:796–802. [DOI] [PubMed] [Google Scholar]
- 40.Dooley JM, Bobbitt SA, Cummings EA. The impact of deflazacort on puberty in Duchenne muscular dystrophy. Pediatr Neurol 2013; 49:292–293. [DOI] [PubMed] [Google Scholar]
- 41.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559. [DOI] [PubMed] [Google Scholar]
- 42.Welle S, Jozefowicz R, Forbes G, Griggs RC. Effect of testosterone on metabolic rate and body composition in normal men and men with muscular dystrophy. J Clin Endocrinol Metab 1992; 74:332–335. [DOI] [PubMed] [Google Scholar]
- 43.■.Bell JM, Shields MD, Watters J, et al. Interventions to prevent and treat corticosteroid-induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy. Cochrane Database Syst Rev 2017; 1:CD010899.Systematic review of published clinical trials addressing the prevention and treatment of osteoporotic fractures in DMD
- 44.Wood CL, Cheetham TD, Guglieri M, et al. Testosterone treatment of pubertal delay in Duchenne muscular dystrophy. Neuropediatrics 2015; 46:371–376. [DOI] [PubMed] [Google Scholar]
- 45.Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 2000; 20:71–74. [PubMed] [Google Scholar]
- 46.King WM, Ruttencutter R, Nagaraja HN, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology 2007; 68:1607–1613. [DOI] [PubMed] [Google Scholar]
- 47.McDonald DG, Kinali M, Gallagher AC, et al. Fracture prevalence in Duchenne muscular dystrophy. Dev Med Child Neurol 2002; 44:695–698. [DOI] [PubMed] [Google Scholar]
- 48.■.Ma J, McMillan HJ, Karaguzel G, et al. The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporos Int 2017; 28:597–608.Retrospective longitudinal study highlighting that glucocorticoid-treated boys with DMD who undergo routine monitoring for vertebral fractures through lateral spine radiographs present with on average after 2 years of glucocorticoid therapy and with minimal signs of vertebral collapse. Boys without routine spine monitoring present later, with more advanced and painful vertebral collapse. In this pain, there was no evidence for medication-unassisted vertebral body reshaping
- 49.■.Singh A, Schaeffer EK, Reilly CW. Vertebral fractures in Duchenne muscular dystrophy patients managed with deflazacort. J Pediatr Orthop 2018; 38:320–324.Retrospective study showing a high frequency of vertebral fractures in DMD, going against earlier views that deflazacort may be bone-sparing
- 50.Alos N, Grant RM, Ramsay T, et al. High incidence of vertebral fractures in children with acute lymphoblastic leukemia 12 months after the initiation of therapy. J Clin Oncol 2012; 30:2760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodd C, Lang B, Ramsay T, et al. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: a national observational study. Arthritis Care Res 2012; 64:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cummings EA, Ma J, Fernandez CV, et al. Incident vertebral fractures in children with leukemia during the four years following diagnosis. J Clin Endocrinol Metab 2015; 100:3408–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LeBlanc CM, Ma J, Taljaard M, et al. Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders. J Bone Miner Res 2015; 30:1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siminoski K, Lee KC, Jen H, et al. Anatomical distribution of vertebral fractures: comparison of pediatric and adult spines. Osteoporos Int 2012; 23:1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sbrocchi AM, Rauch F, Jacob P, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int 2012; 23:2703–2711. [DOI] [PubMed] [Google Scholar]
- 56.■■.Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 2018; 17:347–361.Updated Centres for Disease Control guidances for the diagnosis and management of osteoporosis in DMD. This paper underscores the importance of monitoring for vertebral fractures as one of the early signs of bone fragility in glucocorticoid-treated DMD. This paper also endorses treatment with intravenous bisphosphonate therapy at the first sign of vertebral or long bone fractures in DMD
- 57.Halton J, Gaboury I, Grant R, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res 2009; 24:1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.■.Ward LM, Konji VN, Ma J. The management of osteoporosis in children. Osteoporos Int 2016; 27:2147–2179.Recent review of the diagnosis and treatment of osteoporosis in children, including principles related to the treatment of osteoporosis in chronic diseases such as DMD
- 59.Bishop N, Arundel P, Clark E, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 pediatric official positions. J Clin Densitom 2014; 17:275–280. [DOI] [PubMed] [Google Scholar]
- 60.Medeiros MO, Behrend C, King W, et al. Fat embolism syndrome in patients with Duchenne muscular dystrophy. Neurology 2013; 80:1350–1352. [DOI] [PubMed] [Google Scholar]
- 61.McAdam LC, Rastogi A, Macleod K, Douglas Biggar W. Fat embolism syndrome following minor trauma in Duchenne muscular dystrophy. Neuromuscul Disord 2012; 22:1035–1039. [DOI] [PubMed] [Google Scholar]
- 62.Gordon KE, Dooley JM, Sheppard KM, et al. Impact of bisphosphonates on survival for patients with Duchenne muscular dystrophy. Pediatrics 2011; 127:e353–e358. [DOI] [PubMed] [Google Scholar]
- 63.■.Tian C, Wong BL, Hornung L, et al. Bone health measures in glucocorticoid-treated ambulatory boys with Duchenne muscular dystrophy. Neuromuscul Disord 2016; 26:760–767.Bone mineral density natural history changes in a large cohort of boys with DMD, showing that total body and hip bone density decline significantly with loss of motor function
- 64.Gatti D, Antoniazzi F, Prizzi R, et al. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res 2005; 20:758–763. [DOI] [PubMed] [Google Scholar]
