Abstract
Otitis media (OM) is one of the most common diseases of childhood, and nontypeable Haemophilus influenzae (NTHI) is the predominant causative agent of chronic and recurrent OM, as well as OM for which treatment has failed. Moreover, NTHI is now as important a causative agent of acute OM as the pneumococcus. NTHI colonizes the human nasopharynx asymptomatically. However, upon perturbation of the innate and physical defenses of the airway by upper respiratory tract viral infection, NTHI can replicate, ascend the Eustachian tube, gain access to the normally sterile middle ear space, and cause disease. Bacterial biofilms within the middle ear, including those formed by NTHI, contribute to the chronic and recurrent nature of this disease. These multicomponent structures are highly resistant to clearance by host defenses and elimination by traditional antimicrobial therapies. Herein, we review several strategies utilized by NTHI in order to persist within the human host and interventions currently under investigation to prevent and/or resolve NTHI-induced diseases of the middle ear and uppermost airway.
Keywords: phasevarion, Type IV pilus, PilA, eDNA, DNABII proteins, integration host factor, EPS
Otitis media burden
Otitis media (OM) is one of the most common diseases of children <15 years of age, with peak incidence between 9 and 15 months.1 As a result, OM is the primary cause for hearing loss in childhood, which can have a notable impact on behavior, language and educational development.2–5 In developed countries, therapeutic and prophylactic antibiotic treatment is typically relied upon for management of acute OM, and clinical practice guidelines recommend a period of ‘watchful waiting’ for children with less severe disease.6–9 However, OM is the primary reason for a child to be prescribed an antimicrobial, a fact that is driving the emergence of antibiotic resistance among those bacteria frequently identified as disease-causing agents and not just those that are predominant pathogens of OM.10–12 Worldwide, 709 million cases of acute OM, and 31 million cases of chronic suppurative OM occur yearly, and while morbidity is uncommon in developed countries, ~21,000 children die each year in developing countries as a consequence of this disease.13
Surgical management of OM involves insertion of tympanostomy tubes into the tympanic membrane and is the most common surgical procedure for children under the age of 15 in the US.14 While effective to relieve pressure and pain due to fluid accumulation in the middle ear space, tube insertion does not prevent OM. Moreover, between 10 and 70% of children develop post-tympanostomy tube otorrhea, a complication for which there is no consensus on effective treatment.15,16 Therefore, there is an obvious need to develop more effective approaches to the management and prevention of OM. To do so requires an understanding of the strategies employed by potential otopathogens, including nontypeable Haemophilus influenzae (NTHI), that promote persistence within the human nasopharynx during colonization and survival in the middle ear upon induction of disease.
Bacterial biofilms
One strategy that promotes persistence of NTHI within its host is biofilm formation. The ability of NTHI to build a biofilm contributes to the chronic character of diseases caused by this bacterium, including bronchitis, exacerbations of chronic obstructive pulmonary disease, conjunctivitis, sinusitis and OM. Moreover, biofilms are associated with prolonged drainage from the middle ear that results from perforation of the tympanic membrane in chronic suppurative OM and following tympanostomy tube insertion.15–18 Biofilms are characterized as a community of bacteria, single- or multi-species in nature, often adherent to a surface and encased in an extracellular polymeric substance (EPS).19 Biofilm-resident bacteria exhibit a reduced metabolism and an altered proteome compared to their planktonic counterparts, features that contribute to their recalcitrance against typical antimicrobial therapies.20 Clinically, biofilms are present within middle ear specimens and within the discharge collected from patients with otorrhea.21–23
The EPS that surrounds and supports bacteria within a biofilm is complex in both molecular composition and structure. EPS shields bacteria from host immune responses and antimicrobials, mitigates the efficacy of surfactants, sequesters nutrients, concentrates cell-to-cell signaling molecules and slows desiccation (see reviews,24,25). As such, development of therapeutic strategies to eradicate bacterial biofilms in the middle ear or the design of vaccines to prevent their formation requires a thorough understanding of the EPS structure and composition. Specific components of the EPS can vary among bacterial species; however, EPS is generally comprised of proteins, polysaccharides and nucleic acids.25–27 We and others have investigated the composition of the NTHI biofilm EPS and showed that NTHI proteins OMP P5 and Type IV pilus (Tfp), OMP P2 porin, OMP P6 lipoprotein and lipooligosaccharide are distributed throughout biofilms formed in vitro and in vivo.28–33 In addition, extracellular DNA (eDNA) is found in abundance within most bacterial biofilms and is thought to protect against host-derived antimicrobials and other cationic molecules.34 Moreover, the abundance of eDNA and its unique lattice-like organization observed in vitro and within specimens collected from the middle ear during experimental NTHI infection [Fig. 1] led to the discovery that eDNA also serves as a critical structural component of biofilms formed by NTHI and other medically- and environmentally-important bacterial species.29,35
Figure 1.
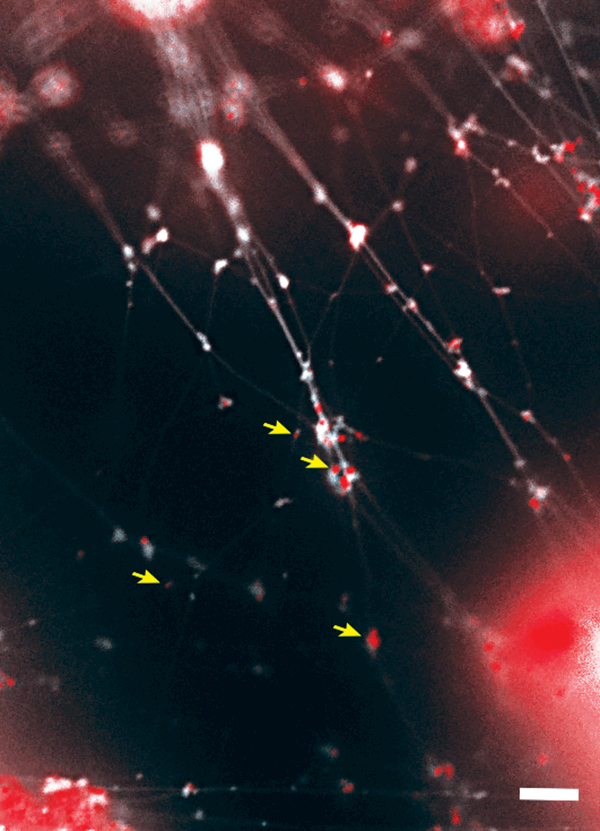
NTHI biofilms formed within the chinchilla middle ear during experimental OM contain abundant eDNA and DNABII proteins in association. Crossed strands of eDNA (white) form a lattice-like structure within the biofilm EPS. The eDNA is stabilized by members of the DNABII family of DNA-binding proteins (red, indicated by yellow arrows) which bind at the vertices of DNA strands. Scale bar, 5 μm.
