Abstract
The central amygdala (CeA) has a unique role in integrating stress and the rewarding effects of ethanol (EtOH) and plays a major role in the development of EtOH dependence via signaling of corticotropinreleasing factor (CRF). A recent report by Herman and colleagues (2013) entitled “Novel Subunit-Specific Tonic GABA Currents and Differential Effects of Ethanol in the Central Amygdala of CRF Receptor-1 Reporter Mice” is the first study to investigate inhibitory tonic currents in relation to CRF signaling in the CeA. The findings of that study significantly enhance our understanding of inhibitory tonic currents in the CeA and give insight into how EtOH may differentially affect CRF signaling within the CeA, leading to the development of EtOH dependence. This commentary will focus on the recent findings of Herman and colleagues and will discuss the effects of EtOH on the entire anxiety/ emotion circuitry.
Keywords: Tonic Currents, Ethanol, Amygdala, CRF
A DELICATE BALANCE of excitatory and inhibitory inputs orchestrates proper signaling within the mammalian central nervous system (CNS). Activation of Cl−-permeable γ-aminobutyric acid (GABA) type A receptors (GABAARs) mediates the majority of inhibitory signaling in the CNS. Vesicular release of GABA activates postsynaptic GABAARs, resulting in a rapid hyperpolarization of the membrane potential leading to phasic inhibition. In addition, extrasynaptic GABAARs can be activated by ambient levels of GABA, resulting in tonic inhibitory currents that continuously hyperpolarize the membrane potential and are only revealed in the presence of GABAAR antagonists (Brickley and Mody, 2012). Synaptically released GABA can contribute to ambient GABA levels, but this seems to be unique to specific brain regions, such as the cerebellum (Diaz et al., 2013) and the dentate gyrus (Glykys and Mody, 2007).
GABAergic tonic currents have been described in many brain regions and are mediated by GABAARs with unique subunit composition. Most commonly, GABAARs containing the δ-subunit are found extrasynaptically and mediate tonic currents, whereas γ-subunit-containing receptors are localized to the synapse and mediate phasic currents. However, recent studies have shown that tonic currents can be mediated by both γ- and δ-containing GABAARs. Tonic currents in hippocampal dentate granule cells, neocortical layer 2/3 pyramidal cells, thalamic relay neurons, and striatal medium spiny neurons are mediated by α4βδ GABAARs, tonic currents in cerebellar granule cells are mediated byα6βδ GABAARs, while GABAergic interneurons in the hippocampus and neocortex display tonic currents mediated by α1βδ GABAARs. In contrast to the δ-containing extrasynaptic GABAARs, α5βγ2 GABAARs mediate tonic currents in hippocampal and neocortical layer 5 pyramidal cells (for review, see Brickley and Mody, 2012). More recently, the amygdala has been added to this growing list of brain regions that express GABAergic tonic currents. In the basolateral amygdala (BLA), GABAergic tonic currents are mediated by α3-containing GABAARs in glutamatergic pyramidal neurons (the primary neuron type within the BLA) and by GABAARs of an unknown subunit composition in GABAergic interneurons (Marowsky et al., 2012).
In a recent paper in The Journal of Neuroscience, Herman and colleagues (2013) characterized 2 novel forms of GABAergic tonic currents that are anatomically and functionally distinct within the central amygdala (CeA). To understand the integration of corticotropin-releasing factor (CRF) signaling and ethanol (EtOH) addiction, Herman and colleagues used CRF receptor 1:GFP reporter mice to show that CRF1 positive neurons (CRF1+) exhibit tonic currents that are dependent on synaptically released GABA. Further, they were potentiated by zolpidem (an allosteric modulator for GABAARs with higher selectivity for the α1/γ2 interface), but insensitive to THIP (a highly selective agonist for δ-containing GABAARs), suggesting that they are mediated by an α1, δ-lacking GABAaR (presumably containing α1βγ). Conversely, CRF1 negative neurons (CRF1−) did not exhibit a tonic current under basal conditions, but could be induced by elevating extracellular GABA levels with a GABA transporter blocker or by pharmacological activation of δ-containing GABAARs with THIP (presumably containing αxβδ). These findings were further supported by immunohistochemical detection of α1- and δ-subunit expression in CRF1+ and CRF1−, respectively.