- 65.Antoniazzi F, Zamboni G, Lauriola S, et al. Early bisphosphonate treatment in infants with severe osteogenesis imperfect. J Pediatr 2006; 149:174–179. [DOI] [PubMed] [Google Scholar]
- 66.Aström E, Jorulf H, Söderhäll S. Intravenous pamidronate treatment of infants with severe osteogenesis imperfecta. Arch Dis Child 2007; 92:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rauch F, Munns CF, Land C, et al. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Bone Mineral Res 2009; 24:1282–1289. [DOI] [PubMed] [Google Scholar]
- 68.Sakkers R, Kok D, Engelbert R, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet 2004; 363:1427–1431. [DOI] [PubMed] [Google Scholar]
- 69.Ward LM, Glorieux FH, Rauch F, et al. A randomized, placebo-controlled trial of oral alendronate in children and adolescents with osteogenesis imperfecta. Bone 2005; 36:0–18. [Google Scholar]
- 70.Ward LM, Denker AE, Porras A, et al. Single-dose pharmacokinetics and tolerability of alendronate 35- and 70-milligram tablets in children and adolescents with osteogenesis imperfecta type I. J Clin Endocrinol Metab 2005; 90:4051–4056. [DOI] [PubMed] [Google Scholar]
- 71.■■.Ward LM, Hadjiyannakis S, McMillan HJ, et al. Bone health and osteoporosis management of the patient with Duchenne muscular dystrophy. Pediatrics 2018; 142:S34–S42.Companion paper to the updated Centres for Disease Control guidances on the care of DMD. This publication provides an in-depth review of the rationale and approaches to the diagnosis and treatment of osteoporosis in DMD, including practical, evidence-based guidelines for the treating clinician
- 72.Hofbauer LC, Zeitz U, Schoppet M, et al. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthr Rheum 2009; 60:1427–1437. [DOI] [PubMed] [Google Scholar]
- 73.Amgen. Prolia (Denosumab) Product Monograph. 2018.
- 74.■.Boulanger Piette A, Hamoudi D, Marcadet L, et al. Targeting the muscle-bone unit: filling two needs with one deed in the treatment of Duchenne muscular dystrophy. Curr Osteoporos Rep 2018; 16:541–553.This paper describes the rationale for the use of denosumab to treat or prevent osteoporosis in DMD, given evidence in animal models for a positive effect on the dystrophinopathy
- 75.Semler O, Netzer C, Hoyer-Kuhn H, et al. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskeletal Neuronal Interact 2012; 12:183–188. [PubMed] [Google Scholar]
- 76.Hoyer-Kuhn H, Netzer C, Koerber F, et al. Two years’ experience with denosumab for children with osteogenesis imperfecta type VI. Orphanet J Rare Dis 2014; 9:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karras NA, Polgreen LE, Ogilvie C, et al. Denosumab treatment of metastatic giant-cell tumor of bone in a 10-year-old girl. J Clin Oncol 2013; 31:e200–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lange T, Stehling C, Frohlich B, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J 2013; 22:1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyce AM, Chong WH, Yao J, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res 2012; 27:1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.■.Kumaki D, Nakamura Y, Sakai N, et al. Efficacy of denosumab for glucocorticoid-induced osteoporosis in an adolescent patient with Duchenne muscular dystrophy: a case report. JBJS Case Connect 2018; 8:e22.First case report on the use of denosumab to treat DMD, showing positive effects on BMD but with an increase in bone turnover markers leading up to each six monthly dose. This observation warrants further scrutiny in view of the ‘rebound phenomenon’ observed in patients receiving denosumab, once the effect of this antibody-based therapy wanes
- 81.Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011; 96:972–980. [DOI] [PubMed] [Google Scholar]
- 82.■.Lamy O, Gonzalez-Rodriguez E, Stoll D, et al. Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab 2017; 102:354–358.Description of the ‘rebound phenomenon’ in adults arising from the use of denosumab
- 83.■.Trejo P, Rauch F, Ward L. Hypercalcemia and hypercalciuria during denosumab treatment in children with osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact 2018; 18:76–80.Description of the ‘rebound phenomenon’ in children arising from the use of denosumab
- 84.Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis - 2016. Endocr Pract 2016; 22:1–42. [DOI] [PubMed] [Google Scholar]
- 85.■.Misof BM, Roschger P, McMillan HJ, et al. Histomorphometry and bone matrix mineralization before and after bisphosphonate treatment in boys with Duchenne muscular dystrophy: a paired transiliac biopsy study. J Bone Miner Res 2016; 31:1060–1069.Bone histormophometric changes before and after bisphosphonate use in boys with DMD, showing that bone turnover is low pretreatment, and falls further with antiresorptive therapy. The study provides rationale for studying anabolic therapy for the treatment or prevention of osteoporosis in DMD
- 86.Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol 2002; 30:312–321. [DOI] [PubMed] [Google Scholar]
- 87.■.Catalano A, Vita GL, Russo M, et al. Effects of teriparatide on bone mineral density and quality of life in Duchenne muscular dystrophy related osteoporosis: a case report. Osteoporos Int 2016; 27:3655–3659.Case report describing the response to anabolic therapy for the treatment of osteoporosis in DMD
- 88.Obermayer-Pietsch BM, Marin F, McCloskey EV, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 2008; 23:1591–1600. [DOI] [PubMed] [Google Scholar]
- 89.■■.Weber DR, Hadjiyannakis S, McMillan HJ, et al. Obesity and endocrine management of the patient with Duchenne muscular dystrophy. Pediatrics 2018; 142:S43–S52.Companion paper to the 2018 Centres for Disease Control updated guidances on the care of patients with DMD, detailing the rationale and the approach to growth, puberty, obesity and adrenal suppression management