Key to the structural integrity of the eDNA lattice is a family of DNA-binding proteins, the DNABII family, which includes integration host factor (IHF) and histone-like protein (HU). Whereas IHF and HU are classically known to bind and stabilize pre-bent DNA and cruciform structures intracellularly36–41, they also play an important role in the stabilization of the eDNA structure within biofilms formed by NTHI and other bacterial species [Fig. 1].35,42 We’ve shown that antibodies directed against DNABII proteins induce catastrophic collapse of biofilms formed by many bacterial pathogens in vitro. This disruption is attributed to the sequestration of DNABII proteins from the extracellular milieu as the proteins rapidly cycle between ‘eDNA-bound’ and ‘free’ states.31,33–41 The resulting equilibrium imbalance promotes dissociation of DNABII proteins from the eDNA matrix, destabilization of the eDNA lattice and subsequent collapse of the biofilm structure [Fig. 2A].42 As biofilms are the preferred lifestyle for many bacterial species, including NTHI, efforts to understand the composition of the EPS and environmental factors that stimulate the formation of biofilms is an active area of investigation.
Figure 2.
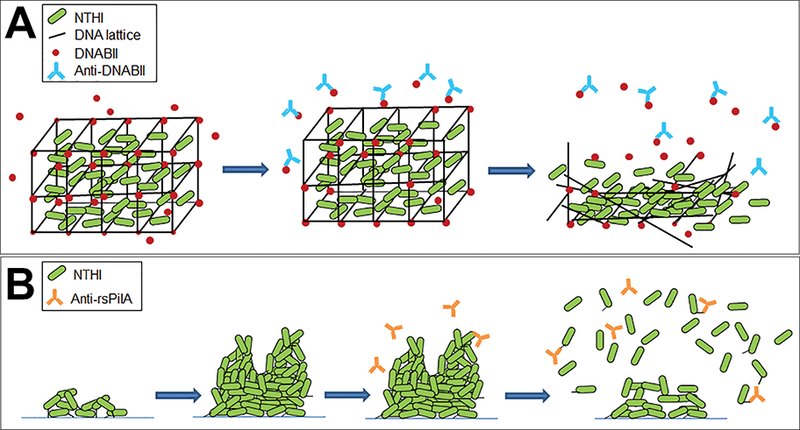
Two distinct mechanisms to disrupt NTHI biofilms. (A) Biofilms formed by many bacterial human pathogens, including NTHI incorporate eDNA (black lines) and DNABII proteins (red circles) within the EPS. Antibodies against DNABII proteins (blue) bind and sequester DNABII molecules from the extracellular milieu and induce an equilibrium imbalance. Release of DNABII proteins from the eDNA scaffold results in catastrophic collapse of the biofilm structure and exposure of resident bacteria. (B) NTHI utilize Tfp to adhere, organize and form a biofilm. Antibodies directed against the majority subunit of NTHI Tfp, PilA, (orange) induce a ‘top-down’ dispersal event that is dependent on quorum signaling.
Environmental factors influence biofilm formation
As we and others examine strategies to break down or prevent bacterial biofilms, it is important to understand the factors that influence the formation of these structures, particularly those relevant to the human host. The majority of work on biofilm biology is performed under standard lab conditions (i.e. 37°C, 5% CO2, humidified atmosphere, rich medium), in order to promote bacterial growth in vitro. However, in the human host, bacteria resident within the nasopharynx experience an average temperature of 34°C, neutral pH, and mechanical and shear stresses due to air and liquid movement in addition to nutrient limitation.43–45 In contrast, at the site of disease in the middle ear, the temperature is typically 37°C or greater if fever is present, and middle ear effusions from patients with chronic OM are uniformly alkaline in pH.46,47 Marks et al. observed temperature-dependent variations in transformation efficiency and biofilm dispersal by the nasopharyngeal commensal bacterium and OM pathogen, Streptococcus pneumoniae.48,49 As NTHI also colonizes the human nasopharynx, we examined whether the 3-degree temperature difference between 34°C and 37°C affected the expression kinetics of the NTHI Type IV pilus (Tfp).
Tfp are essential for NTHI adherence, twitching motility, and biofilm formation in vitro and within the middle ears of chinchillas during experimental NTHI-induced OM.50–53 An additional function attributed to expression of Tfp is competence, and the presence of each gene in the pil and com operons is required for uptake of exogenous DNA.54 Antibodies against an N-terminally truncated, recombinant variant of NTHI PilA, (called rsPilA, for recombinant and soluble PilA), prevent adherence of NTHI to human respiratory tract epithelial cells and inhibit biofilm formation in vitro.55 Moreover, incubation of pre-formed NTHI biofilms with anti-rsPilA antibody induces a ‘top-down’ dispersal of bacteria that is dependent on quorum signaling, a process of bacterial communication facilitated by secretion and detection of self-produced signaling molecules [Fig. 2B].56,57 Of note, the mechanism for anti-rsPilA-induced biofilm ‘top-down’ dispersal is distinct from catastrophic biofilm collapse via anti-IHF antibodies (compare Fig. 2A and B).
Tfp expression (as estimated by pilA promoter activity) is significantly greater in biofilms formed at 34°C compared to those formed at 37°C [Fig. 3].55 Moreover, twitching motility mediated by Tfp is also significantly increased at 34°C compared to that observed at 37°C. Thus temperature likely contributes to the regulation of Tfp expression and twitching motility and facilitates NTHI adherence and organization into a biofilm under conditions that mimic the dynamic and stressful environment of the human nasopharynx. This conclusion is further supported by evidence that a clinical isolate of NTHI, strain 86–028NP, colonizes the nasopharynx of chinchillas during experimental OM significantly longer than its isogenic pilA mutant.50
Figure 3.