A surprising finding of this study was that α1βγ-containing GABAARs mediated tonic currents in CRF1+. As mentioned above, tonic currents mediated by γ-containing GABAARs have been previously described in CA1 and CA3 pyramidal neurons of the hippocampus. Specifically, these extrasynaptic receptors are composed of α5βγ2, and it is thought that the α5-subunit can somehow overcome the localization of γ2 to postsynaptic densities (Farrant and Nusser, 2005). Therefore, the study by Herman and colleagues (2013) is the first report of a tonic current mediated by α1βγ GABAARs. γ-containing GABAARs have a lower affinity for GABA than δ-containing receptors and are thought to mediate tonic currents only when ambient GABA levels are relatively high (Brickley and Mody, 2012). Herman and colleagues found that CRF1+ display a high frequency of phasic GABAergic spontaneous inhibitory postsynaptic currents (sIPSCs), indicating that these neurons receive significant phasic GABA release that could contribute to the generation of relatively high ambient GABA levels ideal for the activation of γ-containing extrasynaptic GABAARs. A more detailed analysis of the presence of the γ-subunit in CRF1+ would be beneficial to further understand the pharmacology and physiology of these neurons.
Another interesting finding of the study of Herman and colleagues (2013) is that the tonic currents in CRF1+ were entirely mediated by action-potential-dependent GABA release, as application of tetrodotoxin (TTX) completely blocked the tonic current. To date, this is a unique characteristic of the CeA, because in other brain regions, only a portion of the tonic current is mediated by action-potential-dependent GABA release [~30% in cerebellar granule cells (Diaz et al., 2013) and 65% in hippocampal dentate granule cells (Glykys and Mody, 2007)].
ETHANOL-INDUCED POTENTIATION OF TONIC CURRENTS
A growing list of neuronal populations have been shown to exhibit GABAergic tonic currents, and recent studies linking these currents to behavioral and pathological states have highlighted the importance of tonic inhibition (Brickley and Mody, 2012). Important for this commentary, GABAARs and tonic inhibitory currents are particularly sensitive to acute EtOH and may contribute to the behavioral effects of EtOH (Brickley and Mody, 2012).
Several studies have shown that GABAergic tonic currents are potentiated by acute EtOH. However, the mechanism underlying this effect differs depending on the brain region and the GABAAR subunit composition. In cerebellar granule cells, acute EtOH robustly increases both tonic currents and phasic sIPSCs by increasing presynaptic Golgi cell firing, in part by inhibiting the Na+/K+-ATPase and depolarizing the membrane potential of Golgi cells (Botta et al., 2010; Diaz et al., 2013). It has been suggested that EtOH can also directly enhance α6-containing GABAaR function in cerebellar granule cells (Hanchar et al., 2004), but this has been a controversial finding (Baur et al., 2009; Botta et al., 2007). In contrast to a presynaptic effect in the cerebellum, postsynaptic α1βδ (Glykys et al., 2007) and α4βδ (Wei et al., 2004) GABAARs that mediate tonic currents in hippocampal molecular layer interneurons and dentate granule cells, respectively, have been shown to be highly sensitive to the potentiating effect of EtOH. α4βδ receptors that mediate tonic currents in thalamic relay neurons are also sensitive to moderate concentrations of EtOH (Jia et al., 2008). Conversely, tonic currents mediated by α5βγ2 receptors in hippocampal pyramidal neurons are insensitive to EtOH (Wei et al., 2004), suggesting that the presence of the δ-subunit confers sensitivity to EtOH.