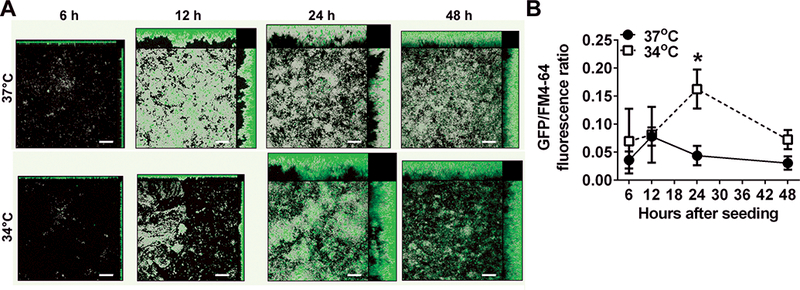
Expression of Tfp, as estimated by pilA promoter activity, reaches a significantly higher maximum value in biofilms formed at 34°C compared to biofilms formed at 37°C in vitro. (A) Biofilms formed with NTHI wherein the pilA promoter drives expression of green fluorescent protein. Total biomass (as indicated by FM-464 fluorescent membrane stain) is shown in gray, and green areas indicate pilA promoter activity. Promoter activity is greatest near the base of the biofilms early on, but as the biofilms mature, regions of intense fluorescence become more prevalent towards the apex of towers. (B) Peak pilA promoter activity is 3.7 times higher in biofilms formed at 34°C vs. 37°C as measured by fluorescent intensity, and this effect is independent of the amount of biomass. * P≤ 0.05. Scale bars, 20 μm. Copyright © American Society for Microbiology, [Journal of Bacteriology, 198, 2016, 2619–2630. doi: 10.1128/JB.01022-15].
NTHI Tfp expression and twitching motility are also induced under alkaline conditions. This result is particularly relevant in OM, as the pH of chronic middle ear effusions is typically greater than 8.0.46,47 Interestingly, alkaline pH also provides an optimal environment for mixed NTHI-S. pneumoniae biofilms. Tikhomirova et al. showed that whereas both bacterial species thrived within a mixed biofilm when grown in medium at a pH of 8.0, NTHI did not survive co-culture with S. pneumoniae at a pH 7.4.58 The interaction between NTHI and S. pneumoniae is complex, however, and displays either synergy or antagonism, depending on the model system and growth conditions. In addition to pH, nutrient availability and the growth phase of the bacterial inoculum also affects NTHI survival.
NTHI has an absolute requirement for iron to survive, however the human host normally sequesters this molecule such that it is not freely available. This iron-restricted status changes upon host inflammatory response due to infection, as damage or death of host immune or epithelial cells results in release iron into the microenvironment. Szelestey et al. examined the outcome of shifts in iron availability specific to NTHI biofilm formation in vitro and in vivo.59 NTHI initially cultured medium depleted of heme-iron, then transition into medium supplemented with heme-iron (i.e. transiently iron-restricted) formed biofilms with a substantially greater peak height and increased architectural complexity compared to NTHI grown continuously in supplemented medium in vitro. Inoculation of chinchilla middle ears with transiently iron-restricted NTHI mixed 1:10 with bacteria maintained in supplemented medium revealed persistence of 99% of the transiently iron-restricted population after 4 days with less severe middle ear pathology, due to an observed increase in number of intracellular NTHI, compared to middle ears inoculated with NTHI maintained in supplemented medium. These results indicated that changes in heme-iron availability can alter the phenotype of NTHI biofilms and promote NTHI survival and persistence in vivo.
It is clear that traditional culture methods that utilize standard laboratory conditions of temperature, pH, and nutrient availability designed to maximize bacterial growth do not faithfully replicate the environments in which bacterial pathogens exist in vivo, and that changes in these environmental factors can significantly influence biofilm biology and virulence factor expression. In order to fully understand the survival strategies and expression of virulence determinants by organisms such as NTHI, temperature, pH and nutrient availability must be considered, particularly in the analysis of potential vaccine targets.
Regulation of biofilm and virulence determinants
NTHI utilizes a variety of mechanisms to regulate the expression of virulence factors required for colonization, immune evasion and biofilm formation. One important mechanism involves transcriptional regulators that alter bacterial gene expression in response to key environmental changes such as oxidative stress. Reactive oxygen species (ROS), such as hydrogen peroxide and superoxide, are among the primary antimicrobial agents produced by macrophages and neutrophils to kill respiratory tract pathogens. In addition, bacteria themselves produce hydrogen peroxide as a respiratory byproduct, which can reach toxic levels at high bacterial density, such as in a biofilm. In response to oxidative stress, the transcriptional regulator OxyR upregulates the expression of proteins that mitigate damage due to ROS. Proteins regulated by OxyR include catalase, an enzyme that breaks down hydrogen peroxide and Dps which protects DNA from damage by oxygen radicals.60–62 OxyR is well-characterized as an oxidative stress-responsive transcriptional regulator in many bacteria and is important for NTHI survival and pathogenesis in animal models of disease.61–63
In addition to active gene regulation and environmental sensing, human-adapted pathogens including NTHI have genes that undergo phase variation, a random and reversible change in gene expression (see review, 64). Phase variation of virulence factors allows NTHI to rapidly adapt to microenvironmental changes and evade host immune defenses. Similar to antigenic variation, phase variation results in a subset of bacteria with a unique phenotype not present within the greater population. Dependent on the mutation and microenvironmantal stresses, one phenotype will be more advantageous than the other. Phase variable virulence factors include the Hia autotransporter and high molecular weight (HMW) 1 & 2 adhesive proteins.65–68 Whereas increased expression of Hia facilitates NTHI adherence and nasopharyngeal colonization in experimental models, reduced Hia expression protects the bacterium against opsonophagocytic killing.67 Similarly, the phase variable promoter region of the NTHI HMW genes controls the expression of these adhesive proteins, which in turn influences bacterial adherence.68,69 While variable expression of virulence factors is beneficial to the bacterium, phase variation of potential vaccine targets can greatly decrease vaccine efficacy.
NTHI has additionally evolved a novel epigenetic system of rapid adaptation which regulates a switch in the expression of multiple virulence factors simultaneously. This unique mechanism is employed by multiple human pathogens and is termed the phasevarion, for phase variable regulon.70 Whereas phase variation results in a change in the expression of a single gene, the phasevarion simultaneously regulates the expression of many genes across the genome. This occurs by phase variation of a single DNA methyltransferase (ModA) independent of environmental cues. When expressed, this methyltransferase binds to and methylates sequence-specific sites on the bacterial chromosome, which, in turn, alters expression of genes in the regulon. The result is two phenotypically distinct subpopulations, modA ON and modA OFF. Within a collection of over 200 NTHI clinical isolates retrieved from the nasopharynx and/or middle ears of healthy and OM-prone children, we identified 21 distinct modA alleles.71 Five phase variable modA alleles accounted for over two-thirds of clinical isolates. As modA2 was the most prevalent allele, we focused our studies thereon.
The phasevarions of multiple NTHI strains control the expression of several outer membrane adhesive proteins, including HMW proteins.71 Transcriptional analysis of the NTHI strain 723 ModA2 phasevarion also revealed regulation of multiple genes required for iron uptake.71 NTHI has a strict requirement for heme-iron and maintenance of iron homeostasis is required for survival and pathogenesis in vivo.62,63 As discussed, availability of heme-iron also influences biofilm formation.59 In a chinchilla model of experimental OM, Atack et al. showed a clear selection for the modA2 ON subpopulation within the middle ear.71 Furthermore, a shift from modA2 OFF status to modA2 ON status within the chinchilla middle ear results in significantly greater disease severity compared to populations that do not shift status.72 Middle ears in which NTHI shifted modA2 status had increased mucosal hyperplasia and edema, and significantly greater NTHI biofilm biomass. Work to identify phasevarion-specific regulation of virulence determinants that contribute to biofilm formation and pathogenesis is necessary and ongoing.
As we continue to develop new methods to treat and prevent bacterial infections, including those due to NTHI, it is critical to understand the regulation of potential vaccine targets under physiological- and disease-relevant conditions. Mechanisms such as phase variable regulation of individual virulence factors and genome-wide regulation by the phasevarion must be considered. Much is still not known about the phasevarions of NTHI and other human pathogens, and phase variation of a vaccine target can severely limit its effectiveness. Continued studies in this exciting new area will be crucial for future vaccine development.
Vaccine strategies for NTHI-induced OM
At present, an NTHI-specific vaccine for OM is not yet available; however prevention of OM has the potential to not only limit disease, but also avert the development of OM-associated sequellae.73–75 Many NTHI surface-exposed proteins, or portions thereof, and lipooligosaccharides are under investigation as potential vaccine candidates. These include several NTHI adhesin proteins (OMP P5, Type IV pilus, Protein E, HMW 1 and 2, and Hia), major NTHI porin protein (OMP P2); outer membrane lipoproteins (OMP P6 and Protein D) and a Skp-like chaperone protein (OMP 26)(see review, 76). The POET pediatric clinical trial, wherein NTHI Protein D served as a carrier molecule for a pneumococcal conjugate vaccine, showed 35.3% vaccine efficacy against OM due to NTHI 77, and whereas these data were the first to demonstrate that immunization against NTH-induced disease was possible, they also indicated that additional antigens, or combinations thereof, may be necessary to achieve greater protection.
A long-standing approach for vaccine development by our laboratory is to target both adhesive proteins expressed by this bacterium as well as those proteins essential to the formation and structural stability of its biofilms.78–82 We’ve developed three immunogens that demonstrate efficacy against NTHI both in vitro and pre-clinically in animal models of disease. These include 1) an NTHI Type IV pilus (Tfp)-derived recombinant protein called ‘rsPilA’, which is designed to inhibit NTHI adherence, twitching motility and biofilm formation 29,50–52,55,57,83; 2) a chimeric immunogen that targets both NTHI OMP P5 and Tfp, called ‘chimV4’, which is designed to block adherence and pathogenesis of NTHI as mediated by two important adhesive proteins/ virulence determinants 83–86 and 3) IHF (integration host factor), a DNA-binding protein that serves as a critical structural element to the extracellular DNA scaffold within the extracellular polymeric substance incorporated into biofilms formed by many bacterial species 17,35,42,87–93.
To date, we’ve shown that parenteral immunization with rsPilA or chimV4 prevents experimental OM caused by NTHI, likely due to inhibition of adherence and twitching motility, which is mediated by pilus- and OMP P5-specific antibodies present at the respiratory mucosal surface.83 As an alternative, but potentially equally efficacious strategy, we’ve also explored the utility of transcutaneous immunization (TCI), the placement of vaccine formulations on to intact skin. TCI offers multiple advantages as an immunization strategy. TCI induces both systemic and mucosal immune responses, an important feature as the mucosae represent critical defensive barriers that also respond immunologically to insult.94–98 It is also noninvasive, which may aid in acceptance and compliance. With all of these advantages, TCI could promote vaccine distribution beyond developed countries.99,100 Thus, TCI exhibits potential as an efficacious and simple method to induce protective immune responses and thereby limit disease.
We considered practical application of TCI to humans, particularly to very young children, and envisioned the use of a small adhesive bandage to administer vaccine formulations on to intact skin. In animal models, the post-auricular region (skin just behind the ear) was specifically targeted as the anatomical location for placement of a circular bandaid. The stratum corneum at this location is uniquely organized in a vertically linear stacked arrangement, in contrast to the more typical ‘brick-and-mortar’ stratification found in skin elsewhere on the body [Fig. 4].101,102 TCI via bandaid to deliver chimV4, rsPilA or IHF admixed with a potent adjuvant, a derivative of E. coli heat-labile enterotoxin LT(R192G/L211A) or ‘dmLT ‘ to potentiate the immune response induced by these antigens103, induces an immune response that resolves NTHI-induced OM.35,57,104–106 Equally important is that TCI via bandaid with chimV4, rsPilA or IHF also prevented the onset of OM in a polymicrobial chinchilla model that mimics the natural progression of disease in children wherein an upper respiratory tract viral infection predisposes to development of bacterial OM.107–110
Figure 4.
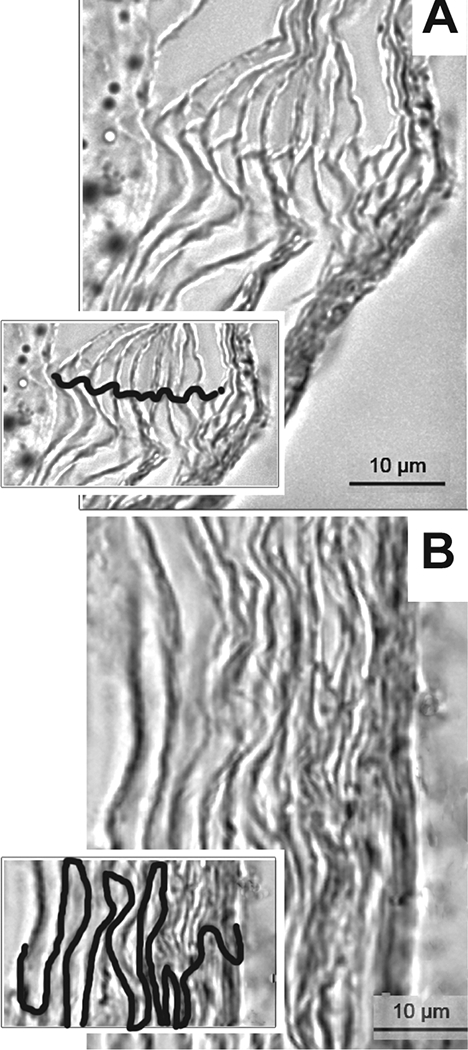
The stratum corneum at the post-auricular region is uniquely organized. To understand any differences in efficacy achieved by TCI via bandaid as related to anatomical placement, skin from the post-auricular region and the nape of the neck on chinchillas was collected and the organization of the cells within the stratum corneum examined by microscopy.106 (A) The corneocytes at the post-auricular region were linearly aligned whereas (B) at the nape, a classic ‘brick-and-mortar’ arrangement was observed. Insets, visualization of cell stratification with cellular junctions traced. Scale bars, 10 μm. Copyright © American Society for Microbiology, [Clinical and Vaccine Immunology, 22, 2015, 867–874. doi:10.1128/CVI.00090-15].