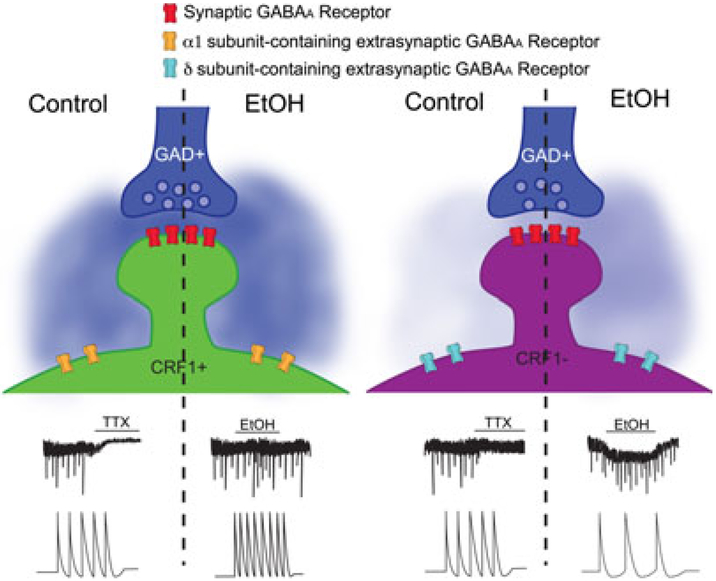
Data presented by Herman and colleagues (2013) support the notion that the δ-subunit is necessary for extrasynaptic GABAAR sensitivity to EtOH. Given that tonic currents in the CeA are mediated by either δ- or γ-containing GABAARs, it is not surprising that the acute application of EtOH (44 mM) differentially modulated CRF1+ and CRF1− within the CeA. As shown in Fig. 1, acute EtOH induced tonic currents and increased the frequency of phasic sIPSCs in CRF1− (expressing αxβδ-containing GABAARs), but had no effect on tonic or phasic currents in CRF1+ (expressing α1βγ-containing GABAARs). Consistent with the increase in GABAergic signaling onto CRF1−, EtOH decreased the spontaneous firing rates of these neurons. Surprisingly, EtOH increased the spontaneous firing rates of CRF1+, independent of glutamatergic activity. The effects of EtOH on CRF1+ and CRF1− could be blocked by a GABAAR antagonist, supporting previous findings suggesting that acute EtOH increases GABAergic transmission within the CeA (Roberto et al., 2012).
Fig. 1.
Effects of ethanol (EtOH) on GABA transmission at CRF1+ and CRF1 − neurons. As demonstrated in the study by Herman and colleagues (2013), CRF1+ and CRF1− receive inputs from GABAergic neurons (represented as glutamic acid decarboxylase (GAD)+ neurons). As depicted on the left, CRF1+ express a TTX-sensitive tonic current (shown on the control side) that is unaffected by the application of EtOH. In CRF1+, EtOH induces an increase in phasic sIPSC frequency (upper traces) and spontaneous firing of CRF1+ (bottom traces); surprisingly, tonic currents are not affected by EtOH application. As depicted on the right, CRF1− neurons do not exhibit a basal tonic current. EtOH application induces a tonic current (shown on the EtOH side, upper trace) and reduces spontaneous firing of CRF1− (bottom traces). CRF1−, CrF1 negative neurons; CRF+, CRF1 positive neurons; sIPSCs, spontaneous inhibitory postsynaptic currents; TTX, tetrodotoxin.