The means for TCI-induced efficacy are multifold. Placement of the immunizing bandaid at the post-auricular region takes advantage of an atypical cellular arrangement at this anatomical location that permits underlying antigen-presenting cells greater access to topically-applied antigens.111,112 Directed migration of activated antigen-presenting cells, specifically dermal dendritic cells, to the rodent equivalent of the human Waldeyer’s ring of lymphoid tissues in the nasopharynx (the nasal-associated lymphoid tissue or NALT), facilitates the induction of an immune response in close proximity to the site of disease within the middle ear.113 Secretion of IFN-γ and IL-17A by activated CD4+ T-cells within the NALT promotes antibody production by plasma cells and chemotaxis of neutrophils to sites of inflammation (i.e. the infected middle ear) subsequent to bacterial clearance.7,114,115 Our strategy of using a traditional small circular bandaid placed directly onto the intact skin just behind the ear as a delivery device may provide the opportunity to expand the reach of vaccines against NTHI-induced diseases.
Conclusion
Whereas the licensure and broad use of several pneumococcal conjugate vaccines has indeed had an impact on preventing acute OM due to those serotypes of Streptococcus pneumoniae included in the vaccine formulations, the impact of these vaccines on OM due to NTHI is much more limited. In some studies, when pneumococcal conjugates vaccines (PCVs) are delivered early in life, prevention of first episodes of AOM has been shown to limit subsequent more complex OM due NTHI.73 However, if these vaccines are given after the first episode of OM, there is no measurable effect on NTHI-induced OM, and in fact, PCVs are not designed to prevent NTHI-induced OM. Thereby, a broadly protective vaccine to prevent NTHI-induced OM is still of critical need, as is the development of novel therapeutic approaches to treat NTHI-associated diseases of the upper respiratory tract. NTHI are highly adept at biofilm formation, a phenotype that contributes significantly to the chronic, recurrent and recalcitrant nature of the OM induced by this highly heterogeneous Gram-negative bacterium. As studies being conducted by laboratories all over the world contribute to our improved understanding of the unique pathobiology of NTHI-induced OM, including how it adheres, builds biofilms and responds to both unique micro-environmental cues encountered as it ascends from the nasopharynx to the middle ear as well as to host immune effectors, the knowledge gained will foster the ability to develop highly targeted approaches for disease prevention and treatment. Multiple technological advances, including genomics, proteomics, metabolomics, transcriptomics, high resolution imaging, more sophisticated animal modeling and appreciation for the polymicrobial nature of OM, in addition to many others make this an exciting time for OM-focused research.
Acknowledgements
We are grateful to Jennifer Neelans for assistance in manuscript preparation. Work performed by our laboratory was funded by NIDCD/NIH R01 003915, R01 DC011818, R01 DC015688, NHMRC Project 1034401 and 1071659.
Footnotes
Competing interest
LAN, KLB, EMM and JAJ have no competing interests. LOB is a Scientific Advisor to, and has equity in, ProclaRx, LLC, to whom technology related to the DNABII proteins is licensed. LOB is an inventor of technology related to PilA-derived immunogens which is licensed to GlaxoSmithKline Biologicals.
References
- 1.Pichichero ME. Otitis media. Pediatric clinics of North America. April 2013;60(2):391–407. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin RL. Effects of otitis media on child development. The American journal of otology. November 1993;14(6):601–604. [PubMed] [Google Scholar]
- 3.Hunter LL, Margolis RH, Giebink GS. Identification of hearing loss in children with otitis media. Ann Otol Rhinol Laryngol Suppl. May 1994;163:59–61. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DL, McCormick DP, Baldwin CD. Early middle ear effusion and language at age seven. J Commun Disord. Jan-Feb 2008;41(1):20–32. [DOI] [PubMed] [Google Scholar]
- 5.Li SF, Kumar A, Thomas S, et al. Safety and efficacy of intravenous combination sedatives in the ED. The American journal of emergency medicine. September 2013;31(9):1402–1404. [DOI] [PubMed] [Google Scholar]
- 6.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. April 2010;29(4):304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Family P, American Academy of O-H, Neck S, American Academy of Pediatrics Subcommittee on Otitis Media With E. Otitis media with effusion. Pediatrics. May 2004;113(5):1412–1429.15121966 [Google Scholar]
- 8.Hoiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. May 2015;21 Suppl 1:S1–25. [DOI] [PubMed] [Google Scholar]
- 9.Leibovitz E, Broides A, Greenberg D, Newman N. Current management of pediatric acute otitis media. Expert review of anti-infective therapy. February 2010;8(2):151–161. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrobial agents and chemotherapy. 2014;58(3):1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. Jama. August 19 2009;302(7):758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song JH, Dagan R, Klugman KP, Fritzell B. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine. April 05 2012;30(17):2728–2737. [DOI] [PubMed] [Google Scholar]
- 13.Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PloS one. 2012;7(4):e36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfeld RM, Schwartz SR, Pynnonen MA, et al. Clinical practice guideline: Tympanostomy tubes in children. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. July 2013;149(1 Suppl):S1–35. [DOI] [PubMed] [Google Scholar]
- 15.Hochman J, Blakley B, Abdoh A, Aleid H. Post-tympanostomy tube otorrhea: a meta-analysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. July 2006;135(1):8–11. [DOI] [PubMed] [Google Scholar]
- 16.Oberman JP, Derkay CS. Posttympanostomy tube otorrhea. Am J Otolaryngol. Mar-Apr 2004;25(2):110–117. [DOI] [PubMed] [Google Scholar]
- 17.Idicula WK, Jurcisek JA, Cass ND, et al. Identification of biofilms in post-tympanostomy tube otorrhea. The Laryngoscope. August 2016;126(8):1946–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syed MI, Suller S, Browning GG, Akeroyd MA. Interventions for the prevention of postoperative ear discharge after insertion of ventilation tubes (grommets) in children. Cochrane Database Syst Rev. April 30 2013(4):CD008512. [DOI] [PubMed] [Google Scholar]
- 19.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. May 21 1999;284(5418):1318–1322. [DOI] [PubMed] [Google Scholar]
- 20.Sauer K The genomics and proteomics of biofilm formation. Genome biology. 2003;4(6):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakaletz LO. Bacterial biofilms in the upper airway - evidence for role in pathology and implications for treatment of otitis media. Paediatric respiratory reviews. September 2012;13(3):154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. Jama. July 12 2006;296(2):202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Post JC. Direct evidence of bacterial biofilms in otitis media. 2001. The Laryngoscope. September 2015;125(9):2003–2014. [DOI] [PubMed] [Google Scholar]
- 24.Flemming HC. EPS-Then and Now. Microorganisms. November 18 2016;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunn JS, Bakaletz LO, Wozniak DJ. What’s on the Outside Matters: The Role of the Extracellular Polymeric Substance of Gram-negative Biofilms in Evading Host Immunity and as a Target for Therapeutic Intervention. The Journal of biological chemistry. June 10 2016;291(24):12538–12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karatan E, Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiology and molecular biology reviews : MMBR. Jun 2009;73(2):310–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. February 22 2002;295(5559):1487. [DOI] [PubMed] [Google Scholar]
- 28.Greiner LL, Watanabe H, Phillips NJ, et al. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N-acetylneuraminic acid that may mimic sialylated O-linked glycans. Infect Immun. July 2004;72(7):4249–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jurcisek JA, Bakaletz LO. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol. May 2007;189(10):3868–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy TF, Kirkham C, Sethi S, Lesse AJ. Expression of a peroxiredoxin-glutaredoxin by Haemophilus influenzae in biofilms and during human respiratory tract infection. FEMS immunology and medical microbiology. April 01 2005;44(1):81–89. [DOI] [PubMed] [Google Scholar]
- 31.Gallaher TK, Wu S, Webster P, Aguilera R. Identification of biofilm proteins in non-typeable Haemophilus Influenzae. BMC microbiology. July 19 2006;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webster P, Wu S, Gomez G, Apicella M, Plaut AG, St Geme JW, 3rd. Distribution of bacterial proteins in biofilms formed by non-typeable Haemophilus influenzae. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. July 2006;54(7):829–842. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Baum MM, Kerwin J, et al. Biofilm-specific extracellular matrix proteins of nontypeable Haemophilus influenzae. Pathogens and disease. December 2014;72(3):143–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuri R, Shprung T, Shai Y. Defensive remodeling: How bacterial surface properties and biofilm formation promote resistance to antimicrobial peptides. Biochimica et biophysica acta. November 2015;1848(11 Pt B):3089–3100. [DOI] [PubMed] [Google Scholar]
- 35.Goodman SD, Obergfell KP, Jurcisek JA, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. November 2011;4(6):625–637. [DOI] [PubMed] [Google Scholar]
- 36.Swinger KK, Rice PA. Structure-based analysis of HU-DNA binding. Journal of molecular biology. January 26 2007;365(4):1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swinger KK, Rice PA. IHF and HU: flexible architects of bent DNA. Current opinion in structural biology. February 2004;14(1):28–35. [DOI] [PubMed] [Google Scholar]
- 38.Rice PA, Yang S, Mizuuchi K, Nash HA. Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn. Cell. December 27 1996;87(7):1295–1306. [DOI] [PubMed] [Google Scholar]
- 39.Goodman SD, Nash HA. Functional replacement of a protein-induced bend in a DNA recombination site. Nature. September 21 1989;341(6239):251–254. [DOI] [PubMed] [Google Scholar]
- 40.Goodman SD, Nicholson SC, Nash HA. Deformation of DNA during site-specific recombination of bacteriophage lambda: replacement of IHF protein by HU protein or sequence-directed bends. Proceedings of the National Academy of Sciences of the United States of America. December 15 1992;89(24):11910–11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamashev D, Rouviere-Yaniv J. The histone-like protein HU binds specifically to DNA recombination and repair intermediates. The EMBO journal. December 01 2000;19(23):6527–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brockson ME, Novotny LA, Mokrzan EM, et al. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Molecular microbiology. September 2014;93(6):1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb P Air temperatures in respiratory tracts of resing subjects in cold. Journal of applied physiology. November 1951;4(5):378–382. [DOI] [PubMed] [Google Scholar]
- 44.Keck T, Leiacker R, Riechelmann H, Rettinger G. Temperature profile in the nasal cavity. The Laryngoscope. April 2000;110(4):651–654. [DOI] [PubMed] [Google Scholar]
- 45.Brunworth JD, Mahboubi H, Garg R, Johnson B, Brandon B, Djalilian HR. Nasopharyngeal acid reflux and Eustachian tube dysfunction in adults. Ann Otol Rhinol Laryngol. June 2014;123(6):415–419. [DOI] [PubMed] [Google Scholar]
- 46.Nuutinen J, Torkkeli T, Penttila I. The pH of secretions in sinusitis and otitis media. The Journal of otolaryngology. April 1993;22(2):79–82. [PubMed] [Google Scholar]
- 47.Wezyk MT, Makowski A. [pH of fluid collected from middle ear in the course of otitis media in children]. Otolaryngologia polska = The Polish otolaryngology. 2000;54(2):131–133. [PubMed] [Google Scholar]
- 48.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio. July 23 2013;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marks LR, Reddinger RM, Hakansson AP. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. mBio. 2012;3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jurcisek JA, Bookwalter JE, Baker BD, et al. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Molecular microbiology. September 2007;65(5):1288–1299. [DOI] [PubMed] [Google Scholar]
- 51.Bakaletz LO, Baker BD, Jurcisek JA, et al. Demonstration of Type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect Immun. March 2005;73(3):1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jurcisek JA, Novotny LA, Armbruster C, Swords WE, Bakaletz LO. Targeting a Haemophilus influenzae protein expressed during biofilm growth in vivo for development of a vaccine for otitis media. Paper presented at: Abst. 5th ASM Conference on Biofilms2009; Cancun, Mexico. [Google Scholar]