IMPLICATIONS OF TONIC CURRENTS AND THE EFFECTS OF ETHANOL ON THE ANXIETY/EMOTION CIRCUITRY
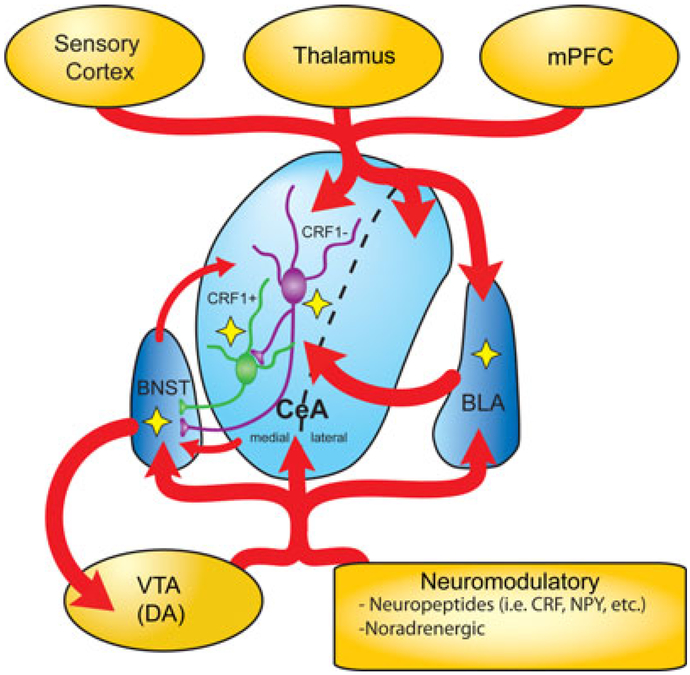
The anxiety/emotion circuitry is highly sensitive to EtOH exposure, as shown in c-fos studies (see review by Vilpoux et al., 2009), and given the intimate association between stress/anxiety and addiction, it has been suggested to play a major role in the development of EtOH addiction. Each brain region within the network has been shown to differentially integrate emotional states, thereby producing either appropriate (normal state) or inappropriate (addicted state) behaviors. As illustrated in Fig. 2, the BLA receives cortical and sensory information from various brain regions, including medial prefrontal cortex (mPFC), thalamus, and hippocampus (not shown in figure). The BLA processes that information and relays it to the CeA. The CeA can be subdivided into medial (CeM) and lateral (CeL), and these subregions have recently been shown to be functionally distinct from one another (Ciocchi et al., 2010; Tye et al., 2011). Using optogenetic approaches, Tye and colleagues (2011) and Ciocchi and colleagues (2010) clearly identified that the CeL and CeM modulate different behaviors and are highly connected. Excitatory inputs from the BLA and thalamus activated GABAergic neurons of the CeL that then inhibited GABAergic neurons in the CeM (Ciocchi et al., 2010; Tye et al., 2011). Furthermore, activation or inhibition of the BLA was sufficient to reduce or induce anxiety-like behavior, respectively (Tye et al., 2011), and activation of the CeL is required for fear acquisition, whereas activity from the CeM drives conditioned fear responses (Ciocchi et al., 2010). Unfortunately, the effects of EtOH in these functionally distinct regions of the CeA have been largely understudied. The studies of Herman and colleagues (2013) noted that the majority of the CRF1+ were located in the CeM and restricted their studies to this region. However, it would be interesting to know whether the CRF1+ and CRF1− of the CeL are similarly affected by EtOH. Both regions of the CeA integrate inputs from the BLA, cortex, thalamus, and the nucleus accumbens (not shown in figure) and are under modulation by numerous stress-regulated neuromodulatory systems (i.e., CRF, nociceptin, neuropeptide Y, endocannabinoids, and opioids; see review by Roberto et al., 2012). While this highly processed information can be projected to many brain regions, one of the primary targets of the CeA is the bed nucleus of the stria terminalis (BNST).
Fig. 2.
Connectivity of the central amygdala (CeA). The amygdala is composed of several nuclei, including the basolateral amygdala (BLA) and CeA. The BLA and CeA receive glutamatergic connections from the sensory cortex, thalamus, and medial prefrontal cortex (mPFC). The BLA sends glutamatergic projections to the CeA. The CeA is comprised of 2 subdivisions that can independently integrate signals from the sensory cortex, thalamus, mPFC, with that from the BLA, and sends GABAergic projections to the bed nucleus of the stria terminalis (BNST). The BNST forms reciprocal connections with the CeA and projects to other regions, including the ventral tegmental area (VTA). Activity within the amygdala and the BNST is regulated by neuropeptides (i.e., corticotropin-releasing factor [CRF] and neuropeptide Y [NPY]), and modulatory neurotransmitter systems such as norepinephrine and dopamine (DA). Yellow stars highlight where the actions of ethanol on synaptic transmission have been described in the context of this commentary. CRF1−, CRF1 negative neurons; CRF+, CRF1 positive neurons.