- 53.Novotny LA, Bakaletz LO. Intercellular adhesion molecule 1 serves as a primary cognate receptor for the Type IV pilus of nontypeable Haemophilus influenzae. Cellular microbiology. February 9 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carruthers MD, Tracy EN, Dickson AC, Ganser KB, Munson RS, Jr., Bakaletz LO. Biological roles of nontypeable Haemophilus influenzae type IV pilus proteins encoded by the pil and com operons. J Bacteriol. April 2012;194(8):1927–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mokrzan EM, Ward MO, Bakaletz LO. Type IV Pilus Expression Is Upregulated in Nontypeable Haemophilus influenzae Biofilms Formed at the Temperature of the Human Nasopharynx. J Bacteriol. October 01 2016;198(19):2619–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol. August 11 2016;14(9):576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novotny LA, Jurcisek JA, Ward MO Jr., Jordan ZB, Goodman SD, Bakaletz LO. Antibodies against the majority subunit of type IV Pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Molecular microbiology. April 2015;96(2):276–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tikhomirova A, Trappetti C, Paton JC, Kidd SP. The outcome of H. influenzae and S. pneumoniae inter-species interactions depends on pH, nutrient availability and growth phase. International journal of medical microbiology : IJMM. December 2015;305(8):881–892. [DOI] [PubMed] [Google Scholar]
- 59.Szelestey BR, Heimlich DR, Raffel FK, Justice SS, Mason KM. Haemophilus responses to nutritional immunity: epigenetic and morphological contribution to biofilm architecture, invasion, persistence and disease severity. PLoS pathogens. 2013;9(10):e1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison A, Dyer DW, Gillaspy A, et al. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol. July 2005;187(13):4627–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison A, Ray WC, Baker BD, Armbruster DW, Bakaletz LO, Munson RS Jr. The OxyR regulon in nontypeable Haemophilus influenzae. J Bacteriol. February 2007;189(3):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitby PW, Morton DJ, Vanwagoner TM, et al. Haemophilus influenzae OxyR: characterization of its regulation, regulon and role in fitness. PloS one. 2012;7(11):e50588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison A, Santana EA, Szelestey BR, Newsom DE, White P, Mason KM. Ferric uptake regulator and its role in the pathogenesis of nontypeable Haemophilus influenzae. Infect Immun. April 2013;81(4):1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou K, Aertsen A, Michiels CW. The role of variable DNA tandem repeats in bacterial adaptation. FEMS microbiology reviews. January 2014;38(1):119–141. [DOI] [PubMed] [Google Scholar]
- 65.Winter LE, Barenkamp SJ. Antibodies to the HMW1/HMW2 and Hia adhesins of nontypeable haemophilus influenzae mediate broad-based opsonophagocytic killing of homologous and heterologous strains. Clinical and vaccine immunology : CVI. May 2014;21(5):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winter LE, Barenkamp SJ. Antibodies specific for the Hia adhesion proteins of nontypeable Haemophilus influenzae mediate opsonophagocytic activity. Clinical and vaccine immunology : CVI. July 2009;16(7):1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atack JM, Winter LE, Jurcisek JA, Bakaletz LO, Barenkamp SJ, Jennings MP. Selection and Counterselection of Hia Expression Reveals a Key Role for Phase-Variable Expression of Hia in Infection Caused by Nontypeable Haemophilus influenzae. J Infect Dis. August 15 2015;212(4):645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis GS, Marino S, Marrs CF, Gilsdorf JR, Dawid S, Kirschner DE. Phase variation and host immunity against high molecular weight (HMW) adhesins shape population dynamics of nontypeable Haemophilus influenzae within human hosts. Journal of theoretical biology. August 21 2014;355:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giufre M, Carattoli A, Cardines R, Mastrantonio P, Cerquetti M. Variation in expression of HMW1 and HMW2 adhesins in invasive nontypeable Haemophilus influenzae isolates. BMC microbiology. May 29 2008;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srikhanta YN, Maguire TL, Stacey KJ, Grimmond SM, Jennings MP. The phasevarion: a genetic system controlling coordinated, random switching of expression of multiple genes. Proceedings of the National Academy of Sciences of the United States of America. April 12 2005;102(15):5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atack JM, Srikhanta YN, Fox KL, et al. A biphasic epigenetic switch controls immunoevasion, virulence and niche adaptation in non-typeable Haemophilus influenzae. Nature communications. 2015;6:7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brockman KL, Jurcisek JA, Atack JM, Srikhanta YN, Jennings MP, Bakaletz LO. ModA2 Phasevarion Switching in Nontypeable Haemophilus influenzae Increases the Severity of Experimental Otitis Media. J Infect Dis. September 01 2016;214(5):817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dagan R, Pelton S, Bakaletz L, Cohen R. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. The Lancet. Infectious diseases. April 2016;16(4):480–492. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Impact of Widespread Introduction of Pneumococcal Conjugate Vaccines on Pneumococcal and Nonpneumococcal Otitis Media. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. September 1 2016;63(5):611–618. [DOI] [PubMed] [Google Scholar]
- 75.Marom T, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions in acute otitis media. Current allergy and asthma reports. December 2012;12(6):551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy TF. Vaccines for Nontypeable Haemophilus influenzae: the Future Is Now. Clinical and vaccine immunology : CVI. May 2015;22(5):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. March 04 2006;367(9512):740–748. [DOI] [PubMed] [Google Scholar]
- 78.Ngo CC, Massa HM, Thornton RB, Cripps AW. Predominant Bacteria Detected from the Middle Ear Fluid of Children Experiencing Otitis Media: A Systematic Review. PloS one. 2016;11(3):e0150949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy TF. Respiratory infections caused by non-typeable Haemophilus influenzae. Current opinion in infectious diseases. April 2003;16(2):129–134. [DOI] [PubMed] [Google Scholar]
- 80.Murphy TF, Faden H, Bakaletz LO, et al. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J. January 2009;28(1):43–48. [DOI] [PubMed] [Google Scholar]
- 81.Swords WE. Nontypeable Haemophilus influenzae biofilms: role in chronic airway infections. Frontiers in cellular and infection microbiology. 2012;2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bakaletz LO. Bacterial biofilms in otitis media: evidence and relevance. Pediatr Infect Dis J. October 2007;26(10 Suppl): S17–19. [DOI] [PubMed] [Google Scholar]
- 83.Novotny LA, Adams LD, Kang DR, et al. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine. December 10 2009;28(1):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Novotny LA, Bakaletz LO. The fourth surface-exposed region of the outer membrane protein P5-homologous adhesin of nontypeable Haemophilus influenzae is an immunodominant but nonprotective decoying epitope. J Immunol. August 15 2003;171(4):1978–1983. [DOI] [PubMed] [Google Scholar]