There is growing literature demonstrating the robust influence of the BNST in regulating this network, particularly its role in mediating learned fear responses (see review by Stamatakis et al., 2013). Similar to the previously discussed brain regions, the BNST is heavily innervated by numerous brain regions and in turn projects to various regions including the ventral tegmental area, hypothalamus, and parabrachial nucleus (not shown in figure) (Kim et al., 2013; Stamatakis et al., 2013). Interestingly, a recent study demonstrated that glutamatergic inputs from BLA to the anterodorsal BNST diminished anxiety-like responses, including respiratory rate (Kim et al., 2013). Importantly, the BLA, CeA, and BNST are regulated not only by the stress-regulated neuromodulatory, but by other modulatory systems such as the dopaminergic and noradrenergic systems, which play a major role in the addiction process (Stamatakis et al., 2013). There are numerous studies demonstrating that EtOH exposure and EtOH addiction can robustly alter the normal function of each brain region within this anxiety/emotion circuitry. How these distinct brain regions function to orchestrate emotional states remains unclear, but understanding how EtOH affects this circuit can give us insight into the pathophysiology of alcoholism. Moreover, the findings of Herman and colleagues suggest novel mechanisms by which EtOH may modulate the activity of this network.
As previously described, the CeA is under modulation of CRF and is predominantly comprised of GABAergic neurons that project to many brain regions, including the dorsolateral portion of the BNST (dlBNST). Within the CeA, Herman and colleagues (2013) show for the first time that some CRF1− innervate local CRF1+ possibly contributing to the action-potential-dependent inhibitory tonic currents in these neurons. The connection between CRF1− and CRF1+ explains the opposite effects of acute EtOH on the firing rates of these neurons. As shown in Fig. 1, acute EtOH induces a tonic current mediated by δ-containing GABAARs in CRF1−, resulting in a decrease in spontaneous firing. This leads to a disinhibition of CRF1+ (observed as an increase in spontaneous firing rate) and an inhibition of the downstream CRF1+ targets, similar to connections between the CeL and CeM (Ciocchi et al., 2010). Importantly, acute EtOH increased spontaneous firing rates of dlBNST-projecting CRF1+ and CRF1−. Indeed, it is difficult to predict the overall effects of EtOH in the CeA, because the upstream BLA is also under tonic and phasic inhibition. Given that the CeA’s complex microcircuitry has only recently begun to be characterized and that most physiological studies in the EtOH field have focused on the CeM (Herman et al., 2013; Roberto et al., 2012), we can only speculate how the actions of EtOH on all of these networks result in the observed effects. Assuming that acute EtOH can also potentiate tonic currents in the BLA, in addition to increasing phasic inhibition (Silberman et al., 2008), this would result in robust suppression of BLA excitability and decreased input into the CeA, particularly the CeL (Tye et al., 2011). The BLA also projects to a cluster of interneurons (intercalated cell masses or medial paracapsular cells [mpcs]) that provide GABAergic feedforward inhibition to the CeA (reviewed by Stamatakis et al., 2013). Specifically, ventral mpcs project to the CeM where Herman and colleagues performed recordings. It is possible that these interneurons provide GABAergic input onto CRF1−, and their input is potentiated by acute EtOH exposure. Interestingly, the lateral amygdala has projections to somatostatin-positive (SOM+) interneurons in the CeL that can drive fear responses independently of the CeM (Li et al., 2013). Although these SOM+ neurons are unlikely to be the source of GABA in CRF1−, it is important to acknowledge their contributions to this multifaceted circuitry, as these neurons may play a role in the EtOH addiction process.