- 85.Novotny LA, Jurcisek JA, Pichichero ME, Bakaletz LO. Epitope mapping of the outer membrane protein P5-homologous fimbrin adhesin of nontypeable Haemophilus influenzae. Infect Immun. April 2000;68(4):2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Novotny LA, Pichichero ME, Denoel PA, et al. Detection and characterization of pediatric serum antibody to the OMP P5-homologous adhesin of nontypeable Haemophilus influenzae during acute otitis media. Vaccine. October 4 2002;20(29–30):3590–3597. [DOI] [PubMed] [Google Scholar]
- 87.Brandstetter KA, Jurcisek JA, Goodman SD, Bakaletz LO, Das S. Antibodies directed against integration host factor mediate biofilm clearance from Nasopore. The Laryngoscope. November 2013;123(11):2626–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Devaraj A, Justice SS, Bakaletz LO, Goodman SD. DNABII proteins play a central role in UPEC biofilm structure. Molecular microbiology. June 2015;96(6):1119–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freire MO, Devaraj A, Young A, et al. A bacterial-biofilm-induced oral osteolytic infection can be successfully treated by immuno-targeting an extracellular nucleoid-associated protein. Molecular oral microbiology. Feb 2017;32(1):74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gustave JE, Jurcisek JA, McCoy KS, Goodman SD, Bakaletz LO. Targeting bacterial integration host factor to disrupt biofilms associated with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. July 2013;12(4):384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Novotny LA, Amer AO, Brockson ME, Goodman SD, Bakaletz LO. Structural stability of Burkholderia cenocepacia biofilms is reliant on eDNA structure and presence of a bacterial nucleic acid binding protein. PloS one. 2013;8(6):e67629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Novotny LA, Jurcisek JA, Goodman SD, Bakaletz LO. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine. August 2016;10:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rocco CJ, Davey ME, Bakaletz LO, Goodman SD. Natural antigenic differences in the functionally equivalent extracellular DNABII proteins of bacterial biofilms provide a means for targeted biofilm therapeutics. Molecular oral microbiology. March 14 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mishra D, Mishra PK, Dubey V, Nahar M, Dabadghao S, Jain NK. Systemic and mucosal immune response induced by transcutaneous immunization using Hepatitis B surface antigen-loaded modified liposomes. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. April 23 2008;33(4–5):424–433. [DOI] [PubMed] [Google Scholar]
- 95.Lawson LB, Clements JD, Freytag LC. Mucosal immune responses induced by transcutaneous vaccines. Curr Top Microbiol Immunol. November 9 2012;354:19–37. [DOI] [PubMed] [Google Scholar]
- 96.Glenn GM, Scharton-Kersten T, Alving CR. Advances in vaccine delivery: transcutaneous immunisation. Expert Opin Investig Drugs. June 1999;8(6):797–805. [DOI] [PubMed] [Google Scholar]
- 97.Frech SA, Dupont HL, Bourgeois AL, et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet. June 14 2008;371(9629):2019–2025. [DOI] [PubMed] [Google Scholar]
- 98.Russell MW, Ogra PL. Mucosal decisions: tolerance and responsiveness at mucosal surfaces. Immunol Invest. 2010;39(4–5):297–302. [DOI] [PubMed] [Google Scholar]
- 99.Giudice EL, Campbell JD. Needle-free vaccine delivery. Adv Drug Deliv Rev. April 20 2006;58(1):68–89. [DOI] [PubMed] [Google Scholar]
- 100.Warger T, Schild H, Rechtsteiner G. Initiation of adaptive immune responses by transcutaneous immunization. Immunol Lett. March 15 2007;109(1):13–20. [DOI] [PubMed] [Google Scholar]
- 101.Christophers E Some observations on stratum corneum. Current medical research and opinion. 1982;7(Suppl 2):26–28. [PubMed] [Google Scholar]
- 102.Christophers E, Kligman AM. Visualization of the cell layers of the stratum corneum. J Invest Dermatol. June 1964;42:407–409. [DOI] [PubMed] [Google Scholar]
- 103.Norton EB, Lawson LB, Freytag LC, Clements JD. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clinical and vaccine immunology : CVI. April 2011;18(4):546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Novotny LA, Clements JD, Bakaletz LO. Transcutaneous immunization as preventative and therapeutic regimens to protect against experimental otitis media due to nontypeable Haemophilus influenzae. Mucosal Immunol. July 2011;4(4):456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Novotny LA, Clements JD, Bakaletz LO. Kinetic analysis and evaluation of the mechanisms involved in the resolution of experimental nontypeable Haemophilus influenzae-induced otitis media after transcutaneous immunization. Vaccine. July 25 2013;31(34):3417–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Novotny LA, Clements JD, Bakaletz LO. Therapeutic Transcutaneous Immunization with a Band-Aid Vaccine Resolves Experimental Otitis Media. Clinical and vaccine immunology : CVI. August 2015;22(8):867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giebink GS. Otitis media: the chinchilla model. Microb Drug Resist. Spring 1999;5(1):57–72. [DOI] [PubMed] [Google Scholar]
- 108.Bakaletz LO. Developing animal models for polymicrobial diseases. Nat Rev Microbiol. July 2004;2(7):552–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bakaletz LO. Viral potentiation of bacterial superinfection of the respiratory tract. Trends Microbiol. March 1995;3(3):110–114. [DOI] [PubMed] [Google Scholar]
- 110.Bakaletz LO. Chinchilla as a robust, reproducible and polymicrobial model of otitis media and its prevention. Expert Rev Vaccines. August 2009;8(8):1063–1082. [DOI] [PubMed] [Google Scholar]
- 111.Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Experimental dermatology. June 2000;9(3):165–169. [DOI] [PubMed] [Google Scholar]
- 112.Tan G, Xu P, Lawson LB, et al. Hydration effects on skin microstructure as probed by high-resolution cryo-scanning electron microscopy and mechanistic implications to enhanced transcutaneous delivery of biomacromolecules. Journal of pharmaceutical sciences. February 2010;99(2):730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brandtzaeg P Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. American journal of respiratory and critical care medicine. June 15 2011;183(12):1595–1604. [DOI] [PubMed] [Google Scholar]
- 114.Mucida D, Salek-Ardakani S. Regulation of TH17 cells in the mucosal surfaces. The Journal of allergy and clinical immunology. May 2009;123(5):997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Connor RA, Taams LS, Anderton SM. Translational mini-review series on Th17 cells: CD4 T helper cells: functional plasticity and differential sensitivity to regulatory T cell-mediated regulation. Clinical and experimental immunology. February 2010;159(2):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]