Herman and colleagues (2013) demonstrate the presence of tonic currents in the CeA, further illustrating the robust and complex GABAergic-mediated inhibition within this emotion-/anxiety-mediating circuitry. Given that the GABAergic system is highly sensitive to EtOH, it is possible that tonic inhibition plays a crucial role in environmental influences on homeostasis (i.e., allostasis), a process disrupted in EtO addiction (Koob, 2009). Interestingly, most tonic currents in this circuit are primarily mediated by benzodiazepine-sensitive GABAAR subunits (α3 in glutamatergic pyramidal neurons of the BLA and α1 in CRF1+ of the CeA), making these subunit combinations favorable pharmaceutical targets for the treatment for anxiety-dependent disorders and EtOH addiction.
In summary, the recent report by Herman and colleagues (2013) describes novel GABAergic tonic currents within the CeA that are functionally distinct between CRF1+ and CRF1−. Furthermore, EtOH differentially modulates these tonic currents and could have significant implications on anxiety and EtOH-related behaviors. While future studies are needed to examine the roles of these tonic currents on behavioral responses to acute EtOH and their involvement in EtOH addiction, we pose several important issues that should be addressed to more clearly understand this system. Are ventral mpcs a source of GABAergic inhibition for CRF1− and, if so, are they sensitive to EtOH? Many studies have demonstrated that CRF and acute EtOH have opposing effects on GABAergic neurons within the CeA and that the disruption of CRF signaling may, in part, underlie EtOH dependence. Therefore, how are the tonic currents in CRF1+ and CRF1− affected by prolonged EtOH exposure and/or EtOH dependence? The studies of Herman and colleagues show that EtOH differentially affects CRF1+ and CRF1− within the CeA, but it remains unclear how CRF and EtOH interactions would affect the downstream signaling to the BNST. Furthermore, are there inhibitory tonic currents in the dlBNST and, if so, do these CRF1+ and CRF1− contribute to them? These questions will give us insight into how EtOH may suppress amygdala output, alleviate stress, trigger the reward pathway, and lead to EtOH dependence.
ACKNOWLEDGMENTS
We would like to thank Drs. C. Fernando Valenzuela, Jonathan L. Brigman, and L. Donald Partridge for critically reading the manuscript.
REFERENCES
- Baur R, Kaur KH, Sigel E (2009) Structure of alpha6 beta3 delta GABA(A) receptors and their lack of ethanol sensitivity. J Neurochem 111:1172–1181. [DOI] [PubMed] [Google Scholar]
- Botta P, de Souza FM, Sangrey T, De Schutter E, Valenzuela CF (2010) Alcohol excites cerebellar Golgi cells by inhibiting the Na+/K+ ATPase. Neuropsychopharmacology 35:1984–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta P, Mameli M, Floyd KL, Radcliffe RA, Valenzuela CF (2007) Ethanol sensitivity of GABAergic currents in cerebellar granule neurons is not increased by a single amino acid change (R100Q) in the alpha6 GABAA receptor subunit. J Pharmacol Exp Ther 323:684–691. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I (2012) Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A (2010) Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468:277–282. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Wadleigh A, Kumar S, De Schutter E, Valenzuela CF (2013) Na+/K+-ATPase inhibition partially mimics the ethanol-induced increase of the Golgi cell-dependent component of the tonic GABAergic current in rat cerebellar granule cells. PLoS ONE 8:e55673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I (2007) The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol 582:1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I (2007) A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci 10:40–48. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW (2004) Alcohol effects on gamma-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? LifeSci 76:1–8. [DOI] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M (2013) Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci 33:3284–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Chandra D, Homanics GE, Harrison NL (2008) Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther 326:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K (2013) Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2009) Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 42(Suppl 1):S32–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B (2013) Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 16:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Rudolph U, Fritschy JM, Arand M (2012) Tonic inhibition in principal cells of the amygdala: a central role for alpha3 subunit-containing GABAA receptors. J Neurosci 32:8611–8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR (2012) The central amygdala and alcohol: role of gamma-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med 2:a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL (2008) Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther 324:251–260. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD (2013) Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology doi: 10.1016/j.neuropharm.2013.05.046 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M (2009) Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res 33:945–969. [DOI] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I (2004) Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci 24:8379–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]